Abstract
Purpose: Povidone-iodine (P-I) is being touted as a topical antiviral treatment for eye infections caused by adenovirus (Ad). This study evaluated the in vitro antiviral activity of the several P-I concentrations previously used in clinical studies against multiple ocular Ad types commonly associated with eye infections.
Methods: The antiviral activity of four concentrations of P-I was compared to vehicle for seven types of Ad after incubating the P-I with Ad at 33°C for various lengths of time. Following incubation and neutralization of the P-I with sodium thiosulfate, viral titers were determined for each Ad type and time point.
Results: Virucidal (99.9%, ≥3-Log10) reductions in titers were produced for 5%, 2%, and 0.4% P-I at 1 min for types Ad5 and Ad7a. Similar reductions were produced at 5 min for types Ad3, Ad4, and Ad8. For type Ad19/64, virucidal reductions took 60 min for 5% P-I and 15 min for 2% and 0.4%. For type Ad37, 60 min (5%), 15 min (2%), and 5 min (0.4%) were required to produce virucidal reductions. There were no virucidal reductions in titers produced by 0.001% P-I.
Conclusions: P-I produced greater than 3-Log10 reductions of titers at 1–5 min for most of the ocular types tested (types Ad3, Ad4, Ad5, Ad7a, and Ad8). However, it took longer (15–60 min) for these reductions to be produced for types Ad19/64 and Ad37. The antiviral activity of P-I may be Ad type dependent.
Keywords: povidone-iodine, adenovirus, epidemic keratoconjunctivitis, in vitro, antiviral
Introduction
Molecular iodine has been known to be an effective antiseptic for more than a century.1 However, its intolerability to mucosal surfaces rendered it unacceptable for certain applications.2 In 1955, a stable chemical complex of polyvinylpyrrolidone (povidone, PVP) and elemental iodine (I) was developed at the Industrial Toxicology Laboratories in Philadelphia.3 The toxicity of this new chemical complex was favorably evaluated in animal models.3 Following clinical trials, povidone-iodine (P-I) became a common preoperative antiseptic as well as a treatment for microbial skin infections caused by bacteria, yeasts, molds, fungi, viruses, and protozoans.4 In addition, bacteria do not develop a resistance to P-I.5 P-I is a commonly used antiseptic for ocular and general surgeries, often sold in 5% or 10% solutions known commercially as Betadine. P-I is a polymeric iodophor that reacts with oxygen containing functional groups of bacteria and viruses, causing oxidation of their proteins.6 This inexpensive nonprescription solution has been long proposed as a topical treatment for adenoviral (Ad) eye infections.
Previous studies have demonstrated that P-I produces variable antiviral activity against Ad in vitro. Monnerat et al.7 demonstrated that 0.8% P-I eliminated all extracellular Ad8 in 10 min, while it produced little antiviral activity against Ad contained in host cells. A second study by Rutala et al.8 showed that 10% P-I was not effective against desiccated Ad8, while a third study by Sauerbrei et al.9 demonstrated variable antiviral activity among 17 Ad types with several P-I concentrations and reaction times. The majority of Ad types used in this study are not commonly isolated from eye infections.9
Several P-I concentrations have been tested for potential clinical use. Five percent P-I has been evaluated in an internet-based, investigator-managed trial. The “Betadine for EKC Study” (www.betadineforekc.com) sought to recruit physicians to participate in a study using a 1-min eye wash of 5% P-I to treat EKC (epidemic keratoconjunctivitis). Two percent P-I was evaluated in a clinical trial involving 61 patients with EKC (NCT01179412). Patients were treated with 2% P-I four times daily for a week. The study concluded that the 2% P-I was tolerable and relieved ocular discomfort in 75% of the patients after 1 week.10 However, no conclusions of the antiviral efficacy of P-I can be made from this study since there was no placebo control group and no virological outcome measures were used; 0.4% P-I has been evaluated in a small clinical study11 and as part of the 0.4% P-I/0.1% dexamethasone combination (clinical trial NCT01470664).
