Abstract
Conservative kidney management is increasingly accepted as an appropriate treatment option for patients with eGFR category 5 CKD who are unlikely to benefit from dialysis and/or who choose a nondialysis care option. However, there remains great variation in the delivery of their care. As part of the development of a conservative kidney management pathway that is undergoing evaluation, a set of recommendations specific to conservative kidney management for managing the complications of CKD and common symptoms was developed. These recommendations focus on the patient’s values and preferences and aim to optimize comfort and quality of life. Explanations for the interventions are provided to support the shared decision-making process between health care professionals, patients, and family members. The recommendations generally emphasize the preservation of function (cognitive, physical, and kidney) and address symptom burden, acknowledging that management priorities can change over time. The recommendations should be used in conjunction with other key elements of conservative kidney management, including clear communication and shared decision making for choosing conservative kidney management, advance care planning, and psychosocial support. Although there are limitations to the existing evidence specific to conservative kidney management, these recommendations are intended as a starting point toward reaching consensus and generating further evidence.
Keywords: Advance Care Planning, chronic kidney disease, Cognition, Consensus, Conservative Kidney Management, Conservative Treatment, Decision Making, end stage kidney disease, glomerular filtration rate, Humans, Palliative Care, quality of life, Recommendations, renal dialysis, Renal Insufficiency, Chronic, Symptoms
Introduction
Conservative kidney management is a treatment option for patients with eGFR category 5 CKD who, through shared decision making and holistic patient-centered care, emphasizes quality of life (QOL), active symptom management, and advance care planning. It includes interventions to delay the progression of kidney disease, but it does not include dialysis (1). Although conservative kidney management is increasingly recognized as an appropriate treatment option for patients who are unlikely to benefit from dialysis and/or who choose a nondialysis care option, there is still great variation in how this care is conceptualized and delivered (2).
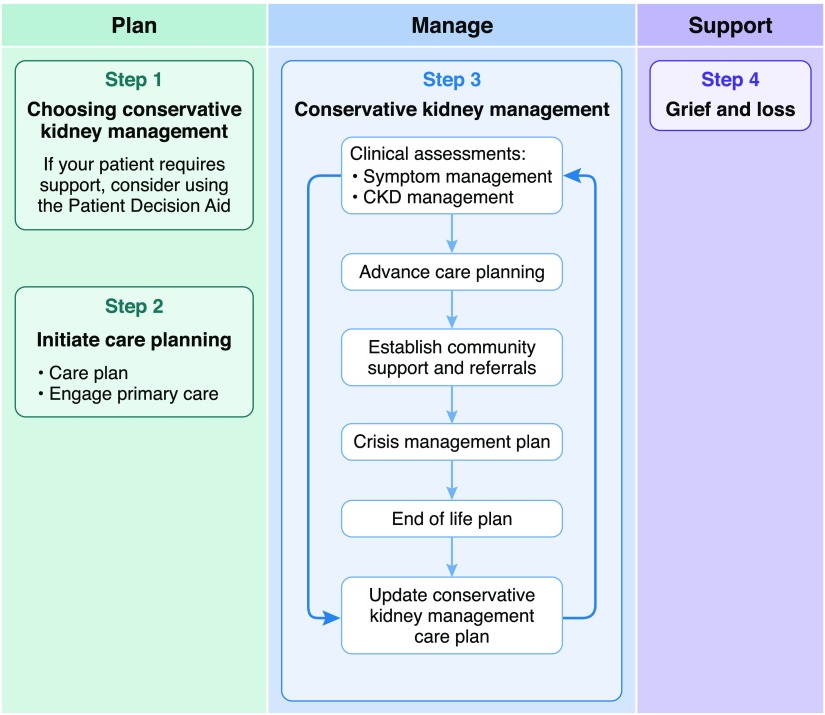
As part of a 3-year plan to develop, implement, and evaluate an interactive conservative kidney management pathway across Alberta, Canada (3), stakeholders and end users, including patients and family, identified the need to include recommendations for managing (1) the complications of CKD and (2) symptoms that were specific to conservative kidney management. Although guidelines exist for CKD management in general and some guidance is available for symptom management for patients with advanced CKD, there are no clear guidelines or recommendations for the care of patients who choose conservative kidney management. The Conservative Kidney Management Pathway (3) is a web-based tool that consists of two main components: (1) an interactive Patient Decision Aid to support shared decision making around dialysis versus conservative kidney management and (2) integrated care plans designed to be individualized. The recommendations on the management of CKD complications and common symptoms are just one part of these care plans (Figure 1). Other key elements include advance care planning and goals of care, establishing community support and appropriate referrals, preparing for crises and the end of life, and grief and loss. The purpose of this article is to describe the development of the conservative kidney management–specific recommendations for both CKD management and symptom control. It should be noted that these recommendations represent expert opinion backed by evidence where it exists. However, evidence is currently insufficient to undergo a rigorous clinical guideline development process. The key principles that were used to guide the development of the recommendations and the conservative kidney management pathway as a whole are outlined in Supplemental Table 1. The recommendations were written for the wide variety of health care professionals (e.g., family physicians, home care nurses, pharmacists, and dieticians) who provide care for these patients. They will be updated as further evidence emerges in the best practices of care for these vulnerable patients.
Figure 1.
Key components of the Conservative Kidney Management Pathway. Reprinted from the Kidney Supportive Care Research Group (3), with permission.
