Abstract
The glomerulus is a highly specialized microvascular bed that filters blood to form primary urinary filtrate. It contains four cell types: fenestrated endothelial cells, specialized vascular support cells termed podocytes, perivascular mesangial cells, and parietal epithelial cells. Glomerular cell-cell communication is critical for the development and maintenance of the glomerular filtration barrier. VEGF, ANGPT, EGF, SEMA3A, TGF-β, and CXCL12 signal in paracrine fashions between the podocytes, endothelium, and mesangium associated with the glomerular capillary bed to maintain filtration barrier function. In this review, we summarize the current understanding of these signaling pathways in the development and maintenance of the glomerulus and the progression of disease.
Keywords: VEGF, angiopoietin, podocyte, endothelial cell
INTRODUCTION
The glomerulus of the kidney is a highly developed microvascular bed that acts as a filter, allowing small molecules, such as water, sugars, electrolytes, and small proteins, to pass through while retaining high-molecular-weight proteins and cells in the circulation. Glomerular development proceeds through coordinated cross talk between various cell types for spatially ordered and sequential recruitment, proliferation, assembly, and differentiation of endothelial cells (ECs), mesangial cells, and epithelial progenitor cells. Mature glomeruli contain four cell types: parietal epithelial cells, which form Bowman’s capsule; podocytes, which cover the outermost layer of the glomerular filtration barrier; glycocalyx-coated fenestrated ECs, which are in direct contact with blood; and mesangial cells, which support the capillary loops. This review discusses vascular growth factors critical for coordinating glomerular development and function and the role of these factors in glomerular disease.
Overview of the Glomerulus and Filtration Barrier
The epithelial cells of the glomerular barrier, podocytes, are highly differentiated cells. Podocytes line the outside of the glomerular capillaries and face the primary urine and Bowman’s capsule (Figure 1). They have a large cell body, which bulges into the urinary space, and long cytoplasmic primary processes that extend along the capillaries with numerous secondary foot processes. The foot processes of neighboring podocytes regularly interdigitate and are separated by a filtration slit that is bridged by an extracellular structure known as the slit diaphragm. The molecular components of slit diaphragms have been extensively studied, and their proteins are vital for normal glomerular permselectivity. The basal cell membrane, i.e., the soles of the foot processes, mediates the connection to the glomerular basement membrane (GBM). Matrix molecules in the GBM are ligands for transmembrane receptors of the podocyte foot process.
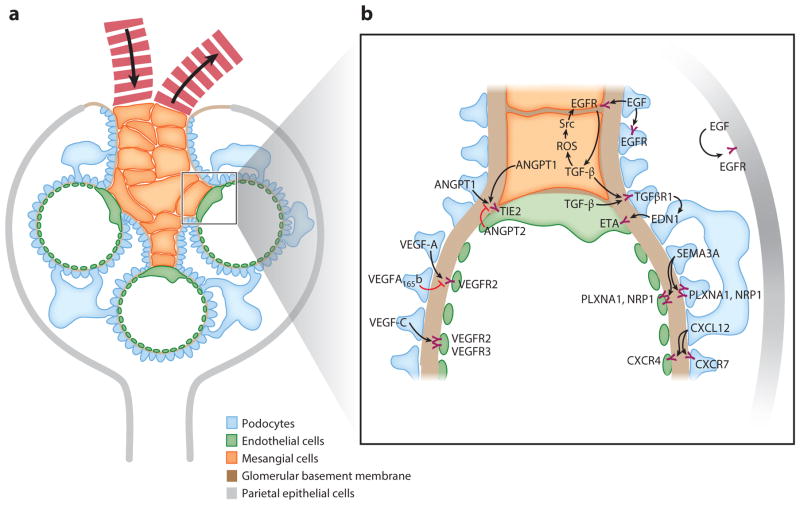
Figure 1.
Schematic of glomerular structure and signaling. (a) A mature glomerulus in cross section. Fewer capillary loops than normal are shown for clarity, and the image is not to scale. The four major cell types of the glomerulus are podocytes, mesangial cells, endothelial cells, and parietal epithelial cells. The glomerulus has a network of capillary loops with mesangial cells forming a nexus at the base of the capillary network. The glomerular basement membrane lies between the podocytes and the endothelial cells and divides the glomerulus into an inner compartment containing capillaries and mesangial cells and an outer one containing podocytes and Bowman’s space, into which the filtrate passes. The arrows in the capillaries indicate the flow of blood into and out of the glomerulus. (b) Summary of signaling pathways between the different cellular compartments of the glomerulus discussed in this review. Abbreviations: ANGPT1, angiopoietin 1; ANGPT2, angiopoietin 2; CXCL12, C-X-C chemokine ligand 12; CXCR, C-X-C chemokine receptor; EDN1, endothelin-1; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; ETA, endothelin-1 receptor A; NRP1, neuropilin-1; PLXNA1, plexin-A1; ROS, reactive oxygen species; SEMA3A, semaphorin 3A; Src, Src tyrosine kinase; TGF-β, transforming growth factor-β; TGFβR1, transforming growth factor-βreceptor 1; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
ECs line the inner surface of the glomerular capillaries and form an antithrombotic barrier between blood and tissues. The glomerular ECs are highly flattened around the capillary loop periphery, where ECs and adjacent podocytes share a common GBM. To allow for high permeability of water and small solutes, the glomerular ECs have large fenestrations constituting 20–50% of the entire endothelial surface (Figure 2). The EC body is thicker, nonfenestrated, and usually located near the hilum of the capillary loop, which is often in direct contact with mesangial cells. More recently, evidence is accumulating that ECs and their fenestrae are covered by a relatively thick layer of negatively charged proteins. Some of these negatively charged proteins are anchored in the EC plasma membrane and form a glycocalyx, whereas other such proteins are noncovalently associated proteins from plasma or ECs and form the endothelial surface layer [reviewed by Haraldsson et al. (1)].
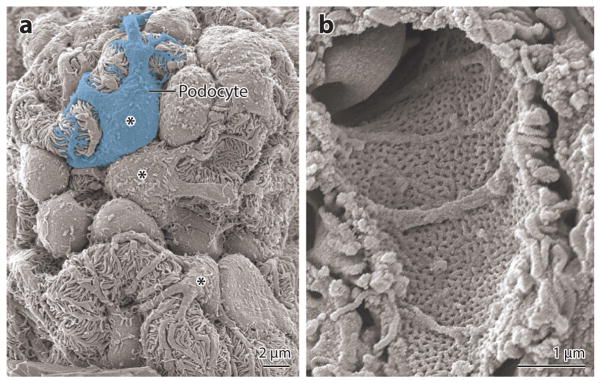
Figure 2.
Scanning electron micrographs of mouse glomeruli. (a) View from the urinary space showing several capillary loops and podocyte cell bodies (marked by asterisks) with their foot processes wrapping around capillaries. (b) View from the capillary lumen showing a fenestrated glomerular capillary.
The GBM is a specialized extracellular matrix that supports its adherent cells, the glomerular ECs and podocytes, and separates the vasculature from the urinary space. During development, the GBM is formed from fusion of two distinct basement membranes, one synthesized by presumptive podocytes and the other by glomerular ECs. Similar to all basement membranes, the GBM is a fibrous network consisting of laminin, collagen type IV, nidogens, and proteoglycans such as agrin and perlecan.
The glomerular mesangium maintains the structure and function of the capillary loops and is located between the capillaries (2). Mesangial cells are surrounded by their secreted extracellular matrix, which is composed mainly of collagen IV, laminin, fibronectin, and proteoglycans (3). Mesangial cells can respond to injury by changing to a myofibroblastic phenotype, resulting in altered mesangial matrix (4). Switching to a myofibroblastic phenotype results in the production of matrix components other than collagen IV and has significant pathological consequences. This switch represents a major factor in the progression to glomerulosclerosis because glomeruli lack the necessary machinery (matrix metalloproteinases) to degrade the newly synthesized matrix components (2).
Glomerular Cell-Cell Communication
Glomerular cell-cell communication is essential for adequate development and maintenance of the glomerular barrier. Several factors discussed in this review exhibit a similar paracrine signaling paradigm (Figure 1b). The podocytes act as vascular support cells and produce factors that are ligands for receptors expressed by the glomerular endothelium. Several studies in the last decade have shown the importance of the vascular endothelial growth factor A (VEGF-A)–VEGF receptor 2 (VEGFR2) paracrine system in glomerular development and maintenance. More recently, angiopoietin 1 (ANGPT1)-induced receptor tyrosine kinase (TIE2; also termed Tek) activation and C-X-C chemokine ligand 12 [CXCL12, also known as stromal cell–derived factor 1 (SDF1)] activation of C-X-C chemokine receptor type 4 (CXCR4) on ECs have been found to be important for the development of glomerular capillaries. Angiopoietin 2 (ANGPT2) signals in an endocrine or an autocrine fashion and is produced and released by ECs. It also binds TIE2 but can act as an agonist or antagonist for TIE2, depending on the context. Increased levels of ANGPT2 have been implicated in vascular diseases and appear to be correlated to adverse outcomes. Another newly implicated system in podocyte–to–endothelial cell cross talk is transforming growth factor-β receptor (TGFRβ)–induced endothelin-1 expression. Endothelin-1 from podocytes binds the endothelin-1 receptor A (ETA) expressed by adjacent ECs and induces oxidative stress and EC dysfunction.
Cross-communication also occurs between other cells within the glomerulus. The glomerular ECs express platelet-derived growth factor-β (PDGF-β), which interacts with its receptor (PDGFRβ) expressed by mesangial cells. This signal is especially important during glomerular development. Additional cross-communication between ECs and mesangial cells is likely to occur, as is communication between mesangial cells and podocytes.
