Abstract
Background:
Congestive heart failure (HF) is a major cause of morbidity, mortality, and cost. Disease management programs have shown promise, but lack firm evidence of effectiveness and scalability. We describe the motivation, design, and planned analyses of EMPOWER, a randomized clinical trial of an innovative intervention incorporating behavioral economic principles with remote monitoring technology embedded within a health care system.
Methods and Results:
EMPOWER is an ongoing, pragmatic, randomized clinical trial comparing usual care to an “automated hovering” intervention that includes patient-level incentives for daily weight monitoring and diuretic adherence combined with automated feedback into the clinical care pathway, enabling real-time response to concerning clinical symptoms. Identification of eligible patients began in May 2016, and implementation of the intervention is feasible. Trial processes are embedded into existing clinical pathways. The primary outcome is time to readmission for any cause. Cost-effectiveness analyses are planned to evaluate the health care costs and health outcomes of the approach.
Conclusions:
The EMPOWER trial incorporates leading edge approaches in human motivation, derived from behavioral economics, with contemporary technology to provide scale and exception handling at low cost. The trial is also implemented within the naturalized environment of a health system, as much as possible taking advantage of the existing journeys of patients and workflows of clinicians. A goal of this pragmatic design is to limit resource utilization, and also to test an intervention that would need minimal modification to be translated from research into a new way of practice.
Keywords: Behavioral economics, medication adherence, readmission, randomized controlled trial
INTRODUCTION
About 6.5 million Americans suffer from heart failure (HF), a major cause of morbidity, mortality, and cost.1, 2 HF is also one of the most common reasons for hospital readmission.3 As value-based payment approaches have increasingly put health systems at financial risk for the costs of readmissions, many health systems have developed and tested interventions to prevent recently discharged patients from returning to the hospital with exacerbations of their illness. It is a challenging problem: HF is a chronic disease requiring a complex array of medications, intensive follow-up care, and daily self-management including significant lifestyle changes. A few missed doses of a diuretic or a small increase in dietary sodium can easily start a vicious cycle that leads to readmission.
Indeed, the combined need for clinical management and patient self-management may be what makes HF such a challenge. Past approaches relied primarily on patients seeking care after they were symptomatic – typically too late to intervene without hospitalization. Newer approaches recognize that outcomes are driven largely by activities at home, out of the view of providers. Yet, trials of self-management – using patients as their own workforce through intensive training – have shown no significant reductions in mortality or rehospitalizations.4 Complex disease management programs that combine clinical and self-management, often staffed by clinical nurses and sometimes including remote monitoring, have been shown in small studies to reduce readmissions and improve quality of life. These programs, however, are typically too costly and labor-intensive to effectively scale, and the promise seen in the small studies hasn’t been realized in the larger trials that followed.5–9 Patients offered such support may not participate at all, and many of those who do participate may quickly lose interest.10 Additionally, many of the trials do not effectively integrate monitoring into the electronic health record or clinical practice workflow, creating ineffective alerts and substantial additional effort for providers that results in unsustainable clinical burden.
The lack of practical success is frustrating because the mainstays of HF management are well understood. Effective management of HF depends upon medication adherence and sodium restriction.11 Poor adherence to HF medications is associated with more frequent admissions and higher mortality rates.12, 13 While sodium intake is difficult to monitor, increases in body weight, reflecting increases in fluid, are situated on the mediating pathway toward exacerbations and are also easily measured. Often the earliest sign of decompensation is a weight gain of a few kilograms (i.e., several pounds), signaling pending clinical deterioration that can be avoided with early intervention.14 Adherence to weight monitoring and subsequent diuretic self-adjustment have been found to be associated with lower adjusted odds of HF-related emergency department (ED) visits and hospitalization.13 Thus, the hope has been to create an approach that persistently engages patients in self-monitoring (because patients with HF have the disease for their lifetimes) and that also efficiently engages clinicians in the care of those patients outside of office visits. For these reasons, the EMPOWER (Electronic Monitoring of Patients Offers Ways to Enhance Recovery) trial intervention focuses on adherence to both daily weight monitoring and daily diuretic use. A remote monitoring strategy focused on these twin elements – with relevant information transmitted to nurses empowered to act on it – could be highly effective if participation and adherence rates can be maintained at high levels and over long periods of time.
