Abstract
The mechanisms underlying the pathophysiology and treatment of depression and stress-related disorders remain unclear, but studies in depressed patients and rodent models are beginning to yield promising insights. These studies demonstrate that depression and chronic stress exposure cause atrophy of neurons in cortical and limbic brain regions implicated in depression, and brain imaging studies demonstrate altered connectivity and network function in the brains of depressed patients. Studies of the neurobiological basis of the these alterations have focused on both the principle, excitatory glutamate neurons, as well as inhibitory GABA interneurons. They demonstrate structural, functional, and neurochemical deficits in both major neuronal types that could lead to degradation of signal integrity in cortical and hippocampal regions. The molecular mechanisms underlying these changes have not been identified but are thought to be related to stress induced excitotoxic effects in combination with elevated adrenal glucocorticoids and inflammatory cytokines, (as well as other environmental factors). Transcriptomic studies are beginning to demonstrate important sex differences and together with genomic studies are starting to reveal mechanistic domains of overlap and uniqueness with regards to risk and pathophysiological mechanisms with schizophrenia and bipolar disorder. These studies also implicate GABA and glutamate dysfunction as well as immunologic mechanisms. While current antidepressants have significant time lag and efficacy limitations, new, rapid acting agents that target the glutamate and GABA systems address these issues and offer superior therapeutic interventions for this widespread and debilitating disorder.
In brief:
The review by Duman and colleagues discusses evidence that depression is characterized by deficits of excitatory and inhibitory neurons, while new rapid acting agents that target the glutamate and GABA systems address these issues and offer superior therapeutic interventions.
Introduction
Over 300 million people are affected by major depressive disorder (MDD) and limitations in the access to and effectiveness of MDD treatment have made it the leading cause of disability world-wide (WHO, 2017). Despite long-standing efforts to identify the pathophysiology of depression, the underlying neurobiological determinants remain largely undefined. The relatively low rate of heritability, approximately 37%, the absence of variants with substantial impact on depression risk, the polygenic nature of depression risk, and the heterogeneity of depression, have contributed to the difficulty in identifying genetic determinants of susceptibility (Flint and Kendler, 2014; Sullivan et al., 2018). This heterogeneity likely reflects multiple underlying genetic, metabolic, endocrine, and neurobiological factors that contribute to depression and that are expressed in changing ways across the lifespan. The clear sexual dimorphism, with two-times higher rates of depression in women compared to men also remains unexplained, although recent postmortem transcriptome studies have begun to identify sex specific network differences. The public health burden of depression is substantially increased by the low rates of remission associated with the current antidepressant treatments, the delay in efficacy after initiating treatment of weeks to months (Trivedi et al., 2006) and high rates of relapse to depression once treatment response is obtained. These limitations in antidepressant effectiveness may also demoralize patients and contribute to suicide risk (Murray et al., 2013).
Environmental factors, such as trauma and stressful life events, contribute to depression risk through altering brain structure, chemistry, and function. Chronic exposure to social, psychological, or physical stressors provide useful contexts for studying how the brain transduces environmental stress exposure into depression. Preclinical studies in rodents and nonhuman primates have reported structural alterations in chronic stress models, including atrophy of neurons in the hippocampus and prefrontal cortex (PFC) (Duman et al., 2016; McEwen et al., 2015; McEwen and Morrison, 2013). Structural and functional alterations have also been reported in depressed patients, including decreased volume and connectivity of both the hippocampus and PFC (MacQueen and Frodl, 2011; Savitz and Drevets, 2009). Disruption of glutamate-excitatory projection neurons in these brain regions that are responsible for circuit level information transmission could contribute to these structural alterations. In addition, there is extensive evidence that stress and depression disrupt the function GABA, the major inhibitory neurotransmitter system in the brain that is responsible for overall control and fine tuning of excitatory transmission. (Fee et al., 2017; Ghosal et al., 2017; Krystal et al., 2002; Luscher and Fuchs, 2015; Luscher et al., 2011). Reductions in synaptic connectivity and deficits in inhibitory tuning may undermine the functionality of neural circuits, reducing the precision, efficiency, and integrity of the information-bearing functions of these networks.
This review will discuss evidence that stress and depression are associated with deficits of both excitatory and inhibitory neurons, and the functional consequences of these changes. We also discuss the mechanisms underlying the reductions of glutamate and GABA systems, including the role of genetic polymorphisms, environment, sex, and activity dependent loss of synaptic function and plasticity. An important question is whether one of these major systems, glutamate or GABA, is more vulnerable to stress and depression, and thereby increases vulnerability to the other system. We also discuss novel treatments that act either directly or indirectly to modulate the function of glutamate and/or GABA systems to produce fast and efficacious antidepressant responses. This includes novel rapid acting agents that reset both excitatory and inhibitory neurotransmitter systems, as well as agents that act as allosteric modulators of glutamate or GABA receptor complexes.
Structural and functional connectivity alterations in depression
Brain imaging studies have demonstrated structural as well as functional alterations in depression (Evans et al., 2018; MacQueen and Frodl, 2011; Savitz and Drevets, 2009). Briefly, these studies demonstrate decreased volume of the hippocampus and PFC (subgenual and anterior cingulate cortex) in depressed patients that are related to the length and severity of illness and time of treatment. Alterations of other regions implicated in depression have also been reported, including decreased insula (Demenescu et al., 2017), as well as some evidence of increased amygdala volumes (McEwen et al., 2016). There is also evidence of increases of hippocampal volume with successful treatment indicating reversibility (MacQueen et al., 2008; Sheline, 2003). The plasticity of these effects is supported by preclinical studies demonstrating that atrophy of principle neurons in the hippocampus and PFC are reversible after discontinuation of stress exposure (Liu and Aghajanian, 2008; McEwen et al., 2015; McEwen et al., 2016).
The cellular determinants underlying these structural alterations are unclear but postmortem studies have provided some evidence of altered neuronal and glial number and morphology. These studies report decreased soma size of neurons, as well decreased glial number in the PFC (Rajkowska and Stockmeier, 2013; Sanacora et al., 2008). There is also evidence of increased packing of pyramidal neurons in the hippocampus (Stockmeier et al., 2004). There is one report of decreased synapse number in dlPFC of a small number of depressed subjects, as well as decreased expression of synaptic markers (Kang et al., 2012). Further postmortem studies are required to confirm these findings and to examine other cellular determinants that could contribute to the decreased volume of hippocampus and PFC in depression. In addition, new brain imaging approaches that allow for analysis of specific neuronal elements, such as the new PET ligand for a synaptic vesicle protein 2a (SV2A) could further define the structural alterations at the cellular level that contribute to depression (Finnema et al., 2016). Preliminary studies using this ligand demonstrate a reduction in SV2A ligand binding levels in PFC and hippocampus of severely depressed patients (Holmes et al., 2018).
Functional brain imaging studies have also reported abnormalities in depression using a combination of approaches. The major functional networks include the default mode network (DMN), responsible for resting state introspection and ruminations, the salience network (SAL), which processes salient information from external sources, and the central executive network (CEN), responsible for working memory and attention (Menon, 2011). The DMN is comprised of connections between the dlPFC, posterior and anterior cingulate cortex, amygdala, and hippocampus. Studies demonstrate an increase in the activity of the DMN in depressed patients and decreases in SAL and CEN, consistent with increased rumination and introspection and decreased association with external inputs in depressed patients (Evans et al., 2018; Greicius et al., 2007; Kaiser et al., 2015). Considering the DMN includes PFC subregions, it is interesting to speculate on the relationship between increased DMN activity and the structural alterations observed in depression. Could sustained long-term increases of DMN activity lead to excitotoxic effects and atrophy of glutamate principle neurons in the PFC? Could the elevated DMN activity be related to reduced GABA inhibitory control (Fee et al., 2017)? Could stress-related effects on glial function in the PFC place both GABA and glutamate neurons at increased risk for excitotoxic effects (Rajkowska and Stockmeier, 2013)? Preclinical studies would help answer these questions, although characterization of rodent equivalents of these networks would be required.
Global brain connectivity with global signal regression (GBCr) using resting state blood oxygen level dependent (BOLD) fMRI is considered a valid marker of global intrinsic network activity that correlates with brain function and regional cerebral blood flow (Cole et al., 2012; Liang et al., 2013). Studies of depressed patients report that GBCr is decreased in the PFC, but increased in the posterior cingulate, precuneus, and lingual gyrus, and find dysconnectivity between PFC/subcortex and the rest of the brain (Abdallah et al., 2017). The authors hypothesize that decreased connectivity could be related to atrophy of glutamate principle neurons and loss of synaptic connections in the PFC. They also report that ketamine administration increased GBCr in PFC, caudate and insula in responders, and reversed the dysconnectivity of PFC with the rest of the brain. The authors note that decreased GBCr is not specific to MDD as similar decreases are reported in schizophrenia, suggesting that there is overlap at the network levels between MDD and other psychiatric illnesses. The possibility that psychiatric illnesses share functional abnormalities is consistent with evidence from genomic and transcriptome overlap (discussed below).
Glutamate alterations in MDD and stress
Changes in brain connectivity in MDD could be related to altered levels of the major excitatory and inhibitory systems in the brain. Unlike the modulatory monoamine systems that have more circumscribed roles and provide extrinsic inputs to the cerebral cortex, glutamate and GABA are the primary excitatory and inhibitory neurotransmitters with both intrinsic and extrinsic control of information flow in the brain. Findings of abnormalities within the glutamate and GABA systems in the brains of individuals with mood disorders have led to new theories on the pathophysiology and treatment of these disorders (Abdallah et al., 2014; Fee et al., 2017; Sanacora et al., 2012) (Figure 1).
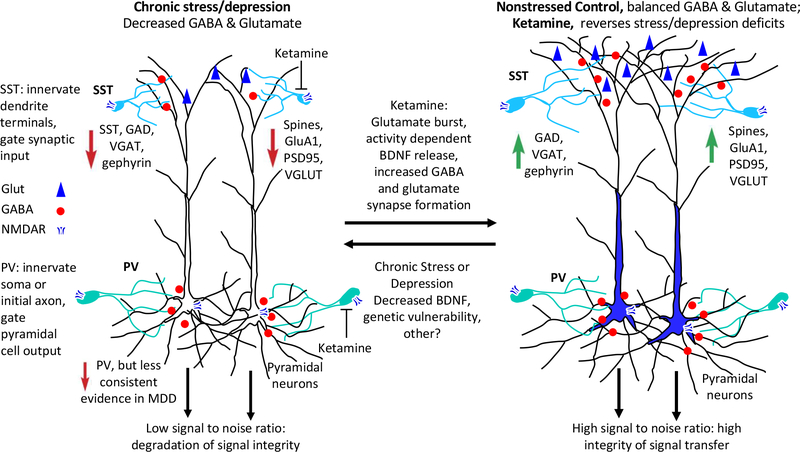
Figure 1. Stress and depression decrease both glutamate and GABA neurotransmitter systems in cortical and limbic brain regions: Reversal by ketamine.
