Chronic kidney disease affects 1 in 9 Americans and more than 300 million persons worldwide. Of these millions, approximately 2.5% have end-stage kidney failure and require treatment with dialysis or transplantation to survive. Kidney transplantation is the preferred form of renal replacement therapy; 85% of those receiving a transplant survive more than 5 years, as compared with approximately 40% of those who undergo dialysis. But there is a critical shortage of donor organs, with wait-list times exceeding 5 years in many U.S. centers. Furthermore, transplant recipients must take potent immunosuppressive agents to prevent rejection of the graft. Advances in stem-cell biology and regenerative medicine fuel hope that one day, tissue-engineered organs with low immunogenicity will solve the organ-shortage problem. Although rapid advances have occurred in the regeneration of certain tissues and cell types (e.g., pancreatic islets and trachea),1,2 the kidney presents some specific challenges.
The mature kidney is an exquisitely complex organ containing more than 20 specialized types of cells. In addition to being made up of multiple differentiated cell types, each adult human kidney contains approximately 1 million individual filtering units known as nephrons, which receive approximately 20% of the cardiac output and produce 180 liters of primary urinary filtrate each day. This filtering process ensures that excess salt and fluids, metabolic waste products, and exogenous toxins are excreted. In addition, the kidney and its specialized cell types orchestrate many other critical physiologic functions, including the regulation of blood pressure, pH, and the production of red cells and hormones needed for bone mineral health.
Classic embryologic experiments performed by Grobstein in the 1950s identified two types of progenitor cells — the metanephric mesenchyme (MM) and ureteric bud epithelium (UB) — that are necessary and sufficient to form all subsequent, mature cell types of the nephron and collecting-duct system. These two populations of founding cells derive from intermediate mesoderm of the fetus. A major advance in tissue regeneration was provided by the identification of four genes — OCT4 (also called POU5F1), KLF4, MYC (also called CMYC), and SOX2 — which encode the so-called Yamanaka factors that are capable of reprogramming somatic, differentiated cells into induced pluripotent stem cells (iPSCs). Such cells can proliferate and give rise to any cell type in the body. Investigators subsequently determined the cocktail of genes and growth factors needed to coax iPSCs to become MM or UB cells.3–5 Using knowledge from these earlier findings, researchers could then grow components of the mature kidney in the laboratory, but a major hurdle remained: how to persuade these two cell types to differentiate, in a coordinated fashion, to produce a functional kidney.
By altering the exposure time, dose, and presence of key growth factors, Takasato and colleagues6 have recently reported a method to trigger nephron formation from human iPSCs through the generation of kidney-like structures called organoids in three-dimensional cultures that contain up to 500 individual nephrons (Fig. 1). Using molecular markers, the investigators confirmed the presence of early UB and MM populations and showed that these progenitor cells differentiated into specialized cell types of the nephron, including podocytes, proximal tubule cells, thick ascending limb of the loop of Henle, and distal nephrons. The same growth-factor cocktail promoted the growth of endothelial vascular cells, some of which grew into glomeruli, which are essential for filtration. In addition, stromal cells, located between nephrons, were also present.
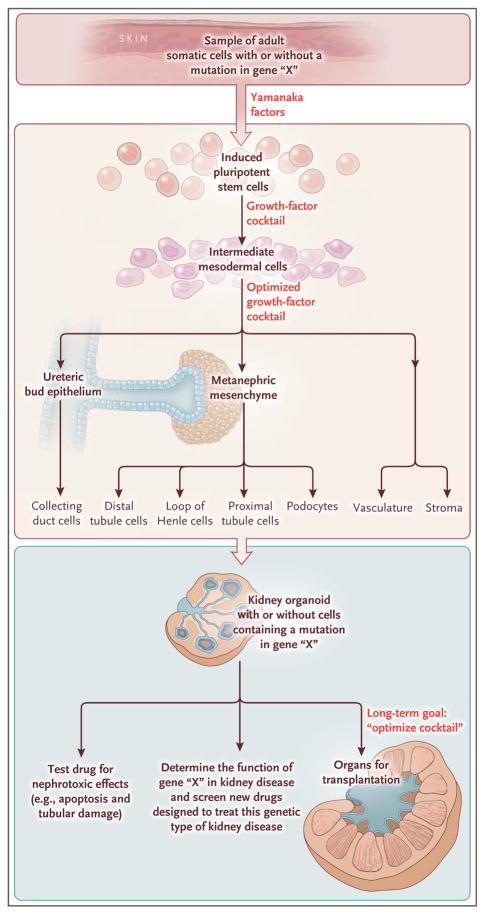
Figure 1. Creating a Kidney Organoid.