New insights from the field of behavioral economics offer promise for sustaining this kind of patient engagement.15–18 Research has shown that common decision errors that normally lead people away from healthy behaviors can be used to instead promote healthy behavior.19, 20 For example, “present bias,” the tendency to overvalue present costs and benefits (the tendency that leads patients to eat a salty snack while knowing that it could set off a HF exacerbation later) typically works against healthy behaviors. The benefits of those behaviors (e.g., better health, fewer hospitalizations) are delayed and often intangible, but the challenges (e.g., resisting temptation) and opportunities (e.g., enjoying the snack) are immediate. To counteract this balance, incentives for healthy behaviors can create immediate benefits from activities whose benefits would otherwise be delayed; for example, small but tangible and frequent positive feedback or rewards can be offered to promote daily medication adherence and weight monitoring.20–22 Behavioral economics also has shown that the structure and timing of incentives can be as important as their magnitude.10 Rather than merely paying people for medication adherence in a transactional way, daily lotteries have been shown to improve medication adherence and weight loss; these have incorporated anticipated regret (people are notified whether they won or would have won) and frequent small payoffs and infrequent large payoffs, which are effective because people tend to overweigh small probabilities and find variable rewards fun.23 Lottery-based incentives with frequent feedback thus have the potential to encourage sustained behavior change among patients with HF when incentives such as simple fixed payments might be less effective.
Here, we describe the design of EMPOWER, a pragmatic randomized trial to evaluate whether automated hovering – a term coined to synthesize the concepts of remote but constant engagement and evaluation – can improve outcomes among HF patients recently discharged from the hospital and prevent readmissions.24 The trial is innovative in its use of remote monitoring technologies to connect with patients, its use of behavioral economics to inform the structure and content of the intervention, and its integration with a health care delivery system to provide data and insights that are actionable within the existing context of clinical care. The EMPOWER intervention is deployed via a novel software platform that allows daily monitoring of weights and medication adherence in a user-friendly way.25 The intervention is enhanced with a series of behavioral economic tools to promote behavior change, including a regret lottery and social support.26 Importantly, the intervention is embedded into routine clinical practice within a health care delivery system, and is implemented with existing clinical resources with direct integration into the electronic health record (EHR).
METHODS
Screening and eligibility.
A purpose-built automated dashboard screening tool identifies potentially eligible participants by flagging recently discharged patients at three urban campuses within Penn Medicine (Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, and Pennsylvania Hospital).27 Study staff then perform additional screening to confirm eligibility. Eligible participants must be between 18 and 80 years of age and have either a primary discharge diagnosis of HF, a secondary discharge diagnosis of HF with IV diuretics administered during their inpatient stay, or HF appearing on their problem list with IV diuretics administered during their hospital stay. Participants are eligible to enroll for up to 30 days post-discharge. To be eligible, participants must also be prescribed a daily diuretic and plan to have their heart failure monitored by a Penn Medicine clinician (a primary care provider, cardiologist, or nurse practitioner). Exclusion criteria include receipt of heart transplant, listed or under evaluation for heart transplant, receipt of ventricular assist device (VAD), listed or under evaluation for VAD, end-stage renal disease (ESRD), a glomerular filtration rate (GFR) less than 25 mL/min, hemodialysis, inotrope dependence, palliative care, hospice care, or participation in other telemonitoring programs. In addition, participants are ineligible if they have a history of uncontrolled cognitive or psychiatric conditions that would affect study participation.
Recruitment.
