Abstract
Feeding strategies are dependent on multi-modal sensory processing, that integrates visual, chemosensory, and mechanoreceptive cues. In many fish species, local environments and food availability dramatically influence the evolution of sensory and morphological traits that underlie feeding. The Mexican cavefish, Astyanax mexicanus, have developed robust changes in sensory-dependent behaviors, but the impact on prey detection and feeding behavior is not known. In the absence of eyes, cavefish have evolved enhanced sensitivity of the lateral line, comprised of mechanosensory organs that sense water flow and detect prey. Here, we identify evolved differences in prey capture behavior of larval cavefish that are dependent on lateral line sensitivity. Under lighted conditions, cavefish strike Artemia prey at a wider angle than surface fish; however, this difference is diminished under dark conditions. In addition, the strike distance is greater in cavefish than surface fish, revealing an ability to capture, and likely detect, prey at greater distances. Experimental ablation of the lateral line disrupts prey capture in cavefish under both light and dark conditions, while it only impacts surface fish under dark conditions. Together, these findings identify an evolutionary shift towards a dependence on the lateral line for prey capture in cavefish, providing a model for investigating how loss of visual cues impacts multi-modal sensory behaviors.
1. Introduction
The ability to locate and acquire food is central to survival and successful reproduction (Illius et al., 2002). Animals have evolved diverse foraging and prey capture mechanisms, with different organisms utilizing a variety of sensory systems to localize food sources (Catania, 2012; Daghfous et al., 2012; Moss and Shettleworth, 1996). Many species, from humans to fish, heavily utilize visual systems to find food (Bianco and Engert, 2015; Troscianko et al., 2011). In the absence of visual cues, organisms must rely on alternative sensory input to locate food sources. For instance, nocturnal species seek food in dimly lit areas, relying almost exclusively on auditory cues (Payne, 1971; Wagner et al., 2013), and many rodents with poor visual acuity are largely reliant on olfactory sensory cues (Doty, 1986; Rattazzi et al., 2015; Yao et al., 2016). The diversity of sensory processes used in foraging and prey detection suggests that the sensory basis for foraging is under stringent evolutionary pressure. While diverse strategies utilizing sensory systems in foraging behavior are well described, much less is known about how these systems evolve in response to environmental perturbation.
The blind Mexican cavefish, Astyanax mexicanus, provide a unique opportunity to study how foraging strategies evolve in response to strong environmental pressures. A. mexicanus exist in two distinct morphological forms: an eyed surface-dwelling form found in above-ground rivers and streams of northeast Mexico and parts of Southern Texas, and 29 populations of cave-dwelling forms, mostly found within the Sierra del Abra region of northeast Mexico (Gross, 2012; Jeffery, 2001; Mitchell et al., 1977). Many of these populations of cavefish are geographically and hydrologically restricted, suggesting they evolved independent of one another (Bradic et al., 2013; Mitchell et al., 1977; Ornelas-García et al., 2008). Moreover, cave-dwelling fish have converged on a suite of morphological and behavioral traits, such as eye loss, which evolved via different genetic mechanisms (Borowsky, 2008a; Duboué et al., 2011; Wilkens and Strecker, 2003; Yoshizawa et al., 2012), making this emerging model organism a powerful system to study the principles of convergent evolution.
Contrasting dramatically with the ecology of the surface rivers and streams, the perpetually dark caves have relatively few primary producers; therefore, the cavefish diet is primarily restricted to small organic matter, bat guano, and insects (Espinasa et al., 2017; Mitchell et al., 1977). To compensate, cavefish have developed a number of behavioral adaptations amenable to the subterranean environment, including increased vibration-associated behavior, hyperphagia, and changes in feeding angle (Aspiras et al., 2015; Kowalko et al., 2013; Wilkins, 1988; Yoshizawa et al., 2010). In addition, cavefish have evolved enhanced mechanosensory, olfactory, and taste sensitivity, presumably to compensate for reduced reliance on visual cues (Bibliowicz et al., 2013; Schemmel, 1967; Varatharasan et al., 2009; Yoshizawa et al., 2010). Despite these enhanced sensory changes, the contributions to evolved differences in feeding behavior remain poorly understood.
Here, we describe a prey capture assay that we developed for studying feeding strategies in larval A. mexicanus, to investigate how different sensory modalities contribute to feeding behavior. Prey capture has been extensively studied in zebrafish, and functional imaging in transgenic zebrafish has been used to map retinal, motor, and central brain circuits required for prey detection and capture (Bianco and Engert, 2015; Muto et al., 2017). In zebrafish larvae, this behavior is almost exclusively driven by visual inputs, yet other fish species are reliant on mechanosensation and olfaction (Bianco and Engert, 2015; Braubach et al., 2009; Schwalbe et al., 2012). We demonstrate that both surface and cave forms of A. mexicanus have the potential to utilize the lateral line in sensing and finding food. In surface fish, the visual system is dominant over the lateral line, yet in cave forms, the lateral line is the primary sensory system by which fish can detect food. Our results demonstrate that several foraging strategies exist within single organisms for finding food, and adaptation to the cave environment resulted in dependence on mechanosensation for prey capture.
2. Results
To determine whether prey-seeking behavior differs between surface fish and cavefish, we recorded 29–33 day post-fertilization (dpf) surface fish and Pachón cavefish larvae (Fig. 1A) consuming Artemia brine shrimp using a high-speed camera set at 100 frames per second. To quantify prey capture behavior, we measured the strike angle by quantifying the angle between the brine shrimp target and the fish’s center point along the midline (Fig. 1B). In addition, we calculated the strike distance by determining the distance between the closest location on the fish head to the Artemia at the start of the strike motion (Fig. 1B). Serial time-lapse images of single prey capture events revealed that surface fish approach prey directly, bending the most caudal region of their tail (Fig. 1C). In zebrafish, this movement has been classified as a J turn (McElligott and O’Malley, 2005). Conversely, Pachón cavefish approach prey using a C-bend turn that involves turning the head toward adjacent prey (Fig. 1D). Quantification under lighted conditions began at zeitgeber time 0 (ZT0), the onset of lights on, when the overall activity is most similar between surface fish and Pachón cavefish (Duboué et al., 2011). Analysis revealed strike angle and strike distance were significantly greater in Pachón cavefish than surface fish, indicating that cavefish both bend their heads towards prey and attack from a greater distance (Fig. 1E, F). Differences in strike angle (Fig. S1A), but not distance (Fig. S1B), emerged as early as 8 dpf, indicating a developmental component to lateral line mediated prey capture.
Fig. 1. Evolved differences in prey capture between surface and Pachón cavefish.
