Summary
Physical activity is a strong positive physiological modulator of adult neurogenesis in the hippocampal dentate gyrus. Although the underlying regulatory mechanisms are still unknown, systemic processes must be involved. Here we show that platelets are activated after acute periods of running, and that activated platelets promote neurogenesis, an effect that is likely mediated by platelet factor 4. Ex vivo, the beneficial effects of activated platelets and platelet factor 4 on neural precursor cells were dentate gyrus specific and not observed in the subventricular zone. Moreover, the depletion of circulating platelets in mice abolished the running-induced increase in precursor cell proliferation in the dentate gyrus following exercise. These findings demonstrate that platelets and their released factors can modulate adult neural precursor cells under physiological conditions and provide an intriguing link between running-induced platelet activation and the modulation of neurogenesis after exercise.
Keywords: exercise, neurogenesis, neural precursor cell, dentate gyrus, platelets, platelet activation, adult mouse
Graphical Abstract

Highlights
-
•
Activated platelets increase hippocampal neurogenesis in physiological conditions
-
•
Platelet-depleted mice lack the running-induced increase in precursor proliferation
-
•
PF4-treated mice have more immature neurons
-
•
Pro-neurogenic effects of activated platelets and PF4 are dentate gyrus-specific
Using the neurogenesis-promoting stimulus of physical activity, Walker and colleagues show that platelets are activated after acute running periods and that activated platelets and their released protein PF4 following exercise, increase neurogenesis. They show that the pro-neurogenic effects of platelets are dentate gyrus specific and that platelet depletion in mice abolishes the running-induced increase in precursor proliferation in the dentate gyrus.
Introduction
Adult neurogenesis is the life-long generation of functional new neurons in the adult brain. In the hippocampal dentate gyrus (DG), one of the major adult neurogenic niches, this process is responsive to external stimuli. A strong positive physiological modulator of neural precursor cell (NPC) proliferation in the DG is physical activity (Kronenberg et al., 2003, van Praag et al., 1999a, van Praag et al., 1999b). We have previously shown that 4 days of running are sufficient to significantly increase the number of proliferating NPCs in the DG (Overall et al., 2013); however, which factors regulate this early response is mainly unknown. Exercise is associated with changes in the blood, and the direct contact of neural stem and progenitor cells with blood vessels (Filippov et al., 2003, Moss et al., 2016, Sun et al., 2015) allows crosstalk between neural stem cells and peripheral modulators. Accordingly, blood-borne factors have been shown to affect adult hippocampal neurogenesis under physiological conditions, including during aging (Castellano et al., 2017, Villeda et al., 2011, Villeda et al., 2014), and in conditions that promote neurogenesis, including physical activity. Following exercise, circulating growth factors, such as peripheral vascular endothelial growth factor (VEGF) (Fabel et al., 2003) and insulin-like growth factor-1 (IGF-1) (Trejo et al., 2001), influence NPC proliferation. Furthermore, muscle-derived cathepsin B mediates running-induced neurogenic effects, particularly improved spatial memory function (Moon et al., 2016).
In this study, our aim was to identify alternative systemic molecular mechanisms by which the early proliferative response of NPCs following exercise is regulated. We hypothesized that systemic factors are released into the blood after acute periods of physical activity. We used a proteomic screening approach to identify running-induced changes in the blood composition that could contribute to the regulation of the NPC response. From this, we identified platelets and their released factors as potential candidates. Platelets are short-lived, small blood cells that primarily regulate hemostasis. However, recently platelets have also gained recognition for their function in a number of other regulatory processes, suggesting a much broader systemic functionality than previously assumed. Signal-dependent translation from stable platelet mRNAs allows these non-nucleated cells to rapidly modify their proteome, thereby adjusting their function (Weyrich et al., 2009, Wicki et al., 1989). With the capacity to synthesize and release selective sets of proteins in response to distinct stimuli (Berthet et al., 2012, Coppinger et al., 2007, Italiano et al., 2008), platelets are proficient at sensing, and therefore responding to environmental changes. Following activation, platelets release a range of bioactive molecules, many of which are capable of increasing hippocampal neurogenesis. Among these, VEGF (Fabel et al., 2003), IGF-1 (Trejo et al., 2001), and serotonin (Klempin et al., 2013) are required for the increase in neurogenesis observed after exercise. In the context of injury and insult, platelets can also promote neurogenesis in the subventricular zone (SVZ) (Hayon et al., 2012, Hayon et al., 2013, Kazanis et al., 2015), highlighting a regulatory role for platelets in another neurogenic niche.
In this study, we investigated whether platelets are involved in regulating adult neurogenesis under baseline physiological circumstances and in response to physical activity.
Results
Acute Exercise-Induced Peripheral Changes Are Platelet-Related and Increase the Number of DG-Derived Neurospheres
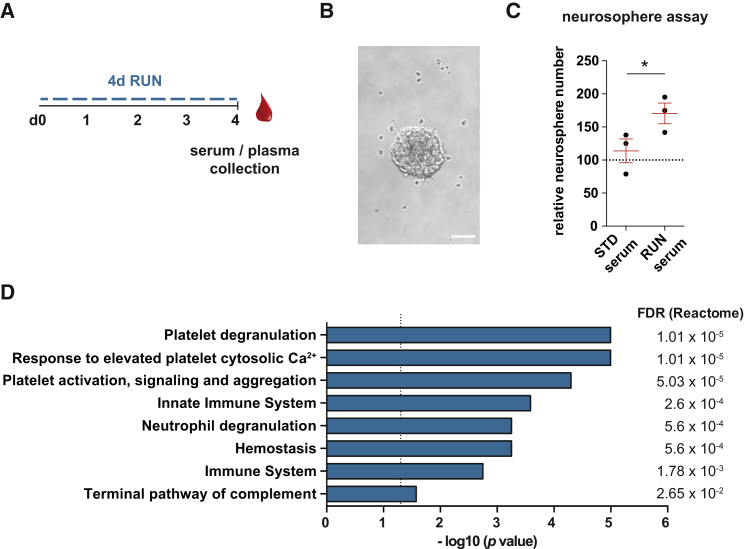
In a previous study, we demonstrated that short periods of exercise (4 days) are sufficient to significantly increase NPC proliferation in the DG (Overall et al., 2013). To test whether factors that are released into the blood following an acute period of running could initiate the increase in NPC proliferation in the DG, we isolated serum from standard-housed (STD) mice or animals housed with a running wheel for 4 days (4d RUN) and performed neurosphere assays on DG-derived primary cells (Figures 1A and 1B). We found significantly more neurospheres in cultures supplemented with 4d RUN serum compared with cultures containing STD serum (170.5% ± 15.6% versus 112.2% ± 15.6% of control, p = 0.04; Figure 1C), indicating that running-induced systemic factors can activate latent stem cells in vitro (Walker et al., 2008).
Figure 1.