The Conservative Kidney Management Clinical Working Group
In June 2015, stakeholders from various disciplines and areas of specialty formed a Conservative Kidney Management Clinical Working Group under the leadership of the Kidney Supportive Care Research Group (KSCRG) (4) and Alberta’s provincial Conservative Kidney Management Steering Committee. The steering committee was responsible for ensuring a high level of engagement of health care providers, patients, and family throughout the province with respect to the delivery of quality conservative kidney management. These patients tend to be older with multimorbidity, including a high prevalence of geriatric syndromes, such as frailty, and they have high annual mortality rates. The recommendations, therefore, needed to incorporate geriatrics and palliative care approaches. To reflect this, the working group consisted of professionals from nephrology, palliative care, primary care, and geriatric medicine, and it represented the disciplines of pharmacy, dietetics, nursing, medicine, and social work. These disciplines are representative of the team members typically involved in the care of patients with ESKD being managed conservatively without dialysis. They each have important roles in managing the complications of CKD and optimizing symptom management and QOL, and they offered important perspectives.
Evidence for the Conservative Kidney Management Recommendations
There are few studies specific to the care of patients choosing conservative kidney management. The recommendations presented here were developed using both existing evidence and expert opinion from nephrology, geriatric medicine, and palliative care. We searched the literature for guidelines on conservative kidney management and evidence on each of the specific topics described in Tables 1 and 2. Electronic databases searched included MEDLINE, CINAHL, UptoDate, the Cochrane Library, and Dynamed. Search terms included conservative kidney management, supportive kidney care, supportive kidney management, ESKD, guidelines, and recommendations. These terms were then used in searches for each specific CKD and symptom management topic. Results also incorporated prior literature searches that had been done for the Kidney Disease Improving Global Outcomes (KDIGO) Controversies Conference (1). Using the Johns Hopkins Nursing Evidence-Based Practice Rating Scale (5), three of the authors (S.N.D., B.T., and B.A.W.) considered the search results and prioritized them for inclusion depending on the strength of evidence. Searches of the literature as well as Micromedex, Lexicomp, and RxTx were also done for appropriate dosing, available dosage forms, and approved indications (versus off-label uses) of drugs, which were included throughout the recommendations.
Table 1.
Summary of recommendations for managing the progression and metabolic complications of CKD for patients receiving conservative kidney management
| Clinical Issue | Rationale for Recommendations | Recommended Interventions |
|---|---|---|
| BP (9,34,35) | The primary goal of BP control in conservative kidney management is to optimize physical and cognitive function, minimize the risk of falls, and avoid very high readings; decisions about specific medications will depend on the patient’s comorbidities; diuretics are a unique consideration and are aimed primarily at the treatment of volume overload that exacerbates hypertension and causes breathlessness or symptomatic peripheral edema | BP targets can be relaxed for most patients on conservative kidney management to <160/90; this applies to patients with diabetes as well |
| Dyslipidemia (9,10) | Patients are unlikely to benefit from treatment of dyslipidemia in the last year or two of life; patients may gain improvement in quality of life from stopping statin medications | Care providers, in discussion with the patient, can discontinue statins |
| Sodium restriction | Sodium intake may be associated with volume overload, leading to breathlessness and symptomatic peripheral edema; sodium restriction, however, influences palatability; strategies should balance symptom management and the patient’s goals of care and other issues, such as appropriate nutrition | Diuretics and dietary sodium restriction to assist with volume control if volume overload is contributing to symptom burden |
| Anemia (36) | Anemia can contribute to fatigue and breathlessness; the purpose of treating anemia is to reduce these symptoms as opposed to reducing cardiac mortality or morbidity; when patients start spending most of their time confined to bed or chair and/or are near the end of life, it is no longer appropriate to manage fatigue and breathlessness by addressing anemia, and anemia treatment can be stopped | Interventions include the use of erythropoiesis-stimulating agents and iron supplements; anemia-related bloodwork every 3 mo is appropriate but should be on the basis of patient preference and symptoms |
| Acidosis (37,38) | Metabolic acidosis can contribute to fatigue, bone loss, muscle wasting, and malnutrition (36–39); treatment is aimed at improving these symptoms; if the patient finds the pill burden too great or when the patient can no longer swallow, treatment should be stopped | Oral sodium bicarbonate; it is reasonable to monitor CO2 every 3 mo if the patient is being treated or is symptomatic |
| Calcium and phosphorus balance (12–14) | Changes in the metabolism of calcium and phosphorus can contribute to (1) myalgias, arthralgias, and pseudogout and (2) restless legs syndrome; interventions would only be promoted for possible benefit of these symptoms; the primary focus should be on maintaining adequate nutrition and enhancing QOL by liberalizing the diet | Interventions include a phosphorus-restricted diet and the use of phosphate binders, monitoring for the effect of phosphorus restriction on overall quality of life and nutritional status. It is reasonable to check calcium and phosphorus levels every 3 mo if patients are being treated or are symptomatic |
| Vitamin D (13) | Vitamin D deficiency may play a role in the symptoms of fatigue, weakness, and muscle loss | Practitioners may recommend low-dose active vitamin D (vitamin D analog) replacement; there is no additional benefit in monitoring parathyroid hormone levels |
| Hyperkalemia (39) | Hyperkalemia predisposes patients to cardiac arrhythmias and sudden death; acute treatment of hyperkalemia is appropriate if consistent with the patient’s goals; if the patient wishes to liberalize potassium intake, the risks of lifting the restriction must be explained clearly | Interventions include a potassium-restricted diet and potassium binding resins, such as patiromer; consider sodium polystyrene sulfonate for life-threatening hyperkalemia; for those aiming to maintain normal potassium levels, it is reasonable to monitor potassium levels monthly |
There are limited data about the potential of sodium polystyrene sulfonate to bind to other oral medications. QOL, quality of life.