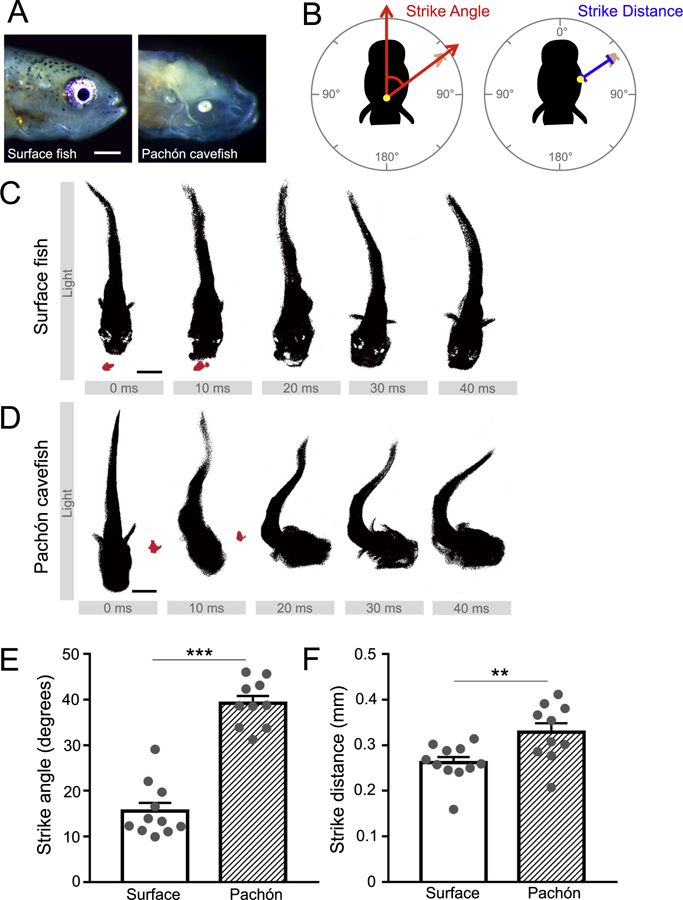
(A) Comparison of morphological differences between larval surface fish and Pachón cavefish morphs. Cavefish (right) exhibit altered cranial structure compared to surface fish (left), and lack pigmentation and functional eyes. Scale bar = 0.5 mm. (B) Quantification of strike angle (left) and strike distance (right) used for behavior analysis. (C) Example time-lapse of a stereotypical surface fish strike, in lighted conditions. Surface fish move towards their prey head-on and propel themselves forward to capture. Scale bar = 1 mm. (D) Example time-lapse of a stereotypical cavefish strike, in lighted conditions. Cavefish remain immobile prior to striking nearby prey, using a lateral C-shaped motion. Scale bar = 1 mm (E) Strike angle in larval surface (N = 11) and cavefish (N = 10; Unpaired t-test, t = 9.772, df=19, P < 0.0001). (F) Strike distance in larval surface (N = 11) and cavefish (N = 10; Unpaired t-test, t = 2.884, df=19, P = 0.0095). Error bars represent + /− standard error of the mean. ** denotes P < 0.01, *** denotes P < 0.001.
It is possible that the reduced attack angle and strike distance during prey capture in surface fish is the result of reliance on visual cues that are absent in cavefish. To investigate this in animals lacking visual cues, we measured prey capture in complete darkness, while maintaining the time of testing at the onset of lights on (ZT0) used for measurements under lighted conditions. We compared images from the high-speed camera in light and dark conditions and found that under dark conditions, the prey capture behavior between surface and cavefish appeared similar (Fig. 2A, B). Unlike lighted conditions, the strike angle of surface fish was significantly greater in dark conditions, while darkness had no effect on Pachón cavefish (Fig. 2C). Further, under dark conditions, the strike angle did not differ between surface fish and cavefish (Fig. 2C). These findings suggest that differences between surface and Pachón cavefish observed under lighted conditions are primarily due to use of visual cues in surface fish. Strike distance was significantly reduced in the dark in surface fish but not cavefish (Fig. 2D), suggesting optimal foraging in surface fish occurs during the light, and cavefish may have improved ability to detect prey compared to surface fish in the dark. Quantification of successful capture rate revealed no differences between light and dark conditions in either surface or cavefish (Fig. 2E). However, the total number of attempts were significantly greater under both conditions in Pachón cavefish (Fig. 2F). Further, in surface fish, the strike rate was reduced in the dark, suggesting they are less successful at capturing prey during dark conditions (Fig. 2F). These findings reveal different foraging strategies where attempts are increased in cavefish with reduced success rate.
Fig. 2. Surface fish alter their strike dynamics in dark conditions.
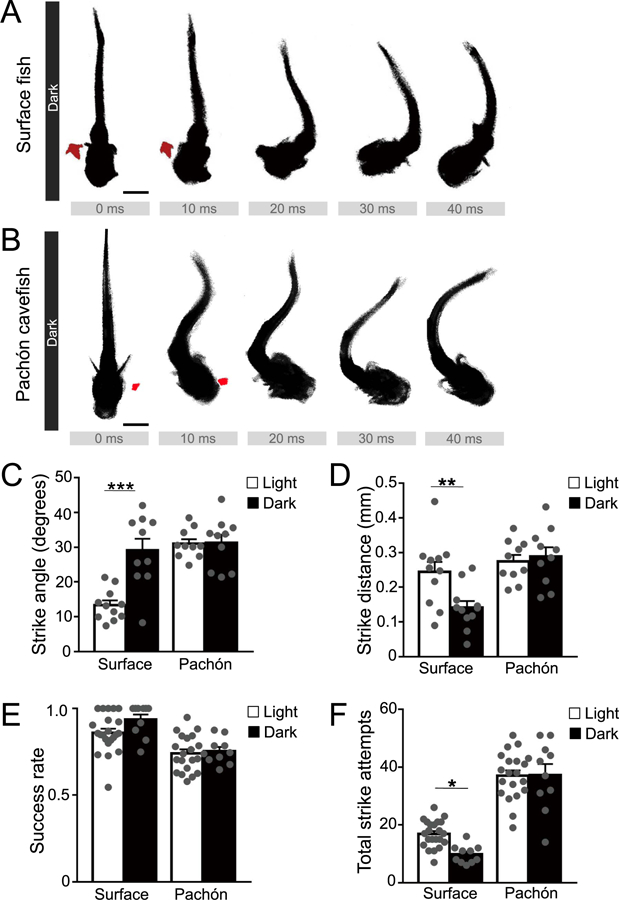
(A) Example time-lapse of a stereotypical surface fish strike, in dark conditions. In the absence of light, surface fish alter their strike dynamics to use a C-shaped movement, indicating a reliance on alternate sensory cues. Scale bar = 1 mm. (B) Example time-lapse of a stereotypical cavefish strike, in dark conditions. Cavefish do not alter their strike pattern in the dark relative to lighted conditions, highlighting their reliance on nonvisual cues. Scale bar = 1 mm. (C) Strike angle in surface fish and Pachón cavefish between lighted and dark conditions. Surface fish light (N = 11) vs. dark (N = 10; P < 0.0001). Pachón cavefish light (N = 10) vs. dark (N = 10; P = 0.9996); Two-Way ANOVA, F(1,37) = 12.59, P = 0.0011. (D) Strike distance in surface fish and Pachón cavefish between lighted and dark conditions. Surface fish light (N = 11) vs. dark (N = 10; P = 0.0068). Pachón cavefish light (N = 10) vs. dark (N = 10; P = 0.9994); Two-Way ANOVA, F(1,37) = 6.325, P = 0.0164. (E) Ratio of successful to unsuccessful strikes over two minutes, in lighted and dark conditions. Surface fish light (N = 22) vs. dark (N = 12; P = 0.1379); Pachón cavefish light (N = 20) vs. dark (N = 10; P = 0.9851). Two-Way ANOVA, F(1, 60) = 32.8, P < 0.0001. (F) Total number of strikes at prey over two minutes, in lighted and dark conditions. Surface fish light (N = 22) vs. dark (N = 12; P = 0.0362). Pachón cavefish light (N = 20) vs. dark (N = 10; P = 0.9997). Two-Way ANOVA, F(1, 60) = 157.6, P < 0.0001. Error bars represent + /- standard error of the mean. * denotes P < 0.05, ** denotes P < 0.01, *** denotes P < 0.001.
