Abstract
Objective:
Postoperative atrial fibrillation (POAF) is the most common complication after cardiac surgery. Multiple studies have shown significantly increased risks of stroke, myocardial infarction and death with POAF. Current prophylaxis strategies are inadequate to eliminate this problem. We examined preclinical efficacy and safety of KCNH2-G628S gene transfer to prevent POAF.
Methods:
Domestic pigs received AdKCNH2-G628S by epicardial atrial gene painting, and atrial pacemaker implantation for continuous burst pacing to induce atrial fibrillation. In an initial dose-ranging evaluation, 4 pigs received 5×1010–5×1011 virus particles. In the formal study, 16 pigs were randomized to 3 groups: (a) 5×1011 virus particles of AdKCNH2-G628S with 20% pluronic P407 in saline, (b) 20% pluronic in saline with no virus, and (c) saline alone. Animals were followed with daily efficacy and safety evaluations through the period of peak adenovirus-mediated transgene expression. After 14 days, pacing was discontinued, and the animals were followed in sinus rhythm for an additional 14 days to assess any longer-term toxicity.
Results:
In the primary efficacy analysis, G628S animals had a significant increase in the average time in sinus rhythm (59 ± 7%) compared to the pluronic control group (14 ± 6%, p = 0.009). There was no significant difference between the pluronic and saline controls (32 ± 12%, p = 0.16). Safety assessment showed improved left ventricular function in the G628S animals; otherwise there were no significant differences between groups in any safety measure.
Conclusions:
These data indicate that KCNH2-G628S gene therapy successfully and safely reduces AF risk.
INTRODUCTION
In the United States, approximately 700,000 cardiac surgical procedures are performed each year.1 POAF continues to be the most common complication after cardiac surgery, affecting 30% of patients on average and more than 75% of patients with risk factors (valve surgery, heart failure, older age, hypertension, pulmonary or renal dysfunction).2–4 POAF occurs predominately over the first few days after surgery, with almost all cases occurring before post-operative day 14.2 In spite of this time-limited risk, studies have consistently shown that POAF increases risks of stroke, myocardial infarction and death.2–8 Clinical trials have shown reduction in POAF with amiodarone, β-blockers, anti-oxidant diet, drug-releasing hydrogels, sotalol, or bi-atrial pacing.3,5,9–11 The continuing occurrence rate of 30% indicates the need for better POAF prevention.7
We propose gene therapy with AdKCNH2-G628S for prevention of POAF. The adenoviral vector induces temporary transgene expression over a 2–3 week interval that mirrors the time frame of POAF risk.12–14 KCNH2-G628S (G628S) is a dominant negative mutation that eliminates function of a potassium channel that plays a central role in cardiac myocyte repolarization.15 In prior study, we showed efficacy with atrial painting of AdG628S with 200 mg/ml Pluronic P407 and 5 mg/ml trypsin.16 The use of trypsin is potentially problematic in the clinical environment due to the narrow therapeutic window (a concentration of 5 mg/ml is needed for transmural penetration of the gene transfer vector and a concentration of 10 mg/ml affects atrial structural integrity).17 We have previously shown that eliminating trypsin from the painting solution allows surface gene transfer but no penetration of the vector beyond the epicardial layer.17 Uncertainty about the efficacy of epicardial only G628S expression motivated the current study.
MATERIALS AND METHODS
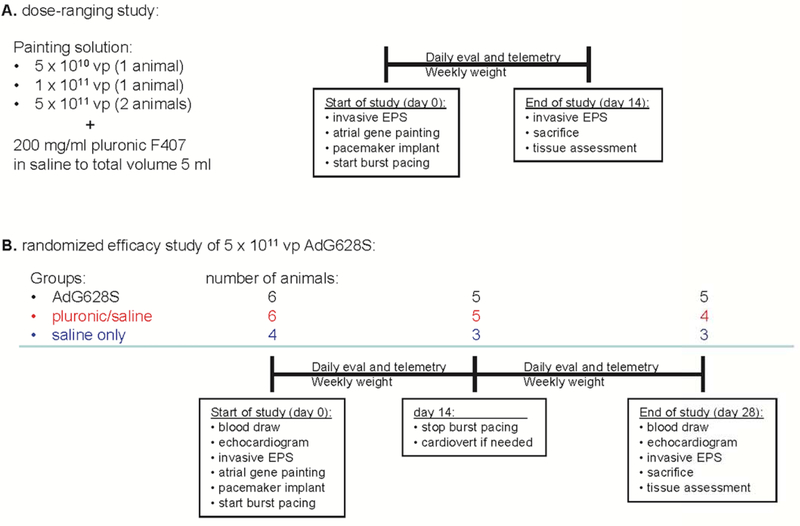
Detailed study methods are in the online supplement. In brief, the study had 2 components: a preliminary dose-ranging assessment in 4 animals, and a randomized, double-blinded, placebo controlled study in 16 animals (Figure 1). All animals underwent an initial surgery that included the gene painting procedure (online supplement video) and implantation of an atrial burst pacemaker for induction of AF. Burst pacing was activated immediately and animals were followed daily for rhythm analysis and safety assessment. The preliminary study animals were sacrificed 14 days after gene transfer to verify transgene expression. The randomized study animals had the atrial burst pacing discontinued at day 14 and they were followed for an additional 14 days of extended safety assessment.