Table 2.
Summary of symptom management guidelines for patients receiving conservative kidney management (3)
| Address Possible Contributing Factors | Nonpharmacologic | Pharmacologic |
|---|---|---|
| Restless legs syndrome (29,40) | ||
| Anemia and iron deficiency | Abstinence from stimulants (e.g., alcohol, caffeine, and nicotine) | First line: gabapentin (50–300 mg daily) 1–2 h before sleep; similarly, pregabalin before sleep (25–75 mg) is also an option |
| Hyperphosphatemia | Mental alerting activities (e.g., puzzles or games) | |
| Medications, such as dopamine antagonists, antidepressants, and opioids | Optimized sleep hygiene | Second line: nonergot-derived dopamine agonists (pramipexole and rotigotine transdermal patch) 2 h before sleep |
| Exercise | ||
| Uremic pruritus (22,23) | ||
| Anemia and iron deficiency | Appropriate skin care and moisturizers (e.g., baths with lukewarm water, pat dry and moisturize with aqueous emollients within 2 min; gentle soaps with no fragrances or additives) | Topical: capsaicin 0.025% or 0.03% ointment; pramoxine 1%; menthol/camphor/phenol 0.3% each either separately or in combination with each other; γ-linolenic acid cream 2.2% |
| Other: xerosis, drug hypersensitivities, allergies, infestations, contact dermatitis, or inflammation | Keep skin cool | |
| Humid environment | ||
| Avoid scratching—maintain short, smooth fingernails; encourage gentle massage; wear gloves at night | ||
| Consider complimentary therapies (e.g., phototherapy [UVB]) three times weekly for a 3-wk trial; acupuncture; very little evidence exists for these alternative therapies | ||
| Systemic: first line: gabapentin (50–300 mg daily) 1–2 h before sleep; similarly, pregabalin before sleep (25–75 mg) is also an option | ||
| Second line: tricyclic antidepressant, such as doxepin 10 mg daily at night | ||
| Nausea and vomiting (30,31) | ||
| Metabolic disturbances | Manage constipation | Recommendations are very specific depending on patient’s status but include the following |
| Medications (e.g., opioids and antidepressants) | Encourage proper oral hygiene | First line: ondansetron 4–8 mg every 8 h as needed |
| Gastrointestinal disturbances (e.g., constipation and delayed gastric emptying) | Smaller, more frequent meals; eat slowly | Second line: metoclopramide 2.5 mg every 4 h as needed |
| Avoid alcohol | Third line: olanzapine 2.5 mg every 8 h as needed or haloperidol 0.5 mg every 8 h as needed | |
| Avoid foods that are greasy, spicy, or excessively sweet | Fourth line: for persistent and severe nausea, consider increasing haloperidol to 1.0 mg (maximum 5 mg in 24 h) or replacing with methotrimeprazine 5 mg orally or 6.25 mg subcutaneously every 8 h as needed; methotrimeprazine may also be beneficial if there is concomitant anxiety, insomnia, or neuropathic pain | |
| Minimize aromas (e.g., cooking odors, perfumes, and smoke) | ||
| Encourage relaxed, upright position after eating to facilitate digestion | ||
| Loose-fitting clothing | ||
| Consider complementary therapies (e.g., relaxation techniques, acupressure, and the use of ginger) | ||
| Breathlessness (41,42) | ||
| Anxiety | Sit in an upright position (e.g., 45°) | If patient is intravascularly overloaded: furosemide |
| Anemia | Position by a window or use a fan to blow air gently across the face | Low doses of opioids, especially nearer to the end of life; because of its fast action, fentanyl works well in patients with breathlessness that is predictable; for shortness of breath that is more constant or unpredictable in nature, consider low-dose hydromorphone (e.g., 0.5 mg PO [0.2 mg subcutaneously] every 4 h around the clock and every hour as needed |
| Infection | Maintain a humid environment | |
| Pulmonary edema | Pursed lip breathing | |
| Supplemental oxygen | ||
| Complementary therapies (e.g., relaxation techniques and music) | ||
| Consider role of diet: fluids and sodium | ||
| Fatigue and sleep disturbances (43–45) | ||
| Fatigue | Fatigue and sleep disturbances | Sleep disturbances |
| Vitamin D deficiency | Exercise | First line: consider low-dose gabapentin (50–300 mg at night), especially if the patient has concomitant neuropathic pain, restless legs syndrome, or uremic pruritus |
| Metabolic acidosis | Nutrition and hydration management | |
| Hypothyroidism, hyperthyroidism, adrenal insufficiency | Cognitive and psychologic approaches (e.g., relaxation therapy and delegating and setting limits) | Second line: doxepin 10 mg at bedtime, especially if concomitant pruritus or neuropathic pain |
| Anemia | Energy conservation strategies | |
| Mood disorder (e.g., anxiety and depression) | Optimized sleep hygiene (e.g., avoid stimulants later in the day, avoid napping, and save the bedroom for sleep) | Third line: cautiously consider mirtazapine 7.5 mg or zopiclone 3.75–5 mg at night or melatonin 2–5 mg at night |