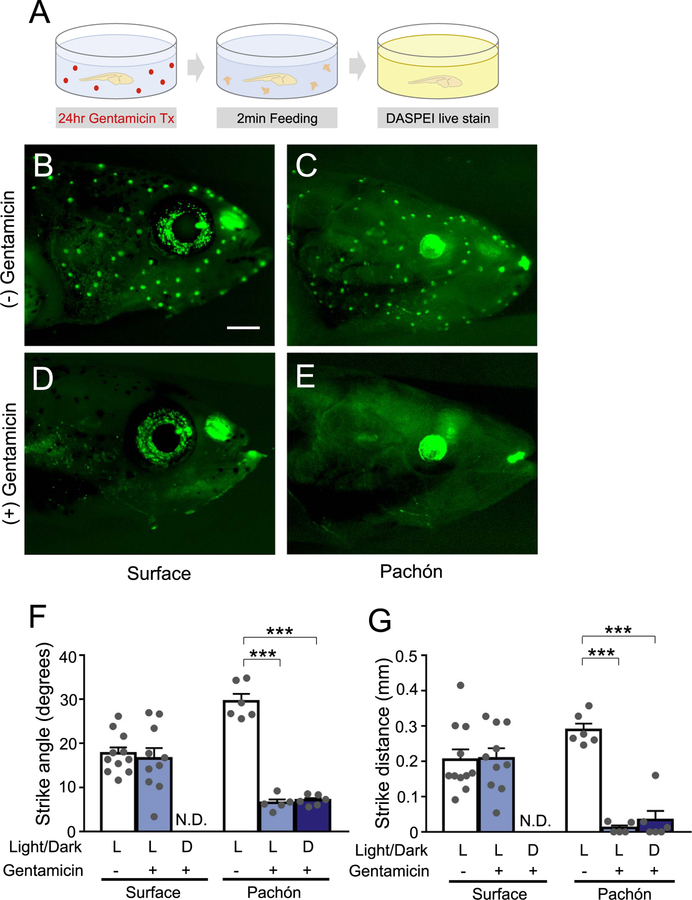
To determine whether the lateral line modulates prey capture, we ablated the lateral line and measured the effects on prey capture. Bathing fish in the antibiotic gentamicin efficiently ablates all lateral line neuromasts by disrupting hair cells (Van Trump et al., 2010). Neuromasts were ablated 24hrs prior to behavioral testing, and the effectiveness of ablation was confirmed by staining fish with the mitochondrial marker DASPEI following prey capture tests (Fig. 3A). Gentamicin treatment fully ablated the lateral line in surface fish and Pachón cavefish (Fig. 3B–E). Ablating the lateral line did not impact strike angle or distance in surface fish under light conditions, however, all prey capture was abolished under dark conditions, indicating that surface fish are dependent on the lateral line for prey capture in dark conditions (Fig. 3F, G). In cavefish, ablation of the lateral line significantly reduced both strike angle and distance under both light and dark conditions, revealing a critical role for the lateral line under both conditions (Fig. 3F, G).
Fig. 3. Ablation of lateral line in Pachón cavefish reduces strike angle and distance.
(A) Diagram of experimental paradigm for gentamicin treatment. Fish are treated for 24 h in dissolved gentamicin sulfate, followed by a 2-min feeding/behavioral recording, and 1 h in a DASPEI solution to confirm complete ablation of neuromasts. (B-E) Visualization of lateral line neuromasts in surface fish (left) and Pachón cavefish (right) before (top) and after (bottom) gentamicin treatment. Scale bar = 0.5 mm. (F) Strike angle of surface fish and Pachón cavefish, with or without prior gentamicin treatment, in lighted or dark conditions. Surface fish control(N = 11) vs. light/gentamicin(N = 10; Unpaired t-test, t = 0.4196, df=19, P = 0.6795). Pachón cavefish control (N = 6) vs. light/gentamicin(N = 5; P < 0.0001). Pachón cavefish control vs dark/gentamicin(N = 6; P < 0.0001; One-Way ANOVA, F(2, 14) = 136.9, P < 0.0001. (G) Strike distance of surface fish and Pachón cave fish, with or without gentamicin treatment, in lighted or dark conditions. Surface fish control(N = 11) vs. light/ gentamicin(N = 10; Unpaired t-test, t = 0.09121, df=19, P = 0.9283). Pachón cavefish control(N = 6) vs. light/gentamicin(N = 5; P < 0.0001). Pachón cavefish control vs. dark/ gentamicin(N = 6; P < 0.0001; One-Way ANOVA, F(2, 14) = 63.46, P < 0.0001. N.D. = No Data/Strikes. Error bars represent + /− standard error of the mean. *** denotes P < 0.001.
The antennae strokes of a moving Artemia generate oscillations of approximately 7 Hz, and based on previous literature, we reasoned that the lateral line may detect water movement induced by these oscillations (Kirchner et al., 2014). To test this hypothesis, we presented surface and Pachón larvae with dead Artemia that had been flash-frozen, then thawed immediately prior to the experiment, in accordance with published protocols (Schwalbe et al., 2012). Under lighted conditions, cavefish and surface fish displayed a reduced attack angle, and all prey capture was abolished in surface fish under dark conditions (Fig. 4A). While cavefish were able to capture prey under light and dark conditions, the strike angle was dramatically reduced compared to previous experiments using live Artemia, and was similar to findings in gentamicin treated fish (Fig. 4A). A significant reduction in strike angle was observed in surface fish under lighted conditions, presumably because the use of dead Artemia eliminates the effect of prey movement on strike angle. Strike distance in surface fish was unaffected by the absence of moving Artemia under lighted conditions, while cavefish showed reduced strike distance under both lighted and dark conditions (Fig. 4B). Taken together, these findings suggest prey capture of dead Artemia largely phenocopies lateral line ablation, supporting the notion that prey capture is induced by detection of Artemia movements by the lateral line.