None of the previous in vitro antiviral efficacy studies have specifically evaluated the P-I concentrations that have been proposed for clinical use against a panel of common ocular Ad types. Therefore, the goal of this study was to evaluate the in vitro virucidal efficacy of several concentrations of P-I that have been proposed for the clinical treatment of Ad ocular infections.
Methods
Experimental agent
Ten percent P-I solution United States Pharmacopeia (USP) was obtained from the Ricca Chemical Company (Arlington, TX). The three highest concentrations (5%, 2%, and 0.4%) used in this study were based on the concentrations tested in the clinical trials described above. The 0.001% concentration was used to determine whether the P-I could be diluted out to produce no virucidal reductions in Ad titers. The concentrations tested were prepared in sterile, deionized water from the 10% stock solution.
Viruses and cells
Clinical isolates of Ad types 3, 4, 5, 7a, 8, and 19/64 (originally called type 19, but now considered type 64) were collected from patients presenting with Ad conjunctivitis at the Charles T. Campbell Ophthalmic Microbiology Laboratory at the UPMC Eye Center, University of Pittsburgh (Pittsburgh, PA), and frozen at −70°C. The viruses are part of a clinical collection of Ad isolates. The isolates were deidentified and stored for diagnostic test validations. The Ad types of the isolates were determined using serum neutralization. No clinical isolates of Ad37 were recovered, so the ATCC (American Type Culture Collection, Manassas, VA) reference strain of Ad37 was used. The isolates selected represent the most common Ad types that cause ocular infections (Ad8, Ad19/64, Ad37 [EKC], Ad3, Ad4, and Ad7a [follicular conjunctivitis and pharyngeal conjunctival fever])12 and a type that can replicate in the eyes of New Zealand White rabbits (Ad5).13
A549 human lung carcinoma cells were used to prepare the Ad stocks and for the determination of viral titers for Ad. The cells were grown in tissue culture media containing Eagle's Minimum Essential Medium (MEM) supplemented with 10% fetal bovine serum (Sigma Cell Culture Reagents, St. Louis, MO).
In vitro log reduction virucidal assay
This study was conducted in duplicate using ∼104–106 plaque-forming units per milliliter (PFU/mL) of the Ad types. One hundred fifty microliters of each of the test viruses was added to 1.35 mL of P-I concentrations of 5.555%, 2.222%, 0.444%, and 0.00111% prepared in sterile water. This yielded final P-I concentrations of 5%, 2%, 0.4%, and 0.001%. The virus/P-I mixtures were incubated at 33°C to simulate the temperature of the ocular surface. At 1, 5, 15, and 60 min of incubation, 250 μL aliquots were removed and added to 250 μL aliquots of 0.2 N sodium thiosulfate solution to neutralize the P-I. These mixtures were incubated at room temperature for 5 min, after which 500 μL of tissue culture media was added.
Determination of viral titers (plaque assay)
Immediately after the addition of the tissue culture medium, the samples were serially diluted for five 10-fold dilutions and inoculated onto duplicate wells of 24-well multiplates containing A549 cells. After 3 h of adsorption, the wells were filled with 1 mL of tissue culture medium containing 0.5% methylcellulose, except for Ad8 for which the wells were filled with 1 ml standard tissue culture medium. After 7–10 days of incubation, the cells were fixed and stained with 0.5% gentian violet and the number of plaques was counted. The viral titers were then calculated and expressed as PFU/mL.
Data analysis
The viral titers (PFU +1) were Log10 converted and Log10 reductions in titers from the negative control were calculated for each trial. The mean ± standard deviation (SD) Log reduction in titer for each time point and virus was calculated for the two trials. The results were plotted as Log10 vs. time. Mean reductions in titer of 3-Log10 (99.9%) were considered virucidal reductions.