The EMPOWER study began enrolling patients in May 2016 and is expected to complete accrual in the spring of 2019. The study is a two-arm randomized controlled trial with a total enrollment goal of 566 participants, evenly randomized to receive usual care (UC) or the automated hovering intervention (I). Randomization is stratified by study site (Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, and Pennsylvania Hospital). Both UC and intervention participants receive $25 for enrolling in the study. Participants randomized to UC have no further interaction with the study team and receive usual medical care. Intervention participants receive two wireless devices, electronic pill bottles to measure adherence to their daily diuretic medication, and an electronic scale to measure their daily morning weight. For taking the time to set up these devices, intervention participants receive an additional $25. Intervention participants’ managing providers are notified of their patient’s enrollment in the study via email and as an in-basket message in the electronic health record (EHR). Intervention participants are asked to provide the name and contact information of a family member or friend to serve as a support partner in the case of extended non-adherence to either device. Participant engagement is managed through Way to Health, a research software platform that integrates with various devices and provides automated feedback.25 Upon recruitment, the preferred contact method for both participants and their support partners is registered. Participants choose whether to receive daily feedback about their medication and weight adherence and lottery winnings via text, email, or interactive voice recording phone call. Participants non-adherent with opening their pill bottle and/or registering a weight measurement for 2 days receive an automated message stressing the importance of taking the medication and weighing in; the participant’s support partner also receives an automated message. After 3 days of non-adherence, study staff call the participant. After 4 days of non-adherence, study staff call the support partner. After 5 days of non-adherence, study staff send a note to the participant’s clinical provider through the EHR. The process is designed with these thresholds for escalation to emphasize self-monitoring and reduce burdens on clinicians.28
Intervention participants are assigned a 2-digit number to be used for daily lottery-based engagement incentives in which eligibility to win is conditional on both daily medication adherence and registering a weight measurement. If adherent to both devices, the participant has an 18 in 100 (roughly 1 in 5) chance of winning $5 or 1 in 100 chance of winning $50 each day for an expected value of $1.40. Over the course of their year-long participation in the trial, intervention participants who are consistently adherent to both medication and weight monitoring are expected to earn approximately $500. Additionally, participants in the intervention arm receive intermittent educational/motivational messages to encourage ongoing healthy behaviors to manage their congestive heart failure.29
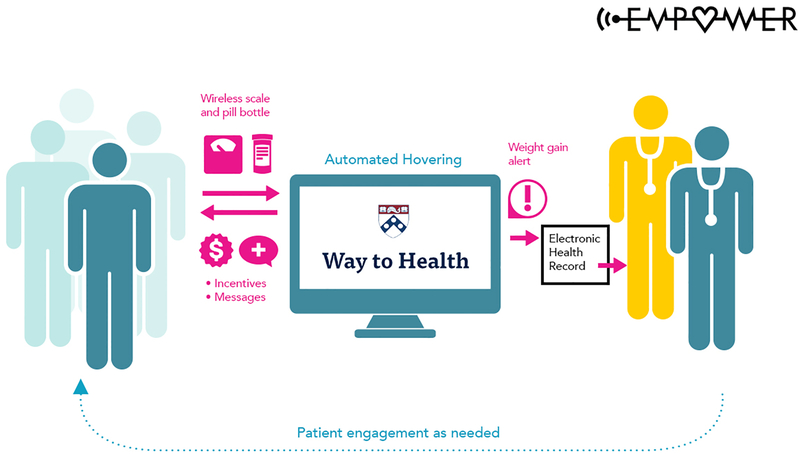
Each participant’s weight is monitored daily and automatically compared to pre-specified weight change thresholds, which are an increase of 1.4kg (i.e. three pounds) in 24 hours or 2.3kg (i.e. five pounds) in 72 hours. If a weight gain alert is triggered, study staff call the participant to validate the weight increase and assess symptoms using a standardized questionnaire. Study staff attempt to reach the participant for 3 consecutive days. If it is determined that the participant had a true weight increase, the coordinator sends the weight alert and symptom questionnaire responses to the participant’s managing clinician via the Way to Health platform to the EHR. If the participant is unreachable, study staff send a message to the participant’s managing provider indicating that the participant had an unconfirmed weight gain. Information regarding a participant’s weight gain is routed as an abnormal result to his/her designated provider and this result is viewable to anyone on the participant’s care team. As part of the integration of the Way to Health platform with the EHR, daily weights are also sent from the Way to Health platform into a flowsheet in the participant’s record on a weekly basis, viewable by any of their Penn Medicine clinicians. Figure 1 illustrates the flow of communication among patients, providers, and the study team.
