Abstract
Background:
Transplant-associated thrombotic microangiopathy (TA-TMA) after allogeneic hematopoietic cell transplantation (HCT) has not been well-characterized in large population studies with clinically adjudicated cases.
Methods:
We performed a retrospective cohort study of adults who underwent allogeneic HCT between 2006 and 2015 to determine the incidence of and risk factors for TA-TMA, and to describe its natural history and response to immunosuppressant withdrawal management.
Results:
Among 2145 patients in this study, 192 developed TA-TMA with a cumulative incidence of 7.6% by 100 days post-transplant. Independent pre-transplant risk factors included the receipt of a second (or third) allogeneic HCT, HLA-mismatched donor, and myeloablative conditioning with or without total body irradiation (TBI); post-transplant risk factors included the antecedent development of acute graft-versus-host disease (GVHD), diffuse alveolar hemorrhage (DAH), bacteremia, invasive aspergillosis, BK viremia, and higher sirolimus trough level. Among TA-TMA patients, 27% achieved hematologic resolution and 57% remained alive as of 90 days following diagnosis. Antecedent risk factors stratified patients into different survival groups and immunosuppressant withdrawal alone did not improve patient outcomes.
Conclusion:
TA-TMA is a heterogenous disease that occurs after allogeneic transplantation. Management with immunosuppressant withdrawal does not impact patient outcomes. Until further evidence becomes available, the management of TA-TMA should focus on the treatment of underlying diseases.
Introduction:
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative treatment modality for patients with hematologic malignancies. Despite improvement in patient outcomes due to improved regimen selection and supportive care, acute regimen-related toxicities remain a major source of morbidity and mortality. Transplant-associated thrombotic microangiopathy (TA-TMA) is a known complication of allogeneic HCT. It is an endothelial disorder that manifests with microangiopathic hemolytic anemia (MAHA), thrombocytopenia, and microvascular thrombosis1 and is a member of the family of thrombotic microangiopathies including thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS). TA-TMA is associated with high mortality.1,2
Since the initial recognition of TA-TMA in the 1980s, the reported incidence and risk factors have varied significantly.3 A systematic review in 2004 summarized 35 published case series of TA-TMA to date. The cumulative incidence of the disease varied from 0.5% to 63.6% (average 8.2%) and the case-fatality varied from 0% to 100%.4 Cohort studies since 2004 have reported an incidence ranging from 4–39% and a case fatality of 50% or more in allogeneic transplant recipients.5–11 An admixture of source populations with different indications for allogeneic HCT, misclassification of TA-TMA outcomes, and the small number of patients likely all contribute to the imprecise estimates of incidence and the inconsistent associations with risk factors described in earlier studies.12 The above notwithstanding, the presence of GVHD has been observed to be associated with the development of TA-TMA with varying magnitude. In addition, administration of calcineurin inhibitors (CNI) or sirolimus has been implicated in some but not all studies as a potential risk factor.8,13–15 An ongoing debate is whether TMA is caused by GVHD or CNI/sirolimus given the associated management differs drastically.
In addition to the lack of understanding of the etiologies, there is no Food and Drug Administration approved treatment. The management of TA-TMA is mostly supportive, including the withdrawal of suspected medications and treatment of underlying infections and graft-versus-host disease (GVHD).1 Existing clinical guidelines recommend discontinuation of calcineurin inhibitors (CNI) as the primary intervention after initial diagnosis.16 However, there is no evidence that this strategy actually improves patient outcomes and may risk inciting or exacerbating GVHD. A dedicated comparative effectiveness study using the experience of a historical cohort without TMA-directed treatment is needed.
To better understand the roles of GVHD and immunosuppressive regimen on the development and resolution of TA-TMA, we performed a retrospective cohort study of 2145 adult patients who underwent allogeneic HCT at a single center.
Methods:
Study Design and Setting
We studied patients who underwent an allogeneic HCT from 1/1/2006 to 12/31/2015 at the Fred Hutchinson Cancer Research Center (FHCRC). The study was approved by the FHCRC institutional review board. Consecutive adult patients were followed from the time of hematopoietic cell infusion until the time of death, loss-to-follow-up (30-day laboratory-free and visit-free gap), or the end of study on 1/1/2017. For patients with multiple HCTs during the study period, only the first HCT observation period was included. For patients with an allogeneic HCT prior to 2006 or performed at an outside institution, their first HCT during this observation period was termed a “subsequent HCT.”
TMA Case Definition, Ascertainment and Validation
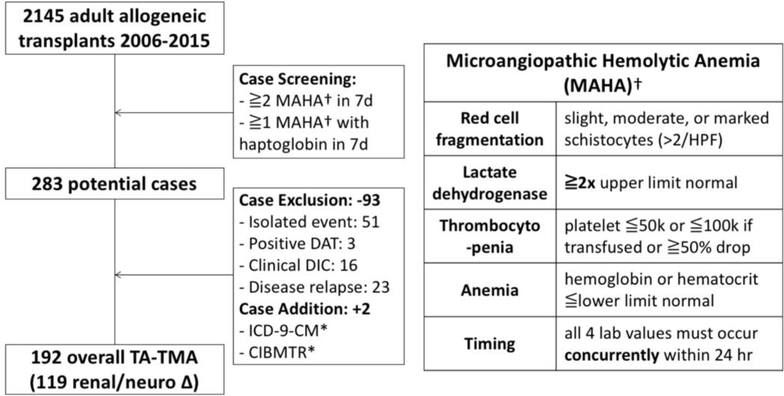
We defined TA-TMA as persistent microangiopathic hemolytic anemia (MAHA) without coagulopathy related to disseminated intravascular coagulation (DIC); this was further sub-classified as overall-TMA and definite-TMA according to prior definitions where definite-TMA was characterized by the presence of acute kidney injury (AKI as ≥2 times pre-conditioning creatinine) or neurologic dysfunction (global or focal neurologic changes that prompted further neurologic imaging and/or admission) within 30 days of disease onset.1,12 In order to ascertain overall-TMA outcome, we conducted a three-step approach with laboratory screening, clinical chart review and adjudication, and cross-reference (Figure 1, Table S1). Laboratory measures as part of screening including complete blood count, red cell morphology assessment (schistocytes), and lactate dehydrogenase (LDH) were measured daily as inpatient or three-times weekly as outpatient.