Acute Exercise-Induced Peripheral Changes Are Platelet-Related and Increase the Number of DG-Derived Neurospheres
(A) Animals ran for 4 days followed by serum and plasma collection in the morning of day 5.
(B) Representative image of a neurosphere cultured with 0.01% mouse serum. Scale bar, 50 μm.
(C) More neurospheres formed after the addition of 0.01% 4d RUN serum. Dashed line represents control cultures normalized to 100%. n = 3 independent experiments, ∗p < 0.05, one-way ANOVA with Tukey test. Data represent the means ± SEM.
(D) Enrichment of biological pathways of proteins with significantly increased plasma levels in 4d RUN mice (see also Table S1). Graph represents result of Reactome pathway analysis. Dashed line indicates false discovery rate (FDR) of 0.05.
To identify potential running-induced protein changes in the blood, we next performed a mass spectrometry-based proteomic screen of plasma isolated from 4d RUN and STD mice (Table S1). This and all further experiments were performed with plasma, as plasma, in comparison with serum, more closely resembles the physiological, non-coagulated blood composition in vivo and could be applied to potential future downstream therapeutic approaches. From the proteomic screen, we identified 38 proteins with significantly higher levels in RUN animals. To investigate a potential interplay between these proteins and to determine potential biological pathways underlying the systemic changes, we performed a Reactome pathway analysis (Fabregat et al., 2018). From this, we identified the top three significant pathway terms as “Platelet degranulation,” “Response to elevated platelet cytosolic Ca2+,” and “Platelet activation, signaling and aggregation” (Figure 1D), indicating that platelets are activated after running and that platelet-associated proteins are secreted into the plasma of running mice.
Platelets Are Activated and Change Their Proteomic Profile after Acute Periods of Running
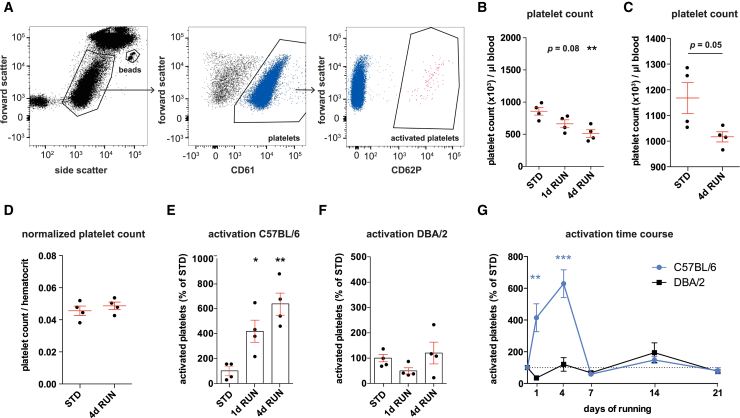
The results from the proteomic pathway analysis suggested that platelets are responsive to exercise. To determine whether platelets are indeed activated after short periods of running, we determined the platelet count and activation state using flow cytometry (Figure 2A). For this, whole blood was collected from STD mice and animals that were running for 1 day (1d RUN) or 4 days (4d RUN). We found that 4d RUN mice had a lower platelet count than STD controls (STD 858.5 ± 60.75 × 103 versus 4d RUN 512.5 ± 59.19 × 103 platelets/μL blood, p = 0.005; Figure 2B), a finding that was confirmed when the platelet count was determined in a different cohort of mice using a Sysmex automated hematology counter (Figure 2C). This result is consistent with what is known to occur in humans following exercise and is most likely due to an increase in plasma volume (Fellmann, 1992). We corroborated this assumption by normalizing the platelet count of individual mice to their hematocrit levels measured by the Sysmex system, which abolished the running-induced decrease in platelet count (Figure 2D).
Figure 2.
Platelets Are Activated after Acute Periods of Running
(A) Representative flow cytometry plots of platelet count and activation. Left: platelets and reference beads were first gated based on forward and side scatter. Platelets and activated platelets were defined by the expression of CD61 (middle) and CD62P (right), respectively.
(B) Platelet count in STD and RUN mice measured by flow cytometry. n = 4 mice per group, ∗∗p < 0.01, one-way ANOVA with Dunnett test.
(C and D) Platelet count (C) and normalized platelet count (D) in a different cohort of mice determined by the Sysmex system. n = 4 mice per group, Student's t test.
(E and F) Platelet activation in (E) C57BL/6 and (F) DBA/2 mice after running. n = 4 mice per group, ∗p < 0.05, ∗∗p < 0.01, one-way ANOVA with Dunnett test.
(G) Time course of platelet activation. n = 4 mice per group, ∗∗p < 0.01, ∗∗∗p < 0.001, one-way ANOVA with Dunnett test. All data represent the means ± SEM.
To address platelet activation in the blood of the same animals, the percentage of activated platelets (CD62P+) within the total platelet population (CD61+) was determined. This revealed that more platelets were activated in the blood of RUN mice compared with STD controls (Figure 2E). A statistically significant difference was already observed after 1d RUN (414% ± 88.1% of control, p = 0.03) with a further increase after 4 days of running (628.7% ± 87.7% of control, p = 0.001). These data show that platelets are responsive to the physiological stimulus of exercise, particularly after acute periods of running.
To test whether running-induced platelet activation is associated with increased levels of NPC proliferation, and to ensure that this was not a side effect of physical exercise per se, we also investigated the activation state of platelets in a different mouse strain. We had previously shown that DBA/2 mice do not show increased NPC proliferation after short periods of running (Overall et al., 2013). Similarly, DBA/2 mice exhibited no increase in platelet activation after 1d RUN and 4d RUN (Figure 2F), even though they ran the same distance as the C57BL/6 mice.
In a time course experiment, we found that after longer periods of running, the level of platelet activation in C57BL/6 mice returned to those observed in STD mice, indicating that running-induced platelet activation is an acute, transient effect (Figure 2G).
To determine whether platelets change their proteomic signature after running, we next performed a proteomic screen on platelets isolated from STD and 4d RUN animals. We identified 16 proteins that were significantly different between running mice and sedentary animals (Figure 3; Table S2). This suggests that the running-induced activation of platelets could lead to changes in protein content and platelet function. Among the differentially expressed proteins were members of the 14-3-3 protein family, regulatory molecules that are highly expressed in the brain. The isoform 14-3-3γ showed the most significantly increased protein levels after running (p < 0.001; Figure 3).
Figure 3.
Platelets Change Their Proteomic Signature after Acute Periods of Running
Quantitative proteomic analysis of platelets from 4d RUN and STD mice revealed distinct platelet protein levels after physical activity (see also Table S2). Log10 p values were plotted against effect size (difference of means). 14-3-3γ showed the most significant increase after 4 days of running. The insert shows the log2-transformed and scaled protein values for 14-3-3γ. n = 4 mice per group, ∗∗∗p < 0.001, Student's t test. Data represent the means ± SEM.