Development of the Glomerular Microvasculature
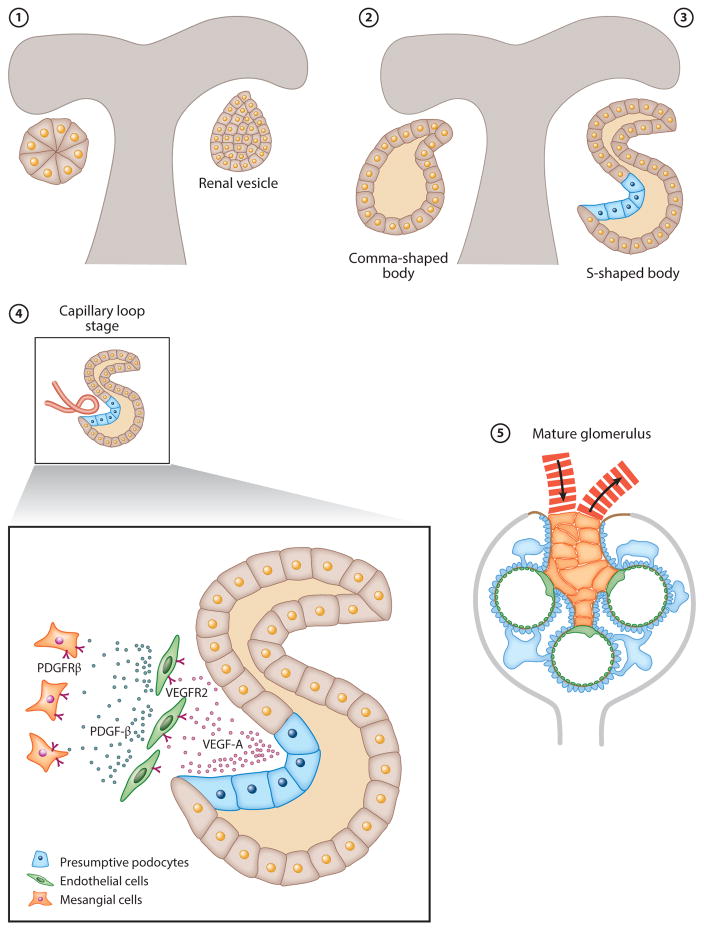
Glomerular development is generally described in five steps: vesicle, comma-shaped body, S-shaped body, glomerular capillary loop stage, and mature glomerulus (Figure 3). The vesicle, the first epithelial structure, consists of polarized cells surrounded by a basement membrane. On one side, the vesicle joins with the ureteric bud, forming a continuous lumen between the vesicle and the duct. On the opposite side, a cleft is formed and produces a comma-shaped or an S-shaped body. The lip beneath this cleft is established by a prominent crescent-shaped layer of epithelial cells that ultimately differentiate into podocytes. The podocyte precursor cells are simple, polygonal cells that undergo significant proliferation. During this early stage of glomerular development, the presumptive podocytes are connected by apical junctions. The expression of VEGF-A by presumptive podocytes induces migration of VEGFR2-positive EC precursors present in the renal mesenchyme (Figure 3). ECs migrate into the vascular cleft and proliferate and differentiate in intimate association with the VEGF-A-positive podocytes (5). Once inside the vascular cleft, ECs proliferate and aggregate, forming precapillary cords. The ECs are initially very large, and the precapillary cords are devoid of vascular lumen. Lumenation develops later through selective apoptosis of ECs, a process that is dependent on TGF-β signaling (6). ECs continue to differentiate into an extremely flattened monolayer that is densely perforated with fenestrae. During development, glomerular EC fenestrae have diaphragms that disappear as the glomerular capillaries mature (7). Studies in mice have shown that reduced Vegf-a signaling from podocytes results in loss of EC migration and proliferation and in decreased survival and thus results in the absence of functional glomerular filtration barriers (8).
Figure 3.
Schematic of glomerular development. Glomerular development is generally described in five steps: (1) vesicle, (2) comma-shaped body, (3) S-shaped body, (4) glomerular capillary loop stage, and (5) mature glomerulus. During the capillary loop stage, presumptive podocytes express VEGF-A, which induces the migration of VEGFR2-positive endothelial cell precursors in the renal mesenchyme. Endothelial cells migrate into the vascular cleft and proliferate and differentiate in intimate association with VEGF-A-producing podocytes. Mesangial cells express PDGFRβ and are attracted into the developing glomerular tuft by PDGF-β-expressing glomerular endothelial cells. Connections to the tubule system were omitted for clarity. Abbreviations: PDGF-β, platelet-derived growth factor-β; PDGFRβ, platelet-derived growth factor-βreceptor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
During nephrogenesis, mesangial cells are identified by their expression of several markers, including desmin, α-smooth muscle actin, and PDGFRβ (9). PDGFRβ-expressing mesangial cells migrate into the cleft by the chemotactic effect of PDGF-β produced by ECs and locate adjacent to ECs of the comma- and S-shaped bodies and maturing glomeruli. The appearance of mesangial cells in the glomerulus is dependent on PDGF-β and its receptor PDGFRβ. Mice carrying null mutations in the Pdgf-β or Pdgfrβ genes or EC-specific knockout of Pdgf-β lack glomerular mesangial cells (10, 11). Interestingly, mice with Vegf-a deficiency in podocytes demonstrate that mesangial cells depend on podocyte-produced Vegf-a for migration and survival, either directly or through modulation of factors produced by glomerular ECs (12). As maturation proceeds, the single first capillary loop divides into six to eight loops, and podocytes extend themselves around the loops. Looping of glomerular capillaries will not proceed in the absence of mesangial cells or in glomeruli with basement membrane defects that prevent adhesion of mesangial cells (9).
VASCULAR ENDOTHELIAL GROWTH FACTORS AND THEIR RECEPTORS
The mammalian family of VEGFs includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor. Each member of the VEGF family facilitates cellular responses by binding to cell surface tyrosine kinase receptors. The VEGF receptors are composed of seven extracellular immunoglobulin-like domains, a transmembrane region, and an intracellular tyrosine kinase domain. Ligand binding induces receptor dimerization and activation through transphosphorylation. Each member of the VEGF family has preferential binding to one or more of the VEGFRs. VEGF-A binds to VEGFR1 and VEGFR2. VEGF-B binds to VEGFR1. Both VEGF-C and VEGF-D bind to VEGFR3 and VEGFR2. Signaling through VEGFRs can also be modulated by coreceptors neuropilin-1 and neuropilin-2 (13, 14). The neuropilins bind ligand and potentiate signaling through the VEGFRs but have no intrinsic signaling capabilities. VEGFR2 is thought to be the principal receptor for VEGF-A signaling, and Vegfr2 knockout in mice results in early embryonic death, resulting in a phenotype similar to that of Vegf-a knockouts (15). In contrast, VEGFR1 may be a decoy receptor sequestering excessive extracellular VEGF-A. Vegfr1 knockout mice die early embryonically due to Vegf-a hyperactivity (16). Furthermore, in mice, a mutant form of Vegfr1 with an inactivated tyrosine kinase domain is sufficient to induce normal blood vessel formation (17). A soluble isoform of VEGFR1 produced endogenously (sVEGFR) may sequester VEGF-A in the endothelial environment to sharpen VEGF-A gradients (18). VEGFR3 is best known for its role in lymphangiogenesis. However, mice that lack a functional Vegfr3 gene die before the emergence of the lymphatic vessels, with defects in large blood vessel development, suggesting that the actions of VEGFR3 are not limited to lymphatic endothelium (19).
VEGF-A
VEGF-A is the best-characterized member of the VEGF family and the major inducer of physiological and pathological angiogenesis. VEGF-A actions have been implicated in tumor angiogenesis, wound healing, diabetic retinopathy, age-related macular degeneration, and glomerular diseases.
VEGF-A isoforms
Differential splicing of the eight-exon VEGF-A gene gives rise to at least five different isoforms in humans: VEGF121, VEGF145, VEGF165, VEGF189, and VEGF206. Rodent VEGF-A isoforms are shorter by one amino acid (e.g., the rodent counterpart to human VEGF121 is VEGF120). VEGF121, VEGF165, and VEGF189 are the most abundantly expressed. Each isoform displays different properties regarding diffusibility and binding to heparan sulfate, neuropilin-1, and neuropilin-2 (20). VEGF121 lacks a heparin-binding domain and is considered to be the most diffusible isoform. In contrast, VEGF165, VEGF189, and VEGF206 are largely found sequestered in the extracellular matrix and at the cell surface. The most abundant, and most mitogenic, isoform expressed by the kidney is VEGF165. All VEGF-A isoforms bind to VEGFR1 and VEGFR2.
Biological activities of VEGF-A
VEGF, initially named vascular permeability factor, was first discovered as a factor that was secreted by carcinoma cell lines and that increased fluid accumulation in tumors. The biological activities of VEGF-A are dependent on its temporal and spatial expression. VEGF-A is involved in vasculogenesis (de novo blood vessel formation) and angiogenesis (blood vessel growth from existing vasculature). VEGF-A regulates the proliferation, migration, specialization, and survival of ECs. VEGF-A can facilitate matrix remodeling through induction of plasminogen activator, plasminogen activator inhibitor-1, and interstitial collagenase. VEGF-A decreases systemic blood pressure and resistance through endothelium-dependent vasodilation due to the acute release of nitric oxide. In monocytes, VEGF-A stimulates the migration and expression of adhesion molecules.
Role of VEGF during the development and maintenance of the glomerular microvasculature
Beginning at the S-shaped stage, all isoforms of VEGF-A are expressed by podocytes. The major signaling receptor, VEGFR2, is expressed by ECs as they migrate into the vascular cleft adjacent to the presumptive podocytes. Global reduction of Vegf-a in mice through the use of neutralizing antibodies results in mesangiolysis and in arrested kidney development (21, 22). Mice expressing only Vegf120, i.e., not expressing Vegf164 and Vegf188, have severely impaired glomerular capillaries and renal function (23). Podocyte-specific loss of Vegf-a in mice results in arrested development of the glomerulus and in the absence of glomerular endothelium (8). Inactivation of a single Vegf-a allele in podocytes also leads to endothelial defects, including endotheliosis (swelling of the endothelium), loss of endothelium, and lysis of mesangial cells (8, 12). In fact, any podocyte decrease in Vegf-a during development results in an endothelial defect leading to end-stage renal failure. Overexpression of Vegf164 in podocytes leads to collapsing glomerulopathy shortly after birth (8, 24). In the mature glomerulus, VEGF-A inhibition in patients or postnatal podocyte-specific Vegf-a deletion in mice causes renal thrombotic microangiopathy (TMA) and highlights the importance of proper dosage of VEGF-A within the mature kidney (25).
The renal phenotype of whole-body postnatal deletion of Vegfr2 is similar to that of podocyte-specific Vegf-a knockouts (24). Although this similarity suggests a model in which VEGF-A from podocytes signals in a paracrine manner through VEGFR2 expressed by glomerular ECs, reports also show signaling through VEGFR2 in podocytes (26, 27). However, deletion of Vegfr2 in podocytes does not result in glomerular developmental defects or in functional defects of the glomerular barrier, strongly suggesting that glomerular structure and function require paracrine and not autocrine VEGF-A/VEGFR2 signaling (24).
A recent finding is that podocytes in mature glomeruli express sVEGFR1 and that it is located primarily at the basal aspect of podocyte foot processes and in endosomes (28). Increased levels of sVEGFR1 play a role in the pathogenesis of preeclampsia, resulting in hypertension, endothelial dysfunction, and proteinuria. Mice with podocyte-specific deletion of Vegfr1 have profound reorganization of podocyte architecture and proteinuria by 6 weeks of age. Interestingly, this phenotype is rescued by the addition of a kinase-dead Vegfr1 capable of expressing sVegfr1, demonstrating dispensability of the full-length isoform (28). Binding of sVEGFR1 to glycosphingolipid monosialodihexosylganglioside, also known as GM3, in lipid rafts of the podocyte activates intracellular signaling pathways, promoting adhesion and rapid actin reorganization (28).
Anti-VEGF therapy
VEGF-A is commonly overexpressed by a wide variety of human tumors, and overexpression has been correlated with increased progression, invasion, metastasis, and microvessel density and with poorer survival and prognosis in patients. VEGF-A and VEGFR2 are currently the main targets for antiangiogenic therapies, as illustrated by the development of highly specific inhibitors of both VEGF-A ligand (e.g., bevacizumab, aflibercept, ranibizumab) and VEGFR (e.g., cediranib, pazopanib, sorafenib, sunitinib, vandetanib, axitinib, telatinib, semaxanib, motesanib, vatalanib). To date, five of these agents (i.e., aflibercept, bevacizumab, ranibizumab, sunitinib, sorafenib) are commonly used for the treatment of cancer, age-related macular degeneration, or diabetic retinopathy.
Although anti-VEGF therapy has become a standard treatment for numerous cancers, there are still various challenges to overcome. First, there is modest or no benefit in advanced cancers. Second, therapeutic resistance almost inevitably occurs (tumors acquire resistance to antiangiogenic treatment), or patients do not respond at all (tumors are inherently resistant to antiangiogenic treatment). Third, antiangiogenic agents may induce significant side effects, including hypertension, proteinuria, TMAs, and impaired wound healing (25).