The EMPOWER automated hovering intervention: Communication among patients, providers, and study team.
STATISTICAL CONSIDERATIONS
Design considerations.
We designed the trial to maximize its pragmatic features. These include automated identification of potentially eligible patients using the built-in EHR screening tool, and embedding of the intervention within existing processes of clinical care. The components of the composite outcome, as well as daily diuretic adherence and weight measurement, are captured in the EHR. Participants randomized to the usual care arm receive routine clinical care and are never contacted again by the study staff following enrollment, making this truly a “usual care” arm. In the intervention arm, triggered weight alerts are sent, after verification, to the provider team via the EHR, and are therefore embedded into existing care pathways without requiring any special connection of clinicians to the study team or another electronic platform. Finally, the clinical outcomes are important to the hosting health system and the intervention was seen as feasibly implementable if successful.
Outcomes.
Our primary outcome is time to readmission for any cause; note that participants may experience multiple readmissions, and each will contribute to the analysis as described below. Outcomes will be observed using electronic health record data from Penn Medicine, supplemented with state data on admissions from Pennsylvania, New Jersey, and Delaware to ensure that all admissions are captured. Secondary outcomes include death, medication adherence, and costs. We will obtain information on death from tri-state area death indices. In the intervention group, we will also measure adherence using the proportion of days adherent to medication, to weight measurement, or to both. These are defined as proportion of days on which the electronic pill bottles were opened or the scale registered a weight. We will report number of alerts triggered, the proportion of those alerts that were verified and sent to the clinical care team, and responses to those alerts. We will measure costs in both groups as described below. Note that all outcomes measured in both study arms are obtained from administrative sources and do not require re-contacting participants; for this reason, we do not obtain direct information on quality of life from study participants.
Sample size.
Prior to study initiation, we estimated the 1-year readmission rate in the usual care group to be approximately 47%, and designed the trial to provide 80% power to detect a hazard ratio of 0.73, corresponding to a 10 percentage point decrease in the readmission rate in the intervention group. With the assumptions of uniform accrual over time, exponentially distributed readmission times, a censoring rate of up to 10%, and using an exponential maximum likelihood estimator test, we intend to accrue 566 patients (approximately 283 subjects in each arm) over the 24-month enrollment period and follow them for 12 months.30 The primary hypothesis test will use a two-sided Type I error rate of 0.05. Note that the power calculations are based on the time to first readmission; if patients experience multiple readmissions, statistical power will be increased.
Analytic plan.
The primary analysis will consist of an unadjusted intent-to-treat hypothesis test using the Andersen-Gill formulation of the Cox proportional hazards model to compare the times to hospitalizations in the two groups; these models properly adjust for the correlation of multiple repeated events within individuals.31,32 We will also estimate multivariate regression models adjusted for the stratification variable (study site), trial arm, and other covariates of interest that may be imbalanced by study arm despite randomization (such as patient sex, income, race, baseline ejection fraction, and other clinical factors), retaining those given evidence of confounding or predictive ability.33 We will assess interaction terms between the intervention indicators and a priori potential effect modifiers such as income level, race, and baseline ejection fraction. All hypothesis tests will be two-sided and models will be assessed using standard diagnostic techniques.34
Because some patients will discontinue follow-up at Penn Medicine during the study period, we may not have complete follow-up data; we expect these changes to be comparable in both arms and to be for reasons unrelated to the study. We will compare dropout rates by arm, attempt to find the reasons for missing data, and compare baseline characteristics in participants with complete vs. incomplete follow-up. In secondary analyses, we will investigate the sensitivity to modeling assumptions using imputation models and inverse-probability-weighted estimating equations and models that adjust for informative missing data.