Figure 1. Flow diagram for population and case selection.
This flow diagram shows the cohort (eligible transplant patients) and case (TA-TMA) selection for this study. TA-TMA was defined as persistent microangiopathic hemolytic anemia (MAHA) without coagulopathy related to diffuse intravascular coagulation (DIC). MAHA was defined as a combination of red cell fragmentation (schistocytosis), elevated lactate dehydrogenase, thrombocytopenia, and anemia occurring within 24 hours. Cases were selected from a combination of electronic screening mechanism and clinical chart review validation. Cases with isolated laboratory events, positive DAT, clinical diagnosis of DIC, or early relapsed disease were excluded during the validation to avoid incorrect attribution to TA-TMA. Additional cases were added from ICD-9-CM codes for “hemolytic uremic syndrome” and “thrombotic microangiopathy,” and CIBMTR registry reports of “post-transplant microangiopathy” from forms 2100 and 2200. * DAT: direct antiglobulin test, DIC: diffuse intravascular coagulation, ICD-9-CM: International Classification of Diseases, Ninth Revision, Clinical Modification, CIBMTR: Center for International Blood and Marrow Transplant Research
Risk Factor Ascertainment
We extracted information on baseline characteristics of the donor and the recipient. These included age, sex, and race for both, and for the patient, comorbidity index (HCT-CI),17,18 disease type, donor match and graft source, conditioning regimen including the use of total body irradiation (TBI), initial graft-versus-host disease (GVHD) prophylaxis regimen, and pre-conditioning laboratory values.
We obtained information on post-transplant complications from transplant databases. Acute GVHD was defined and graded according to established criteria.19,20 Diffuse alveolar hemorrhage (DAH) was defined by the presence of serial lavage blood returns on bronchoscopy plus a suspected clinical and/or radiographic diagnosis without an attributable bacterial, fungal or viral cause. Infections – including bacteremia, aspergillosis, cytomegalovirus (CMV) reactivation, BK viremia, adenovirus infection, human herpesvirus 6 (HHV6) infection, or Epstein-Barr virus (EBV) infection - were ascertained from a prospectively collected surveillance database according to standardized definitions.
TMA Cohort Characteristics
For patients meeting the diagnostic criteria for overall-TMA, we extracted patient and laboratory information at the onset of disease and clinical outcomes within 30 days of diagnosis. We also documented whether there was a strong suspicion or diagnosis of TMA by the clinical team, as well as any preceding clinical events within a 14-day window that could have triggered the onset of the disease. The antecedent conditions ascertained a-priori included infection causing systemic sepsis or shock, diffuse alveolar hemorrhage (DAH), acute and/or refractory graft-versus-host disease (GVHD), and idiopathic/drug (Table S2). For patients with multiple possible competing events, we assigned the most salient clinical condition in a descending order of infection, DAH, GVHD, and idiopathic/drug category.
TMA Management and Outcomes
We enumerated the management strategies employed, including CNI or sirolimus withdrawal, eculizumab administration, or plasma exchange within 30 days of diagnosis. We classified patients as the CNI/sirolimus withdrawal cohort if they had switched or stopped their existing drug within 30 days after TA-TMA diagnosis. Hematologic resolution was defined as concurrent and ongoing achievement of LDH <1.5 times ULN, platelet >50,000/uL (or transfusion independence), and no schistocytosis within 90 days or discharge, whichever occurred first. We assessed overall survival from all available records and censored at the time of last patient contact.
Statistical Methods
The incidence of TA-TMA during the first 100 days following HCT was determined by the cumulative incidence function while treating both death and relapse as competing risks. The risk factors for TA-TMA were assessed by cause-specific Cox regression models. Pre-transplant baseline variables tested included number of prior transplants, age, sex, disease type, co-morbidity index, donor type and HLA-match, conditioning regimen, and GVHD prophylaxis. Donor source (bone marrow, peripheral blood, cord blood) was not assessed due to strong collinearity with HLA-match. Post-transplant variables tested included antecedent acute GVHD, DAH, and various systemic infections. In the adjusted multivariable analyses using cause-specific Cox models, the association of each pretransplant risk factor was only adjusted by other baseline covariates; all post-transplant risk factors were treated as time-varying covariates and adjusted by both baseline covariates and other time varying covariates. Interactions were not assessed. The proportionality assumption for Cox models was checked by Schoenfeld residuals.
To examine the impact of the time-varying levels of immunosuppressant drug on TMA, patients were divided into 3 subgroups based on the choice of initial GVHD prophylaxis. For each subgroup, CNI/sirolimus exposure was either defined as an average of the previous 7-day trough levels (continuous variable) or as time-above-peak (binary variable for tacrolimus >15 ng/mL, cyclosporine >450 ng/mL, or sirolimus >10 ng/mL). Drug trough levels were usually measured 3 times weekly as inpatient or outpatient until time of final discharge from the FHCRC transplant service. Interval trough levels between checks were imputed via linear interpolation. Extended Cox regression models were built to examine the time-varying association between CNI/sirolimus exposures and TMA after adjusting for the onset and grade of GVHD.
