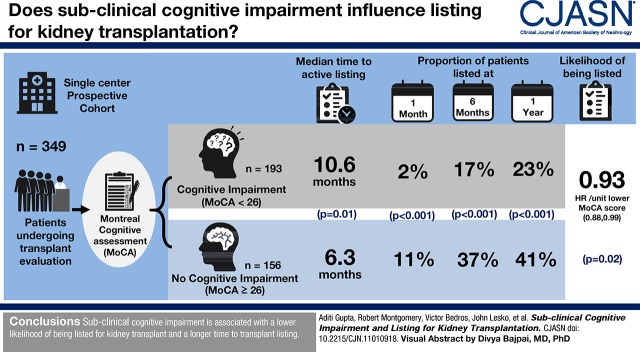
Visual Abstract
Keywords: cognition, eligibility, ESKD, Cohort Studies, Cognitive Dysfunction, coronary artery disease, kidney transplantation, Kaplan-Meier Estimate, Proportional Hazards Models, Smokers, Longitudinal Studies, Cognition, diabetes mellitus, Kidney Diseases, Smoking, Mental Status and Dementia Tests
Abstract
Background and objectives
Cognitive impairment is common in patients with kidney disease and can affect physicians’ perception and/or patients’ ability to complete the pretransplant evaluation. We examined whether cognitive impairment influences the likelihood for transplant listing and whether patients with cognitive impairment take longer to be listed.
Design, setting, participants, & measurements
We conducted a single-center longitudinal cohort study. Patients presenting for their index kidney transplant evaluation were screened for cognitive impairment using the Montreal Cognitive Assessment. A score <26 indicated cognitive impairment. The transplant selection committee was blinded to the scores. Kaplan–Meier analysis assessed time to active listing by level of cognition. A Cox proportional hazards model that included age, sex, race/ethnicity, smoking, coronary artery disease, and diabetes was constructed to evaluate the association between Montreal Cognitive Assessment score and listing for transplant.
Results
In total, 349 patients who underwent Montreal Cognitive Assessment testing at their initial visit were included in the analysis. Patients with cognitive impairment were more likely to be older, black, and smokers. The time to listing in patients with cognitive impairment was longer than the time to listing in those with no cognitive impairment (median time, 10.6 versus 6.3 months; log rank test P=0.01). Cognitive impairment was independently associated with a lower likelihood of being listed for transplant (hazard ratio, 0.93 per unit lower Montreal Cognitive Assessment score; 95% confidence interval, 0.88 to 0.99; P=0.02). A lower proportion of patients with cognitive impairment were listed compared with patients without cognitive impairment at 1 month (2% versus 11%), 6 months (17% versus 37%), and 1 year (23% versus 41%), (P<0.001 for all).
Conclusions
Cognitive impairment is associated with a lower likelihood of being listed for kidney transplant, and is associated with longer time to transplant listing.
Introduction
Cognitive impairment is common in patients with ESKD on dialysis and patients with a kidney transplant (1–3). Cognitive impairment negatively affects activities of daily living, quality of life, medical adherence, health care costs, morbidity, and mortality (1,4–9). Kidney transplantation is the treatment of choice for ESKD, and it is associated with better survival and quality of life (10). To be successfully listed for kidney transplantation, patients must undergo a thorough evaluation process, including several tests and clinic visits. Post-transplant, the care of a transplant recipient is complex and involves multiple clinic visits; titration of immunosuppression; frequent laboratory tests; dietary restrictions; and medications, vaccines, and education for prevention of infection. Cognitive impairment can potentially affect the patients’ ability to complete pretransplant evaluation as well as comprehend and comply with post-transplant care. Cognitive impairment is indeed associated with nonadherence to immunosuppression and adverse outcomes (11,12).
Several factors, such as age, women, education, non-English–speaking background, minority race, socioeconomic status, obesity, and certain medical diagnoses such as diabetes have been known to influence transplant eligibility (13–15). Pretransplant cognition can be perceived as a barrier to transplantation due to concern for adverse outcomes post-transplant. However, no data are available on the association of kidney transplant eligibility and cognition. With the high prevalence of cognitive impairment in ESKD and the unprecedented increase in CKD and ESKD (16,17), it is imperative to examine the effect of cognitive impairment on transplant eligibility. Early detection of cognitive impairment can timely identify patients who might need additional support or more detailed instructions, and it may shorten the time to listing, thus facilitating better access to transplantation for many patients with ESKD. In this study, we examined how cognitive impairment is associated with the likelihood of being listed and time to listing for kidney transplant.
Materials and Methods
Study Design
We conducted a single-center longitudinal cohort study. We screened pretransplant patients with ESKD for cognitive impairment using the Montreal Cognitive Assessment (MoCA) at the initial clinic visit for evaluation for kidney transplant. Patients were followed longitudinally for transplant eligibility. This was performed as a quality improvement project. It was reviewed by the institutional review board and deemed to not constitute human subjects research; hence, it was exempt from institutional review board approval.