| Medication toxicity (e.g., benzodiazepines, antidepressants, and opioids) | Complementary treatments (e.g., acupressure, massage, and acupuncture) | |
| Sleep disorders | ||
| Sleep disturbances | ||
| Other symptoms as described here | ||
| Cognitive impairment | ||
| Medications | ||
| Generalized insomnia | ||
| Mood disorders | ||
| Apnea | ||
| Nociceptive pain (46–48) | ||
| Determine cause for pain and consider appropriate investigations | Physical therapies (e.g., physical therapy, aerobic exercise, massage, acupressure, and acupuncture) | Recommendations depend on severity of pain; proceed stepwise from nonopioid analgesics to weak opioids and strong opioids |
| Behavioral therapies (e.g., cognitive behavioral therapy [most commonly used behavioral therapy], biofeedback, relaxation techniques, and psychotherapy/individual or group counseling) | Step 1: acetaminophen, maximum of 3 g daily; if pain is localized to a small joint, consider a topical NSAID (e.g., dicofenac gel 5% or 10%) two to three times daily | |
| Interventional and surgical (e.g., ablative techniques, nerve blocks, and trigger point injections) | Step 2: there are no recommended step 2 analgesics for nociceptive pain | |
| Step 3: add a strong opioid to step 1; hydromorphone starting at 0.5 mg PO (0.2 mg subcutaneously) every 4–6 h; other options include buprenorphine/fentanyl/methadone | ||
| Neuropathic pain (32,46) | ||
| Determine cause for pain and consider appropriate investigations | Same as for nociceptive pain | Start with adjuvant therapy |
| First line: gabapentin, pregabalin (calcium channel-α 2δ-ligands) | ||
| Second line: tricyclic antidepressants, amitriptyline starting at 10–25 mg daily or doxepine starting at 10 mg daily | ||
| If additional analgesia is required in addition to adjuvant therapy, add a nonopioid and then proceed stepwise as required | ||
| Step 2: add a weak opioid or opioid-like analgesic to step 1; tramadol 25–50 mg, maximum 100 mg daily | ||
| Step 3: add a strong opioid to step 1; hydromorphone starting at 0.5 mg PO (0.2 mg subcutaneously) every 4–6 h; other options include buprenorphine/fentanyl/methadone |
UVB, ultraviolet B; PO, per os; NSAID, nonsteroidal anti-inflammatory drug.
Guidelines for the management of CKD, such as those developed by KDIGO, were considered to be evidence based and best practice. We adapted existing provincial guidelines that are aligned with KDIGO recommendations with two primary objectives: (1) to make them more appropriate for patients undergoing conservative kidney management and (2) to make them more accessible to the health care professionals caring for these patients outside of nephrology.
No comprehensive symptom management guidelines specific for conservative kidney management were identified. Current symptom algorithms proposed for patients on dialysis may not always be appropriate for those with category 5 CKD managed without dialysis. There are, however, examples of end of life (terminal care) algorithms for patients with advanced CKD, regardless of dialysis management (6,7).
Conservative Kidney Management Recommendations Review Process
The KSCRG wrote the initial drafts of the recommendations. Working group meetings were used to discuss all areas of uncertainty and reach consensus. After consensus, all recommendations were circulated to a wider audience of relevant stakeholders, which included practitioners in nephrology continuing care (including home care, long-term care, and assisted living), primary care, acute care, and emergency services and also involved nurse practitioners, respiratory therapy, and spiritual care.
Incorporation of Clinical Condition into Conservative Kidney Management Recommendations
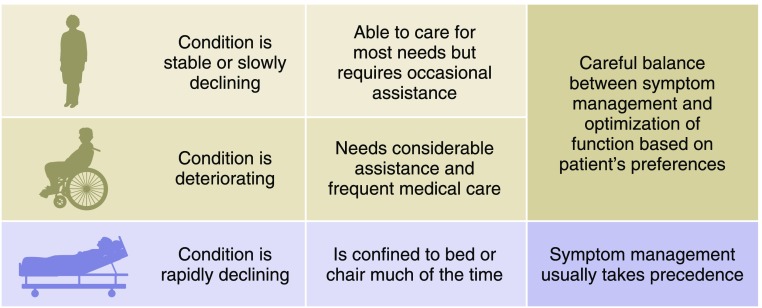
The illness trajectory of patients being cared for conservatively can be highly variable, and the patient’s eGFR does not necessarily predict the overall clinical picture. A patient with an eGFR of 15–10 ml/min per 1.73 m2 or indeed, even lower may remain functional and stable for years, although some may deteriorate over a few months (8). A patient who has an eGFR of 5 ml/min per 1.73 m2 typically has a life expectancy measured in weeks to short months. Comprehensive conservative kidney management recommendations, although focused predominantly on the patient’s goals of care and preferences, should also take into account the patient’s general condition and prognosis, because these may be important factors for patients in the shared decision-making process (Figure 2). Earlier in the disease trajectory, maximizing QOL likely requires a careful balance between preserving function and addressing symptom burden, whereas control of symptoms and comfort generally take precedence in the last weeks and days of life.
Figure 2.
