Abstract
Tumor-specific immune response is an important aspect of disease prognosis and ultimately impacts treatment decisions for innovative immunotherapies. The Atypical Chemokine Receptor 1 (ACKR1/DARC) gene, plays a pivotal role in immune regulation and harbors several Single Nucleotide Variants (SNVs) that are specific to Sub-Saharan African Ancestry. In this study, we report the clinical relevance of DARC/ACKR1 tumor expression in breast cancer, in the context of a tumor immune response that may be associated with Sub-Saharan African Ancestry. We found that in infiltrating carcinomas from The Cancer Genome Atlas (TCGA) breast cohort (n=838), African-Americans (AA) have a higher proportion of tumors with low DARC/ACKR1- expression when compared to White Americans (WA), and DARC/ACKR1 tumor expression is positively correlated with pro-inflammatory chemokine, CCL2/MCP-1 (p<0.0001) and negatively correlated with CXCL8/IL-8 (p<0.0001). DARC/ACKR1 mutations that are specific to Sub-Saharan African ancestry appear to drive these correlations in newly diagnosed breast cancer cases, as shown in our case-control cohort (n=221) of DARC/ACKR1 genotype and phenotype associations. Overall and Relapse-free survival were significantly longer in patients with high levels of DARC/ACKR1 tumor expression (p<2.2×10−6) and p<1.0 × 10−16, respectively), even within molecular tumor subtypes. Using CIBERSORT, we detected a distinct immune cell profile associated with DARC/ACKR1 tumor expression. The immune landscape was distinct in race groups, with increased macrophage and regulatory T-cells in the DARC/ACKR1-high tumors of AAs.
Introduction
Breast cancer disparities have been well-documented for over 50 years, indicating significant differences in rates of incidence and mortality among women of varying self-reported race and ethnicity [1, 2]. While the focus of disparities research has traditionally been studied through a lens of health care and/or socioeconomic barriers [3], we and others have recently identified additional biological factors that are associated with disparate clinical outcomes, including disproportionate burdens of aggressive tumor subtypes [4–8] (i.e. triple-negative basal-like) in women of west African descent [9–12]. Overall, women of African descent are more likely to be diagnosed with the worst types of breast cancer, including Triple-Negative Breast Cancer (TNBC), at earlier ages with the poorest prognosis indications [8, 10, 13–15], despite having access to proper screening and standard treatments [16]. There is a clear need to better understand how the molecular dynamics of tumor progression may differ among women of African descent, compared to women of European descent.
Some evidence of these differences in pathological/histological tumor variables indicate that breast cancer in women of African Ancestry has a distinct genetic signature [4, 17] and distinct associations with immune responses [18–20] even within the TNBC subtype. These differences are the likely causes for increased prevalence of refractory tumors in certain patient groups. With the advent of genomic technologies and increased genetic diversity in multi-ethnic studies, differences in these tumor characteristics can now be investigated to fully comprehend dynamics of ancestry-related immunological interactions and how immunotherapy may or may not work for certain groups [21]. Recent studies have begun to directly address this question from the perspective of genetic risk [22]; however, we sought to identify key biological pathways that are systemically altered in West African populations, which may drive immunological differences in tumor biology in these patients. In terms of genetic risk, the typical focus of genetic background studies, these genetic drivers might not alter tumor etiology, per se, but rather tumor phenotypes. We utilized data from The Cancer Genome Atlas (TCGA) to uncover expression pattern differences in immunological pathway genes that were associated with self-identified race and identified an enrichment in chemokine signaling. The study presented here describes our in-depth investigation of a key regulator of these chemokine signaling pathways, Atypical Chemokine Receptor 1 (ACKR1, aka DARC), in a breast cancer case – control cohort.
The DARC/ACKR1 is a seven-transmembrane G-protein-coupled receptor that is found on the surface of red blood cells (RBC)/erythrocytes, endothelial cells lining post-capillary venules, and most recently has been shown to be expressed on lymphoblast cells [7]. DARC/ACKR1 functions as a decoy receptor for a variety of CXC and CC chemokines, including those with pro-malignant and pro-inflammatory effects, such as CCL2 and CXCL8 (IL-8) [23]. It is considered a decoy receptor because it binds and internalizes these chemokines, and targets them for lysosomal degradation, efficiently sequestering their signaling capabilities [24, 25]. In addition, DARC/ACKR1 receptor has also been shown to regulate transcytosis of the bound ligands in endothelial cells [26], influencing leukocyte trafficking and the ability of the receptor to maintain homeostatic chemokine levels [27]. Erythrocyte-specific DARC/ACKR1 expression plays a key role in inflammation by sequestering chemokines from circulation to recover homeostatic levels during inflammatory responses [26]. A population-private DARC/ACKR1 gene mutation, defined as DARC/ACKR1es (erythrocyte silent), removes expression of DARC/ACKR1, specifically on erythrocytes, and is also known as the extensively characterized “Duffy-null blood group” [28, 29]. This mutation is mostly restricted to populations of West African descent and remains fixed (100% allele frequency) in present-day populations within west and central Sub-Saharan Africa.
Our study defines a set of significant clinical associations of DARC/ACKR1 among patient demographics, population-private alleles and tumor phenotype characteristics that provide greater insight to ancestry-specific differences in tumor biology, particularly immunological responses that are associated with clinical outcomes and that may ultimately inform the decisions for oncologic use of immunotherapy treatments [19, 20, 30]. We anticipate that this research will aid in decreasing the tumor-subtype disparities in incidence and mortality among race groups.
Materials and Methods
Ethics Statement, Study Design, Biospecimens and Cohort Summary
This study is a human subjects study and all biospecimens used in this study were obtained after approval from one of two Institutional Review Board (IRB) approved protocols from either the University of Georgia (IRB ID: MOD00003730) or Henry Ford Health Systems (IRB ID: 4825). All research was performed in accordance with the IRB guidelines, in accordance with an filed assurance of the IRB by the U.S. Department of Health and Human Services. All participants were informed of the study purpose and procedures and have signed an informed consent prior to participation and donation of blood, saliva, and/or tissue for this study.
Gene Expression and DARC/ACKR1 Subtype Analyses.
