Abstract
Background
Stroke results from an acute lack of blood supply to the brain and becomes a chronic health condition for millions of survivors around the world. Self management can offer stroke survivors a pathway to promote their recovery. Self management programmes for people with stroke can include specific education about the stroke and likely effects but essentially, also focusses on skills training to encourage people to take an active part in their management. Such skills training can include problem‐solving, goal‐setting, decision‐making, and coping skills.
Objectives
To assess the effects of self management interventions on the quality of life of adults with stroke who are living in the community, compared with inactive or active (usual care) control interventions.
Search methods
We searched the following databases from inception to April 2016: the Cochrane Stroke Group Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, PsycINFO, SCOPUS, Web of Science, OTSeeker, OT Search, PEDro, REHABDATA, and DARE. We also searched the following trial registries: ClinicalTrials.gov, Stroke Trials Registry, Current Controlled Trials, World Health Organization, and Australian New Zealand Clinical Trials Registry.
Selection criteria
We included randomised controlled trials of adults with stroke living in the community who received self management interventions. These interventions included more than one component of self management or targeted more than a single domain of change, or both. Interventions were compared with either an inactive control (waiting list or usual care) or active control (alternate intervention such as education only). Measured outcomes included changes in quality of life, self efficacy, activity or participation levels, impairments, health service usage, health behaviours (such as medication adherence or lifestyle behaviours), cost, participant satisfaction, or adverse events.
Data collection and analysis
Two review authors independently extracted prespecified data from all included studies and assessed trial quality and risk of bias. We performed meta‐analyses where possible to pool results.
Main results
We included 14 trials with 1863 participants. Evidence from six studies showed that self management programmes improved quality of life in people with stroke (standardised mean difference (SMD) random effects 0.20, 95% confidence interval (CI) 0.00 to 0.41, P = 0.05; low quality evidence) and improved self efficacy (SMD, random effects 0.33, 95% CI 0.04 to 0.61, P = 0.03; low quality evidence) compared with usual care. Individual studies reported benefits for health‐related behaviours such as reduced use of health services, smoking, and alcohol intake, as well as improved diet and attitude. However, there was no superior effect for such programmes in the domains of locus of control, activities of daily living, medication adherence, participation, or mood. Statistical heterogeneity was mostly low; however, there was much variation in the types and delivery of programmes. Risk of bias was relatively low for complex intervention clinical trials where participants and personnel could not be blinded.
Authors' conclusions
The current evidence indicates that self management programmes may benefit people with stroke who are living in the community. The benefits of such programmes lie in improved quality of life and self efficacy. These are all well‐recognised goals for people after stroke. There is evidence for many modes of delivery and examples of tailoring content to the target group. Leaders were usually professionals but peers (stroke survivors and carers) were also reported ‐ the commonality is being trained and expert in stroke and its consequences. It would be beneficial for further research to be focused on identifying key features of effective self management programmes and assessing their cost‐effectiveness.
Plain language summary
Self management programmes for people living with the long‐term effects of stroke
Review question
What are the effects of self management programmes for people who have had a stroke?
Background
A stroke is caused by an interruption in the blood supply to parts of the brain resulting in damage that affects people's lives and changes their ability to live independently and with quality. It has been proposed that special training, called 'a self management programme', teaches people about stroke, helps them develop the skills to work with their problems and challenges, and helps them identify and achieve their own goals and help themselves.
Study characteristics
We found 14 studies up to April 2016 involving 1863 participants that looked at the benefits of these programmes for people with stroke. They were conducted in a variety of countries in a variety of formats ‐ sometimes in groups, sometimes individually, and for varying time periods.
Key results
We found that such programmes may improve the quality of life after stroke. People with stroke reported improvements in their ability to live the way they wanted and that they felt more empowered to take charge of their lives, rather than be dependent on other people for their happiness and satisfaction with life. There were no reports of any risks or negative effects.
Quality of the evidence
The majority of the studies were well conducted and represent credible evidence that self management programmes may benefit people with stroke who are living in the community.
Summary of findings
Summary of findings for the main comparison. Self management programmes compared with usual care for stroke.
| Self management programmes compared with usual care for stroke | ||||
|
Patient or population: adults with stroke Settings: community Intervention: self management programmes Comparison: either an inactive control intervention (usual care, wait list control), or an active control intervention (generic Chronic Condition Self‐Management programme; a component of the intervention programme; coping skills; or physical activity sessions only) | ||||
| Outcomes | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
|
Quality of life Change scores/post intervention SF‐12 or ‐36: physical or mental functioning EuroQol; SAQoL; SSQoL |
SMD 0.20 (0.00 to 0.41) |
469 (6) | ⊕⊕⊝⊝ low | Based on consistent findings across TIA: 6 studies in the meta‐analysis, and further individual studies using single QoL measures, we believe further research may improve our confidence in the estimate of effect. One study has results counter to the main body of evidence ‐ this study has potential risks due to very small numbers, potential differences at baseline and questions of dosage in the control group: removal of this study strengthens confidence in the positive finding. |
|
Self efficacy Change scores/postintervention Stroke self efficacy Locus of control |
Self efficacy SMD 0.33 (0.04 to 0.61) Locus of control SMD 0.02 (‐0.26 to 0.29) |
403 (6) | ⊕⊕⊝⊝ low | We believe that further research is likely to have an impact on the currently reported estimate of effect by increasing the power of the meta‐analysis |
|
Activity limitations Change scores/post intervention FAI, NEADL, or BI |
SMD 0.22 (‐0.03 to 0.46) |
160 (4) | ⊕⊕⊕⊝ moderate | Based on the effect estimate and the stated aims of the interventions, we believe further evidence may change this finding further towards significance |
|
Impairments Change scores/post intervention HADS |
MD ‐0.56 (‐1.27 to 0.15) |
648 (6) |
⊕⊕⊝⊝ low |
We believe there may be a trend towards significance in this meta‐analysis and that further research may clarify this |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
BI: Barthel Index; FAI: Frenchay Activities Index; HADS: Hospital Anxiety and Depression Scale; MD: mean difference; NEADL: Nottingham Extended Activities of Daily Living Scale; QoL: quality of life; SAQoL: Stroke and Aphasia Quality of Life; SF‐12: 12‐Item Short‐Form; SF‐36: 36‐Item Short Form Health Survey; SMD: standardised mean difference; SSQoL: Stroke Specific Quality of Life; TIA: transient ischaemic attack
Background
Description of the condition
Stroke is a sudden health event that has a considerable impact on individuals, families, and the greater community. A stroke occurs when the blood supply to a part of the brain is compromised, causing damage to the brain and often affecting functions such as movement of body parts, vision, swallowing, and communication. The World Health Organization (WHO) defines stroke as rapidly developing clinical signs of focal (at times global) disturbance of cerebral function, lasting more than 24 hours (unless interrupted by surgery or death) with no apparent cause other than that of vascular origin (Hatano 1976).
Although stroke occurs as an acute event, it is then a chronic health condition for the stroke survivor and is a leading cause of long‐term physical disability (Begg 2007; Muntner 2002; Wolfe 2000). The most common types of disability resulting from stroke are restriction in physical activities, incomplete use of limbs, difficulty gripping or holding items, and speech difficulties (AIHW 2011). Stroke is an ongoing burden to the individuals affected, and also to health systems. Approximately 50,000 Australians have a stroke per annum (Deloitte Access Economics 2014). During the first year after a first‐ever stroke, the estimated mean cost of care in Australia was AUD 18,956 (in 1997), or USD 14,361 per case, including informal and formal carer time costs (Dewey 2001). Furthermore, the majority of stroke survivors have chronic stroke‐related disabilities and require ongoing lifetime support. For example, in Australia, it is estimated that just over a third (131,100) of Australians with stroke had a disability from their stroke and were significantly more likely to be profoundly limited ("always need help") in core activities (56%) than people with other disabilities (AIHW 2013).
The main process of adjustment and learning to cope with a new disability after stroke takes place outside of formal rehabilitation settings (Cott 2007; Pound 1998). People with stroke may develop their own practical strategies for self management in the longer term (Pound 1998). However, many people with stroke will experience disappointment when they fail to make a full recovery or experience other setbacks (Dowswell 2000), and this could place them at a greater risk of developing depression (Jones 2006). Ongoing lifestyle risk factors can also put people at risk of a secondary stroke (AIHW 2013).
Recovery from stroke is not dependent solely on improvements in stroke‐related impairments; mood, cognition, motivation, and social support are also important factors (Hackett 2005). Approximately one‐third of stroke survivors have mood disorders, with depression and anxiety most frequently measured (Lees 2012). Carers of stroke survivors report disturbances in mood as the most stressful stroke‐related problem (Haley 2009), and post‐stroke depression is associated with increased disability (Pohjasvaara 2001). These factors combine in a complex interplay whereby physical, functional, social, and mental factors combine to influence quality of life (QoL) (Jeong 2012). QoL is frequently reported to be lower in stroke survivors compared with normative values (Cerniauskaite 2012). Furthermore, participation in life roles and engagement in activities in community settings are frequently reduced following stroke; in part due to transport and mobility issues, but also due to problems with communication and fatigue. Conversely, increased participation is associated with improved QoL (Mayo 2002).
Description of the intervention
Self management interventions for people with chronic disease aim to allow participants to make informed choices, to adopt new perspectives and generic skills that can be applied to new problems as they arise, to practice new health behaviours, and to maintain or regain emotional stability (Lorig 1993). They seek to facilitate behaviour change rather than provide a purely educational programme (Jones 2011), or teach compliance with specific treatment recommendations (Walker 2003). Self management interventions are distinct from simple patient education or skills training in that they are designed to encourage people with chronic diseases to take an active part in the management of their own condition (Foster 2007). Components of a self management intervention after stroke may include problem‐solving, goal‐setting, decision‐making, self monitoring, coping with the condition, or interventions that sustain or progress physical and psychological functioning (Walker 2003). Self management programmes can be provided by health professionals or lay leaders, and can be generic or condition‐specific. They can be delivered to individuals one‐to‐one or in a group format, and can have varying delivery styles such as face‐to‐face or online communication, written materials, or telephone. A self management intervention typically consists of a number of sessions to deliver the components of the intervention (rather than a single session).
How the intervention might work
Stroke is a chronic condition that can have long‐term psychological and social, as well as physical, sequelae for the affected person. Self management interventions focus on teaching skills so that individuals can better manage their chronic illness and thereby optimise their health and well‐being (Walker 2003). A premise of self management is that individuals who have a greater expectation that they are capable of performing a behaviour to produce a given outcome are seen as having greater 'self efficacy' (Bandura 1986). These expectations reflect a person's perceived, rather than actual, capabilities, and it is this self efficacy and not one's true abilities that often influences behaviour (Strecher 1986). For the person with stroke, self efficacy has been reported to be positively associated with outcomes including QoL (or perceived health status), depression, ability to perform activities of daily living (ADL), and walking ability (Jones 2011). Self management interventions for people after stroke that aim to increase individuals' abilities to solve problems, make decisions, and construct action plans for specific functional targets, could help prevent some of the difficulties that people with stroke face when discharged from rehabilitative health care (Jones 2006). Some programmes offer support and training for the carers of stroke survivors but these cannot be considered self management in the context of the person with stroke.
Why it is important to do this review
Provision of self management training is recommended in international stroke guidelines (Lindsay 2010; NSF 2010; Winstein 2016). However, there has not yet been a definitive review of the effectiveness of such interventions in this population to inform practice. Previous literature reviews of the effectiveness of self management interventions after stroke have been limited in the scope of articles retrieved ‐ for example excluding studies that provided a general chronic disease self management for stroke survivors (Jones 2011), or only considering interventions delivered by a nurse (Korpershoek 2011). The topic area would benefit from a comprehensive review of self management interventions after stroke that critically appraises the included studies and considers the application of statistical techniques to determine any possible treatment effect (Jones 2011).
Objectives
To assess the effects of self management interventions on the quality of life of adults with stroke who are living in the community, compared with inactive or active (usual care) control interventions.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), randomised at the individual participant level or via clusters with appropriate methods.
Types of participants
We included studies of adults (18 years and older) with stroke living in the community (own homes or independent living units). There were no restrictions according to gender, comorbidity, or length of time since stroke. We used the definition of stroke from the WHO as rapidly developing clinical signs of focal (at times global) disturbance of cerebral function, lasting more than 24 hours (unless interrupted by surgery or death) with no apparent cause other than that of vascular origin (Hatano 1976). If the sample group included mixed diagnoses (e.g. transient ischaemic attack or traumatic head injury), we contacted the authors for data specific to the stroke cohort.
Types of interventions
We included both self management interventions that were specific to stroke and those that were generic, so long as the participant group for the generic self management intervention included adults with stroke whose data were available separately for inclusion in our analysis. We included interventions provided by health professionals or lay leaders, or a combination of both. The self management interventions could be delivered to a group of participants or on an individual basis, and may have had a variety of delivery formats including, but not limited to, face‐to‐face, postal, or online delivery. To be included in our review, the intervention must have contained at least one of the following components: problem‐solving, goal‐setting, decision‐making, self monitoring, coping with the condition, or an alternative method designed to facilitate behaviour change and improvements in physical and psychological functioning. We excluded interventions that provided education only or exercise only to participants.
We included studies that compared a self management intervention with either an inactive control intervention (e.g. usual care, waiting list control), or an active control intervention (e.g. information only, or alternative intervention that was not considered self management).
Types of outcome measures
We included the following time points of outcome measurement in the review: 'end of intervention', 'first‐scheduled follow‐up', and 'end of scheduled follow‐up'.
Primary outcomes
Quality of life (QoL): health‐related, such as measured by the 36‐item Short Form (SF‐36) version 2, EuroQol (ED‐5D); or general, such as measured by the World Health Organization Quality of Life (WHOQOL)‐BREF.
Secondary outcomes
Self efficacy (usually measured by self report scales such as the General Self‐Efficacy Scale).
Activity limitations (including mobility and both basic and instrumental ADL, such as measured by the Functional Independence Measure or the Barthel Index).
Participation restrictions (including social, vocational, and recreational roles, such as measured by the Life Habits Instrument: LIFE‐H).
Impairments (including: mood, such as measured by the Hospital Anxiety and Depression Scale (HADS), Depression Anxiety Stress Scale; physical, such as measured by the Fugl‐Meyer Assessment of Sensorimotor Recovery After Stroke; cognition, such as measured by the Montreal Cognitive Assessment; speech and language such as measured by the Boston Assessment of Severe Aphasia).
Health service usage (including hospital readmissions, general practitioner attendance, emergency department visits).
Cost‐effectiveness of intervention (such as measured by the median cost of the intervention per quality‐adjusted life year (QALY)).
Participant satisfaction (such as measured by a Likert Satisfaction Scale).
Adverse events (type and frequency).
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged for the translation of relevant articles where necessary. The first date for searches was July to August 2013 and we updated the searches in April 2016.
Electronic searches
We developed the MEDLINE search strategy (Appendix 1) with the help of the Cochrane Stroke Group Information Specialist and adapted it for the other databases as follows.
MEDLINE (from 1948; Appendix 1).
EMBASE (from 1980; (Appendix 2).
CINAHL (from 1982; Appendix 3).
PsycINFO (from 1806; Appendix 3).
SCOPUS (www.scopus.com/home.url; Appendix 4).
Web of Science, Science Citation Index Expanded (from 1900; Appendix 5).
Web of Science Conference Proceedings Citation Index‐Science (from 1990; Appendix 5).
OTseeker (www.otseeker.com/; Appendix 6).
OTSearch (www1.aota.org/otsearch/; Appendix 6).
Physiotherapy Evidence database (PEDro) (www.pedro.org.au/; Appendix 7).
REHABDATA (www.naric.com/research/rehab/; Appendix 8).
Database of Abstracts of Reviews of Effects (DARE; www.crd.york.ac.uk/CRDWeb/AboutDare.asp): we searched this resource to identify potentially relevant reviews and screened the reference lists to identify primary studies (Appendix 9).
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (April 2016), and the trials registers of the Cochrane Stroke Group (Appendix 10) and the Cochrane Effective Practice and Organisation of Care (EPOC) Group (Appendix 11). In addition, we also searched the Proquest Dissertation and Theses (Appendix 12).
We also searched the following ongoing trials registers.
Australian New Zealand Clinical Trials Registry (www.anzctr.org.au/; Appendix 13).
ClinicalTrials.gov (www.clinicaltrials.gov/; Appendix 14).
Current Controlled Trials (www.controlled‐trials.com; Appendix 15).
Stroke Trials Registry (www.strokecenter.org/trials/; Appendix 16).
WHO International Clinical Trials Registry Platform (www.who.int/ictrp/en/; Appendix 17).
Searching other resources
We screened the reference lists of relevant studies to identify studies for potential inclusion in the review. We also used Science Citation Index Cited Reference Search for forward tracking of relevant articles.
Data collection and analysis
Selection of studies
Two review authors (MM and JL) independently assessed the titles and available abstracts of all records identified from the searches of the electronic databases and excluded clearly irrelevant studies. We obtained the full text of the remaining studies, and two review authors (CF and MM; CF and JL) assessed these for inclusion in the review according to the eligibility criteria. We included both published and unpublished trials and contacted authors for further information as required. We resolved disagreements by consensus, and by arbitration by a third review author (SH) if required. We provided reasons for exclusion for potentially relevant studies that, after further consideration, we excluded from the review.
Data extraction and management
Two of three review authors (CF, JL, MM) independently extracted data from the included trials using a standardised data extraction form specifically designed and piloted for this review. Extracted data included the following information from the included studies:
methods: including aim, design, unit of allocation;
participants: including inclusion/exclusion criteria, number randomised, withdrawals and exclusions, sample characteristics;
-
self management intervention: we collected the following information for each self management intervention:
intended audience (people with stroke, cardiovascular disease, chronic disease, or a mixed/combination audience);
theoretical rationale of the intervention (if one was reported and, when available, details of the rationale);
mode (delivered on a one‐to‐one basis or to groups of participants, with the size of the group recorded);
personnel (led by health professionals or trained facilitators, or combination of both; the number of personnel involved and qualifications/training/experience of personnel recorded);
delivery method (face‐to‐face, written such as workbook or pamphlet, audio, video, telephone, Internet; all methods used in intervention recorded);
language in which the intervention was delivered; content/topics covered by the intervention (problem‐solving, goal‐setting, decision‐making, self monitoring, coping with the condition);
location (hospital, general practitioner clinic, community setting, home); and
duration (number and frequency of sessions, hours per session, time between sessions, total duration of the intervention).
outcomes: including time points measured, unit of measurement, power;
other: source of funding, possible conflicts of interest;
risk of bias assessment: including details of sequence generation, allocation concealment, blinding, completeness of outcome data, selective outcome reporting; and
data and analysis: including length of follow‐up, loss to follow‐up, unit of analysis, statistical methods used. When a study had reported results for a self management intervention that included people with a range of chronic conditions, we contacted the study authors to request results specific to the participants with stroke.