Clinical condition is incorporated into the conservative kidney management recommendations.
Recommendations for Managing the Complications of CKD for Patients Receiving Conservative Kidney Management
The recommendations for managing the complications of CKD are summarized in Table 1. The full recommendations are available freely online and provide more specific information on dosing (3). The approach to CKD management in this population represents a shift from disease-focused treatment to symptom- and patient-specific, goal-focused interventions. Medications are used with the intention of improving symptoms and protecting kidney function, but the latter is only if it will also enhance QOL and/or help a patient achieve his or her goals. Three examples will be discussed in more detail, but the principles extend to the other recommendations.
BP
Although optimal BP control is important in slowing the progression of CKD, this is likely not the focus of care for most patients undergoing conservative kidney management who tend to be frail. Rather, the aim of BP management for these patients is to optimize QOL, preserve physical and cognitive function, and minimize the risk of orthostatic hypotension and falls. For these reasons, we recommend relaxing BP targets for most patients who are being conservatively managed, reflecting these priorities of QOL and safety over CKD progression. The geriatric literature suggests that a target systolic BP of 160–190 mm Hg is reasonable for frail patients near the end of life (9). Our recommendations have identified the lower end of this target as being appropriate for conservative kidney management.
Dyslipidemia
There is no evidence that statins provide benefit in the context of significant frailty or decreased life expectancy (9,10). A randomized trial of discontinuing statin therapy in patients with life-limiting illness suggested that cessation was not only safe but that it improved QOL (10). Statin therapy is an example of an intervention that takes years to accrue benefits. Advanced age and multimorbidity are risk factors for adverse effects of statins, which can include myopathy and a potential link to cognitive deficits. Therefore, we feel that it is appropriate to discontinue statins for patients undergoing conservative kidney management (11).
Calcium and Phosphorus Metabolism
Current CKD guidelines aim to normalize serum calcium, phosphate, and parathyroid hormone levels to prevent bone abnormalities and vascular calcification. However, it is not clear that there are benefits to normalizing these factors in patients being cared for conservatively in the last years of life. Rather, there is the possibility of harm in promoting lower protein intake in patients already at high risk for protein malnutrition (12). Instead, the focus is on promoting QOL. This includes maintaining adequate nutrition, avoiding malnutrition and inflammation, and liberalizing the diet. In addition, serum phosphorous, calcium, or parathyroid hormone levels do not seem to be major drivers behind uremic pruritus (13). For these reasons, we recommend not focusing on normalizing biochemistry but rather, emphasizing adequate nutrition. There does seem to be an association between low albumin and pruritus severity, suggesting an association between pruritus and malnutrition-inflammation complex syndrome and reinforcing the need for adequate nutrition (14). Parathyroid hormone levels do not affect decision making within this conservative approach to care; therefore, there is no need to monitor.
For all of the recommendations in Table 1, the intent is to ensure that interventions are aimed at optimizing QOL and providing comfort, which is in alignment with the patient’s preferences.
Recommendations for Managing Symptoms in Patients Receiving Conservative Kidney Management
Recommendations for the management of symptoms for patients on a conservative kidney management pathway are summarized in Table 2. All recommendations follow a similar approach. The first step is to assess the patient and rule out contributing reversible factors. The second step is to consider nonpharmacologic interventions. The third step is to use pharmacologic interventions. The aim of treatment is to ameliorate symptoms that are adversely affecting the patient’s QOL, because it is not always necessary or possible to resolve them completely. Patients under-report symptoms unless they are assessed explicitly (15). Therefore, in alignment with KDIGO recommendations (1), we recommend routine screening at each consultation for physical and emotional symptoms using a validated tool, like the Edmonton Symptom Assessment System Revised: Renal (16) or the Integrated Palliative Care Outcome Scale Renal (17). The pain recommendations also suggest using a validated pain assessment tool, such as the Brief Pain Inventory, to help categorize and classify the pain and to assess response to management (18). For all recommendations, dose adjustments and side effects are mentioned, and off-label uses are noted.
Uremic Pruritus
The pathophysiology of uremic pruritus is not fully understood, and multiple complex mechanisms likely play a role. Uremic neuropathy (19), skin or nerve inflammation in the context of chronic systemic inflammation (20), and an increase in activity of μ-opioid receptors have all been implicated (21,22). A recent systematic review found low-dose gabapentin or pregabalin to be effective for uremic pruritus; however, evidence for other interventions remains weak (23).
Restless Legs Syndrome
The precise pathogenic mechanisms responsible for uremic restless legs syndrome (RLS) are unknown. The most widely accepted hypotheses for idiopathic RLS are dysfunction of the dopaminergic system, iron deficiency, and anemia (24,25). It is felt that these mechanisms may play a role in uremic RLS (26). The associations between RLS and iron deficiency and anemia are inconsistent in patients on hemodialysis, perhaps due to regular treatment with erythropoietin and iron. However, it may be beneficial to address these abnormalities in patients who are conservatively cared for to determine if they receive symptom relief. High serum phosphorus levels have also been independently associated with the presence of RLS in patients on hemodialysis (26). First-line treatment for idiopathic RLS is dopamine agonists. Dopamine agonists have shown benefit in patients on hemodialysis, but their use seems to be associated with decreased response compared with that in patients with idiopathic RLS (27). Subclinical peripheral nerve abnormalities have been implicated in RLS (28). In ESKD, gabapentin was associated with reduced RLS compared with placebo and the dopamine agonist levodopa. Because of a short duration of action, rebound, and augmentation with levodopa treatment as well as adverse effects of severe vomiting, headaches, dry mouth, and gastrointestinal symptoms, our recommendations suggest gabapentin as first-line treatment for uremic RLS (29).