Activated Platelets Increase Neurogenesis and Act Directly on Hippocampal NPCs Ex Vivo
The current literature on alternative platelet function, and the fact that platelets are activated after short periods of running, led us to hypothesize that running-activated platelets might have the capacity to stimulate adult hippocampal neurogenesis. To investigate this, we isolated platelet-rich plasma (PRP) (“rich”) and platelet-poor plasma (PPP) (“poor”) from STD and 4d RUN mice and performed neurosphere assays. Freshly isolated PRP and PPP did not affect DG neurosphere numbers (Figure 4A). We next determined whether platelets activated by freeze-thawing had an effect on NPCs. It has previously been reported that low temperatures induce platelet activation (Roffi et al., 2014), and this was confirmed by our measurements after only one cycle of freeze-thawing (Figure 4B). In cold-activated samples, 8.7% ± 0.7% of platelets were activated compared with only 0.4% ± 0.1% in untreated control samples (p < 0.001). This was as efficient as phorbol-12-myristate-13-acetate-induced platelet activation, which was used as a positive control (8.6% ± 1.3%, p < 0.001). Moreover, co-isolated cell populations, such as erythrocytes, monocytes, and leukocytes, were significantly reduced in the cold-activated samples (Figure 4C), resulting in a purer population of activated platelets (fresh 170,373 ± 8,945 versus frozen 284 ± 45 co-isolated cells per 1,000 bead events; n = 4 samples, p < 0.001).
Figure 4.
Activated Platelets Increase Neurogenesis and Directly Act on Hippocampal NPCs Ex Vivo
(A) Neurosphere number after the addition of freshly isolated PPP and PRP. n = 7 independent experiments.
(B) Platelet activation in the plasma of STD mice through cold and with phorbol-12-myristate-13-acetate (PMA). n = 4 samples of four individual mice, ∗∗∗p < 0.001, one-way ANOVA with Dunnett test.
(C) Representative flow cytometry plots showing cell populations as determined by forward and side scatter in freshly isolated (left) and cold-activated samples (right). Co-isolated cells are gated in P1, whereas P2 yields platelets.
(D) Neurosphere assays with activated platelets in PPP and PRP. n = 11 independent experiments, ∗p < 0.05, one-way ANOVA with Dunnett test.
(E) Representative image of a neurosphere in control conditions (top) and a large neurosphere in RUN rich conditions (bottom). Scale bars, 100 μm.
(F) Neurospheres (≥130 μm) cultured with activated platelets. n = 11 independent experiments, ∗p < 0.05, one-way ANOVA with Dunnett test.
(G) Flow cytometry plots of gating strategy to isolate lysophosphatidic acid receptor 1 (LPA1)+ cells. Left: viable DG cells from LPA1-GFP mice were determined by forward and side scatter. Right: plot of sorted LPA1-GFP+ and LPA1-GFP- cells.
(H) Neurosphere assay using LPA1-GFP+ cells with activated PPP and PRP from STD mice. n = 7 independent experiments, ∗∗p < 0.01, one-way ANOVA with Dunnett test.
(I) Representative image of differentiated neurospheres showing GFAP+ astrocytes (green) and β-tubulin+ neurons (red). Scale bar, 50 μm.
(J and K) Quantification of GFAP+ astrocytes is shown in (J) and β-tubulin+ neurons in (K). n = 4 independent experiments, ∗p < 0.05, one-way ANOVA with Dunnett test. Dashed lines represent control cultures normalized to 100%. All data represent the means ± SEM.
Treatment of primary DG cells with cold-activated PPP and PRP resulted in more neurospheres in the PRP samples from both STD and RUN mice compared with untreated control cultures (Figure 4D). STD-rich plasma increased the neurosphere number to 136.1% ± 13.4% of that in control cultures (p = 0.06). An even greater and significant effect was observed in primary cultures supplemented with RUN-rich plasma (145.0% ± 11.1% of control, p = 0.01). Although there was no significant difference in the response to treatment with cold-activated PRP from STD and RUN animals, the data show a clear effect of activated platelets on NPCs. In contrast, the number of neurospheres in cultures treated with PPP did not differ from that in untreated controls, further confirming that the increase in neurosphere number was due to PRP rather than an effect of the plasma treatment itself. In addition, cultures treated with PRP generated more large neurospheres than untreated control cultures, indicating that more early NPCs were proliferating in the presence of activated platelets (Figures 4E and 4F).
To determine whether PRP directly affects adult hippocampal neural stem and progenitor cells, we next used the lysophosphatidic acid receptor 1 (LPA1) as a marker to specifically isolate type 1 and type 2a cells (Gong et al., 2003, Walker et al., 2016). LPA1-GFP+ and LPA1-GFP− cells (Figure 4G) were cultured as neurospheres with activated PPP and PRP. Treatment of LPA1-GFP+ cells with PRP significantly increased the number of neurospheres to 248.7% ± 34.9% of control cultures (p = 0.006; Figure 4H), indicating that activated platelets in PRP directly act on neural stem and progenitor cells from the adult DG without the support of other cell types.
After assessing their activation and proliferation potential, the neurospheres were differentiated and stained for the expression of β-tubulin and glial fibrillary acidic protein (GFAP) (Figure 4I). The number of GFAP+ astrocytes was similar in all conditions (Figure 4J); however, an increase in the number of β-tubulin+ cells was observed in cultures treated with activated PRP, indicating a specific effect of activated platelets on cells committing to the neuronal lineage (percent of total cells: control: 6.7% ± 0.5%; STD poor: 7.0% ± 1.2%; STD rich: 9.9% ± 1.0%; RUN poor: 6.5% ± 0.7%; RUN rich: 10.1% ± 0.9%; control versus STD rich: p = 0.04, control versus RUN rich: p = 0.03; Figure 4K). Together these data indicate that activated platelets stimulate neurogenesis, not only through the activation and/or proliferation of NPCs, but also by promoting neuronal differentiation.
Activated Platelets Do Not Influence Ex Vivo Neurogenesis in the SVZ
The above data suggest that platelets play a role in the regulation of running-induced neurogenesis in the hippocampal DG. Physical activity, however, was shown to affect the two neurogenic niches differently, resulting in a robust running-induced increase in NPC proliferation in the SGZ (Kronenberg et al., 2003, van Praag et al., 1999a, van Praag et al., 1999b) but not in the SVZ (Brown et al., 2003). To validate this finding, we investigated the effect of three different bouts of physical activity on proliferation in the two neurogenic niches and found that 4, 10, and 28 days of running significantly increased the number of proliferating cells in the DG but not in the SVZ of the same animals (Figures 5A and S1). Fitting with our hypothesis that activated platelets induce hippocampal NPC proliferation and neurogenesis following exercise, activated platelets did not increase the number of neurospheres in any of the SVZ culture conditions compared with untreated controls (Figure 5B). Furthermore, and in contrast to the DG-derived neurosphere cultures, PRP treatment did not alter the differentiation potential of SVZ neurospheres (Figures 5C–5E). These data suggest that, comparable with the differential effect of physical exercise on hippocampal and SVZ neurogenesis, the effect of activated platelets under physiological conditions is specific to DG-derived NPCs.