Ocular anti-VEGF therapy represents one of the most exciting areas in ocular research and clinical ophthalmology. In the eye, anti-VEGF therapies are administered by direct injection into the vitreous; such administration helps to reduce angiogenesis and the associated retinal edema. The incidence and severity of side effects of this treatment are low, and the benefits seem to outweigh the risks. However, less is known about the long-term effect of using these drugs, and there might be reason to raise a note of caution. Mice with a genetic deletion of Vegf-a from retinal pigment epithelial cells show dramatic and rapid loss of ECs of the choriocapillaries and severe vision loss secondary to cone cell death (29). However, a genetic deletion removes most of the VEGF-A produced, whereas drug inhibition may be more modest, and drug-free periods could allow for recovery of damaged endothelium.
In contrast, induction of VEGF-A expression may be beneficial for diseases with ischemia in which angiogenesis is important for establishing collateral circulation and reperfusion. A variety of preclinical models of chronic ischemia have shown that VEGF-A protein and gene delivery is highly effective. Early studies of VEGF-A proangiogenic therapy in ischemic heart disease were promising and were subsequently followed by several clinical trials (30). One of the limitations of this therapy is the delivery method of angiogenic factors. It is therefore uncertain whether the lack of benefit is due to lack of efficacy of proangiogenic factors or an inability to achieve high enough levels of VEGF-A in the ischemic heart.
VEGF in glomerulopathies
In addition to the well-established role of VEGF-A in tumor and retinal vascularization, VEGF-A is a critical player in the regulation of the glomerular filtration barrier. Modulation of VEGF signaling may have potentially therapeutic roles in preeclampsia and thrombotic microangiopathies, diabetic nephropathy, HIV-associated nephropathy (HIVAN), crescentic glomerulonephritis, and membranoproliferative glomerulonephritis (MPGN).
Preeclampsia and thrombotic microangiopathy
Three to five percent of pregnancies are complicated by preeclampsia, a syndrome consisting of hypertension, proteinuria, edema, and reduced glomerular filtration rate. Proteinuria is associated with a pathological renal lesion known as endotheliosis, in which the ECs of the glomerulus swell and fenestrations are lost (31). Circulating factors released by the activated endothelium in women with clinical preeclampsia include endothelin-1, ANGPT2, fibronectin, von Willebrand factor, thrombomodulin, markers of oxidative stress, and inflammatory cytokines. In addition, there is a deficiency of vasodilatory factors such as prostacyclin and nitric oxide in preeclamptic women.
More recently, a probable cause of endothelial dysfunction in preeclampsia was attributed to the release of excessive amounts of antiangiogenic factors by the placenta into the maternal circulation. Both sVEGFR1 and soluble endoglin (sENG) are produced by the placenta to balance the proangiogenic factors needed in pregnancy. ENG is an endothelium-specific type III TGFβR that reduces the binding of TGF-β1 to its receptor and that blocks TGF-β1-induced vasodilation, likely through downregulation of eNOS (32).
In preeclampsia, sVEGFR1 levels begin to rise at least 5 weeks before the onset of preeclampsia and remain elevated (33, 34). As discussed above, sVEGFR1 can sequester VEGF-A, which limits the amount of free VEGF-A in the circulation. Adenoviral administration of sVegfr1 to rats induced hypertension, proteinuria, and glomerular endotheliosis (35). In mice, podocyte-specific haploinsufficiency of Vegf-a results in proteinuria, endotheliosis, and eventually loss of ECs, recapitulating the classic renal lesion seen in preeclampsia (8). Other animal models also implicate VEGFR1 in the pathogenesis of preeclampsia (36, 37). Furthermore, some patients given neutralizing VEGF-A antibodies develop glomerular endothelial injury with proteinuria and endotheliosis (38). HELLP syndrome is a variant of preeclampsia that affects several organ systems. When sVegfr1 and sEng are coadministered, all features of severe preeclampsia and HELLP are observed in rats, even in the absence of pregnancy (32).
TMAs are a group of related disorders in which formation of intracapillary and intra-arteriolar platelet thrombi lead to end-organ ischemia and infarction particularly affecting the kidney and brain. Hemolytic uremic syndrome is a type of TMA and is characterized by the formation of fibrin-platelet thrombi and EC injury, including swelling, detachment, and endotheliosis. Interestingly, TMAs can be seen in the glomerulus in biopsies of a subset of patients receiving treatment with anti-VEGF agents for cancer. It has been estimated that proteinuria induced by anti-VEGF therapy, even if weak and without associated renal insufficiency, may reflect a renal TMA in 35% of cases (39). Furthermore, deletion of Vegf-a from podocytes in adult mice results in profound thrombotic glomerular injury (25). These observations provided evidence that VEGF-A has a role in TMAs.
Diabetic nephropathy
Diabetic nephropathy (DN) develops in approximately 30% of diabetic patients and is the leading cause of end-stage renal disease worldwide. Polymorphisms in VEGF-A are associated with DN and retinopathy (40–42). During the early angiogenic phase of DN, VEGF-A levels are elevated in the glomerulus. Experimental models of early diabetes have shown glomerular upregulation of VEGF-A and its receptors (43–45), and markers of DN can be attenuated by inhibiting VEGF-A in rodents (27, 46–49). Furthermore, transgenic overexpression of Vegf-a in podocytes results in features of DN such as thickening of the GBM and proteinuria (24, 50, 51).
There are several mechanisms by which VEGF-A may enhance progression of DN. First, excess VEGF-A in diabetes causes foot process effacement and nephrin downregulation and increases endothelial fenestrations leading to disruption of the glomerular filtration barrier (52). Second, there is cross talk and positive feedback between VEGF-A and nitric oxide pathways (53). Through PI3K/Akt signaling, VEGF-A activates endothelial nitric oxide synthase, leading to nitric oxide production. Vegf-a expression is upregulated in eNOS-null mice, which develop advanced DN (52, 54). Finally, VEGF-A stimulates TGF-β activation and collagen IV synthesis in podocytes and mesangial cells and directly induces mesangial cell proliferation. Any or all of these pathways could exacerbate DN and are potential therapeutic targets.
Because VEGF-A is absolutely necessary for glomerular development and maintenance, the upregulation in diabetes may be a protective measure to limit endothelial injury and dysfunction. Diabetic mice with podocyte-specific loss of Vegf-a after the induction of diabetes exhibited significantly greater proteinuria, profound glomerular scarring, and increased apoptosis of glomerular ECs (55).
HIVAN
HIVAN is the classical renal complication observed in African-American patients with human immunodeficiency virus (HIV) and is characterized by collapsing focal segmental glomerulosclerosis. In mice, podocyte-specific overexpression of Vegf-a results in a similar collapsing glomerulopathy, suggesting that VEGF may play a role in the pathogenesis of HIVAN (8). Furthermore, HIV-1 transgenic mice and patients with HIVAN have upregulated VEGF-A expression (56, 57). In vitro, the HIV viral protein Nef stimulates HIF-2α, which transcriptionally upregulates VEGF, VEGFR2, and neuropilin-1 (57). VEGFR2-neutralizing antibodies can reverse the proliferation and dedifferentiation of podocytes infected with HIV-1 (57). An association was recently reported between ApoL risk alleles and HIVAN in African-American patients (58, 59). It will be interesting to explore links between ApoL and VEGF pathway regulation in future studies.
Crescentic glomerulonephritis
Rapidly progressive glomerulonephritis (RPGN) is a group of devastating glomerular diseases characterized by glomerular crescents on renal biopsy and by the rapid loss of renal function over a short period of time. Crescent formation represents a nonspecific response to injury of the glomerular capillary wall, and inflammation causing cellular crescents is usually followed by the development of fibrotic crescents. Patients with crescentic glomerulonephritis have significantly higher serum and urine levels of VEGF than do controls (60). In contrast, loss of capillaries in glomerulonephritis is associated with reduced VEGF-A (61), and inhibition of Vegf expression results in massive proteinuria and in reduced expression of nephrin in nephrotic rats (62). Damage to the endothelium may induce the local release of VEGF, possibly reconciling these apparently contradictory observations.
Membranoproliferative glomerulonephritis
MPGN is an uncommon cause of nephritis that occurs primarily in children and young adults. It is defined by its pathological appearance and may be caused by a variety of different mechanisms. In human mesangial cells, VEGFR1, VEGFR2, and neuropilin-1 are expressed, and VEGF-A can induce mesangial cell proliferation (63). Administration of a VEGF-A165 antagonist aptamer to rats with MPGN increased EC death, whereas mesangial cell proliferation and matrix accumulation were unaffected, suggesting that the major role of VEGF-A is to protect the endothelium (64). In a mouse model of MPGN, glomerular Vegf mRNA and protein expression was increased when the glomeruli were healing. This finding suggests that VEGF-A may play a role in repair of glomerular damage (65). Similarly, in rats with severe experimental MPGN, VEGF165 treatment significantly enhanced EC proliferation and capillary repair in glomeruli, with significant improvement of renal function (66). These studies suggest that new therapeutic strategies for glomerulonephritis could be identified to increase capillary repair, potentially by enhancing VEGF-A actions.
VEGF-Axxxb: The Antiangiogenic VEGF
As mentioned above, multiple isoforms of VEGF-A are formed as a result of alternative splicing in exons 6, 7, and 8. Two families of VEGF-A proteins can be generated on the basis of the splicing of exon 8, the terminal exon. These two families, named VEGF-Axxxa and VEGF-Axxxb, differ only in six unique C-terminal amino acids. The VEGF-Axxxb family was initially discovered in 2002 and includes VEGF-A165b, VEGF-A121b, VEGF-A189b, and VEGF-A145b (67). VEGF-A165b binds VEGFR2 with similar affinity as VEGF-A but lacks the proangiogenic properties of VEGF-A. In vitro phosphopeptide mapping demonstrated that VEGF-A165b is less efficient than VEGF-A at inducing phosphorylation of the stimulatory Y1052 residue in VEGFR2 (68). Additionally, the ability of VEGF-A isoforms to induce angiogenesis correlates with neuropilin-1 binding, suggesting that lack of VEGFR2/neuropilin-1-complex formation leads to antiangiogenic phenotypes (68). Anti-VEGF antibody therapies such as bevacizumab are not isoform specific and also bind VEGF-A165b (69). Isoform-specific antibodies, generated against the C terminus of VEGF-A, may improve therapeutic efficacy in the future by scavenging proangiogenic VEGF while antiangiogenic VEGF remains active (70).
Role of VEGF-A165b in glomerular development
In the adult human renal cortex, VEGF-Axxxb accounts for 45% of total VEGF expression (71). During glomerular development, VEGF-Axxxb is expressed in all stages from the condensing vesicle onward. However, in the glomerular cleft, the site to where ECs will migrate, VEGF-Axxb expression is diffuse until in mature glomeruli VEGF-Axxxb is expressed in a subpopulation of differentiated podocytes (71, 72). In HUVEC and podocyte culture, VEGF-A165b inhibits EC migration in response to VEGF-A and increases podocyte survival by reducing apoptosis (71). Thus, the downregulation of VEGF-Axxxb at the time of EC influx suggests that it may prevent aberrant or excessive EC population. Additionally, because VEGF-A165b is expressed in mature podocytes, but not in dedifferentiated immature podocytes, the developmental switching of VEGF isoform balance may play a role in glomerular maturation (72). Denys-Drash syndrome (DDS) is a rare disorder caused primarily by missense mutations in the gene encoding the transcription factor Wilms’ tumor-1 (WT1) and results in renal failure and pseudohermaphroditism. Glomeruli in DDS are immature, with defects in podocyte maturation, immature mesangial cells, endotheliosis, and incomplete basement membrane formation (73). In DDS, podocytes fail to produce VEGF-A165b while retaining high levels of proangiogenic VEGF-A (73). Lack of VEGF-A165b production is caused by the loss of inhibition of SR kinase-1 by mutant WT1, which regulates VEGF-A165 isoform switching (74), and highlights the importance of these counteracting VEGF isoforms in glomerular development.