SST interneurons innervated dendrites of principle neurons and gate synaptic input while PV interneuron subtypes innervate the soma (basket cells) and initial axon (chandelier cells) of principle neurons and gate output. Under normal nonstressed conditions, GABA and glutamate transmission is balanced and optimized to provide high signal to noise ratio and integrity of signal transfer. Clinical and preclinical studies report that depression and chronic stress cause deficits of both GABA and glutamate systems. Clinical studies demonstrate reductions in the volume of prefrontal cortex (PFC) and hippocampus, reductions in synaptic markers, decreased SST interneuron markers, as well as decreased levels of glutamatic acid decarboxylase (GAD). There is less consistent evidence for deficits of PV interneurons. Preclinical studies demonstrate that chronic stress exposure causes atrophy of pyramidal neuron dendrites in PFC and hippocampus, reductions of synapse number and proteins (PSD95, GluA1), and decreased levels of the vesicular glutamate transporter (VGLUT); chronic stress also causes reductions of GABA neuronal markers, notably SST, the vesicular GABA transporter (VGAT), GAD, and gephyrin. Reductions in GABA and glutamate neurotransmitter systems would decrease signal to noise ratio and result in degradation of signal integrity in these regions.
In contrast, a single dose of ketamine, which causes rapid and efficacious antidepressant actions in depressed patients, rapidly increases both GABA and glutamate systems and reverses the deficits caused by chronic stress exposure. Preclinical studies show that ketamine causes a burst of glutamate via blockade of NMDARs on GABA interneurons. GABA interneurons are more sensitive to ketamine because their tonic firing removes Mg+2 from the channel, allowing ketamine to enter and block the channel. This glutamate burst causes activity dependent release of BDNF and increased synapse number and function in layer 5 pyramidal neurons in the medial prefrontal cortex (mPFC), as well as increased synaptic markers (PSD95, GluA1, VGLUT). Recent studies show that ketamine rapidly increases GABA function in the mPFC including increased levels the VGAT, GAD, and gephyrin. Up-regulation of GABA and glutamate neurotransmitter systems reverses the effects of chronic stress exposure, and thereby increases signal to noise and enhances integrity of signaling in these regions
Early studies reported alterations of glutamate in blood, CSF, and brain tissue, although with mixed results. More recent studies using in vivo proton magnetic resonance spectroscopy (MRS) have more consistently reported decreased levels of glutamate metabolites in regions of the medial frontal cortex (Moriguchi et al., 2018). Glutamate reductions could have multiple determinants, including altered synthesis, metabolism, and/or reuptake into neurons and glia (Abdallah et al., 2014; Lener et al., 2017; Sanacora et al., 2012). Studies combining MRS and fMRI show that decreased glutamate in the subgenual anterior cingulate cortex (ACC) is associated with reduced connectivity with insula and decreased BOLD response to emotional stimuli in MDD patients (Lener et al., 2017). These imaging studies point to reductions in glutamate transmission in PFC subregions of MDD patients and are supported by postmortem studies of decreased synapse number and expression of synaptic markers in dlPFC of MDD subjects (Kang et al., 2012). There are also postmortem studies reporting alterations of glutamate receptor subtypes providing further evidence of altered glutamate transmission (Akil et al., 2018; Feyissa et al., 2009; Gray et al., 2015; Karolewicz et al., 2009).
Preclinical studies also report altered levels of glutamate, synaptic markers, and dendrite formation in rodent models following both acute and chronic stress procedures. (Duman et al., 2016; McEwen et al., 2015; McEwen and Morrison, 2013; Popoli et al., 2011; Sanacora et al., 2012). Acute stress increases extracellular glutamate in the mPFC and hippocampus, and this has led the hypothesis that glutamate-mediated excitotoxicity via actions at extrasynaptic N-methyl-D-aspartate receptors (NMDARs) are responsible for the atrophy of neurons in these brain regions (Popoli et al., 2011). The effects of stress can also be mimicked by long-term administration of adrenal glucocorticoids indicating a role for the HPA axis (Popoli et al., 2011). Less is known about the influence of chronic stress exposure on glutamate levels.13C-MRS studies demonstrate that chronic stress exposure decreases the cycling and metabolism of glutamate and glutamine, as well as GABA in rat PFC, effects attributed to a reduction in glial metabolism (Banasr et al., 2010). Conversely, rapid acting antidepressant such as ketamine cause a transient increase in glutamate cycling in the mPFC (Chowdhury et al., 2017).
The strongest evidence of altered glutamate transmission under chronic stress conditions comes from morphological studies of excitatory principle neurons (Figure 1). These studies show that exposure to chronic stress decreases dendrite length and branching of hippocampus CA3 pyramidal neurons and mPFC layers II/II and V pyramidal neurons (Duman et al., 2016; McEwen et al., 2015; McEwen and Morrison, 2013; Morrison and Baxter, 2012). Chronic stress also decreases the number and function of spine synapses of pyramidal cells (Li et al., 2011; Liu and Aghajanian, 2008) as well as levels of synaptic markers, including GluA1, PSD95, and synapsin 1 in the mPFC (Li et al., 2011). Together these studies indicate that chronic stress decreases the structure and function of glutamate neurons and are consistent with the hypothesis that atrophy of glutamate neurons contributes to the reduction in volume of cortical and limbic structures implicated in depression.
While these studies show clear evidence of glutamate neuronal atrophy in the PFC and hippocampus, there is evidence of hypertrophy in the amygdala. Preclinical studies show that chronic stress increases dendrite length and complexity in principle glutamate neurons in the basolateral nucleus of the amygdala (McEwen et al., 2016; Roozendaal et al., 2009). The BLA is involved in control of emotional valence processing, both positive and negative and is associated with increased anxiety and depressive behaviors (Tye, 2018). There is also evidence that social defeat and chronic stress increase mesolimbic dopamine system activity and that these changes are associated with reduction in social interaction and depressive behaviors (Russo and Nestler, 2013). These studies demonstrate the complex, region and circuit specific effects of stress that could be relevant to increased and decreased connectivity reported in depressed patients.
GABA alterations in MDD and stress
While GABA neurons make up a smaller fraction of the total neuronal population, approximately 15–20 percent compared to glutamate, inhibitory neurotransmission and balance with excitatory transmission are critical for normal brain function (Figure 1). Based on evidence of altered glutamate and functional connectivity it is not surprising that there is also evidence of disrupted GABA neurotransmission that contributes to the neurobiology of MDD (Ghosal et al., 2017; Lener et al., 2017; Lin and Sibille, 2015; Luscher et al., 2011; Northoff and Sibille, 2014). Early studies reported decreased levels of GABA in cerebral spinal fluid of MDD patients (Fee et al., 2017; Gold et al., 1980). Brain MRS studies also report reduced GABA levels in cortical brain regions of depressed patients, and normalization of GABA with remission (Godfrey et al., 2018; Hasler et al., 2005; Hasler et al., 2007; Price et al., 2009; Sanacora et al., 1999; Schur et al., 2016). Studies of transcranial magnetic stimulation also report a reduction in GABA function in depressed patients, determined by analysis of cortical inhibition (Bajbouj et al., 2006; Levinson et al., 2010). This includes evidence for deficits of short- and long-interval cortical inhibition, as well as the cortical silent period, which indicates deficits in the function of both ionotropic GABAA and metabotropic GABAB receptors (GABAAR and GABABR) (Fee et al., 2017; Radhu et al., 2013). Postmortem studies report evidence of decreased levels of the GABA synthetic enzyme GAD67 in the PFC (Guilloux et al., 2012; Karolewicz et al., 2010). However, reductions of GABA markers are also observed in other psychiatric illnesses, including schizophrenia and bipolar disorder, indicating that GABA dysfunction is a more general vulnerability factor for illness (Fee et al., 2017). GABA alterations across psychiatric disorders are also consistent with evidence of shared global connectivity (above) as well as shared transcriptomic and genomic determinants of illness (see below).
Studies have also begun to examine markers of the three major largely nonoverlapping GABA interneuron subtypes that can be classified based on morphological, electrophysiological, and molecular characteristics, particularly the expression of somatostatin (SST), parvalbumin (PV), or 5-HT3a receptors (DeFelipe et al., 2013). PV interneurons are the most prominent (40%), and are made up of two subgroups, the chandelier and basket cells that target the initial axons and soma, repectively of principle neurons (Tremblay et al., 2016). SST interneurons make up approximately 30% of the GABA population and target dendrites; most are referred to as Martinotti cells with axonal plexus in layer 1. The 5-HT3a receptor subtype is further defined by expression of vasoactive intestinal peptide (VIP) and can be bipolar or multipolar (DeFelipe et al., 2013). GABA interneurons also express other calcium binding proteins and neuropeptides, including calbindnin (CB), calretin (CR), cholecystokinin (CCK), and neuropeptide Y (NPY) although expression of these proteins is found in different interneuron subtypes and cannot be used for subclassification. Interneuron subtypes can also be further defined by their electrophyiological and functional properties, resulting in a large diversity of interneuron subtypes that are still being delineated. Circuit function is further complicated by interconnections, for example VIP innervation of SST, and SST innervation of PV interneurons, as well as regional and laminar differences in these effects (Tremblay et al., 2016; Xu et al., 2013). Most of the work in the field of depression and stress has focused on the PV and SST subtypes, as well as expression of other neuropeptides and calcium binding proteins. Future studies are needed to further characterize the role of other interneuron subtypes in depression, including single cell transcriptomic studies of postmortem MDD and rodent chronic stress models.
Postmortem studies report a reduction in markers of SST/calbindin interneurons in the PFC and other cortical regions (Maciag et al., 2010; Rajkowska et al., 2007), as well as decreased levels of SST mRNA expression in the dlPFC, ACC, and amygdala of depressed subjects (Douillard-Guilloux et al., 2017; Seney et al., 2015; Tripp et al., 2011) (Figure 1). There is less evidence of consistent alterations of PV and VIP interneurons in MDD (Fee et al., 2017; Rajkowska et al., 2007; Sibille et al., 2011; Tripp et al., 2011). Alterations of PV interneurons have been more consistently reported in postmortem schizophrenia (Dienel and Lewis, 2018), demonstrating a level of interneuron divergence in these disorders. Aberrant hyperconectivity resulting from loss or dysfunction of SST interneurons, could also be exacerbated by loss of the electrical coupling of SST interneurons via gap junctions, which has been reported for interneurons of the same class (Tremblay et al., 2016).
Preclinical studies of chronic stress also report reductions in levels of GABA synthetic enzymes and neuropeptides in medial PFC and other cortical brain regions (Banasr et al., 2017; Fee et al., 2017; Ghosal et al., 2017; Luscher and Fuchs, 2015; Luscher et al., 2011; Ma et al., 2016). Stress also causes a loss of GABA function via a shift in the reversal potential resulting from decreased function of the transmembrane potassium chloride anion co-transporter (Hewitt et al., 2009; MacKenzie and Maguire, 2015). This causes a depolarizing shift in the reversal potential of GABAAR mediated responses that significantly decreases inhibitory GABA inputs. We have also found that chronic unpredictable stress (CUS, 3 week) exposure decreases pre- and postsynaptic GABA markers, including levels of GAD65/67 and gephyrin in the PFC (Ghosal et al., 2018). Studies of SST and PV markers have been mixed, with reports that CUS for 16 d causes a selective reduction of SST/calbindin interneurons in the PFC and hippocampus with no change in PV (Nowak et al., 2010; Zadrozna et al., 2011), and other reports that long-term chronic mild stress (9 week) decreases PV, but not SST in PFC of susceptible rats (Czeh et al., 2005; Czeh et al., 2018; Czeh et al., 2015). Another recent study finds that 5 weeks of CUS decreases protein levels of GAD67, as well as SST and NPY, with a trend for PV in the PFC and/or hippocampus (Banasr et al., 2017); levels of SST and NPY mRNA in the PFC were also decreased. The reasons for these discrepancies are unclear but could be due to the length of stress exposure, type of stressors, and the method for analyzing interneuron markers. In contrast to these effects of stress, administration of SST is reported to produce anxiolytic as well as antidepressant actions in rodent models possibly via actions in the amygdala and septum (Engin et al., 2008; Engin and Treit, 2009; Yeung et al., 2011).