Figure 5.
Activated Platelets Do Not Increase Neurogenesis in SVZ-Derived Cultures
(A) Animals were running for 4 days and received a single BrdU injection 12 h prior to perfusion. Graphs show quantification of BrdU+ cells in the DG and SVZ after 4 days of running. n = 10–13 mice per group. ∗p < 0.05, Student's t test. See also Figure S1.
(B) Neurosphere assays with activated platelets in PPP and PRP. n = 10 independent experiments, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, one-way ANOVA with Dunnett test.
(C) Representative image of differentiated SVZ neurospheres with GFAP+ astrocytes (green) and β-tubulin+ neurons (red). Scale bar, 100 μm.
(D and E) Quantification of β-tubulin+ neurons (D) and GFAP+ astrocytes (E). n = 4 independent experiments. All data represent the means ± SEM.
Platelet Factor 4 Increases Neurogenesis in the DG
We next wanted to determine whether platelet factors, which are released into the bloodstream following platelet activation, such as platelet factor 4 (PF4); (Brandt et al., 2000, Rucinski et al., 1990), can modulate NPCs. Using an ELISA, we found significantly higher plasma PF4 levels in mice that ran for only one night (2,379 ± 98.9 ng/mL versus 835.2 ± 189.9 ng/mL, n = 4 animals per group, p = 0.0004). This result was consistent with the significant increase in platelet activation observed early (1d and 4d RUN) after the onset of exercise (Figure 2E). Moreover, from our proteomic analysis (Table S1), we identified significantly higher levels of plasma PF4 in running mice after 4 days of exercise (p = 0.04; Figure 6A). The exact function of PF4 in vivo, particularly in the brain, is not yet understood. In brain tissue under physiological conditions, PF4 was detected in the lumen of blood vessels inside non-nucleated cells, most likely platelets (de Jong et al., 2008). We therefore next sought to determine whether PF4 could be responsible, at least in part, for the pro-neurogenic effects of PRP described above. Using a resazurin reduction cell viability assay any potential toxic effects of PF4 on NPCs in culture were first excluded (Figures 6B and 6C).
Figure 6.
Platelet Factor 4 Increases Neurogenesis in the DG
(A) Relative plasma PF4 levels in STD and 4d RUN mice. n = 5 mice per group, ∗p < 0.05, Student's t test.
(B) Representative image of proliferating NPCs in monolayer culture. Scale bar, 50 μm.
(C) Viability assay in NPC cultures with PF4. n = 5 independent experiments.
(D) Neurosphere assays with different concentrations of PF4. n = 12 independent experiments, ∗p < 0.05, one-way ANOVA with Dunnett test.
(E and F) Quantification of β-tubulin+ neurons (E) and GFAP+ astrocytes (F) in differentiated neurosphere cultures treated with PF4. n = 3 independent experiments, ∗∗p < 0.01, Student's t test.
(G) Experimental setup of in vivo PF4 administration directly into the hippocampus of C57BL/6 mice.
(H) Representative image of BrdU+ cells in the DG. Scale bar, 50 μm.
(I) Quantification of BrdU+ cells in the SGZ of PF4-treated mice. n = 8 mice per group.
(J) Representative images of newborn immature neurons stained with DCX. Scale bars, 50 μm.
(K) Quantification of DCX+ cells in the DG of PF4-treated mice. n = 8 mice per group, ∗p < 0.05, Student's t test. All data represent the means ± SEM.
Next, we showed that the addition of PF4 significantly increased neurosphere numbers in DG-derived cultures (10 ng/mL 137.9% ± 13.8% of control, p = 0.04 and 100 ng/mL 137.8% ± 12.3% of control, p = 0.04; Figure 6D). No effect of PF4 on neurosphere size was observed (Figure S2). Consistent with the finding that activated PRP treatment promotes neuronal differentiation (Figure 4K), neurospheres cultured in 100 ng/mL PF4 generated 1.5-fold more β-tubulin+ neurons than untreated control cultures (percent of total cells: control: 5.6% ± 0.2%; PF4: 8.9% ± 0.1%; p < 0.001; Figure 6E). The number of GFAP+ astrocytes remained similar to that in control cultures (Figure 6F). Similar to the results obtained with activated platelet-containing plasma, we found no effect of PF4 on SVZ primary neurosphere cultures (Figure S2).
To determine whether NPCs also respond to PF4 treatment in vivo, we used mini-osmotic pumps to administer PF4 at a concentration of 100 ng/mL for 7 days directly into the hippocampus of STD C57BL/6 mice. On the last night, a single bromodeoxyuridine (BrdU) injection was given to label proliferating cells (Figures 6G and 6H). Quantification of BrdU+ cells in the SGZ of the DG revealed that PF4 administration had no effect on NPC proliferation (Figure 6I). Interestingly, despite showing no increase in proliferating cells, PF4-treated animals had significantly more doublecortin (DCX+) cells per DG than PBS-treated controls (32,166 ± 1,529 cells versus 26,834 ± 1,763 cells, p = 0.04; Figures 6J and 6K), indicating that PF4 alone stimulated the survival of immature neurons in vivo.
In this study, the effect of systemic PF4 administration on NPCs was not assessed. However, our findings that plasma PF4 levels are increased following exercise and that NPCs can respond to PF4 suggest that this platelet-derived factor may play a role in regulating the exercise-induced increase in NPC proliferation and differentiation.
In Vivo Platelet Depletion Abolishes the Running-Induced Increase in Precursor Cell Proliferation in the DG
To investigate the ability of platelets to regulate exercise-induced adult hippocampal NPC proliferation in vivo, we induced thrombocytopenia in running adult C57BL/6 mice by the specific depletion of circulating platelets with anti-platelet serum (Langer et al., 2012; Figure 7A). Platelet-depleted mice and control serum-treated animals were housed in cages with a running wheel for 10 days, a time period in which NPC proliferation is robustly increased in the DG (Kronenberg et al., 2006) (Figure S1). As shown in Figure 7B and Table S3, anti-platelet serum-treated mice had a significantly lower platelet count, confirming the efficacy of this treatment. NPC proliferation at baseline did not differ between platelet-depleted and control serum-treated animals (Figures 7C and 7D), suggesting that platelets are not required for NPC proliferation under basal conditions. As expected, running increased NPC proliferation in the SGZ of control mice (6,530 ± 332 versus 4,832 ± 409 Ki67+ cells, p = 0.009; Figure 7D). However, mice depleted of platelets did not exhibit the running-induced increase in NPC proliferation (5,528 ± 454 versus 4,597 ± 442 Ki67+ cells, p = 0.3; Figure 7D). Collectively, these data suggest that platelets contribute to the regulation of hippocampal neurogenesis following physical activity, likely through factors released after platelet activation.
Figure 7.