Study Population
Adults evaluated for kidney transplantation between February 2015 and March 2018 were screened for cognitive impairment using the MoCA. Patients were excluded if (1) they had any hearing or visual impairment that would prohibit them from taking the MoCA; (2) they were unable to read, write, speak, or understand English (because the MoCA was administered in English); (3) they already had a chart diagnosis of dementia or mental retardation; or (4) they had uncontrolled psychosis or active seizure disorder.
Data were recorded in the Research Electronic Data Capture, a web-based electronic data capture tool hosted on a secure, password-protected, Health Insurance Portability and Accountability Act-compliant server (18). Baseline demographics, medical history, dates for transplant evaluation, transplant selection committee discussion, and kidney transplantation were obtained from the patients’ electronic medical records.
Standardized Screening for Cognitive Impairment
All eligible patients were screened for cognitive impairment using the MoCA (19). The MoCA is a clinic-based tool that has been validated in ESKD (20). MoCA scoring ranges from zero to 30 (maximum score of 30), with higher scores indicating better cognition. The test samples from seven domains of cognition (visuospatial/executive, naming, memory [delayed recall], attention, language, abstraction, and orientation) and takes <10 minutes to complete. We used the original English version 7.1 (http://www.mocatest.org/papertests/moca-test-full/). We chose the MoCA over the more commonly used Mini-Mental State Examination (MMSE), because the MoCA has a high sensitivity for detecting mild cognitive impairment (90% compared with 18% for the MMSE) and a better ability to detect vascular dementia (21,22). A cutoff score of 26 was recommended for detection of mild cognitive impairment in the original validation study on the MoCA (19). Other studies have suggested a lower cutoff of 18 (22). We used a cutoff score of ≥26 to describe normal cognition and a cutoff score of ≤18 to describe severe cognitive impairment, and we used the MoCA as a continuous variable for the Cox proportional hazards model. The MoCA scores were adjusted for education, with allocation of one point for ≤12 years of education (19).
The MoCA assessments were performed during the patients’ kidney transplant evaluation visit. The MoCA was administered in a private room to minimize distraction and assure confidentiality. The test was administered by medical assistants at the transplant clinic who underwent training that included a detailed review of online instructions on the MoCA and practice sessions on mock patients. The transplant team and the transplant selection committee involved in the decision to list patients for transplant were blinded to MoCA scores.
Transplant Evaluation Process
After the initial visit for transplant evaluation, patients are discussed in the weekly transplant selection committee meeting, and a decision is made regarding listing for kidney transplant. The committee may make one of the following decisions: (1) list as active for transplant, (2) list as inactive for transplant, (3) defer for listing for patients requiring additional testing, and (4) ineligible for the patients with too many barriers or uncorrectable conditions for listing. After they are listed, patients can be delisted in cases of change in medical, social, or financial situations. After the initial evaluation, patients are seen in the clinic periodically. If they are not listed in the initial committee meeting, they may be discussed again in the selection committee, and their status may be changed from deferred to listed. Thus, patient status may change over time depending on changes in medical, social, or financial status.
Statistical Analyses
For descriptive statistics, we divided our cohort into three groups on the basis of their MoCA scores: MoCA score ≤18 (severe cognitive impairment), MoCA score 19–25 (mild-moderate cognitive impairment), and MoCA score ≥26 (normal cognition). Baseline characteristics were compared in the three groups using one-way ANOVA when the measure was a continuous variable (e.g., age) or a chi-squared test when the measure was a categorical variable (e.g., sex). If the assumptions of a parametric test were violated, a nonparametric test was used (the Kurskal–Wallis test for time on dialysis [Table 1] and the Mann–Whitney U test). Coronary artery disease was defined as history of percutaneous coronary intervention or coronary artery bypass graft procedure, diabetes was defined as current or past use of insulin or oral hypoglycemic agent, and smoking was defined as current or past history of smoking. The “other” causes of ESKD included interstitial nephritis, drug toxicity (including lithium toxicity), nephrectomy, congenital abnormalities, and reflux disease.
Table 1.