The median follow-up time was determined by the reverse Kaplan-Meier method. The overall survival of TA-TMA patients was assessed by Kaplan-Meier curves where different antecedent conditions were compared by multigroup log-rank test. The impact of CNI/sirolimus withdrawal on TA-TMA outcomes was assessed after non-parametric calibration inverse weighting (R package ATE). The calibration weighting approach is similar to but more robust compared to the inverse propensity score of treatment weighting (IPTW) approach.21 Potential confounders included in the weighting model are shown in Table S3. The pre- and post-calibration weighted balances were checked by standardized differences (SD).22 Hematologic resolution was assessed as a binary average treatment effect (ATE) using the calibration model-specific estimator. Overall survival was compared in the treated and untreated groups using weighted Cox regression models with robust variance estimator. To prevent potential immortal time bias, immunosuppressant withdrawal was treated as a time-varying covariate in the final adjusted model. Statistical analyses were performed in Stata 14.2 and R 3.4.4.
Results:
Transplant Population and TA-TMA Patients
Over the 10-year period, 2145 consecutive adult allogeneic HCT patients were identified from the FHCRC database, of whom 80% had their first-ever allogeneic HCT (Table 1). The indication for HCT was myeloid malignancy in 61%, lymphoid malignancy in 36%, and non-malignant conditions in 4% of patients. The mean age was 51 years, and 70% had one or more comorbidities. Ninety percent of patients were white, and 42% were female. Approximately 42% of patients received a reduced intensity conditioning regimen. Nearly all patients received CNI-based GVHD prophylaxis (98%), and 6% of patients received concurrent sirolimus with CNI.
Table 1.
Baseline patient characteristics
| Population (n=2145) | |
|---|---|
| Demographics | |
| __first allogeneic transplant, % (n) | 80% (1712) |
| __age in years, mean (sd) | 51 (13.3) |
| __female, % (n) | 42% (894) |
| __white, % (n) | 90% (1833) |
| Comorbidity Index, % (n) | |
| __HCT-CI score 0 | 13% (276) |
| __HCT-CI score 1–2 | 25% (543) |
| __HCT-CI score >2 | 45% (966) |
| __missing | 17% (360) |
| Disease Type, % (n) | |
| __myeloid | 61% (1299) |
| __lymphoid | 36% (770) |
| __non-malignant | 4% (75) |
| Donor Match, % (n) | |
| __matched related (MRD) | 31% (674) |
| __matched unrelated (MUD) | 42% (907) |
| __mismatched related (MMRD) | 6% (129) |
| __mismatched unrelated (MMUD) | 12% (250) |
| __umbilical cord blood (UCB) | 9% (185) |
| Conditioning Regimen, % (n) | |
| __non-myeloablative (reduced intensity) | 42% (909) |
| __myeloablative without high-dose TBI | 41% (883) |
| __myeloablative with high-dose TBI (≧1200 cGy) | 16% (353) |
| GVHD Prophylaxis, % (n) | |
| __tacrolimus + | 53% (1136) |
| __cyclosporine + | 39% (838) |
| __sirolimus + tacrolimus or cyclosporine | 6% (133) |
| __cyclophosphamide | 2% (38) |
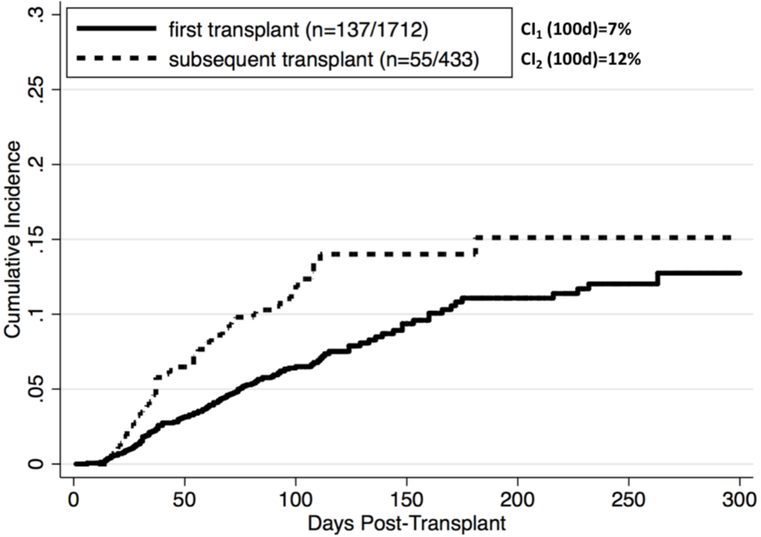
The median follow-up time for the cohort was 99 days for TA-TMA ascertainment (with a follow-up time of 781 days for the entire cohort). The initial laboratory screening methods identified 283 potential cases, and subsequent clinical chart review confirmed 192 validated overall-TMA cases (and 119 definite-TMA cases) (Figure 1). The positive predictive values (PPV) of the primary and alternative outcome ascertainment methods are shown in Table S1. The median time to overall-TMA onset was 59 days (IQR 33–90 days). The cumulative incidence of TA-TMA was 7% and 12% by 100 days for first and subsequent allogeneic HCT recipients, respectively, with an overall incidence of 7.6% (Figure 2). The demographics of 192 patients diagnosed with TA-TMA are shown in Table 4. At initial TA-TMA presentation, 24% of patients had neurologic dysfunction necessitating imaging or further work-up; 36% of patients had a serum creatinine ≥1.5-fold from baseline (and 16% with ≥2-fold increase), and 68% had proteinuria on dipstick. None of the patients tested had severe deficiency of ADAMTS13 activity and 71% had low or undetectable haptoglobin. All patients were receiving CNI or sirolimus at the onset of TA-TMA (52% tacrolimus, 35% cyclosporine, 13% sirolimus plus either tacrolimus or cyclosporine). The mean highest troughs over the preceding 2-week window were 15.2 ng/mL, 423 ng/mL, and 9.0 ng/mL for tacrolimus, cyclosporine, and sirolimus, respectively.
Figure 2. Incidence of TA-TMA after allogeneic transplantation.