We extracted a description of the separate components within each self management intervention for all of the included studies ‐ see Table 2.
1. Components of self management programmes.
| Reference | Problem solving | Goal setting | Decision‐making | Self monitoring |
Coping with the condition |
Additional self management strategies |
| Bishop 2014 | X | ‐ | ‐ | X | ‐ |
|
| Cadilhac 2011 | X | ‐ | ‐ | X | X |
|
| Evans‐Hudnall 2014 | X | X | X | X | X |
|
| Frank 2000 | X | ‐ | ‐ | ‐ | X |
|
| Harwood 2012 | X | X | X | X | ‐ |
|
| Hoffman 2014 | X | X | ‐ | X | X |
|
| Johnston 2007 | ‐ | X | ‐ | ‐ | X |
|
| Jones 2016 | X | X | ‐ | X | X |
|
| Kendall 2007 | X | X | ‐ | ‐ | X |
|
| Kim 2013 | ‐ | ‐ | ‐ | X | X |
|
| Lund 2012 | ‐ | X | ‐ | X | ‐ |
|
| McKenna 2015 | X | X | ‐ | X | X |
|
| Sabariego 2013 | X | X | ‐ | X | X |
|
| Tielemans 2015 | X | X | X | X | X |
|
In order to assess the effects of the intervention, we extracted data for the outcomes of interest (means and standard deviations for continuous outcomes and number of events for dichotomous outcomes) where available in the published reports.
Assessment of risk of bias in included studies
Two review authors (CF and MM) independently assessed the risk of bias in each included study against key criteria: random sequence generation, allocation concealment, blinding of outcomes, incomplete outcome data, and selective outcome reporting. We conducted assessments using Cochrane's tool for assessing risk of bias (Higgins 2011).
We judged selective outcome reporting based on whether all outcomes assessed in a trial had been reported. Where possible, we obtained trial protocols for comparison of planned outcome assessment to the outcome data available from each trial.
We explicitly judged each of the criteria assessed for risk of bias as: low risk of bias, high risk of bias, or unclear risk of bias (either lack of information or uncertainty over the potential for bias). We resolved disagreements by consensus, and consulted a third review author (SH) to resolve disagreements if necessary.
Measures of treatment effect
We calculated point estimates and 95% confidence intervals (CI) for outcomes of individual RCTs wherever possible. We expressed point estimates for dichotomous outcomes as odds ratios (OR). For continuous outcomes, we summarised results as mean difference (MD) where studies used the same tool to measure the same outcome across separate studies. Alternatively, we summarised treatment effects using the standardised mean difference (SMD) where studies measured the same outcome but employed different tools. If it was not possible to summarise results as above, we reported them as 'other data' narratively, but did not include them in the meta‐analysis (Deeks 2011).
Unit of analysis issues
We incorporated results of cluster randomised trials into meta‐analyses using the generic inverse variance method in Review Manager 5 (RevMan 2014). We estimated the intracluster correlation coefficient (ICC) for cluster randomised trials based on cluster number and mean cluster size (M). We used this to calculate the design effect using the formula: design effect = 1 + (M ‐ 1) ICC. Sample sizes for these trials were divided by the design effect (Higgins 2011).
Dealing with missing data
We sought data from authors for outcomes that were measured but not reported (Kirkham 2010), or that were not reported as data able to be incorporated in meta‐analyses, via email to the corresponding author. We also contacted authors for clarification of descriptions of interventions (e.g. setting, mode of delivery, format, duration, etc.) or trial conduct (e.g. method of random sequence generation, method of allocating participants to treatment groups, blinding of trial personnel). We considered intention‐to‐treat analysis as part of the risk of bias assessment and recorded loss to follow‐up.
Assessment of heterogeneity
Prior to meta‐analysis, we first assessed studies for clinical heterogeneity such as variations in interventions, comparisons, outcome measures, and assessment time points. We assessed statistical heterogeneity by visually inspecting the forest plots and then by using the I2 statistic as an indication of the proportion of heterogeneity. We used the following as a guide for interpretation of the I2 statistic: 0% to 14% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% represents considerable heterogeneity (Deeks 2011). In cases of substantial to considerable heterogeneity (defined as I2 > 50%), we would have explored the data further by comparing the characteristics of individual studies and reported any differences when interpreting the results of this review.
Assessment of reporting biases
The risk of publishing bias was mitigated by our comprehensive search strategies, checking all reference lists, and searching all major trial registries. We assessed selective outcome reporting using the approach described previously in Higgins 2011 (see Assessment of risk of bias in included studies). We would have further explored the potential for small‐study effects in the main outcomes of the review using funnel plots if a meta‐analysis included at least 10 studies.
Data synthesis
Where we considered studies to be sufficiently similar, we conducted a meta‐analysis by pooling the appropriate data using Review Manager 5 (RevMan 2014). We used random‐effects models with generic inverse‐variance method for all meta‐analyses (see Measures of treatment effect). Where data were not available or were of unacceptable heterogeneity, we provide a narrative summary of study results rather than a meta‐analysis.
Subgroup analysis and investigation of heterogeneity
If sufficient data were available, we would have performed subgroup analyses to establish effectiveness relative to:
study population characteristics ‐ including age, gender, and severity of stroke;
self management intervention ‐ including content, intended audience, mode, personnel, delivery method, location, and duration; and
study design ‐ including RCTs, cluster RCTs, and cross‐over trials.
We would have performed subgroup analyses using the independent variables for meta‐regression if the appropriate data had been available.
Sensitivity analysis
We would have performed sensitivity analyses to evaluate the influence of elements of risk of bias if we included sufficient studies, for example, based on whether participants were randomly allocated and group assignments were adequately concealed.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies tables.
Results of the search
The initial search strategy for this review was in July and August 2013. We repeated the search strategy for all databases, CENTRAL and Cochrane Stroke Group Trials register in February 2015 and again in April 2016 to update the review prior to publication.
The combined searches retrieved 18,950 records of trials after we removed duplicates. We selected 157 records for full‐text assessment, or for follow‐up with trial investigators if there were no published results, and we included 14 studies in the quantitative synthesis (Bishop 2014; Cadilhac 2011; Evans‐Hudnall 2014; Frank 2000; Harwood 2012; Hoffman 2014; Johnston 2007; Jones 2016; Kendall 2007; Kim 2013; Lund 2012; McKenna 2015; Sabariego 2013; Tielemans 2015). Figure 1 shows the flowchart of the combined results of the searches.
1.
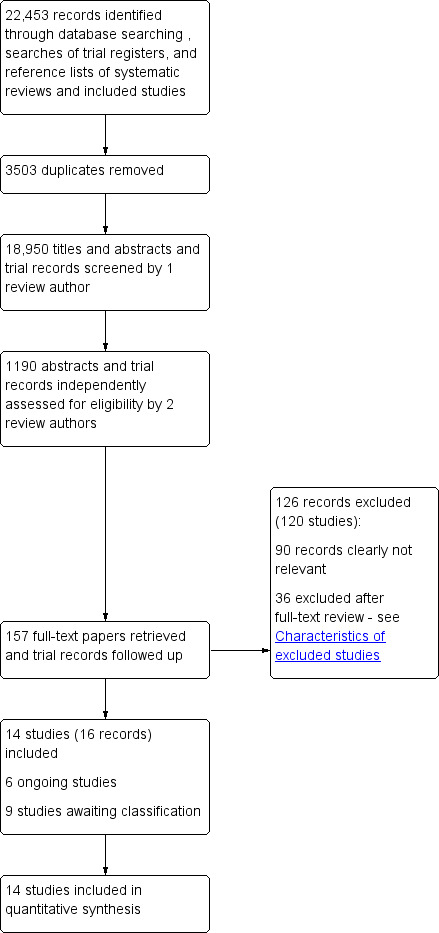
Flow diagram illustrating combined results of searches
We identified six ongoing trials; they did not yet have any results or published material to be considered. Nine trials are awaiting classification and we will assess them for inclusion in the next review update.
Included studies
The 14 included RCTs were all conducted between 2000 and 2015; four in the UK; three in the USA; two in Australia; and one each from New Zealand, the Netherlands, Korea, Norway, and Germany. There were 1863 participants; all adults post‐stroke, and sample sizes varied from 25 to 600. Stroke latency varied when reported from one month post‐stroke to one year or more. Stroke aetiology and severity, when reported, were also highly heterogeneous and reflected the expected mix of infarction/haemorrhage and severity from mild to moderate/severe.
The settings for the intervention were all community‐, home‐, or outpatient‐based. All studies investigated the effects of some form of programme that contained more than one component of self management as identified in our review criteria (see Types of interventions and Differences between protocol and review). We summarised the extracted components for each intervention (Table 2). In all studies, the audience was people with stroke and four studies included carers/significant others (Bishop 2014; Harwood 2012; Kim 2013; Tielemans 2015). Theoretical rationales varied from family systems to lifestyle‐ and occupation‐based approaches. All study reports included statements related to improving self efficacy, knowledge, beliefs, and confidence with a view to self management. Intervention mode varied from one‐to‐one (nine studies) or group (five studies) and all were delivered face‐to‐face except Bishop 2014, which used telephone contact. The programmes commonly used resources and workbooks to promote the material. Personnel were predominantly trained stroke‐allied health professionals conducting the programmes (13 RCTs), or co‐led with peer leaders (Cadilhac 2011). In some instances, the ethnic mix of the participants was matched in the programme leader, particularly for language and cultural considerations (Harwood 2012). Content and topics routinely consisted of stroke‐related education (including secondary prevention), self ratings, problem identification, reinforcing resources and capabilities, self efficacy and control, social support, stress management, goal setting, and problem‐solving. Duration of programmes varied from four weeks to six months, with number and timing of sessions differing between several to weekly.
Comparison groups involved an alternate 'active’ intervention in four studies: a generic Chronic Condition Self‐Management (CCSM) programme (Cadilhac 2011); components of the intervention programme (e.g. a DVD only or face‐to‐face session only: Harwood 2012; Tielemans 2015), coping skills (Hoffman 2014), or physical activity sessions only (Lund 2012). All other trials had an inactive, usual care, or wait list control group.
All studies used a battery of measures related to stroke recovery and health including tests of QoL (eight studies), activity limitations (10 studies), or self efficacy (seven studies). Tests for impairment were all related to mood (depression or anxiety, or both) (eight studies). Only three studies included measures of participation restrictions (Cadilhac 2011; McKenna 2015; Tielemans 2015), and one trial investigated medical adherence as part of a healthy behaviours battery (Evans‐Hudnall 2014). One study reported costs (Jones 2016) and one reported adverse events (Cadilhac 2011). Other measures used within the remit of this review included satisfaction, stroke knowledge, health competence, feasibility, and health service utilisation. All trials assessed outcomes at baseline and post‐intervention (four weeks to six months depending on the duration of the intervention), and the majority also conducted follow‐up measurement at between three and 12 months' post‐intervention.
Excluded studies
We found 120 studies (126 records) at full‐paper review or follow‐up of trial register entry that were clearly not relevant for reasons including inappropriate study design (non‐controlled) or interventions that did not meet our definition of self management, that is the interventions addressed only one aspect of the identified components of a self management programme or addressed only one stroke deficit or risk factor. For trials where the participant sample receiving the intervention included people with stroke and people with other chronic conditions, we attempted to gain separated data for the stroke participants but were unsuccessful.
We excluded See Characteristics of excluded studies table for individual reasons for study exclusion, other than studies that were not RCTs.
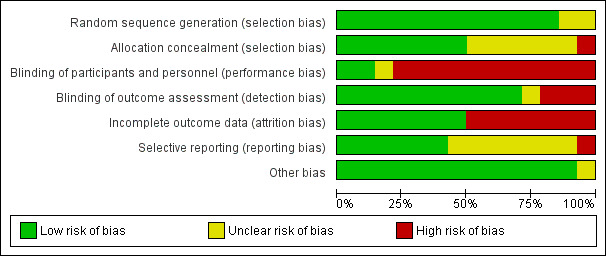
Risk of bias in included studies
We assessed the overall risk of bias as low. Figure 2 shows that the trials together covered a wide range of methodological quality, with the worst performance in the area of performance bias (only two studies achieving blinding of participants and some personnel). Figure 3 (individual trials) shows again that the majority of studies achieved a low risk of bias. No studies achieved low risk in all criteria, with individual scores ranging from achieving low risk on three out of seven to six out of seven areas.
2.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
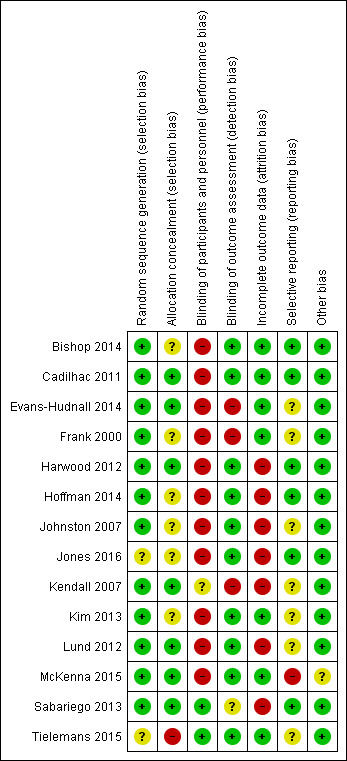
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Twelve studies reported appropriate sequence generation methods while two studies did not report their method (Jones 2016; Tielemans 2015). Concealed allocation was moderately well reported with eight of the studies confirming this was achieved.
Blinding
Only two studies achieved blinding of participants by concealing the nature of the intervention versus the comparison (Sabariego 2013; Tielemans 2015), and no studies achieved blinding of personnel who delivered the interventions; however, the majority reported satisfactory blinding of outcome assessors (11 studies). Where measures were self reported, we interpreted the blinding as pertaining to the administrator not the participant.
Incomplete outcome data
We identified incomplete reporting of outcome data for half the studies with differences in the proportion of drop‐outs or missing data between groups.
Selective reporting
We deemed just over half of the studies at low risk of selective reporting with the remainder judged as unclear (no protocols available to compare), or one not reporting data for secondary measures (McKenna 2015).
Other potential sources of bias
This criterion was at low risk for most studies: McKenna 2015 had missing data on dosage of intervention for five of the 11 intervention participants and we judged this to present an unclear or unknown risk of bias.
Effects of interventions
See: Table 1
Sufficient clinical homogeneity allowed us to pool study data, comparing self management interventions versus predominantly usual care intervention(s). We pooled trials with both usual care controls and control groups that incorporated a small active component of the intervention package (such as the education component only) and checked results using a post hoc subgroup analysis as this was not foreseen a priori. We used outcome data from similar time points post‐intervention: this was the immediate post‐intervention time given the interventions ran for weeks. However, in instances where the intervention was short (e.g. days), we compared with a more clinically comparable time point based on weeks/months that may have been the follow‐up period. Heterogeneity using the I2 statistic was 0% to 1% for all meta‐analyses.
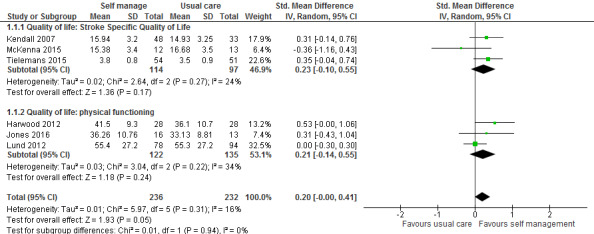
Quality of life
QoL scores were available for 469 participants from six trials (26% of overall participants included in the review). Three trials reported QoL scores from the SF‐36 physical functioning and mental functioning (Harwood 2012; Jones 2016; Lund 2012); and three used the Stroke Specific Quality of Life scale (SSQoL: Kendall 2007; McKenna 2015; Tielemans 2015). Several trials used more than one of these measures; we only included one trial in each measure. We did not include measures that were only used by one trial. The random‐effects pooled estimate for all trials was a SMD of 0.20 (95% CI 0.00 to 0.41; P = 0.05; low quality evidence; Analysis 1.1, Figure 4). Therefore, participants who received self management interventions had a significantly better QoL than those who received usual care or an intervention with a small active component. Removal of the active control trials (Harwood 2012; Lund 2012), as a post hoc subgroup analysis, strengthened the effect (SMD random effects 0.44, 95% CI 0.05 to 0.82; P = 0.03). Jones 2016 was a cluster randomised trial ‐ based on the number of clusters (four) and mean size of clusters (20) an ICC of 0.08 was estimated giving a design effect of 1.6 to be applied to the sample size. McKenna 2015 reported change scores in the published paper, but supplied post‐intervention scores for the 3 month follow‐up on request ‐ the latter are included in the meta‐analysis. It was noted that the baseline QoL scores were different between the self‐management versus control group (not significantly because of large standard deviations and small numbers), and whilst both groups improved over time the self‐management improved at a higher rate. Also of note the follow‐up for Kendall 2007 and Tielemans 2015 was at 6‐9 months compared to three months for mcKenna. Because of this, a sensitivity analysis was run, removing McKenna 2015. This gave a SMD of 0.23 (95% CI 0.04 to 0.41; P=0.02) and thus slightly strengthening the effect in favour of self‐management.
1.1. Analysis.
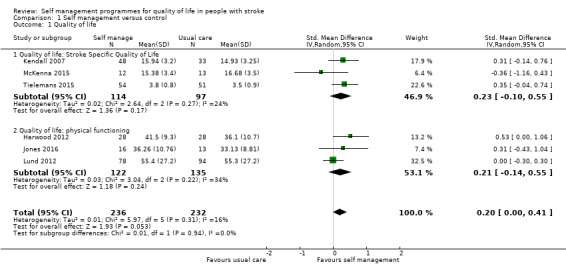
Comparison 1 Self management versus control, Outcome 1 Quality of life.
4.
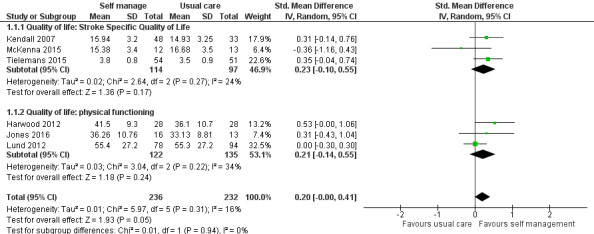
Forest plot of comparison: 1 Self management versus control, outcome: 1.1 Quality of life.