Figure 1:
Study design.
RESULTS
Preliminary dose-ranging study of 4 animals:
To inform the prospective design of the formal efficacy study, we first evaluated escalating virus dose in 4 animals: 1 each received 5 × 1010 and 1 × 1011 vp and 2 received 5 × 1011 vp of AdG628S in 5 ml total volume of pluronic P407/saline (without trypsin). Atrial pacemakers were implanted at the time of gene therapy, and the AF-inducing burst pacing protocol remained active for the entire study. Rhythm was assessed daily from the off-pacing segments of the 2 second on/off cycles of the burst pacing protocol (supplemental figure 1). Over the 14 day duration of the study, the animal receiving 5 × 1010 vp of AdG628S had 6 days with sinus rhythm. The animal receiving 1 × 1011 vp had 3 days with sinus rhythm, and both animals receiving 5 × 1011 vp had 9 days with sinus rhythm. Expression and functional analyses showed increased atrial monophasic action potential duration and KCNH2 expression in all animals (Supplemental figure 2).
Efficacy of AdKCNH2-G628S for atrial fibrillation:
Based on the results of the dose-ranging study, we designed a randomized, controlled trial comparing AdG628S at a concentration of 1 × 1011 vp/ml (for a total of 5 × 1011 vp in 5 ml) to 20% poloxamer/saline controls and saline only controls. Group size was initially 5 pigs each for the AdG628S and poloxamer/saline groups, and 3 for the saline only group. Animals that died during the burst pacing portion of the study were replaced after completion of the initial 13 animal cohort by use of an extended randomization table (generated for 30 animals), so that all animals in the study were prospectively randomized.
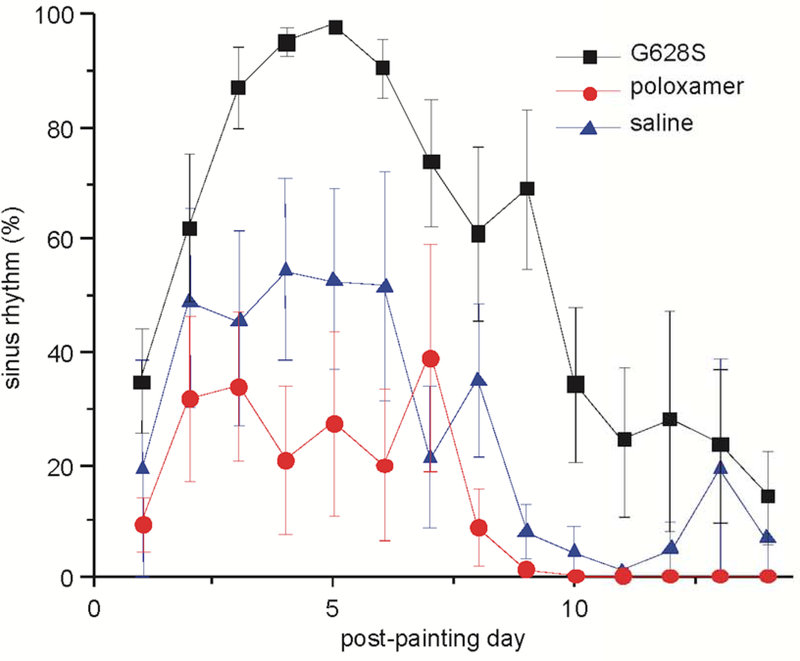
We assessed the percentage of time in sinus rhythm (SR%) on daily telemetry recordings averaged over the 14-day burst pacing period (Figure 2). Animals in the G628S group had significantly increased SR% (59 ± 7%) compared to the pluronic control group (14 ± 6%, p = 0.009). There was no significant difference between the pluronic and saline controls (32 ± 12%, p = 0.44). Even though the saline control group was not powered to determine differences with the G628S group and that comparison was not part of the predetermined analysis, the difference between these groups nearly reached statistical significance (p = 0.068).