How does loss of SST and PV interneuron activity translate functionally? SST interneurons target dendrites and regulate dendritic excitability, thereby gating the pattern of functional connectivity inputs (Chiu et al., 2018). SST interneurons are recruited via NMDA receptor activation, and loss of SST function or blockade of SST-NMDARs reduces input selectivity, thereby causing abberant hyperconnectivity of principal neurons (Chiu et al., 2013); this is consistent with the increase in cortical functional connectivity produced by acute NMDAR blockade in humans (Driesen et al., 2013). The precision of neural representations is maintained through the inhibitory sculpting of principal neural activity and is degraded by this form of NMDAR blockade induced hyperconnectivity (Krystal et al., 2017; Murray et al., 2014). PV interneurons target the soma and initial axon of pyramidal neurons and play a critical role in coordinating the temporal fidelity of neural oscillatory activity, which would be impaird by reduced PV function or blockade PV-NMDARs (Dienel and Lewis, 2018), Thus, deficits in GABA signaling compromise the signal to noise properties of the cerebral cortical neurons, undermining many important features of cortical function (Krystal et al., 2017).
The impact of altered GABA levels has been examined using mutant mouse models, as well as chemo- and optogenetic approaches. Models of stress suggest that behavioral phenotypes relevant to depression, such as anhedonia and neophobia, are observed in GABAA receptor (GABAAR) mutant mice (Luscher and Fuchs, 2015; Ren et al., 2016) or in mutant mice with a 50% tissue reduction in hippocampal and cortical GABA (Kolata et al., 2018). Global heterozygous deletion of the GABAAR γ2 subunit (γ2+/−), a constitutive subunit of the synaptic GABAAR receptor complex causes depressive behaviors and homeostatic reduction of glutamate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) and NMDAR function (Ren et al., 2016). A role for SST is supported by studies of Sst deletion mutant mice, which have a depressive like phenotype in anxiety and depression associated behaviors, as well as decreased expression of brain derived neurotrophic factor (BDNF) and GAD67 (Fuchs et al., 2017). Chemogenetic studies using Gi DREADDs to inhibit SST interneuron function demonstrate that acute inhibition increases anxiety and depressive behaviors while chronic inhibition has the opposite effect, probably due to adaptations to chronic inhibition (Soumier and Sibille, 2014).
The mechanisms underlying stress susceptibility of SST interneurons is not clear but there is evidence of age associated loss of SST neurons that is accelerated in MDD (Fee et al., 2017). There is also evidence that CUS (6 weeks) causes selective effects on SST interneurons compared to pyramidal neurons, notably increased EIF2 kinase signaling and increased ER stress that could reduce SST interneuron function (Lin and Sibille, 2015). ER stress is characterized by overload of protein translation systems, leading to misfolding and accumulation of nonfunctional proteins. Decreased neurotrophic factor expression is another possible mechanism as the maintenance and survival of SST interneurons is dependent on BDNF, which is decreased by stress (Duman and Monteggia, 2006; Grosse et al., 2005; Martinowich et al., 2011). There is also evidence that BDNF-regulated genes, including markers of SST interneurons are decreased in MDD (Tripp et al., 2012). Postmortem studies demonstrate that BDNF mRNA isoforms that target dendrites are decreased in PFC of MDD subjects and are correlated with reduction of SST expression (Oh et al., 2018). This study found that chronic stress causes a similar reduction of dendrite targeting BDNF mRNA isoforms and knockdown of these isoforms was sufficient to cause dendrite retraction, decreased Sst expression, and depressive/anxiety behaviors (Oh et al., 2018).
Genetics of depression: identification of loci related to neurotransmission
Modest rates of heritability combined with disease heterogeneity have slowed efforts to identify the gene loci associated with depression. In addition, it is well accepted that complex illnesses such as depression have many common gene loci, each with small effect size further confounding genetic studies (Sullivan et al., 2018). These factors have resulted in the requirement of large sample sizes (100,000 to 200,000, or more) to identify common genetic risk variants, as well as rare variants of disease (Akil et al., 2018; Sullivan et al., 2018). The Psychiatric Genomics Consortium (PGC), a global effort to combine and create large data sets, was formed to address this issue. These combined large genome wide association studies (GWAS) are making progress and report a strong association with gene loci related to synaptic transmission, as well as other gene loci. One early study of 60,000 cases reported significant associations of major psychiatric illnesses, including schizophrenia, bipolar disorder and MDD with immune, neuronal signaling, synaptic density, and histone pathways (Network and Pathway Analysis Subgroup of Psychiatric Genomics, 2015). A more recent study by the PGC MDD working group examined 135,458 patients with depression (cohorts were defined by different criteria) and 344,901 controls and identified 44 genetic loci that included genes involved in brain development and HPA axis control (RBFOX1), presynaptic differentiation and neuroinflammation (LRFN5), presynaptic vesicle trafficking (PCLO), calcium signaling (CACNA1E, CACNA2D1), and dopamine (DRD2) and glutamate receptors (GRIK5, GRM5) (Wray et al., 2018). Expression of these genes is enriched in brain, particularly frontal cortex and anterior cingulate, and cell type enrichment analysis identifies neurons as the highest represented cell type overall. A UK study that included 322,580 Biobank participants with 3 depression related phenotypes identified 17 loci, with gene sets enriched in excitatory transmission, mechanosensory behavior, neuronal spines, and dendrite function (Howard et al., 2018).
Together these studies provide strong evidence of gene loci related to synaptic transmission in depression and other psychiatric disorders. Many of these gene loci are associated with glutamate excitatory systems, although indirect effects on inhibitory systems cannot be ruled out. The impact of these gene polymorphisms on excitatory and inhibitory transmission, as well as functional connectivity could be interesting. For example, the BDNF Val66Met polymorphism, which blocks the processing and activity dependent release of BDNF, is associated with increased covariance of amygdala-cortical networks implicated in depression in adolescent female Met allele carriers (Wheeler et al., 2018). This study also found that the Met allele is associated with stronger resting state functional connectivity and increased chance of depression in adolescent females.
An important finding of these genetic studies is the overlap in gene loci across psychiatric illnesses. This was noted in one of the early pathways analysis studies (Network and Pathway Analysis Subgroup of Psychiatric Genomics, 2015), as well as the more focused depression genetic study (Wray et al., 2018). Another recent study of 25 brain disorders that included 265,218 patients and 784,683 controls also reports sharing of common risk variants across psychiatric disorders, including ADHD, bipolar disorder, MDD, and schizophrenia (Brainstorm et al., 2018) (Figure 2). This contrasts with neurological disorders which do not overlap with each other or with psychiatric disorders. This polygenic overlap of gene loci between psychiatric illnesses indicates that common genetic variants can lead to diverse illnesses.
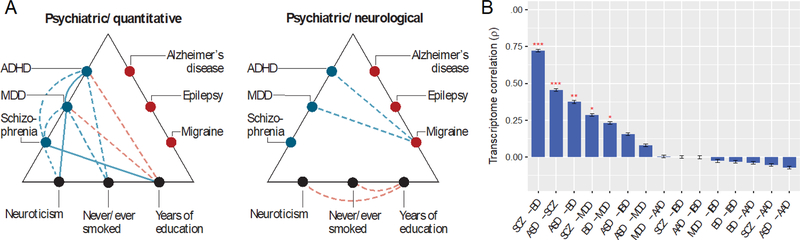
Figure 2. Genome and transcriptome overlap between psychiatric disorders.
(A) The psychiatric genomics consortium (PGC) reported common variant risk was significantly correlated for psychiatric illnesses, most notably attention hyperactivity disorder (ADHD), BD, MDD, and schizophrenia (Brainstorm Consortium, 2018). There was little or no overlap with neurological disorders, including Alzheimer’s disease, epilepsy, and migraine. Both psychiatric and neurological disease groups show correlations with neurocognitive quantitative phenotypes. Blue indicates positive, and red negative correlations; solid lines indicate higher degree of correlation. The first panel shows correlations between psychiatric conditions and with quantitative measures of neuroticism, smoking and education. The second panel shows correlations between psychiatric and neurological disorders. (B) A meta-analysis found significant overlap of transcriptome profiles between schizophrenia (SCZ), bipolar disorder (BD), autism spectrum disorder (ASD) and major depressive disorder (MDD) (Gandal et al., 2018). There was no significant overlap with irritable bowel syndrome (IBD) and alcoholism (AAD).
Overlap between psychiatric disorders has also been reported in large scale postmortem transcriptomic studies. A recent meta-analysis reports shared gene expression patterns, with the highest degrees of overlap between schizophrenia, bipolar disorder and MDD (Gandal et al., 2018) (Figure 2). They also report that the transcriptome overlap is significantly related to polygenic overlap of single nucleotide polymorphisms reported for these disorders. The exact nature of the behavioral phenotype and classification could be dependent on the complexity of the genetic architecture, including a large number of interacting gene loci, both susceptible and protective, as well as sex, metabolic and environmental interactions. These issues are the focus of ongoing large genomic studies of depression and other psychiatric illnesses (Sullivan et al., 2018).
Most of genome association and transcriptome studies have not examined sex as a separate covariate, even though rates of depression are two-fold higher in women. While variants with strong effect might not be influenced by sex, it is possible that the impact of certain common gene variants with small effect size might be dependent on endocrine or environmental factors that are specific to women, or that X-linked polymorphisms play a role in susceptibility. More recent transcriptome studies have reported sex differences (see below).
Stress and neurotrophic factors: Mechanisms underlying synaptic loss
Studies have begun to investigate the mechanisms underlying the loss of synapses in preclinical models of depression. The HPA axis and elevation of adrenal glucocorticoids, the hallmark endocrine response to stress play an important role in the development of MDD, as well as other psychiatric disorders. Preclinical studies demonstrate that administration of glucocorticoids is sufficient to cause atrophy of dendrites and decreased synaptic number in the hippocampus and PFC (Marrocco and McEwen, 2016; McEwen et al., 2016). There is also evidence that administration of adrenal glucocorticoids can lead to deficits of GABA neurotransmission (Ghosal et al., 2017; Luscher et al., 2011; Northoff and Sibille, 2014). Several possible downstream targets of glucocorticoids have been examined, including BDNF, a major neurotrophic factor that contributes to the maintenance and survival of neurons, as well as activity dependent regulation of synapse number and function (Hedrick et al., 2016; Martinowich et al., 2007; Song et al., 2017). Levels of BDNF are decreased in postmortem hippocampus and PFC of depressed subjects (Duman and Monteggia, 2006; Fee et al., 2017), implicating this factor in the reduced volume of these brain regions in MDD (Figure 1). Further evidence comes from identification of a common polymorphism, the BDNF Val66Met allele, which blocks the processing and activity dependent release of BDNF (Chen et al., 2006). Carriers of the BDNF Met allele have reduced hippocampal volume and decreased executive function (Frodl and O’Keane, 2013; Frodl et al., 2007), and are at greater risk for depression when exposed to early life stress or trauma (Gatt et al., 2009; Kaufman et al., 2006; Kim et al., 2007).