Cumulative incidence (CI) was assessed using the competing risk method where death and disease relapse were treated as competing risks. The overall CI was 7.6% by day 100 post-transplant. CI (100d) was 7% for 1712 patients with first allogeneic transplant and 12% for 433 patients with subsequent transplant. The incidence rates (IR) were highest in the first 100 days. IR (100d) was 73/100,000 person-days in first transplant and 137/100,000 person-days in subsequent transplant.
Table 4.
Patient characteristics at the onset of TA-TMA
| Overall TMA (n=192) | |
|---|---|
| Demographics | |
| __first allogeneic transplant, % (n) | 71% (137) |
| __age in years, mean (sd) | 50 (13) |
| __female, % (n) | 44% (84) |
| __white, % (n) | 85% (153) |
| __HCT-CI score >2, % (n) | 59% (113) |
| __lymphoid malignancy, % (n) | 44% (83) |
| __mismatched donor, % (n) | 41% (79) |
| __myeloablative conditioning, % (n) | 60% (115) |
| Characteristics at onset of TMA | |
| __time after transplant (days), median (iqr) | 59 (33–90) |
| __proportion meeting “definite” TMA, % (n) | 62% (119) |
| __neurologic deficit, % (n) | 24% (46) |
| __creatinine (mg/dL), mean (sd) | 1.4 (0.9) |
| __lactate dehydrogenase (U/L), mean (sd) | 613 (279) |
| __platelet (x10^9/L), mean (sd) | 42 (21) |
| __hemoglobin (g/dL), mean (sd) | 9.7 (1.1) |
| __total bilirubin (g/dL), mean (sd) | 2.5 (4.1) |
| __international normalized ratio (INR), mean (sd) | 1.3 (0.6) (n=167) |
| __haptoglobin low/undetectable, % (n) | 71% (79) (n=111) |
| __direct Coombs test negative, % (n) | 100% (67) (n=67) |
| __ADAMTS13 activity <10, % (n) | 0% (0) (n=8) |
| __proteinuria (1–3+ on urine dipstick), % (n) | 68% (110) (n=162) |
| GVHD prophylaxis at onset of TMA | |
| __tacrolimus (TAC), % (n) | 51% (97) |
| ___highest TAC trough within last 2 weeks (ng/mL) | 15 (6) |
| __cyclosporine (CSP), % (n) | 35% (68) |
| ___highest CSP trough within last 2 weeks (ng/mL) | 421 (170) |
| __sirolimus (SIR) + TAC or CSP, % (n) | 13% (25) |
| ___highest SIR trough within last 2 weeks (ng/mL) | 9 (4) |
Risk Factors for TA-TMA
Several pre-transplant risk factors were independently associated with TA-TMA (Table 2): the receipt of a subsequent allogeneic HCT compared to first-ever HCT (HR 2.24, 1.48–3.41), the use of a mismatched donor (HR 2.74, 1.47–5.12 for mismatched related; HR 2.41, 1.52–3.83 for mismatched unrelated; HR 2.13, 1.19–3.80 for umbilical cord blood) compared to a matched related donor, and the receipt of myeloablative conditioning (HR 2.14, 1.38–3.32 for myeloablative without high-dose TBI; HR 2.81, 1.57–5.02 for myeloablative with high-dose TBI) compared to a reduced-intensity conditioning (RIC) regimen. There was no difference for patients with or without TBI. None of the different baseline GVHD prophylaxis regimens (tacrolimus, cyclosporine, sirolimus), patient demographics or comorbidities was associated with TA-TMA.
Table 2.
TA-TMA occurrence in relation to pre- and post-transplant risk factor exposures
| Crude HR (95% CI) for TMA | Adjusted HR (95% CI) for TMA | |
|---|---|---|
| Number of Transplant | ||
| __first allogeneic (n=1712) | 1 | 1 |
| __subsequent allogeneic (n=433) | 1.67 (1.22–2.28) | 2.24 (1.48–3.41) |
| Age | ||
| __Age (continuous increase for every 10 years) | 0.95 (0.85–1.05) | 1.09 (0.96–1.25) |
| Gender | ||
| __male (n=1250) | 1 | 1 |
| __female (n=894) | 1.08 (0.82–1.44) | 1.06 (0.80–1.43) |
| Disease Type | ||
| __myeloid (n=1299) | 1 | 1 |
| __lymphoid (n=769) | 1.37 (1.03–1.84) | 1.24 (0.84–1.82) |
| __non-malignant (n=75) | 1.63 (0.83–3.23) | 1.91 (0.93–3.91) |
| Co-Morbidity Index (HCT-CI) | ||
| __0 (n=276) | 1 | 1 |
| __1 (n=543) | 1.41 (0.81–2.45) | 1.28 (0.73–2.22) |
| __2+ (n=966) | 1.52 (0.91–2.56) | 1.41 (0.84–2.39) |
| __missing (n=360) | 1.51 (0.85–2.70) | 1.46 (0.82–2.63) |
| Donor Match | ||
| __matched related (MRD) (n=674) | 1 | 1 |
| __matched unrelated (MUD) (n=907) | 1.22 (0.84–1.79) | 1.24 (0.84–1.83) |
| __mismatched related (MMRD) (n=129) | 2.24 (1.28–3.94) | 2.74 (1.47–5.12) |
| __mismatched unrelated (MMUD) (n=250) | 2.27 (1.46–3.55) | 2.41 (1.52–3.83) |
| __mismatched umbilical cord blood (UCB) (n=185) | 2.49 (1.53–4.06) | 2.13 (1.19–3.80) |
| Conditioning Regimen | ||
| __non-myeloablative (reduced intensity) (n=908) | 1 | 1 |
| __myeloablative without high-dose TBI (n=883) | 1.04 (0.76–1.42) | 2.14 (1.38–3.32) |
| __myeloablative with high-dose TBI (≧ 1200 cGy) (n=353) | 1.34 (0.91–1.96) | 2.81 (1.57–5.02) |