Self efficacy
Self efficacy scores were available for 403 participants from six trials (22% of overall participants included in the review). Four trials reported scores from the Stroke Self‐Efficacy Questionnaire (SSEQ) (Hoffman 2014; Jones 2016; Kendall 2007; McKenna 2015), and two studies used the Recovery Locus of Control Scale (RLOCS) (Frank 2000; Johnston 2007). The random‐effects pooled estimate for the four trials evaluating self efficacy was an SMD of 0.33 (95% CI 0.04 to 0.61; P = 0.03; low quality evidence; Analysis 1.2). Therefore, participants who received self management interventions had significantly better self efficacy than those who received usual care or an intervention with a small active component. The random‐effects pooled estimate for the two trials evaluating locus of control was an SMD of 0.02 (95% CI ‐0.26 to 0.29; P = 0.91; Analysis 1.2). Therefore, participants who received self management interventions did not have a significantly different locus of control compared with participants who received usual care.
1.2. Analysis.
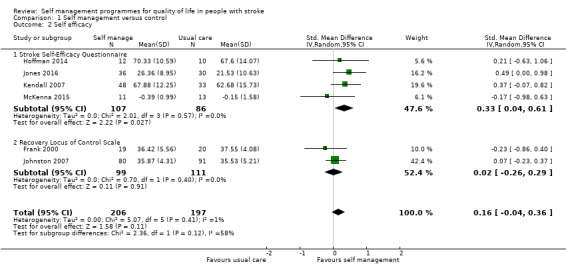
Comparison 1 Self management versus control, Outcome 2 Self efficacy.
Activity
Activity limitation scores were available for 260 participants (14% of overall participants included in the review). Four trials used the Barthel Index (Harwood 2012; Hoffman 2014; Johnston 2007; McKenna 2015). The random‐effects pooled estimate for all trials was an SMD of 0.22 (95% CI ‐0.03 to 0.46; P = 0.08; moderate quality evidence; Analysis 1.3). Therefore, participants who received self management programmes did not have significantly different levels of activity limitation compared with participants who received usual care, although the result does approach significance.
1.3. Analysis.
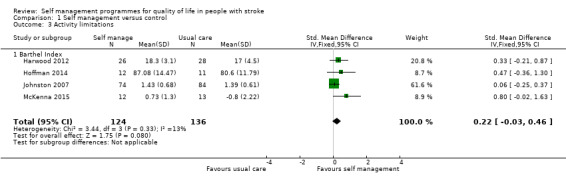
Comparison 1 Self management versus control, Outcome 3 Activity limitations.
Impairment
Mood scores were available for 648 participants (35% of overall participants). Six trials used the Hospital and Anxiety Depression Scale post‐intervention (Hoffman 2014; Johnston 2007; Jones 2016; Lund 2012; Sabariego 2013; Tielemans 2015). The random‐effects pooled estimate for all trials was an MD of ‐0.56 (95% CI ‐1.27 to 0.15; P = 0.12; low quality evidence). This pooled analysis used MD as there was only one type of measure (Analysis 1.4). Therefore, participants who received self management programmes did not have significantly different anxiety or depression levels compared with participants who received usual care.
1.4. Analysis.
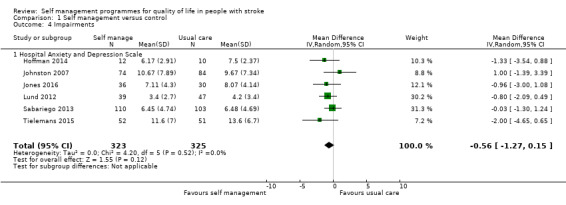
Comparison 1 Self management versus control, Outcome 4 Impairments.
Miscellaneous outcomes: single trial effects
All other measures listed in the inclusion section were only used in single trials and therefore were not pooled. Two studies had recognised measures of participation; McKenna 2015 used the Subjective Index of Physical and Social Outcome (SIPSO), which measures community integration, and Tielemans 2015 administered subscales of the USER‐Participation instrument. Neither found any difference in effect between their stroke self management programme and usual care for participation.
Evans‐Hudnall 2014 evaluated medication adherence as part of the global US Behavioral Surveillance Survey (BRFSS). They reported this item did not change significantly as a result of the 'STOP program' self management intervention. Several studies looked at other health behaviours including reductions in secondary risk factors and adoption of positive activity. Kim 2013 reported a positive effect on several such behaviours including reduced smoking and alcohol intake, improved diet and exercise levels, and greater control and motivation attitudes. With regard to health service usage, Bishop 2014 reported a reduction in use of services post‐intervention using a self report telephone checkup to record visits to health practitioners.
None of the studies performed a full cost‐effectiveness analysis. However, some studies simply reported costs of the actual programme. Johnston 2007 captured satisfaction with the self management programme and reported positive findings. They found no difference between education and self help programme and usual care. Cadilhac 2011 monitored adverse events, which reported no events were attributable to the self management programme.
Discussion
Summary of main results
The primary aim of this review was to investigate the effectiveness of self management programmes for adults with stroke, living in the community. For our primary outcome measure of QoL, we found that overall there was some supporting evidence; in a meta‐analysis pooling six studies, self management interventions were effective in improving health‐related QoL. No one study or measure offered evidence reaching significance; however, the superior sample size of combining six studies (469 participants) gave an effect size of 0.20 (SMD). We justified combining studies with an active control group with those with usual care control groups as from the descriptions 'usual care' did not equate to 'no or inactive' intervention and a post‐hoc subgroup analysis strengthened the result.
QoL is a complex construct and we originally wished to make a distinction between general QoL measures and those that were considered health‐related. From our included studies, the majority used health‐related QoL (e.g. the SF‐36 or 12‐item Short Form (SF‐12) or the SSQoL). Therefore, we made the decision to pool both categories for greater power and used SMD and random effects in acknowledgement that we were combining measures that may be conceptually somewhat different. It should be noted that individual studies used other measures that we did not include in the meta‐analysis, for example the Assessment of Quality of Life (AQoL) (general ‐ Cadilhac 2011) or WHOQOL (Sabariego 2013) or the health‐related General Health Questionnaire‐28 (GHQ‐28); the majority of these reported significant improvements. Pickard 2005 reviewed QoL measures for people with stroke and concluded a change score of 0.03 could be interpreted as clinically important. Therefore, the SMD of 0.20 (or 0.23 in the sensitivity analysis) can be interpreted as somewhat meaningful, and is strengthened by individual studies reporting this level of change.
A meta‐analysis for a secondary outcome of improved self efficacy found in favour of self management programmes, using the specific SSEQ but not the RLOCS. Self efficacy is a complex personality trait that involves a sense of ownership and agency over one's life. It has been reported as modifiable through intervention in some literature (Jones 2011), and is an obvious target domain for self management programmes. Self efficacy (characterised by generalised viewpoints such as 'when I make plans, I am certain I can make them work') has been viewed as related but different to locus of control (characterised by statements relative to internal or external states being the source of power such as 'my life is determined by my own actions') and both have been reported to act as dependent variables within the personality matrix (Judge 2002), along with other traits such as self esteem and emotional stability. Sabariego 2013 analysed the self management trial outcome data using multi‐level models of change and concluded that among other factors, loci of control was a significant predictor of self efficacy. Further investigation into the interpretation of self efficacy is warranted.
Activity limitations were variously captured by several studies using composite measures of functional (in)dependence. We were able to pool data from four studies using the Barthel Index. Our pooled analysis showed no significant effects in favour of either group, however, this did approach significance. This is not entirely unexpected as the evidence that activity performance improves with activity (task) practice is reasonably strong. It is not the intention of self management programmes to practice tasks in this way but rather to promote the overall management and coping capacity of people. Having said that, other individual studies did report some significant positive changes in activity, such as Johnston 2007 using the Observer Assessed Disability scale and McKenna 2015 using the Nottingham Extended Activities of Daily Living Scale (NEADL) and Barthel Index in favour of self management programmes. This interesting trend requires further investigation.
The only impairment level measures employed in the included studies were those related to mood; specifically depression and anxiety. We were able to pool six studies using the HADS and found a potential effect (MD ‐0.56) in favour of self management programmes reducing anxiety and depression post‐stroke but this did not reach significance. To add strength to a more positive interpretation, other included studies that used alternate measures, such as Cadilhac 2011 (Mood Scale) and McKenna 2015 (GHQ‐28), also reported significant positive improvements in mood after self management programmes.
Considering the remainder of our secondary measures, we were unable to perform further meta‐analyses due to the paucity or heterogeneity of measures. There were promising but inconsistent results in several of the single studies around improved health behaviours, such as better blood pressure control, improved diet and exercise, smoking/alcohol reductions, and reduced healthcare usage, but no evidence for improved participation. The low numbers and inconclusive findings suggest further studies powered for these questions are required.
Overall completeness and applicability of evidence
The content, format, and settings for the intervention were all highly variable and explain some of the inconsistent findings. There were insufficient studies to explore the factors that might be responsible for success but simple inspection of the formats does suggest that minimalist interventions such as workbooks need more support and engagement (Frank 2000). However, other low‐cost interventions, such as telephone tracking (Bishop 2014) or Internet‐based programmes (Kim 2013), can have a positive effect on stroke survivor and family functioning, and health behaviours, respectively. Factors such as intensity or personal (face‐to‐face) contact need further investigation to confirm their value.
Several studies had the direct aim of investigating the applicability of the self management programme tailored to specific racial or cultural groups such as Maori and Pacific New Zealanders (Harwood 2012) or under‐served racial and ethnic minority groups in the USA (Evans‐Hudnall 2014). The other studies spanned across several different countries. Therefore, there is emerging evidence that the format and content of self management programmes is able to be tailored and transferred to different communities and needs.
Quality of the evidence
The overall quality of the evidence was low to moderate. We believe the results can be considered to be somewhat indicative despite the relatively small numbers in the individual trials. Where there were higher risks of bias these were in effect acceptable as it is difficult, if not impossible, to blind personnel delivering personal interventions and likewise the participants can only be blinded to the intervention of interest, not to the fact that they are receiving an intervention.
Potential biases in the review process
We do not consider there to be any overt biases in the review process. All of the authors are experienced stroke clinicians and researchers but none have been involved in the conduct of self management programmes in a clinical setting or trials investigating self management programmes.
Agreements and disagreements with other studies or reviews
There are two other published systematic reviews of self management programmes for people after stroke (Lennon 2013; Warner 2015). However, the reviews differed in the information they considered primarily due to differences in design and timing of the review conduct. The other reviews qualitatively synthesised evidence from RCTs (Lennon 2013) and a combination of RCT and non‐controlled trials (Warner 2015), while this review identified and pooled evidence both quantitatively and qualitatively from RCTs only (14 studies, 1863 participants). Lennon 2013 included 15 studies (1233 participants) and Warner 2015 included nine studies (total number of participants not given). We excluded four RCTs included by Lennon 2013 and three RCTS included by Warner 2015 from our review due to differences in inclusion criteria. Whereas our review used the criterion of a complex intervention focusing on more than one deficit or risk and including at least two self management components, both Lennon 2013 and Warner 2015 used a broader criterion of accepting any studies in which the authors had referred to the intervention as 'self management'. Our review also differed in our decision not to include data from adults with transient ischaemic attack (not stroke) or inpatient participant populations. There were several RCTs included in our review that were published since the journal acceptance of the other two reviews; and we included six RCTs in our review that were not included in the other two reviews for reasons unknown (two RCTs Lennon 2013, six RCTs Warner 2015), perhaps due to differences in search strategy.
Our meta‐analysis supported the qualitative findings from Lennon 2013 that self management programmes can improve QoL and self efficacy for people with stroke. Our review did not support the suggestion by Warner 2015 that self management programmes can improve functional ability and participation of people with stroke. Unfortunately, questions still remain as all three reviews have observed gaps regarding the optimal content, timing, mode of delivery, target outcomes, and mechanisms for change in self management interventions for people after stroke, due to the large heterogeneity in the investigated interventions. The reviews also agreed that despite the increasing amount of published evidence about self management programmes after stroke, the wide range of outcome measures and frequency of assessments used in studies of this topic hampers the ability to synthesise the evidence to determine effect. Both this review and Lennon 2013 have called for cost‐effectiveness of the intervention to be investigated in future research of self management programmes for people after stroke.
Authors' conclusions
Implications for practice.
The current evidence indicates that self management programmes may benefit people with stroke in the community. Benefits may include improved quality of life and self efficacy. We observed trends to improve mood (reduce anxiety and depression) and independence in activities but these were not significant. These are all well‐recognised goals for people after stroke. There is evidence for many modes of delivery and the opportunity to tailor content to the target group. Leaders can be peers or professionals but their commonality is being trained and expert in stroke and its consequences.
Implications for research.
Further research is required to understand the complex effects on quality of life and the relationship between self efficacy, recovery, and locus of control. Identification of key features of the programmes is required, for example, what is the ideal frequency, duration, and mode of sessions? Cost‐effectiveness analyses will help service providers to make choices about provision of such programmes. Potential areas of benefit from self management programmes, such as health behaviours, participation, and other impairments, would be useful to investigate.
Feedback
New Feedback, 16 November 2018
Summary
| Comments | Review authors response |
| On behalf of Dr Faye Wray and Dr Tom Crocker (Academic Unit of Elderly Care and Rehabilitation, University of Leeds and Bradford Institute for Health Research) | On behalf of the review author team: Prof Susan Hillier, Dean: Research, University of South Australia. In consultation with the Editorial team, Cochrane Stroke. |
| We wish to express our concerns about the data used in this systematic review in Meta‐Analysis 1.1 (Quality of Life, sub‐group Stroke Specific Quality of Life). The data from McKenna 2015 used in this meta‐analysis is erroneous as this data represents the mean change score from program completion/six weeks to three month follow‐up instead of the mean change score from baseline. Using this data (which has a very large effect size) suggests that the pooled results significantly favour the intervention (self‐management) group. However, the data from baseline to three‐month follow‐up suggests that the difference between the intervention and control group is marginal. | Based on this comment, we have reviewed the data extracted from the McKenna paper. Indeed we did make an error (of oversight). The change score was indeed not calculated from baseline to post intervention or baseline to follow‐up (as assumed), but from post‐intervention to follow‐up. We have obtained the means and standard deviations (SDs) at each time point from the trialists. It is clear that the change scores were used because the two intervention groups were not similar at baseline. This was not significant because of large SDs and small numbers but was consistent across all measures. |
| Furthermore, the use of change scores is problematic as the meta‐analysis uses standardised mean differences. This is problematic because the standard deviations, used to standardise the scores to a uniform scale, do not reflect differences in the measurement scale in the case of change scores (Cochrane Handbook, p.270). | Again this is our error (of analysis). The Cochrane Handbook does confirm that delta means (SDs) and post‐intervention means (SDs) can be combined in meta‐analysis if using Mean Difference analyses but not SMDs (which we did). |
| Finally, the wrong number of participants has been entered, slightly inflating the weight for this study. | The error (of entry) was one participant. Corrected with no change to analysis. |
| A number of options are available to correct these errors and the authors should consider which is most appropriate based upon the following points: 1) Timepoint: the authors should consider which timepoint is most appropriate for this meta‐analysis. In the text of the review, the authors specify that outcome data will be used from the immediate timepoint post‐intervention except for instances where the intervention was short (e.g. days). It may, therefore, be appropriate to use data from baseline to six weeks (programme completion). On the other hand, the authors may wish to include the three‐month data as this is more comparable to the timepoints reported by other interventions in the meta‐analysis. |
We have considered the timepoints. Quality of life is a construct that is unlikely to change immediately post‐intervention, therefore we have continued to use the follow‐up data from all relevant studies. In the QoL meta‐analysis this is between three to six months across the studies. And we have kept it so. |
| 2) Mean change score versus raw means: in either case, the authors should consider whether it is appropriate to use mean change scores or raw post‐intervention means in their analysis. If the authors choose to include the mean change score it should be the change from baseline. The Cochrane Handbook is not clear as to whether mean change or raw mean scores are preferred. It suggests that either can give an indication of the effects of an intervention but that care should be taken to ensure that bias is not introduced by picking more favourable data. Data from McKenna 2015 suggests that the use of raw mean versus mean change score will vary the outcome with regards to whether the intervention or control is favoured in the meta‐analysis (although the effects of the intervention are likely to remain non‐significant overall). For example, at three months follow‐up, mean change scores favour the intervention but raw mean scores favour the control. Obtaining unpublished data from McKenna 2015 may be helpful for a precise estimation of variance for the intervention and control groups. | We have obtained the group means and SDs for the follow‐up timepoints. We have re‐analysed and amended. With a question mark over the McKenna trial – risk of baseline imbalance and a difference in timepoint measure of three months follow‐up versus six to nine months follow‐up – there is a case for either not including OR a sensitivity analysis, i.e. reporting the McKenna data in and out. On discussion with Cochrane Stroke’s Editorial team we agreed to include and then do a sensitivity analysis. As we had reported originally for the overall QoL result, the effect remains significant (P = 0.05) but the SMD is smaller and potentially not as clinically significant (Figure 9). Overall QoL effect is 0.20 (0.00 to 0.41) P = 0.05 We have also downgraded the GRADE to low (from moderate) and added in the sensitivity analysis with an explanation as to why we did this – this increases the SMD slightly to 0.23 (0.04 to 0.41) P = 0.02 (Figure 10) If you consider the results from ONLY 1.1.1 (which we don’t in the review) the effect sizes are greater: 0.23 (‐0.10 to 0.55) P = 0.17 OR in the sensitivity analysis 0.33 (0.04 to 0.63) P = 0.03. |
| 3) Considerations if mean change scores are used: if the authors wish to include change from baseline and post‐intervention scores in the same meta‐analysis they should combine scores using the mean difference (rather than the standardised mean difference). In this case it would be necessary to separate the studies in meta‐analysis 1.1 into separate meta‐analyses according to the measurement scale: the Stroke Specific Quality of Life scale (SSQOL) (Kendall 2007; McKenna 2015), the short version of the Stroke‐Specific Quality of Life Scale (Tielemans 2015), the Short Form (SF) 36 Physical Component Summary (PCS) (Harwood 2012), SF‐12 PCS (Jones 2016), and the SF‐36 Physical Functioning scale (Lund 2012). If the authors wish to combine standardised mean differences, the data from McKenna 2015 should be replaced with post‐intervention raw means from unpublished data or imputed from the data available at baseline. | This was not a necessary option as we could obtain the means (SD) and retain the SMD approach to allow a more powerful pool of data. |
| Correction of these errors using the options outlined above is likely to have a significant effect on the outcome of this meta‐analysis and result in a non‐significant pooled estimate of effect for Stroke Specific Quality of Life. Retaining the authors’ current approach of combining standardised mean differences and data for the other five studies but replacing the data for McKenna 2015 with imputed post‐intervention raw means (using a standard deviation pooled from the baseline data) produces an overall pooled standardised mean difference of 0.16 (95% confidence interval (CI) ‐0.08 to 0.41) with six‐week data or 0.17 (95% CI ‐0.02 to 0.37) with three‐month data. In light of this, the authors may not only need to update their analyses but will also need to update the summary of findings and conclusions to reflect a lack of evidence for the effect of self‐management interventions on stroke survivors’ quality of life. | As above: the QoL meta‐analysis, as we had reported for the overall QoL result, remains significant (P = 0.05) but the SMD is smaller and potentially not as clinically significant. This has been amended and we have also downgraded the GRADE to low (from moderate) and added in the sensitivity analysis with an explanation as to why we did this – this increases the SMD slightly to 0.23 from 0.20. Therefore we have not amended the summary of findings beyond this, nor the conclusions. It remains that we need more robust and properly powered studies to be confident. |
5.