Baseline characteristics compared across groups stratified by Montreal Cognitive Assessment scores
| Patient Characteristics | All Patients, n=349 | Severe Cognitive Impairment: MoCA≤18, n=21 | Mild-Moderate Cognitive Impairment: MoCA=19–25, n=172 | No Cognitive Impairment: MoCA≥26, n=156 |
|---|---|---|---|---|
| Age, yra | 54 (14) | 62 (11) | 55 (13) | 51 (14) |
| Sex | ||||
| Men | 147 (42) | 8 (5) | 67 (46) | 72 (49) |
| Women | 202 (58) | 13 (6) | 105 (52) | 84 (42) |
| Raceb | ||||
| White | 254 (73) | 11 (4) | 116 (46) | 127 (50) |
| Black | 74 (21) | 7 (9) | 44 (59) | 23 (31) |
| Other | 21 (6) | 3 (14) | 12 (57) | 6 (29) |
| Education level, yra | ||||
| ≥15 | 93 (27) | 1 (1) | 37 (40) | 55 (59) |
| 13–15 | 120 (35) | 3 (2) | 61 (51) | 56 (47) |
| ≤12 | 135 (39) | 16 (13) | 74 (54) | 45 (33) |
| BMI, kg/m2 | ||||
| ≥30 | 163 (47) | 9 (6) | 87 (53) | 67 (41) |
| <30 | 186 (53) | 12 (6) | 85 (46) | 89 (48) |
| Cause of ESKD | ||||
| Diabetes | 146 (42) | 5 (3) | 81 (55) | 60 (41) |
| Hypertension | 127 (36) | 9 (7) | 67 (53) | 51 (40) |
| GN | 18 (5) | 2 (11) | 7 (39) | 9 (50) |
| ADPKD | 37 (10) | 2 (5) | 10 (27) | 25 (68) |
| Unknown | 15 (4) | 1 (7) | 7 (47) | 7 (47) |
| Other | 98 (28) | 4 (4) | 52 (53) | 42 (43) |
| Coronary artery disease | 39 (11) | 3 (8) | 23(59) | 13 (33) |
| H/o smokinga | 162 (46) | 16 (10) | 85 (52) | 61 (38) |
| H/o stroke | 19 (5) | 1 (5) | 8 (42) | 10 (53) |
| Time on dialysis, yr | 3.2 (4.4) | 3.5 (4.1) | 2.8 (4.0) | 3.5 (5.0) |
MoCA, Montreal Cognitive Assessment; BMI, body mass index; ADPKD, autosomal dominant polycystic kidney disease; H/o, history of.
P<0.001.
P<0.05.
We fit several Kaplan–Meier incidence [1−S(t)] plots (referred to as Kaplan–Meier plots from now on) to further examine differences on the basis of cognitive impairment specifically for time to active listing and time to be declared ineligible or removed from the waitlist. We also created Kaplan–Meier curves for different age groups within the same MoCA score range. We used a log rank test to evaluate the difference between the Kaplan–Meier curves. We used the Cox proportional hazards model to assess the covariates affecting listing for kidney transplant. We used MoCA scores as a continuous variable for the Cox model. The covariates age, sex, race, coronary artery disease, diabetes, and smoking were selected on the basis of current knowledge and biologic plausibility. For sensitivity analysis, using MoCA as a categorical variable, we analyzed time to listing in patients with and without cognitive impairment as well as in patients with severe cognitive impairment, mild-moderate cognitive impairment, and no cognitive impairment. We evaluated the proportional hazards assumption of the model by graphical evaluation of the residuals and testing whether the Schoenfeld residuals were constant over time for each covariate, and the proportional hazards assumption was not violated. A P value of <0.05 was considered significant. Because the patients included in the analysis can have several possible outcomes (active listing, inactive listing, ineligible, removed from the list, and transplanted) and can change status during the follow-up, we calculated the proportion of patients in these categories at the end of 1 month, 6 months, and 1 year after their initial evaluation. The only missing data were the education level for one patient in the patient group with MoCA score ≤18. We excluded this patient from the specific statistic in Table 1 but included him in the Cox model. Two patients lost to follow-up after their transplant were included in the analysis.
Results
Three hundred forty-nine patients with MoCA assessment during their first evaluation visit were included in the analysis (Supplemental Figure 1). There was a high prevalence of cognitive impairment, with 193 patients (55%) with MoCA<26. Table 1 describes the baseline characteristic of the patients stratified into three groups by their MoCA scores. The patients with no cognitive impairment, mild-moderate cognitive impairment, and severe cognitive impairment differed in age, race, and history of smoking. Patients with cognitive impairment were older, and a higher proportion was nonwhite. There were no differences detected between the three groups for the time on dialysis or the cause for ESKD. However, among patients with ESKD secondary to polycystic kidney disease, a higher proportion of patients had normal cognition (MoCA≥26; P=0.01). Because a higher proportion of black patients (69% compared with 50% white patients) had cognitive impairment (P<0.01), we further analyzed this subgroup to determine whether a difference in age is leading to this difference in MoCA score (Supplemental Figure 2, Supplemental Table 1). Black patients were in fact younger than white patients in our cohort (P=0.02) and had lower MoCA scores compared to white patients (Table 1).