Results
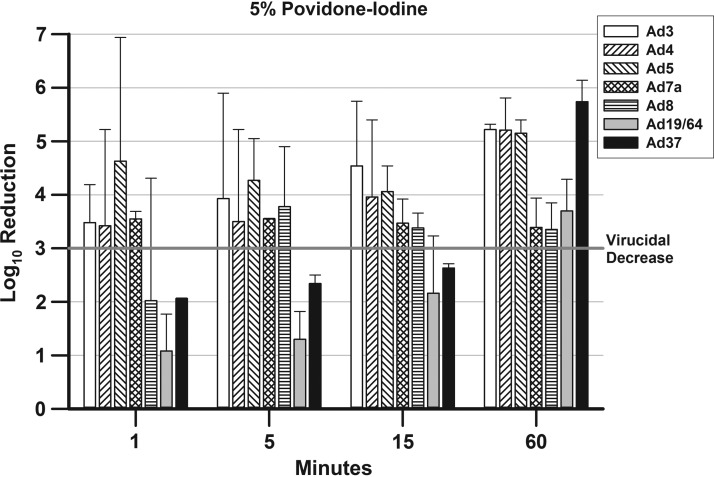
The mean ± SD Log10 reductions in titers for all Ad types and P-I concentrations are presented in Figures 1–4. The line at 3-Log10 decreases in the graphs denotes virucidal decreases. Five percent P-I (Fig. 1) produces virucidal reductions in viral titers for four Ad types at 1 min (Ad3, Ad4, Ad5, and Ad7a), one Ad type at 5 min (Ad8), and two Ad types at 60 min (Ad19/64 and Ad37). The decreases in viral titer continued over time for Ad19/64 and Ad37, ultimately reaching virucidal decreases between the 15- and 60-min time points.
FIG. 1.
presents the mean ± SD Log10 decreases in viral titers for the Ad types after treatment with 5% P-I. A 3-Log10 (99.9%) decrease in viral titer is indicated by a bar rising above the line at 3-Log10 point in the graph. Ad, adenovirus; P-I, povidone-iodine; SD, standard deviation.
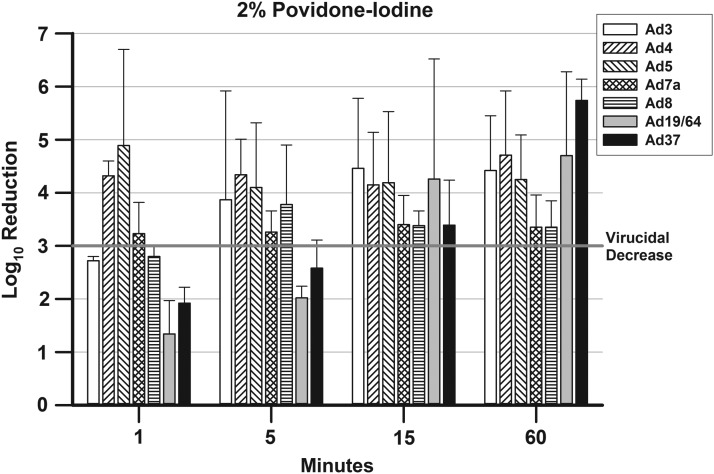
FIG. 2.
presents the mean ± SD Log10 decreases in viral titers for the Ad types after treatment with 2% P-I. A 3-Log10 (99.9%) decrease in viral titer is indicated by a bar rising above the line at 3-Log10 point in the graph.
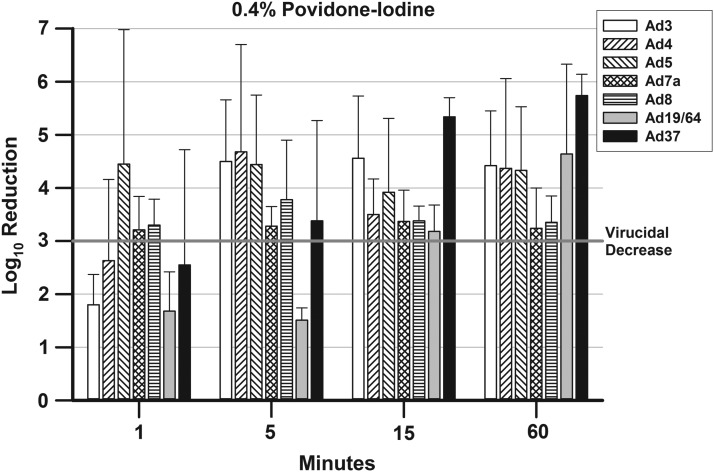
FIG. 3.
presents the mean ± SD Log10 decreases in viral titers for the Ad types after treatment with 0.4% P-I. A 3-Log10 (99.9%) decrease in viral titer is indicated by a bar rising above the line at 3-Log10 point in the graph.