Role of VEGF-A165b in diabetic nephropathy
The role of VEGF-A165b in renal disease is poorly understood compared with that of the well-characterized VEGF-A165a. Mice with over-expression of human VEGF-A165b in podocytes are healthy, with normal glomerular filtration rate, protein excretion, and renal histology (75). However, such mice have reduced glomerular permeability, which is associated with reduced endothelial fenestrations (75). In the presence of high VEGF-A165b and VEGF-A, VEGF-A165b is capable of preventing changes in glomerular structure and permeability induced by VEGF-A (76). In humans, VEGF-A165b is upregulated in diabetic patients with intact renal function and is not upregulated in patients with progressive disease, suggesting that compensatory regulation of VEGF-A165b occurs in disease settings (77). Mice with podocyte-specific overexpression of VEGF-A165b are also protected against diabetic damage, as are mice treated with an exogenous systemic administration of human VEGF-A165b (77). Specifically, VEGF-A165b normalized permeability by reducing VEGFR2 activation and reversed damage to the glycocalyx (77). These studies suggest that podocyte-derived VEGF-A165b acts in the endothelium to protect blood vessels and that systemic administration of VEGF-A165b can have therapeutically relevant benefits.
VEGF-C
VEGF-C was discovered in 1996 and has since been implicated in several pathological conditions. Reduced VEGF-C expression is associated with hereditary lymphedema, whereas VEGF-C overexpression can promote tumor angiogenesis and metastasis.
VEGF-C expression in the kidney
VEGF-C is a dimeric, secreted protein member of the VEGF family. The primary role of VEGF-C is regulation of lymphangiogenesis through VEGFR3 activation. In lymphatic ECs, VEGF-C interacts with neuropilin-2, which complexes with VEGFR3 to facilitate signaling (78). VEGF-C can also regulate the permeability and growth of blood vessels through VEGFR3 and/or VEGFR2. In the kidney, VEGF-C is produced by glomerular podocytes and proximal tubular epithelial cells (79–81). Its primary receptor, VEGFR3, is also expressed in podocytes and fenestrated glomerular ECs (80, 82, 83). Thus, VEGF-C may have both autocrine and paracrine actions within the glomerulus.
Role of VEGF-C in podocyte survival, vascular permeability, and glomerular diseases
In cultured human and murine podocytes, VEGF-C reduces the cytotoxic effect of serum starvation. This effect is similar to that seen by VEGF-A treatment and is accomplished by increasing anti-apoptotic P13K/Akt signaling and reducing proapoptotic p38 MAPK signaling (79). Treatment with a VEGFR3 kinase inhibitor can decrease protection against cytotoxicity (80). In glomerular ECs, VEGF-C increases transendothelial electrical resistance and reduces albumin flux, possibly through VEGFR3/VEGFR2 heterodimers (83). VEGF-C can also reduce permeability by modulating the glycocalyx on the apical surfaces of glomerular ECs. VEGF-C increases the charge density of glycosaminoglycan proteoglycans and promotes hyaluronic acid synthesis (84). In this context, VEGF-C opposes the actions of VEGF-A, which induces shedding of charged glycosaminoglycans and increases permeability (84). In pediatric glomerulopathies, podocyte VEGF-C is upregulated in proteinuric, steroid-resistant idiopathic nephrotic syndromes, suggesting that VEGF-C may contribute to impaired barrier function and inflammatory activity (85). Outside of the glomerulus, VEGF-C promotion of lymphangiogenesis may be an important contributor to tubulointerstitial fibrosis. Lymphatics not only are important for fluid drainage, but are also critical for circulating immune surveillance. Injured tubulointerstitial areas of IgA nephropathy, focal glomerulosclerosis, and DN have increased lymphatic proliferation (81). Specific to the case for diabetic settings, lymphatics are also upregulated in periglomerular fibrotic lesions (81). There was a significant association between lymphatic vessel number, grade of the tubulointerstitial lesion, and increased VEGF-C expression in proximal tubule epithelial cells (81). Additionally, macrophage and proximal tubule expression of Vegf-c was increased in the mouse UUO model of tubulointerstitial fibrosis, resulting in increased lymphatic number and fibrotic lesion severity (86).
ANGIOPOIETINS
Angiopoietin Ligands and Their Receptors
The angiopoietins (ANGPTs) bind the tyrosine kinase receptor TIE2, which is expressed primarily by ECs (87, 88). Most studies have focused on the functions of ANGPT1 and ANGPT2, whereas little is known about ANGPT3 or ANGPT4. ANGPT1 and ANGPT2 are ~70-kDa proteins with considerable sequence homology, which consist of a signal peptide, an N-terminal coiled-coil domain, a short linker peptide region, and a C-terminal fibrinogen homology domain. The coiled-coil region is important for multimerization, and both ANGPT1 and ANGPT2 form dimers and oligomers (89). ANGPT1 is produced by podocytes and vascular support cells such as pericytes, whereas ANGPT2 is produced and released from Weibel-Palade bodies in ECs upon stress (90, 91).
ANGPT1 functions as a TIE2 receptor agonist and promotes EC survival and quiescence, whereas ANGPT2 functions mainly as a TIE2 antagonist (92, 93). Targeted disruption of Angpt1 or Tie2 or overexpression of Angpt2 results in embryonic death with similar vascular defects. Embryos have normal primary vascular development, but remodeling and maturation of the vasculature are defective (45, 87, 93, 94). Conditional overexpression of Angpt2 in ECs in mice abrogates physiological Tie2 activation in vivo, supporting the antagonistic effect of Angpt2 (95). In contrast, ANGPT2 can function as a TIE2 agonist under certain conditions (96, 97). Angpt2 is required for the formation of lymphatic vessels, but interestingly, the lymphatic defects in Angpt2 knockout mice can be rescued by Angpt1 (98). Inducible combined Angpt1 and Angpt2 knockout in mice resulted in lymphatic defects and glaucoma, something not seen when Angpt1 or Angpt2 was knocked out individually (99). This finding strongly suggests that ANGPT1 and ANGPT2 have opposing roles in the blood vasculature but function in a similar manner in the lymphatic system.
The TIE2 homolog TIE1 is an orphan receptor but binds TIE2 and regulates its activity (100). Tie1 knockout in mice results in embryonic lethality, with phenotypes in both blood and lymphatic vasculature (101).
ANGPT1/TIE2 signaling appears to be redundant in mature quiescent vessels. However, signaling can inhibit vascular leakage induced by VEGF-A and other inflammatory mediators in various in vivo models (45, 102). ANGPT1/TIE2 signaling promotes junctional integrity and anti-inflammatory actions by suppression of tissue factor, leukocyte adhesion molecules, leukocyte adhesion, monocyte adhesion, NF-κ B, and endothelial transmigration by inflammatory stimuli (103, 104).
ANGPT2 is expressed in activated ECs and counteracts ANGPT1-mediated endothelial stabilization. ANGPT2 expression is regulated by several factors, including VEGF-A, PDGF, TNFα, thrombin, estrogen, leptin, hypoxia, high glucose, and forkhead box transcription factors FOXO1 and FOXC2 (105).
Pharmacological Targeting of the Angiopoietin/TIE2 Pathway
A limited number of studies have targeted the ANGPT/TIE2 pathway in kidney disease. Treatment with ANGPT1 is protective in several experimental models of kidney disease, including DN. However, the ANGPT/TIE2 system is a target of antiangiogenic drug development. This pathway is a challenging target particularly because each ligand can be pro- or antitumorigenic, depending on the context.
Stabilizing tumor vasculature by promoting TIE2 signaling (ANGPT2 blockade or ANGPT1 overexpression) may offer the benefits of reducing new angiogenic sprouting, edema, and tumor cell intravasation. However, it may render established vasculature more resistant to antiangiogenic therapy. ANGPT1 overexpression leads to vasculature that is more mature and normal in appearance and explains the vessel-normalization effect that results from anti-VEGF/VEGFR therapies, because this effect is mediated through ANGPT1 (106).
In contrast, TIE2 inhibition may promote vascular regression, particularly when VEGF-A is absent (107). TIE2-expressing monocytes contribute to tumor angiogenesis and growth in several mouse models (108). In cancer development, TIE2 or ANGPT1 inhibition may block the beneficial anti-inflammatory effects of ANGPT1 signaling. In addition, ANGPT1 is more widely expressed in normal vascular homeostasis, whereas ANGPT2 is present in higher concentrations only at sites undergoing vascular remodeling and in hypoxic tumor microenvironments. The benefits of ANGPT2 targeting in cancer are evident, whereas the benefits of ANGPT1 targeting remain debatable.
To complicate things further, ANGPT2 can bind integrins, and integrin-expressing tumor cells may therefore respond to ANGPT2 independently of the vascular effects of this ligand (109). This relationship has been reported between VEGF-A and integrins in ECs, tumor cells, and tumor angiogenesis (110). Several inhibitors of the ANGPT/TIE2 system are in clinical trials (111, 112).
A novel approach to enhance TIE2 activity is to inhibit vascular endothelial protein tyrosine phosphatase (VE-PTP), which negatively regulates TIE2 phosphorylation. In mouse studies, the VE-PTP inhibitor AKB-9778 delays early growth of mammary tumors and metastases to the lung (113). Additionally, in clinical trials AKB-9778 is well tolerated and improves vision in patients with diabetic macular edema (114).
Role of Angiopoietins in the Development and Maintenance of Glomerular Microvasculature
Angpt1, Angpt2, Tie2, and Tie1 are expressed from the inception of the mouse metanephros (115, 116). In mice, expression of these genes peaks soon after birth, and these genes remain expressed in the adult kidney (117). Tie2 and Tie1 are expressed in mouse metanephric interstitial and glomerular capillaries, whereas Angpt1 is expressed in nephrogenic mesenchyme, differentiating tubule epithelia, and presumptive and mature podocytes (90, 117).
Angpt1 and Tie2 knockout embryos die before metanephric differentiation, which has limited studies of their role in the kidney. In a whole-body inducible system, excision of the Angpt1 gene at E10.5 leads to embryonic lethality shortly before birth. In these embryos, glomeruli have dilated capillary loops, and segments of the GBM are disrupted with numerous folds, suggesting a primary abnormality of the glomerular endothelium and associated matrix. Rounded and poorly matrix-associated ECs are seen in the glomeruli of induced Angpt1 knockout mice and in other vascular beds in conventional Angpt1 knockout mice (45, 94). ANGPTs assemble distinct TIE2 signaling complexes in endothelial cell-cell and cell-matrix contacts. ANGPT1 binding to the extracellular matrix of cultured ECs promotes TIE2 localization to the basal plasma membrane, resulting in endothelium-matrix adhesion and a migratory phenotype (118, 119). Although glomerular maturation continues for 3 weeks after birth in mice, no glomerular phenotype was found in mice with Angpt1 knockdown after E13.5, suggesting that Angpt1 is not critical for maintenance in the healthy glomerulus (45). Transgenic expression of Angpt2 by podocytes in adult mice results in albuminuria and glomerular EC apoptosis (120).
