Abstract
The role of ERα36 in regulating BPA’s effects and its potential as a risk factor for human uterine fibroids were evaluated. BPA at low concentrations (10−6 μM - 10 μM) increased proliferation by facilitating progression of hormonally regulated, immortalized human uterine leiomyoma (ht-UtLM; fibroid) cells from G0-G1 into S phase of the cell cycle; whereas, higher concentrations (100 μM – 200 μM) decreased growth. BPA upregulated ERα36 gene and protein expression, and induced increased SOS1 and Grb2 protein expression, both of which are mediators of the MAPKp44/42/ERK1/2 pathway. EGFR (pEGFR), Ras, and MAPKp44/42 were phosphorylated with concurrent Src activation in ht-UtLM cells within 10 minutes of BPA exposure. BPA enhanced colocalization of phosphorylated Src (pSrc) to ERα36 and coimmunoprecipitation of pSrc with pEGFR. Silencing ERα36 with siERα36 abolished the above effects. BPA induced proliferation in ht-UtLM cells through membrane-associated ERα36 with activation of Src, EGFR, Ras and MAPK nongenomic signaling pathways.
Keywords: Bisphenol A, Leiomyoma cells, Estrogen Receptor alpha36 (ERα36), Nongenomic signaling
1. Introduction
BPA is recognized as an environmental estrogen and a proven endocrine disrupting chemical (EDC) both in vivo and in vitro (Gao, Yang, Li et al., 2015,Peretz, Vrooman, Ricke et al., 2014,Richter, Birnbaum, Farabollini et al., 2007) BPA is pervasive and is found in dust, air, and paper currency and receipts. It is present in human serum, urine, amniotic fluid, and breast milk in the populations of industrialized countries worldwide. In a reference population of 394 adults in the United States, BPA was detected in 95% of human urine samples with a median concentration of 1.28 μg/L (5.6 nM) and in human serum at levels of 0.2–1.6 ng/mL (0.88–7.0 nM) (Gao et al., 2015). Therefore, due to ubiquitous exposures of populations to BPA, it is a public health concern (Gao et al., 2015,Peretz et al., 2014).
BPA is structurally and functionally similar to 17β-estradiol (E2), has estrogenic effects, and interacts differentially with estrogen receptors alpha (ERα) and beta (ERβ) (Ashby and Odum, 2004), but has 2,000–10,000-fold lower binding affinity to classical ERs than E2 (Kuiper, Lemmen, and Carlsson et al., 1998). BPA has also been shown to elicit rapid, nongenomic estrogenic responses via nonclassical membrane-anchored ERs (Wetherill, Akingbemi, and Kanno et al., 2007), such as the transmembrane G protein-coupled receptor, GPR30 (GPER) (Dong, Terasaka and Kiyama, 2011). Another membrane-associated ER and a variant of ERα66, is the truncated ERα36, which is an estrogen-responsive receptor that can activate crosstalk among multiple pathways involved in proliferation, cell survival (anti-apoptotic), and metastatic events in breast cancer (2010,Chaudhri, Olivares-Navarrete, Cuenca et al., 2012,Wang, Zhang, Shen et al., 2006). Also, ERα36 has been implicated in estrogen-stimulated MAPK (ERK) activation (Wang et al., 2006). BPA at low concentrations is reported to increase proliferation and phosphorylation of MAPK in ER-negative breast cancer cells (2010,Song, Zhang, Yang et al., 2015,Zhang, Wang, Liu et al., 2015).
At human exposure levels, BPA induced uterine leiomyomas in adult mice following neonatal exposures (Newbold, Jefferson and Padilla-Banks, 2007). Also, it was reported that human leiomyoma tissue concentrations of BPA were significantly higher than that of myometrial tissue (Othman, Al-Adly, Elgamal et al., 2016). However, the specific molecular mechanisms of BPA’s action on estrogen-responsive uterine leiomyomas in women are not yet known. Due to BPA’s ubiquitous nature and wide-spread human exposures, in addition to its estrogenic activity, ability to induce uterine leiomyomas in mice, and the hormonal dependency of uterine leiomyomas in women, our immortalized human uterine leiomyoma (ht-UtLM; fibroid) cells were used to evaluate the low-dose effects of this xenoestrogen (Gao, Yu, Castro et al., 2010,Watson, Bulayeva, Wozniak et al., 2005,Yu, Moore, Castro et al., 2012). The present study was therefore, designed to determine the rapid nongenomic mechanisms of action of low doses of BPA at human exposure levels, in human fibroid cells, and to evaluate whether BPA’s effects are mediated via the transmembrane receptor, ERα36.
2. Materials and Methods
2.1. Cell culture
Ht-UtLM cells (Carney, Tahara, Swartz et al., 2002) are hormonally responsive and were used for testing cell proliferation, functional endpoints, and nongenomic signaling. The cells were grown and maintained in MEM (Gibco Life Technologies, Grand Island, NY) with supplements at 37°C, with 95% humidity and 5% CO2, as previously described (Yu, Saile, Swartz et al., 2008). For the treatment of cells with various concentrations of BPA (99%; Sigma-Aldrich, Saint Louis, MO) and 17 Beta-estradiol (E2) (Sigma-Aldrich), we used phenol red free DMEM (Gibco Life Technologies) along with 10% Charcoal Dextran treated FBS (CD-FBS) (GE Healthcare Life Science Pittsburgh, PA) for preparing test media.
2.2. Bisphenol A (BPA) Doses
All concentrations for time courses and dose responses were chosen based on previous studies (2010,Jeng, Kochukov and Watson, 2010,Jeng and Watson, 2009,Kochukov, Jeng and Watson, 2009). The chosen concentrations of BPA reflect the range of concentrations likely to be found in the environment (Liao, Liu, Guo et al., 2012,Liao, Liu, Moon et al., 2012). Lower concentrations are of interest to determine how sensitive biological systems are to presumably more widespread exposure concentrations. BPA was solubilized in 0.1% DMSO (Sigma Aldrich) and diluted in treatment medium at required concentrations. A dose-range of 10−6 - 200 μM BPA for the cell proliferation studies, and 10−6, 10−3, and 1 μM BPA for additional studies was selected.
2.3. Cell proliferation assay
To evaluate the effects of BPA on cell proliferation, an MTS (methyltetrazolium sulfate)-based CellTiter 96® Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI) was used according to the manufacturer’s protocol to measure the number of viable cells based on the dye intensity as previously described (Gao et al., 2010). Briefly, the ht-UtLM cells were plated in 96-well plates at 5 × 103 cells/well. Ht-UtLM cells were exposed to BPA at 0, 10−6, 10−5, 10−4, 10−3, 10−2, 10−1, 1, 10, 100, and 200 μM and with E2 at 10−2 μM as a positive control, along with a vehicle control (no BPA, but DMSO in culture medium) for 24, 48, and 72h.
2.4. Cell cycle analysis
Ht-UtLM cells were treated with BPA (10−6, 10−3, 1 μM and E2) along with a vehicle control for 24h for cell cycle analysis. After treatment, cells were fixed with cold 70% ethanol (overnight) prior to staining with Propidium Iodide (PI). Cells were examined by flow cytometry using a FACSort Flow Cytometer (San Jose, CA) equipped with CellQuest Software. An initial gate was set on an PI area versus width dot-plot to identify single cells and 10,000 single cells collected per sample. The data was further analyzed using ModFit software to determine each phase of the cell cycle and are reported as the percentage of G0/G1, S, and G2/M for each sample.
2.5. Real-time RT-PCR analysis
Real-time RT-PCR was used to determine ERα36 gene expression induced by BPA. Ht-UtLM cells were treated for 24h with BPA (10−6, 10−3 μM), or E2 (10−2 μM) or untreated. Cells were harvested with Trizol reagent, and total RNA was extracted with an RNA Purification Kit (Qiagen, Valencia, CA). Two micrograms of total RNA were used to prepare cDNA and primed with ERα36 and GAPDH primers (housekeeping gene as control) and reverse-transcribed with Superscript II (Invitrogen, Carlsbad, CA). The following primer sets specific for ERα36, forward primer 5’-TTGGAAACAAGTGGTTTCCTCG-3’ and ERα36 reverse primer 5’- CTGCCTCAAAACAAAATGTCCC-3’ and the housekeeping gene GAPDH were used for gene expression studies. The data analysis was based on the ΔΔCt method with normalization to GAPDH, and the results were expressed as fold changes as compared to untreated control groups.