Figure 2:
Percentage of time in sinus rhythm per day after gene painting. Compared to pluronic controls, animals exposed to G628S had a significant decrease in AF even with the continued stress of aggressive burst atrial pacing (p = 0.0006). Saline and pluronic controls were not statistically different.
Figure 2 is designated as the central figure. Abbreviated legend: KCNH2-G628S gene transfer decreases atrial fibrillation risk.
The control animals followed a general pattern of progressively increasing AF from the time of burst pacing onset, similar to control animals from our prior published studies.16,18–20 The AdG628S animals showed competing influences of burst-pacing driven AF and antiarrhythmic efficacy, with an initial increase in SR% from the influence of the G628S transgene followed by a loss of SR as transgene expression presumably waned and burst pacing continued to induce AF. The onset of antiarrhythmic efficacy in the AdG628S animals was consistent with the previously observed timing of adenovirus-mediated transgene expression in cardiac myocytes.12 In comparison to our prior report with AdG628S, pluronic P407 and trypsin, the duration of efficacy in this study was attenuated,16 likely due to the reduced gene transfer and therefore reduced transgene expression in the absence of trypsin.17
Safety of AdKCNH2-G628S for atrial fibrillation
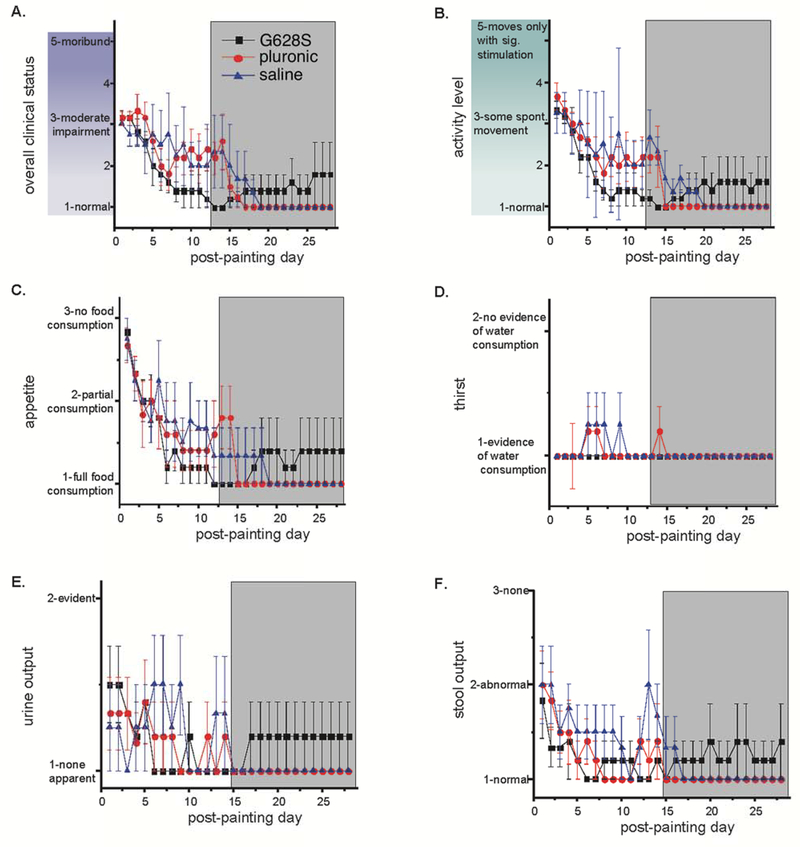
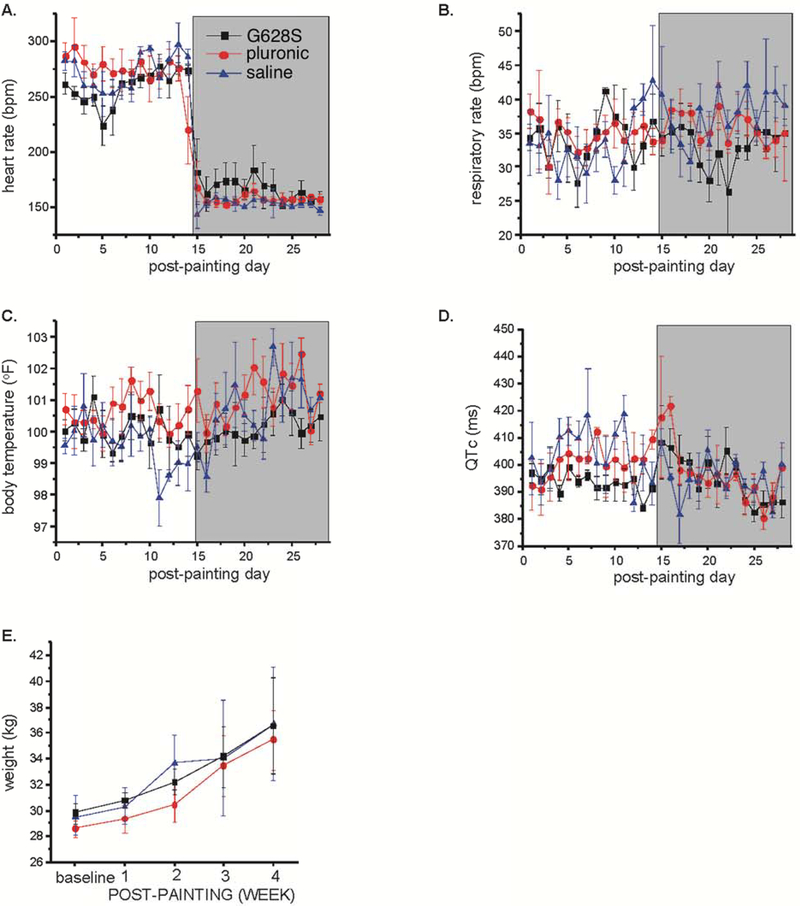
We compared the 3 groups for a number of safety measures including qualitative measures of overall clinical status, activity, appetite, thirst, urine and stool generation, and quantitative measures of average heart rate, respiratory rate, body temperature, and electrocardiographic QT interval measured on a daily basis and body weight measured weekly (Figures 3–4). We also evaluated general labs (Supplementary Table 1), echocardiographic measures of cardiac structure and function and electrophysiological measures of repolarization (Supplementary Table 2) taken at baseline and sacrifice studies, and we assessed cardiac dissection time (as a surrogate for pericardial adhesion formation), organ weights and effusion volumes at sacrifice study (Supplementary Table 3). Other than significantly better left ventricular function at sacrifice for the G628S group (a reflection of the increased SR% for that group), we found no differences between groups for any of these measures.
Figure 3:
Clinical observations: daily assessment of (A) overall clinical status, (B) activity, (C) appetite, (D) thirst, (E) urine and (F) stool output was recorded using a 2–5 point numerical scale with guidance from the descriptors shown on the graphs. The investigator evaluating the animals was masked to study group identity of each animal. The G628S group is the black square. The pluronic group is the red circle. The saline group is the blue triangle. There were no statistically significant differences between groups.
Figure 4:
Clinical measurements: daily measurement of (A) ventricular rate from ECG, (B) respiratory rate (C) body temperature and (D) QTc from ECG were performed. The animals were weighed weekly. The shaded box indicates the period of time after burst pacing was discontinued. There were no statistically significant between group differences for any measurement.
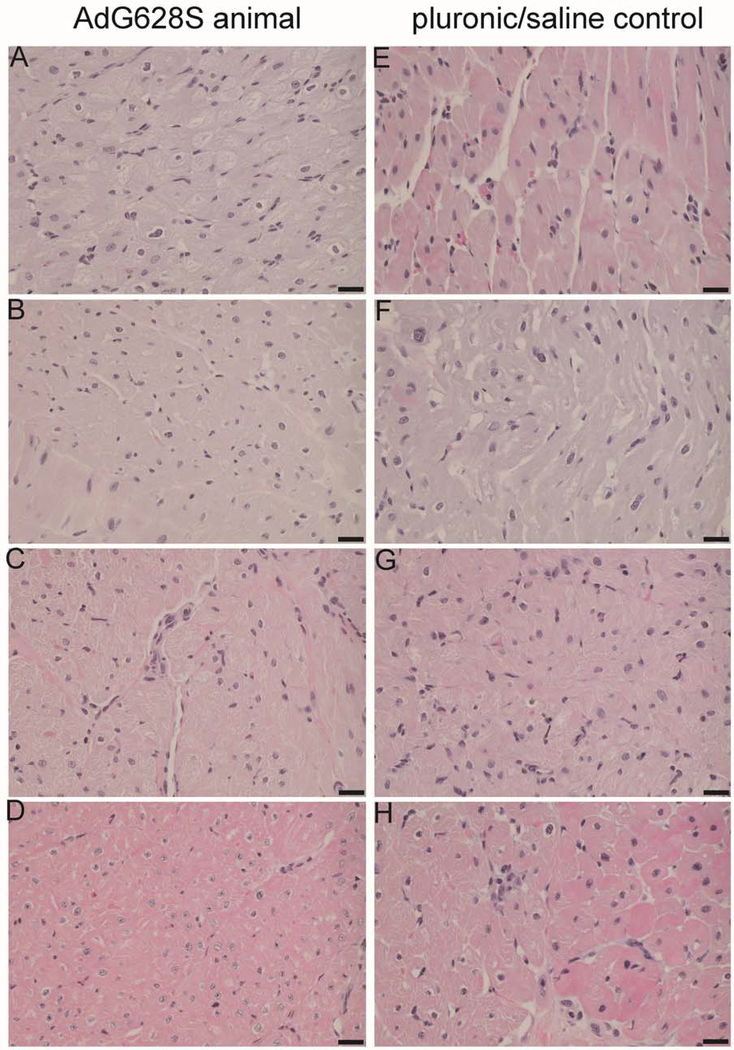
Microscopic evaluation of all tissues collected at necropsy demonstrated findings that were related to the open chest surgery or the tachycardiomyopathy inherent to the model. There were no differences between groups in the incidence of these findings, and there were no findings suggesting any adverse effect of AdG628S treatment (Figure 5).