Gene expression levels were obtained from RNAseq data accessed through the web-portal of The Cancer Genome Atlas Breast Cancer cohort (n=838, 167 African Americans, 671 White Americans), https://cancergenome.nih.gov. After filtering samples for tissue status (removing samples that were normal tissue and metastatic tumors), histological findings (removing samples that were annotated as ‘other’, metaplastic, or non-infiltrating), we conducted linear regression analyses for gene correlations, stratified as indicated for specific contexts of interactions (i.e. molecular subtypes/phenotypes) and nominal logistical regressions across derived tumor status. For DARC/ACKR1 subtype analyses, DARC/ACKR1 expression (Supplemental Figure 1A) was stratified based on quartile ranking and shown as high (upper 30th quartile), medium (intermediate quartile) and low (lower 30th) categories (Supplemental Figure 1B). DARC/ACKR1 subtypes were then measured for associations with specific demographic or clinical variables by stratifying the population according to these variables (e.g. race and molecular breast cancer subtype, Supplemental Figures 1C-D) and comparing distributions of DARC/ACKR1 subtypes within or among each category. We conducted multivariate modeling to assess effect estimates and adjust for demographic variables (i.e. race and age).
Cytokine Analysis with DARC/ACKR1 Subtypes.
The UCSC Xena Browser (http://xenabrowser.net, accessed April 2018) was used to geenerate a heat map of TCGA breast invasive carcinoma RNAseq gene expression data (IlluminaHiSeq, n=399) compared to a user-generated geneset of relevant cytokine genes (n=67, Supplemental Table 1). Dichotomized DARC/ACKR1 positive (red) and negative (blue) subgroups were determined according to the values in Supplemental Table 2. P-values for select cytokines were determined using Welch’s t-test.
Blood and Serum Specimens.
Following informed consent, and at time of enrollment or time of surgery, approximately 4 mL of blood was collected in EDTA-treated vacutainer tubes (BD) from each newly diagnosed patient pre-treatment at UCBC (n=41) and Henry Ford Health System (HFHS) (n=225). Blood samples from non-cancer controls (n=67) and breast cancer survivors (n=17) were collected in a similar manner at time of enrollment at the University of Georgia (UGA) Clinical and Translational Research Unit (CTRU, Athens, GA, n=84). All blood samples were processed within 24 hours of collection. Undiluted plasma was collected through centrifugation, and the remaining sample was separated via a Ficoll-plaque gradient (GE Healthcare) according to manufacturer’s instructions. Serum control samples from pre-menopausal (n=31) and post-menopausal women (n=30) and breast cancer survivors (n=11) were purchased from the Susan G. Komen Tissue Bank (n=72). A flow chart detailing patient numbers and study exclusions can be found in Supplemental Figure 2.
Luminex Human Chemokine Multiplex Assay.
The multiplex assay was completed using patient undiluted plasma and serum. Plasma chemokine levels were quantified using the Bio-Plex Pro™ Human Chemokine Assay Kit (Bio-Rad) that was custom designed to measure the following chemokines and cytokines: CCL2/MCP-1, CXCL8/IL-8, CXCL9/MIG, CXCL1/Gro-α, IL-10. The assay was carried out following manufacturer’s instructions, and the results were analyzed using the Bio-Plex Manager Software version 6.1.1. Statistical multivariate pairwise correlation analyses with these analytes can be found in Supplemental Table 3.
Red Blood Cell Phenotyping by Immunofluorescence.
RBCs isolated from Ficoll-paque blood separation techniques are fixed in 4% PFA and stained with DARC/ACKR1 goat anti-human (Novus Biologicals) primary antibody and Alexa Fluor 488 chicken anti-goat (Invitrogen) fluorescent secondary antibody using standard immunofluorescence techniques. Positive plasma membrane control stains were done using CellMask™ Plasma Membrane Stain (Life Technologies) according to manufacturer’s instructions. RBCs were imaged using a Keyence BZ fluorescent microscope at 40X magnification.
Duffy-Null Genotyping.
Genotyping for the Duffy-Null SNV, rs2814778, was performed using the Taqman™ GTXpress™ Master Mix and variant-specific probes (Applied Biosystems) according to manufacturer’s instructions. The assay was executed using an Applied Biosystems 7500 Fast Real-Time PCR System. Cohort allelic and genotypic frequencies compared to global populations from 1000 genomes data via ENSEMBL (www.ensembl.org) are provided in Supplemental Table 4.
Tumor-Associated Leukocytes Gene Profiling.
The CIBERSORT online platform [31] was used to estimate the absolute fractions of 22 leukocyte populations in TCGA samples denoted as breast primary tumor samples. The analysis was run with 500 permutations, and quantile normalization disabled (as recommended by the tool for RNAseq data). Only those samples with a maximum significance value of p<0.05 were included in the final analysis (n=472).
Survival Analyses.
Patient survival associations with gene expression data was calculated from 3,951 breast cancer patients. The data (Platforms: Affymetrix Microarrays: HG-U133A, HG-U133 Plus 2.0 and HG-U133A 2.0) were downloaded from GEO and AE databases [32] (Supplemental Table 5). Recurrence-Free Survival (RFS) and Overall Survival (OS) was assessed across all breast cancer subtypes and treatment types with dichotomized grouping for expression of DARC/ACKR1 (Probe ID: 208335_s_at), CCL2 (216598_s_at), and CXCL8 (211506_s_at) using the Kaplan-Meier (KM) plot application [33] (http://kmplot.com). Distributions for high and low cutoff values (Supplemental Figure 3), p-values, hazard ratios, and confidence intervals for all survival analyses can be found in Supplemental Materials (Supplemental Table 6).
Clinical Tumor specimens.
Primary breast tumor specimens were acquired from the University Cancer and Blood Center (UCBC, n=8) in Athens, GA, USA. UCBC patients were newly diagnosed (within ~1 month), and following informed consent at time of enrollment, primary tumor samples were collected at local Athens area hospitals. There were no exclusion criteria for this study cohort, which consisted of a racially diverse patient group having various molecular subtypes of breast cancer (Table 1). Tumor grade was determined using WHO guidelines, as well as the Ellis & Elston system of histologic grading. Clinical staging was evaluated using the TNM staging system maintained by AJCC (American Joint Committee on Cancer) and UICC (Union for International Cancer Control) following the most current NCCN guidelines at time of staging. Molecular breast cancer subtypes were determined using immunohisto-chemical (IHC) staining for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) on the surface of the primary breast tumor.
Table 1.