We anticipate that short-term mortality rates will be comparable between treatment groups, and that there will be no differential reporting of deaths because information in both groups will be derived from state death indices. To assess the impact of the intervention on both readmissions and mortality, we will analyze the composite outcome defined by time to the earliest of readmission or death; this will account for potential bias due to differential death rates or reporting, and provide important information to complement the analysis of readmissions.
Regulatory requirements.
The EMPOWER trial was approved by the University of Pennsylvania Institutional Review Board. All participants provide informed consent prior to enrollment. The Data Safety and Monitoring Board (DSMB) comprises three experts with nationally known expertise in clinical trials, heart failure, and biostatistics. The DSMB judged the trial to be low risk but continues to monitor adverse and serious adverse events as well as to review study and staff training protocols. The DSMB receives a regular report that includes all readmissions to Penn Medicine for the enrolled participants. Each readmission is independently adjudicated by two resident physicians at Penn Medicine for its possible relatedness to the study protocol, as well as to gain further insight into the reasons enrolled heart failure patients are rehospitalized. Adjudications that are discordant are given final review and adjudication by two cardiologists who are co-investigators on the study and experts in treating heart failure. The DSMB reviews the report approximately every six months and makes a recommendation regarding study continuation.
Cost-effectiveness.
An important secondary objective is assessment of the cost-effectiveness of the intervention. Because the primary purpose of the cost-effectiveness analysis is to inform potential adopters about what they can expect, health outcomes, costs, and cost-effectiveness will be estimated from the perspective of a hospital or health plan. This reflects the healthcare sector perspective recommended by the Second Panel on Cost-Effectiveness in Health and Medicine.35 Costs will be valued using Medicare’s national base rates for 2019.
The primary outcome of the trial is hospital readmission for any cause. By design, the UC group is not contacted after enrollment, so readmissions will be assessed via the electronic medical record, supplemented with state data on admissions from Pennsylvania, New Jersey, and Delaware to ensure that all admissions are captured. A primary hypothesis of the trial is that the intervention will reduce readmissions among intervention participants, compared to control, possibly by enough to offset the costs of the intervention and of any additional outpatient medical care. This hypothesis will be assessed by costing medical care utilization during the trial in the control and intervention arms, a standard part of a cost-effectiveness analysis conducted from the health care sector perspective. Costing is described in more detail below.
Although cost-effectiveness can show whether an intervention is cost-saving, its main purpose is to show the additional health achieved by an intervention for any additional costs incurred. For the primary cost-effectiveness analysis health will be represented by all-cause readmissions, the primary outcome of the trial. A reduction in readmissions is an intermediate, not a direct, measure of health, and the electronic medical record will give access to two important additional measures. The first, all-cause mortality, is high among HF patients. If all-cause mortality at 12 months differs significantly between the UC and intervention arms, that difference, and projections of its longer-term impact, will serve as an important direct measure of health outcome. As a second measure that can be viewed as a proxy for quality of life, we will use the change over the trial period in the Seattle Heart Failure Model risk score.36,37 The score calculates the risk of all-cause mortality at 1 and 5 years using lab values and clinical assessments available in the EHR. If the intervention is successful, the risk scores of intervention patients are expected to increase less, or even decrease, compared to those of usual care patients.