In Vivo Platelet Depletion Abolishes the Running-Induced Increase in Precursor Cell Proliferation in the DG
(A) Animals received a single injection of anti-platelet serum for 13 days, every second day. From days 3 to 13 the mice had access to a running wheel.
(B) Platelet count of mice treated with anti-platelet and control serum (see also Table S3). n = 14–15 mice per group pooled from three independent experiments, ∗∗∗∗p < 0.0001, one-way ANOVA with Sidak test.
(C) Representative image of Ki67+ cells in the SGZ. Scale bar, 100 μm.
(D) Quantification of Ki67+ cells in the SGZ. n = 14–15 mice per group pooled from three independent experiments, ∗∗p < 0.01, one-way ANOVA with Sidak test. All data represent the means ± SEM.
Discussion
The complex regulatory mechanisms underlying running-induced neurogenesis are largely unknown. In particular, the mode of communication between acute systemic changes in the periphery and the brain remains unclear. Here we show that platelets contribute to the regulation of adult hippocampal neurogenesis, particularly after acute periods of physical activity.
Platelets can exert selective and dosed responses depending on the type and context of stimulation (Berthet et al., 2012, Coppinger et al., 2007, Italiano et al., 2008). We have identified another type of platelet activation in mice that is induced by physical activity. Consistent with studies in humans (Heber and Volf, 2015), acute exercise in mice increased the percentage of activated platelets in the blood. In the adult DG, type 1 cells reside in a vascular niche (Palmer et al., 2000) and directly contact blood vessels (Filippov et al., 2003, Moss et al., 2016), allowing a potential interaction with external factors at the vessel wall. In the developing brain, it has been shown that platelets supply the DG, via the vessels, with external compounds, such as epithelial sonic hedgehog (Choe et al., 2015). Although the authors examined a time point when vessels are still leaky, alterations in blood-brain barrier integrity have also been reported to occur under normal circumstances in the adult brain (Abbott, 2002, Zlokovic, 2008), as well as after physical exercise (Sharma et al., 1991, Van der Borght et al., 2009). The fact that in our study, platelets responded to exercise after just 1 day of running is of particular interest, as proliferation in the DG has been previously shown to increase after a single day of exercise (Steiner et al., 2008). We found that activated platelets in the form of PRP act directly on type 1 and type 2a dentate NPCs ex vivo. Our data suggest that activated platelets may deliver external compounds or relevant cues that result in an increase in the number of proliferating precursor cells. One possible explanation of this effect is that rapidly dividing precursor cells are recruited from quiescent NPCs residing in the niche (Lugert et al., 2010). However, a number of alternative mechanisms which also lead to a net increase in precursor cell numbers have previously been proposed (Overall et al., 2016). These include shortening of the cell-cycle length, an increase in the number of divisions before exiting the cell cycle, and changes in the number of precursor cells undergoing cell death. However, further research is necessary to determine which of these mechanisms underlies the effect of activated platelets on NPC proliferation.
We also show that activated platelets and PF4 promote neuronal differentiation. In DBA/2 mice, running-induced proliferation of NPCs only occurs after approximately 2 weeks of running (Overall et al., 2013). However, the proliferating cells fail to survive and do not differentiate into mature neurons (Overall et al., 2013). Thus, the absence of platelet activation in running DBA/2 mice supports the idea that a link might exist between activated platelets, the factors they release, and neurogenesis after physical exercise.
Our platelet proteomic screen revealed that platelets change their proteomic signature after running, including the levels of 14-3-3 proteins. The exact function of these proteins is not well understood, although previous work has revealed their contribution to neuronal processes, such as neuronal differentiation, synaptic plasticity, and learning and memory (Ramocki et al., 2010, Skoulakis and Davis, 1998, Toyo-oka et al., 2014). Whether 14-3-3 proteins are involved in platelet-brain communication and whether the herein identified isoform 14-3-3γ represents a potential candidate that contributes to neurogenesis remains to be determined. Moreover, platelets have the ability to collect proteins from the plasma through endocytosis (Harrison et al., 1989). Thus, together with their potential to stimulate NPCs, platelets could act as vehicles that sense alterations in the blood and translate the systemic changes that occur after physical activity into plastic changes in the adult hippocampal niche. How and where intercellular communication between platelets and NPCs occurs remains to be established. A potential mechanism could be the release of platelet proteins into the bloodstream, globally or at local sites, e.g., through receptor interaction. In this study, we determined that PF4 is a potential mediator between the blood and the hippocampal stem cell niche in running mice. We found increased levels of PF4 in the plasma of running mice but not in the platelets of exercising mice. Although we did not determine the source of the raised plasma PF4, it is likely that it is released by running-induced activated platelets. We have shown that this increase occurs as early as 1 day after the onset of running, and it is therefore possible that the levels of PF4 do not remain high in the platelets themselves. In vivo, activated platelets represent the primary source of PF4 (Shi et al., 2013). However, we cannot exclude the possibility that the accumulation of PF4 in the plasma is platelet independent, as it has been shown, although not under physiological conditions, that PF4 can be produced by a subset of myeloid cells (Jian et al., 2017) and after brain injury by microglia (de Jong et al., 2008). The precise mechanisms through which PF4 promotes adult hippocampal neurogenesis are unknown. However, in the bone marrow, PF4 was shown to directly affect hematopoietic stem cell quiescence (Bruns et al., 2014), underlining its potential as a regulatory factor in another stem cell niche.
Our results demonstrate that, under physiological conditions, the neurogenesis-enhancing effects of platelets and their secreted factor PF4 are specific to DG NPCs. Although platelets have been shown to promote neurogenesis in the SVZ, the majority of these studies were performed in the context of injury (Hayon et al., 2012, Kazanis et al., 2015). They do, however, emphasize the potential of platelets to mediate regulatory processes in the brain and suggest that these cells could potentially contribute to the creation of an increased pool of NPCs. Under more physiological conditions, platelet lysate treatment in vitro promotes the survival but not the proliferation and differentiation of NPCs from the adult rat SVZ (Kazanis et al., 2015). This is more in line with our work, although an effect of activated PRP treatment on the survival of NPCs was not determined. The observed differential effects of activated platelets on DG and SVZ NPCs in the present study suggest distinct regulatory mechanisms within the two major neurogenic niches under physiological conditions. We have previously shown that NPCs from these niches respond differently to neuronal depolarization (Walker et al., 2008). Furthermore, a robust running-induced increase in neurogenesis is clearly observed in the SGZ (Kronenberg et al., 2003, van Praag et al., 1999a, van Praag et al., 1999b), but not along the lateral ventricles of the SVZ (Brown et al., 2003). Together with the results of the present study, these findings provide an intriguing link between running-induced platelet activation and the capacity of platelets and their released factors to specifically modulate NPC biology in the DG after physical activity.