2.6. Receptor Knock-down with siERα36
To assess ERα36’s involvement in BPA-induced cell proliferation, gene expression, and MAPK activation, a short interfering (si) RNA technique was used to knockdown ERα36 gene expression in ht-UtLM cells. The ERα36 siRNA fragments were predesigned and produced by Ambion (catalog# 4399666; hERα36 ID: s499850; Foster City, CA) The transfection into ht-UtLM cells of siERα36 oligos targeting human ERα36 gene expression (siERα36) and a control scrambled siRNA (catalog# 4390843; siScr, Ambion) with a nonsense sequence designed to have no significant sequence similarity to mouse, rat, or human transcript sequences was done using Lipofectamine (Invitrogen) as a transfection agent and incubated for 24h following the manufacturer’s protocol. Transfected ht-UtLM cells with or without ERα36 silencing (siERα36) were maintained in phenol red free MEM along with CD-FBS medium containing 0.001% of vehicle (DMSO) for 24h, then treated with BPA at various concentrations for cell proliferation, cell cycle and protein expression studies.
2.7. Western Blot Analysis
Expression levels of ERα36 receptor and associated signaling proteins were determined in ht-UtLM cells transfected with siERα36 or siSrc for 24h, then treated with BPA (10−6, 10−3, 1 μM) or E2 for 24h (ERα36, Grb2 and SOS1 expression studies) and for 10 min (phosphorylated EGFR, Src, Ras and MAPKp44/42 expression studies) by western blot analysis as previously described (Yu et al., 2008). The following primary antibodies were used for the western blotting: Rabbit Polyclonal Anti-SOS1 and Anti-Grb2 (1:1000 dilution, Cell Signaling Technology Inc, Danvers, MA); Rabbit Polyclonal Anti-ERα36 (1:1000 dilution, Cell Applications, Inc., San Diego, CA); Rabbit Polyclonal Anti-HPRT (1:500 dilution, Santa Cruz Biotechnology Inc., Santa Cruz, CA); Rabbit Polyclonal Anti-pEGFR(tyr845)/tEGFR, Anti-pSrc(tyr416)/tSrc, Anti-pRas1/tRas1 and Anti-pMAPK/tMAPKp44/42 (1:1000 dilution, Cell Signaling Technology). The blots were incubated at 4°C, overnight. Primary antibodies were detected with horseradish peroxidase-conjugated secondary anti-rabbit or anti-mouse antibodies by incubating at RT for 1h and followed by an ECL Western Blotting detection reagent (GE Healthcare, Buchinghamshire, UK).
2.8. Immunofluorescence and confocal microscopy
Expression of ERα36 in ht-UtLM cells following BPA exposure was determined by immunofluorescence and confocal microscopy. The cells were grown on glass bottom plates at a density of 50,000 cells/plate and transfected with siERα36 or siSrc for 24 h. The cells were then treated with 0 or 10−3 μM of BPA for 24h. For immunofluorescence staining, ht-UtLM cells were fixed in −20°C methanol for 5 min, and blocked with 10% normal goat serum for 30 min on ice. The cells were incubated with primary antibody of ERα36 (1:100 dilution, Cell Applications Inc., San Diego, CA) in 1.5% normal goat serum overnight. The secondary antibody of Alexa Fluor 488 goat anti-rabbit (Invitrogen) was used at 1:3000 dilution for 1h at RT, followed by 3 μM DAPI (Molecular Probes, Eugene, OR) for nuclear staining for 30 min. For negative controls, the cells were incubated with nonimmune rabbit serum (Jackson Immunoresearch, West Grove, PA) at the primary antibody’s concentration.
BPA-induced migration of EGFR to mitochondria in cells was also evaluated. Ht-UtLM cells were grown and transfected with siERα36 or siScr as described above; then treated with BPA at 10−3μM. The cells were co-stained with a rabbit polyclonal anti-EGFR (Santa Cruz Biotechnology Inc.) at a 1:50 dilution, and MitoTracker dye (Thermo Fisher Scientific, Waltham, MA) at 25 nM at 4°C overnight. The cells were costained with Alexa Fluor 488 goat anti-mouse IgG Antibody and/or Alexa Fluor 594 goat anti-Rabbit IgG antibody at a dilution of 1:3000 for 1h. Cells were then counterstained with 3 μM DAPI for 30 min. Confocal images were taken on a Zeiss LSM710-UV (Carl Zeiss Inc, Oberkochen, Germany) using a Plan-Apochromat 63X/1.40 oil DIC M27 objective. The staining intensity was measured by ImageJ.
2.9. Proximity ligation assay (PLA)
Interaction of intermediary proteins of the MAPK signaling pathway with ERα36 was assessed by determining the colocalization of activated Src, an intracellular tyrosine-protein kinase, with ERα36 was measure by PLA (Sigma, DUO92101–1KT) according to manufacturer’s protocol. Briefly, the ht-UTLM cells were grown on glass bottom plates until 70% of confluent and transfected with siERα36 or siSrc for 24 h, treated with 0 or 10−3 μM of BPA for 10min, fixed with 4% of paraformaldehyde for 20min, and permeabilized with 0.1% triton X-100 for 30min. The cells then were incubated with a rabbit polyclonal anti-ERα36 (Cell Application) at a 1:100 dilution and a mouse monoclonal anti-phospho-Src (tyr416) antibody (Millipore, Temecula, CA) at a 1:50 dilution, overnight at 4° C. The cells were only incubated with pSrc antibody in negative control plate). PLUS and MINUS secondary PLA probes against rabbit and mouse IgG were added with incubation at 37° C for 1h, followed by incubation with ligase for 30 min at 37° C. Amplification was then applied for 120 min at 37° C. The coverslips were mounted on plate with Doulink Mounting Medium with Dapi (Sigma, DUO82040). The cells were imaged on a Zeiss LSM780-UV (Carl Zeiss Inc, Oberkochen, Germany) using a Plan-Apochromat 40X/1.40 oil DIC M27 objective. The number of PLA dots (Bustosa V et al., 2017) was measured by Fiji.
2.10. Coimmunoprecipitation
To detect the association of phospho-EGFR(tyr845) with phospho-Src(tyr416), an immunoprecipitation Kit–dynabeads Protein G (ThermoFisher Scientific, Waltham, MA) was used. A total of 500 μg of total protein from cell lysate in different conditions (see western blot analysis) was mixed with 500 μl of binding buffer. The 50 μl of Dynabeads® Protein G was incubated with 10 μg of phospho-Src mouse monoclonal antibody (Merck Millipore, Burlington, MA) for 30 min at RT. The Dynabeads-Ab complex was then resuspended with the cell lysate and incubated on a rotation rocker at RT for 2h. The Dynabeads-Ab-Antigen complexes were washed and eluted according to the manufacturer’s instructions, separated on SDS-PAGE, and analyzed by western blotting with anti-phospho-EGFR (Cell Signaling) and anti-phospho-Src (Merck Millipore).
2.10. Statistical Analyses
Nonparametric tests were used to perform statistical analyses. The Mann-Whitney Exact test (Conover, 1995) was used to compare proliferation and western blot band intensities between treated and untreated. The Mann-Whitney Exact test was also used to compare treated to untreated for staining intensities. The student T-test was used to analyze PLA dots/cell between treated and un treated groups. For the real-time RT-PCR studies, statistical analyses were performed on the normalized values with housekeeping genes using the ΔΔCt method. All data are displayed with standard error of the mean calculated from independent experiments.