Although benzodiazepines are not recommended for RLS in patients undergoing conservative kidney management due to the limited evidence for their use and the significant risks of falls, fractures, and decreased cognition, they may be the only option for a patient who can no longer swallow. This is reflected in the terminal symptom algorithm for restlessness. If the patient is experiencing refractory RLS causing significant sleep disturbance or if benzodiazepines may potentially treat concurrent symptoms (e.g., anxiety), they could be considered.
Nausea and Vomiting
Recommendations for the management of nausea and vomiting place particular emphasis on nonpharmacologic management due to the adverse effects of many of the commonly used agents (30,31). Ondansetron can be constipating. Haloperidol, metoclopramide, and olanzapine are all dopamine antagonists and should not be prescribed together. In addition, they cross the blood-brain barrier, and they can cause extrapyramidal symptoms and exacerbate RLS. Metoclopramide has prokinetic properties and would be first-line treatment for patients with nausea and vomiting due to gastroparesis. Olanzapine has a lower risk of causing extrapyramidal symptoms, but it has a higher risk of causing metabolic side effects, like weight gain and type 2 diabetes. Haloperidol has a higher risk of extrapyramidal symptoms than both metoclopramide and olanzapine.
Chronic Pain Management
Nonpharmacologic therapies have become a vital part of managing chronic pain. They may be used as standalone therapies or to augment pharmacologic treatments. Medication should not be the sole focus of treatment. The choice of an appropriate initial therapeutic strategy for pain is dependent on the type of pain. Neuropathic pain results from damage to the somatosensory nervous system and is commonly described as burning, stabbing, or shooting. Nociceptive pain results from tissue damage causing stimulation of sensory receptors and may be described as sharp, dull, or cramping. Neuropathic pain responds well to adjuvant therapy. For patients undergoing conservative kidney management, the first-line treatment of neuropathic pain involves calcium channel-α 2δ-ligands, such as gabapentin (32,33). Tramadol and opioid medications are considered second line. In contrast, the first-line pharmacologic approach to nociceptive pain involves analgesics. Opioids are recommended only as the last option, and they should be started at low doses, monitored carefully for adverse effects and overall benefit, and titrated slowly.
Five distinct algorithms specific to the end of life were developed for the following symptoms: breathlessness, pain, nausea and vomiting, respiratory secretions, and agitation and restlessness; they can be accessed freely from the online conservative kidney management pathway (3). As a patient’s condition deteriorates, certain nonpharmacologic interventions will become less realistic (e.g., exercise). It is important to ensure that appropriate supports are in place to assist with activities of daily living and that nursing care is available as needed. These end of life algorithms emphasize the importance of anticipatory prescribing and having an alternate route to oral. They also highlight when referral to a specialist palliative care team may be appropriate. Drowsiness may increase as the end of life approaches due to disease progression and/or medications; some patients and families may even prefer increased drowsiness if the patient remains comfortable.
Conclusions
Although conservative kidney management is increasingly recognized as an appropriate treatment option for patients unlikely to benefit from dialysis, there is still great variation in the current understanding and acceptance of conservative kidney management as a distinct modality choice (1,2). With this variation comes great differences in clinical practice and standards of care. It is hoped that the recommendations presented here will serve as a starting point for a standardized approach to care that can be refined as evidence for best practice accumulates. Our ongoing evaluation of these recommendations includes patient and family caregiver perspectives and the effect on outcomes, such as overall symptom burden, preservation of physical and cognitive function, satisfaction with care, and QOL. This is an important step toward furthering knowledge and optimizing patient outcomes. The recommendations, however, serve another purpose. By including the philosophy behind each intervention, it is hoped that they will support conversations between care professionals, patients, and families to more clearly elicit goals of care and better understand not only the patient’s symptom burden but also, what matters most to them. Many patients undergoing conservative kidney management present with complex health scenarios, and most recommendations have to be personalized. By explaining the rationale and possible benefits and drawbacks of interventions for CKD and symptoms, patients can become active participants in a shared decision-making process, ensuring that all interventions are aligned appropriately with their values, preferences, and prognosis.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by funding from Alberta Innovates Health Solutions grant 201400400.
The authors would like to acknowledge the contributions of the Kidney Health Strategic Clinical Network and the additional members of the Clinical Guidelines Working Group: Lisa Blacklock, Ruth deBoer, Pat Holmes, Jodi Kerr, Neil Thompson, Julie Yuen (Southern Alberta Renal Program, Alberta Health Services), Amanda Brisebois, Elisa Mori-Torres (University of Alberta), Gloria Finkbeiner, Lana Marchinko, Janice McKenzie, Kari McKnight, Nualla Virga (Northern Alberta Renal Program, Alberta Health Services), and Maureen McCall, (Family Practice, Alberta Medical Association).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10510917/-/DCSupplemental.