Case-Control cohort baseline characteristics
| Characteristic | Cases, n (%) n=266 | Controls, n (%) n=156 |
|---|---|---|
| Age (years) | ||
| Mean (±SD) | 62.69 (±11.79) | 46.10 (±16.34) |
| Median (Range) | 63 (33–92) | 45 (20–79) |
| Ethnicity | ||
| White-American | 190 (71.43) | 111 (71.15) |
| African-American | 76 (28.57) | 45 (28.85) |
| Molecular Subtype | ||
| Lum A | 174 (65.41) | - |
| Lum B | 25 (9.40) | - |
| HER2+ | 37 (13.91) | - |
| Basal-like | 25 (9.40) | - |
| HR+ | 5 (1.88) | - |
| Clinical Stage | ||
| I | 183 (68.80) | - |
| II | 62 (23.31) | - |
| III | 10 (3.76) | - |
| IV | 5 (1.88) | - |
| Other | 5 (1.88) | - |
| Not Available | 1 (0.37) | - |
| DARC/ACKR1 Genotype (rs2814778) | ||
| AA | 157 (73.71) | 110 (73.33) |
| AG | 23 (10.80) | 15 (10.00) |
| GG | 33 (15.49) | 25 (16.67) |
| DARC/ACKR1 RBC Phenotype | ||
| Positive | 145 (69.05) | 42 (89.36) |
| Intermediate | 41 (19.52) | 3 (6.38) |
| Negative | 24 (11.43) | 2 (4.26) |
Lum A, Luminal A; Lum B, Luminal B; HER2+, Human Epidermal Growth Factor Receptor; HR, Hormone Receptor; RBC, Red Blood Cell
Immunohistochemistry.
Formalin-Fixed Paraffin-Embedded tumor blocks were obtained from local Athens-area hospitals through UCBC. Subsequent slide preparations were conducted through the University of Georgia’s Histology Core, using standard operating protocols: (FFPE) blocks used to cut 4m sections onto glass slides. DARC/ACKR1 staining was done using a goat anti-human polyclonal antibody (Novus Biologicals, NB100–2421, Isotype IgG). CCL2 and CXCL8 staining were both done using mouse anti-human monoclonal antibodies (R&D Systems, MAB2791, Isotype IgG2B and Lifespan Biosciences, LS-B6427, Isotype IgG1, respectively).
Statistics.
Statistical analyses were done using appropriate functions in JMP (SAS) Pro v. 13.0.0. All student’s t-test performed were two-tailed and the appropriate absolute p-values are indicated in the figure legends of each statistical analysis. F values are reported for ANOVA analyses and p-values are reported for linear regression or paired t-test analyses. Numbers of included data points and degrees of freedom are also shown for the appropriate analyses.
Results
DARC/ACKR1 tumor expression is significantly different across race groups and tumor molecular subtypes.
Our previous studies of genomic signatures, in breast cancer subtypes indicated an enrichment in immune-related gene pathways [34]. We identified a pattern of dichotomous variation, measured by first-generation microarray platforms from Gene Expression Omnibus (GEO) [35, 36], in a chemokine receptor that regulates immune cell signaling molecules, the DARC/ACKR1 gene. To quantify these findings more robustly, we investigated DARC/ACKR1 expression differences at a higher resolution, using the TCGA breast infiltrating carcinoma (BrC) RNAseq dataset (n=838; 167 AAs, 671 WAs), and found a broad range and distribution of DARC/ACKR1 expression across the cohort (Supplemental Figure 1A-B). A statistical response screen across anthropomorphic and clinical variables suggested that DARC/ACKR1 tumor expression was distinct across race and tumor phenotypes (Supplemental Figure 1 C-D). Our analysis revealed that WAs have a nearly equal distribution of DARC/ACKR1 tumor expression subgroups, with the highest proportion being DARC/ACKR1-high (35.3%), AAs have the highest proportion of DARC/ACKR1-low tumors (40.1%) (Figure 1A, top). These race-related trends indicate a distinct regulation of DARC/ACKR1 that is likely linked to geographic genetic ancestry, which is closely associated with race.
Figure 1. DARC/ACKR1 is significantly associated with VWF, breast cancer molecular subtypes, and pro-inflammatory chemokines in TCGA data.
TCGA Breast Cancer RNAseq data (n = 838) was used to compare (A, top) the distribution of DARC/ACKR1 expression subgroups (high, n = 289, pink; medium, n = 268, yellow; low, n = 281, purple) and race (African-Americans, n = 167, AA; White Americans, n = 671, WA). (A, bottom) same as (A, top) but with VWF expression subgroups (VWF Low, n = 336; VWF Mid, n = 309; VWF High, n = 193, p = 0.06, ANOVA ) by race. (B, top) Distribution of DARC/ACKR1 expression subgroups compared to molecular breast cancer subtypes (Basal-like, n = 74; HER2+, n = 24; Luminal A, n = 196; Luminal B, n = 58, p<0.0001 ). (B, bottom) same as (B, top) but broken down by race. (C) Heat map (UCSC Xena Browser) of TCGA breast invasive carcinoma RNAseq gene expression data (IlluminaHiSeq) shows cytokine expression after creating dichotomized DARC/ACKR1 positive (red) and negative (blue) subgroups. Gene expression was assessed in 399 breast tumors against a panel of 67 genes associated with known cytokines, blue = low expression, red = high expression. Welch’s t-test was performed on CCL2 (p = 0.0000, t = 10.28) and CXCL8 (p = 0.0003, t = −3.644). (D) DARC/ACKR1 high and low categories compared to CCL2 (left, student’s t-test, p<0.0001, DF = 438.05) and CXCL8 (right, student’s t-test, p<0.0001, DF = 376.25) gene expression (mean ± SEM).
However, because DARC/ACKR1 expression occurs across all organ systems within endothelial cells of post-capillary venules [24, 37], we also considered the relative expression of an endothelial cell-type marker, Von-Wilderbrand Factor (VWF) within the tumor samples. When tumors were stratified by DARC/ACKR1 status and VWF (Figure 1A, bottom), the differences of DARC/ACKR1 status between race groups were more evident (p=0.06), just above the threshold for significance. Interestingly, the correlation of DARC/ACKR1 to VWF was very significant (p<0.0001, Supplemental Figure 1F), which suggests that a significant portion of DARC expression within these tumor samples might be derived from endothelial tissue. However, even within tumors with low VWF, we still find high expression of DARC/ACKR1, particularly in WA patients.
In the context of current standards of breast tumor molecular phenotypes, we also found that DARC/ACKR1 tumor status (e.g. DARC-high vs DARC-low) was significantly distinct across the PAM50 [36] subtype categories (p<0.0001), as defined by RNAseq gene expression profiles (Figure 1B, top). Specifically, nearly 40% of the PAM50 Luminal A subtypes are DARC/ACKR1-high tumors, which is the largest proportion of DARC/ACKR1-high tumors across all subtypes. Contrarily, the highest proportion of DARC/ACKR1-low tumors are within the Basal-like subtype, making up over 60% of this category. When the PAM50 subtypes are also stratified for [self-reported] race (Figure 1B, bottom), we observed clear distinctions in the distributions of DARC/ACKR1 tumor status between AA and WA, though these differences did not pass thresholds for statistical significance.