Costs of the intervention over the 12-month trial period include the electronic pill bottles and scales; incentive payments to patients; staff time to enroll patients, trouble shoot devices, and monitor alerts; the Way to Health platform that enrolls patients and calculates their incentives; and medical utilization during the trial, which captures services in response to alerts and the intervention’s success in reducing hospital admissions. Device and incentive costs are available from the trial records. To ensure that only the time required for the intervention itself is costed, and not time involved in trial- and research-related activities, staff will record their time, by activity, for a sample of weeks using time logs. Medical utilization will be measured using the billing and service utilization data systems of Penn Medicine and costed using national Medicare payment rates; this information includes Penn Medicine inpatient and outpatient hospital services, and services at Penn Medicine outpatient practices. We will have information on inpatient prescriptions but not outpatient prescriptions, making it difficult to calculate a measure of adherence in both arms. We will also obtain information on admissions outside of the Penn Medicine system using state hospital discharge records from Pennsylvania, New Jersey, and Delaware. We will estimate confidence intervals around these values and perform sensitivity analyses to reflect underlying uncertainty in inputs and assumptions.
As is standard for cost-effectiveness analysis, the analyses will report: (a) a comparison of the costs in the usual care and intervention arms, which will show whether the intervention reduced medical costs, a primary aim of the trial; (b) estimates of years of life gained by participants in the intervention arm compared to usual care, based on differences in mortality across arms, differences in Seattle Heart Failure Model scores across arms, or both, and; (c) if the intervention costs more than usual care, cost-effectiveness ratios that show the additional cost per readmission prevented or year of life gained.
DISCUSSION
A scalable approach to substantially reduce the risk of readmission among patients hospitalized for HF has remained elusive, despite considerable understanding of the physiologic mechanisms of the disease. The most promising models for a successful approach include remote monitoring of behavioral risk factors paired with timely clinical remediation. However, sustainable approaches require keeping patients engaged in the process for their lifetimes, and scalable approaches require keeping costs, as well as the burden on busy clinicians, low.
EMPOWER is designed to meet these requirements by combining leading edge approaches in human motivation, derived from behavioral economics, with contemporary technology to provide scale and exception handling at low cost. The trial is implemented within the naturalized environment of a health system, as much possible accommodating the existing journeys of patients and the workflows of clinicians. This prevents clinicians from having to access separate systems but rather allows all data to flow into the common EHR and allow management within the “context of care.” A goal of this pragmatic design is to limit resource utilization, and also to test an intervention that would need minimal modification to move from an experiment to a new way of practice.
Indeed, EMPOWER is an experiment. It is being fielded at a time when health systems around the nation are so desperately aiming to reduce the readmission rates for their patients with HF and other chronic illnesses that many are pursuing interventions that, while well-meaning and often well-designed, are rolled out on the basis of their intuitive promise without control and with little planned evaluation. Pharmaceuticals are similarly designed based on their intuitive promise, but we would never prescribe them to our patients without first testing them for safety and efficacy. Nonetheless, compound clinical policy interventions, like new approaches to reduce HF rehospitalizations, are often released on large populations of patients without extensive prior testing. Most of those that have been tested have not been effective. EMPOWER aims to provide a careful test of a cutting-edge intervention.
EMPOWER concludes patient enrollment in the spring of 2019 and concludes patient follow up in the spring of 2020. Soon after that, we will learn the results.
CLINICAL PERSPECTIVES.
Congestive heart failure is one of the leading reasons for hospitalization and rehospitalization. Successful management depends critically on patient behavior. The EMPOWER trial tests a promising intervention blending our understanding of physiology and behavioral economics with contemporary technology. If successful, this trial can serve as a model for the many other common conditions where patient engagement is essential for good clinical outcomes.
TRANSLATIONAL OUTLOOK.
Pragmatic trials aim to rigorously test interventions while including a broadly representative patient group, so that the results will be generalizable. EMPOWER is designed with these features in mind: we are recruiting a representative sample of HF patients. In addition, a critical feature of the EMPOWER intervention is its integration into the existing context of care and clinical workflow, which will facilitate effective translation of the approach, if successful, into standard practice.