Fig. 4. Effect of immobile prey on strike dynamics in light and dark conditions.
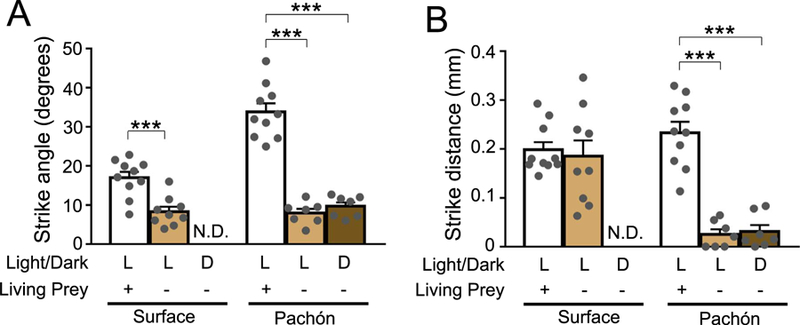
Fish were fed either live brine shrimp or non-living (flash-frozen) shrimp. (A) Strike angle in surface and Pachón cavefish, between lighted and dark conditions, on mobile (living) or immobile (dead) prey. Surface fish control(N = 10) vs. light/immobile prey(N = 9; Unpaired t-test, t = 4.372, df=17, P = 0.0004). Pachón cavefish control(N = 10) vs. light/immobile prey(N = 7; P < 0.0001). Pachón cavefish control vs dark/immobile prey(N = 7; P < 0.0001; One-Way ANOVA, F(2, 21) = 74.38, P < 0.0001. (B) Strike distance in surface and Pachón cave fish, between lighted and dark conditions, on mobile (living) or immobile (dead) prey. Surface fish control(N = 10) vs. light/immobile prey(N = 9; Unpaired t-test, t = 0.3753, df=17, P = 0.7120). Pachón cavefish control(N = 10) vs. light/immobile prey(N = 7; P < 0.0001). Pachón cavefish control vs dark/immobile prey(N = 7; P < 0.0001; One-Way ANOVA, F(2, 21) = 45.56, P < 0.0001. N.D. = No Data/Strikes. Error bars represent + /− standard error of the mean. *** denotes P < 0.001.
3. Discussion
We used high-speed imaging to examine state-dependent and population-specific differences in prey capture behavior in A. mexicanus. This assay provides quantifiable metrics of strike angle, which likely reflects the sensory modality used to detect prey, lateral line sensitivity in prey detection, or a stereotyped motor response induced by prey detection. In addition, we quantify strike distance, which likely reflects the sensitivity of prey detection. Further, we identify state-dependent modulation of prey capture behavior in A. mexicanus surface fish. These findings reveal that A. mexicanus surface fish have the ability to modulate between prey capture behaviors in accordance with light availability, uniquely suiting them for adaptation to the dark cave environment.
3.1. Evolutionary shift in prey capture behavior
Comparative analysis of prey capture behavior in surface fish and Pachón cavefish revealed an evolutionary shift towards dependence on non-visual cues in cavefish, indicative of evolution in a perpetually dark environment. Our findings reveal a greater strike distance in cavefish fry under dark conditions, suggesting that cavefish are more attuned to foraging in the dark. A number of factors may account for this increase in strike distance, including enhanced detection of prey, or increased motivation to feed, that have previously been documented in cavefish (Aspiras et al., 2015; Yoshizawa et al., 2010). Suction also plays a critical role in prey capture, and it is possible that increased suction in cavefish contributes to the greater strike distance we observed in Pachón cavefish (Camp et al., 2015). Supporting the notion that feeding is enhanced in cavefish, a competition assay in 17 dpf fry revealed cavefish consumed more Artemia than surface fish under dark conditions (Espinasa et al., 2014). Further, this is not related to the presence of eyes because there was no effect of eye-ablation on food consumption in surface fish (Espinasa et al., 2014). In addition, multiple lines of evidence in adult fish reveal enhanced foraging capabilities in adult cavefish. In a competition assay, Pachón cavefish were able to out-compete surface fish under dark, but not lighted conditions, and this was later related to the degree of vibration attraction behavior in individual fish (Hüppop, 1987; Yoshizawa et al., 2010). Further, the expansion of lateral line superficial neuromasts surrounding the eye orbit were found to increase vibration attraction behavior in cavefish, revealing an adaptive function of lateral line expansion (Yoshizawa et al., 2010). While vibration attraction behavior was not detected in 30 dpf fry (Yoshizawa et al., 2010), it is possible that alternative changes in the lateral line allow for increased strike distance and angle in cavefish.
3.2. Relation to stereotyped turning behavior in zebrafish
High resolution imaging of prey capture in A. mexicanus reveals robust differences in stereotyped turning behavior, which are dependent on light availability and differ between surface and cavefish. The stereotypic capture behavior of zebrafish larvae in response to prey has been studied extensively (Budick and O’Malley, 2000; Gahtan et al., 2005). This pathway has primarily been studied under lighted conditions, since zebrafish demonstrate a significant reduction in prey capture behavior both in darkness and when vision is impaired (Gahtan et al., 2005). This visually driven behavior in zebrafish results in a stereotypic J-turn movement, initiated by the tail (Patterson et al., 2013). In A. mexicanus, surface fish exhibit a J-turn under lighted conditions, while the stereotyped movement shifts to C-shaped movements in dark conditions. Further, cavefish use C-shaped movements to attack prey under both light and dark conditions. In zebrafish, C and J turns are dependent on distinct neural circuits and are activated by distinct sensory inputs (Fajardo et al., 2013; Liu and Fetcho, 1999), supporting the notion that independent neural circuits regulate context and population-specific foraging in A. mexicanus.
The findings that A. mexicanus surface and cavefish readily detect prey in the dark are notable because conflicting accounts exist on the ability of zebrafish to detect prey in the absence of visual cues. While it was previously reported that prey capture in zebrafish is entirely dependent on visual processing (McElligott and O’Malley, 2005), other studies suggest there is consumption under dark conditions (Gahtan et al., 2005; Westphal and O’Malley, 2013). Interestingly, little is known about the mechanism of prey detection under dark conditions, and one study suggests it is dependent on tactile cues through direct contact between paramecium prey and larvae (Patterson et al., 2013). Further, this study suggests zebrafish larvae use the same J-turn behavior under light and dark conditions (Patterson et al., 2013). In contrast, findings in A. mexicanus indicates surface fish larvae have two different foraging strategies under light and dark conditions, and are fully capable of capturing prey in the dark. These data reveal the presence of light-dependent plasticity of prey capture behavior that is not present, or has yet to be identified, in zebrafish.