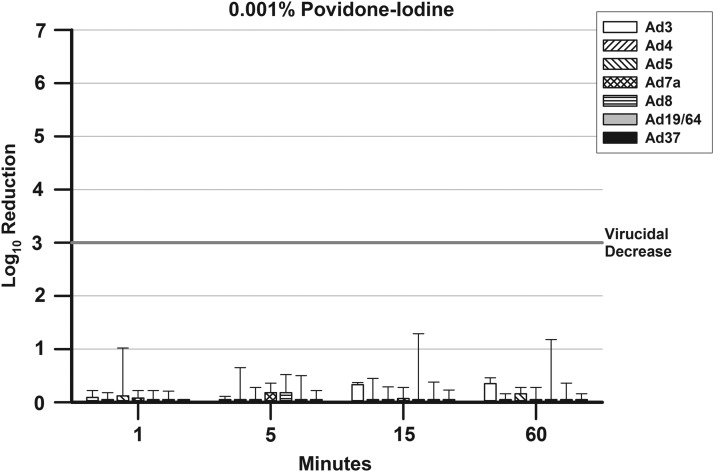
FIG. 4.
presents the mean ± SD Log10 decreases in viral titers for the Ad types after treatment with 0.001% P-I. A 3-Log10 (99.9%) decrease in viral titer is indicated by a bar rising above the line at 3-Log10 point in the graph.
The results for 2% P-I (Fig. 2) vary from those of 5% P-I in that only three Ad types reached virucidal decreases at 1 min (Ad4, Ad5, and Ad7a). The time to reach a virucidal decrease for Ad3 increased to 5 min with 2% P-I compared with 5% P-I, whereas the times for Ad19/64 and Ad37 decreased from 60 to 15 min. The time to reach a virucidal decrease for Ad8 was the same for 2% P-I and 5% P-I (5 min).
Figure 3 presents the results for 0.4% P-I. As with the 2% P-I, there was a reduction in the number of Ad types, for which there was a virucidal decrease in titers when the P-I concentration was decreased. With 0.4% P-I, only two Ad types reached virucidal decreases at 1 min (Ad5 and Ad7a). The time to virucidal decrease increased for Ad4 from 1 min for 5% and 2% P-I to 5 min for 0.4% P-I. However, the time to virucidal decrease was reduced from 60 min with 5% P-I and 15 min with 2% P-I to 5 min with 0.4% P-I for Ad37. The times to virucidal decreases demonstrated by 0.4% P-I remained the same compared with 2% P-I for Ad3 and Ad8 (5 min).
There were no virucidal decreases for any Ad type for all the concentrations and time points tested for 0.001% P-I (Fig. 4).
Discussion
An effective treatment of Ad eye infections (EKC, follicular conjunctivitis, and pharyngeal conjunctival fever) is an unmet medical need in ophthalmology. Treatment to reduce the ocular sequelae of these infections along with the length of infections and the potential infectivity to others is of utmost importance. Many antivirals have been proposed and evaluated for treatments for these infections,14–18 but none have been Food and Drug Administration or European Medicines Agency approved for this use.
P-I is a commonly used antiseptic for ocular and general surgeries. It has been proposed as a topical treatment for Ad eye infections with several clinical trials performed. The goal of this study was to evaluate the in vitro virucidal efficacy of several concentrations of P-I that have been proposed for the clinical treatment of Ad ocular infections.