Experimental Procedures
Mice
Animals were housed on a 12/12-h light/dark cycle in standard cages of an individually ventilated cage system from Tecniplast. Standard mouse chow (Sniff) and water was provided ad libitum. All animals were female, except for LPA1-GFP mice, and were 8–10 weeks old at the beginning of the experiment. C57BL/6JRj and DBA/2JRj mice were purchased from Janvier Labs, France. LPA1-GFP mice (STOCK Tg(Lpar1-EGFP)GX193Gsat/Mmucd) (Gong et al., 2003, Walker et al., 2016); were initially purchased from the Mutant Mouse Resource & Research Center and maintained as a hemizygous breeding colony. All mouse experimental tasks were conducted on approval by the local ethics committee (Landesdirektion Sachsen) and in accordance with the European and national regulations (Tierschutzgesetz).
Proteomic Profiling of Mouse Plasma and Platelets
Whole blood from C57BL/6JRj mice that were either STD or housed with a running wheel for 4 days (4d RUN) was collected into EDTA-coated tubes (Sarstedt). Either plasma was prepared, or platelets isolated, and mass spectrometry-based proteomics profiling performed. The resulting spectrometry peak data of each screen were analyzed and annotated to the corresponding proteins. Any determined protein with expression data for fewer than four samples per group (STD or 4d RUN) was discarded. A linear model was fitted to the data for each resulting protein and moderated p values were calculated using the functions lmFit and eBayes from the R package limma.
Reactome Pathway Analysis
Pathway analysis was performed using the Reactome online analysis tool (Fabregat et al., 2018) at https://reactome.org/ as of December 5, 2017. Uniprot accessions of proteins, which were determined to be significantly elevated in the plasma of mice after 4d RUN were analyzed using standard settings of the online version for identifiers from Mus musculus.
Platelet Count and Platelet Activation State
The platelet count and activation state were determined using flow cytometry following an optimized protocol based on Alugupalli et al. (2001). In brief, 5 μL of tail blood was collected into 45 μL 0.11 M sodium citrate (Merck) in 0.01 M PBS, pH 6.5. Diluted whole blood (10 μL) was stained with 5 μL each of anti-mouse CD61 PE (Thermo Fisher Scientific) and Alexa Fluor 647-conjugated anti-mouse CD62P (BD Pharmingen) for 30 min. The samples were fixed with 975 μL platelet fixation solution (0.01 M PBS containing 0.2% BSA [Sigma-Aldrich], 0.1% D-glucose [AppliChem], and 0.1% formalin [Sigma-Aldrich]). A dose of 5 μL of Sphero 8-peak Rainbow Calibration Particles (3.0–3.4 μm diameter; BD Biosciences) was added as an internal standard to determine the platelet count. A total of 30,000 platelet events were analyzed by flow cytometry. Activated platelets were identified as CD62P+ cells within the CD61+ population.
Preparation of PPP and PRP
Whole blood was collected transcardially using a 21G7/8 needle with a blunt tip, then transferred into EDTA-coated tubes. Blood samples were centrifuged at 200× g for 20 min at room temperature. The plasma and buffy coat were collected into a new centrifugation tube. A second centrifugation step was performed at 800× g for 15 min at room temperature to obtain a platelet gradient. The upper two-thirds of the supernatant was collected as PPP, and the lower one-third of the volume was resuspended as PRP. Depending on the experimental setup, PPP were either used immediately as freshly isolated plasma or stored at −80°C for no longer than 2 weeks.
Neurosphere Culture
Neurosphere cultures were prepared from mouse primary cells seeded at a density of the DG's from one mouse per 96-well plate according to Bernas et al. (2017), which results in an average of one DG-derived neurosphere per well. The cultures were supplemented with either 0.01% mouse serum, 0.01% mouse PPP or PRP, or PF4 diluted in 0.5% BSA in 0.01 M PBS (10–500 ng/mL; BioLegend). Control cultures of PF4 neurosphere experiments were treated with 0.5% BSA in 0.01 M PBS. SVZ- and DG-derived cultures were incubated at 37°C for 7 and 12 days, respectively. The neurospheres were counted and DG neurospheres measured. Then, the neurospheres were differentiated at 37°C for 7 days until adherent. The differentiated cells were fixed with 4% paraformaldehyde (Sigma-Aldrich) in 0.01 M PBS at room temperature for 15 min. The differentiated cells were immunostained with antibodies against GFAP (1:500; Dako Cytomation) and β-III-tubulin (1:2,000; Promega). Neurosphere counts per condition were normalized to the value of an untreated control of the respective experiment. The raw counts of each neurosphere experiment are presented in Tables S5–S11.
In Vivo PF4 Infusion
Mini-osmotic pumps (ALZET; model 1007D) were used to deliver 100 ng/mL PF4 (BioLegend) supplemented with 0.1% BSA or vehicle solution (0.9% PBS containing 0.1% BSA) for 7 days into the hippocampus of 8-week-old C57BL/6JRj female mice. The pump cannula was inserted to reach the hilar region (coordinates relative to bregma: anterior-posterior −1.3 mm; medial-lateral +1.0 mm; ventral-dorsal −2.2 mm). Twelve hours prior to perfusion, the mice received a single intraperitoneal (i.p.) injection of BrdU (50 mg/kg, Sigma-Aldrich).
Platelet Depletion with Anti-platelet Serum
Platelets were depleted in 8-week-old pair housed C56BL/6JRj mice using rabbit anti-platelet serum (1:2 in PBS; Accurate Chemical & Scientific Corporation). The mice were given i.p. injections every second day for 2 weeks, making seven injections in total. The control group received injections of normal rabbit serum (1:2 in PBS; Accurate Chemical & Scientific Corporation). On day 3, the cages were equipped with a running wheel that remained in the cage for the following 10 days.
Histology
Immunostaining was performed on a complete series of 40-μm sections, 240 μm apart, using primary antibodies against BrdU (1:500; AbD Serotec), Ki67 (1:1,000; eBioscience), and DCX (1:500; Santa Cruz; see also Table S4). Counting was performed blinded to the experimental groups at 400× magnification.
Statistical Analysis
All analyses were carried out using Graph Pad Prism 6 or R/Bioconductor. All data are represented as means ± SEM. Comparison of two groups was performed using unpaired, two-tailed Student's t tests with assumed equal variances. When comparing more than two groups, a one-way ANOVA with post hoc Dunnett, Tukey, or Sidak comparison was performed, as appropriate. Conventional statistical significance was set at p < 0.05.
Author Contributions
Conceptualization, O.L., G.K., and T.L.W.; Formal Analysis, O.L. and R.W.O.; Investigation, O.L., S. Seidemann, S. Schallenberg, B.R., T.G., N.R., and T.L.W.; Resources, B.W.; Data Curation, R.W.O.; Writing – Original Draft, O.L. and T.L.W.; Writing – Review & Editing, O.L., R.W.O., T.G., G.K., and T.L.W.; Supervision, G.K., O.L., and T.L.W.; Funding Acquisition, G.K., S. Schallenberg, O.L., and T.L.W.