Figure 5:
Example microsections from hearts of animals receiving AdG628S (left) and pluronic/saline (right). There were no differences between groups when comparing pathological findings in right atria (a,e), right ventricles (b,f), left atria (c,g) or left ventricles (d,h). Pathological findings of hypertrophy and myolysis in all chambers from all animals were consistent with structural remodeling from AF and heart failure. No treatment-specific pathological findings were observed.
During the course of the study, 2 animals were found dead, a G628S animal with cardiac tamponade from atrial lead perforation and a pluronic animal with severe heart failure that never recovered from the initial surgical procedure. Interestingly, the pluronic animal had a low-level positive troponin on baseline study suggesting that the animal had subclinical myocarditis that may have contributed to its intolerance of the AF-heart failure. We terminated 2 animals (1 pluronic animal and 1 saline animal) early due to intractable, severe heart failure that is a described component of the model.18 In-life clinical observations, gross and microscopic pathology in these animals were assessed for any treatment-related cause. Based on these data, all deaths were determined to be procedural or model-related and not product-related.
DISCUSSION
This preclinical study was designed to evaluate AdKCNH2-G628S gene therapy for prevention of POAF. It should be considered in the context of our prior work showing that atrial gene painting with reporter genes caused epicardial only gene transfer in the absence of trypsin and dense transmural gene transfer with 0.5% trypsin, and that gene painting with AdG628S and trypsin reliably increased atrial APD and prevented AF at peak gene expression with dissipation of efficacy over 2–3 weeks.16,17 In the current study, we found effective AF prevention without any observable toxicity in animals receiving AdG628S in the absence of trypsin.
Porcine model of AF and heart failure
There is no animal model that reliably produces POAF. In order to test AF therapies in an aggressive preclinical environment, we developed and validated a porcine AF-heart failure model.16,18–20 Independent use of this model by other labs has verified reliability.21 The basic protocol includes burst right atrial pacing to induce persistent AF and an uncontrolled ventricular rate to cause a cardiomyopathy. Animals have progressively increasing non-sustained AF over the first few days before developing persistent AF a median of 5±1 days after burst pacing onset.18 The tachycardiomyopathy progressively worsens over time, with LVEF decreasing to 53±4% after 1 week, 28±2% after 3 weeks and 22±3 after 5 weeks.18–20 Along with this decrease in cardiac function, progressive dilation occurs in all cardiac chambers, and histological findings of cellular hypertrophy, myolysis, apoptosis, inflammation, and fibrosis progress over the same time frame. To test efficacy against POAF, we lacked facilities and expertise to put the animals on cardiopulmonary bypass and perform coronary bypass or valve surgery. As such, our animals didn’t have “typical” POAF, but we did test efficacy in the setting of median sternotomy and hands-on manipulation of the heart (in addition to the persistent AF and structural remodeling components of the model), and we found efficacy in an aggressive AF-inducing post-operative environment.
KCNH2-G628S
KCNH2 is the alpha subunit of an ion channel that generates IKr, one of the principal cardiac repolarizing currents. The KCNH2-G628S mutation replaces a glycine with a serine at a position within the channel pore. The larger serine blocks the pore.22 In vitro study showed no current if equal quantities of wild type and G628S messenger RNA were injected, and only 12% of the expected current if a ratio of 5 wild type KCNH2 to 1 G628S were injected.22 This dominant negative action is required for therapeutic efficacy because patients would presumably express normal endogenous KCNH2. We do not have the tools available to quantify the percentage of IKr channels in each myocyte that contained the transgene-derived mutant channel, but the stoichiometry of wild type to mutant channels is undoubtedly a component in the overall time course of efficacy for our therapy.