Preclinical studies have also provided evidence for a role of BDNF in depression and treatment response (Figure 1). Mice with a knockin of the BDNF Val66Met allele have reduced dendrite arbor and decreased synapse number and function in the PFC and hippocampus, similar to chronic stress (Chen et al., 2006; Magarinos et al., 2011). In addition, there is no further reduction of dendrite complexity in Met mice exposed to chronic stress (Magarinos et al., 2011). BDNF deletion mutant mice or Met knockin mice do not respond to antidepressant treatments, indicating a requirement for functional BDNF (Liu et al., 2012; Monteggia et al., 2007). Clinical studies also report that BDNF Met carriers have a significant reduction in the response to ketamine (Laje et al., 2012), although this was not observed in an Asian population suggesting that there are race specific interacting gene loci (Su et al., 2017). The synaptic actions of BDNF are mediated by a number of signaling pathways, including mTORC1, which plays a role in activity dependent protein translation and synapse formation (Castren and Kojima, 2017). Chronic stress decreases mTORC1 signaling via up-regulation of a negative regulator, REDD1, which is sufficient to cause synaptic loss in the PFC (Ota et al., 2014).
There is also evidence that BDNF plays a role in the maintenance and survival of SST interneurons (Grosse et al., 2005; Martinowich et al., 2011), and that BDNF-regulated genes, including markers of SST interneurons are decreased in MDD (Tripp et al., 2012). Expression of BDNF decreases with age, and expression of genes involved in both inhibitory and excitatory neurotransmission are also decreased and are significantly correlated with BDNF expression (Oh et al., 2016). Blockade of BDNF-TrkB signaling causes a similar reduction of GABA, but not glutamate markers, leading to the conclusion that BDNF reduction is directly linked to regulation of GABA, which subsequently causes a homeostatic decrease in glutamate function (Oh et al., 2016).
Sex differences in GABA and Glutamate function
Women are at a two-fold higher risk for depression compared to men, and twin studies report a higher genetic risk in women (40 percent) relative to men (30 percent) (Flint and Kendler, 2014). These findings suggest sex specific alleles but also raise the possibility of polymorphisms that interact with sex specific endocrine factors, as well as environmental and behavioral differences. For example, social stress is a greater risk factor for women whereas low self-esteem is more relevant to men (Kendler and Gardner, 2014). A sex difference in connectivity was reported in adolescents, with an affective go/no-go task and fMRI reporting differences in supramarginal gyrus and posterior cingulate (Chuang et al., 2017). There have been few fMRI connectivity studies of sex differences although nothing definitive has emerged and it is possible that brain imaging studies are underpowered to identify sex differences. There is some evidence from postmortem studies demonstrating differences in the expression of glutamate receptor subtypes in cortical brain regions (Gray et al., 2015), and SST gene expression deficits in the ACC and sgPFC are greater in women compared to men (Seney et al., 2015; Tripp et al., 2011). Studies in Sst deletion mutant mice also report greater effects on measures of behavioral emotion in females compared to males (Lin and Sibille, 2015).
Large fluctuations in ovarian steroids have been linked to increased risk of depression in women (McEwen and Milner, 2017; Rubinow, 2011; Schiller et al., 2015). This includes fluctuations in levels of the neuroactive steroid allopregnanolone with specific actions at the GABAAR complex. In particular, allopregnanolone levels are high during pregnancy and precipitously drop at birth, giving rise to overall reduced GABA function that is thought to underlie postpartum depression (Zorumski et al., 2013). This has led to the development of a novel, efficacious treatment for postpartum depression, that has received breakthrough status from the FDA (Kanes et al., 2017).
There have been two high profile postmortem transcriptome studies reporting sex differences in cortical structures of MDD subjects (Labonte et al., 2017; Seney et al., 2018). Labonte and colleagues reported sex specific disease transcriptome modules in postmortem cortical brain regions and found that manipulation of these modules in mice produces depressive behaviors but only in the corresponding sex (DUSP in females, EMX1 in males). Moreover, they found convergence of these sex specific modules at the level of activity of excitatory neurons in the PFC that could underlie the observed convergence on depressive behaviors (Labonte et al., 2017). The effects of these modules on GABA interneuron function was not tested. Seney and colleagues reported overlap of transcriptome profiles of dlPFC and sgPFC but in the opposite direction in men compared to women: synapse related genes were decreased in men and increased in women, whereas oligodendrocytes and microglia markers were increased in men and decreased in women (Seney et al., 2018).
There is also evidence from rodent studies that chronic restraint stress causes sex specific alterations of dendrite morphology of layer II/III pyramidal neurons in the infralimbic PFC; in females, there is a reduction in spine density of neurons projecting to the BLA that is not observed in males, although males display decreases in spine density in a larger population of pyramidal neurons in the infralimbic PFC (Shansky et al., 2009; Shansky and Morrison, 2009). Ovarian steroids are also reported to significantly regulate dendrite and synapse number in the hippocampus and PFC and abnormal fluctuations of these hormones could lead to alterations of synaptic connectivity (Marrocco and McEwen, 2016; McEwen and Milner, 2017).
Novel antidepressants treatments that influence glutamate
Evidence from brain imaging, MRS, and postmortem studies as well as chronic stress models demonstrate deficits of both glutamate and GABA neurotransmitter systems in cortical and limbic brain regions. These deficits are associated with MDD and based on preclinical studies are causally related to depressive-like behaviors, indicating that treatments that reverse or normalize glutamate and GABA neurotransmission should improve depressive symptoms. Agents that directly target the major excitatory and inhibitory systems should also be more effective than typical antidepressants that block the reuptake of modulatory monoamine neurotransmitters, which could account for the slow onset and modest efficacy of these agents. However due to their ubiquitous nature, drugs targeting the primary excitatory and inhibitory systems in the brain may also produce more side effects that would have to be balanced with potential increased efficacy.
Based in part on evidence of excitatory neuronal deficits, agents that act as glutamate AMPAR positive allosteric modulators (PAMs) have been developed and shown to have efficacy in rodent models (Sanacora et al., 2008). However, clinical use has been limited as AMPAR PAMs with greater activity also have more side effects and finding the right balance has been difficult. Further evidence for glutamatergic agents is provided by studies of mGluR2/3 antagonists that regulate presynaptic release of glutamate (Xi et al., 2002). Preclinical studies demonstrate that mGluR2/3 antagonists increase extracellular glutamate in the PFC and produce rapid antidepressant behavioral actions in rodent models (Koike et al., 2011). These agents also increase GluA1, as well as other synaptic proteins in the PFC and the antidepressant behavioral actions require BDNF and mTORC1 signaling (Dwyer et al., 2012; Koike et al., 2011). However, initial clinical trials with mGluR2/3 antagonists have been negative, possibly due to trial methodology and extremely high placebo response rates.
There is also growing evidence from studies of rapid acting antidepressants, notably ketamine, of increased glutamate connectivity and signaling. Infusion of a low nonanesthetic dose of ketamine (0.5 mg/kg, i.v., 40 min) leads to a rapid (80 min) and sustained (up to 7 d) antidepressant response even in patients considered treatment resistant (Berman et al., 2000; Zarate et al., 2006). This ketamine treatment regimen is also reported to produce rapid and sustained thereapeutic effects in a small trial (12 patients) of anxiety disorder (Glue et al., 2017). Although ketamine is an NMDAR channel blocker, it produces a paradoxical burst of glutamate in the rodent PFC (Moghaddam et al., 1997) (Figures 1, 3). Increased glutamate signaling is supported by MRS studies in rodents and humans, demonstrating dose dependent increases in glutamate cycling (Chowdhury et al., 2017). Importantly, these studies demonstrate that the burst of glutamate is rapid (within minutes) and transient, which is critical to limit the excitotoxic effects of ketamine (Chowdhury et al., 2017; Moghaddam et al., 1997). The cellular trigger for this burst of glutamate is thought to involve blockade of NMDAR on tonic firing GABA interneurons, leading to disinhibition of glutamate transmission (Duman et al., 2016; Miller et al., 2016). Tonic activity of GABA interneurons would allow for removal of the Mg2+ block of the NMDAR channel thereby increasing sensitivity of these interneurons to ketamine blockade compared to less active glutamate neurons (Figure 1). Recent slice electrophysiology studies demonstrating that ketamine incubation decreases IPSCs on hippocampal principle neurons supports this hypothesis (Widman and McMahon, 2018). In addition, preliminary studies demonstrate that knockdown of the NMDAR GluN2B subunit on GABA, but not glutamate neurons blocks the antidepressant actions of ketamine (Gerhard and Duman, 2018). The muscarinic receptor antagonist scopolamine, which also produces rapid antidepressant actions in MDD patients, is dependent on blockade of M1 receptors on GABAergic interneurons in the mPFC and disinhibition of glutamate transmission (Wohleb et al., 2016).
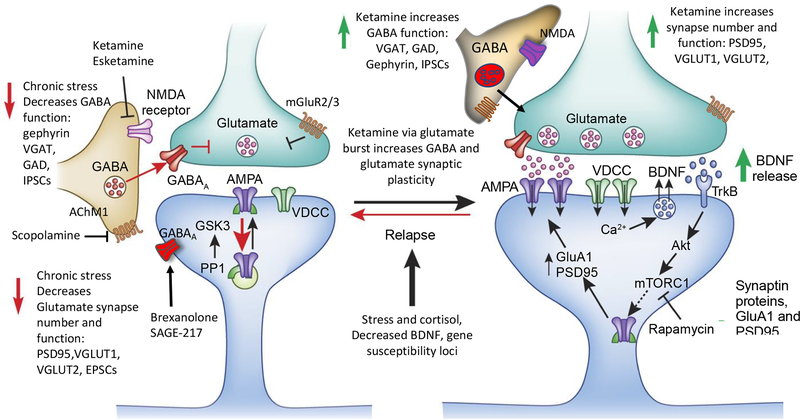
Figure 3. Chronic stress and depression decrease and rapid acting antidepressants increase glutamate and GABA neurotransmitter function.
Chronic stress and depression are associated with reductions of both glutamate and GABA function, including atrophy of pyramidal neurons and reductions of glutamate and GABA neuronal markers in the medial PFC and hippocampus. Rapid acting antidepressants, notably ketamine and esketamine that produce rapid antidepressant actions in depressed patients, rapidly up-regulate glutamate synapse density, synaptic protein levels (i.e., PSD95, GluA1, VGLUTs), and function, and also increase GABA function, including increased VGAT, GAD, and gephyrin. Ketamine causes a transient burst of glutamate, resulting from blockade of NMDA receptors on tonic firing GABA interneurons. This glutamate burst causes synaptic remodeling and resetting of glutamate and GABA systems. Other rapid acting antidepressants, including the muscarinic receptor antagonist scopolamine and mGluR2/3 antagonists also produce rapid antidepressant actions via transient stimulation of glutamate. The glutamate burst stimulates BDNF release, which activates downstream signaling, including the Akt-mTORC1 pathway that increases synaptic proteins synthesis. Blockade of this pathway with the selective mTORC1 inhibitor rapamycin blocks the synaptic and antidepressant behavioral actions of ketamine. Deficits of GABA, combined with evidence that fluctuations of neuroactive steroids contribute to depression in women have led to development of GABAergic agents, including formulations of alloprenanolone (brexanolone and SAGE-217), which act as positive allosteric modulators of the GABAAR complex.