The in vitro studies previously performed, evaluating the efficacy of P-I for reducing Ad titers, used a variety of methods to determine efficacy and used Ad types that are not commonly found in eye infections.7–9 In this study, we sought to simplify the assay by using an in vitro log reduction virucidal assay that simply mixes the virus and the various P-I concentrations and incubates the mixtures at 33°C to simulate the temperature on the ocular surface. At the predetermined time points, aliquots are removed from the mixtures and are combined with an agent that neutralizes the P-I, but has no effect on Ad titers. Viral titers were then performed to determine the amount of residual virus after treatment. To determine the in vitro efficacy of P-I against Ads that may be encountered during a conjunctivitis clinical trial, we used a panel of Ad types that are commonly isolated from ocular infections. Furthermore, we simplified the main outcome measure to a virucidal reduction as the determination of efficacy.
The results of this study demonstrated that P-I, in the concentrations proposed for use for the treatment of Ad ocular infections (5%, 2%, and 0.4%), was virucidal to all seven of the Ad types tested over the experimental time period. The 0.001% concentration of P-I was ineffective in reducing Ad titers. However, there was some variability among the effective P-I concentrations and the Ad types in the time it took to reach those virucidal decreases.
In general, there appeared to be dose-dependent virucidal activity at 1 min among the three effective P-I concentrations. Five percent P-I produced virucidal reductions in 5 of 7 Ad types at 1 min, while 2% P-I produced virucidal reductions in 3 of 7 Ad types, and 0.4% P-I produced virucidal reductions in 2 of 7 Ad types. The three effective P-I concentrations produced virucidal decreases in Ad5 and Ad7a at 1 min.
By 5 min, 5 of 7 Ad types had virucidal decreases produced by the three effective P-I concentrations (Ad3, Ad4, Ad5, Ad7a, and Ad8). The 0.4% concentration also produced a virucidal decrease in Ad37 titers at 5 min. In contrast, it took between 5 and 15 min for 2% and 0.4% P-I to produce virucidal decreases for Ad19/64 and for 2% P-I to produce a virucidal decrease for Ad37. Finally, it took between 15 and 60 min for the highest concentration of P-I tested, 5%, to produce virucidal decreases for Ad19/64 and Ad37. There appeared to be an inverse of the normal dose-response of activity for these two EKC Ad types. Additional studies must be performed to investigate this inverse dose-response for Ad19/64 and Ad37. It must be noted that even if there was no virucidal decrease at a specific time point and P-I concentration, there were decreases in viral titers of one to two logs of virus.
This study demonstrates that proposed steady-state clinical concentrations of P-I are effective at producing virucidal reductions of common ocular Ad types. However, the time it takes to reach these virucidal decreases varies among the concentrations and Ad types tested. It appears that the follicular conjunctivitis and pharyngeal conjunctival fever types (Ad3, Ad4, and Ad7a), as well as the follicular conjunctivitis and the rabbit model type (Ad5) are inactivated more rapidly than the EKC types of Ad19/64 and Ad37. This may have clinical ramifications in the treatment of EKC caused by Ad19/64 and A37 due to the potentially short residence time that P-I may have on the ocular surface. Only data from clinical trials will demonstrate the efficacy of P-I for the treatment of Ad ocular infections.
Acknowledgments
This study was supported by internal funding from The Charles T. Campbell Ophthalmic Microbiology Laboratory. Additional departmental funding was provided to the University of Pittsburgh Department of Ophthalmology by NIH CORE Grant for Vision Research EY08098, The Eye and Ear Foundation of Pittsburgh, and Research to Prevent Blindness. RMQS was supported by a Career Development Award from Research to Prevent Blindness. The Corresponding Author (E.G.R.) of this article containing original data confirms that he had full access to all the data in the study and takes responsibility for the integrity of data and the accuracy of data analysis, as well as the decision to submit for publication.
Author Disclosure Statement
The authors have no significant financial interests in the subject matter of this article and have no conflict of interests regarding this article. This study was not supported by any outside commercial entity.