Insight into arrhythmia mechanisms
The mechanism of post-operative AF remains controversial. Prior studies have implicated a variety of factors including pre-existing atrial fibrosis, expression levels of various ion channels, inflammation, metabolic or oxidative stress on myocytes, alterations in connexin expression, adrenergic, purinergic and/or cholinergic stimulation.23–29 These associations could potentially support either triggered or reentrant mechanisms for POAF. Manipulations to prolong action potential duration (APD) could help distinguish between these mechanisms. Triggered activity by early afterdepolarization mechanisms would worsen with APD prolongation because the increased repolarization time would give more opportunity for early afterdepolarizations to occur. Delayed afterdepolarizations and automatic activity would not be directly affected by APD. IKr block is a well-established method to prolong APD. The efficacy of IKr block, both in our study and in the sotalol clinical trials, suggests that reentry is the underlying mechanism of POAF.
The contribution of pulmonary veins to AF was established by Hassaiguerre et al. and has since become an accepted paradigm for paroxysmal AF.30 Decidedly less efficacy has been found for pulmonary vein ablation in patients with longstanding persistent AF and/or structural heart disease suggesting that the pulmonary vein contribution is less relevant in those settings.31 The pulmonary veins in the pig are not accessible from a pericardial approach, so our intervention did not affect pulmonary vein function. Nonetheless we saw efficacy with our intervention, suggesting that POAF is likely an intra-atrial and not pulmonary venous process. That conclusion is supported by Ryu et al. who found decreased intra-atrial connexin43 expression in dogs with POAF,27 data from Zaman et al. showing reduced right atrial sarcolipin, myosin heavy chain and SERCA2a in patients with POAF.32, and data from Dizon et al. showing POAF occurs in patients with double lung transplantation in spite of pulmonary vein isolation.33
Additional insight into the AF mechanism can be gleaned from the efficacy in our current study where trypsin was no longer included in the gene painting solution. We have previously shown that transgene expression was limited to the epicardial layer when trypsin is not part of the adenoviral painting solution.17 Those observations combined with our current study results indicate that epicardial only expression of G628S is sufficient to prevent AF. These data suggest that the epicardium is an obligatory component in the reentry circuits that sustain AF. Overall, our data indicate that POAF is a reentrant process, that it is not dependent on pulmonary vein activity, but it is critically dependent on conduction through the epicardium.
Safety of the G628S gene therapy
In extensive safety analysis, we found no evidence of any adverse effects of therapy. We performed a comprehensive safety analysis, with 36 observation variables including overall clinical status, a number of specific measures of animal condition, daily QTc and rhythm analysis. The only between group differences suggested benefit of therapy (likely due to the reduced heart failure from the overall lower average heart rate and increased sinus rhythm in the active treatment group). We saw no suggestion from any of these measures that there might be any potential harm from therapy. In particular, we saw no evidence of QT prolongation or ventricular arrhythmias, consistent with the atrial specificity of the delivery method. Our overall assessment of safety for this therapy should be considered in the context of Kikuchi et al. and Amit et al., where 18 pigs received AdKCNH2-G628S by atrial painting with trypsin (a more effective delivery method than the current trypsin-free protocol). These animals had no QT prolongation and no significant adverse events relative to the 21 control animals in those studies.16,17
Study limitations
Our data present compelling evidence for efficacy and safety of G628S gene painting for prevention of POAF. In addition to the lack of cardiopulmonary bypass discussed above, other considerations for these data include the younger substrate and starting good health of the pigs relative to the typical elderly cardiac surgery patient. Age, hypertension, heart failure, diabetes, and pulmonary disease are consistent risk factors for AF overall, and POAF in particular. Our model does include severe heart failure that develops from the uncontrolled ventricular rate during either burst pacing or AF.18 Antiarrhythmic efficacy is maintained in the setting of heart failure, suggesting that the therapy may withstand these other comorbidities. In the end, only clinical testing will answer the question of efficacy and safety in the clinical environment.
Conclusion
Overall, we find that G628S is safe and effective in preclinical study. Animals in the G628S group had significantly reduced AF burden and no toxicity attributable to the test article. These data support further study of G628S in a Phase I clinical trial to prevent post-operative atrial fibrillation.
Supplementary Material
PERSPECTIVE STATEMENT.
POAF is a common and significant complication of cardiac surgery. Stroke, heart failure and death risk are increased in patients who have post-operative atrial fibrillation. We found that KCHN2-G628S gene transfer reduced AF burden without any evident toxicity. The timing of adenovirus-mediated gene expression is such that the method could be directly applicable to preventing post-operative atrial fibrillation.