Feedback graph: full analysis
6.
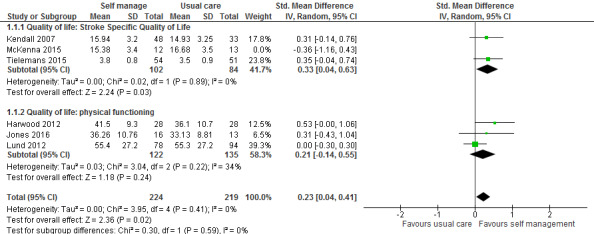
Feedback graph: sensitivity analysis – McKenna removed
Reply
See above
Contributors
See above
What's new
| Date | Event | Description |
|---|---|---|
| 5 March 2019 | Feedback has been incorporated | Analysis 1.1 amended in response to feedback |
| 30 January 2019 | Amended | Change scores for McKenna 2015 were replaced with mean (SD) scores at follow‐up for both groups in analysis 1.1. The results for an effect in favour of self‐management were somewhat weakened so we downgraded the level of evidence to 'low'. A sensitivity analysis removing McKenna 2015 strengthened the results towards favouring the intervention. |
Acknowledgements
The authors acknowledge and thank Brenda Thomas from the Cochrane Stroke Group for her assistance with the search strategy and Hazel Fraser also from the Cochrane Stroke Group for her patient, expert, and timely support.
Appendices
Appendix 1. MEDLINE search strategy
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/ or vertebral artery dissection/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ or exp paresis/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. brain injuries/ or brain injury, chronic/ 8. exp Gait Disorders, Neurologic/ 9. or/1‐8 10. self efficacy/ or self care/ 11. self administration/ or self‐assessment/ or self concept/ 12. patient compliance/ or patient education as topic/ or patient participation/ or patient satisfaction/ 13. consumer health information/ or consumer participation/ 14. attitude to health/ or health behavior/ or health education/ or health knowledge, attitudes, practice/ or health promotion/ 15. life style/ or disease management/ or risk reduction behavior/ 16. adaptation, psychological/ or motivation/ or goals/ or problem solving/ or exp decision making/ 17. health plan implementation/ 18. (self care or self‐care or self management or self‐management or self efficacy or self‐efficacy or self monitor$ or self‐monitor$).tw. 19. ((self or oneself) adj3 care).tw. 20. ((patient$ or consumer$ or client$) adj5 (educat$ or participat$ or behaviour$ or behavior$ or compliance or centered)).tw. 21. (health adj5 (promot$ or educat$ or behav$)).tw. 22. (risk adj3 reduc$ adj3 behav$).tw. 23. ((patient$ or consumer$ or client$) adj5 manag$ adj5 disease$).tw. 24. (((behav$ adj3 chang$) or (problem$ adj3 solv$) or (goal$ adj3 setting) or (decision$ adj3 mak$) or coping) adj5 (patient$ or consumer$ or client$)).tw. 25. or/10‐24 26. Randomized Controlled Trials as Topic/ 27. random allocation/ 28. Controlled Clinical Trials as Topic/ 29. control groups/ 30. clinical trials as topic/ 31. double‐blind method/ 32. single‐blind method/ 33. Placebos/ 34. placebo effect/ 35. Research Design/ 36. Program Evaluation/ 37. randomized controlled trial.pt. 38. controlled clinical trial.pt. 39. clinical trial.pt. 40. (random$ or RCT or RCTs).tw. 41. (controlled adj5 (trial$ or stud$)).tw. 42. (clinical$ adj5 trial$).tw. 43. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 44. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 45. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 46. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 47. placebo$.tw. 48. sham.tw. 49. (assign$ or allocat$).tw. 50. controls.tw. 51. or/26‐50 52. 9 and 25 and 51 53. exp animals/ not humans.sh. 54. 52 not 53
Appendix 2. EMBASE search strategy
| 1. | cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/ or vertebral artery dissection/ |
| 2. | (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. |
| 3. | ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. |
| 4. | ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. |
| 5. | hemiplegia/ or exp paresis/ |
| 6. | (hemipleg$ or hemipar$ or paresis or paretic).tw. |
| 7. | brain injuries/ or brain injury, chronic/ |
| 8. | exp Gait Disorders, Neurologic/ |
| 9. | OR/1‐8 |
| 10. | self efficacy/ or self care/ |
| 11. | self administration/ or self‐assessment/ or self concept/ |
| 12. | patient compliance/ or patient education as topic/ or patient participation/ or patient satisfaction/ |
| 13. | consumer health information/ or consumer participation/ |
| 14. | attitude to health/ or health behavior/ or health education/ or health knowledge, attitudes, practice/ or health promotion/ |
| 15. | life style/ or disease management/ or risk reduction behavior/ |
| 16. | adaptation, psychological/ or motivation/ or goals/ or problem solving/ or exp decision making/ |
| 17. | health plan implementation/ |
| 18. | (self care or self‐care or self management or self‐management or self efficacy or self‐efficacy or self monitor$ or selfmonitor$).tw. |
| 19. | ((self or oneself) adj3 care).tw. |
| 20. | ((patient$ or consumer$ or client$) adj5 (educat$ or participat$ or behaviour$ or behavior$ or compliance or centered)).tw. |
| 21. | (health adj5 (promot$ or educat$ or behav$)).tw. |
| 22. | (risk adj3 reduc$ adj3 behav$).tw. |
| 23. | ((patient$ or consumer$ or client$) adj5 manag$ adj5 disease$).tw. |
| 24. | (((behav$ adj3 chang$) or (problem$ adj3 solv$) or (goal$ adj3 setting) or (decision$ adj3 mak$) or coping) adj5 (patient$ or consumer$ or client$)).tw. |
| 25. | OR/10‐24 |
| 26. | Randomized Controlled Trials as Topic/ |
| 27. | random allocation/ |
| 28. | Controlled Clinical Trials as Topic/ |
| 29. | control groups/ |
| 30. | clinical trials as topic/ |
| 31. | double‐blind method/ |
| 32. | single‐blind method/ |
| 33. | Placebos/ |
| 34. | placebo effect/ |
| 35. | Research Design/ |
| 36. | Program Evaluation/ |
| 37. | randomized controlled trial/ |
| 38. | *controlled clinical trial/ |
| 39. | clinical trial/ |
| 40. | (random$ or RCT or RCTs).tw. |
| 41. | (controlled adj5 (trial$ or stud$)).tw. |
| 42. | (clinical$ adj5 trial$).tw. |
| 43. | ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. |
| 44. | (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. |
| 45. | ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. |
| 46. | ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. |
| 47. | placebo$.tw. |
| 48. | sham.tw. |
| 49. | (assign$ or allocat$).tw. |
| 50. | controls.tw. |
| 51. | OR/26‐50 |
| 52. | 9 and 25 and 51 |
| 53. | exp animals/ not humans.sh. |
| 54. | 52 not 53 |
Appendix 3. CINAHL and PsycInfo search strategy
| 1. | (MH “cerebrovascular disorders”) or (MH “basal ganglia cerebrovascular disease+”) or (MH “brain ischemia+”) or (MH “carotid artery diseases+”) or (MH “intracranial arterial diseases+”) or (MH "intracranial embolism and thrombosis+") or (MH “intracranial hemorrhages+”) or (MH stroke) or (MH “brain infarction+”) or (MH “vasospasm, intracranial”) or (MH “vertebral artery dissection”) |
| 2. | (stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva* or apoplex* or SAH) |
| 3. | ((brain* or cerebr* or cerebell* or intracran* or intracerebral) N5 (isch#emi* or infarct* or thrombo* or emboli* or occlus*)) |
| 4. | ((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) N5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)) |
| 5. | (hemipleg* or hemipar* or paresis or paretic) |
| 6. | (MH “brain injuries”) or (MH “brain damage, chronic+”) |
| 7. | MH “Gait Disorders, Neurologic+” |
| 8. | OR/1‐7 [S13] |
| 9. | (MH "Self‐Efficacy") or (MH "Self Care") |
| 10. | (MH "Self Administration") or (MH "Self Assessment") or (MH "Self Concept") |
| 11. | (MH "Patient Compliance") or (MH "Patient Education") or (MH "Consumer Participation") or (MH "Patient Satisfaction") |
| 12. | (MH "Consumer Health Information") |
| 13. | (MH "Attitude to Health") or (MH "Health Behavior") or (MH "Health Education") or (MH "Attitude to Health") or (MH "Health Knowledge and Behavior (Iowa NOC) (Non‐Cinahl)") or (MH "Health Promotion") |
| 14. | (MH "Life Style") or (MH "Disease Management") |
| 15. | (MH "Adaptation, Psychological") or (MH "Motivation") or (MH "Goals and Objectives") or (MH "Problem Solving") or (MH "Decision Making+") |
| 16. | “health plan implementation” |
| 17. | |
| 18. | (self care or self‐care or self management or self‐management or self efficacy or self‐efficacy or self monitor* or selfmonitor*) [S50] |
| 19. | ((self or oneself) N3 care) |
| 20. | ((patient# or consumer# or client#) N5 (educat* or participat* or behaviour? or behaviour? or compliance or centered)) |
| 21. | (health N5 (promot* or educat* or behav*)) |
| 22. | (risk N3 reduc* N3 behav*) |
| 23. | ((patient# or consumer# or client#) N5 manag* N5 disease#) |
| 24. | (((behav* N3 chang*) or (problem# N3 solv*) or (goal* N3 setting) or (decision# N3 mak*) or coping) N5 (patient? or consumer? or client?)) |
| 25. | OR/9‐24 {rerun ] |
| 26. | (MH "Randomized Controlled Trials") |
| 27. | (MH "Random Assignment") |
| 28. | (MH "Clinical Trials") |
| 29. | (MH "Control Group") |
| 30. | (MH "Double‐Blind Studies") |
| 31. | “single‐blind method” |
| 32. | (MH "Placebos") |
| 33. | (MH "Placebo Effect") |
| 34. | (MH "Study Design") |
| 35. | (MH "Program Evaluation") [S68] 149059 |
| 36. | (random* or RCT or RCTs) |
| 37. | (controlled N5 (trial? or stud*)) |
| 38. | (clinical? N5 trial?) |
| 39. | ((control or treatment or experiment? or intervention) N5 (group? or subject? or patient?)) |
| 40. | (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*) |
| 41. | ((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*)) [S74] 184168 |
| 42. | ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*)) |
| 43. | placebo? |
| 44. | sham |
| 45. | (assign* or allocat*) |
| 46. | controls |
| 47. | OR/26‐46 [S |
| 48. | 9 and 25 and 47 |
| 49. | (MH "Animals+") |
| 50. | 48 not 49 |
Appendix 4. SCOPUS search strategy
((TITLE‐ABS‐KEY((strokeOR poststroke OR post‐stroke OR cerebrovasc* OR brain vasc* OR cerebral vasc*OR cva* OR apoplex* OR sah)) AND SUBJAREA(mult OR medi OR nurs OR veteOR dent OR heal)) OR (TITLE‐ABS‐KEY(((brain* OR cerebr* OR cerebell* OR intracran* OR intracerebral) W/5 (isch?emi* OR infarct* OR thrombo* OR emboli* OR occlus*))) AND SUBJAREA(mult OR medi OR nursOR vete OR dent OR heal)) OR (TITLE‐ABS‐KEY(((brain* OR cerebr* OR cerebell* OR intracerebral OR intracranial OR subarachnoid) W/5 (haemorrhage* OR hemorrhage* OR haematoma* OR hematoma* OR bleed*))) AND SUBJAREA(mult OR medi OR nursOR vete OR dent OR heal)) OR (TITLE‐ABS‐KEY((hemipleg*OR hemipar* OR paresis OR paretic)) AND SUBJAREA(mult OR medi OR nurs OR veteOR dent OR heal))) AND ((TITLE‐ABS‐KEY((self care OR self‐care OR self managementOR self‐management OR self efficacy OR self‐efficacy OR self monitor* OR selfmonitor*)) AND SUBJAREA(mult OR medi OR nurs OR vete OR dentOR heal)) OR (TITLE‐ABS‐KEY(((selfOR oneself) W/3 care)) AND SUBJAREA(mult OR medi OR nurs OR vete OR dentOR heal)) OR (TITLE‐ABS‐KEY(((patient*OR consumer* OR client*) W/5 (educat* OR participat* OR behaviour* OR behavior* OR compliance OR centered))) AND SUBJAREA(mult OR medi OR nurs OR vete OR dentOR heal)) OR (TITLE‐ABS‐KEY((healthW/5 (promot* OR educat* OR behav*))) AND SUBJAREA(mult OR medi OR nurs OR veteOR dent OR heal)) OR (TITLE‐ABS‐KEY((risk W/3 reduc*W/3 behav*)) AND SUBJAREA(multOR medi OR nurs OR vete OR dent OR heal)) OR (TITLE‐ABS‐KEY(((patient* OR consumer*OR client*) W/5 manag* W/5 disease*)) AND SUBJAREA(mult OR medi OR nurs OR veteOR dent OR heal)) OR (TITLE‐ABS‐KEY((((behav* W/3 chang*) OR (problem* W/3 solv*) OR (goal* W/3 setting) OR (decision* W/3 mak*) OR coping) W/5 (patient* OR consumer* OR client*))) AND SUBJAREA(mult OR medi OR nursOR vete OR dent OR heal))) AND (((TITLE‐ABS‐KEY((random*OR rct OR rcts)) AND SUBJAREA(mult OR medi OR nursOR vete OR dent OR heal)) OR (TITLE‐ABS‐KEY((controlledW/5 (trial* OR stud*))) AND SUBJAREA(mult OR medi OR nurs OR vete OR dentOR heal)) OR (TITLE‐ABS‐KEY((clinical*W/5 trial*)) AND SUBJAREA(multOR medi OR nurs OR vete OR dent OR heal)) OR (TITLE‐ABS‐KEY(((control OR treatmentOR experiment* OR intervention) W/5 (group* OR subject* OR patient*))) AND SUBJAREA(mult OR medi OR nurs OR veteOR dent OR heal)) OR (TITLE‐ABS‐KEY((quasi‐random* OR quasirandom* OR pseudo‐random* OR pseudo random*)) AND SUBJAREA(mult OR medi OR nursOR vete OR dent OR heal)) OR (TITLE‐ABS‐KEY(((control OR experiment* OR conservative) W/5 (treatment OR therapy OR procedure OR manage*))) AND SUBJAREA(mult OR medi OR nursOR vete OR dent OR heal)) OR (TITLE‐ABS‐KEY(((singl* OR doubl* OR tripl* OR trebl*) W/5 (blind* OR mask*))) AND SUBJAREA(mult OR medi OR nurs OR vete OR dentOR heal))) OR ((TITLE‐ABS‐KEY(placebo*OR sham) AND SUBJAREA(mult OR medi OR nurs OR veteOR dent OR heal)) OR (TITLE‐ABS‐KEY((assign* OR allocat*)) AND SUBJAREA(mult OR medi OR nurs OR vete OR dentOR heal)) OR (TITLE‐ABS‐KEY(controls) AND SUBJAREA(mult OR medi OR nurs OR vete OR dentOR heal)))
Excluded: Subject areas biochemistry, genetics, molecular biology, Engineering, Chemical Engineering, Physics and Astronomy, Veterinary; Source type book series, trade publications, Document types editorials.
