Summary
Tissue-specific stem cells have unique properties and growth requirements, but a small set of juxtacrine and paracrine signals have been identified that are required across multiple niches. Whereas insulin-like growth factor II (IGF-II) is necessary for prenatal growth, its role in adult stem cell physiology is largely unknown. We show that loss of Igf2 in adult mice resulted in a ∼50% reduction in slowly dividing, label-retaining cells in the two regions of the brain that harbor neural stem cells. Concordantly, induced Igf2 deletion increased newly generated neurons in the olfactory bulb accompanied by hyposmia, and caused impairments in learning and memory and increased anxiety. Induced Igf2 deletion also resulted in rapid loss of stem and progenitor cells in the crypts of Lieberkühn, leading to body-weight loss and lethality and the inability to produce organoids in vitro. These data demonstrate that IGF-II is critical for multiple adult stem cell niches.
Keywords: neural stem cell, IGF-II, intestinal stem cell, subventricular zone, subgranular zone, crypt of Lieberkühn, neurogenesis
Highlights
-
•
IGF-II maintains neural stem cells in the subventricular and subgranular zones
-
•
Adult loss of IGF-II increases new neurons and causes behavioral deficits
-
•
IGF-II is produced locally within the adult intestinal stem cell niche
-
•
Induced Igf2 deletion causes rapid intestinal stem cell loss
In this article, Levison, Wood and colleagues demonstrate that IGF-II expression in the adult mouse is necessary for maintaining multiple adult stem cell niches including in the subventricular and subgranular zones of the brain and in the crypts of Lieberkühn of the gut.
Introduction
Stem cells have long been of interest to developmental biologists; however, these cells have now gained the interest of the broader scientific community because of their potential to repair degenerating tissues. For example, neural stem cells (NSCs) and the new neurons that they produce are now recognized as necessary for learning and memory, for promoting cognitive function, and for their potential to replace neurons and glial cells lost as a consequence of brain injury or disease (Chojnacki et al., 2012, Lim and Alvarez-Buylla, 2014, Zhao et al., 2008). Similarly, adult intestinal stem cells (ISCs), which reside at the bottom of the crypt of Lieberkühn, constantly replenish the epithelial sheet for a lifespan, contributing to tissue homeostasis as well as mucosal regeneration after injury (Tetteh et al., 2014).
Stem cells naturally reside within specific niches where they receive local signals that maintain them in a primitive state. The niches for each adult stem cell population differ; thus, most of the factors that maintain tissue-specific stem cells exert local effects restricted to that niche. Only a limited number of juxtacrine or paracrine signaling factors have been identified that maintain stem cells residing in multiple niches; these include epidermal growth factor (EGF), Notch, Wnts, fibroblast growth factors (FGFs), Shh, and transforming growth factor β (Lander et al., 2012).
Insulin-like growth factor II (IGF-II) is highly expressed in the embryo and is necessary for normal embryonic and fetal growth (Baker et al., 1993, DeChiara et al., 1990, Liu et al., 1993, Ren et al., 2008, Shinar et al., 1993). In contrast, IGF-II is downregulated in most adult tissues, with notable exceptions including the brain and the intestine. In the adult CNS, IGF-II is produced by the choroid plexus, leptomeninges, endothelial cells, and stem/progenitor cells in the hippocampal subgranular zone (SGZ) (Bracko et al., 2012, Buono et al., 2015b, Ferron et al., 2015, Logan et al., 1994a, Logan et al., 1994b, Stylianopoulou et al., 1988). Due to production by the choroid plexus, IGF-II protein is found at high levels in the cerebral spinal fluid (Bunn et al., 2005). The sites of IGF-II expression place it in close proximity to the NSCs residing in the two major NSC niches: the subventricular zone (SVZ) of the lateral ventricle and the hippocampal SGZ. Moreover, although the Igf2 gene is imprinted such that it is expressed only from the paternal allele in most tissues (DeChiara et al., 1991, Ferguson-Smith et al., 1991, Giannoukakis et al., 1993), it is biallelically expressed in choroid plexus, leptomeninges, and brain endothelial cells (Charalambous et al., 2004, DeChiara et al., 1991, Feil et al., 1994, Ferron et al., 2015).