Acknowledgments
This study was supported by National Institute of Health grants R01-HL93486, R01-HL130376 and R21-AG42701.
Glossary of Abbreviations
- AF
atrial fibrillation
- APD
action potential duration
- EPS
electrophysiology study
- G628S
Ad-KCNH2-G628S
- KCNH2
Potassium voltage- gated channel subfamily H member 2
- LVEF
left ventricular ejection fraction
- MAP
monophasic action potential
- POAF
postoperative atrial fibrillation
- Pluronic P407
polyoxyethylene/polyoxypropylene block copolymer referred variably in the literature as either poloxamer, pluronic, Lutrol® or Kolliphor® and either F127 or P407
- QRS
the combination of Q, R, and S wave
- QT
the interval between Q and T wave
- QTc
QT interval corrected for heart rate
- SR
sinus rhythm
- VP
viral particles
Footnotes
Disclosure: ZL, BR, YA, and KLD have no conflicts of interest to declare. JKD is inventor on gene therapy patents issued to Johns Hopkins University. Otherwise JKD has no conflicts to declare.
CENTRAL MESSAGE
G628S gene transfer safely and effectively prevents POAF, supporting further study in a Phase I clinical trial.
Literature Cited:
- (1).Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics––2014 update: a report from the american heart association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–9. [DOI] [PubMed] [Google Scholar]
- (3).Echahidi N, Pibarot P, O’Hara G, Mathieu P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51:793–801. [DOI] [PubMed] [Google Scholar]
- (4).Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226:501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Burgess DC, Kilborn MJ, Keech AC. Interventions for prevention of post-operative atrial fibrillation and its complications after cardiac surgery: a meta-analysis. Eur Heart J. 2006;27:2846–57. [DOI] [PubMed] [Google Scholar]
- (6).Horwich P, Buth KJ, Legare JF. New onset postoperative atrial fibrillation is associated with a long-term risk for stroke and death following cardiac surgery. J Card Surg. 2013;28:8–13. [DOI] [PubMed] [Google Scholar]
- (7).Almassi GH, Pecsi SA, Collins JF, Shroyer AL, Zenati MA, Grover FL. Predictors and impact of postoperative atrial fibrillation on patients’ outcomes: a report from the Randomized On Versus Off Bypass trial. J Thorac Cardiovasc Surg. 2012;143:93–102. [DOI] [PubMed] [Google Scholar]
- (8).Kaw R, Hernandez AV, Masood I, Gillinov AM, Saliba W, Blackstone EH. Short- and long-term mortality associated with new-onset atrial fibrillation after coronary artery bypass grafting: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2011;141:1305–12. [DOI] [PubMed] [Google Scholar]
- (9).Wang W, Mei YQ, Yuan XH, Feng XD. Clinical efficacy of epicardial application of drug-releasing hydrogels to prevent postoperative atrial fibrillation. J Thorac Cardiovasc Surg. 2016;151:80–5. [DOI] [PubMed] [Google Scholar]
- (10).Ad N, Holmes SD, Shuman DJ, Pritchard G, Miller CE. Amiodarone after surgical ablation for atrial fibrillation: Is it really necessary? A prospective randomized controlled trial. J Thorac Cardiovasc Surg. 2016;151:798–803. [DOI] [PubMed] [Google Scholar]
- (11).Costanzo S, De CA, di N, V, Olivieri M, Morena M, De Filippo CM, et al. Postoperative atrial fibrillation and total dietary antioxidant capacity in patients undergoing cardiac surgery: The Polyphemus Observational Study. J Thorac Cardiovasc Surg. 2015;149:1175–82. [DOI] [PubMed] [Google Scholar]
- (12).Wu JC, Inubushi M, Sundaresan G, Schelbert HR, Gambhir SS. Optical imaging of cardiac reporter gene expression in living rats. Circulation. 2002;105:1631–4. [DOI] [PubMed] [Google Scholar]
- (13).French B, Mazur W, Geske R, Bolli R. Direct in vivo gene transfer into porcine myocardium using replication-deficient adenoviral vectors. Circulation. 1994;90:2414–24. [DOI] [PubMed] [Google Scholar]
- (14).Muhlhauser J, Jones M, Yamada I, Cirielli C, Lemarchand P, Gloe TR, et al. Safety and efficacy of in vivo gene transfer into the porcine heart with replication-deficient, recombinant adenovirus vectors. Gene Therapy. 1996;3:145–53. [PubMed] [Google Scholar]