Angiopoietin and Tie2 in Glomerulopathies
ANGPTs are critical during development for differentiation of the vasculature and angiogenesis and participate in maintenance of blood vessels in adulthood. ANGPTs and TIE2 are expressed in the normal developing kidney and have been implicated in glomerular diseases and nephropathies associated with tubulointerstitial lesions.
Altered expression in glomerular disease
Several studies show a dysregulation of ANGPT1 and ANGPT2 in kidney diseases. Increased serum levels of ANGPT2 and decreased levels of ANGPT1 are typically observed. Endothelial stress induces release of ANGPT2 from Weibel-Palade bodies in the endothelium; such release impairs endothelial function by inhibiting ANGPT1/TIE2 signaling. Serum levels of ANGPT2 can predict mortality in chronic kidney disease patients and are a marker for early cardiovascular disease in children on chronic dialysis (121, 122).
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by multisystem involvement and is associated with the production of autoantibodies and immune complex vasculitis with EC damage. ANGPT1 levels are decreased and ANGPT2 levels are increased in serum of SLE patients compared with healthy controls. ANGPT2 levels also show a significant independent correlation with proteinuria in SLE patients, but ANGPT2 levels are not distinguishable between proliferative and nonproliferative lupus nephritis (123–125). The same trend is seen in patients suffering from TMAs and anti-GBM disease. Plasma exchange may effectively lower elevated ANGPT2 levels while leaving ANGPT1 levels decreased (126). It remains to be seen whether ANGPT2 removal is enough to ameliorate endothelial damage in these diseases.
Angiopoietin, TIE2, and diabetic nephropathy
In recent years, the ANGPT/TIE2 system has gained interest in the contexts of diabetes and endothelial dysfunction. Growing evidence suggests an involvement of ANGPT2 in the pathophysiology of several vascular and inflammatory diseases, including type I and type II diabetes, acute myocardial infarction, arteriosclerosis, hypertension, chronic kidney disease, sepsis, malaria, multiple trauma, and acute lung injury. More importantly, increased ANGPT2/ANGPT1 levels appear to be associated with adverse outcomes.
Experimental diabetes models in rodents show that Angpt1, Angpt2, and Tie2 expression is upregulated in kidneys during the early phase of diabetes and that, whereas Angpt1 expression eventually returns to control levels or below, Angpt2 and Tie2 expression remains high (43, 127). Cell fractions from isolated diabetic glomeruli show an upregulation of Angpt2 expression in glomerular ECs, whereas Angpt1 expression was unchanged in podocytes (45). Furthermore, transgenic overexpression of Angpt2 in podocytes causes proteinuria and glomerular EC apoptosis, presumably by antagonizing Angpt1/Tie2 signaling (120). Adenoviral delivery of COMP-Angpt1 (a modified form of Angpt1) in the db/db model of diabetes reduces albuminuria, mesangial expansion, and GBM thickening (128). This COMP-Angpt1 delivery is associated with a significant improvement in hyperglycemia, which may account for the amelioration of nephropathy. However, a recent paper reported that transgenic podocyte repletion of Angpt1 in experimental diabetes resulted in reduced albuminuria without changes in hyperglycemia (129).
In support of a protective role of ANGPT1, diabetic Angpt1-deficient mice have decreased survival, increased proteinuria, and increased glomerulosclerosis compared with diabetic controls (45). The ANGPT/TIE2 system may prove to be a useful target for therapeutics in endothelial dysfunction by inhibiting ANGPT2 or enhancing TIE2 phosphorylation and signaling.
ADDITIONAL GROWTH FACTORS
Epidermal Growth Factor
Epidermal growth factors (EGFs) stimulate mitogenesis, differentiation, and apoptosis. The EGF family of proteins includes EGF, HB-EGF, TGF-α, amphiregulin, epiregulin, and neuregulin. EGFs mediate their effects by binding to epidermal growth factor receptor (EGFR), a prototypical cell surface tyrosine kinase receptor, with high affinity. In addition to direct extracellular activation by its ligands, EGFR can be activated in trans by stimuli such as angiotensin II, high glucose, ROS, TGF-β1, and endothelin-1. This transactivation can occur via EGFR phosphorylation by intracellular Src and PKC kinases or via activation of proteases that release EGF ligands. EGFR is widely expressed in the kidney, including within glomeruli, proximal tubules, and collecting ducts. Furthermore, EGFR activation can be beneficial or detrimental, depending on the setting.
In acute kidney injury, EGFR enhances renal recovery. In mice, proximal tubule cell deletion of Egfr or treatment with an Egfr inhibitor delays functional recovery of ischemia-reperfusion-induced injury, likely as a result of reduced proliferation and regeneration (130). In contrast, EGFR promotes renal fibrosis and injury in DN and RPGN. EGFR activity is a well-established mechanism causing increased tubulointerstitial fibrosis. ROS-mediated activation of Src kinase and subsequent phosphorylation of EGFR lead to the activation of fibroblasts and the expression of profibrotic cytokine genes, such as the TGF-β1 gene. Additionally, in diabetic glomeruli, high glucose transactivates EGFR, leading to TGF-β upregulation in mesangial cells and to increased collagen I expression (131, 132).
Crescentic glomerulonephritis or RPGN injury involves the proliferation of glomerular epithelial cells and the accumulation of inflammatory cells, which form a crescent. HB-EGF expression in podocytes and parietal epithelial cells is increased in humans and mice with RPGN, leading to enhanced activation of EGFR and to increased proliferation and migration (133). Furthermore, global deletion of HB-Egf or podocyte-specific deletion of Egfr improves the severity of nephrotoxic serum-induced RPGN in mice (133). Most promisingly, pharmacological blockade of EGFR markedly improved histological and functional RPGN outcomes, even when administered after induction of injury (133), suggesting that the HB-EGF/EGFR pathway may have therapeutic potential.
Finally, EGF and HB-EGF ligands are also upregulated in diabetes-induced nephropathy (134, 135). In rats, Egfr blockade attenuated glomerular hypertrophy and increased podocyte survival (136, 137). Furthermore, podocyte-specific Egfr deletion led to reduced albuminuria and to podocyte loss in type I DN via reduced apoptosis and blunted induction of TGF-β1, Smad2/3 signaling, and fibronectin deposition. Diabetic conditions in vivo and in vitro increase glomerular ROS production, leading to enhanced EGFR activity via Src transactivation and induction of TGFβ/Smad2/3 signaling, which can further enhance ROS production. This vicious cycle can potentially be halted by blockade of ROS production or of EGFR signaling (138).
TGF-β
TGF-β is a superfamily of cytokines that has a multitude of cellular effects, including regulation of cell growth, differentiation, apoptosis, and inflammation. TGF-β is a well-recognized mediator of glomerulosclerosis and DN. Increased levels of TGF-β in chronic kidney disease are well documented and are associated with podocyte loss, mesangial matrix expansion, and interstitial fibrosis. Until recently, there was limited knowledge of whether TGF-β is synthesized in all glomerular cells and whether TGF-β signals in an autocrine or a paracrine fashion. Inducible podocyte overexpression of Tgfβr1 in mice resulted in a phenotype with albuminuria and glomerulosclerosis (139). Interestingly, the glomerular ECs were altered before there was any evidence of foot process effacement. Expression profiling showed that activation of Tgfβr1 signaling leads to increased expression of endothelin-1 in podocytes. Endothelin-1 mediates mitochondrial oxidative stress and dysfunction in adjacent ECs via ETA, which in turn promotes podocyte apoptosis (139). Inhibition of ETA or scavenging of mitochondrial oxidative stress prevents podocyte loss, albuminuria, glomerulosclerosis, and renal failure (139).
Semaphorins
Semaphorins are guidance molecules initially identified to have axon-repellent properties. They can be membrane bound, secreted, or glycosyl phosphatidylinositol linked. In the adult kidney, the secreted class 3 semaphorin (SEMA3A) is produced by podocytes and collecting ducts. SEMA3A signals through a receptor complex such that ligand binds to neuropilin-1 and Plexin-A1 mediates signaling. These receptors are expressed in podocytes and ECs. During development, SEMA3A modulates kidney vascular patterning through its inhibitory effects on EC migration and on ureteric bud branching (140, 141). In addition to its developmental role, SEMA3A plays a role in proteinuric glomerular disease (142). Inducible podocyte-specific overexpression of Sema3a in adult mice results in reversible proteinuria accompanied by expansion of the mesangial matrix, by EC swelling, by thickening of the GBM, and by podocyte foot process effacement (143). These effects appear to be mediated, at least in part, by downregulation of nephrin, leading to the disruption of slit diaphragms and to increased permeability of the filtration barrier. Additionally, overexpression of Sema3a results in reduced αvβ3 integrin activity that is similar to that seen in podocyte-specific knockout of Vegf-a, suggesting an interaction between semaphorin signaling and VEGF signaling (144). In podocyte-specific overexpression of Vegf-a at baseline and in the setting of type I diabetes, there is a compensatory increase in podocyte Sema3a expression (52). Furthermore, administration of exogenous Sema3a in mice, which results in podocyte foot process effacement and proteinuria, caused downregulation of Vegfr2 signaling, and damage was rescued by Vegf-a coadministration (145). Indeed, both VEGF and SEMA3A can signal through neuropilin-1 coreceptor–dependent mechanisms, suggesting a critical balance between SEMA3A and VEGF for the maintenance of podocyte integrity.
CXCL12
Chemokines are a family of structurally related chemoattractant cytokines. Among them, CXCL12 is an indispensable morphogen that signals through its receptor, CXCR4 (146). Knockout mice for Cxcl12 and Cxcr4 show similar, lethal phenotypes before or around birth (147). Cxcl12 is expressed in the developing glomerulus, and Cxcr4 knockout mice show vascular congestion in their kidney. Indeed, the CXCL12/CXCR4 system is essential for blood vessel formation in the kidney and, in particular, in the glomerulus. Cxcr4 and Cxcl12 knockout mice show defective blood vessel formation and capillary ballooning of the glomerular tufts (148). CXCL12 expression is detected in the stromal cells surrounding the developing nephrons and blood vessels. Podocytes start to express CXCL12 in developing glomeruli and continue to do so as they mature (148).
At an early embryonic stage, CXCR4 is strongly expressed in ureteric buds and metanephric mesenchymal cells. Later, expression switches to the cap mesenchyme and finally disappears completely from these epithelial components in the S-shaped stage. CXCL12-expressing podocytes are in close proximity to CXCR4-expressing ECs within the vascular cleft at the S-shaped stage of glomerular development. In mature glomeruli, both podocytes and glomerular ECs continue to express CXCL12 and CXCR4, respectively.
CXCR7 was recently identified as a second receptor for CXCL12 (149). CXCR7 is expressed in ureteric buds, the cap mesenchyme, and pretubule aggregates. In contrast to CXCR4, CXCR7 continues to be expressed in epithelial structures in a pattern similar to that of its ligand, CXCL12, including podocytes in the mature glomerulus (150). CXCR7 modulates CXCL12/CXCR4-dependent cell migration by acting as a scavenger, generating local CXCL12 gradients (151). Most Cxcr7 knockout mice die perinatally due to heart malformations, and their kidneys have ballooning of glomerular capillaries similar to that seen in Cxcl12 and Cxcr4 knockout mice (152).