To investigate whether status of DARC/ACKR1 tumor expression is connected to genes that function in DARC/ACKR1-related chemokine signaling pathways, we investigated over 60 chemokine genes (Supplemental Table 1) and found that there were significant differences in chemokine gene expression, correlated with DARC/ACKR1 status (Figure 1C, Supplemental Table 2). Two of these genes, CCL2 and CXCL8, are high-affinity DARC/ACKR1 binding targets [38, 39] and have significant expression in breast tumors (Supplemental Figure 4A) correlated with DARC/ACKR1 expression (Supplemental Figure 4 C-D). When compared across DARC/ACKR1 tumor types, we found that DARC/ACKR1-high tumors have significantly correlated high levels of CCL2 (p<0.0001) but significantly anti-correlated lower levels of CXCL8 in primary tumors (p<0.0001, Figure 1D).
Circulating levels of DARC/ACKR1-related chemokines, CCL2 and CXCL8, are associated with disease status, Duffy-null phenotype, self-reported race and African ancestry-specific alleles of DARC/ACKR1.
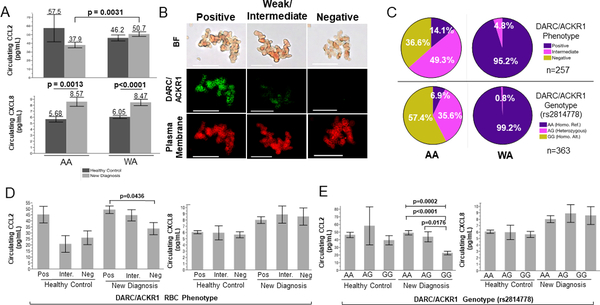
We conducted a Luminex analysis in an independent breast cancer case-control cohort (case, n=266, controls, n=156) to identify associations among DARC/ACKR1-related chemokines and histopathological variables in our cohort. When comparing race between cases and controls overall, we identified a significant difference in circulating CCL2 levels between AA cases and WA cases, where AA had significantly lower CCL2 than WA (p=0.0031, Figure 2A, top). We did not observe a significant difference between cases and controls in AA, but CCL2 levels were decreased in AA cases only, a trend we did not observe in WA. We also observed a significant increase in circulating CXCL8 levels in AA cases (p = 0.0013, Figure 2A, bottom) and WA cases (p<0.0001, Figure 2A, bottom) compared to their respective controls. These lower circulating CCL2 protein expression levels in AAs compared to WAs is a trend that indicates a potential race-specific circulatory inflammatory response, however this same trend was not observed in the tumor gene expression data from TCGA (Supplemental Figure 4B).
Figure 2. DARC/ACKR1 phenotype and genotype associated with race and pro-inflammatory chemokines in case-control cohort (n=422).
(A) Case-control comparison of circulating CCL2 (top) and CXCL8 (bottom) levels by race (student’s t-test, p=0.0031, DF=182.09). (B) Representative images of DARC/ACKR1 phenotyping on RBCs; BF = bright field, DARC/ACKR1 = green, plasma membrane control = red, scale = 50μm. (C) Distribution of DARC/ACKR1 phenotype (top, positive = purple, intermediate = pink, negative = yellow, n (AA) = 71, n (WA) = 186) and genotype (bottom, rs2814778; AA = purple, homozygous reference; AG = pink, heterozygous; GG = yellow, homozygous alternate, n (AA) = 101, n (WA) = 262) compared to race. (D) DARC/ACKR1 phenotype on RBCs compared to circulating CCL2 (left, student’s t-test, p = 0.0436, DF = 42.88) and CXCL8 levels (right); Inter. = intermediate. (E) Same as (D) but with DARC/ACKR1 genotype (rs2814778; CCL2, left, ANOVA, F = 0.0002, DF = 212, student’s t-test, p < 0.0001, DF = 137.31, student’s t-test, p = 0.0176, DF = 27.47, CXCL8, right).
To determine the distribution and impact of the Duffy-null phenotype on cancer-related inflammatory response in our cohort, we tested our cases and controls for the erythrocyte-silent phenotype (Figure 2B), as well as the Duffy-null gene mutation (rs2814778). As expected, we found both a higher frequency of the DARC/ACKR1es/Duffy-null blood group and the Duffy-null/ACKR1es allele (rs2814778) frequency in AAs (Figure 2C). These distributions were in concordance with published global and population-specific allele frequencies (Supplemental Table 4). We then compared circulating levels of CCL2 and CXCL8 with the DARC/ACKR1 RBC phenotype and Duffy-null genotype for case and control groups (Figure 2D-E). For phenotype, we found significant differences in CCL2 levels between those with positive DARC/ACKR1 erythrocyte expression and negative expression among the newly diagnosed breast cancer cases only (p=0.0436, Fig 2D, left). When considering genotype, we found significant differences in CCL2 levels across all newly diagnosed cases (p = 0.0002, Fig 2E, left). We also observed individual associations between those that are homozygous for the reference allele (AA) and homozygous for the alternate Duffy-null allele (GG, p<0.0001, Fig 2E, left), and those that are heterozygous and homozygous for the Duffy-null allele (p=0.0176, Fig 2E, left) in newly diagnosed cases. This correlation was similar when the phenotype groups were broken down by race (Supplemental Figure 5). Interestingly, the significant differences in CCL2 circulating levels increased among the DARC/ACKR1 allele carriers (Figure 2E) indicating that the genetic allele is a more robust indicator of these circulating chemokine levels than the RBC phenotype, in newly diagnosed patients.
DARC/ACKR1 tumor gene expression is associated with distinct Tumor-Associated Leukocyte gene expression profiles.
Because the chemokines under regulation of DARC/ACKR1 direct the infiltration of immune cells during inflammatory responses, we investigated the differences in immune cell responses, relative to DARC/ACKR1 tumor expression. Using TCGA RNA-sequence data, we employed a cell type deconvolution algorithm, CIBERSORT, and determined that overall leukocyte infiltration (Total TAL score) is greater in DARC/ACKR1-high tumors (Figure 3A) [31]. These counts were directly correlated with quantified DARC/ACKR1 gene expression (Supplemental Figure 6A), suggesting that DARC/ACKR1 could be a marker that predicts the level of tumor immunogenicity. The increase of total immune cell infiltrates was also correlated with increasing CCL2, but to a lesser extent (Supplemental Figure 6B), indicating that CCL2 has some influence on, but is not sufficient for, the total observed immune cell profiles.
Figure 3. DARC/ACKR1 expression levels are positively and significantly associated with TAL abundance in breast tumors.