Appendix 5. Web of Science search strategy
| 1. | (stroke OR poststroke OR post‐stroke OR cerebrovasc* OR brain vasc* OR cerebral vasc* OR cva* OR apoplex* OR SAH) |
| 2. | ((brain* OR cerebr* OR cerebell* OR intracran* OR intracerebral) NEAR/5 (isch$emi* OR infarct* OR thrombo* OR emboli* OR occlus*)) |
| 3. | ((brain* OR cerebr* OR cerebell* OR intracerebral OR intracranial OR subarachnoid) NEAR/5 (haemorrhage* OR hemorrhage* OR haematoma* OR hematoma* OR bleed*)) |
| 4. | (hemipleg* OR hemipar* OR paresis OR paretic) |
| 5. | OR/1‐4 |
| 6. | (self care OR self‐care OR self management OR self‐management OR self efficacy OR self‐efficacy OR self monitor* OR selfmonitor*) |
| 7. | ((self OR oneself) NEAR/3 care) |
| 8. | ((patient* OR consumer* OR client*) NEAR/5 (educat* OR participat* OR behaviour* OR behavior* OR compliance OR centered)) |
| 9. | (health NEAR/5 (promot* OR educat* OR behav*)) |
| 10. | (risk NEAR/3 reduc* NEAR/3 behav*) |
| 11. | ((patient* OR consumer* OR client*) NEAR/5 manag* NEAR/5 disease*) |
| 12. | (((behav* NEAR/3 chang*) OR (problem* NEAR/3 solv*) OR (goal* NEAR/3 setting) OR (decision* NEAR/3 mak*) OR coping) NEAR/5 (patient* OR consumer* OR client*)) |
| 13. | OR/6‐12 |
| 14. | (random* OR RCT OR RCTs) |
| 15. | (controlled NEAR/5 (trial* OR stud*)) |
| 16. | (clinical* NEAR/5 trial*) |
| 17. | ((control OR treatment OR experiment* OR intervention) NEAR/5 (group* OR subject* OR patient*)) |
| 18. | (quasi‐random* OR quasi random* OR pseudo‐random* OR pseudo random*) |
| 19. | ((control OR experiment* OR conservative) NEAR/5 (treatment OR therapy OR procedure OR manage*)) |
| 20. | ((singl* OR doubl* OR tripl* OR trebl*) NEAR/5 (blind* OR mask*)) |
| 21. | placebo* |
| 22. | sham |
| 23. | (assign* or allocat*) |
| 24. | controls |
| 25. | OR/14‐23 |
| 26. | 5 and 13 and 25 |
| 27. | Exclude letters, editorials, books, notes |
| 28. | 26 or 27 |
Appendix 6. OTseeker search strategy
| 1 | (stroke OR cerebrovascular OR cerebro‐vascular OR CVA OR hemiplegia OR hemiparesis) AND (“self care” OR “self management” OR self‐management OR “self efficacy” OR self‐efficacy OR “self monitoring”) |
| 2 | (stroke OR cerebrovascular OR cerebro‐vascular OR CVA OR hemiplegia OR hemiparesis) AND (“health promotion” OR “health education” OR “health behaviour” OR “health behavior” OR “patient education” OR “patient behaviour” OR “patient behavior”) |
Appendix 7. PEDro search strategy
| 1. | Abstract & title: | *stroke Self‐management |
| Method | Clinical trial |
Appendix 8. REHABDATA search strategy
| 1. | Abstract & title: | stroke AND “Self‐management” |
Appendix 9. DARE search strategy
| 1. | MeSH DESCRIPTOR Stroke EXPLODE ALL TREES |
| 2. | MeSH DESCRIPTOR Hemiplegia EXPLODE ALL TREES |
| 3. | MeSH DESCRIPTOR Paresis EXPLODE ALL TREES |
| 4. | MeSH DESCRIPTOR cerebrovascular trauma EXPLODE ALL TREES |
| 5. | MeSH DESCRIPTOR Brain injury EXPLODE ALL TREES |
| 6. | MeSH DESCRIPTOR Brain injury, Chronic EXPLODE ALL TREES |
| 7. | MeSH DESCRIPTOR Gait Disorders, EXPLODE ALL TREES |
| 8. | OR/1‐7 |
| 9. | MeSH DESCRIPTOR self efficacy EXPLODE ALL TREES |
| 10. | MeSH DESCRIPTOR self care EXPLODE ALL TREES |
| 11. | MeSH DESCRIPTOR self administration EXPLODE ALL TREES |
| 12. | MeSH DESCRIPTOR self‐assessment EXPLODE ALL TREES |
| 13. | MeSH DESCRIPTOR self concept EXPLODE ALL TREES |
| 14. | MeSH DESCRIPTOR patient compliance EXPLODE ALL TREES |
| 15. | MeSH DESCRIPTOR patient participation EXPLODE ALL TREES |
| 16. | MeSH DESCRIPTOR patient satisfaction EXPLODE ALL TREES |
| 17. | MeSH DESCRIPTOR Consumer Participation EXPLODE ALL TREES |
| 18. | MeSH DESCRIPTOR Consumer Health Information EXPLODE ALL TREES |
| 19. | MeSH DESCRIPTOR Health Behavior EXPLODE ALL TREES |
| 20. | MeSH DESCRIPTOR Attitude to Health EXPLODE ALL TREES |
| 21. | MeSH DESCRIPTOR Health Education EXPLODE ALL TREES |
| 22. | MeSH DESCRIPTOR Health Knowledge, Attitudes, Practice EXPLODE ALL TREES |
| 23. | MeSH DESCRIPTOR Health Promotion EXPLODE ALL TREES |
| 24. | MeSH DESCRIPTOR Life Style EXPLODE ALL TREES |
| 25. | MeSH DESCRIPTOR Disease Management EXPLODE ALL TREES |
| 26. | MeSH DESCRIPTOR Risk Reduction Behavior EXPLODE ALL TREES |
| 27. | MeSH DESCRIPTOR Adaptation, Psychological EXPLODE ALL TREES |
| 28. | MeSH DESCRIPTOR Motivation EXPLODE ALL TREES |
| 29. | MeSH DESCRIPTOR Goals EXPLODE ALL TREES |
| 30. | MeSH DESCRIPTOR problem solving EXPLODE ALL TREES |
| 31. | MeSH DESCRIPTOR Decision making EXPLODE ALL TREES |
| 32. | MeSH DESCRIPTOR Health plan implementation EXPLODE ALL TREES |
| 33. | self care or self‐care or self management or self‐management or self efficacy or self‐efficacy or self monitor* or selfmonitor* |
| 34. | (self or oneself) NEAR3 care |
| 35. | ((patient* or consumer* or client*) NEAR5 (educat* or participat* or behaviour* or behaviour* or compliance or centered)) |
| 36. | (health NEAR5 (promot* or educat* or behave*)) |
| 37. | (((behave* NEAR3 chang*) or (problem* NEAR3 solv*) or (goal* NEAR3 setting) or (decision* NEAR3 mak*) or coping) NEAR5 (patient* or consumer* or client*)) |
| 38. | OR/9‐37 |
| 39. | MeSH DESCRIPTOR Controlled Clinical Trials as Topic EXPLODE ALL TREES |
| 40. | MeSH DESCRIPTOR Random allocation EXPLODE ALL TREES |
| 41. | MeSH DESCRIPTOR control groups EXPLODE ALL TREES |
| 42. | MeSH DESCRIPTOR Clinical Trials as Topic EXPLODE ALL TREES |
| 43. | MeSH DESCRIPTOR double‐blind method EXPLODE ALL TREES |
| 44. | MeSH DESCRIPTOR single‐blind method EXPLODE ALL TREES |
| 45. | MeSH DESCRIPTOR Placebos EXPLODE ALL TREES |
| 46. | MeSH DESCRIPTOR placebo effect EXPLODE ALL TREES |
| 47. | MeSH DESCRIPTOR Research Design EXPLODE ALL TREES |
| 48. | MeSH DESCRIPTOR Program Evaluation EXPLODE ALL TREES |
| 49. | randomized controlled trial OR controlled clinical trial OR clinical trial OR random* or RCT* OR (controlled NEAR5 (trial* or stud*)) OR (clinical* NEAR5 trial*) |
| 50. | ((control or treatment or experiment* or intervention) NEAR5 (group* or subject* or patient*)) |
| 51. | (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*) |
| 52. | ((control or experiment* or conservative) NEAR5 (treatment or therapy or procedure or manage*)) |
| 53. | ((singl* or doubl* or tripl* or trebl*) NEAR5 (blind* or mask*)) |
| 54. | Placebo* |
| 55. | sham |
| 56. | assign* or allocate* |
| 57. | controls |
| 58. | OR/39‐57 |
| 59. | 8 and 38 and 58 |
| 60. | MeSH DESCRIPTOR animals EXPLODE ALL TREES |
| 61. | 59 not 60 |
Appendix 10. Cochrane Centre Register of Controlled Trials (CENTRAL) search strategy
| 1. | MeSH descriptor [stroke] explode all trees |
| 2. | MeSH descriptor [hemiplegia] explode all trees |
| 3. | MeSH descriptor [paresis] explode all trees |
| 4. | MeSH descriptor [cerebrovascular trauma] explode all trees |
| 5. | MeSH descriptor [brain injury] explode all trees |
| 6. | MeSH descriptor [brain injury, chronic] explode all trees |
| 7. | MeSH descriptor [gait disorders] explode all trees |
| 8. | OR/1‐7 |
| 9. | MeSH descriptor [self efficacy] explode all trees |
| 10. | MeSH descriptor [self care] explode all trees |
| 11. | MeSH descriptor [self administration] explode all trees |
| 12. | MeSH descriptor [self‐assessment] explode all trees |
| 13. | MeSH descriptor [self concept] explode all trees |
| 14. | MeSH descriptor [patient compliance] explode all trees |
| 15. | MeSH descriptor [patient participation] explode all trees |
| 16. | MeSH descriptor [patient satisfaction] explode all trees |
| 17. | MeSH descriptor [consumer participation] explode all trees |
| 18. | MeSH descriptor [consumer health information] explode all trees |
| 19. | MeSH descriptor [health behavior] explode all trees |
| 20. | MeSH descriptor [attitude to health] explode all trees |
| 21. | MeSH descriptor [health education] explode all trees |
| 22. | MeSH descriptor [health knowledge, attitudes, practice] explode all trees |
| 23. | MeSH descriptor [health promotion] explode all trees |
| 24. | MeSH descriptor [life style] explode all trees |
| 25. | MeSH descriptor [disease management] explode all trees |
| 26. | MeSH descriptor [risk reduction behaviour] explode all trees |
| 27. | MeSH descriptor [adaptation, psychologicall] explode all trees |
| 28. | MeSH descriptor [motivation] explode all trees |
| 29. | MeSH descriptor [goals] explode all trees |
| 30. | MeSH descriptor [problem solving] explode all trees |
| 31. | MeSH descriptor [decision making] explode all trees |
| 32. | MeSH descriptor [health plan implementation] explode all trees |
| 33. | self care or self‐care or self management or self‐management or self efficacy or self‐efficacy or self monitor* or selfmonitor* |
| 34. | (self or oneself) NEAR/3 care |
| 35. | ((patient* or consumer* or client*) NEAR/5 (educat* or participat* or behaviour* or behaviour* or compliance or centered)) |
| 36. | (health NEAR/5 (promot* or educat* or behave*)) |
| 37. | (((behave* NEAR/3 chang*) or (problem* NEAR/3 solv*) or (goal* NEAR/3 setting) or (decision* NEAR/3 mak*) or coping) NEAR/5 (patient* or consumer* or client*)) |
| 38. | OR/9‐37 |
| 39. | MeSH descriptor [controlled clinical trials] as topic explode all trees |
| 40. | MeSH descriptor [random allocation] explode all trees |
| 41. | MeSH descriptor [control groups] explode all trees |
| 42. | MeSH descriptor [clinical trials as topic] explode all trees |
| 43. | MeSH descriptor [double‐blind method] explode all trees |
| 44. | MeSH descriptor [single‐blind method] explode all trees |
| 45. | MeSH descriptor [placebos] explode all trees |
| 46. | MeSH descriptor [placebo effect] explode all trees |
| 47. | MeSH descriptor [research design] explode all trees |
| 48. | MeSH descriptor [program evaluation] explode all trees |
| 49. | randomized controlled trial OR controlled clinical trial OR clinical trial OR random* or RCT* OR (controlled NEAR/5 (trial* or stud*)) OR (clinical* NEAR/5 trial*) |
| 50. | ((control or treatment or experiment* or intervention) NEAR/5 (group* or subject* or patient*)) |
| 51. | (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*) |
| 52. | ((control or experiment* or conservative) NEAR/5 (treatment or therapy or procedure or manage*)) |
| 53. | ((singl* or doubl* or tripl* or trebl*) NEAR/5 (blind* or mask*)) |
| 54. | Placebo* |
| 55. | sham |
| 56. | assign* or allocate* |
| 57. | controls |
| 58. | OR/39‐57 |
| 59. | 8 and 38 and 58 |
| 60. | MeSH descriptor animals explode all trees |
| 61. | 59 not 60 |
Appendix 11. Cochrane Effective Practice and Organisation of Care search strategy
The terms used in the search were: {stroke} OR {brain infarc} OR {cerebral infarc} OR {brain stem infarc} OR {brain vascular accident*} OR {vascular accident brain} OR {Cerebrovascular Accident} OR {apoplexy} in All Fields.
Appendix 12. Proquest Dissertations and Theses search strategy
Stroke
(Chronic disease management) MeSH
Appendix 13. Australian New Zealand Clinical Trials Registry (ANZCTR) search strategy
| 1. | stroke (as condition category) (limiters= ‘allocation to intervention’ randomized, ‘age group’ adults 18 years and over) |
| 2. | stroke OR CVA |
| 3. | self care OR self‐care OR self management OR self‐management OR self monitoring OR selfmonitoring |
| 4. | 1 and 3 |
| 5. | lifestyle OR life style OR health behavior |
| 6. | 1 and 5 |
| 7. | behavior change OR problem solving OR goal setting OR decision making OR coping |
| 8. | 1 and 7 |
| 9. | Patient education or patient participation OR consumer education OR consumer participation OR client education OR client participation |
| 10. | 1 and 9 |
Appendix 14. ClinicalTrials.gov search strategy
| stroke (as search term); Limiters = study type ‘interventional’, age group ‘Adult (18‐65) and Senior (66+)’ |
| stroke AND (self care OR self‐care OR self management OR self‐management OR self monitoring OR selfmonitoring) |
| stroke AND (self efficacy OR self‐efficacy OR motivation OR motivational) NOT (self care OR self‐care OR self management OR self‐management OR self monitoring OR selfmonitoring) |
| stroke AND (life style OR lifestyle OR disease management OR health behaviour OR health behaviour) NOT (self care OR self‐care OR self management OR self‐management OR self monitoring OR selfmonitoring OR self efficacy OR self‐efficacy OR motivation OR motivational OR self efficacy OR self‐efficacy OR motivation OR motivational) |
| stroke AND (lifestyle OR life style OR health behavior) |
| stroke AND (behavior change OR problem solving OR goal setting OR decision making OR coping) |
| stroke AND (Patient education or patient participation OR consumer education OR consumer participation OR client education OR client participation) |
Appendix 15. Current Controlled Trials search strategy
| 1. | stroke OR CVA or cerebrovascular disease (as search terms) |
| 2. | stroke OR CVA |
| 3. | self care OR self‐care OR self management OR self‐management OR self monitoring OR selfmonitoring |
| 4. | 1 and 3 |
| 5. | lifestyle OR life style OR health behavior |
| 6. | 1 and 5 |
| 7. | behavior change OR problem solving OR goal setting OR decision making OR coping |
| 8. | 1 and 7 |
| 9. | Patient education or patient participation OR consumer education OR consumer participation OR client education OR client participation |
| 10. | 1 and 9 |
Appendix 16. Stroke Trials Registry (The Internet Stroke Center) search strategy
| 1. | limiter= ‘allocation to intervention’ randomized |
| 2. | 1 and stroke (as condition) |
| 3. | 1 and self management or self‐management (as keywords) |
| 4. | 1 and self care OR self‐care OR self management OR self‐management OR self monitoring OR selfmonitoring (as keywords) |
| 5. | 1 and self‐directed program (drop‐down interventions term) |
| 6. | 1 and ‘chronic disease self management course’ (drop‐down interventions term) |
| 7. | 1 and ‘Self management education programme’ (drop‐down interventions term) |
| 8. | 1 and ‘Evaluation of stroke self management’ |
| 9. | 1 and ‘education’ (as keyword) |
| 10. | 1 and ‘problem solving’ (as keyword) |
| 11. | 1 and ‘goal setting’ (as keyword) |
| 12. | 1 and ‘coping’ |
Appendix 17. WHO International Clinical Trials Registry Platform (ICTRP) search strategy
| 1. | Stroke OR brain injur* (in title field) |
| 2. | Stroke OR brain injur* OR cerebrovascu* OR cva OR poststroke OR post‐stroke OR hemipleg* OR hemipar* |
| 3. | self care OR self‐care OR self management OR self‐management OR self monitor* OR selfmonitor* |
| 4. | 2 AND 3 |
| 5. | 4 AND 1 |
| 6. | self efficacy OR self‐efficacy OR motivation* |
| 7. | 2 AND 6 |
| 8. | Life Style OR Disease Management OR Health Behav* |
| 9. | 2 AND 8 |
| 10. | 9 AND 1 |
| 11. | Patient educat* or patient participat* OR consumer educat* OR consumer particip* OR client educat* OR client particip* |
| 12. | 2 AND 11 |
| 13. | 12 AND 1 |
| 14. | behav* chang* OR problem solv*OR goal* setting OR decision mak* OR coping |
| 15. | 14 AND 2 |
Data and analyses
Comparison 1. Self management versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life | 6 | 468 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.00, 0.41] |
| 1.1 Quality of life: Stroke Specific Quality of Life | 3 | 211 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.10, 0.55] |
| 1.2 Quality of life: physical functioning | 3 | 257 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.14, 0.55] |
| 2 Self efficacy | 6 | 403 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.04, 0.36] |
| 2.1 Stroke Self‐Efficacy Questionnaire | 4 | 193 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [0.04, 0.61] |
| 2.2 Recovery Locus of Control Scale | 2 | 210 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.26, 0.29] |
| 3 Activity limitations | 4 | 260 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.03, 0.46] |
| 3.1 Barthel Index | 4 | 260 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.03, 0.46] |
| 4 Impairments | 6 | 648 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.27, 0.15] |
| 4.1 Hospital Anxiety and Depression Scale | 6 | 648 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.27, 0.15] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bishop 2014.
| Methods | RCT | |
| Participants | Adults with stroke, living in community and their cares | |
| Interventions | Intervention: FITT plus standard medical follow‐up: n = 23
Control: standard medical follow‐up: n = 26 |
|
| Outcomes | Primary and secondary: global outcomes for healthcare utilisation (doctor and hospital visits, total therapy hours), family functioning (Family Assessment Device, Perceived Criticism Scale) and general functioning (FAI, FIM, GDS) Assessed at baseline, 3/12, and 6/12 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomisation, ensured balanced distribution of gender, age, and marital status |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither able to be blinded to group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data collectors blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No group differences in drop‐outs observed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | n/a |
Cadilhac 2011.
| Methods | RCT | |
| Participants | Adults with stroke, living in community | |
| Interventions | Intervention: stroke self management programme: n = 48
Control 1: Generic Stanford (CCSM): n = 47
Control 2: standard care: n = 48
|
|
| Outcomes | Primary: feasibility (enrolment, access, and completion rates) Secondary: Health Education Impact Questionnaire (domain of engagement in life); AQoL; Mood Scale Assessed at baseline, 3/12, and 6/12 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a remote Internet‐based telephone randomisation service |
| Allocation concealment (selection bias) | Low risk | Allocated by the stroke educator |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither able to be blinded to group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research staff unaware of allocation group for assessments and data processing |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported via intention‐to‐treat and as per protocol (50% programme completed) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | n/a |
Evans‐Hudnall 2014.
| Methods | RCT | |
| Participants | Adults with stroke, living in community, primarily African‐American and Hispanic of low socioeconomic status | |
| Interventions | Intervention: secondary stroke prevention program (STOP): n = 30
Control: usual care: n = 30 |
|
| Outcomes | Primary: US BRFSS: stroke knowledge, servings of fruit and vegetables, exercise, tobacco use, alcohol use, medication adherence Secondary: BSI‐18: subscales anxiety and depression Assessed at baseline and 4/52 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation, prior to assessment, centrally generated |
| Allocation concealment (selection bias) | Low risk | Performed by an independent statistician |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither able to be blinded to group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "The RA conducted all the ...assessments". Blinding not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Same number of participants analysed at baseline and follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | n/a |
Frank 2000.