3.3. A role for the lateral line in prey capture behavior
Our results reveal an essential role for the lateral line in cavefish prey capture behavior, as well as in surface fish when visual cues are not present. While to our knowledge, these results are the first to document a role for the lateral line in A. mexicanus larvae, the adult lateral line regulates vibration attraction behavior (VAB) that promotes foraging, particularly under dark conditions (Yoshizawa et al., 2010). In adult cavefish, ablation of the lateral line with gentamicin abolishes vibration attraction behavior, and this effect was localized to a population of superficial neuromasts near the eye-orbit that are present in greater number in cavefish (Yoshizawa et al., 2010). Examination of VAB over the course of development revealed VAB is not present in fish under five months old, suggesting the prey capture observed in this study and VAB are regulated by distinct processes (Yoshizawa et al., 2010). These discrepancies are likely explained by the assays measuring different aspects of foraging behavior. While the VAB assay focuses on the attraction to a vibrating source, our prey capture analysis focuses on the mechanics of striking at prey. Early in development, fish likely consume smaller animals, and therefore the lateral line may be involved in the initiation and execution of strikes at nearby prey, but dispensable for responding to vibrations at longer distances. Alternatively, it is possible that differences in frequency account for discrepancies in lateral line dependent behavior. Significant VAB is detected at 10–50 Hz in adult fish while Artemia are estimated to generate 7 Hz frequency (Kirchner et al., 2014; Yoshizawa et al., 2010). Identification of lateral line neuromasts contributing to prey detection would allow for further investigation of how prey-sensation is represented within the brain, and how it integrates with other sensory modalities.
While our findings reveal a critical role for the lateral line in prey capture, a small number of prey capture events remained in cavefish lacking functional lateral lines; these tended to occur when food was immediately in front of the fish, perhaps indicating the presence of an alternative enhanced sensory modality, such as taste or olfaction, that allowed larvae to detect prey. Supporting this, cave morphs of A. mexicanus have previously been reported to have both increased numbers of taste buds (Varatharasan et al., 2009; Yamamoto et al., 2009) and enhanced chemosensory response (Bibliowicz et al., 2013) compared to their surface counterparts.
A number of other species use the lateral line to detect prey (Boord and Montgomery, 1989; Coombs et al., 2012). In the peacock cichlid, Aulonocara stuartgranti, the lateral line is required for detecting movements generated by Artemia (Schwalbe et al., 2012). A. stuartgranti consume live and dead Artemia equally in light conditions, yet nearly all consumption was abolished in both light and dark conditions following lateral line ablation (Schwalbe et al., 2012). Similarly, we find that consumption of dead Artemia is dramatically reduced in both surface fish and cavefish under dark conditions, accompanied by reduced strike angle and distance. These findings largely phenocopy the effect of gentamicin treatment, suggesting that the lateral line is detecting Artemia movement.
This assessment can be tested by examining response to non-nutritive foods. For example, zebrafish will approach and consume air bubbles (Muto and Kawakami, 2013). Testing consumption of air bubbles in lateral line ablated fish would test the hypothesis that residual feeding in cavefish is due to gustatory or olfactory processing. An alternative possibility is that increased locomotion previously observed in cavefish at 30 dpf (Duboué et al., 2011, 2012) may result in an increased number of random contacts with dead Artemia, resulting in consumption. Therefore, these findings lay the groundwork for future studies examining the requirements of altered sensory modalities in prey capture behavior.
3.4. Multi-modal processing of prey-seeking behavior
Prey capture serves as a model for examining innate multimodal behavior and decision making. This process is described as having multiple steps that include object recognition, approach, a decision to attempt a strike, object capture, and assessment, where the animal decides to consume or expel the object (Muto and Kawakami, 2013). The response to objects is dependent on their size and angle, resulting in approach or avoidance (Bianco et al., 2011). Functional imaging in partially immobilized zebrafish has identified a visual pathway in the pre-tectal region that is activated by prey-stimulus and required for the initiation of prey capture behavior (Semmelhack et al., 2014), but it is unclear how this pathway interfaces with decision making processes or other sensory modalities. Studies examining the neural basis of prey capture primarily focus on visual circuitry in zebrafish (Muto and Kawakami, 2013), and our findings that surface morphs of A. mexicanus integrate visual and mechanosensory stimuli provide the unique opportunity to examine how different sensory processes are integrated to generate a stereotyped J-turn behavior under lighted conditions, and C-shaped movements under dark conditions. Whereas J-bends are driven through activation of midbrain neurons receiving input from tectal and pretectal areas (Fajardo et al., 2013), C-bends are largely thought to arise through activation of Mauthner cells (Burgess and Granato, 2007; Lee and Eaton, 1991). Since Mauthner cells receive input from cells of the inner ear and the lateral line (Eaton et al., 1988), and ablation of the lateral line reduces prey capture in cavefish, this suggests a possible lateral-line to Mauthner mechanism for prey capture in cavefish, and a mostly tectal or pre-tectal one for surface fish. Further analysis in A. mexicanus, such as ablation of Mauthner cells may provide insight as to how the integration of these processes evolved in cavefish.
The ability to detect prey in darkness, and the differences in strike angle and distance between surface fish and cavefish, may have important consequences for foraging ability in natural conditions. The lack of reported prey capture in zebrafish larvae under dark conditions suggests these fish exclusively feed during the day. By contrast, both surface and cave populations of A. mexicanus larvae appear capable of detecting and capturing prey in dark conditions. While Artemia do not represent a natural food source for cavefish, these findings are likely to be ecologically relevant, as stomach content analysis of fry from the Pachón cave revealed consumption of diverse crustaceans including water fleas, copepods, and isopods (Espinasa et al., 2017), though a different study on adult cavefish from the Micos caves suggested fish consume bat guano (Mitchell et al., 1977). A better understanding of the diets in surface fish and cavefish would provide insight into the evolutionary basis for differences in prey-seeking behavior.
While little is known of A. mexicanus foraging behavior in the wild, these findings raise the possibility that A. mexicanus surface and cavefish feed during the day and night. Adult cavefish lack behavioral and molecular locomotor rhythms in their natural cave environment, but behavioral rhythms are present under light-dark conditions (Beale et al., 2013; Yoshizawa et al., 2015). Further, bat colonies in caves may provide circadian and nocturnal rhymes, resulting in the necessity of nighttime foraging (Mitchell et al., 1977). In the Somalian cavefish, Preatichtys andruzzi, food-entrainable oscillators are present that can both synchronize the clock and behavioral rhythms with feeding (Cavallari et al., 2011). Taken together, these findings raise the possibility that nighttime foraging in cavefish may impact circadian function in A. mexicanus.