Induced pluripotent stem cells (iPSCs) are derived from adult tissues (e.g., skin) by the introduction of four genes that encode the so-called Yamanaka factors (OCT4 [also called POU5F1], KLF4, MYC [also called CMYC], and SOX2). If the skin-cell donor carries a mutation in a gene, every single iPSC will carry the same mutation. Such cells are pluripotent and have the potential to develop into any cell type of the body, which is determined by the specific cocktail of growth factors that are provided. Takasato and colleagues6 have identified the combination of factors needed to coax iPSCs to become kidney progenitors (intermediate mesoderm) that in turn can give rise to many different types of mature kidney cells. This process ultimately generates a kidney organoid containing 500 nephrons or filtering units. Immediate clinical applications may include the testing of the nephrotoxicity of current or new drugs by treating the organoid with the compound and looking for kidney injury. In addition, the organoid will carry the same genetic mutation in cells obtained from the original donor, permitting investigators to study the function of such genes and screen new drugs to treat the disease arising from the mutation in a physiologically relevant kidney structure. The long-term goal is to optimize the growth-factor cocktail to grow larger and even more complex kidney tissues that would be suitable for organ transplantation.
The clinical potential of this discovery is obvious. The ability to produce kidney structures with representative cell populations from all three compartments (nephrons, collecting duct, and stroma) together with blood vessels brings us much closer to realizing the goal of generating tissue-engineered kidneys for transplantation. The generation of kidneys from iPSCs obtained from individual patients could provide a source of autologous organs compatible with the patient’s own immune system. Although there are additional studies and steps needed to produce kidneys with fully functional filtration and vascular systems capable of handling the demands of endogenous kidneys, this study represents a major step toward that goal.
An immediate application is the use of kidney organoids to screen for nephrotoxic effects of new or current drugs, potentially avoiding the need for expensive in vivo studies in animals. In their study, the investigators show the feasibility of this application: they observed tubular injury and apoptosis on exposure of the kidney organoid to cisplatin, a known nephrotoxic agent. Another exciting opportunity is the prospect of developing organoids from cells obtained from patients with genetic kidney diseases, which would provide a complex tissue model (physiologically and genetically identical to patients from whom the iPSCs were derived) for determining pathogenic mechanisms and the testing of new therapies.
Footnotes
Disclosure forms provided by the author are available with the full text of this article at NEJM.org.
References
- 1.Pagliuca FW, Millman JR, Gürtler M, et al. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428–39. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonfiotti A, Jaus MO, Barale D, et al. The first tissue-engineered airway transplantation: 5-year follow-up results. Lancet. 2014;383:238–44. doi: 10.1016/S0140-6736(13)62033-4. [DOI] [PubMed] [Google Scholar]
- 3.Mae S, Shono A, Shiota F, et al. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat Commun. 2013;4:1367. doi: 10.1038/ncomms2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam AQ, Freedman BS, Morizane R, Lerou PH, Valerius MT, Bonventre JV. Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J Am Soc Nephrol. 2014;25:1211–25. doi: 10.1681/ASN.2013080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taguchi A, Kaku Y, Ohmori T, et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14:53–67. doi: 10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Takasato M, Er PX, Chiu HS, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–8. doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]