| Methods | RCT | |
| Participants | Adults with stroke, living in community | |
| Interventions | Intervention: workbook group: n = 19
Control: usual care (wait list): n = 20 |
|
| Outcomes | Primary: Functional Limitations Profile Secondary: RLOCS, Perceived Health Competence Scale Assessed baseline and 1/12 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither able to be blinded to group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Intervention and assessment by 1 researcher |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Equal number of drop‐outs per group (1 each) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | n/a |
Harwood 2012.
| Methods | RCT | |
| Participants | Adults with stroke, living in community (Maori and Pacific Islander) | |
| Interventions | Overall:
Intervention 1: DVD ‐ inspirational stories and advice from same ethnic group: n = 48
Intervention 2: TCS: n = 46
Intervention 3: DVD and TCS: n = 39
Control: usual care
|
|
| Outcomes | Primary: SF‐36 Secondary: BI, FAI, Carer Strain Index, mRS, use of rehabilitation services Assessed at 6/12 and 12/12 |
|
| Notes | Included unpublished data from author: mean scores SF‐36, BI, FAI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table with stratification by ethnic group |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes to conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither able to be blinded to group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments by RAs masked to allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data were not missing at random |
| Selective reporting (reporting bias) | Low risk | Only 12/12‐month data in journal article, authors supplied 6/12 data |
| Other bias | Low risk | n/a |
Hoffman 2014.
| Methods | RCT | |
| Participants | Adults with stroke, admitted to large tertiary hospital stroke unit | |
| Interventions | Intervention: self management: n = 12
Intervention 2: coping skills: n = 11
Control: standard care: n = 10
|
|
| Outcomes | Primary: MADRS Secondary: HADS, SSEQ, NEADL, Stroke Knowledge Scale; SAQoL‐g; modified BI Assessed at baseline; post‐intervention i.e. 2/12 post discharge; 5/12 postdischarge |
|
| Notes | Included unpublished data from author: mean scores MADRS, HADS, modified BI, SAQoL, NEADL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Predetermined computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | Concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither able to be blinded to group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessed by RA blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear how much missing data; 'last observation carried forward' method |
| Selective reporting (reporting bias) | Low risk | All reported either in email or draft paper |
| Other bias | Low risk | n/a |
Johnston 2007.
| Methods | RCT | |
| Participants | Adults with stroke, living in community | |
| Interventions | Intervention: control cognition: n = 103
Control: normal care: n = 100 |
|
| Outcomes | Primary: BI Secondary: OAD, HADS, satisfaction (0‐10); RLOCS, confidence in recovery (0‐10) Assessed at baseline, 2nd and 3rd interview (5/52) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation schedules generated by statistician |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither able to be blinded to group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | RAs who administered the interviews were kept blind to randomisation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unequal drop‐outs across groups |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | n/a |
Jones 2016.
| Methods | RCT (cluster allocation) | |
| Participants | Adults with stroke, living in community | |
| Interventions | Intervention: self management programme: n = 40
Control: usual care: n = 38 |
|
| Outcomes | Primary: SAQoL Secondary: NEADL; SSEQ; HADS; SF‐12 Assessed at baseline, 6/52, and 3/12 |
|
| Notes | Included unpublished data from authors ‐ mean scores NEADL, HADS, SAQoL, SF‐12, SSEQ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither able to be blinded to group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors masked to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unequal drop‐outs across groups, not explained |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Other bias | Low risk | n/a |
Kendall 2007.
| Methods | RCT | |
| Participants | Adults with stroke, living in community | |
| Interventions | Intervention: Chronic Disease Self Management Program: n = 58
Control: usual care: n = 42 |
|
| Outcomes | Primary: SSQoL Secondary: SSEQ Assessed at baseline, 3/12, 6/12, and 12/12 |
|
| Notes | Included unpublished data from authors: mean scores SSQoL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 2 dice roll |
| Allocation concealment (selection bias) | Low risk | Conducted by researcher who had no information about the participant at the time of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Neither able to be blinded to group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Considerable attrition from both groups not always with reasons, no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | None apparent |
Kim 2013.
| Methods | RCT | |
| Participants | Adults with stroke, living in community | |
| Interventions | Intervention: Internet‐based education programme: n = 18
Control: usual care: n = 18 |
|
| Outcomes | Primary: health behaviours (questionnaire) Secondary: Mastery Scale; Health Motivation Scale; Care‐Giving Mastery Scale; feasibility (completion of sessions; occurrence of technical problems) Assessed at baseline and 3/12 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random code |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither able to be blinded to group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment by RA not involved in the programme |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Equal number of drop‐outs both groups (1, with reasons) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | n/a |
Lund 2012.
| Methods | RCT | |
| Participants | Adults with stroke, living in community | |
| Interventions | Intervention: lifestyle course and PA: n = 48
Control: PA only: n = 51 Completed over 9/12, 1 x 30‐ to 60‐minute group session per week (36 sessions) ‐ non‐specific physical activity Open to all seniors regardless of diagnosis |
|
| Outcomes | Primary: SF‐36 Secondary: COPM, HADS, Timed Up and Go; Trail making A and B Assessed at baseline and 9/12 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation list in blocks of 10, stratified to centres |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes opened by researcher |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither able to be blinded to group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Imbalance in lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | n/a |
McKenna 2015.
| Methods | RCT | |
| Participants | Adults with stroke, living in community | |
| Interventions | Intervention: Bridges SSMP: n = 12
Control: usual care: n = 13 |
|
| Outcomes | Primary: EuroQol Secondary: SSQoL, SES, SSEQ, BI, NEADL, GHQ‐28, SIPSO Assessed at baseline, 6/52, and 4.5/12 (3 month follow‐up) |
|
| Notes | Data supplied by authors for mean (SD) at all timepoints | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation generated |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes prepared |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither able to be blinded to group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | RA blinded to group allocation assessed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant withdrew |
| Selective reporting (reporting bias) | High risk | Only qualitative reporting of results for secondary measures |
| Other bias | Unclear risk | Intervention logs not clear on amount of intervention in control group |
Sabariego 2013.
| Methods | RCT | |
| Participants | Adults with stroke (ICD‐10), living in community, BI 35‐65 | |
| Interventions | Intervention: ICF‐based education programme: n = 130
Control: active: n = 130
|
|
| Outcomes | Primary: Liverpool Self‐efficacy scale Secondary: WHOQOL, SIS, EQ VAS, HADS Assessed at baseline, postintervention, and 6/12 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in blocks with externally generated list (6 permutations ‐ 1 chosen by throw of dice) |
| Allocation concealment (selection bias) | Low risk | External |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded, personnel conducting could not be |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 47 participants did not receive allocation; a further 14 were lost to follow‐up for unstated reasons |
| Selective reporting (reporting bias) | Low risk | All intended outcomes reported |
| Other bias | Low risk | n/a |
Tielemans 2015.
| Methods | RCT | |
| Participants | Adults with stroke, living in community | |
| Interventions | Intervention: self management intervention: n = 58
Control: education: n = 55
|
|
| Outcomes | Primary: UPCC Secondary: USER‐Participation instrument, GSES, HADS; life satisfaction; SSQoL |
|
| Notes | Unpublished data obtained from authors ‐ mean scores GSES, SSQoL12, HADS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Blocks of 8 participants, then participants selected 1 out of 8 blank envelopes containing an invitation for 1 of the interventions. Generation not stated |
| Allocation concealment (selection bias) | High risk | Allocation not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded to intervention of interest. Both interventions were plausible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A blinded RA assisted in the completion of outcome measures at all time points after randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants allocated were analysed; followed intention‐to‐treat principles. 4 participants lost to follow‐up in both groups, reasons provided |
| Selective reporting (reporting bias) | Unclear risk | Excluded life satisfaction from full reporting, only reported "important" estimated mean differences |
| Other bias | Low risk | No other sources of bias noted |
ADL: activities of daily living; AQoL: Assessment of Quality of Life; BI: Barthel Index; BRFSS: Behavioural Surveillance Survey; BSI‐18: Brief Symptom Inventory; CBT: cognitive behavioural therapy; CCSM: chronic condition self management; COPM: Canadian Occupational Performance Measure; EQ VAS: EQ visual analogue scale; FAI: Frenchay Activities Index; FIM: Functional Independence Measure; FITT: Family Intervention: Telephone Tracking; GDS: Geriatric Depression Scale; GHQ‐28: General Health Questionnaire‐28; GSES: General Self‐efficacy Scale; HADS: Hospital Anxiety and Depression Scale; ICD‐10: International Classification of Diseases; ICF: International Classification of Functioning, Disability and Health; MADRS: Montgomery and Åsberg Depression Rating Scale; mRS: modified Rankin Score; n: number of participants; n/a: not applicable; NEADL: Nottingham Extended Activities of Daily Living Scale; PA: physical activity; OAD: Observer Assessed Disability; OT: occupational therapist; RA: research assistant; RCT: randomised controlled trial; RLOCS: Recovery Locus of Control Scale; SAQoL: Stroke and Aphasia Quality of Life; SES: Self‐Efficacy Scale; SF‐12: 12‐item Short‐Form; SF‐36: 36‐Item Short Form Health Survey; SIPSO: Subjective Index of Physical and Social Outcome; SIS: Stroke Impact Scale; SMP: ; SSEQ: Stroke Self‐Efficacy Questionnaire; SSMP: Stroke Self Management Program; SSQoL: Stroke Specific Quality of Life; TCS: Take Charge Session; UPCC: Utrecht Proactive Coping Competence scale; WHOQOL: World Health Organization Quality of Life.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aben 2013 | Single stroke deficit targeted (memory) |
| Allen 2009 | Not self management intervention as per review definition |
| Andrea 2003 | Unable to locate reference |
| Backhaus 2010 | Separate stroke data not available |
| Boter 2004 | Not self management intervention as per review definition |
| Brown 2012 | Participants not adults with stroke |
| Byers 2010 | Participants were inpatients |
| Chang 2011 | Participants were inpatients |
| Claiborne 2006 | Not self management intervention as per review definition |
| Damush 2011 | Participants included adults with TIA |
| Eames 2013 | Not self management intervention as per review definition |
| Egan 2007 | Single discipline (occupational therapy) |
| Ellis 2005 | Not self management intervention as per review definition |
| Flemming 2013 | Participants included adults with TIA |
| Forster 1996 | Not self management intervention as per review definition |
| Friedland 1992 | Not self management intervention as per review definition |
| Fu 2003 | Separate stroke data not available |
| Gray 2011 | Single stroke deficit targeted (depression) |
| Guidetti 2010 | Single discipline (occupational therapy) |
| Harrington 2010 | Unable to isolate effect of self management intervention from exercise |
| Jones 2015 | Not self management intervention as per review definition |
| Kang 2004 | Unable to access English translation from Korean |
| Kim 2011 | Not self management intervention as per review definition |
| Kronish 2014 | Participants included adults with TIA |
| Logan 1997 | Not self management intervention as per review definition |
| Lorig 1999 | Separate stroke data not available |
| Markle‐Reid 2011 | Not self management intervention as per review definition |
| Marsden 2010 | Not self management intervention as per review definition |
| Rodgers 1999 | Not self management intervention as per review definition |
| Sahebalzamani 2009 | Not self management intervention as per review definition |
| Smith 2004 | Participants were inpatients |
| Thrift 2014 | Not self management intervention as per review definition |
| van der Ploeg 2007 | Participants were inpatients |
| Vluggen 2012 | Unable to isolate effect of self management from other intervention components |
| Wang 2013 | Not self management intervention as per review definition |
| Wolfe 2010 | Not self management intervention as per review definition |
TIA: transient ischaemic attack.
Characteristics of studies awaiting assessment [ordered by study ID]
Damush 2013.
| Methods | RCT |
| Participants | Adults (≥ 18 years) with diagnosis of acute ischaemic stroke or TIA in the past 12 months |
| Interventions | Stroke self management programme delivered via 6 x biweekly telephone calls for the first 3 months, then 3 x monthly group sessions during months 4 to 6 |
| Outcomes | Primary: SSQoL |
| Notes |
Donnellan 2014.
| Methods | RCT |
| Participants | People post‐stroke |
| Interventions | Control: standard care Intervention: REsources And LIfe Strategy Management (REALISM) training programme REALISM will involve providing participants with a training programme on managing short‐ and long‐term effects at 4/52, 3/12, and 6/12 poststroke using a goal setting and attainment care plan based on the adaptive strategies selection, optimisation, and compensation |
| Outcomes | Primary: metacognition, self regulation, executive function Secondary: functional ability, health‐related quality of life, mood |
| Notes |
Leistner 2013.
| Methods | RCT |
| Participants | Age > 18 years Acute patients with TIA or minor stroke (mRS ≤ 2 at time of screening and visible DWI lesion in MRI) within 14 days of study inclusion Evaluated in a dedicated stroke unit or clinic At least 1 of the following risk factors: arterial hypertension, diabetes mellitus, atrial fibrillation, smoking n = 2082 |
| Interventions | Information about pathophysiology of the individual risk for recurrent event of stroke or TIA and potentials of vascular risk reduction Motivational interviewing to develop an agreed individual plan regarding risk reduction targets and medication The person's motivation will be enhanced using feedback strategies regarding measured risk factors Assistance in finding peer groups and group therapies (e.g. Nordic walking, INR self measurement and smoking cessation programmes) |
| Outcomes | Follow‐up 2 years Primary: recurrence of stroke or other cardiovascular events Secondary: total mortality, rate of participants who meet the recommended guideline targets regarding risk factors, frequency of hospital admissions for vascular diseases, number of days "alive and at home" |
| Notes |
Lo 2014.
| Methods | RCT |
| Participants | Community‐dwelling stroke survivors who have had a stroke in the past year |
| Interventions | Intervention includes 1 individual home visit, 2 group sessions, and 2 follow‐up telephone calls |
| Outcomes | Self efficacy, outcome expectation, self management behaviours, quality of life, depressive symptoms, and community reintegration |
| Notes |
MacKay‐Lyons 2010.
| Methods | Multicentre RCT |
| Participants | Adults (> 17 years) within 90 days of being diagnosed with non‐disabling stroke (NIHSS < 6) or TIA n = 250 |
| Interventions | Multi‐modal model, case‐managed programme of exercise and stroke risk management education Use of positive reinforcement (encouragement, positive feedback) Use of adult learning strategies (interactive educational sessions, participant involvement in content selection) |
| Outcomes | Primary: stroke risk factors ‐ blood pressure, waist girth, biochemical analysis Secondary: HADS, health‐related quality of life ‐ medical outcomes SF‐36, fitness and activity measures ‐ peak oxygen uptake, 6‐Minute Walk Test, accelerometers, International Physical Activity Questionnaire, Fatigue Assessment Scale, Montreal Cognitive Assessment, Healthcare utilisation and medication adherence and tobacco use ‐ self report using a health passport, Pittsburgh Sleep Quality Index, Health‐related goals Goal Attainment Scaling Secondary vascular events: health record abstraction |
| Notes |
NCT01550822.
| Methods | RCT |
| Participants | Completed 12‐month SUSTAIN trial (SUSTAIN criteria: age ≥ 40 years, acute TIA or ischaemic stroke within the previous 1 month and systolic blood pressure > 120 mmHg) |
| Interventions | Group clinics addressing smoking cessation, healthy eating, physical activity, and risk factors of stroke |
| Outcomes | Primary: physical activity (timeframe: 6/12), diet (≥ 5 servings fruits/vegetables/day), body mass index Secondary: change in waist circumference |
| Notes |
NCT02156778.
| Methods | RCT |
| Participants | Adults with acute ischaemic stroke or high‐risk TIA |
| Interventions | Comparator: standard care In‐hospital training (education of participants, next of kin and carers on risk factor management and assessment, lifestyle improvement, and compliance) Complimentary provision of a book/information material dealing with participant and carer relevant aspects of stroke care Advice from a dietitian (general advice and individualised recommendations in people with diabetes and obesity) Standardised information materials (e.g. for oral anticoagulant or new oral anticoagulant therapy). Support for smoking cessation and weight reduction if necessary or requested. Detailed medical reports (doctor's letter for the general practitioner and participant) at discharge containing target levels for risk factor management Atrial fibrillation detection at the Stroke Unit (1‐5 days' monitoring) or at the ward (24‐hour ECG), or both 3/12 telephone interview and 12/12 clinical visit and outcome assessment Intervention: standard care plus Extended training with access to weekly educational lectures (education of participants and relatives), implementation of "My Stroke Card" containing an adopted version of the 'post‐stroke checklist' (ascertainment of post‐stroke complications), self administered Internet‐based tools for risk factor monitoring and reinforcement of target level achievement, and information and educational materials 3‐month outpatient appointment with standardised assessment of risk factors and screening for complications, health problems and residual deficits, estimation of the participant's demand for nursing services and support, guideline‐conform secondary prevention with full achievement of target levels, assessment of participant adherence to drug prescriptions 6‐month and 9‐month visits on the discretion of the study team in case of medical needs 12‐month clinical visit and outcome assessment |
| Outcomes | Primary: major recurrent (post‐discharge) cardiovascular events, health‐related quality of life Secondary: recurrent stroke, death from all causes, functional outcome, quality of life, target level achievement in secondary prevention, cost‐effectiveness, number of out‐of‐schedule consultations of physicians and outpatient hospital services, and out‐of‐schedule hospital admissions |
| Notes |
NCT02207023.
| Methods | Pilot RCT |
| Participants | Has experienced a stroke in the last 12 months Age ≥ 50 years Living in the community with telephone access Able to walk independently at least 10 feet or 3 metres Able to communicate in English |
| Interventions | No intervention: Memory Training Program: participants will participate in 7 memory self efficacy training coaching sessions (2 in the first month) over a 6‐month period. The coaching sessions will be administered by telephone Experimental: Healthy Lifestyle Training Program: participants will participate in 7 lifestyle coaching sessions (2 in the first month) over a 6‐month period. The coaching sessions will be administered by telephone |
| Outcomes | Primary: lifestyle behaviour (Health Promoting Lifestyle Profile II) Secondary: physical activity, dietary behaviour, medication adherence, depression, cognition, body composition, health‐related quality of life, health and social service utilisation |
| Notes |
UMIN000007808.
| Methods | RCT |
| Participants | People aged 40 to 80 years Discharged from acute hospital with mild ischaemic stroke (Japanese mRS 0‐3) or TIA n = 308 |
| Interventions | Long‐term participant education, training and counselling on stroke self management (no details found) |
| Outcomes | Primary: recurrence rate and mortality caused by the stroke Secondary: Framingham Risk Score: cardiovascular disease, physiological indicators (blood pressure, glycated haemoglobin, etc.), psychological indicators (self efficacy, depression, quality of life), attainment rate of behaviour modification |
| Notes |
DWI: diffusion‐weighted imaging; ECG: electrocardiogram; HADS: Hospital Anxiety and Depression Scale; INR: International Normalized Ratio; MRI: magnetic resonance imaging; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; RCT: randomised controlled trial; SF‐36: 36‐Item Short Form Health Survey; SSQoL: Stroke Specific Quality of Life; TIA: transient ischaemic stroke.