ACKNOWLEDGMENTS
We are grateful to the participating patients, as well as to the dedicated EMPOWER staff, including Sophia Anderson, Michael Josephs, and Akriti Mishra. We also thank the EMPOWER DSMB members, Dr. Lynne Warner Stevenson (chair), Dr. William Yancy, and Dr. Patrick Heagerty.
FUNDING
Funding is from NHLBI R01 HL128465 (Asch and Volpp, MPIs).
Footnotes
Clinical Trial Registration Information: ClinicalTrials.gov Identifier: NCT02708654
DISCLOSURES
Drs. Asch and Volpp are partners at VAL Health and Dr. Troxel is on VAL Health’s Scientific Advisory Board.
REFERENCES
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–44. [DOI] [PubMed] [Google Scholar]
- 3.Suter LG, Li SX, Grady JN, Lin Z, Wang Y, Bhat KR, Turkmani D, Spivack SB, Lindenauer PK, Merrill AR, Drye EE, Krumholz HM, Bernheim SM. National patterns of risk-standardized mortality and readmission after hospitalization for acute myocardial infarction, heart failure, and pneumonia: update on publicly reported outcomes measures based on the 2013 release. J Gen Intern Med. 2014;29:1333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powell LH, Calvin JE Jr., Richardson D, Janssen I, Mendes de Leon CF, Flynn KJ, Grady KL, Rucker-Whitaker CS, Eaton C, Avery E. Self-management counseling in patients with heart failure: the heart failure adherence and retention randomized behavioral trial. JAMA. 2010;304:1331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peikes D, Chen A, Schore J, ad Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301:603–18. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, Phillips CO, Hodshon BV, Cooper LS, Krumholz HM. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363:2301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inglis SC, Clark RA, McAlister FA, Stewart S, Cleland JG. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: Abridged Cochrane Review. European Journal of Heart Failure. 2011;13:1028–40. [DOI] [PubMed] [Google Scholar]
- 8.Cleland JGF, Louis AA, Rigby AS, Janssens U, Balk AHMM. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death. The Trans-European Network-Home-Care Management System (TEN-HMS) study. J Am Coll Cardiology. 2005;45:1654–1664. [DOI] [PubMed] [Google Scholar]
- 9.Ong MK, Romano PS, Edgington S, Aronow HU, Auerbach AD, Black JT, De Marco T, Escarce JJ, Evangelista LS, Hana B, Ganiats TG, Greenberg MH, Greenfield S, Kaplan SH, Kimchi A, Liu H, Lombardo D, Mangione CM, Sadeghi B, Sadeghi B, Sarrafzadeh M Tong K, Fonarow GC. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: The better effectiveness after transition–heart failure (beat-hf) randomized clinical trial. JAMA Internal Medicine. 2016;176:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sen AP, Sewell TB, Riley EB, Stearman B, Bellamy SL, Hu MF, Tao Y, Zhu J, Park JD, Loewenstein G, Asch DA, Volpp KG. Financial incentives for home-based health monitoring: a randomized controlled trial. J Gen Intern Med. 2014;29:770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu JR, Moser DK, Chung ML, Lennie TA. Objectively measured, but not self-reported, medication adherence independently predicts event-free survival in patients with heart failure. Journal of Cardiac Failure. 2008;14:203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granger BB, Swedberg K, Ekman I, Granger CB, Olofsson B, McMurray JJ, Yusuf S, Michelson EL, Pfeffer MA. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet. 2005;366:2005–11. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgerald AA, Powers JD, Ho PM, Maddox TM, Peterson PN, Allen LA, Masoudi FA, Magid DJ, Havranek EP. Impact of medication nonadherence on hospitalizations and mortality in heart failure. Journal of Cardiac Failure. 2011;17:664–9. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation. 2007;116:1549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loewenstein G, Brennan T, Volpp KG. Asymmetric paternalism to improve health behaviors. JAMA. 2007;298:2415–7. [DOI] [PubMed] [Google Scholar]