References
- 1.Davison SN, Levin A, Moss AH, Jha V, Brown EA, Brennan F, Murtagh FE, Naicker S, Germain MJ, O’Donoghue DJ, Morton RL, Obrador GT; Kidney Disease: Improving Global Outcomes: Executive summary of the KDIGO controversies conference on supportive care in chronic kidney disease: Developing a roadmap to improving quality care. Kidney Int 88: 447–459, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Roderick P, Rayner H, Tonkin-Crine S, Okamoto I, Eyles C, Leydon G, Santer M, Klein J, Lily Yao G, Murtagh F, Farrington K, Caskey F, Tomson C, Loud F, Greenwood R, O’Donoghue D: A National Study of Practice Patterns in UK Renal Units in the Use of Dialysis and Conservative Kidney Management to Treat People Aged 75 Years and Over with Chronic Kidney Failure, Southampton, United Kingdom, Queen’s Printer and Controller of HMSO, 2015 [PubMed] [Google Scholar]
- 3.Kidney Supportive Care Research Group: Conservative Kidney Management Care Pathway, 2016. Available at: www.ckmcare.com. Accessed September 20, 2017
- 4.Kidney Supportive Care Research Group: KSCRG Website, 2016. Available at: www.ualberta.ca/∼kscrg. Accessed September 20, 2017
- 5.Newhouse R, Dearholt S, Poe S, Pugh LC, White KM: The John Hopkins Nursing Evidence-Based Practice Rating Scale, Indianapolis, IN, The Honor Society of Nursing, Sigma Theta Tau International, 2005 [Google Scholar]
- 6.Royal Liverpool University Hospital and Marie Curie Hospice: Terminal Symptom Algorithms: Liverpool Care Pathway: Pathway for Renal Patients. Available at: http://www.rowcrofthospice.org.uk/web/data/microsoft-word-final-guidelines-renal-impairment-june-2014.pdf. Accessed September 20, 2017
- 7. Russon L, Hicks F: Yorkshire & the Humber Conservative Kidney Management Strategy, North Bristol, United Kingdom, National Health Service, 2012.
- 8.Pacilio M, Minutolo R, Garofalo C, Liberti ME, Conte G, De Nicola L: Stage 5-CKD under nephrology care: To dialyze or not to dialyze, that is the question. J Nephrol 29: 153–161, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Benetos A, Rossignol P, Cherubini A, Joly L, Grodzicki T, Rajkumar C, Strandberg TE, Petrovic M: Polypharmacy in the aging patient: Management of hypertension in octogenarians. JAMA 314: 170–180, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Kutner JS, Blatchford PJ, Taylor DH Jr, Ritchie CS, Bull JH, Fairclough DL, Hanson LC, LeBlanc TW, Samsa GP, Wolf S, Aziz NM, Currow DC, Ferrell B, Wagner-Johnston N, Zafar SY, Cleary JF, Dev S, Goode PS, Kamal AH, Kassner C, Kvale EA, McCallum JG, Ogunseitan AB, Pantilat SZ, Portenoy RK, Prince-Paul M, Sloan JA, Swetz KM, Von Gunten CF, Abernethy AP: Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: A randomized clinical trial. JAMA Intern Med 175: 691–700, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silveira MJ, Kazanis AS, Shevrin MP: Statins in the last six months of life: A recognizable, life-limiting condition does not decrease their use. J Palliat Med 11: 685–693, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Shinaberger CS, Greenland S, Kopple JD, Van Wyck D, Mehrotra R, Kovesdy CP, Kalantar-Zadeh K: Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr 88: 1511–1518, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathur VS, Lindberg J, Germain M, Block G, Tumlin J, Smith M, Grewal M, McGuire D; ITCH National Registry Investigators: A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol 5: 1410–1419, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rayner HC, Larkina M, Wang M, Graham-Brown M, van der Veer SN, Ecder T, Hasegawa T, Kleophas W, Bieber BA, Tentori F, Robinson BM, Pisoni RL: International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol 12: 2000–2007, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman R, Berman N, Reid MC, Roberts J, Shengelia R, Christianer K, Eiss B, Adelman RD: Improving symptom management in hemodialysis patients: Identifying barriers and future directions. J Palliat Med 16: 1528–1533, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davison SN: Edmonton Zone Palliative Care Program and Northern Alberta Renal Program, 2016. Available at: http://www.palliative.org/tools.html. Accessed September 20, 2017
- 17.Cicely Saunders Institute: Palliative Care Outcome Scale, 2012. Available at: https://pos-pal.org/maix/ipos-renal-in-english.php. Accessed September 20, 2017
- 18.Cleeland CS, Ryan KM: Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore 23: 129–138, 1994 [PubMed] [Google Scholar]
- 19.Zakrzewska-Pniewska B, Jedras M: Is pruritus in chronic uremic patients related to peripheral somatic and autonomic neuropathy? Study by R-R interval variation test (RRIV) and by sympathetic skin response (SSR). Neurophysiol Clin 31: 181–193, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Kimmel M, Alscher DM, Dunst R, Braun N, Machleidt C, Kiefer T, Stülten C, van der Kuip H, Pauli-Magnus C, Raub U, Kuhlmann U, Mettang T: The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol Dial Transplant 21: 749–755, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Umeuchi H, Togashi Y, Honda T, Nakao K, Okano K, Tanaka T, Nagase H: Involvement of central mu-opioid system in the scratching behavior in mice, and the suppression of it by the activation of kappa-opioid system. Eur J Pharmacol 477: 29–35, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Jaiswal D, Uzans D, Hayden J, Kiberd BA, Tennankore KK: Targeting the opioid pathway for uremic pruritus: A systematic review and meta-analysis. Can J Kidney Health Dis 3: 2054358116675345, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simonsen E, Komenda P, Lerner B, Askin N, Bohm C, Shaw J, Tangri