Although transient, the ketamine stimulated glutamate burst causes activity dependent synapse formation that is dependent on BDNF release and stimulation of the mTORC1 signaling pathway (Duman et al., 2016; Lepack et al., 2014; Li et al., 2010; Liu et al., 2012) (Figures 1, 3). A single dose of ketamine increases the number and function of spine synapses on layer V pyramidal neurons in the mPFC and these effects are blocked in mice with a knockin of the BDNF Met allele or by infusion of a selective mTORC1 antagonist (Li et al., 2010; Liu et al., 2012) (Figure 3). A single dose of ketamine also rapidly reverses the mPFC synaptic and behavioral deficits caused by CUS (Li et al., 2011). The increase in synapse number and function is accompanied by increased levels of synaptic proteins, including GluA1 in the PFC (Li et al., 2010; Li et al., 2011; Maeng et al., 2008). A role for increased glutamate-AMPAR activity is provided by studies demonstrating that pretreatment with an AMPAR antagonist blocks the antidepressant actions of ketamine in rodents (Li et al., 2010; Maeng et al., 2008). Ketamine also increases protein synthesis in hippocampus via blockade of eukaryotic elongation factor 2 kinase signaling (Autry et al., 2011). Regulation of signaling in hippocampus and requirement for hippocampus projections to PFC have also been reported (Carreno et al., 2016). Additional studies are needed to further investigate the actions of ketamine on other corticolimbic brain structures.
Other rapid acting agents are reported to also have convergent effects on excitatory synapse formation and functional connectivity. This includes ketamine stereoisomers (R and S), ketamine metabolites (S-norketamine and (2R,6R)-hydroxynorketamine), and the muscarinic receptor antagonist scopolamine; all of these agents increase synaptic proteins, including GluA1, and/or synapse number and function (Duman, 2018; Yang et al., 2017; Zanos and Gould, 2018). The S stereoisomer, referred to as Esketamine has received fast track status from the FDA and a 14 to 2 vote in favor of approval. Most of these agents also cause a burst of glutamate and require activity dependent BDNF release. Another agent, rapastinel, a NMDAR PAM, also increases GluA1 and synapse number and function in rodents (Burgdorf et al., 2013; Kato et al., 2018; Liu et al., 2016), and produces rapid antidepressant actions in depressed patients (Preskorn et al., 2015). These studies have also led to the development of a number of other NMDAR agents, including selective GluN2B negative allosteric modulators (NAMs) and glycine site agents (Duman et al., 2016).
Novel antidepressant treatments that act on GABA systems
Therapeutic strategies that target GABA deficits for the treatment of depression are also being developed. Early studies reported beneficial effects of benzodiazepines, which enhance the function of synaptic γ2 containing GABAARs that are expressed throughout the brain (Petty et al., 1995). While these agents are highly effective anxiolytics, they have modest efficacy as long-term antidepressants and are limited by side effects and abuse potential. Further evidence potential therapeutic actions of GABAergic agents is provided by studies of mutant mice with deletion of the GABAAR γ2-subunit in the SST interneurons, resulting in disinhibition of GABA neurotransmission that produces an antidepressant behavioral phenotype (Fuchs et al., 2017).
Based on this and other evidence Luscher and colleagues have proposed that more selective GABAAR modulators could produce more effective antidepressant actions with fewer side effects (Luscher and Fuchs, 2015; Luscher et al., 2011). For example the α5-subunit has a more restricted expression in cortical and limbic brain regions implicated in depression and α5-GABAARs are thought to mediate the inhibitory actions of SST interneurons particularly on dendrite targeting projections (Ali and Thomson, 2008; Fee et al., 2017; Schulz et al., 2018). Preclinical studies demonstrate that acute or chronic administration of an α5-selective PAM produces antidepressant actions in female mice exposed to chronic stress (Piantadosi et al., 2016). Further studies are needed to confirm and extend these findings and identify α5 PAMs that are effective in males. Somewhat surprising, there is evidence that α5 selective NAMs also produce rapid antidepressant actions (Fischell et al., 2015; Zanos et al., 2017). Although this appears contradictory to evidence of a GABA deficit in MDD and the antidepressant actions of the α5 PAM, the α5 NAMs are thought to produce a ketamine-like transient disinhibition of glutamate transmission, resulting in activity dependent induction of synaptic function, including increased GluA1 expression in the hippocampus (Fischell et al., 2015).
Another promising area is neuroactive steroids, specifically allopregnanolone (Zorumski et al., 2013). Allopregnanolone is a GABAAR PAM, and acts on complexes containing most subunits, including synaptic (α, β, and γ) as well as the extrasynaptic δ that mediates tonic inhibition (MacKenzie and Maguire, 2013). Allopregnanolone was initially targeted for its potential in postpartum depression, to replace the precipitous drop that occurs at birth, resulting in reduced GABAAR transmission (Bloch et al., 2000; Schiller et al., 2015). As noted previously, a recent study demonstrates that administration of allopregnanolone formulation, referred to as brexanolone developed by SAGE, produces rapid and robust antidepressant effects in postpartum depressed women lasting for a month or longer following a single 60-hr infusion (Kanes et al., 2017). In addition, an allopregnanolone analogue, SAGE-217 has recently shown rapid antidepressant efficacy in a mixed population (male and female) of MDD patients, indicating more broad effects via enhanced GABAAR function. Because of these encouraging findings, brexanolone and SAGE-217 have received breakthrough status from the FDA.
GABA also acts via metabotropic GABAB receptors (GABABR) that are typically found as heterodimers of GABAB1R and GABAB2R (Cryan and Kaupmann, 2005; Gassmann and Bettler, 2012). TMS studies of cortical inhibition provide evidence of GABABR, as well as GABAAR deficits in depression (Fee et al., 2017; Radhu et al., 2013), and preclinical studies report that antidepressant treatments up-regulate GABABRs (Alexander, 2017). While studies of GABABR agonists have been mixed, there is more consistent evidence that GABABR antagonists produce antidepressant actions (Alexander, 2017).
In addition to these direct GABA targeting approaches, indirect up-regulation of GABA systems contribute to the response to rapid acting antidepressants. Ketamine, as well as the selective GluN2B negative allosteric modulator Ro 25–6981 and scopolamine all produce a transient increase in GABA cycling shortly following administration in rodents (Chowdhury et al., 2017). Ketamine also transiently increases the GABA/W ratio in mPFC during the time of ketamine infusion in depressed humans (Milak et al., 2016). Imaging studies report that hyperactivity of the DMN and altered connectivity with the insula in depressed patients is normalized by ketamine up to 2 days after infusion, possibly due to increased GABA function (Evans et al., 2018). Studies in healthy controls are also consistent with the hypothesis that ketamine enhances GABA inhibition, reporting that ketamine decreases DMN connectivity and reduces reactivity of amygdala-hippocampal circuity in response to emotional stimuli (Evans et al., 2018; Scheidegger et al., 2016; Scheidegger et al., 2012). This effect was observed up to 2 days after ketamine (Evans et al., 2018), although another study reports that decreased global connectivity in MDD patients is normalized one day after a single dose of ketamine (Abdallah et al., 2017), possibly due to type of analysis or response heterogeneity.
While brain imaging studies may be difficult to interpret, genetic engineering studies in rodents provide evidence that ketamine and other rapid acting agents up-regulate GABA signaling. Luscher and colleagues (Ren et al., 2016) demonstrate that mice with a global heterozygous deletion of the GABAAR γ2 (γ2+/−) subunit have a depressive behavioral phenotype and a homeostatic reduction of NMDAR and AMPAR in the PFC and hippocampus. A single dose of ketamine reversed the behavioral and glutamate receptor synaptic deficits, and enhanced GABA synaptic function. Incubation of GABAAR γ2+/− cultured neurons with ketamine also increased the GABA synaptic markers VGAT and gephyrin in (Ren et al., 2016).
In agreement with these studies, we have found that a single dose of ketamine increases pre- and postsynaptic markers of GABA synapses in PFC, including increased GAD65/67, VGAT, and gephyrin, and reversed the deficits in these GABA markers caused by chronic stress exposure (Ghosal et al., 2017) (Figures 1, 3). Ketamine also produced a sustained increase in the function of GABA transmission, demonstrated by increased IPSCs in layer V pyramidal neurons in the PFC. Similar effects have been observed with the NMDA allosteric modulator rapastinel (unpublished), and studies are currently underway to determine if other rapid acting agents produce similar effects. It is interesting to speculate that up-regulation of GABA neurotransmission could play a role in the sustained, as well as rapid antidepressant actions of these agents, and studies are being conducted to test this hypothesis.
Although these findings appear to contradict the acute actions of ketamine to block NMDAR drive of GABA interneurons, these effects are rapid and transient, occurring within the first 30 to 60 minutes after dosing (Chowdhury et al., 2017; Moghaddam et al., 1997). However, the burst of glutamate leads to activity dependent BDNF release and sustained effects on both GABA and glutamate synaptic markers and function (Figure 3). The activity dependent release of BDNF, which enhances both GABA and glutamate synaptic function, could be a key mediator of these effects.
Summary and conclusions
Clinical and preclinical evidence demonstrates deficits in both glutamate and GABA signaling in MDD, raising questions about the vulnerability of these major neurotransmitter systems and whether homeostatic mechanisms play a role in the development and/or progression of illness? Preclinical studies demonstrate the functional impact of glutamate and GABA alterations on synaptic and behavioral responses in rodent models, including induction of depressive-like effects and reversal by rapid acting antidepressants. Some evidence points to GABA as the primary determinant, as manipulation of BDNF signaling leads to initial deficits in GABA, but not glutamate (Oh et al., 2018). Vulnerability of GABA interneurons, particularly SST could be related to the tonic activity of these interneurons in control of excitatory neurons, and age or illness related failure of these neurons would lead to imbalance and further maladaptive changes that cause degradation of signal integrity. Depression could also result from systems level alterations (e.g., hyperactivity of the DMN) in conjunction with genetic susceptibility alleles, many of which influence neurotransmission, as well as environmental (stress and trauma) and sex-related (ovarian steroid fluctuations) risk factors. Overlap of connectivity, gene loci, and transcriptome networks with schizophrenia and bipolar disorder indicates that psychiatric illnesses have common underlying determinants that present as different clinical behavioral phenotypes, which could be dependent on the genetic landscape as well as environmental and endocrine factors. Additional large-scale collaborative studies with increased statistical power are required to identify the genetic determinants and how gene loci interact with other risk factors that together influence functional connectivity, GABA and glutamate transmission, and complex behaviors. These advances will also provide the rationale for informed and targeted therapeutic interventions with greater efficacy and reduced side effects.