- 16.Loewenstein G, Asch DA, Friedman JY, Melichar LA, Volpp KG. Can behavioural economics make us healthier? BMJ. 2012;344:e3482. [DOI] [PubMed] [Google Scholar]
- 17.Kahneman D, Tversky A. Prospect theory: An analysis of decision under risk. Econometrica. 1979;47:263–291. [Google Scholar]
- 18.Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science. 1981;211:453–8. [DOI] [PubMed] [Google Scholar]
- 19.Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357:1340–4. [DOI] [PubMed] [Google Scholar]
- 20.Volpp KG, Pauly MV, Loewenstein G, Bangsberg D. P4P4P: an agenda for research on pay-for-performance for patients. Health Affairs. 2009;28:206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loewenstein G, John L, Volpp K. Using decision errors to help people help themselves In: S. E, ed. Behavioral Foundations of Policy New York: Russell Sage Foundation Press; 2009. [Google Scholar]
- 22.Volpp KG, Loewenstein G, Troxel AB, Doshi J, Price M, Laskin M, Kimmel SE. A test of financial incentives to improve warfarin adherence. BMC Health Services Research. 2008;8:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008;300:2631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asch DA, Muller RW, Volpp KG. Automated hovering in health care--watching over the 5000 hours. N Engl J Med. 2012;367:1–3. [DOI] [PubMed] [Google Scholar]
- 25.Asch DA, Volpp KG. On the Way to Health. LDI issue brief. 2012;17:1–4. [PubMed] [Google Scholar]
- 26.Volpp KG, Troxel AB, Mehta SJ, Norton L, Zhu J, Lim R, Wang W, Marcus N, Terweisch C Caldarella K, Levin T, Relish M, Negin N, Smith-Mclallen A, Syder R, Spetell CM, Drachman B, Kolansky D, Asch DA. Effect of electronic reminders, financial incentives, and social support on outcomes after myocardial infarction: The Heartstrong randomized clinical trial. JAMA Internal Medicine. 2017;179:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi K, Gitelman Y, Asch DA. Subscribing to your patients - reimagining electronic health records. N Engl J Med. 2018;378:1960–2. [DOI] [PubMed] [Google Scholar]
- 28.Asch DA, Terwiesch C, Volpp KG. How to reduce primary care doctors’ workloads while improving care. Harvard Business Review. November 13, 2017. [Google Scholar]
- 29.Chow CK, Redfern J, Hillis GS, Thakkar J, Santo K, Hackett ML, Jan S, Graves N, de Keizer L, Barry T, Bompoint S, Stepien S, Whittaker R, Rodgers A, Thiagalingam A. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: A randomized clinical trial. JAMA. 2015;314:1255–63. [DOI] [PubMed] [Google Scholar]
- 30.Bernstein D, Lagakos SW. Sample size and power determination for stratified clinical trials. Journal of Statistical Computation Simulation. 1978. 8:65–73. [Google Scholar]
- 31.Andersen PK, Gill RD. Cox’s regression model for counting processes: A large sample study. Annals of Statistics. 1982;10:1100–1120. [Google Scholar]
- 32.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer-Verlag; 2000. [Google Scholar]
- 33.Rothman KJ, Greenland S. Modern Epidemiology. 2nd ed Philadelphia: Lippincott-Raven Publishers; 1998. [Google Scholar]
- 34.Harrell JFE Regression Modeling Strategies with Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer-Verlag; 2001. [Google Scholar]
- 35.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA, Salomon JA, Sculpher MJ, Trikalinos TA, Russell LB, Siegel JE, Ganiats TG. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–103. [DOI] [PubMed] [Google Scholar]
- 36.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–33. [DOI] [PubMed] [Google Scholar]
- 37.University of Washington, Seattle Heart Failure Model, https://depts.washington.edu/shfm/?width=1280&height=720, accessed 06 April 2018.