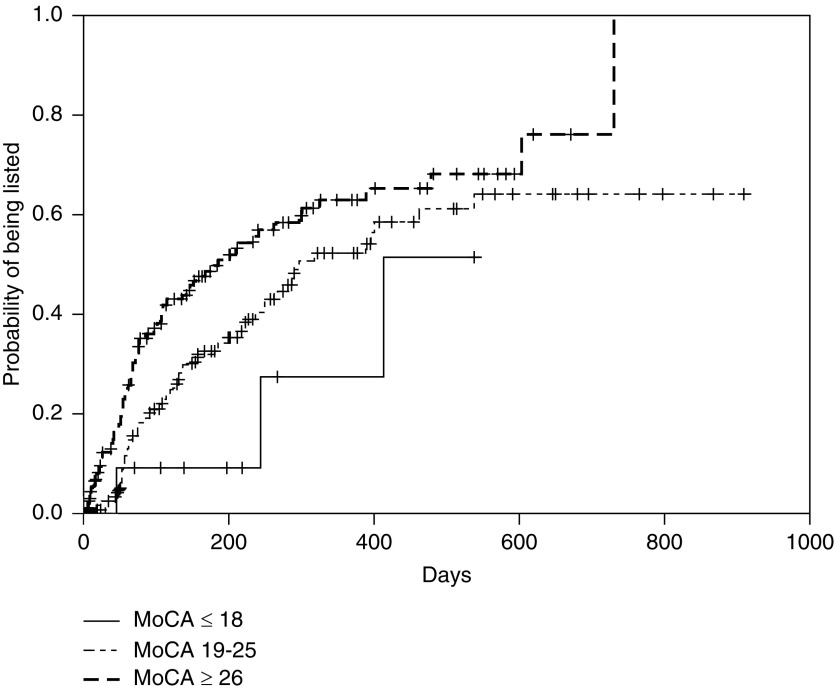
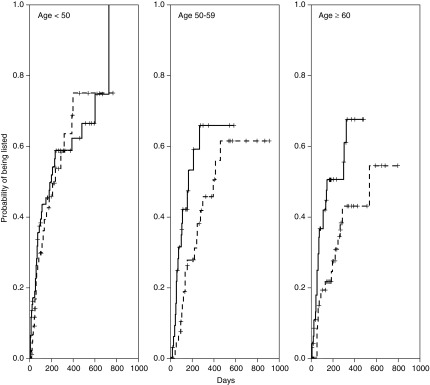
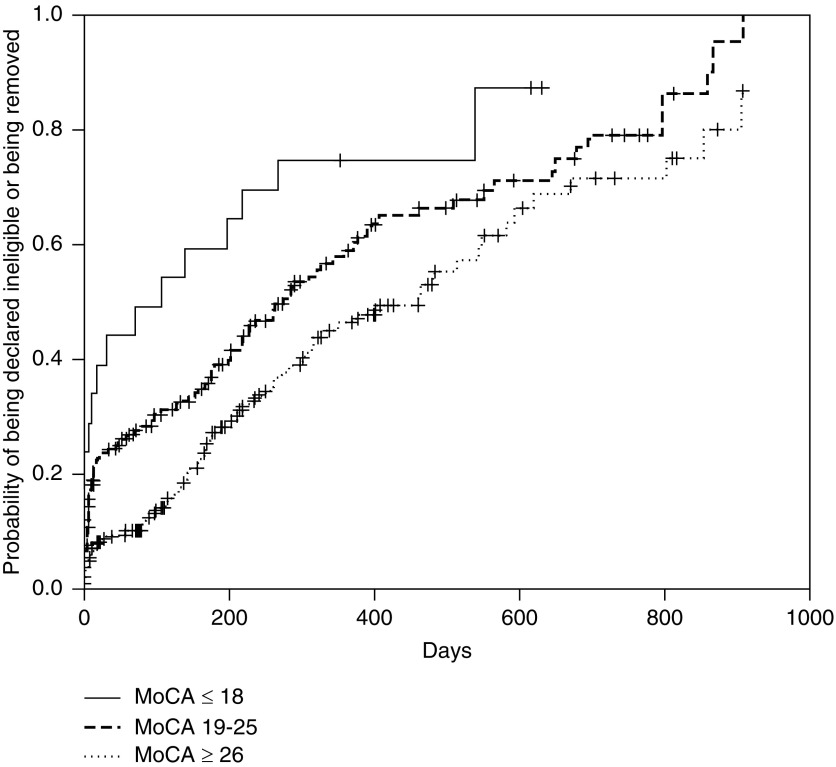
In the Kaplan–Meier analysis, patients with higher MoCA scores were listed earlier, with clear delineation between the three subgroups of no cognitive impairment, mild-moderate cognitive impairment, and severe cognitive impairment (Figure 1). When divided further by age and MoCA score, patients with cognitive impairment took longer for active listing than those with no cognitive impairment in that age group, except in subjects <50 years old (Figure 2). The median time to active listing was higher for those with cognitive impairment (10.6 months; interquartile range, 4.3 to >17.9 months) compared with those with no cognitive impairment (6.3 months; interquartile range, 2–20.1 months). Conversely, patients with cognitive impairment were declared ineligible sooner (8.6 months) compared with those with no cognitive impairment (15.4 months) (Figure 3).
Figure 1.
Time to listing was more for patients with lower Montreal Cognitive Assessment (MoCA) scores. Kaplan–Meier incidence [1-S(t)] plots for time to active listing by MoCA score. The three categories were MoCA scores ≤18, 19–25, and ≥26. Log rank test P=0.01.
Figure 2.
Older patients (>50 years of age) with cognitive impairment took longer to get listed compared to patients without cognitive impairment. Kaplan-Meier incidence [1-S(t)] plots for time to active listing stratified by age and MoCA score. Solid lines represent no cognitive impairment and dotted lines represent cognitive impairment. For patients 50–59 years, log-rank test P=0.05; for patients ≥60 years, P=0.01, and for <50 years, P=0.7.
Figure 3.
Patients with lower Montreal Cognitive Assessment (MoCA) scores were declared ineligible sooner than those with higher MoCA scores. Kaplan–Meier incidence [1-S(t)] plots for time to being declared ineligible or removed from the waitlist by Montreal Cognitive Assessment score. The three categories were MoCA scores ≤18, 19–25, and ≥26. Log rank test P=0.003.