3. Results
3.1. BPA stimulates cell proliferation in human ht-UtLM cells
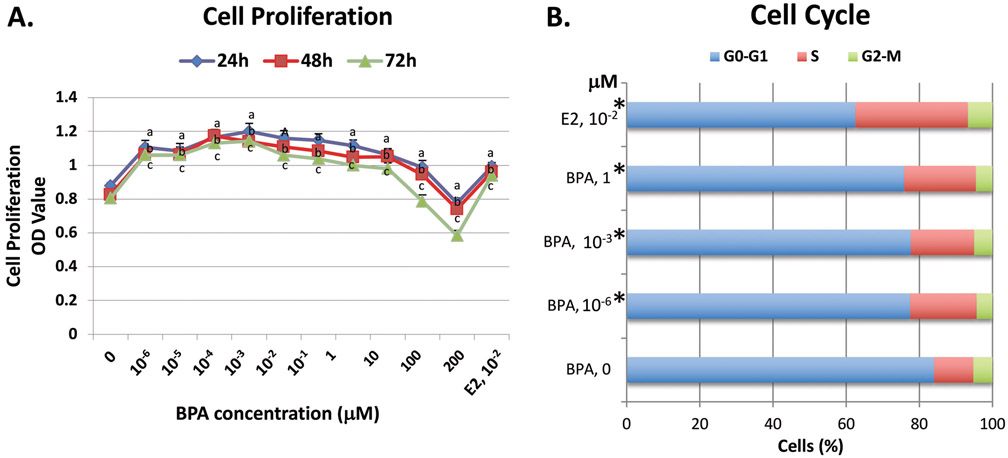
To determine the cell proliferation potential of BPA, ht-UtLM cells were exposed to a wide range of ultra-low to low doses (0, 10−6, 10−5, 10−4, 10−3, 10−2, 10−1, 1, 10, 100 and 200 μM) of BPA for 24, 48 and 72h. Cell proliferation, measured by an MTS assay, was significantly increased (P<0.05) in ht-UtLM cells at doses from 10−6 (1 pM) to 10 μM at 24, 48 and 72h and significantly decreased (P<0.05) at doses of 100 μM at 72h, and 200 μM at all time points compared to the cells without BPA exposure, and formed non-monotonic, inverted U-shaped, curves (Fig. 1A). The proliferative responses to BPA were similar to that of E2 at 10−2 μM (a positive control) (Fig. 1A).
Fig. 1. The effects of BPA on cell proliferation and cell cycle progression in ht-UtLM cells.
(A) BPA at doses of 0, 10−6, 10−5, 10−4, 10−3, 10−2, 10−1, 1, and 10 μM significantly increased cell proliferation in ht-UtLM cells at 24h, 48h, and 72h; whereas, growth was inhibited at 100 and 200 μM as measured by the MTS assay. E2 at 10−2 μM served as a positive control. The absorbance of MTS was expressed as mean±SE of 6 wells in 96-well plates for each treatment condition with experiments done independently three times. aP<0.05 compared to control at 24h; bP<0.05 compared to control at 48h; cP<0.05 compared to control at 72h. (B) The percentage of ht-UtLM cells in the different phases of the cell cycle as determined by flow cytometry. When ht-UtLM cells were treated with 0, 10−6, 10−3, or 1 μM BPA or E2 for 24h, there were more cells in the S phase versus the G0-G1 phase (*P<0.005) in treated cells compared to controls. Cell cycle progression was expressed as the percentage of cells in the different cell cycle phases and represents three independent experiments.
3.2. BPA promotes cell cycle progression of human ht-UtLM cells into the S phase
The percentage of cells in the different phases of the cell cycle were obtained by flow cytometry to evaluate the effects of BPA on cell cycle progression in ht-UtLM cells (Fig. 1B). It appears that BPA at concentrations of 10−6, 10−3, and 1 μM in ht-UtLM cells remarkably altered the cell cycle by shifting cells from G0-G1 to S phase (P<0.005) and increasing the percentage of cells in the S phase, whereas the number of cells in G2-M phase remained unchanged as compared to controls at 24h (Fig. 1B). These data supported the findings of BPA induced increased proliferation in ht-UtLM cells (see Fig. 1A).
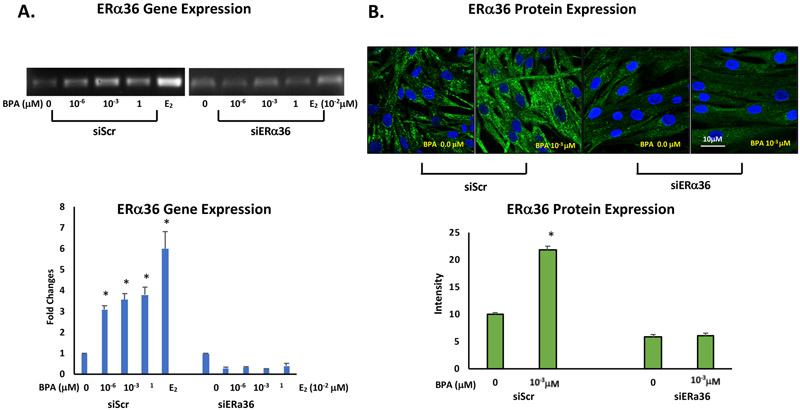
3.3. BPA induces ERα36 gene expression and increases ERα36 protein levels in ht-UtLM cells
The ERα36 gene and protein expression induced by BPA was determined by real time RT-PCR and immunofluorescence confocal microscopy, respectively. Total RNA extracted from BPA-treated (10−3 μM) ht-UtLM cells transfected with siERα36 or siScr fragments was examined for gene expression levels of ERα36. There was a significant increase in ERα36 gene expression with fold changes of 3.09, 3.56 and 3.80 detected for cells treated with BPA at 10−6, 10−3, and 1 μM, respectively, compared to untreated controls 0 μM (p<0.05; Fig. 2A). Additionally, immunofluorescence staining and confocal microscopy revealed ERα36 protein was predominantly localized to the cytoplasm. At 24h, following an exposure to 10−3 μM BPA, ERα36 expression was significantly increased in the cells compared to cells without treatment (p<0.001; Fig. 2B). However, when ERα36 was knocked down by siERα36, the increased expression of both gene and protein levels of ERα36 were attenuated. The data confirmed ERα36 expression at the gene and protein levels is responsive to BPA exposure, and may play a role in BPA induced cell proliferation of ht-UtLM cells.
Fig. 2. BPA induces ERα36 gene and protein expression in ht-UtLM cells.
Gene expression and protein expression of ERα36 in ht-UtLM cells transfected with siScr or siERα36 and treated with BPA at 10−3μM for 24h were evaluated by PT-PCR, and immunofluorescence staining. (A) ERα36 gene expression by RT-PCR presented as fold-changes. There was increased ERα36 gene expression in ht-UtLM cells treated with BPA at 10−6, 10−3, 1 μM compared to untreated cells (*p<0.05). The fold changes in gene expression were expressed as mean±SE in three wells in RT-PCR plates. (B) Representative images of immunofluorescence staining of ERα36 in the ht-UtLM cells treated with BPA at 10−3 μM for 24h (Scale bars = 10 μm); and bar graph comparing the intensity of fluorescent signal in cells treated with BPA showed higher expression of ERα36 compared to the cells without treatment (*p<0.0001). Both treated and untreated ht-UtLM cells stained positive for ERα36 and expression was localized to the cytoplasm of the cells. However, all elevated gene and protein expression levels were diminished when ERα36 was knocked down (siERα36). The graph shows mean ±SE of intensity of 6 fields of confocal images.
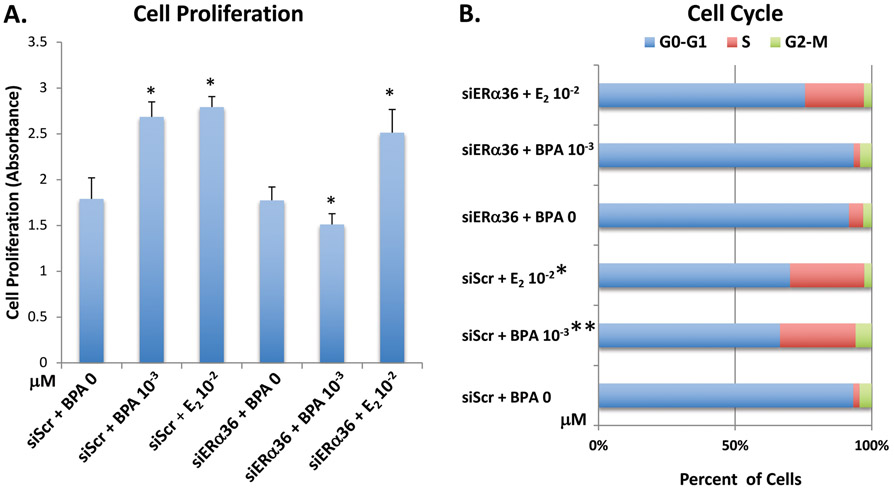
3.4. ERα36 is involved in BPA stimulated cell proliferation and cell cycle progression
To determine the involvement of ERα36 in the ht-UtLM cell proliferation and cell cycle progression, a silencing RNA technique was applied. The cells were transfected with siERα36 or siScr fragments then treated with 10−3 μM of BPA. The proliferative effect of BPA on ht-UtLM cells was abolished when the functional ERα36 was knocked down by siERα36 in cells treated with BPA compared to the cells transfected with siScr (scrambled RNA fragment) and containing a functional ERα36 (p<0.05; Fig. 3A). Additionally, there was an alteration in cell cycle progression when cells were transfected with siERα36 (P<0.0008), in that the progression effects induced by BPA were diminished, and S phase remained unchanged (Fig. 3B). Therefore, these results further support the hypothesis that ERα36 is involved in the proliferative effects observed in ht-UtLM cells induced by BPA.