Characteristics of ongoing studies [ordered by study ID]
Cheng 2011.
| Trial name or title | Systemic Use of STroke Averting INterventions (SUSTAIN) |
| Methods | RCT |
| Participants | Adults with TIA or ischaemic stroke within the past 90 days n = 410 |
| Interventions | Group sessions on:
1‐to‐1 sessions to:
|
| Outcomes | Primary: BP Secondary: LDL level, smoking status, physical activity level, healthcare costs |
| Starting date | Unknown |
| Contact information | Eric M Cheng, MD, MS, VA Greater Los Angeles/UCLA, 11301 Wilshire Blvd, Department of Neurology, ML 127, Los Angeles, CA 90073 E‐mail eric.cheng@va.gov |
| Notes |
Fukuoka 2014.
| Trial name or title | Randomised Trial Assessing the Effects of Disease Management Programs for the Prevention of Recurrent Ischemic Stroke |
| Methods | RCT |
| Participants | Participants aged 40 to 80 years within 1 year from the onset of ischaemic stroke or TIA n = 321 |
| Interventions | 6‐month programme of 2 x face‐to‐face interviews and telephone calls every 2 weeks Educated via interviews and telephone calls to help participants acquire skills for self management and the control of ischaemic stroke risk factors Individualised education booklets on risk factor management A self management record notebook to recorded daily BP, bodyweight, and lifestyle improvement goals |
| Outcomes | 2‐year follow‐up Primary: recurrence or mortality from stroke Secondary: economic indicators: unplanned consultation and days of hospitalisation in conjunction with ischaemic stroke and risk factors; physiological indicators: e.g. BP, cholesterol; psychological indicators: self efficacy scale of health behaviour in people with chronic disease, CES‐D, SF‐36; evaluation of self monitoring and lifestyle improvement actions |
| Starting date | September 2010 |
| Contact information | Yasuko Fukuoka Address: Department of Nursing Science, Graduate School of Biomedical & Health Sciences, Hiroshima University, Hiroshima, Japan Email: yasukofukuoka@hotmail.com |
| Notes |
Graven 2011.
| Trial name or title | From Rehabilitation to Recovery: a Randomised Controlled Trial Evaluating a Goal Based Intervention to Reduce Depression and Facilitate Participation Post‐Stroke |
| Methods | RCT |
| Participants | People admitted to inpatient rehabilitation with the primary diagnosis of acute stroke Primary informal carers if it is envisaged that they will provide at least 5 hours per week assistance to the participant n = 132 |
| Interventions |
|
| Outcomes | Assessed at 3 time points: T1 = rehabilitation discharge, T2 = 6/12 poststroke, T3 = 12/12 poststroke Primary: depression Secondary: participation and activity status, health‐related quality of life, self efficacy |
| Starting date | Unknown |
| Contact information | Christine Graven Address: School of Health Sciences, The University of Melbourne, Parkville, Victoria 3052, Australia Email: Christine.Graven@svhm.org.au |
| Notes |
ISRCTN08913646.
| Trial name or title | HEISS: The Effect of a Health Empowerment Intervention for Stroke Self‐management on the Self‐management Behaviour and Health Outcomes of Stroke Rehabilitation Patients |
| Methods | RCT |
| Participants | Adults (age ≥ 18 years) with haemorrhagic or ischaemic stroke Admitted to the ambulatory stroke rehabilitation programme with no premorbid disability Experiencing poststroke functional difficulties that limit participation in self care activities Chinese ethnicity and Cantonese dialect communicability n = 210 |
| Interventions | HEISS is based on the Theory of Health Empowerment. It consists of:
|
| Outcomes | Primary (measured pretest, 1/52, 3/12, and 6/12 post‐tests): self efficacy, engagement in self management behaviour, functional ability in activities of daily living Secondary: quality of life, unplanned hospital re‐admission rate, stroke recurrent rate |
| Starting date | May 2012 |
| Contact information | Janet Sit Address: The Nethersole School of Nursing Faculty of Medicine The Chinese University of Hong Kong, 6/F, Esther Lee Building, The Chinese University of Hong Kong, Shatin, NT, Hong Kong |
| Notes |
NCT01507688.
| Trial name or title | Stroke Self‐management: Effect on Function and Stroke Quality of Life |
| Methods | RCT |
| Participants | Adults (≥ 18 years) with acute diagnosis of ischaemic stroke or TIA within past 12 months |
| Interventions | Behavioural: stroke self management |
| Outcomes | Change in stroke, specific quality of life (timeframe: baseline, 3/12, 6/12, and 12/12) |
| Starting date | January 2013 |
| Contact information | Gloria T Nicholas Telephone: +1 317‐988‐4388 Email: Gloria.Nicholas@va.gov |
| Notes |
NCT01770184.
| Trial name or title | Clinical Effectiveness of Self‐management Education Post‐mild Stroke |
| Methods | RCT |
| Participants | Adults (18 to 90 years) with mild stroke (NIHSS total scores 0 to 5) Plus identified as having at least 1 other chronic condition besides stroke n = 60 |
| Interventions | Chronic Disease Self‐Management Program |
| Outcomes | Primary: Adapted Illness Intrusiveness Ratings (timeframe: change from baseline to 6 months post‐stroke), Healthcare Utilization Survey Secondary: Activity Card Sort, Chronic Disease Self‐Efficacy Scale, Multidimensional Assessment of Fatigue, Patient Health Questionnaire, Reintegration to Normal Living Index, Stroke Impact Scale, Work Ability Index, World Health Organization Quality of Life |
| Starting date | January 2013 |
| Contact information | Timothy J Wolf, OTD, MSCI, OTR/L Telephone: +1 314‐286‐1683 Email: wolft@wusm.wustl.edu |
| Notes |
BP: blood pressure; CES‐D: Center for Epidemiological Studies‐Depression; GP: general practitioner; LDL; low‐density lipoprotein; NIHSS: National Institutes of Health Stroke Scale; RCT: randomised controlled trial; SF‐36: 36‐item Short Form Health Survey; TIA: transient ischaemic attack.
Differences between protocol and review
In recognition that chronic disease self management is a complex intervention with multiple components (Campbell 2000), we excluded from the review self management interventions that contain only a single component (e.g. only problem‐solving or only decision‐making) and we excluded self management interventions that target only a single stroke deficit or risk factor (e.g. only depression).
At the request of the Cochrane Stroke Group Editorial Board, 'medication adherence' was included as a secondary outcome of the review.
Several studies compared Chronic Condition Self‐Management (CCSM) to usual care, which we had assumed would be passive and equate to no intervention. However, this was not the case; hence, we combined usual care with other active control comparisons. We performed a post hoc subgroup analysis to observe any differences in the combining of usual care controls and active controls.
Contributions of authors
All authors contributed to the review.
CF, JL, and MM searched the literature and extracted data.
SH performed the meta‐analysis and interpretation.
All authors were involved in the writing of the review.
Declarations of interest
Caroline E Fryer: none known.
Julie A Luker: none known.
Michelle N McDonnell: none known.
Susan L Hillier: none known.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Bishop 2014 {published data only}
- Bishop D, Miller I, Weiner D, Guilmette T, Mukand J, Feldmann E, et al. Family Intervention: Telephone Tracking (FITT): a pilot stroke outcome study. Topics in Stroke Rehabilitation 2014;21(1):S63‐74. [DOI] [PubMed] [Google Scholar]
Cadilhac 2011 {published data only}
- Battersby M, Hoffmann S, Cadilhac D, Osborne R, Lalor E, Lindley R. 'Getting your life back on track after stroke': a phase II multi‐centered, single‐blind, randomized, controlled trial of the Stroke Self‐Management Program vs the Stanford Chronic Condition Self‐Management Program or standard care in stroke survivors. International Journal of Stroke 2009;4(2):137‐44. [DOI] [PubMed] [Google Scholar]
- Cadilhac D, Hoffman S, Kilkenny M, Lindley R, Lalor E, Osborne R, et al. A phase II multi‐centred, single‐blinded randomised, controlled trial of the stroke self‐management program. Stroke 2011;42:1673‐9. [DOI] [PubMed] [Google Scholar]
- Cadilhac D, Kilkenny M, Hoffmann S, Osborne R, Lindley R, Lalor E, et al. Developing a self management program for stroke: results of a phase II multi centred, single blind RCT. International Journal of Stroke 2010;5:343. [Google Scholar]
Evans‐Hudnall 2014 {published data only}
- Evans‐Hudnall G, Stanley M, Clark A, Bush A, Resnicow K, Liu Y, et al. Improving secondary stroke self‐care among underserved ethnic minority individuals: a randomised clinical trial of a pilot intervention. Journal of Behavioural Medicine 2014;37(2):196‐204. [DOI] [PubMed] [Google Scholar]
Frank 2000 {published data only}
- Frank G, Johnston M, Morrison V, Pollard B, MacWalter R. Perceived control and recovery from functional limitations: preliminary evaluation of a workbook‐based intervention for discharged stroke patients. British Journal of Health Psychology 2000;5:413‐20. [Google Scholar]
Harwood 2012 {published data only}
- Harwood M, Weatherall M, Talmaitoga A, Barber P, Gommans J, Taylor W, et al. Taking charge after stroke: promoting self‐directed rehabilitation to improve quality of life ‐ a randomised controlled trial. Clinical Rehabilitation 2012;26(6):493‐501. [DOI] [PubMed] [Google Scholar]
Hoffman 2014 {published and unpublished data}
- Hoffman T, Ownsworth T, Eames S, Shum D. Evaluation of brief interventions for managing depression and anxiety symptoms during early discharge period post stroke: a pilot RCT. Topics in Stroke Rehabilitation 2014;22:116‐26. [DOI] [PubMed] [Google Scholar]
Johnston 2007 {published data only}
- Johnston M, Bonetti D, Joice S, Pollard B, Morrison V, Francis J, et al. Recovery from disability after stroke as a target for a behavioural intervention: results of a randomised controlled trial. Disability and Rehabilitation 2007;29:1117‐27. [DOI] [PubMed] [Google Scholar]
Jones 2016 {published and unpublished data}
- Jones F, Gage H, Drummond A, Bhalla A, Grant R, Lennon S, et al. Feasibility study of an integrated stroke self‐management programme: a cluster‐randomised controlled trial. BMJ Open 2016;6(e008900):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kendall 2007 {published and unpublished data}
- Kendall E, Catalano T, Kuipers P, Posner N, Buys N, Charker J. Recovery following stroke: the role of self‐management education. Social Science & Medicine 2007;64(3):735‐46. [DOI] [PubMed] [Google Scholar]
Kim 2013 {published data only}
- Kim J, Lee S, Kim J. Effect of a web‐based stroke education program on recurrence prevention behaviours among stroke patients: a pilot study. Health Education Research 2013;28:488‐501. [DOI] [PubMed] [Google Scholar]
Lund 2012 {published data only}
- Lund A, Michelet M, Sandvik T, Wyller T, Sveen U. A lifestyle intervention as supplement to a physical activity programme in rehabilitation after stroke: a randomised controlled trial. Clinical Rehabilitation 2012;26:502‐12. [DOI] [PubMed] [Google Scholar]
McKenna 2015 {published data only}
- McKenna S, Jones F, Glenfield P, Lennon S. Bridges self‐management program for people with stroke in the community: a feasibility randomised controlled trial. International Journal of Stroke 2015;10:697‐704. [DOI] [PubMed] [Google Scholar]
Sabariego 2013 {published data only}
- Sabariego C, Barrera A, Neubert S, Stier‐Jarmer M, Bostan C, Cieza A. Evaluation of an ICF‐based patient education programme for stroke patients: a randomised, single‐blinded, controlled multicentre trial of the effects on self‐efficacy, life satisfaction and functioning. British Journal of Health Psychology 2013;18(4):707‐28. [DOI] [PubMed] [Google Scholar]
Tielemans 2015 {published and unpublished data}
- Tielemans N, Visser‐Meily J, Schepers V, Passier P, Port I, Vloothuis J, et al. Effectiveness of the RESTORE4STROKE self‐management intervention "plan ahead": a randomised controlled trial in stroke patients and partners. Journal of Rehabilitation Medicine 2015;47:901‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aben 2013 {published data only}
- Aben L, Heijenbrok‐Kal M, Ponds R, Busschbach J, Ribbers G. Long term effects of a memory self‐efficacy training program for stroke patients in the chronic stage: a randomized controlled trial. Brain Injury 2012;26(4‐5):414‐5. [Google Scholar]
- Aben L, Heijenbrok‐Kal MH, Loon EMP, Groet E, Ponds RWHM, Busschbach JJV, et al. Training memory self‐efficacy in the chronic stage after stroke: a randomized controlled trial. Neurorehabilitation and Neural Repair 2013;27(2):110‐7. [DOI] [PubMed] [Google Scholar]
Allen 2009 {published data only}
- Allen K, Hazelett S, Jarjoura D, Hua K, Wright K, Weinhardt J, et al. A randomized trial testing the superiority of a postdischarge care management model for stroke survivors. Journal of Stroke and Cerebrovascular Diseases 2009;18(6):443‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrea 2003 {published data only}
- Andrea S, Kotzabassaki S, Bellou M, Vardaki Z. Evaluation of the effectiveness of a self‐care educational program on activities of daily living, performed by hemiplegic patients. ICUs & Nursing Web Journal 2003;16:7p. [Google Scholar]
Backhaus 2010 {published data only (unpublished sought but not used)}
- Backhaus SL, Ibarra SL, Klyce D, Trexler LE, Malec JF. Brain injury coping skills group: a preventative intervention for patients with brain injury and their caregivers [corrected]. Archives of Physical Medicine & Rehabilitation 2010;91(6):840‐8. [DOI] [PubMed] [Google Scholar]
Boter 2004 {published data only}
- Boter H. Multicenter randomized controlled trial of an outreach nursing support program for recently discharged stroke patients. Stroke 2004;35(12):2867‐72. [DOI] [PubMed] [Google Scholar]
Brown 2012 {published data only}
- Brown DL, Conley KM, Resnicow K, Murphy J, Sanchez BN, Cowdery JE, et al. Stroke Health and Risk Education (SHARE): design, methods, and theoretical basis. Contemporary Clinical Trials 2012;33(4):721‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Byers 2010 {published data only}
- Byers AM, Lamanna L, Rosenberg A. Enhanced stroke education using motivational interviewing increases patient knowledge and satisfaction after discharge from an acute care setting. Canadian Journal of Neuroscience Nursing 2010;32(1):22. [Google Scholar]
- Byers AM, Lamanna L, Rosenberg A. The effect of motivational interviewing after ischemic stroke on patient knowledge and patient satisfaction with care: a pilot study. Journal of Neuroscience Nursing 2010;42(6):312‐22. [DOI] [PubMed] [Google Scholar]
Chang 2011 {published data only}
- Chang K, Zhang HJ, Xia Y, Chen CS. Testing the effectiveness of knowledge and behavior therapy in patients of hemiplegic stroke. Topics in Stroke Rehabilitation 2011;18(5):525‐35. [DOI] [PubMed] [Google Scholar]
Claiborne 2006 {published data only}
- Claiborne N. Effectiveness of a care coordination model for stroke survivors: a randomized study. Health & Social Work 2006;31(2):87‐96. [DOI] [PubMed] [Google Scholar]
- Claiborne N. Efficiency of a care coordination model: a randomized study with stroke patients. Research on Social Work Practice 2006;16(1):57‐66. [Google Scholar]
Damush 2011 {published data only}
- Damush TM, Ofner S, Yu Z, Plue L, Nicholas G, Williams S. Implementation of a stroke self‐management program. Translational Behavioral Medicine 2011;1(4):561‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eames 2013 {published data only}
- Eames S, Hoffman T, Worrall L, Read S, Wong A. Randomised controlled trial of a postdischarge education and support package for clients with stroke and their carers. Australian Occupational Therapy Journal 2011; Vol. 58, issue 51.