Acknowledgments
This work was funded by the Bundesministerium für Bildung und Forschung (G.K.), the Deutsche Forschungsgemeinschaft SFB 655 (G.K. and T.L.W.), a CRTD postdoctoral seed grant (T.L.W. and Sonja Schallenberg) and the Graduate Academy of the Technische Universität Dresden funded by the Excellence Initiative of the Federal and State Governments (O.L.). The authors deeply thank Anne Karasinsky and Sandra Guenther for their expert assistance with animal care and logistics.
Published: March 21, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2019.02.009.
Supplemental Information
References
- Abbott N.J. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alugupalli K.R., Michelson A.D., Barnard M.R., Leong J.M. Serial determinations of platelet counts in mice by flow cytometry. Thromb. Haemost. 2001;86:668–671. [PubMed] [Google Scholar]
- Bernas S., Leiter O., Walker T., Kempermann G. Isolation, culture and differentiation of adult hippocampal precursor cells. Bio Protoc. 2017;7(21):e2603. doi: 10.21769/BioProtoc.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet J., Damien P., Hamzeh-Cognasse H., Arthaud C.-A., Eyraud M.-A., Zéni F., Pozzetto B., McNicol A., Garraud O., Cognasse F. Human platelets can discriminate between various bacterial LPS isoforms via TLR4 signaling and differential cytokine secretion. Clin. Immunol. 2012;145:189–200. doi: 10.1016/j.clim.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Van der Borght K., Kóbor-Nyakas D.E., Klauke K., Eggen B.J.L., Nyakas C., Van der Zee E.A., Meerlo P. Physical exercise leads to rapid adaptations in hippocampal vasculature: temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus. 2009;19:928–936. doi: 10.1002/hipo.20545. [DOI] [PubMed] [Google Scholar]
- Brandt E., Petersen F., Ludwig A., Ehlert J.E., Bock L., Flad H.D. The beta-thromboglobulins and platelet factor 4: blood platelet-derived CXC chemokines with divergent roles in early neutrophil regulation. J. Leukoc. Biol. 2000;67:471–478. doi: 10.1002/jlb.67.4.471. [DOI] [PubMed] [Google Scholar]
- Brown J., Cooper-Kuhn C.M., Kempermann G., Van Praag H., Winkler J., Gage F.H., Kuhn H.G. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur. J. Neurosci. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Bruns I., Lucas D., Pinho S., Ahmed J., Lambert M.P., Kunisaki Y., Scheiermann C., Schiff L., Poncz M., Bergman A. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat. Med. 2014;20:1315–1320. doi: 10.1038/nm.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano J.M., Mosher K.I., Abbey R.J., McBride A.A., James M.L., Berdnik D., Shen J.C., Zou B., Xie X.S., Tingle M. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature. 2017;544:488–492. doi: 10.1038/nature22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe Y., Huynh T., Pleasure S.J. Epithelial cells supply Sonic Hedgehog to the perinatal dentate gyrus via transport by platelets. Elife. 2015;4:e07834. doi: 10.7554/eLife.07834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppinger J.A., O’Connor R., Wynne K., Flanagan M., Sullivan M., Maguire P.B., Fitzgerald D.J., Cagney G. Moderation of the platelet releasate response by aspirin. Blood. 2007;109:4786–4792. doi: 10.1182/blood-2006-07-038539. [DOI] [PubMed] [Google Scholar]
- Fabel K., Fabel K., Tam B., Kaufer D., Baiker A., Simmons N., Kuo C.J., Palmer T.D. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Fabregat A., Jupe S., Matthews L., Sidiropoulos K., Gillespie M., Garapati P., Haw R., Jassal B., Korninger F., May B. The Reactome pathway knowledgebase. Nucleic Acids Res. 2018;46:D649–D655. doi: 10.1093/nar/gkx1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellmann N. Hormonal and plasma volume alterations following endurance exercise. A brief review. Sports Med. 1992;13:37–49. doi: 10.2165/00007256-199213010-00004. [DOI] [PubMed] [Google Scholar]
- Filippov V., Kronenberg G., Pivneva T., Reuter K., Steiner B., Wang L.P., Yamaguchi M., Kettenmann H., Kempermann G. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol. Cell. Neurosci. 2003;23:373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Gong S., Zheng C., Doughty M.L., Losos K., Didkovsky N., Schambra U.B., Nowak N.J., Joyner A., Leblanc G., Hatten M.E. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Harrison P., Wilbourn B., Debili N., Vainchenker W., Breton-Gorius J., Lawrie A.S., Masse J.M., Savidge G.F., Cramer E.M. Uptake of plasma fibrinogen into the alpha granules of human megakaryocytes and platelets. J. Clin. Invest. 1989;84:1320–1324. doi: 10.1172/JCI114300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayon Y., Dashevsky O., Shai E., Varon D., Leker R.R. Platelet microparticles promote neural stem cell proliferation, survival and differentiation. J. Mol. Neurosci. 2012;47:659–665. doi: 10.1007/s12031-012-9711-y. [DOI] [PubMed] [Google Scholar]
- Hayon Y., Dashevsky O., Shai E., Varon D., Leker R.R. Platelet lysates stimulate angiogenesis, neurogenesis and neuroprotection after stroke. Thromb. Haemost. 2013;110:323–330. doi: 10.1160/TH12-11-0875. [DOI] [PubMed] [Google Scholar]
- Heber S., Volf I. Effects of physical (in)activity on platelet function. Biomed. Res. Int. 2015;2015:165078. doi: 10.1155/2015/165078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italiano J.E., Richardson J.L., Patel-Hett S., Battinelli E., Zaslavsky A., Short S., Ryeom S., Folkman J., Klement G.L. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian J., Pang Y., Yan H.H., Min Y., Achyut B.R., Hollander M.C., Lin P.C., Liang X., Yang L. Platelet factor 4 is produced by subsets of myeloid cells in premetastatic lung and inhibits tumor metastasis. Oncotarget. 2017;8:27725–27739. doi: 10.18632/oncotarget.9486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong E.K., de Haas A.H., Brouwer N., van Weering H.R.J., Hensens M., Bechmann I., Pratley P., Wesseling E., Boddeke H.W.G.M., Biber K. Expression of CXCL4 in microglia in vitro and in vivo and its possible signaling through CXCR3. J. Neurochem. 2008;105:1726–1736. doi: 10.1111/j.1471-4159.2008.05267.x. [DOI] [PubMed] [Google Scholar]