Using CIBERSORT absolute mode, the absolute score totals the quantitative abundance of each individual leukocyte population among TCGA breast primary cases. Those cases with significant CIBERSORT results (p<0.05) were included in this analysis (n=472). (A) The total TAL absolute score reported is a total of abundance scores across all 22 tumor-associated leukocyte populations. By plotting total TAL absolute score by DARC/ACKR1 expression status, we see significant increases in TAL abundance as DARC/ACKR1 expression is increasing between low, medium and high categories. (B) Looking at individual TAL populations, panel B shows the 12 leukocyte populations that are significantly different between DARC/ACKR1 expression levels. Student’s t-test was performed comparing each DARC/ACKR1 expression group, and the p-values for the associations are represented by the heat map in panel (C). Highly significant associations = dark red; Less significant associations = dark blue.
We found distinctions in both the proportions and combinations of specific infiltrating immune cell subtypes of DARC/ACKR1-high tumors compared to DARC/ACKR1-low tumors. Specifically, there is an increase in numbers of B cells, T cells (CD8 and CD4), T-regulatory cells and activated macrophages (M1 and M2) (Figure 3B and C). Conversely, DARC/ACKR1-low tumors appear to lack dendritic and B [memory] cells. Differences in the DARC/ACKR1-associated immune cell type profiles are also present among race groups, (p=0.08). Specifically, AA patients with the DARC/ACKR1-low tumor subtype have more infiltrating M0 macrophages and fewer B cells compared to WA patients (Supplemental Figure 6C).
Higher DARC/ACKR1 tumor gene expression is associated with longer survival.
To determine the clinical significance of DARC/ACKR1 gene expression in breast tumors, we investigated publicly available datasets for associations of DARC/ACKR1 tumor gene expression with survival (cohorts are summarized in Supplemental Table 6). We found that when patients have higher DARC/ACKR1 tumor expression, they have significantly longer OS and RFS (Figure 3A–B) (OS p<2.2×10−6, n = 1,402; RFS p<1×10−16, n = 3951). This survival association remained significant for RFS across all molecular subtypes (including; Luminal A, Luminal B, Basal-like), with one exception, HER2+ (Figure 4A-E). Similarly, the <5-year survival outcome in TCGA is also linked to DARC/ACKR1 tumor expression (Supplemental Fig 1, G). The impact of CCL2 (Figure 4G-L) and CXCL8 (Figure 4M-R) can also be seen within certain molecular subtypes of breast cancer, but often to a lesser degree than DARC/ACKR1 associations. In all subtypes, it is clear that inflammatory markers related to DARC/ACKR1 tumor expression have a significant impact on survival. Specifically, CCL2 has the most significant survival impact on basal-like and HER2+ cases (Figure 4K and L) where higher CCL2 is associated with longer survival. Contrarily, CXCL8 also has a significant impact in basal-like cases (Fig 4Q) but in the opposite direction, where low expression of CXCL8 leads to longer survival. This opposing trend of CCL2 and CXCL8 is directly associated with DARC/ACKR1 status, as shown previously in Figure 1D.
High DARC/ACKR1 tumor expression is associated with significantly higher survival in breast cancer.
Kaplan-Meier (KM) survival curves (www.kmplot.com) of breast cancer patients dichotomized into DARC/ACKR1 (208335_s_at, top row), CCL2 (216598_s_at, middle row), and CXCL8 (211506_s_at, bottom row) low (black) and high (red) categories showing overall survival (A, G, M) and relapse-free survival (B-F, H-L, N-R) by all molecular subtypes and four individual subtypes (Lum A = Luminal A, Lum B = Luminal B, Basal-like, and HER2+ = Human Epidermal Growth Factor Receptor 2). Beeswarm distribution plots, hazard ratios, and confidence intervals can be found in Supplementary Materials.
In tumor epithelial cells, DARC/ACKR1 is correlated with higher CCL2 and lower CXCL8 by Immunohistochemistry data.
DARC/ACKR1 has been shown to be expressed on endothelial post-capillary venules, which are present in normal breast tissue (Supplemental Figure 7A) and tumors as well. However, our preliminary data in breast cell lines indicated DARC/ACKR1 was expressed in tumor cells (Supplemental Figure 7B). To confirm the genomic and cell line data, we conducted IHC on a small cohort of primary tumor specimens (n=8) to validate the spatial expression of DARC/ACKR1 in the tumor microenvironment. Our initial results indicate that when DARC/ACKR1 is expressed, it was not limited to endothelial cells but was uniformly expressed within the tumor-specific epithelial cells. This finding validates the VWF-associated DARC expression trends show in Figure 1A and Supplemental Figure 1F. Specifically, our IHC subset suggests that the DARC/ACKR1 gene product is also expressed over the full spectrum of scoring (0–4) in our cohort (Figure 5A), similar to the RNA-seq distributions (Supplemental Figure 1A and B). Despite the small size of the pilot IHC cohort (n=8), we observed a similar trend as the RNAseq findings, where tumors with high DARC/ACKR1 IHC scores showed correlated high CCL2 expression and low CXCL8 expression (Figure 5A).
Figure 5. DARC/ACKR1 may correlate with pro-inflammatory chemokines in breast tumor tissue.
(A) Primary breast tumors stained for DARC/ACKR1 (dark pink), CCL2 (light pink), and CXCL8 (brown) by IHC and scored on a numezrical system of 0–4, bar = 200 uM. (B) Linear correlation between DARC/ACKR1 IHC scores (grey bars) and circulating CCL2 (straight line) and CXCL8 (dotted line) concentrations from Luminex assay (n=8).
Anticipating that the role of DARC/ACKR1 on epithelial cells would be similar to that on endothelial cells, we investigated whether circulating chemokine levels were associated with DARC/ACKR1 tumor scores. We integrated the Luminex chemokine assays from our clinical cohort with a pilot IHC study with a subset of cases and found a significant positive correlation between DARC/ACKR1 tumor scores and circulating levels of CCL2 (p=0.0061) and CXCL8 (p=0.0291) (Figure 5B and Supplemental Table 3). This finding was also aligned with our RNAseq findings for CCL2, CXCL8 and DARC/ACKR1 levels.
Discussion/Conclusions
Distinct tumor immune responses among ancestry groups with cancer disparities.
Disparities in cancer outcomes among race groups parallel distributions of tumor phenotypes, including distinct signatures of tumor-specific gene expression signatures. These findings have shifted the lens of clinical disparities research to now encompass molecular investigations of tumor phenotypes that drive poor prognoses, which can be now be linked to genetic ancestry, particularly West African Ancestry.
Work from our group and others uncovers molecular mechanisms of cancer that parallels the mortality and tumor phenotype incidence among groups of African and AA women [12]. Genomic studies conducted in large AA and/or multi-ethnic cohorts have begun to utilize modified genomic tools in search of population-specific risk alleles. Single Nucleotide Variants (SNVs), structural chromosomal and epigenetic variations are being discovered and associated with a still theoretical genetic predisposition for the distinct tumor biology observed among women of West African descent [40–43]. While more risk alleles are being detected through increased diversity of multi-ethnic GWAS, the specific risk alleles transmitted into non-white populations through African genetic ancestry have not yet been definitively shown [44]. Our current study deepens our understanding of a repeated finding among gene expression studies which describe differences in disease biology rather than risk of disease incidence/predisposition among race groups. Multiple groups have reported African-American (race-specific) expression profiles that implicate immune response pathways in a distinct category of disease progression [45–47], but again without definitive links to African genetic ancestry.