The presence of dark feeding in surface morphs may provide insight into the unique ability of A. mexicanus to inhabit subterranean environments and adapt to cave life. Numerous fish species populate a wide range of freshwater rivers surrounding the caves and are occasionally swept in to the subterranean habitat. Species such as cichlids have been observed residing in these caves; yet A. mexicanus remains nearly exclusive among species that repeatedly colonize the cave environment in NE Mexico (Mitchell et al., 1977). Cave-dwelling A. mexicanus have enhanced lateral line sensitivity relative to surface conspecifics, however some surface fish demonstrate a moderate level of vibration attraction behavior (VAB), suggesting natural populations of surface fish harbor individuals which are suited for finding food in darkness. Further, surface x Pachón cavefish F1 progeny show a higher number of neuromasts and VAB compared to surface fish, suggesting this sensory enhancement is genetic and can dramatically enhance food finding abilities in the dark within a single generation (Teyke, 1990; Yoshizawa et al., 2010). These reports, combined with our findings, suggest A. mexicanus prey capture under dark conditions, through a lateral line-dependent mechanism, allowed for early colonization of the caves.
3.5. Applications for investigating the evolution of prey capture
Our analysis is limited to Pachón cavefish, yet lateral-line or feeding dependent changes have been identified in multiple populations of independently evolved cavefish, including those from the Tinaja and Molino caves (Yoshizawa et al., 2015). It will be of interest to determine whether evolved differences in strike angle and distance identified in cavefish occur in multiple populations of cavefish. In addition, the identification of multimodal regulation of prey capture in A. mexicanus, in combination with well-defined visually-mediated prey capture circuits in zebrafish, provide a unique opportunity for examining how sensory processes are integrated. A recently sequenced genome and the application of gene editing and transgenesis techniques in A. mexicanus (Elipot et al., 2014; Ma et al., 2015; McGaugh et al., 2014) may allow for the application of genetic tools to investigate neural circuits regulating prey-seeking in A. mexicanus. Many of the tools, including methodology for whole-brain Ca2+ imaging and Ca2+ sensors have been developed in zebrafish using technology that is readily transferable to A. mexicanus (Ahrens et al., 2013; Muto et al., 2013). Therefore, our findings identifying multi-modal prey capture in A. mexicanus and an evolutionary shift towards lateral line-dependent prey capture in cavefish provide a system for examining the integration of sensory circuits and how these circuits and behaviors evolve.
4. Materials and methods
4.1. Fish rearing and maintenance
Animal husbandry was carried out as previously described (Borowsky, 2008b) and all protocols were approved by the IACUC Florida Atlantic University. Fish were housed in the Florida Atlantic University core facilities at 21 °C ± 1 °C constant water temperature throughout rearing for behavior experiments (Borowsky, 2008b). Lights were kept on a 14:10 h light-dark cycle that remained constant throughout the animal’s lifetime. Light intensity was kept between 25 and 40 lx for both rearing and behavior experiments. Adult fish were fed a diet of black worms to satiation twice daily at zeitgeber time (ZT) 2 and ZT12, (Aquatic Foods, Fresno, CA,) and standard flake fish food during periods when fish were not being used for breeding (Tetramine Pro). All fry used for experiments were reared on live Artemia through 33 dpf, and fed twice daily.
4.2. Artemia preparation
Approximately 24 h prior to behavioral experiments, Brine shrimp cysts (Artemia salina, S.K.) were prepared by adding to a plastic container with 1.2 L of water at a salinity of 25–30 ppt, pH of 7.5–8.5, temperature of 28–30 °C, and constant aeration. Immediately prior to testing, Artemia were rinsed with fresh water and placed into recording chambers. Only newly hatched Artemia nauplii, of the 1st instar stage, were used in behavioral experiments, to ensure consistency of vibrational stimuli. For experiments using dead brine shrimp, Artemia nauplii were flash-frozen, and then thawed by immersion in fresh water immediately prior to behavioral testing.
4.3. High speed recording of prey-seeking behavior
High speed images were acquired using a USB 3.0 camera (Grasshopper3, FLIR Systems) fitted with a zoom lens (75 mm DG Series Fixed Focal Length Lens, Edmund Optics Worldwide), and recorded with FlyCapture2 software (v2.11.3.163, FLIR Systems). All images were acquired at 100 frames per second. Recording chambers were illuminated with custom-designed infrared LED source (Infrared (IR) 850 nm 5050 LED Strip Light, Environmental Lights). All recordings were performed in 29–33 dpf fry from zeitgeber (ZT) 0 to ZT3, shortly after the onset of lights on. For dark recordings, visible light was turned off, but recordings took place at the same time of day. An IR high-pass filter (Edmund Optics Worldwide) was placed between the camera and the lens to block visible light. For larval fish recordings, individual fish were placed in 24 well tissue culture plates (Cellvis) or custom-made chambers, filled with ~3 mm of water to constrict the larvae to a single focal plane. Fish were allowed to acclimate for 2 min prior to the start of the experiment. To record feeding behavior, approximately 30 Artemia nauplii were added to each well and fish were imaged for 2 min.
4.4. Quantification of prey capture behavior
Recordings were analyzed using ImageJ (NIH, v.1.51). Chamber diameter was set using ImageJ’s native “Set Scale” function, and strike distance and angle were measured for all successful feeding events in the two-minute recordings, using ImageJ’s “Line” and “Angle” tools. Measurements of both strike distance and angle were taken in the frame prior to initiation of movement towards the prey. Strike distance was defined as the shortest distance between the edge of the fish’s body and the prey (Fig. 1B). Strike angle was defined as the angle between a line extending down the fish’s midline, terminating parallel with the pectoral fins, and a line extending from this point to the center of the prey (Fig. 1B). Measurements of each strike were averaged to calculate the mean strike distance and angle for that individual, and any recording with fewer than three feeding events was excluded from analysis.
4.5. Gentamicin ablation of the lateral line
To validate neuromast ablation following gentamicin treatment, fish were treated for 1 h with 0.05% DASPEI (2–4-dimethylaminostyryl-N-ethylpyridinium iodide) solution (Sigma Aldrich), a dye that specifically labels both superficial and canal neuromasts (Van Trump et al., 2010). Although the exact mechanism of DASPEI labeling is unknown, the dye is thought to enter cells through transduction channels and apical endocytosis, allowing its uptake by active hair cells via transduction-dependent mechanisms and making it highly specific for labeling intact neuromasts of the lateral line (Van Trump et al., 2010). After staining, fish were anesthetized and neuromasts were observed using a microscope (Leica M205 FA) set to 40 × magnification, 5.17 mm FOV, with a GFP filter set (excitation 450–490 nm). Photographs were captured with a high-resolution CCD camera (ProgRes C14) with ImagePro software (v.9.1). All images were acquired within ∼4 h of the end of baseline behavior recordings. All experimental fish were placed back in their home tanks and given approximately 24 h to recover before any further testing was done. Images represent tiled images merged in Photoshop CS6 (Adobe).
5. Statistics
Two-way ANOVA tests were carried out to test the effects of light level, lateral line ablation, and/or prey mobility among different groups and populations on behavior. Each was modeled as a function of genotype (Surface and Pachón) and genotype by condition interaction. Significance for all tests was set at p < 0.05. When the ANOVA test detected significance, Tukey’s multiple comparison post-test was carried out to detect differences between individual groups. For comparison of two baseline groups, parametric t-tests were carried out to test for significance. Because surface fish did not strike at prey in some of the conditions (e.g. gentamicin treated fish in the dark), Two-Way ANOVAs were not possible. In these cases, an unpaired t-test was performed to detect differences in the surface fish, while a One-Way ANOVA was performed to detect differences between conditions among Pachón cavefish. All statistical analysis was carried out using InStat software (GraphPad 7.0) or SPSS software 22.0 (IBM).