| Baseline GVHD Prophylaxis | ||
| __tacrolimus + (n=1136) | 1 | 1 |
| __cyclosporine + (n=837) | 1.26 (0.94–1.70) | 1.44 (0.97–2.15) |
| __sirolimus + tacrolimus or cyclosporine (n=133) | 1.29 (0.73–2.26) | 1.59 (0.82–3.07) |
| __cyclophosphamide (n=38) | 1.06 (0.34–3.35) | 1.51 (0.47–4.88) |
| Acute GVHD | ||
| __none or Grade 1 | 1 | 1 |
| __grade 2 (n=1179) | 2.59 (1.66–4.03) | 2.65 (1.67–4.20) |
| __grade 3 (n=190) | 12.24 (7.76–19.30) | 9.54 (5.82–15.64) |
| __grade 4 (n=62) | 37.68 (23.10–61.46) | 26.74 (15.66–45.68) |
| DAH | ||
| __none | 1 | 1 |
| __DAH (n=50) | 13.49 (8.64–21.06) | 7.28 (4.37–12.13) |
| Infections | ||
| __none | 1 | 1 |
| __bacteremia (n=788) | 2.70 (2.02–3.61) | 1.52 (1.11–2.10) |
| __aspergillosis (n=253) | 4.72 (3.43–6.49) | 2.23 (1.56–3.18) |
| __CMV reactivation (n=987) | 1.52 (1.13–2.06) | 1.11 (0.81–1.52) |
| __BK viremia (n=149) | 4.53 (3.02–6.80) | 2.67 (1.74–4.09) |
| __HHV6 infection (n=62) | 3.54 (2.01–6.24) | 1.85 (0.99–3.43) |
| __adenovirus infection (n=40) | 3.34 (1.47–7.60) | 1.03 (0.44–2.45) |
| __EBV reactivation (n=54) | 4.09 (2.14–7.82) | 1.26 (0.61–2.60) |
Pre-transplant risk factors (number of transplants, age, gender, disease type, co-morbidity index, donor match, conditioning regimen, baseline GVHD prophylaxis) were assessed as baseline covariates. The adjusted HR for these covariates showed the adjustment for other baseline covariates only.
Post-transplant risk factors (GVHD, DAH, infections) were assessed as time-varying covariates. The adjusted HR for these covariates showed the adjustment for all baseline covariates and other time-varying covariates.
GVHD: graft-versus-host disease, DAH: diffuse alveolar hemorrhage, CMV: cytomegalovirus, BK: BK polyomavirus, HHV6: human herpesvirus 6, EBV: Epstein-Barr virus
Many post-transplant risk factors were independently associated with TA-TMA (Table 2). The onset of acute GVHD had the strongest association (HR 2.65, 1.67–4.20 for grade 2; HR 9.54, 5.82–15.64 for grade 3; HR 26.74, 15.66–45.68 for grade 4, relative to no GVHD or grade 1 disease). For patients with concurrent GVHD and subsequent TA-TMA, the median time between the two events was 39 days (IQR 18–63). When specific organ involvement was assessed instead of clinical grading in the adjusted analysis, gastrointestinal (HR 3.26, 2.16–4.91) and liver GVHD (HR 2.93, 1.98–4.32) retained their association with TA-TMA whereas skin GVHD did not (HR 1.23, 0.89–1.70). Other notable post-HCT risk factors for subsequent TA-TMA included the presence of DAH (HR 7.28, 4.37–12.13), bacteremia (HR 1.52, 1.11–2.10), invasive aspergillosis (HR 2.23, 1.56–3.18), and BK viremia (HR 2.67, 1.744.09). Both pre- and post-transplant risk factors remained unchanged in a sensitivity analysis where outcomes were restricted to 119 patients with definite-TMA (data not shown).
In the subgroup analysis for individual immunosuppressant drugs, the median (IQR) trough levels for tacrolimus, cyclosporine, and sirolimus were 9 ng/mL (7–12), 283 ng/mL (206–372), and 5 ng/mL (4–7), respectively. With or without adjusting for GVHD, higher trough levels of tacrolimus and cyclosporine (either as an average level or time-above-peak) were not associated with an increased risk of TMA (Table 3). However, higher sirolimus trough levels were associated with an appreciable risk of TMA (HR 1.44, 1.16–1.179, for every 1 ng/mL increase in sirolimus average trough level; HR 3.23, 0.62–16.80, for time above 10 ng/mL).
Table 3.
The association between calcineurin inhibitor and sirolimus trough level over time and the risk of TA-TMA
| Unadjusted HR (95% CI) for TMA | Adjusted HR (95% CI) for TMA* | |
|---|---|---|
| Tacrolimus only patients (n=1136) | ||
| __every 1 ng/mL increase in tacrolimus trough (7d average) | 1.02 (0.95–1.09) | 1.04 (0.97–1.11) |
| __discrete time above tacrolimus trough >15 ng/mL | 1.22 (0.66–2.25) | 1.18 (0.64–2.17) |
| Cyclosporine only patients (n=837) | ||
| __every 100 ng/mL increase in cyclosporine trough (7d average) | 0.96 (0.73–1.27) | 0.97 (0.74–1.27) |
| __discrete time above cyclosporine trough >450 ng/mL | 0.91 (0.45–1.86) | 0.82 (0.41–1.66) |
| Sirolimus + tacrolimus or cyclosporine patients (n=133) | ||
| __every 1 ng/mL increase in sirolimus trough (7d average) | 1.43 (1.16–1.76) | 1.44 (1.16–1.79) |
| __discrete time above sirolimus trough >10 ng/mL | 3.59 (0.77–16.71) | 3.23 (0.62–16.80) |
Adjusted by onset and grade of GVHD.