Acknowledgements
This work is supported by NIMH Grants MH045481 (RSD) and MH093897 (RSD), NIAAA Center for the Translational Neuroscience of Alcohol P50AA12870) (JHK), the CT Department of Mental Health and Addiction Services (RSD, GS) and Yale-New Haven Health (GS), the Yale Center for Clinical Investigation (UL1 RR024139)(JHK), and U.S. Department of Veterans Affairs through their support of the National Center for PTSD and the Consortium to Alleviate PTSD (RSD, JHK),
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
Dr. Duman has received consulting fees from Janssen, Taisho, Naurex, and Aptinyx, and has received research support from Lilly, Taisho, Allergan, Janssen, Naurex, Aptynix, Navitor, and Relmada. He is also listed as a co-inventor with Drs. Abdallah, Krystal, and Sanacora on Combination Therapy for Treating or Preventing Depression or Other Mood Diseases. U.S. Provisional Patent Application No. 047162–7177P1 (00754) filed on August 20, 2018 by Yale University Office of Cooperative Research OCR 7451 US01. Dr. Sanacora has received consulting fees from Allergan, Alkermes, AstraZeneca, Avanier, Axsome Therapeutics, Pharmaceuticals, Biohaven Pharmaceuticals, Bristol-Myers Squibb, Clexio Biosciences, Epiodyne, Intra-Cellular Therapies, Janssen, Merck & Co., Naurex, Navitor, NeruoRx, Novartis, Noven Pharmaceuticals, Otsuka, Perception Neuroscience, Praxis Therapeutics, Sage Pharmaceuticals, Servier Pharmaceuticals, Taisho Pharmaceuticals, Teva, Valeant, and Vistagen therapeutics over the last 36 months. He has also received additional research contracts from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Hoffman La-Roche, Merck, Naurex, and Servier over the last 36 months. Free medication was provided to GS for an NIH-sponsored study by Sanofi-Aventis. In addition, he holds shares in BioHaven Pharmaceuticals Holding Company and is a co-inventor on a patent ‘Glutamate agents in the treatment of mental disorders’ (Patent number: 8778979), and a U.S. Provisional Patent Application No. 047162–7177P1 (00754) filed on August 20, 2018 by Yale University Office of Cooperative Research OCR 7451 US01.
Dr. Krystal has received consulting fees from_AstraZeneca Pharmaceuticals, Biogen, Idec, MA, Biomedisyn Corporation, Bionomics, Limited (Australia) Boehringer Ingelheim International, Concert Pharmaceuticals, Inc., Epiodyne, Inc., Heptares Therapeutics, Limited (UK), Janssen Research & Development, L.E.K. Consulting, Otsuka America Pharmaceutical, Inc., Perception Neuroscience Holdings, Inc., Spring Care, Inc., Sunovion Pharmaceuticals, Inc., Takeda Industries,Taisho Pharmaceutical Co., Ltd. He is listed on the Scientific Advisory Boards of Bioasis Technologies, Inc., Biohaven Pharmaceuticals, BioXcel Therapeutics, Inc. (Clinical Advisory Board), BlackThorn Therapeutics, Inc., Broad Institute of MIT and Harvard, Cadent Therapeutics, Lohocla Research Corporation, Stanley Center for Psychiatric Research at the Broad Institute. Dr. Krystal holds stock in ArRETT Neuroscience, Inc., BlackThorn Therapeutics, Inc., Biohaven Pharmaceuticals Medical Sciences Spring Care, Inc., Sage Pharmaceuticals; Stock Options in Biohaven Pharmaceuticals Medical Sciences, Storm Biosciences, Inc. He receives Income Greater than $10,000 as Editor of Biological Psychiatry. He has Patents and Inventions in 1) Seibyl JP, Krystal JH, Charney DS. Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia. US Patent #5,447,948. September 5, 1995. 2) Vladimir, Coric, Krystal, John H, Sanacora, Gerard – Glutamate Modulating Agents in the Treatment of Mental Disorders US Patent No. 8,778,979 B2 Patent Issue Date: July 15, 2014. US Patent Application No. 15/695,164: Filing Date: 09/05/2017 3) Charney D, Krystal JH, Manji H, Matthew S, Zarate C., - Intranasal Administration of Ketamine to Treat Depression United States Application No. 14/197,767 filed on March 5, 2014; United States application or Patent Cooperation Treaty (PCT) International application No. 14/306,382 filed on June 17, 2014 4) Zarate, C, Charney, DS, Manji, HK, Mathew, Sanjay J, Krystal, JH, Department of Veterans Affairs “Methods for Treating Suicidal Ideation”, Patent Application No. 14/197.767 filed on March 5, 2014 by Yale University Office of Cooperative Research. 5) Arias A, Petrakis I, Krystal JH. – Composition and methods to treat addiction. Provisional Use Patent Application no.61/973/961. April 2, 2014. Filed by Yale University Office of Cooperative Research. 6) Chekroud, A., Gueorguieva, R., & Krystal, JH. “Treatment Selection for Major Depressive Disorder” [filing date 3rd June 2016, USPTO docket number Y0087.70116US00]. Provisional patent submission by Yale University. 7) Gihyun, Yoon, Petrakis I, Krystal JH – Compounds, Compositions and Methods for Treating or Preventing Depression and Other Diseases. U. S. Provisional Patent Application No. 62/444,552, filed on January10, 2017 by Yale University Office of Cooperative Research OCR 7088 US01. 8) Abdallah, C, Krystal, JH, Duman, R, Sanacora, G. Combination Therapy for Treating or Preventing Depression or Other Mood Diseases. U.S. Provisional Patent Application No. 047162–7177P1 (00754) filed on August 20, 2018 by Yale University Office of Cooperative Research OCR 7451 US01. He has NON Federal Research Support AstraZeneca Pharmaceuticals provides the drug, Saracatinib and Novartis provides maveglurant for research related to NIAAA grant “Center for Translational Neuroscience of Alcoholism [CTNA-4].
References
- Abdallah C, Jiang L, De Feyter HM, Fasula M, Krystal JH, Rothman DL, Mason GF, and G. S (2014). Glutamate metabolism in major depressive disorder. Am J Psych 171, 1320–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, DeWilde KE, Wong E, Anticevic A, Tang CY, et al. (2017). Ketamine Treatment and Global Brain Connectivity in Major Depression. Neuropsychopharmacology 42, 1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil H, Gordon J, Hen R, Javitch J, Mayberg H, McEwen B, Meaney MJ, and Nestler EJ (2018). Treatment resistant depression: A multi-scale, systems biology approach. Neuroscience and biobehavioral reviews 84, 272–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander RC (2017). The potential efficacy of GABAB antagonists in depression. Curr Opin Pharmacol 35, 101–104. [DOI] [PubMed] [Google Scholar]
- Ali AB, and Thomson AM (2008). Synaptic alpha 5 subunit-containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex. Cereb Cortex 18, 1260–1271. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, and Monteggia LM (2011). NMDA receptor blockade at rest triggers rapid behavioural anatidepressant responses. Nature 475, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajbouj M, Lisanby SH, Lang UE, Danker-Hopfe H, Heuser I, and Neu P (2006). Evidence for impaired cortical inhibition in patients with unipolar major depression. Biol Psychiatry 59, 395–400. [DOI] [PubMed] [Google Scholar]
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, and Sanacora G (2010). Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry 15, 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Lepack A, Fee C, Duric V, Maldonado-Aviles J, DiLeone R, Sibille E, Duman RS, and Sanacora G (2017). Characterization of GABAergic marker expression in the chronic unpredictable stress model of depression. Chronic Stress (Thousand Oaks) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, and Krystal JH (2000). Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47, 351–354. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, and Rubinow DR (2000). Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 157, 924–930. [DOI] [PubMed] [Google Scholar]
- Brainstorm C, Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L, Escott-Price V, Falcone GJ, Gormley P, et al. (2018). Analysis of shared heritability in common disorders of the brain. Science 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, Gross AL, Kroes RA, and Moskal JR (2013). GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 38, 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno FR, Donegan JJ, Boley AM, Shah A, DeGuzman M, Frazer A, and Lodge DJ (2016). Activation of a ventral hippocampus-medial prefrontal cortex pathway is both necessary and sufficient for an antidepressant response to ketamine. Mol Psychiatry 21, 1298–1308. [DOI] [PubMed] [Google Scholar]
- Castren E, and Kojima M (2017). Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiology of disease 97, 119–126. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, et al. (2006). Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 314, 140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CQ, Lur G, Morse TM, Carnevale NT, Ellis-Davies GC, and Higley MJ (2013). Compartmentalization of GABAergic inhibition by dendritic spines. Science 340, 759–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CQ, Martenson JS, Yamazaki M, Natsume R, Sakimura K, Tomita S, Tavalin SJ, and Higley MJ (2018). Input-Specific NMDAR-Dependent Potentiation of Dendritic GABAergic Inhibition. Neuron 97, 368–377 e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury GM, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, Bristow L, Schaeffer E, Duman RS, Rothman DL, et al. (2017). Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry 22, 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JY, Hagan CC, Murray GK, Graham JME, Ooi C, Tait R, Holt RJ, Elliott R, van Nieuwenhuizen AO, Bullmore ET, et al. (2017). Adolescent Major Depressive Disorder: Neuroimaging Evidence of Sex Difference during an Affective Go/No-Go Task. Front Psychiatry 8, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Yarkoni T, Repovs G, Anticevic A, and Braver TS (2012). Global connectivity of prefrontal cortex predicts cognitive control and intelligence. J Neurosci 32, 8988–8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, and Kaupmann K (2005). Don’t worry ‘B’ happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci 26, 36–43. [DOI] [PubMed] [Google Scholar]
- Czeh B, Simon M, van der Hart MG, Schmelting B, Hesselink MB, and Fuchs E (2005). Chronic stress decreases the number of parvalbumin-immunoreactive interneurons in the hippocampus: prevention by treatment with a substance P receptor (NK1) antagonist. Neuropsychopharmacology 30, 67–79. [DOI] [PubMed] [Google Scholar]
- Czeh B, Vardya I, Varga Z, Febbraro F, Csabai D, Martis LS, Hojgaard K, Henningsen K, Bouzinova EV, Miseta A, et al. (2018). Long-Term Stress Disrupts the Structural and Functional Integrity of GABAergic Neuronal Networks in the Medial Prefrontal Cortex of Rats. Front Cell Neurosci 12, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Varga ZK, Henningsen K, Kovacs GL, Miseta A, and Wiborg O (2015). Chronic stress reduces the number of GABAergic interneurons in the adult rat hippocampus, dorsal-ventral and region-specific differences. Hippocampus 25, 393–405. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S, Burkhalter A, Cauli B, Fairen A, Feldmeyer D, et al. (2013). New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci 14, 202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demenescu LR, Colic L, Li M, Safron A, Biswal B, Metzger CD, Li S, and Walter M (2017). A spectroscopic approach toward depression diagnosis: local metabolism meets functional connectivity. European archives of psychiatry and clinical neuroscience 267, 95–105. [DOI] [PubMed] [Google Scholar]
- Dienel SJ, and Lewis DA (2018). Alterations in cortical interneurons and cognitive function in schizophrenia. Neurobiology of disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douillard-Guilloux G, Lewis D, Seney ML, and Sibille E (2017). Decrease in somatostatin-positive cell density in the amygdala of females with major depression. Depress Anxiety 34, 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D’Souza DC, Gueorguieva R, He G, Ramachandran R, Suckow RF, et al. (2013). Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry 18, 1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS (2018). Ketamine and rapid-acting antidepressants: a new era in the battle against depression and suicide. F1000Research 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, and Krystal JH (2016). Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22, 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, and Monteggia LM (2006). A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59, 1116–1127. [DOI] [PubMed] [Google Scholar]
- Dwyer JM, Lepack AE, and Duman RS (2012). mTOR activation is required for the antidepressant effects of mGluR(2)/(3) blockade. Int J Neuropsychopharmacol 15, 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Stellbrink J, Treit D, and Dickson CT (2008). Anxiolytic and antidepressant effects of intracerebroventricularly administered somatostatin: behavioral and neurophysiological evidence. Neuroscience 157, 666–676. [DOI] [PubMed] [Google Scholar]
- Engin E, and Treit D (2009). Anxiolytic and antidepressant actions of somatostatin: the role of sst2 and sst3 receptors. Psychopharmacology (Berl) 206, 281–289. [DOI] [PubMed] [Google Scholar]