Fig. 3. ERα36 knockdown inhibits the proliferation and cell cycle progression of ht-UtLM cells induced by BPA.
(A) Proliferation of ht-UtLM cells transfected with siScr or siERα36 and treated with BPA at 10−3μM for 24h as measured by the MTS assay. The bar graph shows the mean ± SE values of absorbance of 6 wells of 96-well plates for each treatment group and indicates the increased cell proliferation induced by BPA at 10−3 μM was abolished by siERα36. *P<0.05 versus controls. (B) Relative percentage of ht-UtLM cells with siScr or siERα36 in the different phases of the cell cycle following 10−3μM BPA for 24h by flow cytometry analysis. The values of cell cycle analysis represent the number of cells in the different phases of the cell cycle as a percentage of total cells observed. The data revealed there were more cells in the S phase versus the G0-G1 phase (*P<0.008) following BPA treatment; however, this effect was inhibited by siERα36. The data shown represent three independent experiments.
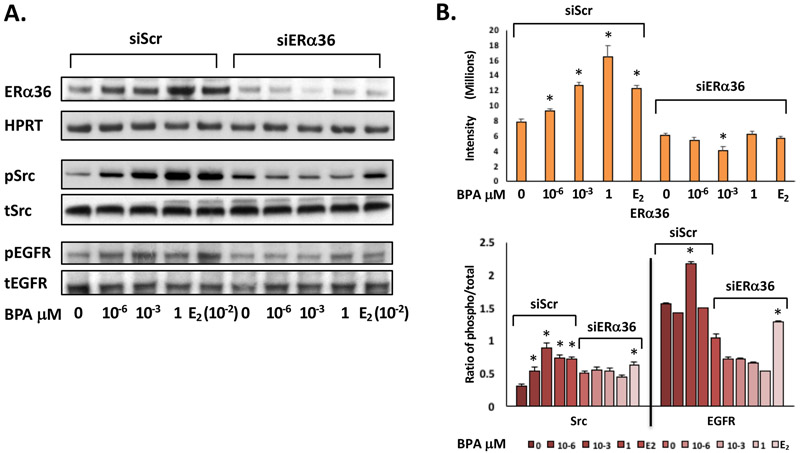
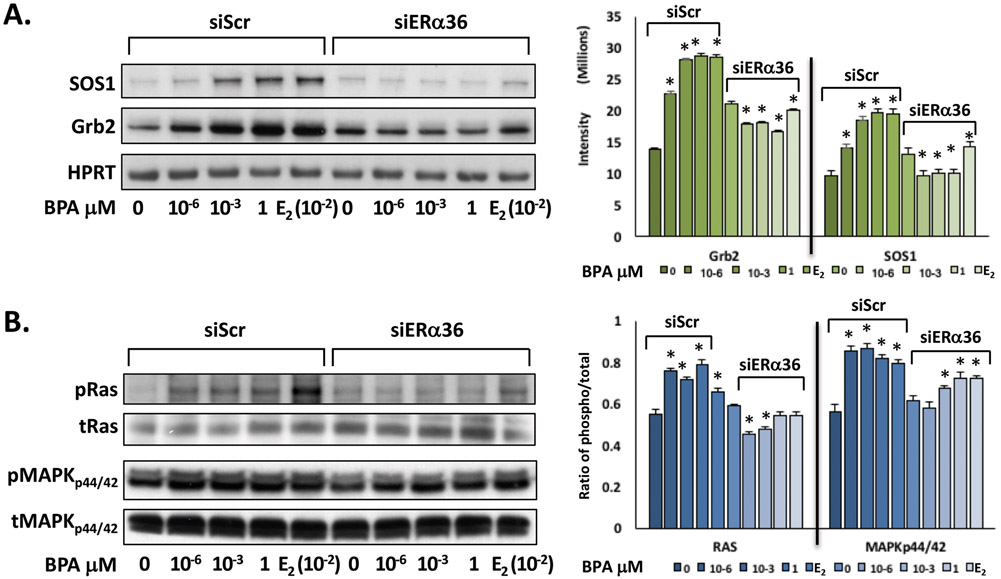
3.5. BPA induces upregulation of ERα36 and MAPK signaling through receptor tyrosine kinase activation via Src phosphorylation in ht-UtLM cells
To determine whether BPA could activate the MAPK pathway through phosphorylation of protein kinases and receptor tyrosine kinases (RTKs), ht-UtLM cells were exposed to BPA (0, 10−6, 10−3, 1 μM) for 10 min, and western blots of cell lysates were probed with anti-phosphorylated and anti-total Src and EGFR. The cells exposed to BPA were found to have increased tyrosine phosphorylation of Src and EGFR. Both phosphorylated protein expression levels were increased significantly in the siScr transfected cells exposed to BPA compared to the controls without BPA exposure (p<0.05; Fig. 4A). However, phosphorylated Src and EGFR expression levels were attenuated in the cells transfected with siERα36 when treated with BPA (Fig. 4A and 4B), which indicated that BPA could induce increased expression of ERα36; concurrently the phosphorylation levels of receptor tyrosine kinase, EGFR and downstream proteins, Src and MAPK were also increased.
Fig 4. ERα36 mediates the activation of Src, EGFR, and MAPK in ht-UtLM cells exposed to BPA.
(A) Representative western blots and (B) band intensity bar graphs of ERα36 and phosphorylated over total (phospho/total) ratios of intermediary proteins (Src) and the receptor tyrosine kinase (EGFR) in ERα36 knockdown (siERα36) or scrambled RNA (siScr) transfected ht-UtLM cells exposed to BPA for 24h or 10 min. There were significantly (*P<0.05) higher expression levels of ERα36, phospho-EGFR (pEGFR), and phospho-Src (pSrc) in the cells treated with BPA with a functional ERα36 (siScr) compared to controls. However, all elevated protein and phosphorylation levels were diminished when ERα36 was knocked down (siERα36). The western band images shown are representative of three independent experiments and the data were expressed as mean±SE done in three independent experiments.
3.6. BPA induces increased expression of downstream MAPK-associated SOS1 and Grb2 signaling molecules
In order to determine the role of ERα36 and receptor-associated transmembrane signaling molecules in ht-UtLM cells following BPA treatment, protein expression of downstream MAPK-associated SOS1 and Grb2 signaling molecules were determined by western blot analysis with or without siERα36. The data indicated that BPA at 10−6, 10−3, 1 μM increased protein expression of ERα36, SOS1 and Grb2 in ht-UtLM cells as determined by western blotting and densitometric analysis (p<0.05; Fig. 5A). However, knockdown of ERα36 gene expression with siERα36 abolished these effects. The base-line expression of human hypoxanthine phosphoribosyl transferase (HPRT) was used as a housekeeping protein.
Fig 5. ERα36 mediates the expression of intermediary proteins of the MAPK signaling pathway in ht-UtLM cells exposed to BPA.
(A) Representative western blots and bar graphs of intermediary proteins (SOS1 and Grb2) and (B) ratios (phospho over total) of signaling proteins (Ras and MAPKp44/42) in the MAPK pathway in ht-UtLM cells with siERα36 or siScr RNA exposed to BPA for 24h or10 min. There were significantly higher expression levels of SOS1 and Grb2, phosphorylated Ras (P<0.04), and MAPKp44/42 (*P<0.05) in the cells treated with BPA with a functional ERα36 (siScr) compared to controls. However, all elevated protein and phosphorylation levels were diminished when ERα36 was knocked down (siERα36). The western band images shown are representative of three independent experiments and the intensity and ratio of phosphor/total are expressed as mean±SE.