- (15).Zhou Z, Gong Q, Ye B, Fan Z, Makiekski J, Rovertson G, et al. Properties of HERG channels stably expressed in HEK293 cells studied at physiological temperature. Biophys J. 1998;74:230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Amit G, Kikuchi K, Greener ID, Yang L, Novack V, Donahue JK. Selective molecular potassium channel blockade prevents atrial fibrillation. Circulation. 2010;121:2263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Kikuchi K, McDonald AD, Sasano T, Donahue JK. Targeted modification of atrial electrophysiology by homogeneous transmural atrial gene transfer. Circulation. 2005;111:264–70. [DOI] [PubMed] [Google Scholar]
- (18).Bauer A, McDonald AD, Donahue JK. Pathophysiological findings in a model of persistent atrial fibrillation and severe congestive heart failure. Cardiovasc Res. 2004;61:764–70. [DOI] [PubMed] [Google Scholar]
- (19).Bauer A, McDonald AD, Nasir K, Peller L, Rade JJ, Miller JM, et al. Inhibitory G protein overexpression provides physiologically relevant heart rate control in persistent atrial fibrillation. Circulation. 2004;110:3115–20. [DOI] [PubMed] [Google Scholar]
- (20).Igarashi T, Finet JE, Takeuchi A, Fujino Y, Strom M, Greener ID, et al. Connexin gene transfer preserves conduction velocity and prevents atrial fibrillation. Circulation. 2012;125:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Soucek R, Thomas D, Kelemen K, Bikou O, Seyler C, Voss F, et al. Genetic suppression of atrial fibrillation using a dominant-negative ether-a-go-go-related gene mutant. Heart Rhythm. 2012;9:265–72. [DOI] [PubMed] [Google Scholar]
- (22).Sanguinetti MC, Curran ME, Spector PS, Keating MT. Spectrum of HERG K+-channel dysfunction in an inherited cardiac arrhythmia. PNAS. 1996;93:2208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Goette A, Juenemann G, Peters B, Klein H, Roessner A, Huth C, et al. Determinants and consequences of atrial fibrosis in patients undergoing open heart surgery. Cardiovasc Res. 2002;54:390–6. [DOI] [PubMed] [Google Scholar]
- (24).Plante I, Fournier D, Mathieu P, Daleau P. A pilot study to estimate the feasibility of assessing the relationships between polymorphisms in hKv1.5 and atrial fibrillation in patients following coronary artery bypass graft surgery. Can J Cardiol. 2008;24:41–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Korantzopoulos P, Kolettis T, Siogas K, Goudevenos J. Atrial fibrillation and electrical remodeling: the potential role of inflammation and oxidative stress. Med Sci Monit. 2003;9:RA225–RA229. [PubMed] [Google Scholar]
- (26).Korantzopoulos P, Kolettis TM, Siogas K, Goudevenos JA. The emerging role of inflammation in atrial fibrillation and the potential of anti-inflammatory interventions. Eur Heart J. 2005;26:2207–8. [DOI] [PubMed] [Google Scholar]
- (27).Ryu K, Li L, Khrestian CM, Matsumoto N, Sahadevan J, Ruehr ML, et al. Effects of sterile pericarditis on connexins 40 and 43 in the atria: correlation with abnormal conduction and atrial arrhythmias. Am J Physiol Heart Circ Physiol. 2007;293:H1231–H1241. [DOI] [PubMed] [Google Scholar]
- (28).Workman AJ, Pau D, Redpath CJ, Marshall GE, Russell JA, Kane KA, et al. Post-operative atrial fibrillation is influenced by beta-blocker therapy but not by pre-operative atrial cellular electrophysiology. J Cardiovasc Electrophysiol. 2006;17:1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Amar D, Zhang H, Miodownik S, Kadish AH. Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. J Am Coll Cardiol. 2003;42:1262–8. [DOI] [PubMed] [Google Scholar]
- (30).Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. [DOI] [PubMed] [Google Scholar]
- (31).Kirchhof P, Calkins H. Catheter ablation in patients with persistent atrial fibrillation. Eur Heart J. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Zaman JA, Harling L, Ashrafian H, Darzi A, Gooderham N, Athanasiou T, et al. Post-operative atrial fibrillation is associated with a pre-existing structural and electrical substrate in human right atrial myocardium. Int J Cardiol. 2016;220:580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Dizon JM, Chen K, Bacchetta M, Argenziano M, Mancini D, Biviano A, et al. A comparison of atrial arrhythmias after heart or double-lung transplantation at a single center: insights into the mechanism of post-operative atrial fibrillation. J Am Coll Cardiol. 2009;54:2043–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.