In this report, we have shown that a west African-specific allele of DARC/ACKR1, a gene that regulates immune and inflammatory response, is strongly associated with both the epithelial expression and circulating levels of CCL 2 and CXCL8, which are pro-inflammatory chemokines previously shown to be related to cancer progression. We have thusly implicated the epithelial tumor expression of DARC/ACKR1 to be a key modulating factor of chemokines in tumors, putatively confirmed in small IHC study within our clinical cohort. These findings suggest that DARC/ACKR1 has a dual role in controlling chemokine gradients that determine tumor immune cell response, specifically by regulating both the levels of chemokines in tumors and in circulation. Interestingly, tumor expression of CXCL8 is negatively correlated with DARC/ACKR1 tumor expression while circulating levels of CXCL8 are positively correlated with DARC/ACKR1 tumor expression. These opposing trends are recapitulated in our survival analyses where high levels of DARC in tumors and/or low levels of CXCL8 yield longer survival. We hypothesize this is an unmasking of a distinction in DARC/ACKR1 control of CXCL8 compared to CCL2 that may be related to DARC/ACKR1 tumor activity involving clearance of CXCL8 from the tumor and/or DARC/ACKR1 isoform functionality, which we are currently investigating.
Previous studies have already shown that the spatial expression of DARC/ACKR1 is governed, at least in part, by the Duffy-null allele (rs2814778). Given that AA cohorts have over 70% allele frequency of this allele [48, 49], we anticipate that most AA’s would carry the allele and therefore have distinct regulation of the gene in certain tissues. Based on our findings, this allele would predispose breast cancer patients who are Duffy-null carriers to having a specific tumor phenotype, defined by the tumor immune cell landscape, creating a tumor immune response that is directed by the DARC/ACKR1 expression in tumors. Our case-control cohort indicates that the Duffy-null genotypes are a stronger indicator of circulating chemokines, compared to correlations that use self-identified race. The differences in chemokine correlations among race groups, compared to strict heterozygote/homozygote allele groups, corresponds to what would be expected within an admixed population where alleles related to genetic ancestry are convoluted within the proxy of race, where a portion of the individuals that may self-identify with African ancestry are harboring a non-African allele. Therefore, the associations of DARC/ACKR1 and chemokine levels in breast cancer cases can be rationally interpreted as ancestry-associated.
While we have found this association in our study, we believe this allele has not been identified in multi-ethnic GWAS that investigate cancer risk among race groups [22, 50] because this gene would not impact tumor etiology, but rather disease progression, and therefore doesn’t qualify as a “disease [onset] risk” allele. However, when prevention fails, survival becomes imperative. This allele is associated strongly with both overall and recurrence-free survival across all breast cancer subtypes and is linked to race through genetic ancestry. Therefore, this important work begins to unravel how African ancestry directly impacts the tumor biology and microenvironment of tumor progression, even systemically. We suspect that the trends of DARC/ACKR1 tumor expression were not quite significant in the TCGA data simply due to lack of true African Ancestry measurements in lieu of self-identified race proxies. The genetic admixture of European genetic inheritance within the AA group has likely confounded DARC/ACKR1 tumor expression race associations. We will seek to follow-up with genetic ancestry estimates in these cases in a subsequent validation cohort.
The cancer-related mechanisms that derive aggressive tumor phenotypes, such as TNBC, have yet to provide clear and actionable markers for therapeutic decisions in clinic. Studies that seek to identify the factors that lead to treatment-refractory tumor conditions have often implicated the tumor microenvironment (TME) [51]. A significant component of the TME includes immune cells that are either associated with or infiltrating into the tumor and tumor-associated stroma [21]. The assortment of immune cells that respond to tumor formation can determine the course of tumor progression [52]. Tumor immunology studies suggest that Tumor Associated Lymphocytes (TALs) can lead to either good or bad prognoses, such as with higher proportions of B cells vs T cells, respectively [53–57]. While our ability to discern immune cell types in the tumor space has great prognostic value, it is a tedious task to quantify each TAL cell type to establish adverse vs favorable immune profiles and is not feasible on a case-by-case basis in most clinics. Our discovery of a single genetic marker that could definitively regulate the course of immune response in tumors and therefore could forecast TAL profiles would be an invaluable biomarker to identify patients suitable for immunotherapies. In our report, we have introduced such a potential biomarker, showing that tumor expression of an atypical chemokine receptor, DARC/ACKR1, is associated with immune response. This association is aligned with the gene’s extensively defined regulation of chemokine levels in circulation, which may now also include the regulation of these levels in tumors as well. This suggests that DARC/ACKR1 can modulate the chemo-attractants in breast cancer patients, ultimately directing the actions of immune cells within the tumors.
With further investigations that characterize and quantify DARC/ACKR1-dependent TAL infiltration, in the context of African-ancestry, we will develop a systematic approach to determine if DARC/ACKR1 status may predict immune response profiles in breast tumors and ultimately predict immunotherapy treatment responses. This current study has established the feasibility of DARC/ACKR1 as a marker to define a novel tumor subtyping that defines its immune status. A subset of our cohort study, reported here, indicates the feasibility of integrating single-marker tumor IHC with a multi-marker peripheral blood assay. In combination, our data has shown that differences in tumor immune cell profiles, that are relevant to race, are associated with the tumor expression of DARC/ACKR1. Specifically, the African-specific Duffy-null variant of DARC/ACKR1, which is already associated with the gene’s spatial expression, determines a unique biological scenario for distinct TAL infiltrates. This landmark finding has high relevance in cancer management, as a functional regulator of pro-inflammatory and chemotactic cytokines that can specify an infiltrating tumor immune cell type profile and specify differences between patients of distinct ancestry. This further implicates DARC/ACKR1 in tumor phenotype distinctions that could significantly drive treatment outcomes that lead to cancer mortality disparities. Indeed, DARC/ACKR1 may also begin to unravel the racial disparities of treatment outcomes within tumor phenotype categories that have effective targeted therapies and the overall outcomes in TNBC [57].