3.7. BPA activates downstream effector molecules of MAPK pathways in ht-UtLM cells
To investigate whether BPA could mediate MAPK activation, downstream of SOS1 and Grb2 in ht-UtLM cells, the cells were exposed to BPA (0, 10−6, 10−3, 1 μM) and evaluated by western blotting for activation of downstream mediators Ras and MAPKp44/42. The data showed that a short exposure period of 10 min to various doses of BPA induced rapid, increased expression of phosphorylated Ras (pRas) and MAPKp44/42 (pMAPKp44/42) in the cells. Knocking down ERα36 with siERα36 resulted in a reduction in phosphorylation of Ras and MAPKp44/42 compared with ht-UtLM cells with siScr exposed to the same amount of BPA (Fig. 5B). Densitometric scanning and ratio of phosphorylated/total protein showed that Ras and MAPKp44/42 followed similar patterns, and were significantly increased in ht-UtLM cells transfected with siScr and treated with BPA compared with untreated ht-UtLM cells (P<0.01–0.05; Fig. 5B); and that phosphorylated Ras and MAPKp44/42 levels in ht-UtLM cells transfected with siERα36 were approximately decreased to the levels seen in controls without BPA treatment (Fig. 5B).
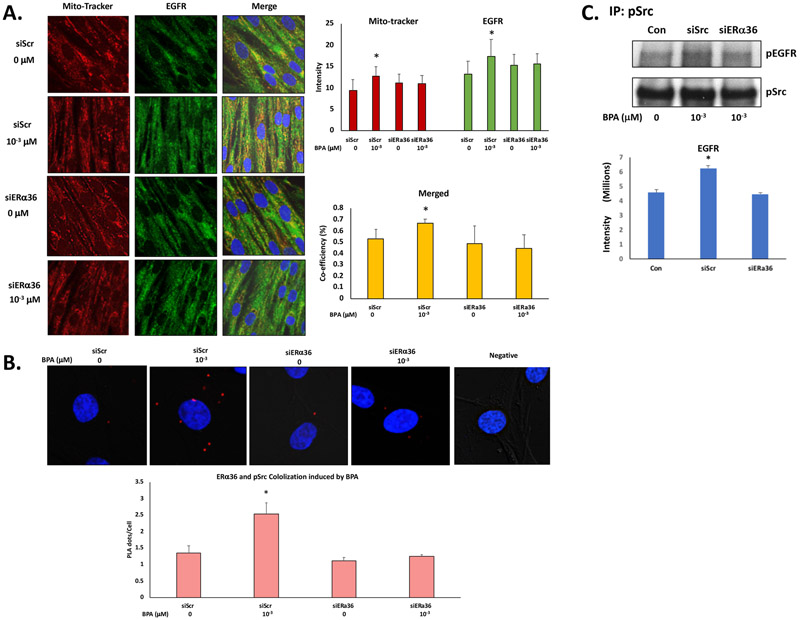
3.8. BPA induces association of phospho-Src with ERα36 and EGFR in ht-UtLM cells
To determine the expression and if there was association of ERα36, EGFR and phosphorylated Src following BPA exposure with or without a functional ERα36, ht-UtLM cells were transfected with siScr or siERα36 and exposed to 10−3 μM of BPA for 10 min, then analyzed by immunofluorescence confocal microscopy, PLA assay and coimmunoprecipitation analysis. EGFR has been previously reported to be located in the mitochondria (Demory, Boerner, Davidson et al., 2009) and we wanted to assess whether BPA treatment could facilitate the localization of EGFR to mitochondria. The cells were stained with DAPI (blue) for nuclei and MitoTracker (Demory et al., 2009) for mitochondria localization, and a specific primary antibody was used for staining EGFR (Fig. 6A). The data showed increased expression of EGFR and partial localization in mitochondria when the cells were treated with BPA (Fig. 6A). These effects were abolished when the ERα36 gene was knocked down by siERα36 fragments (Fig. 6A). Phospho-Src and ERα36 colocalized together in the cells with more PLA dots/cell in the cells with a functional ERα36 induced by BPA compared to the control. When ERα36 gene expression was knocked down by siERα36 fragments, the increased PLA dots/cell of ERα36 and phospho-Src were diminished (Fig. 6B).
Fig. 6. ERα36, EGFR and phospho-Src were coexpressed and colocalized in ht-UtLM cells treated with BPA.
(A) Representative images of immunofluorescence staining and confocal microscopy taken of ht-UtLM cells treated with BPA (10−3 μM) for 10 min following siERα36 or siScr transfections. EGFR=green, MitoTracker=red, DAPI=blue, and colocalization=yellow, Bar graph of semi-quantitated staining intensity and correlation coefficient of colocalization of MitoTracker and EGFR. (B), Representative images of PLA of ERα36 and phosphorylated Src (pSrc) induced by BPA at 10–3 μM for 10min in siScr and siERα36 transfected ht-UtLM cells. The colocaliztion of ERα36 and pSrc revealed by dots = red. The cells were counterstained with DAPI = blue to show the nuclei. The graph shows mean ±SE of red dots counted in 100 – 200 cells. The PLA dots/cell was significantly higher (*P<0.05) in the cells with functional ERα36 (siScr) treated with BPA compared with controls; however, the level PLA dots/cell was diminished by ERα36 knockdown (siERα36). (C) Coimmunoprecipitation of phospho-EGFR and phospho-Src in ht-UtLM cells treated with BPA (10−3 μM) for 10 min in the presence of siERα36 or siScr, Concentrations of phosphorylated EGFR associated with phospho-Src were significantly higher (P < 0.05) in the cells with functional ERα36 (siScr) treated with BPA compared with controls. However, there was no difference in levels of phosphorylated EGFR associated with phospho-Src compared to control when ERα36 was knocked down (siERα36). The band of coimmunoprecipitation and confocal images shown are representative of three independent experiments and the intensity and correlation coefficients are expressed as mean±SE done in three independent experiments;
To determine if there was an association between increased levels of phospho-EGFR and phospho-Src, which could initiate MAPK pathway signaling induced by BPA, an immunoprecipitation technique was applied to pull down phospho-EGFR with phospho-Src (Fig. 6C). We found that more phospho-EGFR was precipitated with the phospho-Src in cells treated with BPA compared to cells without BPA treatment with a functional ERα36 (P < 0.05); however, in cells without a functional ERα36, the increased coimmunoprecipitation was not seen, which suggests that pEGFR interacted with pSrc to activate the MAPK pathway, and ERα36 plays an important role in mediating its activation through crosstalk or independently in the cells treated with BPA.
4. Discussion
BPA is present ubiquitously in the environment and in human tissues due to its widespread use (Gao et al., 2015). The structural and functional similarity of BPA to that of estrogen substantiates BPA’s xenoestrogenic potential. Transplacental exposures to BPA causes alterations of the gene expression profiles in E2-sensitive tissues of rats, particularly ovary and uterus (Naciff, Jump, Torontali et al., 2002). Neonatal exposures in mice have been found to produce uterine tumors, such as leiomyomas, later in life (Newbold, Jefferson and Padilla-Banks, 2009). In our study, we found BPA induced increased proliferation of human uterine leiomyoma cells that was well comparable to that of E2, and provided evidence that BPA is a xenoestrogen that influences leiomyoma cell growth at very low levels. The proliferative responses observed were within a dose-range of pico- to micromolar concentrations in vitro, which is well within the recommended human (children/adult) exposure range (Richter et al., 2007,Wetherill et al., 2007,2010). Our findings are also consistent with other groups that have reported BPA exposures target cell types in the uterus and can induce pathologic changes such as adenomyosis, leiomyoma, atypical hyperplasia, and stromal polyps in rodents at human exposure levels (Newbold et al., 2007). BPA also stimulates proliferation in human breast cancer cells (Wu, Wei and Hao, 2009); therefore, BPA is a xenoestrogen that may impact human health at environmentally relevant concentrations.
BPA also remarkably altered cell cycle progression in this study by significantly increasing the percentage of cells in the S phase (DNA synthesis) while decreasing those in G0-G1 phase in BPA-treated ht-UtLM cells compared to controls. BPA, like E2, also promotes cell proliferation and DNA synthesis in ER-positive ovarian, prostate, and endometrial cancer cells (Liu, Xu, Yin et al., 2010). Likewise, BPA-mediated cell entry into S phase implies the triggering of DNA replication that promotes the cell proliferation observed in leiomyoma cells in the present study.