SUMMARY
In summary, autocrine and paracrine vascular growth factor signaling is critical for the proper development and maintenance of a stable glomerulus. VEGF, ANGPT, EGF, SEMA3A, TGF-β, and CXCL12 communicate between the podocytes, endothelium, and mesangium within the glomerular capillaries to maintain filtration barrier function. These pathways are dysregulated in disease settings, and targeted blockade or stimulation of these pathways may provide new therapeutics in the future.
Acknowledgments
C.S.B. is supported by NIH/NIDDK grant 5T32 DK007169. The laboratory of M.J. is funded by Swedish Research Council grant 521-2012-865; by a Department of Immunology, Genetics, and Pathology Young Investigator grant; by the Åke Wiberg Foundation; and by the Magnus Bergvall Foundation. S.E.Q. holds the Charles H. Mayo Chair of Medicine at Northwestern University Feinberg School of Medicine and a Finnish Distinguished Professorship at Biocenter Oulu. The laboratory of S.E.Q. is funded by NIH/NHLBI grant HL1241200, by Canadian Institute of Health Research grants M0P62931 and M0P77756, by an E-rare Joint Translation Call (JTC 2011) for European Research Projects on Rare Diseases, and by Terry Fox Foundation grant 016002.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88:451–87. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 2.Herrera GA. Plasticity of mesangial cells: a basis for understanding pathological alterations. Ultrastruct Pathol. 2006;30:471–79. doi: 10.1080/01913120600932594. [DOI] [PubMed] [Google Scholar]
- 3.Ohyama K, Seyer JM, Raghow R, Kang AH. Extracellular matrix phenotype of rat mesangial cells in culture. Biosynthesis of collagen types I, III, IV, and V and a low molecular weight collagenous component and their regulation by dexamethasone. J Lab Clin Med. 1990;116:219–27. [PubMed] [Google Scholar]
- 4.Johnson RJ, Floege J, Yoshimura A, Iida H, Couser WG, Alpers CE. The activated mesangial cell: a glomerular “myofibroblast”? J Am Soc Nephrol. 1992;2:S190–97. doi: 10.1681/ASN.V210s190. [DOI] [PubMed] [Google Scholar]
- 5.Abrahamson DR. Glomerulogenesis in the developing kidney. Semin Nephrol. 1991;11:375–89. [PubMed] [Google Scholar]
- 6.Choi ME, Ballermann BJ. Inhibition of capillary morphogenesis and associated apoptosis by dominant negative mutant transforming growth factor-beta receptors. J Biol Chem. 1995;270:21144–50. doi: 10.1074/jbc.270.36.21144. [DOI] [PubMed] [Google Scholar]
- 7.Ichimura K, Stan RV, Kurihara H, Sakai T. Glomerular endothelial cells form diaphragms during development and pathologic conditions. J Am Soc Nephrol. 2008;19:1463–71. doi: 10.1681/ASN.2007101138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Investig. 2003;111:707–16. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindahl P, Hellstrom M, Kalen M, Karlsson L, Pekny M, et al. Paracrine PDGF-B/PDGF-Rβ signaling controls mesangial cell development in kidney glomeruli. Development. 1998;125:3313–22. doi: 10.1242/dev.125.17.3313. [DOI] [PubMed] [Google Scholar]
- 10.Bjarnegård M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, et al. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–57. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- 11.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–87. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- 12.Eremina V, Cui S, Gerber H, Ferrara N, Haigh J, et al. Vascular endothelial growth factor A signaling in the podocyte-endothelial compartment is required for mesangial cell migration and survival. J Am Soc Nephrol. 2006;17:724–35. doi: 10.1681/ASN.2005080810. [DOI] [PubMed] [Google Scholar]
- 13.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–45. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 14.Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165. J Biol Chem. 2000;275:18040–45. doi: 10.1074/jbc.M909259199. [DOI] [PubMed] [Google Scholar]
- 15.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 16.Fong GH, Zhang L, Bryce DM, Peng J. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development. 1999;126:3015–25. doi: 10.1242/dev.126.13.3015. [DOI] [PubMed] [Google Scholar]
- 17.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. PNAS. 1998;95:9349–54. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kearney JB, Kappas NC, Ellerstrom C, DiPaola FW, Bautch VL. The VEGF receptor flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood. 2004;103:4527–35. doi: 10.1182/blood-2003-07-2315. [DOI] [PubMed] [Google Scholar]
- 19.Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, et al. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–49. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- 20.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 21.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, et al. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–59. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 22.Kitamoto Y, Tokunaga H, Tomita K. Vascular endothelial growth factor is an essential molecule for mouse kidney development: glomerulogenesis and nephrogenesis. J Clin Investig. 1997;99:2351–57. doi: 10.1172/JCI119416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattot V, Moons L, Lupu F, Chernavvsky D, Gómez RA, et al. Loss of the VEGF164 and VEGF188 isoforms impairs postnatal glomerular angiogenesis and renal arteriogenesis in mice. J Am Soc Nephrol. 2002;13:1548–60. doi: 10.1097/01.asn.0000013925.19218.7b. [DOI] [PubMed] [Google Scholar]
- 24.Sison K, Eremina V, Baelde H, Min W, Hirashima M, et al. Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling. J Am Soc Nephrol. 2010;21:1691–701. doi: 10.1681/ASN.2010030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–36. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan F, Villegas G, Teichman J, Mundel P, Tufro A. Autocrine VEGF-A system in podocytes regulates podocin and its interaction with CD2AP. Am J Physiol Ren Physiol. 2006;291:F422–28. doi: 10.1152/ajprenal.00448.2005. [DOI] [PubMed] [Google Scholar]
- 27.Ku CH, White KE, Dei Cas A, Hayward A, Webster Z, et al. Inducible overexpression of sFlt-1 in podocytes ameliorates glomerulopathy in diabetic mice. Diabetes. 2008;57:2824–33. doi: 10.2337/db08-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin J, Sison K, Li C, Tian R, Wnuk M, et al. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell. 2012;151:384–99. doi: 10.1016/j.cell.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 29.Kurihara T, Westenskow PD, Bravo S, Aguilar E, Friedlander M. Targeted deletion of Vegfa in adult mice induces vision loss. J Clin Investig. 2012;122:4213–17. doi: 10.1172/JCI65157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eibel B, Rodrigues CG, Giusti II, Nesralla IA, Prates PR, et al. Gene therapy for ischemic heart disease: review of clinical trials. Rev Bras Cir Cardiovasc. 2011;26:635–46. doi: 10.5935/1678-9741.20110056. [DOI] [PubMed] [Google Scholar]
- 31.Stillman IE, Karumanchi SA. The glomerular injury of preeclampsia. J Am Soc Nephrol. 2007;18:2281–84. doi: 10.1681/ASN.2007020255. [DOI] [PubMed] [Google Scholar]
- 32.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–49. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 33.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 34.Hertig A, Berkane N, Lefevre G, Toumi K, Marti HP, et al. Maternal serum sFlt1 concentration is an early and reliable predictive marker of preeclampsia. Clin Chem. 2004;50:1702–3. doi: 10.1373/clinchem.2004.036715. [DOI] [PubMed] [Google Scholar]
- 35.Maynard SE, Min JY, Merchan J, Lim KH, Li J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Investig. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makris A, Thornton C, Thompson J, Thomson S, Martin R, et al. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007;71:977–84. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 37.Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, et al. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstet Gynecol. 2007;196:396, e1–7. doi: 10.1016/j.ajog.2006.12.024. discussion e7. [DOI] [PubMed] [Google Scholar]
- 38.Ranieri G, Patruno R, Ruggieri E, Montemurro S, Valerio P, Ribatti D. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: from the biology to the clinic. Curr Med Chem. 2006;13:1845–57. doi: 10.2174/092986706777585059. [DOI] [PubMed] [Google Scholar]
- 39.Izzedine H, Soria JC, Escudier B. Proteinuria and VEGF-targeted therapies: an underestimated toxicity? J Nephrol. 2013;26:807–10. doi: 10.5301/jn.5000307. [DOI] [PubMed] [Google Scholar]
- 40.Buraczynska M, Ksiazek P, Baranowicz-Gaszczyk I, Jozwiak L. Association of the VEGF gene polymorphism with diabetic retinopathy in type 2 diabetic patients. Nephrol Dial Transplant. 2007;22:827–32. doi: 10.1093/ndt/gfl641. [DOI] [PubMed] [Google Scholar]
- 41.Ray D, Mishra M, Ralph S, Read I, Davies R, et al. Association of the VEGF gene with proliferative diabetic retinopathy but not proteinuria in diabetes. Diabetes. 2004;52:861–64. doi: 10.2337/diabetes.53.3.861. [DOI] [PubMed] [Google Scholar]
- 42.Amle D, Mir R, Khaneja A, Agarwal S, Ahlawat R, et al. Association of 18bp insertion/deletion polymorphism, at −2549 position of VEGF gene, with diabetic nephropathy in type 2 diabetes mellitus patients of North Indian population. J Diabetes Metab Disord. 2015;27:14–19. doi: 10.1186/s40200-015-0144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizkalla B, Forbes JM, Cao Z, Boner G, Cooper ME. Temporal renal expression of angiogenic growth factors and their receptors in experimental diabetes: role of the renin-angiotensin system. J Hypertens. 2005;23:153–64. doi: 10.1097/00004872-200501000-00026. [DOI] [PubMed] [Google Scholar]
- 44.Cooper ME, Vranes D, Youssef S, Stacker SA, Cox AJ, et al. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes. 1999;48:2229–39. doi: 10.2337/diabetes.48.11.2229. [DOI] [PubMed] [Google Scholar]
- 45.Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, et al. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Investig. 2011;121:2278–89. doi: 10.1172/JCI46322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol. 2001;12:993–1000. doi: 10.1681/ASN.V125993. [DOI] [PubMed] [Google Scholar]
- 47.Flyvbjerg A, Dagnaes-Hansen F, De Vriese AS, Schrijvers BF, Tilton RG, Rasch R. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes. 2002;51:3090–94. doi: 10.2337/diabetes.51.10.3090. [DOI] [PubMed] [Google Scholar]
- 48.Sung SH, Ziyadeh FN, Wang A, Pyagay PE, Kanwar YS, Chen S. Blockade of vascular endothelial growth factor signaling ameliorates diabetic albuminuria in mice. J Am Soc Nephrol. 2006;17:3093–104. doi: 10.1681/ASN.2006010064. [DOI] [PubMed] [Google Scholar]
- 49.Schrijvers BF, Flyvbjerg A, Tilton RG, Lameire NH, De Vriese AS. A neutralizing VEGF antibody prevents glomerular hypertrophy in a model of obese type 2 diabetes, the Zucker diabetic fatty rat. Nephrol Dial Transplant. 2006;21:324–29. doi: 10.1093/ndt/gfi217. [DOI] [PubMed] [Google Scholar]
- 50.Veron D, Reidy KJ, Bertuccio C, Teichman J, Villegas G, et al. Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int. 2010;77:989–99. doi: 10.1038/ki.2010.64. [DOI] [PubMed] [Google Scholar]