- Kazanis I., Feichtner M., Lange S., Rotheneichner P., Hainzl S., Öller M., Schallmoser K., Rohde E., Reitsamer H.A., Couillard-Despres S. Lesion-induced accumulation of platelets promotes survival of adult neural stem/progenitor cells. Exp. Neurol. 2015;269:75–89. doi: 10.1016/j.expneurol.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Klempin F., Beis D., Mosienko V., Kempermann G., Bader M., Alenina N. Serotonin is required for exercise-induced adult hippocampal neurogenesis. J. Neurosci. 2013;33:8270–8275. doi: 10.1523/JNEUROSCI.5855-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G., Reuter K., Steiner B., Brandt M.D., Jessberger S., Yamaguchi M., Kempermann G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J. Comp. Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Kronenberg G., Bick-Sander A., Bunk E., Wolf C., Ehninger D., Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol. Aging. 2006;27:1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Langer H.F., Choi E.Y., Zhou H., Schleicher R., Chung K.-J., Tang Z., Göbel K., Bdeir K., Chatzigeorgiou A., Wong C. Platelets contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Circ. Res. 2012;110:1202–1210. doi: 10.1161/CIRCRESAHA.111.256370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugert S., Basak O., Knuckles P., Haussler U., Fabel K., Götz M., Haas C.A., Kempermann G., Taylor V., Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Moon H.Y., Becke A., Berron D., Becker B., Sah N., Benoni G., Janke E., Lubejko S.T., Greig N.H., Mattison J.A. Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab. 2016;24:332–340. doi: 10.1016/j.cmet.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Gebara E., Bushong E.A., Sánchez-Pascual I., O’Laoi R., El M’Ghari I., Kocher-Braissant J., Ellisman M.H., Toni N. Fine processes of Nestin-GFP-positive radial glia-like stem cells in the adult dentate gyrus ensheathe local synapses and vasculature. Proc. Natl. Acad. Sci. U S A. 2016;113:E2536–E2545. doi: 10.1073/pnas.1514652113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall R.W., Walker T.L., Leiter O., Lenke S., Ruhwald S., Kempermann G. Delayed and transient increase of adult hippocampal neurogenesis by physical exercise in DBA/2 mice. PLoS One. 2013;8:e83797. doi: 10.1371/journal.pone.0083797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall R.W., Walker T.L., Fischer T.J., Brandt M.D., Kempermann G. Different mechanisms must be considered to explain the increase in hippocampal neural precursor cell proliferation by physical activity. Front. Neurosci. 2016;10:362. doi: 10.3389/fnins.2016.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer T.D., Willhoite A.R., Gage F.H. Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- van Praag H., Christie B.R., Sejnowski T.J., Gage F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Kempermann G., Gage F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Ramocki M.B., Bartnik M., Szafranski P., Kołodziejska K.E., Xia Z., Bravo J., Miller G.S., Rodriguez D.L., Williams C.A., Bader P.I. Recurrent distal 7q11.23 deletion including HIP1 and YWHAG identified in patients with intellectual disabilities, epilepsy, and neurobehavioral problems. Am. J. Hum. Genet. 2010;87:857–865. doi: 10.1016/j.ajhg.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffi A., Filardo G., Assirelli E., Cavallo C., Cenacchi A., Facchini A., Grigolo B., Kon E., Mariani E., Pratelli L. Does platelet-rich plasma freeze-thawing influence growth factor release and their effects on chondrocytes and synoviocytes? Biomed. Res. Int. 2014;2014:692913. doi: 10.1155/2014/692913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucinski B., Niewiarowski S., Strzyzewski M., Holt J.C., Mayo K.H. Human platelet factor 4 and its C-terminal peptides: heparin binding and clearance from the circulation. Thromb. Haemost. 1990;63:493–498. [PubMed] [Google Scholar]
- Sharma H.S., Cervós-Navarro J., Dey P.K. Increased blood-brain barrier permeability following acute short-term swimming exercise in conscious normotensive young rats. Neurosci. Res. 1991;10:211–221. doi: 10.1016/0168-0102(91)90058-7. [DOI] [PubMed] [Google Scholar]
- Shi G., Field D.J., Long X., Mickelsen D., Ko K., Ture S., Korshunov V.A., Miano J.M., Morrell C.N. Platelet factor 4 mediates vascular smooth muscle cell injury responses. Blood. 2013;121:4417–4427. doi: 10.1182/blood-2012-09-454710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoulakis E.M., Davis R.L. 14-3-3 proteins in neuronal development and function. Mol. Neurobiol. 1998;16:269–284. doi: 10.1007/BF02741386. [DOI] [PubMed] [Google Scholar]
- Steiner B., Zurborg S., Horster H., Fabel K., Kempermann G. Differential 24 h responsiveness of Prox1-expressing precursor cells in adult hippocampal neurogenesis to physical activity, environmental enrichment, and kainic acid-induced seizures. Neuroscience. 2008;154:521–529. doi: 10.1016/j.neuroscience.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Sun G.J., Zhou Y., Stadel R.P., Moss J., Yong J.H.A., Ito S., Kawasaki N.K., Phan A.T., Oh J.H., Modak N. Tangential migration of neuronal precursors of glutamatergic neurons in the adult mammalian brain. Proc. Natl. Acad. Sci. U S A. 2015;112:9484–9489. doi: 10.1073/pnas.1508545112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyo-oka K., Wachi T., Hunt R.F., Baraban S.C., Taya S., Ramshaw H., Kaibuchi K., Schwarz Q.P., Lopez A.F., Wynshaw-Boris A. 14-3-3ɛ and ζ regulate neurogenesis and differentiation of neuronal progenitor cells in the developing brain. J. Neurosci. 2014;34:12168–12181. doi: 10.1523/JNEUROSCI.2513-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo J.L., Carro E., Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda S.A., Luo J., Mosher K.I., Zou B., Britschgi M., Bieri G., Stan T.M., Fainberg N., Ding Z., Eggel A. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda S.A., Plambeck K.E., Middeldorp J., Castellano J.M., Mosher K.I., Luo J., Smith L.K., Bieri G., Lin K., Berdnik D. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 2014;20:659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T.L., White A., Black D.M., Wallace R.H., Sah P., Bartlett P.F. Latent stem and progenitor cells in the hippocampus are activated by neural excitation. J. Neurosci. 2008;28:5240–5247. doi: 10.1523/JNEUROSCI.0344-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T.L., Overall R.W., Vogler S., Sykes A.M., Ruhwald S., Lasse D., Ichwan M., Fabel K., Kempermann G. Lysophosphatidic acid receptor is a functional marker of adult hippocampal precursor cells. Stem Cell Reports. 2016;6:552–565. doi: 10.1016/j.stemcr.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyrich A.S., Schwertz H., Kraiss L.W., Zimmerman G.A. Protein synthesis by platelets: historical and new perspectives. J. Thromb. Haemost. 2009;7:241–246. doi: 10.1111/j.1538-7836.2008.03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicki A.N., Walz A., Gerber-Huber S.N., Wenger R.H., Vornhagen R., Clemetson K.J. Isolation and characterization of human blood platelet mRNA and construction of a cDNA library in lambda gt11. Confirmation of the platelet derivation by identification of GPIb coding mRNA and cloning of a GPIb coding cDNA insert. Thromb. Haemost. 1989;61:448–453. [PubMed] [Google Scholar]
- Zlokovic B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.