- Eames S, Hoffmann T, Worrall L, Read S, Wong A. Randomised controlled trial of an education and support package for stroke patients and their carers. BMJ Open 2013; Vol. 3, issue 4. [DOI] [PMC free article] [PubMed]
- Eames S, Hoffmann T, Worrall L, Wong A, Read S. Evaluation of an innovative post‐discharge education and support package for patients with stroke and their carers. International Journal of Stroke 2010;5:190. [Google Scholar]
Egan 2007 {published data only}
- Egan M, Kessler D, Laporte L, Metcalfe V, Carter M. A pilot randomized controlled trial of community‐based occupational therapy in late stroke rehabilitation. Topics in Stroke Rehabilitation 2007;14(5):37‐45. [DOI] [PubMed] [Google Scholar]
Ellis 2005 {published data only}
- Ellis G, Rodger J, McAlpine C, Langhorne P. The impact of stroke nurse specialist input on risk factor modification: a randomised controlled trial. Age & Ageing 2005;34(4):389‐92. [DOI] [PubMed] [Google Scholar]
- McManus JA, Craig A, McAlpine C, Langhorne P, Ellis G. Does behaviour modification affect post‐stroke risk factor control? Three‐year follow‐up of a randomized controlled trial. Clinical Rehabilitation 2009;23(2):99‐105. [DOI] [PubMed] [Google Scholar]
Flemming 2013 {published data only}
- Flemming KD, Allison TG, Covalt JL, Herzig DE, Brown Jr RD. Utility of a post‐hospitalization stroke prevention program managed by nurses. Hospital Practice 2013;41(3):70‐9. [DOI] [PubMed] [Google Scholar]
Forster 1996 {published data only}
- Forster A, Young J. Specialist nurse support for patients with stroke in the community: a randomised controlled trial. BMJ 1996;312(7047):1642‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedland 1992 {published data only}
- Friedland JF, McColl M. Social support intervention after stroke: results of a randomized trial. Archives of Physical Medicine and Rehabilitation 1992;73(6):573‐81. [PubMed] [Google Scholar]
Fu 2003 {published data only}
- Fu DB, Hua F, McGowan P, Shen YE, Zhu LH, Yang HQ, et al. Implementation and quantitative evaluation of chronic disease self‐management programme in Shanghai, China: randomized controlled trial. Bulletin of the World Health Organization 2003;81(3):174‐82. [PMC free article] [PubMed] [Google Scholar]
Gray 2011 {published data only (unpublished sought but not used)}
- Gray RJ, Myint PK, Elender F, Barton G, Pfeil M, Price G, et al. A Depression Recognition and Treatment package for families living with Stroke (DepReT‐Stroke): study protocol for a randomised controlled trial. Trials 2011; Vol. 12. [DOI] [PMC free article] [PubMed]
Guidetti 2010 {published data only}
- Guidetti S, Andersson K, Andersson M, Tham K, Koch L. Client‐centred self‐care intervention after stroke: a feasibility study. Scandinavian Journal of Occupational Therapy 2010;17(4):276‐85. [DOI] [PubMed] [Google Scholar]
- Guidetti S, Ytterberg C. A randomised controlled trial of a client‐centred self‐care intervention after stroke: a longitudinal pilot study. Disability & Rehabilitation 2011;33(6):494‐503. [DOI] [PubMed] [Google Scholar]
Harrington 2010 {published data only}
- Harrington R, Taylor G, Hollinghurst S, Reed M, Kay H, Wood VA. A community‐based exercise and education scheme for stroke survivors: a randomized controlled trial and economic evaluation. Clinical Rehabilitation 2010;24(1):3‐15. [DOI] [PubMed] [Google Scholar]
Jones 2015 {published data only}
- Jones KM, Bhattacharjee R, Krishnamurthi R, Blanton S, Theadom A, Barker‐Collo S, et al. Methodology of the stroke self‐management rehabilitation trial: an international, multisite pilot trial. Journal of Stroke and Cerebrovascular Diseases 2015;24(2):297‐303. [DOI] [PubMed] [Google Scholar]
Kang 2004 {published data only}
- Kang HS, Kim WO, Kim JW, Wang MJ, Cho JH. Effect of East‐West Self‐help Group program for rehabilitation of post stroke clients. Taehan Kanho Hakhoe Chi 2004;34(7):1351‐61. [DOI] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim CG, Park HA. Development and evaluation of a web‐based education program to prevent secondary stroke. Journal of Korean Academy of Nursing 2011;41(1):47‐60. [DOI] [PubMed] [Google Scholar]
Kronish 2014 {published data only}
- Kronish I, Goldfinger J, Negron R, Tuhrim S, Arniella G, Horowitz C. Effect of peer education on stroke prevention: the prevent recurrence of all inner‐city strokes through education randomised controlled trial. Stroke 2014;45(11):3330‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Logan 1997 {published data only}
- Logan PA, Ahern J, Gladman JR, Lincoln NB. A randomized controlled trial of enhanced Social Service occupational therapy for stroke patients. Clinical Rehabilitation 1997;11(2):107‐13. [DOI] [PubMed] [Google Scholar]
Lorig 1999 {published data only (unpublished sought but not used)}
- Lorig KR, Ritter P, Stewart AL, Sobel DS, Brown BW, Bandura A, et al. Chronic disease self‐management program ‐ 2‐year health status and health care utilization outcomes. Medical Care 2001;39(11):1217‐23. [DOI] [PubMed] [Google Scholar]
- Lorig KR, Sobel DS, Stewart AL, Brown BW, Bandura A, Ritter P, et al. Evidence suggesting that a chronic disease self‐management program can improve health status while reducing hospitalization ‐ a randomized trial. Medical Care 1999;37(1):5‐14. [DOI] [PubMed] [Google Scholar]
Markle‐Reid 2011 {published and unpublished data}
- Markle‐Reid M, Orridge C, Weir R, Browne G, Gafni A, Lewis M, et al. Interprofessional stroke rehabilitation for stroke survivors using home care. Canadian Journal of Neurological Sciences 2011;39(2):317‐34. [DOI] [PubMed] [Google Scholar]
Marsden 2010 {published data only}
- Marsden D, Quinn R, Pond N, Golledge R, Neilson C, White J, et al. A multidisciplinary group programme in rural settings for community‐dwelling chronic stroke survivors and their carers: a pilot randomized controlled trial. Clinical Rehabilitation 2010;24(4):328‐41. [DOI] [PubMed] [Google Scholar]
Rodgers 1999 {published data only}
- Rodgers H, Atkinson C, Bond S, Suddes M, Dobson R, Curless R. Randomized controlled trial of a comprehensive stroke education program for patients and caregivers. Stroke 1999;30(12):2585‐91. [DOI] [PubMed] [Google Scholar]
Sahebalzamani 2009 {published data only}
- Sahebalzamani M, Aliloo L, Shakibi A. The efficacy of self‐care education on rehabilitation of stroke patients. Saudi Medical Journal 2009;30(4):550‐4. [PubMed] [Google Scholar]
Smith 2004 {published data only}
- Smith J, Forster A, Young J. A randomized triad to evacuate an education programme for patients and carers after stroke. Clinical Rehabilitation 2004;18(7):726‐36. [DOI] [PubMed] [Google Scholar]
Thrift 2014 {published data only}
- Thrift AG, Srikanth VK, Nelson MR, Kim J, Fitzgerald SM, Gerraty RP, et al. Risk factor management in survivors of stroke: a double‐blind, cluster‐randomized, controlled trial. International Journal of Stroke 2014;9(5):652‐7. [DOI] [PubMed] [Google Scholar]
van der Ploeg 2007 {published data only}
- Ploeg HP, Streppel KR, Beek AJ, Woude LH, Vollenbroek‐Hutten MM, Harten WH, et al. Successfully improving physical activity behavior after rehabilitation. American Journal of Health Promotion 2007;21(3):153‐9. [DOI] [PubMed] [Google Scholar]
Vluggen 2012 {published data only (unpublished sought but not used)}
- Vluggen TP, Haastregt JC, Verbunt JA, Keijsers EJ, Schols JM. Multidisciplinary transmural rehabilitation for older persons with a stroke: the design of a randomised controlled trial. BMC Neurology 2012;12:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2013 {published data only}
- Wang L, Chen C‐M, Liao W‐C, Hsiao C‐Y. Evaluating a community‐based stroke nursing education and rehabilitation programme for patients with mild stroke. International Journal of Nursing Practice 2013;19(3):249‐56. [DOI] [PubMed] [Google Scholar]
Wolfe 2010 {published data only}
- Wolfe CD, Redfern J, Rudd AG, Grieve AP, Heuschmann PU, McKevitt C. Cluster randomized controlled trial of a patient and general practitioner intervention to improve the management of multiple risk factors after stroke: stop stroke. Stroke 2010;41(11):2470‐6. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Damush 2013 {published data only}
- Damush T, Anderson J, Yu Z, Ofner S, Myers L, Schmid A, et al. Secondary stroke prevention program: effect on stroke specific quality of life. Stroke 2013; Vol. 44, issue 2.
Donnellan 2014 {published data only}
- Donnellan C. REsources And LIfe Strategy Management (REALISM) trial: protocol for a stroke rehabilitation intervention using a goal setting and attainment framework. International Journal of Stroke 2014;9 Suppl 3:284. [Google Scholar]
Leistner 2013 {published data only (unpublished sought but not used)}
- Leistner S, Michelson G, Laumeier I, Ahmadi M, Smyth M, Nieweler G, et al. Intensified secondary prevention intending a reduction of recurrent events in TIA and minor stroke patients (INSPiRE‐TMS): a protocol for a randomised controlled trial. BMC Neurology 2013;13(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lo 2014 {published data only}
- Lo SHS, Chang AM, Chau JPC, Gardner G. Development of a randomized controlled trial to evaluate the effectiveness of a self‐efficacy enhancing stroke self‐management program for stroke survivors. International Journal of Stroke 2014;9 Suppl 3:246. [Google Scholar]
MacKay‐Lyons 2010 {published data only (unpublished sought but not used)}
- MacKay‐Lyons M, Gubitz G, Giacomantonio N, Wightman H, Marsters D, Thompson K, et al. Program of rehabilitative exercise and education to avert vascular events after non‐disabling stroke or transient ischaemic attack. BMC Neurology 2010;10:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01550822 {published data only (unpublished sought but not used)}
- NCT01550822. HEALS (Healthy Eating And Lifestyle After Stroke). clinicaltrials.gov/ct2/show/NCT01550822 (accessed 4 July 2013).
NCT02156778 {published data only}
- NCT02156778. Post‐Stroke Disease Management ‐ Stroke Card, 2014. clinicaltrials.gov/ct2/show/NCT02156778 (accessed 22 March 2016).
- Toell T, Willeit K, Schoenherr G, Tuer A, Pechlaner R, Furtner M, et al. Post‐Stroke Disease Management ‐ Stroke Card: extending the standard care after stroke and high‐risk TIA. International Journal of Stroke 2014;9 Suppl 3:284. [Google Scholar]
NCT02207023 {published data only}
- NCT02207023. Healthy Lifestyles After Stroke (Stroke COACH), 2014. clinicaltrials.gov/ct2/show/NCT02207023 (accessed 22 March 2016).
- Sakakibara BM, Eng JJ, Benavente O, Barr S, Silverberg ND, Goldsmith CH, et al. A telehealth intervention to promote healthy lifestyles after stroke: the Stroke COACH protocol. Stroke 2014;45(12):E285. [DOI] [PMC free article] [PubMed] [Google Scholar]
UMIN000007808 {published data only (unpublished sought but not used)}
- UMIN000007808. Construction of a self‐management patient education program and evidences for preventing recurrence of stroke in community settings. upload.umin.ac.jp/cgi‐open‐bin/ctr/ctr.cgi?function=brows&action=brows&type=summary&recptno=R000009198&language=E (accessed February 2015).
References to ongoing studies
Cheng 2011 {published data only (unpublished sought but not used)}
- Cheng EM, Cunningham WE, Towfighi A, Sanossian N, Bryg RJ, Anderson TL, et al. Randomized, controlled trial of an intervention to enable stroke survivors throughout the Los Angeles County safety net to "stay with the guidelines". Circulation: Cardiovascular Quality and Outcomes 2011;4(2):229‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fukuoka 2014 {published data only}
- Fukuoka Y, Hosomi N, Hyakuta T, Omori T, Ito Y, Uemura J, DMP Stroke Trial Investigators. Baseline feature of a randomized trial assessing the effects of disease management programs for the prevention of recurrent ischemic stroke. Journal of Stroke and Cerebrovascular Diseases 2014;24(3):610‐7. [DOI] [PubMed] [Google Scholar]
Graven 2011 {published data only (unpublished sought but not used)}
- Graven C, Brock K, Hill K, Ames D, Cotton S, Joubert L. From rehabilitation to recovery: protocol for a randomised controlled trial evaluating a goal‐based intervention to reduce depression and facilitate participation post‐stroke. BMC Neurology 2011;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN08913646 {published data only (unpublished sought but not used)}
- ISRCTN08913646. Study to determine whether the empowered stroke patients demonstrate better self‐management behaviour and health outcomes HEISS. isrctn.org/ISRCTN08913646 (accessed 4 July 2013).
NCT01507688 {published data only (unpublished sought but not used)}
- NCT01507688. Stroke self‐management. clinicaltrials.gov/show/NCT01507688 (accessed 4 July 2013).
NCT01770184 {published data only (unpublished sought but not used)}
- NCT01770184. Clinical effectiveness of self‐management education post‐mild stroke. clinicaltrials.gov/show/NCT01770184 (accessed 4 July 2013).
Additional references
AIHW 2011
- AIHW. Health Priority Areas. Canberra: Australian Institute of Health and Welfare, 2011. [Google Scholar]
AIHW 2013
- Australian Institute of Health and Welfare 2013. Stroke and its management in Australia: an update. Cardiovascular Disease Series no. 37. Cat. no. CVD 61: 6‐12. Canberra: AIHW.
Bandura 1986
- Bandura A. The explanatory and predictive scope of self‐efficacy theory. Journal of Social and Clinical Psychology 1986;4(3):359‐73. [Google Scholar]
Begg 2007
- Begg S, Vos T, Barker B, Stevenson C, Stanley L, Lopez A. The Burden of Disease and Injury in Australia 2003. Canberra: Australian Institute of Health and Welfare, 2007. [Google Scholar]
Campbell 2000
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321(7262):694‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cerniauskaite 2012
- Cerniauskaite M, Quintas R, Koutsogeorgou E, Meucci P, Sattin D, Leonardi M, et al. Quality‐of‐life and disability in patients with stroke. American Journal of Physical Medicine and Rehabilitation 2012;91(13 Suppl 1):S39‐47. [DOI] [PubMed] [Google Scholar]
Cott 2007
- Cott CA, Wiles R, Devitt R. Continuity, transition and participation: preparing clients for life in the community post‐stroke. Disability and Rehabilitation 2007;29(20‐21):1566‐74. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG, Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Deloitte Access Economics 2014
- Deloitte Access Economics. Stroke in Australia: No Postcode Untouched. Melbourne: National Stroke Foundation, 2014. [Google Scholar]
Dewey 2001
- Dewey HM, Thrift AG, Mihalopoulos C, Carter R, Macdonell RA, McNeil JJ, et al. Cost of stroke in Australia from a societal perspective: results from the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke 2001;32(10):2409‐16. [DOI] [PubMed] [Google Scholar]
Dowswell 2000
- Dowswell G, Lawler J, Dowswell T, Young J, Forster A, Hearn J. Investigating recovery from stroke: a qualitative study. Journal of Clinical Nursing 2000;9(4):507‐15. [DOI] [PubMed] [Google Scholar]
Foster 2007
- Foster G, Taylor SJC, Eldridge S, Ramsay J, Griffiths CJ. Self‐management education programmes by lay leaders for people with chronic conditions. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD005108.pub2] [DOI] [PubMed] [Google Scholar]
Hackett 2005
- Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: a systematic review of observational studies. Stroke 2005;36(6):1330‐40. [DOI] [PubMed] [Google Scholar]
Haley 2009
- Haley WE, Allen JY, Grant JS, Clay OJ, Perkins M, Roth DL. Problems and benefits reported by stroke family caregivers: results from a prospective epidemiological study. Stroke 2009;40(6):2129‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hatano 1976
- Hatano S. Experience from a multicentre stroke register: a preliminary report. Bulletin of the World Health Organization 1976;54(5):541‐53. [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jeong 2012
- Jeong BO, Kang HJ, Bae KY, Kim SW, Kim JM, Shin IS, et al. Determinants of quality of life in the acute stage following stroke. Psychiatry Investigation 2012;9(2):127‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2006
- Jones F. Strategies to enhance chronic disease self‐management: how can we apply this to stroke?. Disability and Rehabilitation 2006;28(13/14):841‐7. [DOI] [PubMed] [Google Scholar]
Jones 2011
- Jones F, Riazi A. Self‐efficacy and self‐management after stroke: a systematic review. Disability and Rehabilitation 2011;33(10):797‐810. [DOI] [PubMed] [Google Scholar]
Judge 2002
- Judge T, Erez A, Bono J, Thoresen C. Are measures of self‐esteem, neuroticism, locus of control and generalised self‐efficacy indicators of a common core construct?. Journal of Personality and Social Psychology 2002;83:693‐710. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Korpershoek 2011
- Korpershoek C, Bijl J, Hafsteinsdóttir TB. Self‐efficacy and its influence on recovery of patients with stroke: a systematic review. Journal of Advanced Nursing 2011;67(9):1876‐94. [DOI] [PubMed] [Google Scholar]
Lees 2012
- Lees R, Fearon P, Harrison JK, Broomfield NM, Quinn TJ. Cognitive and mood assessment in stroke research: focused review of contemporary studies. Stroke 2012;43(6):1678‐80. [DOI] [PubMed] [Google Scholar]
Lennon 2013
- Lennon S, McKenna S, Jones F. Self‐management programmes for people post stroke: a systematic review. Clinical Rehabilitation 2013;27:867‐78. [DOI] [PubMed] [Google Scholar]
Lindsay 2010
- Lindsay MP, Gubitz G, Bayley M, Hill MD, Davies‐Schinkel C, Singh S, et al. Canadian Best Practice Recommendations for Stroke Care (Update 2010). Ottawa: On behalf of the Canadian Stroke Strategy Best Practices and Standards Writing Group, Canadian Stroke Network, 2010. [Google Scholar]
Lorig 1993
- Lorig KR, Mazonson PD, Holman HR. Evidence suggesting that health education for self‐management in patients with chronic arthritis has sustained health benefits while reducing health care costs. Arthritis & Rheumatism 1993;36(4):439‐46. [DOI] [PubMed] [Google Scholar]
Mayo 2002
- Mayo NE, Wood‐Dauphinee S, Cote R, Durcan L, Carlton J. Activity, participation, and quality of life 6 months post stroke. Archives of Physical Medicine and Rehabilitation 2002;83(8):1035‐42. [DOI] [PubMed] [Google Scholar]
Muntner 2002
- Muntner P, Garrett E, Klag MJ, Coresh J. Trends in stroke prevalence between 1973 and 1991 in the US population 25 to 74 years of age. Stroke 2002;33(5):1209‐13. [DOI] [PubMed] [Google Scholar]
NSF 2010
- Clinical guidelines for stroke rehabilitation and recovery. National Stroke Foundation, Canberra 2010.
Pickard 2005
- Pickard S, Johnson J, Feeny D. Responsiveness of generic health‐related quality of life measures in stroke. Quality of Life Research 2005;14:207‐19. [DOI] [PubMed] [Google Scholar]
Pohjasvaara 2001
- Pohjasvaara T, Vataja R, Leppavuori A, Kaste M, Erkinjuntti T. Depression is an independent predictor of poor long‐term functional outcome post‐stroke. European Journal of Neurology 2001;8(4):315‐9. [DOI] [PubMed] [Google Scholar]
Pound 1998
- Pound P, Gompertz P, Ebrahim S. A patient‐centred study of the consequences of stroke. Clinical Rehabilitation 1998;12:255‐64. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Strecher 1986
- Strecher VJ, Mcevoy B, Becker MH, Rosenstock IM. The role of self‐efficacy in achieving health behaviour change. Health Education and Behaviour 1986;13(1):73‐92. [DOI] [PubMed] [Google Scholar]
Walker 2003
- Walker C, Swerissen H, Belfrage J. Self‐management: its place in the management of chronic illness. Australian Health Review 2003;26(2):34‐42. [DOI] [PubMed] [Google Scholar]
Warner 2015
- Warner G, Packer T, Villeneuve M, Audulv A, Versnel J. A systematic review of the effectiveness of stroke self‐management programs for improving function and participation outcomes: self‐management programs for stroke survivors. Disability and Rehabilitation 2015;37(23):2141‐63. [DOI] [PubMed] [Google Scholar]
Winstein 2016
- Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, the American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016;47:e98‐169. [DOI: 10.1161/STR.0000000000000098] [DOI] [PubMed] [Google Scholar]
Wolfe 2000
- Wolfe CD. The impact of stroke. British Medical Bulletin 2000;56(2):275‐86. [DOI] [PubMed] [Google Scholar]