- 51.Bertuccio C, Veron D, Aggarwal PK, Holzman L, Tufro A. Vascular endothelial growth factor receptor 2 direct interaction with nephrin links VEGF-A signals to actin in kidney podocytes. J Biol Chem. 2011;286:39933–44. doi: 10.1074/jbc.M111.241620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veron D, Bertuccio CA, Marlier A, Reidy K, Garcia AM, et al. Podocyte vascular endothelial growth factor (Vegf164) overexpression causes severe nodular glomerulosclerosis in a mouse model of type 1 diabetes. Diabetologia. 2011;54:1227–41. doi: 10.1007/s00125-010-2034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Investig. 1998;100:3131–39. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437:497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- 55.Sivaskandarajah GA, Jeansson M, Maezawa Y, Eremina V, Baelde HJ, Quaggin SE. Vegfa protects the glomerular microvasculature in diabetes. Diabetes. 2012;61:2958–66. doi: 10.2337/db11-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar D, Konkimalla S, Yadav A, Sataranatarajan K, Kasinath BS, et al. HIV-associated nephropathy: role of mammalian target of rapamycin pathway. Am J Pathol. 2010;177:813–21. doi: 10.2353/ajpath.2010.100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korgaonkar SN, Feng X, Ross MD, Lu TC, D’Agati V, et al. HIV-1 upregulates VEGF in podocytes. J Am Soc Nephrol. 2008;19:877–83. doi: 10.1681/ASN.2007050629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papeta N, Kiryluk K, Patel A, Sterken R, Kacak N, et al. APOL1 variants increase risk for FSGS and HIVAN but not IgA nephropathy. J Am Soc Nephrol. 2011;22:1991–96. doi: 10.1681/ASN.2011040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22:2129–37. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nitta K, Uchida K, Kimata N, Honda K, Horita S, et al. Increased serum levels of vascular endothelial growth factor in human crescentic glomerulonephritis. Clin Nephrol. 1999;52:76–82. [PubMed] [Google Scholar]
- 61.Yuan HT, Tipping PG, Li XZ, Long DA, Woolf AS. Angiopoietin correlates with glomerular capillary loss in anti-glomerular basement membrane glomerulonephritis. Kidney Int. 2002;61:2078–89. doi: 10.1046/j.1523-1755.2002.00381.x. [DOI] [PubMed] [Google Scholar]
- 62.Hara A, Wada T, Furuichi K, Sakai N, Kawachi H, et al. Blockade of VEGF accelerates proteinuria, via decrease in nephrin expression in rat crescentic glomerulonephritis. Kidney Int. 2006;69:1986–95. doi: 10.1038/sj.ki.5000439. [DOI] [PubMed] [Google Scholar]
- 63.Thomas S, Vanuystel J, Gruden G, Rodriguez V, Burt D, et al. Vascular endothelial growth factor receptors in human mesangium in vitro and in glomerular disease. J Am Soc Nephrol. 2000;11:1236–43. doi: 10.1681/ASN.V1171236. [DOI] [PubMed] [Google Scholar]
- 64.Ostendorf T, Kunter U, Eitner F, Loos A, Regele H, et al. VEGF165 mediates glomerular endothelial repair. J Clin Investig. 1999;104:913–23. doi: 10.1172/JCI6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haas CS, Campean V, Kuhlmann A, Dimmler A, Reulbach U, et al. Analysis of glomerular VEGF mRNA and protein expression in murine mesangioproliferative glomerulonephritis. Virchows Arch. 2006;450:81–92. doi: 10.1007/s00428-006-0340-0. [DOI] [PubMed] [Google Scholar]
- 66.Masuda Y, Shimizu A, Mori T, Ishiwata T, Kitamura H, et al. Vascular endothelial growth factor enhances glomerular capillary repair and accelerates resolution of experimentally induced glomerulonephritis. Am J Pathol. 2001;159:599–608. doi: 10.1016/S0002-9440(10)61731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123–31. [PubMed] [Google Scholar]
- 68.Kawamura H, Li X, Harper S, Bates D, Claesson-Welsh L. Vascular endothelial growth factor (VEGF)-A165b is a weak in vitro agonist for VEGF receptor-2 due to lack of coreceptor binding and deficient regulation of kinase activity. Cancer Res. 2008;68:4683–92. doi: 10.1158/0008-5472.CAN-07-6577. [DOI] [PubMed] [Google Scholar]
- 69.Varey AHR, Rennel ES, Qiu Y, Bevan HS, Perrin RM, et al. VEGF165b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer. 2008;98:1366–79. doi: 10.1038/sj.bjc.6604308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carter JG, Gammons MV, Damodaran G, Churchill AJ, Harper SJ, Bates DO. The carboxyl terminus of VEGF-A is a potential target for anti-angiogenic therapy. Angiogenesis. 2015;18:23–30. doi: 10.1007/s10456-014-9444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bevan H, van den Akker N, Qiu Y, Polman J, Foster R, et al. The alternatively spliced anti-angiogenic family of VEGF isoforms VEGFxxxb in human kidney development. Nephron Physiol. 2008;110:57–67. doi: 10.1159/000177614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui TG, Foster RR, Saleem M, Mathieson PW, Gillatt DA, et al. Differentiated human podocytes endogenously express an inhibitory isoform of vascular endothelial growth factor (VEGF165b) mRNA and protein. Am J Physiol Ren Physiol. 2004;286:F767–73. doi: 10.1152/ajprenal.00337.2003. [DOI] [PubMed] [Google Scholar]
- 73.Schumacher VA, Jeruschke S, Eitner F, Becker JU, Pitschke G, et al. Impaired glomerular maturation and lack of VEGF165b in Denys-Drash syndrome. J Am Soc Nephrol. 2007;18:719–29. doi: 10.1681/ASN.2006020124. [DOI] [PubMed] [Google Scholar]
- 74.Amin EM, Oltean S, Hua J, Gammons MV, Hamdollah-Zadeh M, et al. WT1 mutants reveal SRPK1 to be a downstream angiogenesis target by altering VEGF splicing. Cancer Cell. 2011;20:768–80. doi: 10.1016/j.ccr.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qiu Y, Ferguson J, Oltean S, Neal CR, Kaura A, et al. Overexpression of VEGF165b in podocytes reduces glomerular permeability. J Am Soc Nephrol. 2010;21:1498–509. doi: 10.1681/ASN.2009060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oltean S, Neal CR, Mavrou A, Patel P, Ahad T, et al. VEGF165b overexpression restores normal glomerular water permeability in VEGF164-overexpressing adult mice. Am J Physiol Ren Physiol. 2012;303:F1026–36. doi: 10.1152/ajprenal.00410.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oltean S, Qiu Y, Ferguson JK, Stevens M, Neal C, et al. Vascular endothelial growth factor-A165b is protective and restores endothelial glycocalyx in diabetic nephropathy. J Am Soc Nephrol. 2015;26:1889–904. doi: 10.1681/ASN.2014040350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kärpänen T, Heckman C, Keskitalo S, Jeltsch M, Ollila H, et al. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. FASEB J. 2006;20:1462–72. doi: 10.1096/fj.05-5646com. [DOI] [PubMed] [Google Scholar]
- 79.Müller-Deile J, Worthmann K, Saleem M, Tossidou I, Haller H, Schiffer M. The balance of autocrine VEGF-A and VEGF-C determines podocyte survival. Am J Physiol Ren Physiol. 2009;297:F1656–67. doi: 10.1152/ajprenal.00275.2009. [DOI] [PubMed] [Google Scholar]
- 80.Foster RR, Satchell SC, Seckley J, Emmett MS, Joory K, et al. VEGF-C promotes survival in podocytes. Am J Physiol Ren Physiol. 2006;291:F196–207. doi: 10.1152/ajprenal.00431.2005. [DOI] [PubMed] [Google Scholar]
- 81.Sakamoto I, Ito Y, Mizuno M, Suzuki Y, Sawai A, et al. Lymphatic vessels develop during tubulointerstitial fibrosis. Kidney Int. 2009;75:828–38. doi: 10.1038/ki.2008.661. [DOI] [PubMed] [Google Scholar]
- 82.Partanen TA, Arola J, Saaristo A, Jussila L, Ora A, et al. VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. FASEB J. 2000;14:2087–96. doi: 10.1096/fj.99-1049com. [DOI] [PubMed] [Google Scholar]
- 83.Foster R, Slater S, Seckley J, Kerjaschki D, Bates D, et al. Vascular endothelial growth factor-C, a potential paracrine regulator of glomerular permeability, increases glomerular endothelial cell monolayer integrity and intracellular calcium. Am J Pathol. 2008;173:938–48. doi: 10.2353/ajpath.2008.070416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Foster R, Armstrong L, Baker S, Wong D, Wylie E, et al. Glycosaminoglycan regulation by VEGFA and VEGFC of the glomerular microvascular endothelial cell glycocalyx in vitro. Am J Pathol. 2013;183:604–16. doi: 10.1016/j.ajpath.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ostalska-Nowicka D, Zachwieja J, Nowicki M, Kaczmarek E, Siwinska A, Witt M. Vascular endothelial growth factor (VEGF-C1)-dependent inflammatory response of podocytes in nephrotic syndrome glomerulopathies in children: an immunohistochemical approach. Histopathology. 2005;46:176–83. doi: 10.1111/j.1365-2559.2005.02076.x. [DOI] [PubMed] [Google Scholar]
- 86.Lee AS, Lee JE, Jung YJ, Kim DH, Kang KP, et al. Vascular endothelial growth factor-C and -D are involved in lymphangiogenesis in mouse unilateral ureteral obstruction. Kidney Int. 2013;83:50–62. doi: 10.1038/ki.2012.312. [DOI] [PubMed] [Google Scholar]
- 87.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, et al. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 88.Partanen J, Armstrong E, Makela TP, Korhonen J, Sandberg M, et al. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol Cell Biol. 1992;12:1698–707. doi: 10.1128/mcb.12.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davis S, Papadopoulos N, Aldrich TH, Maisonpierre PC, Huang T, et al. Angiopoietins have distinct modular domains essential for receptor binding, dimerization and superclustering. Nat Struct Biol. 2003;10:38–44. doi: 10.1038/nsb880. [DOI] [PubMed] [Google Scholar]
- 90.Satchell SC, Harper SJ, Tooke JE, Kerjaschki D, Saleem MA, Mathieson PW. Human podocytes express angiopoietin 1, a potential regulator of glomerular vascular endothelial growth factor. J Am Soc Nephrol. 2002;13:544–50. doi: 10.1681/ASN.V132544. [DOI] [PubMed] [Google Scholar]
- 91.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150–56. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 92.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–69. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 93.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 94.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 95.Reiss Y, Droste J, Heil M, Tribulova S, Schmidt MH, et al. Angiopoietin-2 impairs revascularization after limb ischemia. Circ Res. 2007;101:88–96. doi: 10.1161/CIRCRESAHA.106.143594. [DOI] [PubMed] [Google Scholar]
- 96.Kim I, Kim JH, Moon SO, Kwak HJ, Kim NG, Koh GY. Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Oncogene. 2000;19:4549–52. doi: 10.1038/sj.onc.1203800. [DOI] [PubMed] [Google Scholar]
- 97.Yuan HT, Khankin EV, Karumanchi SA, Parikh SM. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol. 2009;29:2011–22. doi: 10.1128/MCB.01472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3:411–23. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 99.Thomson BR, Heinen S, Jeansson M, Ghosh AK, Fatima A, et al. A lymphatic defect causes ocular hypertension and glaucoma in mice. J Clin Investig. 2014;124:4320–24. doi: 10.1172/JCI77162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seegar TC, Eller B, Tzvetkova-Robev D, Kolev MV, Henderson SC, et al. Tie1-Tie2 interactions mediate functional differences between angiopoietin ligands. Mol Cell. 2010;37:643–55. doi: 10.1016/j.molcel.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Puri MC, Rossant J, Alitalo K, Bernstein A, Partanen J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 1995;14:5884–91. doi: 10.1002/j.1460-2075.1995.tb00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thurston G, Suri C, Smith K, McClain J, Sato TN, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–14. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 103.Kim I, Oh JL, Ryu YS, So JN, Sessa WC, et al. Angiopoietin-1 negatively regulates expression and activity of tissue factor in endothelial cells. FASEB J. 2002;16:126–28. doi: 10.1096/fj.01-0556fje. [DOI] [PubMed] [Google Scholar]