- Evans JW, Szczepanik J, Brutsche N, Park LT, Nugent AC, and Zarate CA Jr. (2018). Default Mode Connectivity in Major Depressive Disorder Measured Up to 10 Days After Ketamine Administration. Biol Psychiatry 84, 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee C, Banasr M, and Sibille E (2017). Somatostatin-Positive Gamma-Aminobutyric Acid Interneuron Deficits in Depression: Cortical Microcircuit and Therapeutic Perspectives. Biol Psychiatry 82, 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyissa AM, Chandran A, Stockmeier CA, and Karolewicz B (2009). Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Progress in neuro-psychopharmacology & biological psychiatry 33, 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnema SJ, Nabulsi NB, Eid T, Detyniecki K, Lin SF, Chen MK, Dhaher R, Matuskey D, Baum E, Holden D, et al. (2016). Imaging synaptic density in the living human brain. Science translational medicine 8, 348ra396. [DOI] [PubMed] [Google Scholar]
- Fischell J, Van Dyke AM, Kvarta MD, LeGates TA, and Thompson SM (2015). Rapid Antidepressant Action and Restoration of Excitatory Synaptic Strength After Chronic Stress by Negative Modulators of Alpha5-Containing GABAA Receptors. Neuropsychopharmacology 40, 2499–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J, and Kendler KS (2014). The Genetics of Major Depression. Neuron 81, 1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, and O’Keane V (2013). How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiology of disease 52, 24–37. [DOI] [PubMed] [Google Scholar]
- Frodl T, Schule C, Schmitt G, Born C, Baghai T, Zill P, Bottlender R, Rupprecht R, Bondy B, Reiser M, et al. (2007). Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch Gen Psychiatry 64, 410–416. [DOI] [PubMed] [Google Scholar]
- Fuchs T, Jefferson SJ, Hooper A, Yee P-HP, Maguire J, and Luscher B (2017). Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Molecular psychiatry 22, 920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Haney JR, Parikshak NN, Leppa V, Ramaswami G, Hartl C, Schork AJ, Appadurai V, Buil A, Werge TM, et al. (2018). Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 359, 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann M, and Bettler B (2012). Regulation of neuronal GABA(B) receptor functions by subunit composition. Nat Rev Neurosci 13, 380–394. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, Gordon E, Kemp AH, and Williams LM (2009). Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Molecular Psychiatry 14, 681–695. [DOI] [PubMed] [Google Scholar]
- Gerhard DM, and Duman RS (2018). Role of GABAergic interneuron GluN2B subunits on the antidepressant actions of ketamine in male and female mice. In Society for Neuroscience (San Diego, California). [Google Scholar]
- Ghosal S, Bang E, Yue W, Hare BD, Lepack AE, Girgenti MJ, and Duman RS (2018). Activity-Dependent Brain-Derived Neurotrophic Factor Release Is Required for the Rapid Antidepressant Actions of Scopolamine. Biol Psychiatry 83, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S, Hare B, and Duman RS (2017). Prefrontal Cortex GABAergic Deficits and Circuit Dysfunction in the Pathophysiology and Treatment of Chronic Stress and Depression. Current opinion in behavioral sciences 14, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glue P, Medlicott NJ, Harland S, Neehoff S, Anderson-Fahey B, Le Nedelec M, Gray A, and McNaughton N (2017). Ketamine’s dose-related effects on anxiety symptoms in patients with treatment refractory anxiety disorders. J Psychopharmacol 31, 1302–1305. [DOI] [PubMed] [Google Scholar]
- Godfrey KEM, Gardner AC, Kwon S, Chea W, and Muthukumaraswamy SD (2018). Differences in excitatory and inhibitory neurotransmitter levels between depressed patients and healthy controls: A systematic review and meta-analysis. J Psychiatr Res 105, 33–44. [DOI] [PubMed] [Google Scholar]
- Gold BI, Bowers MB Jr., Roth RH, and Sweeney DW (1980). GABA levels in CSF of patients with psychiatric disorders. Am J Psychiatry 137, 362–364. [DOI] [PubMed] [Google Scholar]
- Gray AL, Hyde TM, Deep-Soboslay A, Kleinman JE, and Sodhi MS (2015). Sex differences in glutamate receptor gene expression in major depression and suicide. Mol Psychiatry 20, 1139. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, and Schatzberg AF (2007). Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62, 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse G, Djalali S, Deng DR, Holtje M, Hinz B, Schwartzkopff K, Cygon M, Rothe T, Stroh T, Hellweg R, et al. (2005). Area-specific effects of brain-derived neurotrophic factor (BDNF) genetic ablation on various neuronal subtypes of the mouse brain. Brain Res Dev Brain Res 156, 111–126. [DOI] [PubMed] [Google Scholar]
- Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, Tseng GC, Lewis DA, and Sibille E (2012). Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry 17, 1130–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Neumeister A, van der Veen JW, Tumonis T, Bain EE, Shen J, Drevets WC, and Charney DS (2005). Normal prefrontal gamma-aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biol Psychiatry 58, 969–973. [DOI] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, and Drevets WC (2007). Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 64, 193–200. [DOI] [PubMed] [Google Scholar]
- Hedrick NG, Harward SC, Hall CE, Murakoshi H, McNamara JO, and Yasuda R (2016). Rho GTPase complementation underlies BDNF-dependent homo- and heterosynaptic plasticity. Nature 538, 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SA, Wamsteeker JI, Kurz EU, and Bains JS (2009). Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat Neurosci 12, 438–443. [DOI] [PubMed] [Google Scholar]
- Holmes SE, Scheinost D, Sjoerd FJ, Naganawa M, Davis MT, DellaGioia N, Nabulsi N, Matuskey D, Angarita GA, Pietrzak RH, et al. (2018). Lower synaptic density is associated with depression severity and network. Submitted. [DOI] [PMC free article] [PubMed]
- Howard DM, Adams MJ, Shirali M, Clarke TK, Marioni RE, Davies G, Coleman JRI, Alloza C, Shen X, Barbu MC, et al. (2018). Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat Commun 9, 1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, and Pizzagalli DA (2015). Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry 72, 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, Doherty J, Epperson CN, Deligiannidis KM, Riesenberg R, et al. (2017). Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet 390, 480–489. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, Lepack A, Majik MS, Jeong LS, Banasr M, et al. (2012). Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med 18, 1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, and Rajkowska G (2010). Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol 13, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karolewicz B, Szebeni K, Gilmore T, Maciag D, Stockmeier CA, and Ordway GA (2009). Elevated levels of NR2A and PSD-95 in the lateral amygdala in depression. Int J Neuropsychopharmacol 12, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Fogaca MV, Deyama S, Li XY, Fukumoto K, and Duman RS (2018). BDNF release and signaling are required for the antidepressant actions of GLYX-13. Mol Psychiatry 23, 2007–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, and Gelernter J (2006). Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry 59, 673–680. [DOI] [PubMed] [Google Scholar]
- Kendler KS, and Gardner CO (2014). Sex differences in the pathways to major depression: a study of opposite-sex twin pairs. Am J Psychiatry 171, 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Kim YH, and Yoon JS (2007). Interactions between life stressors and susceptibility genes (5-HTTLPR and BDNF) on depression in Korean elders. Biological Psychiatry 62, 423–428. [DOI] [PubMed] [Google Scholar]
- Koike H, Iijima M, and Chaki S (2011). Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology 61, 1419–1423. [DOI] [PubMed] [Google Scholar]
- Kolata SM, Nakao K, Jeevakumar V, Farmer-Alroth EL, Fujita Y, Bartley AF, Jiang SZ, Rompala GR, Sorge RE, Jimenez DV, et al. (2018). Neuropsychiatric Phenotypes Produced by GABA Reduction in Mouse Cortex and Hippocampus. Neuropsychopharmacology 43, 1445–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Anticevic A, Yang GJ, Dragoi G, Driesen NR, Wang XJ, and Murray JD (2017). Impaired Tuning of Neural Ensembles and the Pathophysiology of Schizophrenia: A Translational and Computational Neuroscience Perspective. Biol Psychiatry 81, 874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, Epperson CN, Goddard A, and Mason GF (2002). Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Molecular Psychiatry 7, S71–S80. [DOI] [PubMed] [Google Scholar]
- Labonte B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, Scarpa JR, Moy G, Loh YE, Cahill M, et al. (2017). Sex-specific transcriptional signatures in human depression. Nat Med 23, 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, Kelmendi B, Simen A, McMahon FJ, Sanacora G, and Zarate C (2012). Brain-Derived Neurotrophic Factor Val66Met Polymorphism and Antidepressant Efficacy of Ketamine in Depressed Patients. Biological psychiatry 72, e27–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, and Zarate CA Jr. (2017). Glutamate and Gamma-Aminobutyric Acid Systems in the Pathophysiology of Major Depression and Antidepressant Response to Ketamine. Biol Psychiatry 81, 886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepack A, Fuchikami M, Dwyer J, Banasr M, Aghajanian G, and Duman R (2014). BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, and Daskalakis ZJ (2010). Evidence of cortical inhibitory deficits in major depressive disorder. Biol Psychiatry 67, 458–464. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, and Duman RS (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329, 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, and Duman RS (2011). Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69, 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zou Q, He Y, and Yang Y (2013). Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci U S A 110, 1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LC, and Sibille E (2015). Somatostatin, neuronal vulnerability and behavioral emotionality. Mol Psychiatry 20, 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, and Aghajanian GK (2008). Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A 105, 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Duman C, Kato T, Hare B, Lopresto D, Bang E, Burgdorf J, Moskal J, Taylor J, Aghajanian G, and Duman RS (2016). GLYX-13 Produces Rapid Antidepressant Responses with Key Synaptic and Behavioral Effects Distinct from Ketamine. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, and Aghajanian GK (2012). Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 71, 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, and Fuchs T (2015). GABAergic control of depression-related brain states. Adv Pharmacol 73, 97–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Shen Q, and Sahir N (2011). The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 16, 383–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K, Xu A, Cui S, Sun MR, Xue YC, and Wang JH (2016). Impaired GABA synthesis, uptake and release are associated with depression-like behaviors induced by chronic mild stress. Transl Psychiatry 6, e910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag D, Hughes J, O’Dwyer G, Pride Y, Stockmeier CA, Sanacora G, and Rajkowska G (2010). Reduced density of calbindin immunoreactive GABAergic neurons in the occipital cortex in major depression: relevance to neuroimaging studies. Biol Psychiatry 67, 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie G, and Maguire J (2013). Neurosteroids and GABAergic signaling in health and disease. Biomol Concepts 4, 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie G, and Maguire J (2015). Chronic stress shifts the GABA reversal potential in the hippocampus and increases seizure susceptibility. Epilepsy Res 109, 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen G, and Frodl T (2011). The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry 16, 252–264. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Yucel K, Taylor VH, Macdonald K, and Joffe R (2008). Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol Psychiatry 64, 880–883. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA Jr., Du J, Schloesser RJ, McCammon J, Chen G, and Manji HK (2008). Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63, 349–352. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Li CJ, Gal Toth J, Bath KG, Jing D, Lee FS, and McEwen BS (2011). Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus 21, 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco J, and McEwen BS (2016). Sex in the brain: hormones and sex differences. Dialogues in clinical neuroscience 18, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Manji H, and Lu B (2007). New insights into BDNF function in depression and anxiety. Nat Neurosci 10, 1089–1093. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Schloesser RJ, Jimenez DV, Weinberger DR, and Lu B (2011). Activity-dependent brain-derived neurotrophic factor expression regulates cortistatininterneurons and sleep behavior. Mol Brain 4, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, and Nasca C (2015). Mechanisms of stress in the brain. Nat Neurosci 18, 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, and Milner TA (2017). Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res 95, 24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, and Morrison JH (2013). The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79, 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, and Gray JD (2016). Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology 41, 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15, 483–506. [DOI] [PubMed] [Google Scholar]