Rapid responses to E2 and E2-BSA are seen in ERα-negative HCC38 and ERα-positive MCF-7 breast cancer cells (Boyan, Sylvia, Frambach et al., 2003). Blockers of ERα/ERβ ICI 182780 failed to inhibit BPA mediated proliferative responses in human normal breast cells and in ER negative breast cancer cells (Wu, Wei, Jiang et al., 2012), which suggests that besides conventional ERs, alternative membrane-associated receptor mediated rapid signaling pathways may operate in both BPA and E2 induced cell proliferation (Wetherill et al., 2007). There are several publications that state BPA, at or below human exposure levels (10−12 to 10−7 M), stimulates proliferation of human testicular JKT-1 cells via a nongenomic action that is independent of classical ERs (Bouskine, Nebout, Brucker-Davis et al., 2009). Another alternative nongenomic signaling pathway is the G protein-coupled estrogen receptor (GPR30 or GPER) found in ER-positive and ER-negative breast cancer cells (Filardo, Quinn, Pang et al., 2007). It is proposed that the ERα36 and GPR30 nongenomic pathways are integrated in that it has been suggested that the GPR30 nongenomic signaling pathway activation of pERK1/2 is directly mediated by ERα36, and not by GPR30, because the activities of GPR30 promoted by E2 are due to induction of ERα36 expression (Kang, Guo, Zhang et al., 2011). In addition to the above observations, which prompted us to look at ERα36, BPA’s binding affinity to ERα and β is a thousand-times lower than E2 (Gao et al., 2015, Kuiper et al., 1998,2010); http://lib.znate.ru/docs/index-20398.html?page=47). A variety of signaling pathways from the cell membrane to the nucleus may be driving BPA-mediated leiomyoma cell proliferation. Several studies have shown that cell proliferation induced by BPA in human breast cancer cells is through signaling (Lee, Hwang, Park et al., 2012) via ERα membrane-associated receptors and/or nongenomic pathways (Wu et al., 2012).
ERα36 is a variant of ERα66 first identified and cloned by Wang’s group in 2005 (Wang, Zhang, Shen et al., 2005). ERα36 is located on the plasma membrane and within the cytoplasm, although there has been controversy as to its exact subcellular localization. We have found that ERα36 is mostly in mitochondria as we have observed significant Pearson’s correlation coefficients for colocalization of ERα36 with mitochondria by confocal microscopy in ht-UtLM cells, and also uterine smooth muscle cells (Yan, Yu, Castro et al., 2017). It is proposed that ERα36 functions as a mediator of rapid membrane-initiated, nongenomic, signal-regulated, kinase mitogenic signaling pathway (2010,Chaudhri et al., 2012,Wang et al., 2006,Wang et al., 2005,Gu, Chen, Lopez et al., 2014,Lin, Yan, Liang et al., 2009). In the present study, low doses of BPA significantly induced gene and protein expression of ERα36 and triggered entry of cells into S phase, and promoted cell proliferation suggesting a nongenomic pathway triggering that led to activation of mitogenic signaling molecules and initiation of cell proliferation.
The elevated level of ERα36 gene and protein expression in BPA treated ht-UtLM cells has driven us to knockdown ERα36 gene expression to observe the effects of BPA on ht-UtLM cells without a functional ERα36. As expected, the promotion of cell proliferation and cell cycle progression induced by BPA was abrogated, which strongly suggests the involvement of ERα36 in the BPA’s mitogenic effects in ht-UtLM cells. To further examine the effects of BPA on nongenomic signaling at concentrations relevant to human exposure levels, ht-UtLM cells with or without a functional ERα36 were used to identify the predominant ERα36 signaling proteins through which BPA initiated nongenomic signaling. We found increased expression of ERα36 in leiomyoma cells and associated increased expression of intermediary proteins, SOS1 and Grb2, in cells, which suggested a coordinated induction and activation of intracellular signaling molecules involved in Ras-ERK/MAP kinase signaling as seen in this study and observed in our previous studies with other xenoestrogens (Di, Yu, Moore et al., 2008). Thus, our findings of BPA mediated initiation and triggering of rapid transmembrane signaling via ERα36 and induction of intracellular signaling molecules provides evidence for nongenomic actions of BPA in leiomyoma cells. Our conclusions were further strengthened by the fact that these signaling molecules were significantly increased and/or activated in BPA-treated cells that had a functional ERα36 compared to untreated control cells, or cells where the functional ERα36 was silenced, and these effects were abrogated. Together, these observations would suggest that the nongenomic MAPK signaling pathway was activated, and contributed to the enhanced cell proliferation and cell cycle progression caused by BPA exposure; and that ERα36 played an important role in initiating MAPK signaling through the intracellular molecules SOS1 and Grb2.
The thought of what mediator or mediators bridged the ERα36 and MAPK pathway activation motivated us to further examine whether ERα36 mediated nongenomic MAPK pathway activation was though the activation of receptor tyrosine kinases (RTKs) via Src phosphorylation. The Src (proto-oncogene c-Src) is a non-receptor tyrosine kinase whose expression and activity has led to a diverse array of biological functions including proliferation, cell growth, differentiation, cell shape, motility, migration, angiogenesis, and survival (Wheeler, Iida and Dunn, 2009). The Src protein contains multiple domains for specific attachment to membranes (Kaplan, Varmus and Bishop, 1990). Several investigators discovered a significant coexpression of ERα36 and epidermal growth factor receptor (EGFR) in primary breast cancers, suggesting that ERα36 took part in EGFR-related carcinogenesis (2010,Su, Xu, Li et al., 2014,Vranic, Gatalica, Deng et al., 2011). A positive feedback loop was confirmed that EGFR signaling activated transcription of ERα36 through an activator-protein-1-binding site in the promoter of ERα36. In turn, ERα36 interacted with the EGFR/Src complex to strengthen the EGFR signaling pathway and stabilize EGFR protein (Zhang, Kang, Ding et al., 2011). It has also been reported that EGFR, Src and ERα36 could be located in mitochondria (2010,Demory et al., 2009,Yan et al., 2017,Hebert-Chatelain, 2013), and Src could function as a switch in ERα36-mediated mitogenic signaling through EGFR phosphorylation in ER-negative breast cancer cells (Zhang, Ding, Kang et al., 2012). In the present study, BPA induced transmembrane ERα36 receptor expression and increased Src and EGFR phosphorylation, which indicated that ERα36 plays an integral positive role in rapid nongenomic signaling in leiomyoma cell proliferation, and this all may be mediated through the activation of EGFR through Src phosphorylation. Activation of Scr/EGFR leads to the signaling cascade of Ras-ERK/MAP kinases (Yu, Moore and Dixon, 2010,McKay and Morrison, 2007). Activated EGFR could interact with the adapter protein, SH2. The SH2-containing collagen-related proteins (Shc) and the growth-factor-receptor binding protein Grb2 initiate signaling via Ras and MAP kinase (Li, Batzer, Daly et al., 1993,Rozakis-Adcock, Fernley, Wade et al., 1993). A Grb2-SOS1 interaction provides a key regulatory mediator that relays signaling from activated RTKs to the downstream effector molecules particularly Ras. Thus, low-dose BPA mediated multiple signaling pathways that originate at the cell membrane by interacting with the membrane / cytosolic ERα36 concurrent with the EGFR and its downstream target and associated proteins and protein complexes to initiate rapid signaling that converges in the nucleus for transcription of proliferation factors and possibly tumor promotion. The colocalization of ERα36 and phosphorylated Src observed by PLA (Bustosa et al., 2017) further provides evidence of a bridge role of Src in ERα36 mediated EGFR/MAPK signaling and pathway activation induced by BPA.