In the Cox proportional hazards model, the significant covariates associated with time to listing were MoCA score, sex, race, smoking, and diabetes (Table 2). A lower MoCA score was associated with a lower chance of active listing (i.e., the likelihood of being listed at any time point was lower with a lower MoCA score; unadjusted hazard ratio, 0.91; 95% confidence interval, 0.87 to 0.96; P<0.001; adjusted hazard ratio, 0.93; 95% confidence interval, 0.88 to 0.99; P=0.02). The likelihood of listing was lower with older age and women. Supplemental Table 2 presents the results when MoCA was used as a categorical variable instead of a continuous variable.
Table 2.
Cox proportional hazards model for time to active listing
| Covariates | β Coefficient | Hazard Ratio/Exp (β Coefficient) | 95% Confidence Interval | P Value |
|---|---|---|---|---|
| MoCA scorea | 0.07 | 1.07 | 1.01 to 1.13 | 0.02b |
| Agec | −0.01 | 0.98 | 0.97 to 0.99 | 0.04b |
| Sex (women) | −0.48 | 0.62 | 0.43 to 0.89 | 0.01b |
| Race (black) | −0.78 | 0.46 | 0.27 to 0.78 | 0.004d |
| Race (other) | 0.10 | 1.11 | 0.52 to 2.35 | 0.78 |
| Coronary artery disease | −0.45 | 0.64 | 0.29 to 1.39 | 0.26 |
| Diabetes | −0.45 | 0.64 | 0.43 to 0.94 | 0.02b |
| Smoking | −0.62 | 0.54 | 0.37 to 0.79 | 0.002d |
References for comparisons used were men, white race/ethnicity, no known coronary artery disease, no diabetes, and no history of smoking. Note that a “hazard” of being listed is a favorable outcome. Exp, exponential; MOCA, Montreal Cognitive Assessment.
For a one-point higher MoCA score.
P<0.05.
For a 1-year older age.
P<0.01.
Table 3 indicates the status of patients at 1 month, 6 months, and 1 year after the first pretransplant evaluation. By the end of 1 year, 23.3% of patients with cognitive impairment were listed for transplant or transplanted (compared with 41% with no cognitive impairment), whereas 43% of patients with cognitive impairment were declared ineligible or removed from the waitlist (compared with 32% with no cognitive impairment). There was no difference between patients who were declared ineligible within a month and those who were declared ineligible after a month, except for a shorter time on dialysis for patients with mild-moderate cognitive impairment who were declared ineligible within a month (P<0.01) (Supplemental Table 3). Lack of social support did not delay listing. Of the 114 patients who were not listed within 1 month, only one patient lacked adequate social support. Supplemental Tables 4 and 5 list the reasons for ineligibility or removal from the waitlist.
Table 3.
Proportion of patients with (Montreal Cognitive Assessment score <26) and without (Montreal Cognitive Assessment score ≥26) cognitive impairment active on the waitlist or transplanted, declared ineligible/removed from the list, inactive on the waitlist, and deferred for decision regarding listing or in evaluation after discussion in the selection committee at 1 month, 6 months, and 1 year after their initial evaluation
| Time from First Evaluation | Total Sample, n=349 | Cognitive Impairment: MoCA<26, n=193 | No Cognitive Impairment: MoCA≥26, n=156 | P Value |
|---|---|---|---|---|
| Listed active or transplanted | ||||
| 1 mo | 20 (6) | 3 (2) | 17 (11) | <0.001a |
| 6 mo | 90 (26) | 32 (17) | 58 (37) | <0.001a |
| 1 yr | 109 (31) | 45 (23) | 64 (41) | <0.001a |
| Ineligible/removed | ||||
| 1 mo | 61 (18) | 48 (25) | 13 (8) | <0.001a |
| 6 mo | 100 (29) | 68 (35) | 32 (21.2) | 0.003b |
| 1 yr | 137 (39) | 88 (46) | 49 (32) | <0.01b |
| Listed inactive | ||||
| 1 mo | 2 (1) | 0 | 2 (1) | 0.38 |
| 6 mo | 15 (4) | 7 (4) | 8 (5) | 0.67 |
| 1 yr | 19 (5) | 9 (5) | 10 (6) | 0.63 |
| Deferred or in evaluation | ||||
| 1 mo | 265 (76) | 142 (74) | 123 (79) | 0.31 |
| 6 mo | 143 (41) | 86 (45) | 57 (37) | 0.16 |
| 1 yr | 83 (24) | 51 (26) | 32 (21) | 0.25 |
MoCA, Montreal Cognitive Assessment.
P<0.001.
P<0.01.
Discussion
This study is the first to describe the association of cognitive impairment with transplant eligibility. Cognitive impairment in pretransplant ESKD is common and influences kidney transplant eligibility. Patients with cognitive impairment take longer to get listed. Even within the same age group, the likelihood of active listing was lower with cognitive impairment. Cognitive impairment is associated with a shorter time to be declared ineligible or delisted from the transplant waitlist.