References
- 1. Gershenfeld L., and Witlin B. Iodine as an antiseptic: Ann. N. Y. Acad. Sci. 53:172–182, 1950 [DOI] [PubMed] [Google Scholar]
- 2. Gottardi W. Iodine and iodine compounds. In: Block S.S., ed. Disinfection, Sterilization, and Preservation. Philadelphia: Lippincott Williams & Wilkins; 2000; p. 159–204 [Google Scholar]
- 3. Sneader W. Drug Discovery: A History. New York: John Wiley & Sons; 2005 [Google Scholar]
- 4. Jayaraja K.K., Jayachandran E., and Hemanth K.R.C. Application of broad spectrum antiseptic povidone iodine as powerful action: a review. J. Pharm. Sci. Tech. 1:48–58, 2009 [Google Scholar]
- 5. Klossner B.L., Widner H.R., and Frey F. Nondevelopment of resistance by bacteria during hospital use of povidone-iodine. Dermatology. 195:10–13, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Sunil K.P., Raja B.P., Jagadish R.G., and Uttam A. Povidone iodine-revisited. Indian J. Dental. Adv. 3:617–620, 2011 [Google Scholar]
- 7. Monnerat N., Bossart W., and Thiel M.A. Povidone-iodine for treatment of adenoviral conjunctivitis: an in vitro study. Klin. Monatsbl. Augenheilkd. 223:349–352, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Rutala W.A., Peacock J.E., Gergen M.F., Sobsey M.D., and Weber D.J. Efficacy of hospital germicides against adenovirus 8, a common cause of epidemic keratoconjunctivitis in health care facilities. Antimicrob. Agents Chemother. 50:1419–1424, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sauerbrei A., Sehr K., Brandstädt A., et al. Sensitivity of human adenoviruses to different groups of chemical biocides. J. Hosp. Infect. 57:59–66, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Trinavarat A., and Atchaneeyasakul L. Treatment of epidemic keratoconjunctivitis with 2% povidone-iodine: a pilot study. J. Ocul. Pharmacol. Ther. 28:53–58, 2012 [DOI] [PubMed] [Google Scholar]
- 11. Pelletier J.S., Stewart K., Trattler W., et al. A combination povidone-iodine 0.4%/dexamethasone 0.1% ophthalmic suspension in the treatment of adenoviral conjunctivitis. Adv. Ther. 26:776–783, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Gordon J.S., Aoki K., and Kinchington P.R. Adenovirus keratoconjunctivitis. In: Pepose J.S., Holland G.N., Wilhelmus K.R., eds. Ocular Infection and Immunity. St Louis, MO: Mosby; 1996; p. 877–894 [Google Scholar]
- 13. Romanowski E.G., Araullo-Cruz T., and Gordon Y.J. Multiple adenoviral serotypes demonstrate host range extension in the New Zealand rabbit ocular model. Invest. Ophthalmol. Vis. Sci. 39:532–536, 1998 [PubMed] [Google Scholar]
- 14. Gordon Y.J., Romanowski E.G., and Araullo-Cruz T. Topical HPMPC inhibits adenovirus type 5 in the New Zealand rabbit ocular replication model. Invest. Ophthalmol. Vis. Sci. 35:4135–4143, 1994 [PubMed] [Google Scholar]
- 15. Romanowski E.G., Yates K.A., Teuchner B., et al. N–Chlorotaurine is an effective antiviral agent against adenovirus in vitro and in the Ad5/NZW rabbit ocular model. Invest. Ophthalmol. Vis. Sci. 47:2021–2026, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Nwanegbo E.C., Romanowski E.G., Gordon Y.J., and Gambotto A. Efficacy of topical immunoglobulins (IG) against experimental adenoviral ocular infection. Invest. Ophthalmol. Vis. Sci. 48:4171–4176, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Romanowski E.G., Yates K.A., and Gordon Y.J. The in vitro and in vivo evaluation of ddC as a topical antiviral for ocular adenovirus infections. Invest. Ophthalmol. Vis. Sci. 50:5295–5299, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Trousdale M.D., Goldschmidt P.L., and Nobrega R. Activity of ganciclovir against human adenovirus type-5 infection in cell culture and cotton rat eyes. Cornea. 13:435–439, 1994 [DOI] [PubMed] [Google Scholar]