- Milak MS, Proper CJ, Mulhern ST, Parter AL, Kegeles LS, Ogden RT, Mao X, Rodriguez CI, Oquendo MA, Suckow RF, et al. (2016). A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder. Mol Psychiatry 21, 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller OH, Moran JT, and Hall BJ (2016). Two cellular hypotheses explaining the initiation of ketamine’s antidepressant actions: Direct inhibition and disinhibition. Neuropharmacology 100, 17–26. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, and Daly D (1997). Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17, 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, and Nestler EJ (2007). Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry 61, 187–197. [DOI] [PubMed] [Google Scholar]
- Moriguchi S, Takamiya A, Noda Y, Horita N, Wada M, Tsugawa S, Plitman E, Sano Y, Tarumi R, ElSalhy M, et al. (2018). Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, and Baxter MG (2012). The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci 13, 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JD, Anticevic A, Gancsos M, Ichinose M, Corlett PR, Krystal JH, and Wang XJ (2014). Linking microcircuit dysfunction to cognitive impairment: effects of disinhibition associated with schizophrenia in a cortical working memory model. Cereb Cortex 24, 859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Network, and Pathway Analysis Subgroup of Psychiatric Genomics, C. (2015). Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci 18, 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, and Sibille E (2014). Cortical GABA neurons and self-focus in depression: a model linking cellular, biochemical and neural network findings. Mol Psychiatry 19, 959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak B, Zadrozna M, Ossowska G, Sowa-Kucma M, Gruca P, Papp M, Dybala M, Pilc A, and Nowak G (2010). Alterations in hippocampal calcium-binding neurons induced by stress models of depression: a preliminary assessment. Pharmacol Rep 62, 1204–1210. [DOI] [PubMed] [Google Scholar]
- Oh H, Lewis DA, and Sibille E (2016). The Role of BDNF in Age-Dependent Changes of Excitatory and Inhibitory Synaptic Markers in the Human Prefrontal Cortex. Neuropsychopharmacology 41, 3080–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Piantadosi SC, Rocco BR, Lewis DA, Watkins SC, and Sibille E (2018). The Role of Dendritic Brain-Derived Neurotrophic Factor Transcripts on Altered Inhibitory Circuitry in Depression. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota KT, Liu RJ, Voleti B, Maldonado-Aviles JG, Duric V, Iwata M, Dutheil S, Duman C, Boikess S, Lewis DA, et al. (2014). REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med 20, 531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty F, Trivedi MH, Fulton M, and Rush AJ (1995). Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry 38, 578–591. [DOI] [PubMed] [Google Scholar]
- Piantadosi SC, French BJ, Poe MM, Timic T, Markovic BD, Pabba M, Seney ML, Oh H, Orser BA, Savic MM, et al. (2016). Sex-Dependent Anti-Stress Effect of an alpha5 Subunit Containing GABAA Receptor Positive Allosteric Modulator. Front Pharmacol 7, 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, and Sanacora G (2011). The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 13, 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM, and Group G-CS (2015). Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. Journal of psychiatric practice 21, 140–149. [DOI] [PubMed] [Google Scholar]
- Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, Murrough JW, Charney DS, and Mathew SJ (2009). Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry 65, 792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhu N, de Jesus DR, Ravindran LN, Zanjani A, Fitzgerald PB, and Daskalakis ZJ (2013). A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders. Clin Neurophysiol 124, 1309–1320. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, and Miguel-Hidalgo JJ (2007). GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology 32, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, and Stockmeier CA (2013). Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Current drug targets 14, 1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, and Luscher B (2016). Bidirectional Homeostatic Regulation of a Depression-Related Brain State by Gamma-Aminobutyric Acidergic Deficits and Ketamine Treatment. Biol Psychiatry 80, 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, and Chattarji S (2009). Stress, memory and the amygdala. Nat Rev Neurosci 10, 423–433. [DOI] [PubMed] [Google Scholar]
- Rubinow D, Girdler SS (2011). Hormones, heart disease, and health: individualized medicine versus throwing the baby out with the bathwater. Depress Anxiety 28, E1–E15. [DOI] [PubMed] [Google Scholar]
- Russo SJ, and Nestler EJ (2013). The brain reward circuitry in mood disorders. Nat Rev Neurosci 14, 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, Berman RM, Charney DS, and Krystal JH (1999). Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry 56, 1043–1047. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, and Popoli M (2012). Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62, 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, and Manji HK (2008). Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nature reviews Drug discovery 7, 426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, and Drevets WC (2009). Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neuroscience and biobehavioral reviews 33, 699–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidegger M, Henning A, Walter M, Lehmann M, Kraehenmann R, Boeker H, Seifritz E, and Grimm S (2016). Ketamine administration reduces amygdalohippocampal reactivity to emotional stimulation. Hum Brain Mapp 37, 1941–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, Boesiger P, Henning A, and Seifritz E (2012). Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One 7, e44799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller CE, Meltzer-Brody S, and Rubinow DR (2015). The role of reproductive hormones in postpartum depression. CNS Spectr 20, 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz JM, Knoflach F, Hernandez MC, and Bischofberger J (2018). Dendrite-targeting interneurons control synaptic NMDA-receptor activation via nonlinear alpha5-GABAA receptors. Nat Commun 9, 3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schur RR, Draisma LW, Wijnen JP, Boks MP, Koevoets MG, Joels M, Klomp DW, Kahn RS, and Vinkers CH (2016). Brain GABA levels across psychiatric disorders: A systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp 37, 3337–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney ML, Huo Z, Cahill K, French L, Puralewski R, Zhang J, Logan RW, Tseng G, Lewis DA, and Sibille E (2018). Opposite Molecular Signatures of Depression in Men and Women. Biol Psychiatry 84, 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney ML, Tripp A, McCune S, Lewis DA, and Sibille E (2015). Laminar and cellular analyses of reduced somatostatin gene expression in the subgenual anterior cingulate cortex in major depression. Neurobiology of disease 73, 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, McEwen BS, and Morrison JH (2009). Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex 19, 2479–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, and Morrison JH (2009). Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest. Brain Res 1293, 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y, Gado MH, and Kraemer HC (2003). Untreated depression and hippocampal volume loss. Am J Psychiatry 160, 1–3. [DOI] [PubMed] [Google Scholar]
- Sibille E, Morris HM, Kota RS, and Lewis DA (2011). GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol 14, 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Martinowich K, and Lee FS (2017). BDNF at the synapse: why location matters. Mol Psychiatry 22, 1370–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumier A, and Sibille E (2014). Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacology 39, 2252–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, and Rajkowska G (2004). Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry 56, 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, Chen MH, Li CT, Lin WC, Hong CJ, Gueorguieva R, Tu PC, Bai YM, Cheng CM, and Krystal JH (2017). Dose-Related Effects of Adjunctive Ketamine in Taiwanese Patients with Treatment-Resistant Depression. Neuropsychopharmacology 42, 2482–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Agrawal A, Bulik CM, Andreassen OA, Borglum AD, Breen G, Cichon S, Edenberg HJ, Faraone SV, Gelernter J, et al. (2018). Psychiatric Genomics: An Update and an Agenda. Am J Psychiatry 175, 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay R, Lee S, and Rudy B (2016). GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 91, 260–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp A, Kota RS, Lewis DA, and Sibille E (2011). Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiology of disease 42, 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, and Sibille E (2012). Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry 169, 1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, et al. (2006). Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163, 28–40. [DOI] [PubMed] [Google Scholar]
- Tye KM (2018). Neural Circuit Motifs in Valence Processing. Neuron 100, 436–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler AL, Felsky D, Viviano JD, Stojanovski S, Ameis SH, Szatmari P, Lerch JP, Chakravarty MM, and Voineskos AN (2018). BDNF-Dependent Effects on Amygdala-Cortical Circuitry and Depression Risk in Children and Youth. Cereb Cortex 28, 1760–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2017). WHO. WHO | Depression [Internet]. Depression. [Google Scholar]
- Widman AJ, and McMahon LL (2018). Disinhibition of CA1 pyramidal cells by low-dose ketamine and other antagonists with rapid antidepressant efficacy. Proc Natl Acad Sci U S A 115, E3007–E3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, Adams MJ, Agerbo E, Air TM, Andlauer TMF, et al. (2018). Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 50, 668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Baker DA, Shen H, Carson DS, and Kalivas PW (2002). Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther 300, 162–171. [DOI] [PubMed] [Google Scholar]
- Xu H, Jeong HY, Tremblay R, and Rudy B (2013). Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron 77, 155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Qu Y, Abe M, Nozawa D, Chaki S, and Hashimoto K (2017). (R)-Ketamine Shows Greater Potency and Longer Lasting Antidepressant Effects Than Its Metabolite (2R,6R)-Hydroxynorketamine. Biol Psych 85, e43–e44. [DOI] [PubMed] [Google Scholar]
- Yeung M, Engin E, and Treit D (2011). Anxiolytic-like effects of somatostatin isoforms SST 14 and SST 28 in two animal models (Rattus norvegicus) after intraamygdalar and intra-septal microinfusions. Psychopharmacology (Berl) 216, 557–567. [DOI] [PubMed] [Google Scholar]
- Zadrozna M, Nowak B, Lason-Tyburkiewicz M, Wolak M, Sowa-Kucma M, Papp M, Ossowska G, Pilc A, and Nowak G (2011). Different pattern of changes in calcium binding proteins immunoreactivity in the medial prefrontal cortex of rats exposed to stress models of depression. Pharmacol Rep 63, 1539–1546. [DOI] [PubMed] [Google Scholar]
- Zanos P, and Gould TD (2018). Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 23, 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Nelson ME, Highland JN, Krimmel SR, Georgiou P, Gould TD, and Thompson SM (2017). A Negative Allosteric Modulator for alpha5 Subunit-Containing GABA Receptors Exerts a Rapid and Persistent Antidepressant-Like Action without the Side Effects of the NMDA Receptor Antagonist Ketamine in Mice. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, and Manji HK (2006). A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63, 856–864. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Paul SM, Izumi Y, Covey DF, and Mennerick S (2013). Neurosteroids, stress and depression: potential therapeutic opportunities. Neuroscience and biobehavioral reviews 37, 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]