Natural History and Prognosis of TA-TMA
There was variability in clinical recognition and disease management of TA-TMA. Only 36% of patients (n=70) were clinically recognized by a provider at the time of TA-TMA onset (Table 4) and the remaining cases were retrospectively recognized based on the diagnostic and adjudication criteria as described above. Interestingly, patients with clinically recognized TA-TMA had similar resolution and survival outcomes as the remaining cases (data not shown). Immunosuppressant withdrawal was the most common management strategy employed. Few patients received adjunct or experimental therapy such as plasma exchange (n=1) or eculizumab (n=2). Over the course of 1 month following the diagnosis of TA-TMA, approximately 29% (n=56) of patients developed neurologic deficit, 46% (n=89) of patients developed AKI, 42% (n=80) of patients were admitted to the intensive care unit (ICU), 29% (n=55) required intubation, and 11% (n=21) underwent hemodialysis.
Hematologic resolution was achieved in 27% (n=51) of patients by 90 days or discharge home although the overall survival was 57% (95% CI 50–64) by 90 days. Antecedent risk factors stratified patients into different prognostic groups. The estimated 90-day survival was 96% (95% CI 75–99) for patients whose TA-TMA onset was precipitated by an unknown reason or immunosuppressant only; 62% (95% CI 52–71) for patients with acute GVHD as the precipitating event; 41% (95% CI 26–55) for those with a systemic infection; and 23% (95% CI 8–41) in those with DAH (Figure 3).
Figure 3. Prognosis (overall survival) for patients diagnosed with TA-TMA according to antecedent conditions.
The most salient antecedent condition at the time of TA-TMA diagnosis stratified patients into different prognostic groups (multigroup log rank test P<0.001).
Effectiveness of Immunosuppressant Withdrawal on TA-TMA Resolution
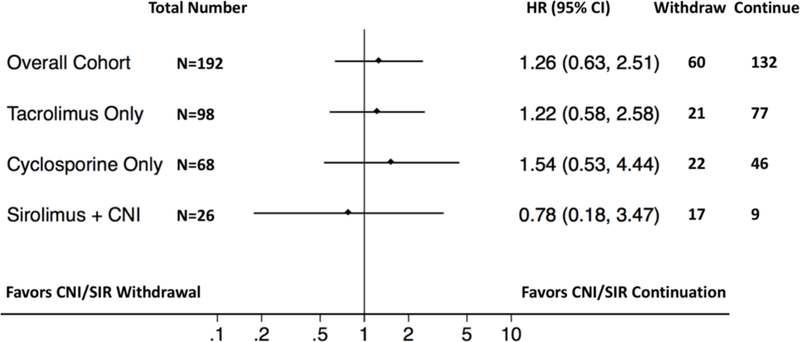
Among patients whose immunosuppressant was withdrawn, cyclosporine was the most commonly stopped drug (n=29) followed by tacrolimus (n=22) and sirolimus (n=9). After initial drug cessation, 29 switched to another type of CNI/sirolimus and 31 stopped the drug completely. Due to the small sample size, we combined the stop and switch into one “withdrawal” group for analysis. The median time from diagnosis to drug withdrawal was 5 days, and 75% stopped within 2 weeks. Prior to calibration weighting, CNI/sirolimus withdrawal was associated (with SD >0.30) with many confounders such as younger age, female, the presence of neurologic deficits, early clinical recognition of TA-TMA, absence of recent infections, and the use of sirolimus combination immunosuppression regimens (Table S3). After calibration weighting adjustment, all confounders were equalized between the two groups. The adjusted analysis showed that hematologic resolution occurred in 28% (95% CI 19–37) and 29% (95% CI 20–37) of patients in the withdrawal and continuation groups, respectively. The HR for mortality for the withdrawal versus continuation groups was not appreciably different in either the unadjusted (HR 0.93, 95% CI 0.65–1.35) or the adjusted analysis (HR 1.26, 95% CI 0.632.51) (Figure 4). In an exploratory subgroup analysis, there was little difference in mortality associated with stopping or continuing individual drugs, though the point estimates tended to favor drug continuation in the tacrolimus (HR 1.22, 95% CI 0.58–2.58) and cyclosporine (HR 1.54, 95% CI 0.53–4.44) subgroups and to favor drug withdrawal in the sirolimus plus CNI dual immunosuppression subgroup (HR 0.78, 95% CI 0.18–3.47).
Figure 4. Outcome (overall survival) in relation to calcineurin inhibitor or sirolimus continuation versus withdrawal in the calibration weighted cohort.
The forest plot shows the relative survival associated with continuation versus withdrawal of immunosuppressants as well as individual subgroup analysis.
Discussion:
In this retrospective cohort study with 2145 adult patients who underwent allogeneic HCT, we discovered a number of independent risk factors that were both diagnostic and prognostic for TA-TMA. Antecedent acute GVHD had the strongest independent association with 10- to 27-fold higher risks in grade 3 and grade 4 compared to patients without GVHD. Some of the baseline risk factors, such as mismatched donor and myeloablative conditioning, likely predispose patients to TA-TMA via the GVHD pathway. Equally importantly, higher CNI (tacrolimus or cyclosporine) trough levels over time were not associated with higher risks of TA-TMA; however, higher sirolimus trough levels (when used in conjunction with CNI) were associated with an increased risk independent of the effect of GVHD. Finally, we did not detect an improvement in hematologic resolution or overall survival for patients treated by withdrawal of CNI or sirolimus. Taken together, these findings provide evidence that TA-TMA is a multifactorial disease and that withdrawal of immunosuppressant alone is not sufficient management.
There is an ongoing controversy on whether the incidence of TA-TMA is higher in patients receiving sirolimus in combination with a CNI versus CNI alone. Two previous observational studies showed a lack of association whereas two others showed a 1.7–2.6 fold increased risk.8,9,11,23 Almost all studies to date have only analyzed the effect of CNI and sirolimus as baseline risk factors when drug levels do not remain static over time. By modeling each drug exposure longitudinally, we were able to observe an increased risk of TA-TMA at higher levels of sirolimus over time. Conversely, we found no association with higher levels of tacrolimus or cyclosporine and no clinical improvement after drug withdrawal (where 85% stopped CNI). Our results raise the question whether CNI exposure alone was truly culpable for the development of TA-TMA.