Although patients referred for transplant are usually healthier (23) than the average dialysis population, the prevalence of cognitive impairment in our study was consistent with that in other studies evaluating cognition in ESKD (1,2). Patients who were older, were nonwhite, had lower education, or had history of smoking were more likely to have cognitive impairment (Table 1). Similar to the general population (24), black patients in our cohort had cognitive impairment at a younger age. Black patients have higher prevalence (25,26) and earlier onset (27) of hypertension, diabetes, and heart disease, all of which are risk factors for cognitive impairment (28). Similar to other studies in ESKD (29), we did not find an association between time on dialysis and cognitive impairment. However, unlike some other studies, we did not see an association between cause of ESKD and cognitive impairment (29), possibly because of differences in the patient population referred for transplantation.
Prior studies have determined an association of pretransplant variables, such as age, race, medical comorbidities, and socioeconomic status, with pretransplant eligibility (13,14). We found similar associations of age, race/ethnicity, and sex with the likelihood of listing. Our study made the novel observation that patients with cognitive impairment had a lower likelihood of being listed. For two patients with the same demographics, a one-point lower MoCA score resulted in a 7% lower instantaneous chance of being listed. When listed, patients with cognitive impairment required a longer time to get listed. As shown in Table 3, very few patients with cognitive impairment were listed within 1 month of their evaluation. Even after 1 year, the proportion of patients who had cognitive impairment and were listed was almost one half of the proportion of patients who had no cognitive impairment. Patients with cognitive impairment also had a higher likelihood being declared ineligible or removed from the waitlist. Cognitive impairment, however, was not associated with time to listing in patients younger than 50 years old (Figure 2). This may be due to more social support, fewer comorbid illnesses, and physicians’ bias toward listing younger patients.
The prevalence of cognitive impairment increases with age. Despite this interaction between older age and cognition and between older age and listing, in our Cox model, we found that cognitive impairment was more strongly associated with transplant eligibility than age (Table 2). Medical conditions, including diabetes, hypertension, and heart disease, have been associated with cognitive impairment. Perhaps cognitive impairment is the resultant effect of all comorbid illnesses that may be difficult to quantify otherwise.
Cognitive impairment is highly prevalent in pretransplant patients with ESKD and kidney transplant recipients (3). Because cognitive impairment is associated with medical nonadherence, a concern for post-transplant outcomes in patients with cognitive impairment seems valid. However, cognition improves post-transplant (30–33), and pretransplant cognition may not be a true reflection of post-transplant cognition or medication adherence. Moreover, with adequate social support, even patients with known mental retardation can be successfully transplanted (34). Cognitive impairment alone should not be a criterion for transplant eligibility. Additional studies should investigate whether there is a cutoff in the MoCA score where post-transplant outcomes are particularly poor or whether there is a group of patients who continue to have significant cognitive impairment affecting post-transplant outcomes. Cognitive impairment was associated with a longer time to listing. The major cause for delay in listing is usually the inability to complete testing required for the transplantation. Perhaps a better approach may be to identify patients with cognitive impairment who might benefit from additional interventions to facilitate transplant evaluation in a timely manner. These interventions may include timely search for support, written or more detailed instructions, streamlining the transplant evaluation process, and assisting patients in completing the evaluation.
Our strengths include blinding of the transplant selection committee to the MoCA scores so that decisions about listing were not on the basis of the MoCA results. There are currently no data to suggest altering the decision to transplant on the basis of pretransplant cognition. We used a clinic-based screening test to assess cognition. The MoCA has a high sensitivity and specificity for detecting cognitive impairment, and it is practical to use in the transplant clinic, where time constraints may not permit detailed neuropsychologic testing. Using a screening test for cognitive impairment, patients can be further referred for a full neuropsychologic assessment. Because of controversy regarding the optimal cutoff of MoCA score in diagnosis of cognitive impairments and the problems associated with categorizing a continuous variable in general, we used MoCA score as a continuous variable for the Cox proportional hazard model (35).
Our study has limitations. First, although the MoCA is a valid screening test for assessment of cognition, it may not be as robust as a detailed assessment of cognition with multiple standard neuropsychologic tests. Second, we obtained the medical history through chart review, which has inherent limitations. However, because patients underwent a detailed transplant evaluation, we believe that the information in the chart is detailed and accurate. Third, this was a single-center study. Although the results may not be completely generalizable, we had a fair demographic representation of white and black patients. We did not control for competing risks, such as death. However, the number of deaths in the cohort was small (n=5) (Supplemental Tables 2 and 3).
In conclusion, cognitive impairment is common in patients presenting for transplant evaluation. Increasing age, history of smoking, and nonwhite race/ethnicity are associated with pretransplant cognitive impairment. Pretransplant cognition is associated with transplant eligibility. Cognitive impairment is associated with a lower likelihood of being listed for kidney transplant, and it is associated with a longer time to listing. Additional understanding of the reasons why cognitive impairment influences time to listing may improve transplant eligibility.