Supplementary Material
Acknowledgements
This work was funded by National Science Foundation Award IOS-125762 to ACK.
Footnotes
Competing interests
There are no competing interests associated with this work.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ydbio.2018.04.027.
References
- Ahrens MB, Orger MB, Robson DN, Li JM, Keller PJ, 2013. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat. Methods 10, 413–420. [DOI] [PubMed] [Google Scholar]
- Aspiras A, Rohner N, Marineau B, Borowsky R, Tabin J, 2015. Melanocortin 4 receptor mutations contribute to the adaptation of cavefish to nutrient-poor conditions. Proc. Natl. Acad. Sci 112, (9688–73). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale A, Guibal C, Tamai TK, Klotz L, Cowen S, Peyric E, Reynoso VH, Yamamoto Y, Whitmore D, 2013. Circadian rhythms in Mexican blind cavefish Astyanax mexicanus in the lab and in the field. Nat. Commun 4, 2769. [DOI] [PubMed] [Google Scholar]
- Bianco IH, Engert F, 2015. Visuomotor transformations underlying hunting behavior in zebrafish. Curr. Biol 25, 831–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco IH, Kampff AR, Engert F, 2011. Prey capture behavior evoked by simple visual stimuli in larval zebrafish. Front. Syst. Neurosci, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibliowicz J, Alié A, Espinasa L, Yoshizawa M, Blin M, Hinaux H, Legendre L, Père S, Rétaux S, 2013. Differences in chemosensory response between eyed and eyeless Astyanax mexicanus of the Rio Subterráneo cave. Evodevo 4, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boord RL, Montgomery JC, 1989. Central mechanosensory lateral line centers. In: Coombs S, Görner P, Münz H (Eds.), The Mechanosensory Lateral Line. Springer-Verlag, New York, 323–340. [Google Scholar]
- Borowsky R, 2008a. Restoring sight in blind cavefish. Curr. Biol 18, R23–R24. [DOI] [PubMed] [Google Scholar]
- Borowsky R, 2008b. Handling Astyanax mexicanus eggs and fry. Cold Spring Harb. Protoc, 3. [DOI] [PubMed] [Google Scholar]
- Bradic M, Teotónio H, Borowsky RL, 2013. The population genomics of repeated evolution in the blind cavefish Astyanax mexicanus. Mol. Biol. Evol 30, 2383–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braubach OR, Wood HD, Gadbois S, Fine A, Croll RP, 2009. Olfactory conditioning in the zebrafish (Danio rerio). Behav. Brain Res 198, 190–198. [DOI] [PubMed] [Google Scholar]
- Budick S. a., O’Malley DM, 2000. Locomotor repertoire of the larval zebrafish: swimming, turning and prey capture. J. Exp. Biol 203, 2565–2579. [DOI] [PubMed] [Google Scholar]
- Burgess H. a., Granato M, 2007. Sensorimotor gating in larval zebrafish. J. Neurosci 27, 4984–4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp AL, Roberts TJ, Brainerd EL, 2015. Swimming muscles power suction feeding in largemouth bass. Proc. Natl. Acad. Sci 112, 8690–8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania KC, 2012. Evolution of brains and behavior for optimal foraging: a tale of two predators. Proc. Natl. Acad. Sci 109, 10701–10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari N, Frigato E, Vallone D, Fröhlich N, Lopez-Olmeda JF, Foà A, Berti R, Sánchez-Vázquez FJ, Bertolucci C, Foulkes NS, 2011. A blind circadian clock in cavefish reveals that opsins mediate peripheral clock photoreception. PLoS Biol 9, e1001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs S, Görner P, Münz H, 2012. The mechanosensory lateral line: neurobiology and evolution Springer Science & Business Media. [Google Scholar]
- Daghfous G, Green WW, Zielinski BS, Dubuc R, 2012. Chemosensory-induced motor behaviors in fish. Curr. Opin. Neurobiol. [DOI] [PubMed] [Google Scholar]
- Doty RL, 1986. Odor-guided behavior in mammals. Experientia [DOI] [PubMed] [Google Scholar]
- Duboué ER, Keene AC, Borowsky RL, 2011. Evolutionary convergence on sleep loss in cavefish populations. Curr. Biol 21, 671–676. [DOI] [PubMed] [Google Scholar]
- Duboué ER, Borowsky RL, Keene AC, 2012. β-adrenergic signaling regulates evolutionarily derived sleep loss in the mexican cavefish. Brain. Behav. Evol 80, 233–243. [DOI] [PubMed] [Google Scholar]
- Eaton RC, DiDomenico R, Nissanov J, 1988. Flexible body dynamics of the goldfish C-start: implications for reticulospinal command mechanisms. J. Neurosci 8, 2758–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elipot Y, Legendre L, Père S, Sohm F, Rétaux S, 2014. Astyanax transgenesis and husbandry: how cavefish enters the laboratory. Zebrafish 11, 291–299. [DOI] [PubMed] [Google Scholar]
- Espinasa L, Bibliowicz J, Jeffery WR, Rétaux S, 2014. Enhanced prey capture skills in Astyanax cavefish larvae are independent from eye loss. Evodevo, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinasa L, Bonaroti N, Wong J, Pottin K, Queinnec E, Rétaux S, 2017. Contrasting feeding habits of post-larval and adult Astyanax cavefish. Subterr. Biol 21, 1–17. [Google Scholar]
- Fajardo O, Zhu P, Friedrich RW, 2013. Control of a specific motor program by a small brain area in zebrafish. Front. Neural Circuits, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahtan E, Tanger P, Baier H, 2005. Visual prey capture in larval zebrafish is controlled by identified reticulospinal neurons downstream of the tectum. J. Neurosci 40, 9294–9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JB, 2012. The complex origin of Astyanax cavefish. BMC Evol. Biol 12, 105 10.1186/1471-2148-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüppop K, 1987. Food-finding ability in cave fish (Astyanax fasciatus). Int. J. Speleol. [Google Scholar]
- Illius AW, Tolkamp BJ, Yearsley J, 2002. The evolution of the control of food intake. Proc. Nutr. Soc 61, 465–472. 10.1079/PNS2002179. [DOI] [PubMed] [Google Scholar]
- Jeffery WR, 2001. Cavefish as a model system in evolutionary developmental biology. Dev. Biol 231, 1–12. [DOI] [PubMed] [Google Scholar]
- Kirchner SR, Fedoruk M, Lohmüller T, Feldmann J, 2014. Analyzing the movement of the Nauplius “Artemia salina” by optical Tracking of plasmonic nanoparticles. J. Vis. Exp, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalko JE, Rohner N, Linden TA, Rompani SB, Warren WC, Borowsky R, Tabin CJ, Jeffery WR, Yoshizawa M, 2013. Convergence in feeding posture occurs through different genetic loci in independently evolved cave populations of Astyanax mexicanus. Proc. Natl. Acad. Sci 110, 16933–16938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RK, Eaton RC, 1991. Identifiable reticulospinal neurons of the adult zebrafish, BrachyDanio rerio. J. Comp. Neurol 304, 34–52. [DOI] [PubMed] [Google Scholar]