In addition to GVHD and sirolimus/CNI combination regimen, various other post-transplant risk factors found in our study (DAH, bacteremia, invasive aspergillosis, BK viremia) not only provide distinct pathophysiological pathways for the development of TATMA, they also appear to be prognostic for predicting patient survival. The median 90-day survival of patients with TA-TMA was 57%; however, that estimate varies widely from 23% to 96% depending on the underlying condition that preceded the development of TA-TMA. This heterogeneity potentially explains the wide range in case fatality reported in prior studies.4 It also raises concerns for the interpretation of the results of any single arm clinical trial used to assess the efficacy of drug interventions. It may be prudent to focus future interventional trials on the 50% of patients with preceding GVHD, rather than intervening on patients with idiopathic/drug conditions who already have favorable outcomes, or those with DAH or serious infections whose adverse outcomes are unlikely to be reversed by TMA-targeted treatment.
We believe there are several distinctive features of the present study that contribute to the understanding of the causes and consequences of TA-TMA. In addition to including a large cohort of allogeneic HCT patients with relatively complete data, we employed clinical validation and adjudication to enhance correct outcome classification. For example, the use of electronic screening criteria of MAHA alone would have identified 13% (similar to another recently published study using the same criteria11) instead of 7.6% of patients with adjudicated TA-TMA; the patients we excluded had isolated MAHA without clinical sequelae, or well-known secondary causes of TMA such as clinical DIC or disease relapse. Second, we assessed not only baseline characteristics, but important post-transplant complications and drug levels that were more likely to be pathogenic for the development of TA-TMA. In contrast to prior studies, we did not find an appreciable association between TA-TMA and age,12,24,25 gender,6,24,26 the use of an unrelated donor,12,24–26 lymphoid malignancy,6 or CMV viremia.12,27 Lastly, we analyzed the impact of immunosuppressant withdrawal management using a calibration inverse weighting approach, allowing us to minimize the influence of potential confounding factors.
Our study has several limitations due to its observational nature. For TA-TMA case ascertainment, we adopted the Cho et al definition12 but used a more stringent threshold for LDH and further excluded cases secondary to clinical DIC or pre-transplant disease relapse. After consulting with the transplant community physicians, we felt that laboratory criteria alone is insufficient to define TA-TMA without excluding known secondary causes of TMA. Nonetheless, we cannot exclude cases with subclinical DIC despite the adjudication process. The possibility of missing true cases is partially mitigated by the use of alternative data sources based on comprehensive chart reviews such as ICD-9-CM and CIBMTR. For the risk factor assessment, the novel association with DAH requires future validation as DAH and TMA may have similar laboratory presentations. Furthermore, we did not have biomarker or genetic data on the complement pathway available on all patients included in the current study; availability of such data might be important for further clarification of the onset and prognosis of the disease. For the assessment of prognosis, we have chosen the most clinically relevant and salient condition in a descending order chosen a-priori due to the frequent occurrence of multiple comorbidities. While admittedly a source of selection bias, we have performed this assessment systematically and blinded to patient outcomes. Finally, for the analysis of the effect of CNI/sirolimus withdrawal, calibration weighting equalizes observable confounders but cannot address unknown confounders at baseline or time-varying confounders and our samples size for each group was small.
Conclusion:
TA-TMA occurred in 7.6% of allogeneic HCT patients within the first 100 days post-HCT. Vigilance for this diagnosis is required if patients received a second HCT, HLA-mismatched donor, myeloablative conditioning, or developed GVHD, DAH, bacteremia, invasive aspergillosis, BK viremia, or exposure to higher sirolimus trough level. We did not find any evidence that withdrawal of CNI or sirolimus was beneficial. Until effective treatments become available, the management of TA-TMA should focus on the treatment of underlying diseases and management of immunosuppressant withdrawal should be considered on an individualized basis.
Supplementary Material
Acknowledgements:
Research reported in this publication was supported by NHLBI T32HL007093 (AL), NCI T32CA009515 (KSK), NCI K24CA184039 (AKG), donations from Frank and Betty Vandermeer (AKG), NIH HL088021 (MLS), ACS No. RSG-13–084-01-CPHPS (MLS), and PCORI No. CE-1304–7451 (MLS). P01 CA018029-43A1 (CD). Ang Li received a 2018 Mentored Research Award from the Hemostasis and Thrombosis Research Society (HTRS), which was supported by an independent medical educational grant from Shire.