Disclosures
M.J.S. reports “other support” from Akebia outside the submitted work. J.M.B. reports clinical trial support to the institution (Lilly, Avid Radiopharmaceuticals, Toyama Chemical Company, Merck, Biogen, AbbVie, vTv Therapeutics, Janssen, Roche, and Astra-Zeneca); personal fees from Stage 2 Innovations for consulting; personal fees from Astra-Zeneca for honoraria for speaking at a symposium; and personal fees from Lilly for serving on a speaker’s bureau in 2016 and 2017 outside the submitted work. A.G., R.N.M., V.B., J.L., J.D.M., S.C., D.D., J.A.K., W.M.B., T.S.T., A.I., P.B., T.M.S., and D.M.C. have nothing to disclose.
Supplementary Material
Acknowledgments
We thank the transplant clinic staff (Adrienne Adams, Teya Bradley, Brandy Allen, Desiree Mack, Ariane Stanley, and Heather Miller) for performing the Montreal Cognitive Assessment and keeping the scores confidential.
This work was supported by National Institutes of Health (NIH) Grant K23-AG055666 (to A.G.), NIH Clinical and Translational Science Award Grant UL1 TR000001 (formerly UL1RR033179; to the University of Kansas Medical Center), the University of Kansas Medical Center Department of Medicine (A.G.), and NIH Grants P30AG035982 (to the University of Kansas Alzheimer’s Disease Center) and P30DK106912 (to the Kidney Institute). J.M.B. reports support from NIH Grants R01AG060157, R01AG052954, R01AG053312, R01AG049740, R01AG043962, and P30 AG035982.
This work was presented at the American Transplant Congress, Seattle, WA, June 2–6, 2018.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11010918/-/DCSupplemental.
Supplemental Figure 1. Flow diagram of paticipants included in the analysis.
Supplemental Figure 2. Kaplan–Meier incidence [1−S(t)] plots for time to active listing in black patients by age.
Supplemental Table 1. Mean age of black patients in the three categories divided by MoCA scores.
Supplemental Table 2. Cox proportional hazards model for time to active listing using MoCA as a categorical variable.
Supplemental Table 3. Comparison of characteristics of patients with severe and mild-moderate cognitive impairment who were deemed ineligible within a month versus later.
Supplemental Table 4. Reasons for ineligibility for listing (before being listed active or inactive; n=168).
Supplemental Table 5. Reasons for delisting (i.e., declared ineligible after being listed previously; n=14).
References
- 1.Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson SL, Li S, Smith GE, Hochhalter AK, Collins AJ, Kane RL: Cognitive impairment in hemodialysis patients is common. Neurology 67: 216–223, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Drew DA, Weiner DE, Tighiouart H, Duncan S, Gupta A, Scott T, Sarnak MJ: Cognitive decline and its risk factors in prevalent hemodialysis patients. Am J Kidney Dis 69: 780–787, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Mahnken JD, Johnson DK, Thomas TS, Subramaniam D, Polshak T, Gani I, John Chen G, Burns JM, Sarnak MJ: Prevalence and correlates of cognitive impairment in kidney transplant recipients. BMC Nephrol 18: 158, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bremer BA, Wert KM, Durica AL, Weaver A: Neuropsychological, physical, and psychosocial functioning of individuals with end-stage renal disease. Ann Behav Med 19: 348–352, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Vargas PA, Tong A, Phoon RK, Chadban SJ, Shen Y, Craig JC: Knowledge deficit of patients with stage 1-4 CKD: A focus group study. Nephrology (Carlton) 19: 234–243, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Drew DA, Weiner DE, Tighiouart H, Scott T, Lou K, Kantor A, Fan L, Strom JA, Singh AK, Sarnak MJ: Cognitive function and all-cause mortality in maintenance hemodialysis patients. Am J Kidney Dis 65: 303–311, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patzer RE, Serper M, Reese PP, Przytula K, Koval R, Ladner DP, Levitsky JM, Abecassis MM, Wolf MS: Medication understanding, non-adherence, and clinical outcomes among adult kidney transplant recipients. Clin Transplant 30: 1294–1305, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yost KJ, DeWalt DA, Lindquist LA, Hahn EA: The association between health literacy and indicators of cognitive impairment in a diverse sample of primary care patients. Patient Educ Couns 93: 319–326, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sehgal AR, Grey SF, DeOreo PB, Whitehouse PJ: Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis 30: 41–49, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Michelon T, Dominguez V, Losekan A, Messias A, Bruno R, Bittar A, Keitel E, Santos A, Goldani J, Bianchinni J, Garcia C, Neumann J, Garcia V: Kidney graft failure due to noncompliance. Transplant Proc 31: 3031–3032, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Cheng CY, Lin BY, Chang KH, Shu KH, Wu MJ: Awareness of memory impairment increases the adherence to immunosuppressants in kidney transplant recipients. Transplant Proc 44: 746–748, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Myaskovsky L, Almario Doebler D, Posluszny DM, Dew MA, Unruh M, Fried LF, Switzer GE, Kim S, Chang CC, Ramkumar M, Shapiro R: Perceived discrimination predicts longer time to be accepted for kidney transplant. Transplantation 93: 423–429, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schold JD, Gregg JA, Harman JS, Hall AG, Patton PR, Meier-Kriesche HU: Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol 6: 