5. Conclusions
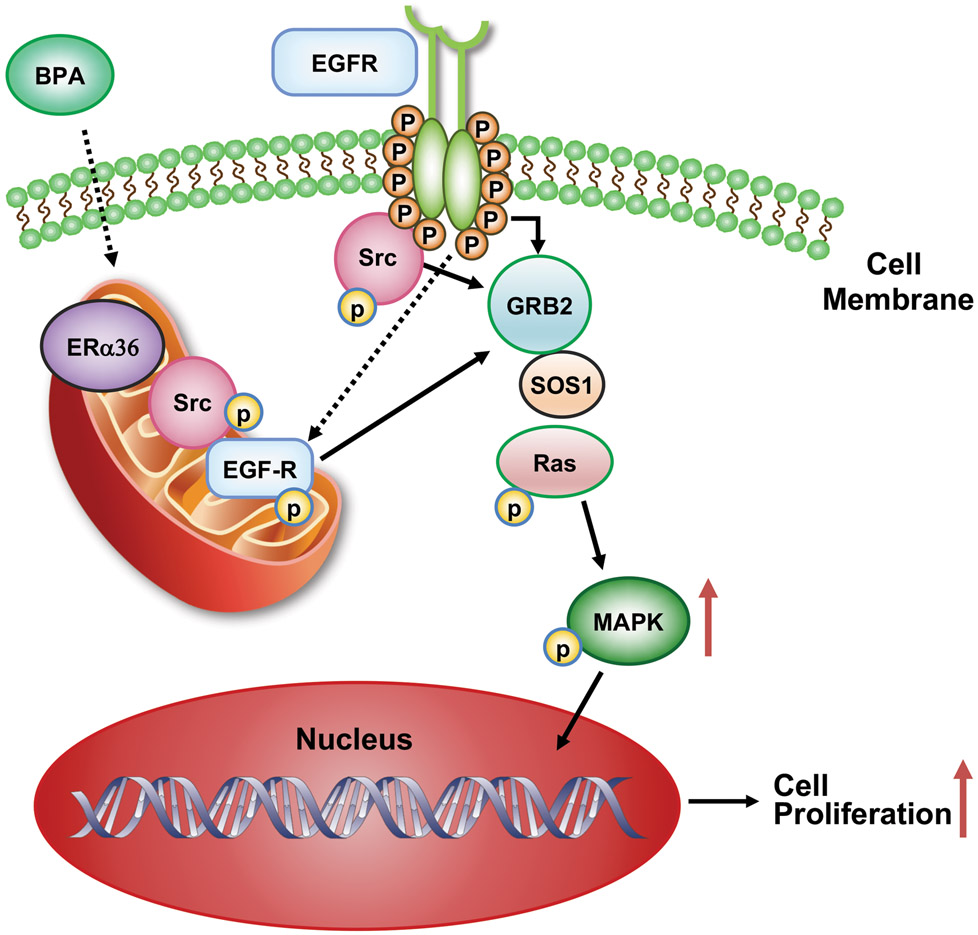
The results of this study demonstrate that the interaction of BPA with the membrane / cytosolic ERα36 receptor presumably is through its ligand binding domain. BPA concurrently activates EGFR through Src phosphorylation, which in turn upregulates associated signaling complexes including the molecules SOS1, Grb2, and Ras that lead to activation of downstream effector MAPKp44/42 resulting in leiomyoma cell cycle progression by inducing cell entry into the S phase from G0-G1 phase leading to cell proliferation (Fig. 7). Thus, ERα36 appears to play a pivotal role in BPA-induced MAPK signaling. Human exposure to environmental concentrations of BPA and at its recommended human exposure levels may be an inducer of hormonally responsive reproductive tract tumors including uterine fibroids, implying as a risk factor for humans.
Fig. 7.
Schematic illustration of proposed nongenomic signaling pathways induced by a low dose of BPA through ERα36 with activation of pSrc, pEGFR, and MAPK.
References:
- [1].Gao H, Yang BJ, Li N, Feng LM, Shi XY, Zhao WH and Liu SJ, 2015. Bisphenol A and hormone-associated cancers: current progress and perspectives, Medicine (Baltimore). 94, e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, Padmanabhan V, Taylor HS, Swan SH, VandeVoort CA and Flaws JA, 2014. Bisphenol a and reproductive health: update of experimental and human evidence, 2007–2013, Environ Health Perspect. 122, 775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR and vom Saal FS, 2007. In vivo effects of bisphenol A in laboratory rodent studies, Reprod Toxicol. 24, 199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ashby J and Odum J, 2004. Gene expression changes in the immature rat uterus: effects of uterotrophic and sub-uterotrophic doses of bisphenol A, Toxicol Sci. 82, 458–67. [DOI] [PubMed] [Google Scholar]
- [5].Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B and Gustafsson JA, 1998. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta, Endocrinology. 139, 4252–63. [DOI] [PubMed] [Google Scholar]
- [6].Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT and Belcher SM, 2007. In vitro molecular mechanisms of bisphenol A action, Reprod Toxicol. 24, 178–98. [DOI] [PubMed] [Google Scholar]
- [7].Dong S, Terasaka S and Kiyama R, 2011. Bisphenol A induces a rapid activation of Erk1/2 through GPR30 in human breast cancer cells, Environ Pollut. 159, 212–218. [DOI] [PubMed] [Google Scholar]
- [8]., 2010. Environment and Human Health: The Role of HHS, Statement of Linda Birnbaum, Committee on Energy and Commerce. US Government Printing Office, Washington DC, pp. 16–21. [Google Scholar]
- [9].Chaudhri RA, Olivares-Navarrete R, Cuenca N, Hadadi A, Boyan BD and Schwartz Z, 2012. Membrane estrogen signaling enhances tumorigenesis and metastatic potential of breast cancer cells via estrogen receptor-alpha36 (ERalpha36), J Biol Chem. 287, 7169–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang Z, Zhang X, Shen P, Loggie BW, Chang Y and Deuel TF, 2006. A variant of estrogen receptor-{alpha}, hER-{alpha}36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling, Proc Natl Acad Sci U S A. 103, 9063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Song H, Zhang T, Yang P, Li M, Yang Y, Wang Y, Du J, Pan K and Zhang K, 2015. Low doses of bisphenol A stimulate the proliferation of breast cancer cells via ERK1/2/ERRgamma signals, Toxicol In Vitro. 30, 521–8. [DOI] [PubMed] [Google Scholar]
- [12].Zhang XL, Wang HS, Liu N and Ge LC, 2015. Bisphenol A stimulates the epithelial mesenchymal transition of estrogen negative breast cancer cells via FOXA1 signals, Arch Biochem Biophys. 585, 10–16. [DOI] [PubMed] [Google Scholar]
- [13].Newbold RR, Jefferson WN and Padilla-Banks E, 2007. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract, Reprod Toxicol. 24, 253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Othman ER, Al-Adly DM, Elgamal DA, Ghandour N and El-Sharkawy S, 2016. Bisphenol A Concentrates Preferentially in Human Uterine Leiomyoma and Induces Proliferation in Rat Myometrium, Reprod Sci. 23, 508–14. [DOI] [PubMed] [Google Scholar]
- [15].Gao X, Yu L, Castro L, Moore AB, Hermon T, Bortner C, Sifre M and Dixon D, 2010. An endocrine-disrupting chemical, fenvalerate, induces cell cycle progression and collagen type I expression in human uterine leiomyoma and myometrial cells, Toxicol Lett. 196, 133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Watson CS, Bulayeva NN, Wozniak AL and Finnerty CC, 2005. Signaling from the membrane via membrane estrogen receptor-alpha: estrogens, xenoestrogens, and phytoestrogens, Steroids. 70, 364–71. [DOI] [PubMed] [Google Scholar]
- [17].Yu L, Moore AB, Castro L, Gao X, Huynh HL, Klippel M, Flagler ND, Lu Y, Kissling GE and Dixon D, 2012. Estrogen Regulates MAPK-Related Genes through Genomic and Nongenomic Interactions between IGF-I Receptor Tyrosine Kinase and Estrogen Receptor-Alpha Signaling Pathways in Human Uterine Leiomyoma Cells, J Signal Transduct. 2012, 204236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Carney SA, Tahara H, Swartz CD, Risinger JI, He H, Moore AB, Haseman JK, Barrett JC and Dixon D, 2002. Immortalization of human uterine leiomyoma and myometrial cell lines after induction of telomerase activity: molecular and phenotypic characteristics, Lab Invest. 82, 719–28. [DOI] [PubMed] [Google Scholar]
- [19].Yu L, Saile K, Swartz CD, He H, Zheng X, Kissling GE, Di X, Lucas S, Robboy SJ and Dixon D, 2008. Differential expression of receptor tyrosine kinases (RTKs) and IGF-I pathway activation in human uterine leiomyomas, Mol Med. 14, 264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jeng YJ, Kochukov M and Watson CS, 2010. Combinations of physiologic estrogens with xenoestrogens alter calcium and kinase responses, prolactin release, and membrane estrogen receptor trafficking in rat pituitary cells, Environ Health. 9, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jeng YJ and Watson CS, 2009. Proliferative and anti-proliferative effects of dietary levels of phytoestrogens in rat pituitary GH3/B6/F10 cells - the involvement of rapidly activated kinases and caspases, BMC Cancer. 9, 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kochukov MY, Jeng YJ and Watson CS, 2009. Alkylphenol xenoestrogens with varying carbon chain lengths differentially and potently activate signaling and functional responses in GH3/B6/F10 somatomammotropes, Environ Health Perspect. 117, 723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liao C, Liu F, Guo Y, Moon HB, Nakata H, Wu Q and Kannan K, 2012. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure, Environ Sci Technol. 46, 9138–45. [DOI] [PubMed] [Google Scholar]