Based on the expression data and our earlier studies on SVZ-derived neurospheres (Ziegler et al., 2012, Ziegler et al., 2014), we hypothesized that IGF-II might be an integral component of the NSC niche. More recently, two studies provided support for IGF-II function in regulating NSCs; however, these studies did not address the function of IGF-II from multiple sources in regulating the adult NSCs. Bracko et al. (2012) demonstrated that Igf2 mRNA is expressed by NSCs in the dentate gyrus (DG) but not by the NSCs in the SVZ. Thus, short hairpin RNA knockdown of Igf2 mRNA reduced proliferation of DG NSCs but not SVZ NSCs. A second report by Ferron et al. (2015) demonstrated that deleting Igf2 systemically beginning embryonically reduced the number of label-retaining cells in the SVZ and SGZ in vivo. However, loss of Igf2 embryonically significantly reduces prenatal brain growth, making it impossible to separate the function of IGF-II in maintaining adult NSCs from a function in establishing NSCs during development. This study further demonstrated that loss of Igf2 from endothelial cells beginning embryonically partially compromises SVZ NSCs but has no effect on the SGZ NSC population (Ferron et al., 2015), thus raising the question as to whether IGF-II is essential for maintaining the NSCs in the adult hippocampus.
In the intestine, IGF-II is found in the colonic mucosa, and Igf2 loss of imprinting (LOI) in Apcmin/+ background increases crypt length (Sakatani et al., 2005). However, no studies to date have established whether IGF-II is an essential niche stem cell factor in the adult intestine. Therefore, to address the functions of IGF-II in multiple adult stem cell niches, we designed experiments to remove Igf2 in young adult mice and evaluate adult NSC and ISC maintenance.
Results
Adult NSCs Require Sustained IGF-II Production
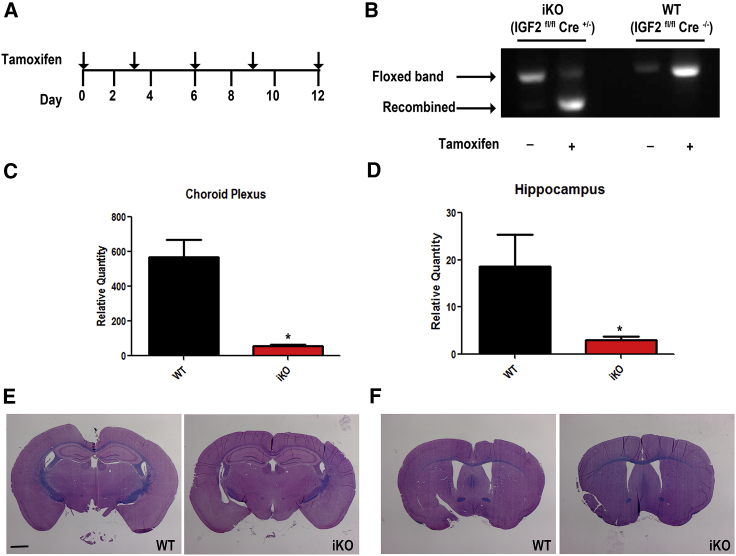
To determine whether IGF-II is necessary for adult stem cell homeostasis, we mated floxed Igf2 mice with a tamoxifen-inducible Rosa 26 CreER driver mouse line (Badea et al., 2003, Haley et al., 2012) to generate littermates for experimental animals that were all Igf2fl/fl and either Cre+ or Cre−. Administering tamoxifen over 5 consecutive days beginning at postnatal day 21 (p21) resulted in death of Igf2 knockout (KO) mice (see below regarding this lethal phenotype); therefore, we administered tamoxifen (75 mg/kg) every 3 days for the studies on the CNS (Figure 1A). PCR analysis 2 weeks after tamoxifen administration showed a recombined band of the expected size indicating excision of exons 4–6 of the Igf2 gene (Figure 1B). Consistent with these results, mRNA levels of Igf2 in choroid plexus and hippocampus were reduced by 90% in heterozygous Cre (Igf2fl/flCre+/−) mice versus wild-type (WT) (Igf2fl/fl) (Figures 1C and 1D). Since Igf2 mRNA levels were similarly reduced in mice heterozygous and homozygous for Cre (Figure S1), heterozygous Cre mice were used for all in vivo studies to exclude possible Cre toxicity (Silver and Livingston, 2001). Luxol fast blue with H&E staining from adult mice 3 months after Igf2 removal revealed no gross anatomical changes in the brain structures surrounding the SVZ or SGZ of the Igf2-inducible KO (iKO) mice (Figures 1E and 1F). Additionally, no differences were observed in organ weights of the heart, lung, liver, kidney, and spleen adjusted to total body weight for WT and iKO mice (data not shown).