Footnotes
Declaration of Interests:
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Jodele S, Laskin BL, Dandoy CE, et al. A new paradigm: Diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015;29(3):191–204. doi: 10.1016/j.blre.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavriilaki E, Sakellari I, Anagnostopoulos A, Brodsky RA. Transplant-associated thrombotic microangiopathy: opening Pandora’s box. Bone Marrow Transplant. 2017;52(10):1355–1360. doi: 10.1038/bmt.2017.39. [DOI] [PubMed] [Google Scholar]
- 3.Powles RL, Clink HM, Spence D, et al. Cyclosporin A to prevent graft-versus-host disease in man after allogeneic bone-marrow transplantation. Lancet (London, England). 1980;1(8164):327–329. http://www.ncbi.nlm.nih.gov/pubmed/6101787. [DOI] [PubMed] [Google Scholar]
- 4.George JN, Li X, McMinn JR, et al. Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome following allogeneic HPC transplantation: A diagnostic dilemma. Transfusion. 2004;44(2):294–304. doi: 10.1111/j.1537-2995.2004.00700.x. [DOI] [PubMed] [Google Scholar]
- 5.Shimoni A, Yeshurun M, Hardan I, Avigdor A, Ben-Bassat I, Nagler A. Thrombotic microangiopathy after allogeneic stem cell transplantation in the era of reduced-intensity conditioning: The incidence is not reduced. Biol Blood Marrow Transplant. 2004;10(7):484–493. doi: 10.1016/j.bbmt.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Oran B, Donato M, Aleman A, et al. Transplant-associated microangiopathy in patients receiving tacrolimus following allogeneic stem cell transplantation: risk factors and response to treatment. Biol Blood Marrow Transplant. 2007;13(4):469–477. doi: 10.1016/j.bbmt.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Sakellari I, Gavriilaki E, Boussiou Z, et al. Transplant-associated thrombotic microangiopathy: an unresolved complication of unrelated allogeneic transplant for hematologic diseases. Hematol Oncol. 2017;35(4):932–934. doi: 10.1002/hon.2346. [DOI] [PubMed] [Google Scholar]
- 8.Cutler C, Henry NL, Magee C, et al. Sirolimus and thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(7):551–557. doi: 10.1016/j.bbmt.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Labrador J, López-Corral L, López-Godino O, et al. Risk factors for thrombotic microangiopathy in allogeneic hematopoietic stem cell recipients receiving GVHD prophylaxis with tacrolimus plus MTX or sirolimus. Bone Marrow Transplant. 2014;49(5):684–690. doi: 10.1038/bmt.2014.17. [DOI] [PubMed] [Google Scholar]
- 10.Jodele S, Davies SM, Lane A, et al. Refined diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a prospective study in children and young adults. Blood. 2014;124(4):645–654. doi: 10.1182/blood-2014-03-564997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Postalcioglu M, Kim HT, Obut F, et al. Impact of Thrombotic Microangiopathy on Renal Outcomes and Survival after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. May 2018. doi: 10.1016/j.bbmt.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho B-S, Yahng S-A, Lee S-E, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2010;90(8):918–926. doi: 10.1097/TP.0b013e3181f24e8d. [DOI] [PubMed] [Google Scholar]
- 13.Shayani S, Palmer J, Stiller T, et al. Thrombotic Microangiopathy Associated with Sirolimus Level after Allogeneic Hematopoietic Cell Transplantation with Tacrolimus/Sirolimus-Based Graft-versus-Host Disease Prophylaxis. Biol Blood Marrow Transplant. 2013;19(2):298–304. doi: 10.1016/j.bbmt.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labrador J, López-Corral L, Vazquez L, et al. Incidence and risk factors for life-threatening bleeding after allogeneic stem cell transplant. Br J Haematol. 2015;169(5):719–725. doi: 10.1111/bjh.13344. [DOI] [PubMed] [Google Scholar]
- 15.Nakamae H, Yamane T, Hasegawa T, et al. Risk factor analysis for thrombotic microangiopathy after reduced-intensity or myeloablative allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2006;81(7):525–531. doi: 10.1002/ajh.20648. [DOI] [PubMed] [Google Scholar]
- 16.Ho VT, Cutler C, Carter S, et al. Blood and Marrow Transplant Clinical Trials Network Toxicity Committee consensus summary: Thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(8):571–575. doi: 10.1016/j.bbmt.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation ( HCT )– specific comorbidity index : a new tool for risk assessment before allogeneic HCT. October 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004.Supported. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood. 2013;121(15):2854–2863. doi: 10.1182/blood-2012-09-455063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, et al. 1994. Consensus Conference on Acute GVHD Grading. In: Bone Marrow Transplantation. Vol 15 ; 1995:825–828. [PubMed] [Google Scholar]
- 20.Thomas ED, Storb R, Clift RA, et al. Bone-Marrow Transplantation. N Engl J Med. 1975;292(16):832–843. doi: 10.1056/NEJM197504172921605. [DOI] [PubMed] [Google Scholar]
- 21.Chan KCG, Yam SCP, Zhang Z. Globally efficient non-parametric inference of average treatment effects by empirical balancing calibration weighting. J R Stat Soc Ser B (Statistical Methodol. 2016;78(3):673–700. doi: 10.1111/rssb.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC. A Tutorial and Case Study in Propensity Score Analysis: An Application to Estimating the Effect of In-Hospital Smoking Cessation Counseling on Mortality. Multivariate Behav Res. 2011;46(1):119–151. doi: 10.1080/00273171.2011.540480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenthal J, Pawlowska A, Bolotin E, et al. Transplant-associated thrombotic microangiopathy in pediatric patients treated with sirolimus and tacrolimus. Pediatr Blood Cancer. 2011;57(1):142–146. doi: 10.1002/pbc.22861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez MT, Bucher C, Stussi G, et al. Transplant-associated microangiopathy (TAM) in recipients of allogeneic hematopoietic stem cell transplants. Bone Marrow Transplant. 2005;36(11):993–1000. doi: 10.1038/sj.bmt.1705160. [DOI] [PubMed] [Google Scholar]
- 25.Willems E, Baron F, Seidel L, Frère P, Fillet G, Beguin Y. Comparison of thrombotic microangiopathy after allogeneic hematopoietic cell transplantation with high-dose or nonmyeloablative conditioning. Bone Marrow Transplant. 2010;45(4):689–693. doi: 10.1038/bmt.2009.230. [DOI] [PubMed] [Google Scholar]
- 26.Uderzo C, Bonanomi S, Busca A, et al. Risk factors and severe outcome in thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Transplantation. 2006;82(5):638–644. doi: 10.1097/01.tp.0000230373.82376.46. [DOI] [PubMed] [Google Scholar]
- 27.Ye Y, Zheng W, Wang J, et al. Risk and prognostic factors of transplantation-associated thrombotic microangiopathy in allogeneic haematopoietic stem cell transplantation: a nested case control study. Hematol Oncol. 2017;35(4):821–827. doi: 10.1002/hon.2310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.