1760–1767, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Gaston RS, Danovitch GM, Adams PL, Wynn JJ, Merion RM, Deierhoi MH, Metzger RA, Cecka JM, Harmon WE, Leichtman AB, Spital A, Blumberg E, Herzog CA, Wolfe RA, Tyan DB, Roberts J, Rohrer R, Port FK, Delmonico FL: The report of a national conference on the wait list for kidney transplantation. Am J Transplant 3: 775–785, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, Bragg-Gresham J, Chen JT, Cope E, Gipson D, He K, Herman W, Heung M, Hirth RA, Jacobsen SS, Kalantar-Zadeh K, Kovesdy CP, Leichtman AB, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, O'Hare AM, Pisoni R, Plattner B, Port FK, Rao P, Rhee CM, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, Eggers PW, Agodoa LY, Abbott KC: US Renal Data System 2014 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 66: S1–S305, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbertson DT, Liu J, Xue JL, Louis TA, Solid CA, Ebben JP, Collins AJ: Projecting the number of patients with end-stage renal disease in the United States to the year 2015. J Am Soc Nephrol 16: 3736–3741, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG: Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42: 377–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H: The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: 695–699, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Tiffin-Richards FE, Costa AS, Holschbach B, Frank RD, Vassiliadou A, Krüger T, Kuckuck K, Gross T, Eitner F, Floege J, Schulz JB, Reetz K: The Montreal Cognitive Assessment (MoCA) - a sensitive screening instrument for detecting cognitive impairment in chronic hemodialysis patients. PLoS One 9: e106700, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai CF, Lee WJ, Wang SJ, Shia BC, Nasreddine Z, Fuh JL: Psychometrics of the Montreal Cognitive Assessment (MoCA) and its subscales: Validation of the Taiwanese version of the MoCA and an item response theory analysis. Int Psychogeriatr 24: 651–658, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Trzepacz PT, Hochstetler H, Wang S, Walker B, Saykin AJ; Alzheimer’s Disease Neuroimaging Initiative : Relationship between the montreal cognitive assessment and mini-mental state examination for assessment of mild cognitive impairment in older adults. BMC Geriatr 15: 107, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thamer M, Hwang W, Fink NE, Sadler JH, Bass EB, Levey AS, Brookmeyer R, Powe NR; CHOICE Study. Choices for Healthy Outcomes in Caring for ESRD : U.S. nephrologists’ attitudes towards renal transplantation: Results from a national survey. Transplantation 71: 281–288, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Lopez OL, Jagust WJ, Dulberg C, Becker JT, DeKosky ST, Fitzpatrick A, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, Kuller LH: Risk factors for mild cognitive impairment in the cardiovascular health study cognition study: Part 2. Arch Neurol 60: 1394–1399, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ: Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988-1994 and 1999-2004. Hypertension 52: 818–827, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Ostchega Y, Dillon CF, Hughes JP, Carroll M, Yoon S: Trends in hypertension prevalence, awareness, treatment, and control in older U.S. adults: Data from the National Health and Nutrition Examination Survey 1988 to 2004. J Am Geriatr Soc 55: 1056–1065, 2007 [DOI] [PubMed] [Google Scholar]
- 27.James SA: John Henryism and the health of African-Americans. Cult Med Psychiatry 18: 163–182, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, Deal JA, McKhann GM, Mosley TH, Sharrett AR, Schneider ALC, Windham BG, Wruck LM, Knopman DS: Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol 74: 1246–1254, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurella M, Mapes DL, Port FK, Chertow GM: Correlates and outcomes of dementia among dialysis patients: The dialysis outcomes and practice patterns study. Nephrol Dial Transplant 21: 2543–2548, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Radić J, Ljutić D, Radić M, Kovačić V, Dodig-Ćurković K, Šain M: Kidney transplantation improves cognitive and psychomotor functions in adult hemodialysis patients. Am J Nephrol 34: 399–406, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Griva K, Thompson D, Jayasena D, Davenport A, Harrison M, Newman SP: Cognitive functioning pre- to post-kidney transplantation--a prospective study. Nephrol Dial Transplant 21: 3275–3282, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Kramer L, Madl C, Stockenhuber F, Yeganehfar W, Eisenhuber E, Derfler K, Lenz K, Schneider B, Grimm G: Beneficial effect of renal transplantation on cognitive brain function. Kidney Int 49: 833–838, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Gupta A, Lepping RJ, Yu AS, Perea RD, Honea RA, Johnson DK, Brooks WM, Burns JM: Cognitive function and white matter changes associated with renal transplantation. Am J Nephrol 43: 50–57, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benedetti E, Asolati M, Dunn T, Walczak DA, Papp P, Bartholomew AM, Smith Y, Washington AW, Pollak R: Kidney transplantation in recipients with mental retardation: Clinical results in a single-center experience. Am J Kidney Dis 31: 509–512, 1998 [DOI] [PubMed] [Google Scholar]
- 35.van Walraven C, Hart RG: Leave 'em alone—why continuous variables should be analyzed as such. Neuroepidemiology 30: 138–139, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.