As with any epidemiological study, limitations of our study include confounding factors within cohorts that are inseparable from variables of interest. Specifically, differences in DARC gene expression between race groups could also be a function of a well-documented bias in occurrence of specific molecular tumor phenotypes across these populations [58]. For instance, women of African descent have a higher relative incidence of TNBC, and we see this same trend in our cohort and included supplemental analysis of race and subtypes in supplemental data. Interestingly, the Duffy-null allele has previously been implicated in other immune-related diseases, such as neutropenia [59], and therefore the distinct chemokine levels we observe in AA patients, associated with the mutation, and now implicates a novel role of DARC/ACKR1 in a breast cancer disparities context. These correlations in chemokine levels were found primarily in newly diagnosed cases, suggesting that DARC/ACKR1 plays a similar role in tumors as what has been shown in endothelial cells - facilitating the entry of tumor-associated chemokines into circulation (i.e. transcytosis) [26] – and so it may be regulating increase in circulating chemokine levels by facilitating chemokine transfer from epithelial-tumor cells. Therefore, despite our limitations, these compelling findings indicate that the African-specific allele of DARC/ACKR1 modulates cancer-driven circulating chemokine levels and thereby may regulate the infiltration of tumor-associated immune cells we observed in tumors expressing DARC/ACKR1 at high compared to the lower numbers of tumor-associated immune cells when DARC/ACKR1 is expressed at low levels, if at all. Our on-going investigations in larger cohorts with higher numbers of non-white cases and better representation of all intrinsic tumor subtypes can further solidify the race/ancestry findings we have uncovered here.
Supplementary Material
Acknowledgments:
We are eternally grateful for the donors and volunteers who have supported our research efforts through participation and donating their blood or tissue for use as cohort samples. These brave women include the participants of the “Be the Research” program at UGA, TCGA patients, Komen Biospecimen Tissue Bank, and the Henry Ford International Breast Registry. B.D.J. is a Howard Hughes Medical Institute Gilliam Fellow. Special thanks to Ben Rybicki and Nancy Manley for helpful discussions on data interpretations, and study scope and impact. This work was supported by grants: R21-CA210237-03 (NIH/NCI) [MD], U54-MD007585-26 (NIH/RCMI) [CY], U54 CA118623 (NIH/NCI) [CY], (NIH/NCI) 1 R21 CA188799-01 [CY], SAC160072 (Komen) [LN].
Footnotes
Ethics statement
All participants provided their written consent to participate in this study. This study was approved by the University of Georgia Institutional Review Board (IRB ID: MOD00003730) and the Henry Ford Health Systems Institutional Review Board (IRB ID: 4825).
Additional Information
The authors declare no competing interest.
References Cited
- 1.Jemal A, et al. , Cancer statistics, 2006. CA Cancer J Clin, 2006. 56(2): p. 106–30. [DOI] [PubMed] [Google Scholar]
- 2.Chlebowski RT, et al. , Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst, 2005. 97(6): p. 439–48. [DOI] [PubMed] [Google Scholar]
- 3.Akinyemiju TF, et al. , Socioeconomic status and incidence of breast cancer by hormone receptor subtype. Springerplus, 2015. 4: p. 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ademuyiwa FO, et al. , Differences in the mutational landscape of triple-negative breast cancer in African Americans and Caucasians. Breast Cancer Res Treat, 2017. 161(3): p. 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tao L, et al. , Breast Cancer Mortality in African-American and Non-Hispanic White Women by Molecular Subtype and Stage at Diagnosis: A Population-Based Study. Cancer Epidemiol Biomarkers Prev, 2015. 24(7): p. 1039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham JE, et al. , Racial differences in the incidence of breast cancer subtypes defined by combined histologic grade and hormone receptor status. Cancer Causes Control, 2010. 21(3): p. 399–409. [DOI] [PubMed] [Google Scholar]
- 7.Davis MB, et al. , Distinct Transcript Isoforms of the Atypical Chemokine Receptor 1 (ACKR1)/Duffy Antigen Receptor for Chemokines (DARC) Gene Are Expressed in Lymphoblasts and Altered Isoform Levels Are Associated with Genetic Ancestry and the Duffy-Null Allele. PLoS One, 2015. 10(10): p. e0140098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeSantis C, et al. , Breast cancer statistics, 2013. CA Cancer J Clin, 2014. 64(1): p. 52–62. [DOI] [PubMed] [Google Scholar]
- 9.Silber JH, et al. , Characteristics associated with differences in survival among black and white women with breast cancer. JAMA, 2013. 310(4): p. 389–97. [DOI] [PubMed] [Google Scholar]
- 10.Amirikia KC, et al. , Higher population-based incidence rates of triple-negative breast cancer among young African-American women : Implications for breast cancer screening recommendations. Cancer, 2011. 117(12): p. 2747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chollet-Hinton L, et al. , Biology and Etiology of Young-Onset Breast Cancers among Premenopausal African American Women: Results from the AMBER Consortium Pubertal growth and adult height in relation to breast cancer risk in African American women. Cancer Epidemiol Biomarkers Prev, 2017. 26(12): p. 1722–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiagge E, et al. , Comparative Analysis of Breast Cancer Phenotypes in African American, White American, and West Versus East African patients: Correlation Between African Ancestry and Triple-Negative Breast Cancer. Ann Surg Oncol, 2016. 23(12): p. 3843–3849. [DOI] [PubMed] [Google Scholar]
- 13.Joslyn SA and West MM, Racial differences in breast carcinoma survival. Cancer, 2000. 88(1): p. 114–23. [DOI] [PubMed] [Google Scholar]
- 14.Lund MJ, et al. , Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat, 2009. 113(2): p. 357–70. [DOI] [PubMed] [Google Scholar]
- 15.Clarke CA, et al. , Age-specific incidence of breast cancer subtypes: understanding the black-white crossover. J Natl Cancer Inst, 2012. 104(14): p. 1094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortel M, et al. , Racial and Ethnic Disparity in Symptomatic Breast Cancer Awareness despite a Recent Screen: The Role of Tumor Biology and Mammography Facility Characteristics. Cancer Epidemiol Biomarkers Prev, 2015. 24(10): p. 1599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keenan T, et al. , Comparison of the Genomic Landscape Between Primary Breast Cancer in African American Versus White Women and the Association of Racial Differences With Tumor Recurrence. J Clin Oncol, 2015. 33(31): p. 3621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burstein MD, et al. , Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res, 2015. 21(7): p. 1688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Teijido P, et al. , Tumor-Infiltrating Lymphocytes in Triple Negative Breast Cancer: The Future of Immune Targeting. Clin Med Insights Oncol, 2016. 10(Suppl 1): p. 31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto H, et al. , Role of inflammatory infiltrates in triple negative breast cancer. J Clin Pathol, 2015. 68(7): p. 506–10. [DOI] [PubMed] [Google Scholar]