- 104.Kim I, Moon SO, Park SK, Chae SW, Koh GY. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circ Res. 2001;89:477–79. doi: 10.1161/hh1801.097034. [DOI] [PubMed] [Google Scholar]
- 105.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–77. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 106.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–63. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 107.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. PNAS. 2002;99:11205–10. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–26. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 109.Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med. 2011;17:347–62. doi: 10.1016/j.molmed.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 110.Serini G, Valdembri D, Bussolino F. Integrins and angiogenesis: a sticky business. Exp Cell Res. 2006;312:651–58. doi: 10.1016/j.yexcr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 111.Cascone T, Heymach JV. Targeting the angiopoietin/Tie2 pathway: cutting tumor vessels with a double-edged sword? J Clin Oncol. 2012;30:441–44. doi: 10.1200/JCO.2011.38.7621. [DOI] [PubMed] [Google Scholar]
- 112.Huang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer. 2010;10:575–85. doi: 10.1038/nrc2894. [DOI] [PubMed] [Google Scholar]
- 113.Goel S, Gupta N, Walcott BP, Snuderl M, Kesler CT, et al. Effects of vascular-endothelial protein tyrosine phosphatase inhibition on breast cancer vasculature and metastatic progression. J Natl Cancer Inst. 2013;105:1188–201. doi: 10.1093/jnci/djt164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Campochiaro PA, Sophie R, Tolentino M, Miller DM, Browning D, et al. Treatment of diabetic macular edema with an inhibitor of vascular endothelial-protein tyrosine phosphatase that activates Tie2. Ophthalmology. 2015;122:545–54. doi: 10.1016/j.ophtha.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 115.Loughna S, Hardman P, Landels E, Jussila L, Alitalo K, Woolf AS. A molecular and genetic analysis of renalglomerular capillary development. Angiogenesis. 1997;1:84–101. doi: 10.1023/A:1018357116559. [DOI] [PubMed] [Google Scholar]
- 116.Kolatsi-Joannou M, Li XZ, Suda T, Yuan HT, Woolf AS. Expression and potential role of angiopoietins and Tie-2 in early development of the mouse metanephros. Dev Dyn. 2001;222:120–26. doi: 10.1002/dvdy.1170. [DOI] [PubMed] [Google Scholar]
- 117.Yuan HT, Suri C, Yancopoulos GD, Woolf AS. Expression of angiopoietin-1, angiopoietin-2, and the Tie-2 receptor tyrosine kinase during mouse kidney maturation. J Am Soc Nephrol. 1999;10:1722–36. doi: 10.1681/ASN.V1081722. [DOI] [PubMed] [Google Scholar]
- 118.Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, et al. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008;10:513–26. doi: 10.1038/ncb1714. [DOI] [PubMed] [Google Scholar]
- 119.Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol. 2008;10:527–37. doi: 10.1038/ncb1715. [DOI] [PubMed] [Google Scholar]
- 120.Davis B, Dei Cas A, Long DA, White KE, Hayward A, et al. Podocyte-specific expression of angiopoietin-2 causes proteinuria and apoptosis of glomerular endothelia. J Am Soc Nephrol. 2007;18:2320–29. doi: 10.1681/ASN.2006101093. [DOI] [PubMed] [Google Scholar]
- 121.David S, John SG, Jefferies HJ, Sigrist MK, Kumpers P, et al. Angiopoietin-2 levels predict mortality in CKD patients. Nephrol Dial Transpl. 2012;27:1867–72. doi: 10.1093/ndt/gfr551. [DOI] [PubMed] [Google Scholar]
- 122.Shroff RC, Price KL, Kolatsi-Joannou M, Todd AF, Wells D, et al. Circulating angiopoietin-2 is a marker for early cardiovascular disease in children on chronic dialysis. PLOS ONE. 2013;8:e56273. doi: 10.1371/journal.pone.0056273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kumpers P, David S, Haubitz M, Hellpap J, Horn R, et al. The Tie2 receptor antagonist angiopoietin 2 facilitates vascular inflammation in systemic lupus erythematosus. Ann Rheum Dis. 2009;68:1638–43. doi: 10.1136/ard.2008.094664. [DOI] [PubMed] [Google Scholar]
- 124.Salama MK, Taha FM, Safwat M, Darweesh HE, Basel ME. The Tie2 receptor antagonist angiopoietin-2 in systemic lupus erythematosus: its correlation with various disease activity parameters. Immunol Investig. 2012;41:864–75. doi: 10.3109/08820139.2012.711407. [DOI] [PubMed] [Google Scholar]
- 125.El-Banawy HS, Gaber EW, Maharem DA, Matrawy KA. Angiopoietin-2, endothelial dysfunction and renal involvement in patients with systemic lupus erythematosus. J Nephrol. 2012;25:541–50. doi: 10.5301/jn.5000030. [DOI] [PubMed] [Google Scholar]
- 126.Lovric S, Lukasz A, Hafer C, Kielstein JT, Haubitz M, et al. Removal of elevated circulating angiopoietin-2 by plasma exchange—a pilot study in critically ill patients with thrombotic microangiopathy and anti-glomerular basement membrane disease. Thromb Haemost. 2010;104:1038–43. doi: 10.1160/TH10-02-0138. [DOI] [PubMed] [Google Scholar]
- 127.Yamamoto Y, Maeshima Y, Kitayama H, Kitamura S, Takazawa Y, et al. Tumstatin peptide, an inhibitor of angiogenesis, prevents glomerular hypertrophy in the early stage of diabetic nephropathy. Diabetes. 2004;53:1831–40. doi: 10.2337/diabetes.53.7.1831. [DOI] [PubMed] [Google Scholar]
- 128.Lee S, Kim W, Moon SO, Sung MJ, Kim DH, et al. Renoprotective effect of COMP-angiopoietin-1 in db/db mice with type 2 diabetes. Nephrol Dial Transpl. 2007;22:396–408. doi: 10.1093/ndt/gfl598. [DOI] [PubMed] [Google Scholar]
- 129.Dessapt-Baradez C, Woolf AS, White KE, Pan J, Huang JL, et al. Targeted glomerular angiopoietin-1 therapy for early diabetic kidney disease. J Am Soc Nephrol. 2014;25:33–42. doi: 10.1681/ASN.2012121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen J, Chen J-K, Harris R. Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney Int. 2012;82:45–52. doi: 10.1038/ki.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu D, Peng F, Zhang B, Ingram AJ, Kelly DJ, et al. PKC-β1 mediates glucose-induced Akt activation and TGF-β1 upregulation in mesangial cells. J Am Soc Nephrol. 2009;20:554–66. doi: 10.1681/ASN.2008040445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu D, Peng F, Zhang B, Ingram AJ, Gao B, Krepinsky JC. Collagen I induction by high glucose levels is mediated by epidermal growth factor receptor and phosphoinositide 3-kinase/Akt signalling in mesangial cells. Diabetologia. 2007;50:2008–18. doi: 10.1007/s00125-007-0721-1. [DOI] [PubMed] [Google Scholar]
- 133.Bollee G, Flamant M, Schordan S, Fligny C, Rumpel E, et al. Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat Med. 2011;17:1242–50. doi: 10.1038/nm.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gilbert RE, Cox A, McNally PG, Wu LL, Dziadek M, et al. Increased epidermal growth factor in experimental diabetes related kidney growth in rats. Diabetologia. 1997;40:778–85. doi: 10.1007/s001250050749. [DOI] [PubMed] [Google Scholar]
- 135.Lee YJ, Shin SJ, Lin SR, Tan MS, Tsai JH. Increased expression of heparin binding epidermal growth-factor-like growth factor mRNA in the kidney of streptozotocin-induced diabetic rats. Biochem Biophys Res Commun. 1995;207:216–22. doi: 10.1006/bbrc.1995.1175. [DOI] [PubMed] [Google Scholar]
- 136.Wassef L, Kelly D, Gilbert R. Epidermal growth factor receptor inhibition attenuates early kidney enlargement in experimental diabetes. Kidney Int. 2004;66:1805–14. doi: 10.1111/j.1523-1755.2004.00955.x. [DOI] [PubMed] [Google Scholar]
- 137.Advani A, Wiggins K, Cox A, Zhang Y, Gilbert R, Kelly D. Inhibition of the epidermal growth factor receptor preserves podocytes and attenuates albuminuria in experimental diabetic nephropathy. Nephrology. 2011;16:573–81. doi: 10.1111/j.1440-1797.2011.01451.x. [DOI] [PubMed] [Google Scholar]
- 138.Chen J, Chen J-K, Harris R. EGF receptor deletion in podocytes attenuates diabetic nephropathy. J Am Soc Nephrol. 2015;26(5):1115–25. doi: 10.1681/ASN.2014020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Daehn I, Casalena G, Zhang T, Shi S, Fenninger F, et al. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Investig. 2014;124:1608–21. doi: 10.1172/JCI71195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–28. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- 141.Reidy K, Tufro A. Semaphorins in kidney development and disease: modulators of ureteric bud branching, vascular morphogenesis, and podocyte-endothelial crosstalk. Pediatr Nephrol. 2011;26:1407–12. doi: 10.1007/s00467-011-1769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tufro A. Semaphorin3a signaling, podocyte shape, and glomerular disease. Pediatr Nephrol. 2014;29:751–55. doi: 10.1007/s00467-013-2743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Reidy K, Aggarwal P, Jimenez J, Thomas D, Veron D, Tufro A. Excess podocyte semaphorin-3A leads to glomerular disease involving plexinA1-nephrin interaction. Am J Pathol. 2013;183:1156–68. doi: 10.1016/j.ajpath.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Veron D, Villegas G, Aggarwal PK, Bertuccio C, Jimenez J, et al. Acute podocyte vascular endothelial growth factor (VEGF-A) knockdown disrupts alphaVbeta3 integrin signaling in the glomerulus. PLOS ONE. 2012;7:e40589. doi: 10.1371/journal.pone.0040589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tapia R, Guan F, Gershin I, Teichman J, Villegas G, Tufro A. Semaphorin3a disrupts podocyte foot processes causing acute proteinuria. Kidney Int. 2008;73:733–40. doi: 10.1038/sj.ki.5002726. [DOI] [PubMed] [Google Scholar]
- 146.Patrussi L, Baldari CT. Intracellular mediators of CXCR4-dependent signaling in T cells. Immunol Lett. 2008;115:75–82. doi: 10.1016/j.imlet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 147.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–94. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 148.Takabatake Y, Sugiyama T, Kohara H, Matsusaka T, Kurihara H, et al. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol. 2009;20:1714–23. doi: 10.1681/ASN.2008060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–66. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 150.Haege S, Einer C, Thiele S, Mueller W, Nietzsche S, et al. CXC chemokine receptor 7 (CXCR7) regulates CXCR4 protein expression and capillary tuft development in mouse kidney. PLOS ONE. 2012;7:e42814. doi: 10.1371/journal.pone.0042814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, et al. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–73. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 152.Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. PNAS. 2007;104:14759–64. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]