- Liu KS, Fetcho JR, 1999. Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron 23, 325–335. [DOI] [PubMed] [Google Scholar]
- Ma L, Jeffery WR, Essner JJ, Kowalko JE, 2015. Genome editing using TALENs in blind Mexican cavefish, Astyanax mexicanus. PLoS One, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott MB, O’Malley DM, 2005. Prey tracking by larval zebrafish: axial kinematics and visual control. Brain. Behav. Evol 66, 177–196. [DOI] [PubMed] [Google Scholar]
- McGaugh S, Gross JB, Aken B, Blin M, Borowsky R, Chalopin D, Hinaux H, Jeffery W, Keene A, Ma L, Minx P, Murphy D, O’Quin K, Rétaux S, Rohner N, Searle S, Stahl B, Tabin C, Volff J, Yoshizawa M, Warren W, 2014. The cavefish genome reveals candidate genes for eye loss. Nat. Commun 5, 5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RW, Russell WH, Elliott WR, 1977. Mexican eyeless characin fishes, genus Astyanax: environment, distribution, and evolution, Special publications the museum Texas Tech University. Texas Tech Press, Texas. [Google Scholar]
- Moss C, Shettleworth S, 1996. NeuroethologicalStudies Of Cognitive And Perceptual Processes 1st edition Routledge, New York. [Google Scholar]
- Muto A, Kawakami K, 2013. Prey capture in zebrafish larvae serves as a model to study cognitive functions. Front. Neural Circuits, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A, Ohkura M, Abe G, Nakai J, Kawakami K, 2013. Real-time visualization of neuronal activity during perception. Curr. Biol 23, 307–311. [DOI] [PubMed] [Google Scholar]
- Muto A, Lal P, Ailani D, Abe G, Itoh M, Kawakami K, 2017. Activation of the hypothalamic feeding centre upon visual prey detection. Nat. Commun 8, 15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornelas-García CP, Domínguez-Domínguez O, Doadrio I, 2008. Evolutionary history of the fish genus Astyanax Baird & Girard (1854) (Actinopterygii, Characidae) in Mesoamerica reveals multiple morphological homoplasies. BMC Evol. Biol 8, 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson BW, Abraham AO, MacIver MA, McLean DL, 2013. Visually guided gradation of prey capture movements in larval zebrafish. J. Exp. Biol 216, 3071–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RS, 1971. Acoustic location of prey by barn owls (Tyto alba). J. Exp. Biol 54, 535–573. [DOI] [PubMed] [Google Scholar]
- Rattazzi L, Cariboni A, Poojara R, Shoenfeld Y, D’Acquisto F, 2015. Impaired sense of smell and altered olfactory system in RAG-1−/− immunodeficient mice. Front. Neurosci, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemmel C, 1967. Vergleichende Untersuchungen an den Hautsinnesorganen ober-und unterirdisch lebender Astyanax-Formen. Z. Morph. Tiere 61, 255–316. [Google Scholar]
- Schwalbe MAB, Bassett DK, Webb JF, 2012. Feeding in the dark: lateral-line-mediated prey detection in the peacock cichlid Aulonocara stuartgranti. J. Exp. Biol 215, 2060–2071. [DOI] [PubMed] [Google Scholar]
- Semmelhack JL, Donovan JC, Thiele TR, Kuehn E, Laurell E, Baier H, 2014. A dedicated visual pathway for prey detection in larval zebrafish. Elife, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyke T, 1990. Morphological differences in neuromasts of the blind cave fish Astyanax hubbsi and the sighted river fish Astyanax mexicanus. Brain. Behav. Evol 35, 23–30. [DOI] [PubMed] [Google Scholar]
- Troscianko T, Benton CP, Lovell GG, Tolhurst DJ, Pizlo Z, 2011. Camouflage and visual perception. Anim. Camoufl.: Mech. Funct, 118–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Trump WJ, Coombs S, Duncan K, McHenry MJ, 2010. Gentamicin is ototoxic to all hair cells in the fish lateral line system. Hear. Res 261, 42–50. 10.1016/j.heares.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Varatharasan N, Croll RP, Franz-Odendaal T, 2009. Taste bud development and patterning in sighted and blind morphs of Astyanax mexicanus. Dev. Dyn 238, 3056–3064. [DOI] [PubMed] [Google Scholar]
- Wagner H, Kettler L, Orlowski J, Tellers P, 2013. Neuroethology of prey capture in the barn owl (Tyto alba L.). J. Physiol. Paris 107, 51–61. 10.1016/j.jphysparis.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Westphal RE, OMalley DM, 2013. Fusion of locomotor maneuvers, and improving sensory capabilities, give rise to the flexible homing strikes of juvenile zebrafish. Front. Neural Circuits 7, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkens H, Strecker U, 2003. Convergent evolution of the cavefish Astyanax (Characidae: teleostei): Genetic evidence from reduced eye-size and pigmentation. Biol. J. Linn. Soc 80, 545–554. [Google Scholar]
- Wilkins H, 1988. Evolution and genetics of epigean and cave Astyanax fasciatus. Evol. Biol, 23. [Google Scholar]
- Yamamoto Y, Byerly MS, Jackman WR, Jeffery WR, 2009. Pleiotropic functions of embryonic sonic hedgehog expression link jaw and taste bud amplification with eye loss during cavefish evolution. Dev. Biol 330, 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao ZG, Jing HY, Wang DM, Lv BB, Li JM, Liu FF, Fan H, Sun XC, Qin YJ, Zhao MQ, 2016. Valproic acid ameliorates olfactory dysfunction in APP/PS1 transgenic mice of Alzheimer’s disease: ameliorations from the olfactory epithelium to the olfactory bulb. Pharmacol. Biochem. Behav 144, 53–59. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M, Gorički Š, Soares D, Jeffery WR, 2010. Evolution of a behavioral shift mediated by superficial neuromasts helps cavefish find food in darkness. Curr. Biol 20, 1631–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa M, Yamamoto Y, O’Quin KE, Jeffery WR, 2012. Evolution of an adaptive behavior and its sensory receptors promotes eye regression in blind cavefish. BMC Biol 10, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa M, Robinson B, Duboué E, Masek P, Jaggard J, O’Quin K, Borowsky R, Jeffery W, Keene A, 2015. Distinct genetic architecture underlies the emergence of sleep loss and prey-seeking behavior in the Mexican cavefish. BMC Biol 20, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.