- 21.Hendry SA, et al. , The Role of the Tumor Vasculature in the Host Immune Response: Implications for Therapeutic Strategies Targeting the Tumor Microenvironment. Front Immunol, 2016. 7: p. 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong CC, et al. , Genetic Variants in Immune-Related Pathways and Breast Cancer Risk in African American Women in the AMBER Consortium. Cancer Epidemiol Biomarkers Prev, 2018. 27(3): p. 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neote K, et al. , Functional and biochemical analysis of the cloned Duffy antigen: identity with the red blood cell chemokine receptor. Blood, 1994. 84(1): p. 44–52. [PubMed] [Google Scholar]
- 24.Peiper SC, et al. , The Duffy antigen/receptor for chemokines (DARC) is expressed in endothelial cells of Duffy negative individuals who lack the erythrocyte receptor. J Exp Med, 1995. 181(4): p. 1311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhuri A, et al. , Expression of the Duffy antigen in K562 cells. Evidence that it is the human erythrocyte chemokine receptor. J Biol Chem, 1994. 269(11): p. 7835–8. [PubMed] [Google Scholar]
- 26.Nibbs RJ and Graham GJ, Immune regulation by atypical chemokine receptors. Nat Rev Immunol, 2013. 13(11): p. 815–29. [DOI] [PubMed] [Google Scholar]
- 27.Pruenster M, et al. , The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat Immunol, 2009. 10(1): p. 101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meny GM, The Duffy blood group system: a review. Immunohematology, 2010. 26(2): p. 51–6. [PubMed] [Google Scholar]
- 29.Lopez GH, et al. , Duffy blood group phenotype-genotype correlations using high-resolution melting analysis PCR and microarray reveal complex cases including a new null FY*A allele: the role for sequencing in genotyping algorithms. Vox Sang, 2015. 109(3): p. 296–303. [DOI] [PubMed] [Google Scholar]
- 30.Hurvitz S and Mead M, Triple-negative breast cancer: advancements in characterization and treatment approach. Curr Opin Obstet Gynecol, 2016. 28(1): p. 59–69. [DOI] [PubMed] [Google Scholar]
- 31.Newman AM, et al. , Robust enumeration of cell subsets from tissue expression profiles. Nat Methods, 2015. 12(5): p. 453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gyorffy B, et al. , An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat, 2010. 123(3): p. 725–31. [DOI] [PubMed] [Google Scholar]
- 33.Szasz AM, et al. , Cross-validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget, 2016. 7(31): p. 49322–49333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis M, et al. , AR negative triple negative or “quadruple negative” breast cancers in African American women have an enriched basal and immune signature. PLoS One, 2018. 13(6): p. e0196909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrett T, et al. , NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res, 2013. 41(Database issue): p. D991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parker JS, et al. , Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol, 2009. 27(8): p. 1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horuk R, The Duffy Antigen Receptor for Chemokines DARC/ACKR1. Front Immunol, 2015. 6: p. 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, et al. , The role of Duffy antigen receptor for chemokines in keloids. Gene, 2015. 570(1): p. 44–9. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, et al. , Chemokines fluctuate in the progression of primary breast cancer. Eur Rev Med Pharmacol Sci, 2013. 17(5): p. 596–608. [PubMed] [Google Scholar]
- 40.Song MA, et al. , Racial differences in genome-wide methylation profiling and gene expression in breast tissues from healthy women. Epigenetics, 2015. 10(12): p. 1177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, et al. , Genetic variants demonstrating flip-flop phenomenon and breast cancer risk prediction among women of African ancestry. Breast Cancer Res Treat, 2018. 168(3): p. 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Q, et al. , Trans-ethnic follow-up of breast cancer GWAS hits using the preferential linkage disequilibrium approach. Oncotarget, 2016. 7(50): p. 83160–83176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruiz-Narvaez EA, et al. , Admixture Mapping of African-American Women in the AMBER Consortium Identifies New Loci for Breast Cancer and Estrogen-Receptor Subtypes. Front Genet, 2016. 7: p. 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newman LA and Carpten J, Integrating the Genetics of Race and Ethnicity Into Cancer Research: Trailing Jane and John Q. Public. JAMA Surg, 2018. 153(4): p. 299–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parada H Jr., et al. , Race-associated biological differences among luminal A and basal-like breast cancers in the Carolina Breast Cancer Study. Breast Cancer Res, 2017. 19(1): p. 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kroenke CH, et al. , Race and breast cancer survival by intrinsic subtype based on PAM50 gene expression. Breast Cancer Res Treat, 2014. 144(3): p. 689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chavez-Macgregor M, et al. , Differences in gene and protein expression and the effects of race/ethnicity on breast cancer subtypes. Cancer Epidemiol Biomarkers Prev, 2014. 23(2): p. 316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hodgson JA, et al. , Natural selection for the Duffy-null allele in the recently admixed people of Madagascar. Proc Biol Sci, 2014. 281(1789): p. 20140930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Howes RE, et al. , The global distribution of the Duffy blood group. Nat Commun, 2011. 2: p. 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yao S, et al. , Genetic ancestry and population differences in levels of inflammatory cytokines in women: Role for evolutionary selection and environmental factors. PLoS Genet, 2018. 14(6): p. e1007368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stakheyeva M, et al. , Role of the Immune Component of Tumor Microenvironment in the Efficiency of Cancer Treatment: Perspectives for the Personalized Therapy. Curr Pharm Des, 2017. 23(32): p. 4807–4826. [DOI] [PubMed] [Google Scholar]
- 52.Gibbons DL and Creighton CJ, Pan-cancer survey of epithelial-mesenchymal transition markers across the Cancer Genome Atlas. Dev Dyn, 2018. 247(3): p. 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inoue H, et al. , Tumor-infiltrating lymphocytes affect the efficacy of trastuzumab-based treatment in human epidermal growth factor receptor 2-positive breast cancer. Breast Cancer, 2018. 25(3): p. 268–274. [DOI] [PubMed] [Google Scholar]
- 54.Choi J, et al. , The role of tumor-associated macrophage in breast cancer biology. Histol Histopathol, 2018. 33(2): p. 133–145. [DOI] [PubMed] [Google Scholar]
- 55.Hendry S, et al. , Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non-Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv Anat Pathol, 2017. 24(6): p. 311–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu X, et al. , Prognostic and predictive value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. Clin Transl Oncol, 2016. 18(5): p. 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stanton SE, Adams S, and Disis ML, Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review. JAMA Oncol, 2016. 2(10): p. 1354–1360. [DOI] [PubMed] [Google Scholar]
- 58.Newman LA and Kaljee LM, Health Disparities and Triple-Negative Breast Cancer in African American Women: A Review. JAMA Surg, 2017. 152(5): p. 485–493. [DOI] [PubMed] [Google Scholar]
- 59.Reich D, et al. , Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS Genet, 2009. 5(1): p. e1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.