N, Rigatto C: Treatment of uremic pruritus: A systematic review. Am J Kidney Dis 70: 638–655, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Zucconi M, Manconi M, Ferini Strambi L: Aetiopathogenesis of restless legs syndrome. Neurol Sci 28[Suppl 1]: S47–S52, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Allen RP: Controversies and challenges in defining the etiology and pathophysiology of restless legs syndrome. Am J Med 120[Suppl 1]: S13–S21, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Takaki J, Nishi T, Nangaku M, Shimoyama H, Inada T, Matsuyama N, Kumano H, Kuboki T: Clinical and psychological aspects of restless legs syndrome in uremic patients on hemodialysis. Am J Kidney Dis 41: 833–839, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Enomoto M, Inoue Y, Namba K, Munezawa T, Matsuura M: Clinical characteristics of restless legs syndrome in end-stage renal failure and idiopathic RLS patients. Mov Disord 23: 811–816, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Marconi S, Scaglione C, Pizza F, Rizzo G, Plazzi G, Vetrugno R, La Manna G, Campieri C, Stefoni S, Montagna P, Martinelli P: Group I nonreciprocal inhibition in restless legs syndrome secondary to chronic renal failure. Parkinsonism Relat Disord 18: 362–366, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Gopaluni S, Sherif M, Ahmadouk NA: Interventions for chronic kidney disease-associated restless legs syndrome. Cochrane Database Syst Rev 11: CD010690, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collis E, Mather H: Nausea and vomiting in palliative care. BMJ 351: h6249, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Murray-Brown F, Dorman S: Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev 11: CD006271, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atalay H, Solak Y, Biyik Z, Gaipov A, Guney F, Turk S: Cross-over, open-label trial of the effects of gabapentin versus pregabalin on painful peripheral neuropathy and health-related quality of life in haemodialysis patients. Clin Drug Investig 33: 401–408, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Solak Y, Biyik Z, Atalay H, Gaipov A, Guney F, Turk S, Covic A, Goldsmith D, Kanbay M: Pregabalin versus gabapentin in the treatment of neuropathic pruritus in maintenance haemodialysis patients: A prospective, crossover study. Nephrology (Carlton) 17: 710–717, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Muller M, Smulders YM, de Leeuw PW, Stehouwer CD: Treatment of hypertension in the oldest old: A critical role for frailty? Hypertension 63: 433–441, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Musini VM, Tejani AM, Bassett K, Wright JM: Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst Rev 4: CD000028, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Roger SD, Jassal SV, Woodward MC, Soroka S, McMahon LP: A randomised single-blind study to improve health-related quality of life by treating anaemia of chronic kidney disease with Aranesp® (darbepoetin alfa) in older people: STIMULATE. Int Urol Nephrol 46: 469–475, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Kopple JD, Kalantar-Zadeh K, Mehrotra R: Risks of chronic metabolic acidosis in patients with chronic kidney disease. Kidney Int Suppl 95: S21–S27, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Raphael KL: Approach to the treatment of chronic metabolic acidosis in CKD. Am J Kidney Dis 67: 696–702, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Packham DK, Rasmussen HS, Lavin PT, El-Shahawy MA, Roger SD, Block G, Qunibi W, Pergola P, Singh B: Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med 372: 222–231, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Giannaki CD, Hadjigeorgiou GM, Karatzaferi C, Pantzaris MC, Stefanidis I, Sakkas GK: Epidemiology, impact, and treatment options of restless legs syndrome in end-stage renal disease patients: An evidence-based review. Kidney Int 85: 1275–1282, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Booth S, Moffat C, Burkin J, Galbraith S, Bausewein C: Nonpharmacological interventions for breathlessness. Curr Opin Support Palliat Care 5: 77–86, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Barnes H, McDonald J, Smallwood N, Manser R: Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev 3: CD011008, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Canadian Agency for Drugs and Technologies in Health: Treatment of Older Adults with Insomnia, Agitation, or Delirium with Benzodiazepines: A Review of the Clinical Effectiveness and Guidelines, Ottawa, Ontario, Canada, Canadian Agency for Drugs and Technologies in Health, 2016. [PubMed]
- 44.Furey SA, Hull SG, Leibowitz MT, Jayawardena S, Roth T: A randomized, double-blind, placebo-controlled, multicenter, 28-day, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J Clin Sleep Med 10: 1101–1109, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yennurajalingam S, Bruera E: Palliative management of fatigue at the close of life: “It feels like my body is just worn out.” JAMA 297: 295–304, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Davison SN, Koncicki H, Brennan F: Pain in chronic kidney disease: A scoping review. Semin Dial 27: 188–204, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Davison SN, Mayo PR: Pain management in chronic kidney disease: The pharmacokinetics and pharmacodynamics of hydromorphone and hydromorphone-3-glucuronide in hemodialysis patients. J Opioid Manag 4: 335–336, 2008 [PubMed] [Google Scholar]
- 48.El Harraqui R, Abda N, Bentata Y, Haddiya I: [Evaluation and analysis of pain in chronic hemodialysis]. Nephrol Ther 10: 500–506, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.