- [24].Liao C, Liu F, Moon HB, Yamashita N, Yun S and Kannan K, 2012. Bisphenol analogues in sediments from industrialized areas in the United States, Japan, and Korea: spatial and temporal distributions, Environ Sci Technol. 46, 11558–65. [DOI] [PubMed] [Google Scholar]
- [25].Conover WJ, 1995. Practical nonparameteric statistics, John wiley & Sons, New York. [Google Scholar]
- [26].Demory ML, Boerner JL, Davidson R, Faust W, Miyake T, Lee I, Huttemann M, Douglas R, Haddad G and Parsons SJ, 2009. Epidermal growth factor receptor translocation to the mitochondria: regulation and effect, J Biol Chem. 284, 36592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Naciff JM, Jump ML, Torontali SM, Carr GJ, Tiesman JP, Overmann GJ and Daston GP, 2002. Gene expression profile induced by 17alpha-ethynyl estradiol, bisphenol A, and genistein in the developing female reproductive system of the rat, Toxicol Sci. 68, 184–99. [DOI] [PubMed] [Google Scholar]
- [28].Newbold RR, Jefferson WN and Padilla-Banks E, 2009. Prenatal exposure to bisphenol a at environmentally relevant doses adversely affects the murine female reproductive tract later in life, Environ Health Perspect. 117, 879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wu S, Wei X and Hao W, 2009. Effect of bisphenol A and diazinon on proliferation of MCF-7 cells, Carcino Terato Muta. 21, 249–253. [Google Scholar]
- [30].Liu W, Xu Q, Yin H, Li C, Sun X, Gu H and Zhang L, 2010. Effects of bisphenol A on the proliferation of three kinds of hormone related tumor cells, Shandong Med. . 50, 9–11. [Google Scholar]
- [31].Boyan BD, Sylvia VL, Frambach T, Lohmann CH, Dietl J, Dean DD and Schwartz Z, 2003. Estrogen-dependent rapid activation of protein kinase C in estrogen receptor-positive MCF-7 breast cancer cells and estrogen receptor-negative HCC38 cells is membrane-mediated and inhibited by tamoxifen, Endocrinology. 144, 1812–24. [DOI] [PubMed] [Google Scholar]
- [32].Wu S, Wei X, Jiang J, Shang L and Hao W, 2012. Effects of bisphenol A on the proliferation and cell cycle of HBL-100 cells, Food Chem Toxicol. 50, 3100–5. [DOI] [PubMed] [Google Scholar]
- [33].Bouskine A, Nebout M, Brucker-Davis F, Benahmed M and Fenichel P, 2009. Low doses of bisphenol A promote human seminoma cell proliferation by activating PKA and PKG via a membrane G-protein-coupled estrogen receptor, Environ Health Perspect. 117, 1053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J and Thomas P, 2007. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane, Endocrinology. 148, 3236–45. [DOI] [PubMed] [Google Scholar]
- [35].Kang L, Guo Y, Zhang X, Meng J and Wang ZY, 2011. A positive cross-regulation of HER2 and ER-alpha36 controls ALDH1 positive breast cancer cells, J Steroid Biochem Mol Biol. 127, 262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lee HR, Hwang KA, Park MA, Yi BR, Jeung EB and Choi KC, 2012. Treatment with bisphenol A and methoxychlor results in the growth of human breast cancer cells and alteration of the expression of cell cycle-related genes, cyclin D1 and p21, via an estrogen receptor-dependent signaling pathway, Int J Mol Med. 29, 883–90. [DOI] [PubMed] [Google Scholar]
- [37].Wang Z, Zhang X, Shen P, Loggie BW, Chang Y and Deuel TF, 2005. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66, Biochem Biophys Res Commun. 336, 1023–7. [DOI] [PubMed] [Google Scholar]
- [38].Yan Y, Yu L, Castro L and Dixon D, 2017. ERalpha36, a variant of estrogen receptor alpha, is predominantly localized in mitochondria of human uterine smooth muscle and leiomyoma cells, PLoS One. 12, e0186078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gu Y, Chen T, Lopez E, Wu W, Wang X, Cao J and Teng L, 2014. The therapeutic target of estrogen receptor-alpha36 in estrogen-dependent tumors, J Transl Med. 12, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lin SL, Yan LY, Liang XW, Wang ZB, Wang ZY, Qiao J, Schatten H and Sun QY, 2009. A novel variant of ER-alpha, ER-alpha36 mediates testosterone-stimulated ERK and Akt activation in endometrial cancer Hec1A cells, Reprod Biol Endocrinol. 7, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Di X, Yu L, Moore AB, Castro L, Zheng X, Hermon T and Dixon D, 2008. A low concentration of genistein induces estrogen receptor-alpha and insulin-like growth factor-I receptor interactions and proliferation in uterine leiomyoma cells, Hum Reprod. 23, 1873–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wheeler DL, Iida M and Dunn EF, 2009. The role of Src in solid tumors, Oncologist. 14, 667–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kaplan JM, Varmus HE and Bishop JM, 1990. The src protein contains multiple domains for specific attachment to membranes, Mol Cell Biol. 10, 1000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Su X, Xu X, Li G, Lin B, Cao J and Teng L, 2014. ER-alpha36: a novel biomarker and potential therapeutic target in breast cancer, Onco Targets Ther. 7, 1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Vranic S, Gatalica Z, Deng H, Frkovic-Grazio S, Lee LM, Gurjeva O and Wang ZY, 2011. ER-alpha36, a novel isoform of ER-alpha66, is commonly over-expressed in apocrine and adenoid cystic carcinomas of the breast, J Clin Pathol. 64, 54–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhang XT, Kang LG, Ding L, Vranic S, Gatalica Z and Wang ZY, 2011. A positive feedback loop of ER-alpha36/EGFR promotes malignant growth of ER-negative breast cancer cells, Oncogene. 30, 770–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hebert-Chatelain E, 2013. Src kinases are important regulators of mitochondrial functions, Int J Biochem Cell Biol. 45, 90–8. [DOI] [PubMed] [Google Scholar]
- [48].Zhang XT, Ding L, Kang LG and Wang ZY, 2012. Involvement of ER-alpha36, Src, EGFR and STAT5 in the biphasic estrogen signaling of ER-negative breast cancer cells, Oncol Rep. 27, 2057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yu L, Moore AB and Dixon D, 2010. Receptor tyrosine kinases and their hormonal regulation in uterine leiomyoma, Semin Reprod Med. 28, 250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].McKay MM and Morrison DK, 2007. Integrating signals from RTKs to ERK/MAPK, Oncogene. 26, 3113–21. [DOI] [PubMed] [Google Scholar]
- [51].Li N, Batzer A, Daly R, Yajnik V, Skolnik E, Chardin P, Bar-Sagi D, Margolis B and Schlessinger J, 1993. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling, Nature. 363, 85–8. [DOI] [PubMed] [Google Scholar]
- [52].Rozakis-Adcock M, Fernley R, Wade J, Pawson T and Bowtell D, 1993. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1, Nature. 363, 83–5. [DOI] [PubMed] [Google Scholar]
- [53].Bustosa V, Pulinaa MV, Bispoa A, Lama A, Flajoleta M, Gorelickb FS, and Greengarda P, 2017. Phosphorylated Presenilin 1 decreases β-amyloid by facilitating autophagosome lysosome fusion, PNAS. vol 114, no.27 7153 [DOI] [PMC free article] [PubMed] [Google Scholar]