Figure 1.
Tamoxifen-Induced Removal of Igf2
Schematic for tamoxifen dosing in C57BL/6 mice (A). PCR for the floxed Igf2 allele at 449 bp in iKO mice (Igf2fl/fl Cre+/−) with no tamoxifen or in WT mice (Igf2fl/fl Cre−/−) or for the recombined allele at 384 bp in iKO mice (Igf2fl/fl Cre+/−) plus tamoxifen (B). qPCR for Igf2 mRNA in choroid plexus (C) and hippocampus (D) from samples collected 2 weeks post tamoxifen administration. See also Figure S1. Representative sections of Luxol fast blue and H&E stains at the level of the septal nuclei and the third ventricle of WT and iKO mice (E and F). Statistical significance: ∗p < 0.05 using ANOVA and Tukey’s post hoc test, n = 5 WT and n = 12 iKO mice. Error bars represent SEM. Scale bar, 1 mm.
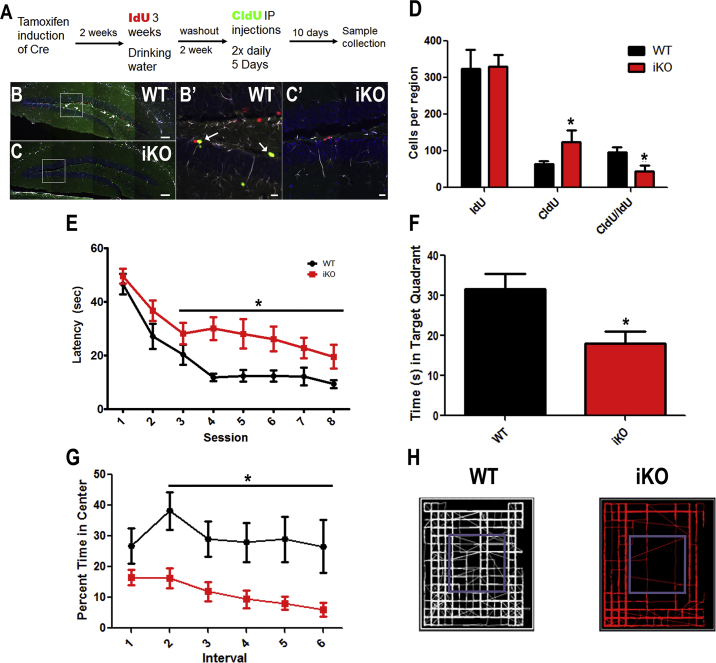
To evaluate the effects of Igf2 deletion on NSCs and progenitors in the SGZ and SVZ, we analyzed the preservation of label-retaining cells using temporally spaced administrations of the thymidine analogs iododeoxyuridine (IdU) and chlorodeoxyuridine (CldU) as depicted in Figure 2A (Llorens-Martin and Trejo, 2011, Vega and Peterson, 2005). Studies were performed to validate lack of cross-reactivity in detecting IdU and CldU (Figure S2). We examined the number of IdU and CldU single- and double-positive cells in the SGZ and SVZ in the WT and iKO mice, where double analog-positive cells are the label-retaining, slowly cycling, NSCs (Figures 2 and 3). Glial fibrillary acidic protein (GFAP), which is expressed by adult NSCs in SVZ and SGZ, was also included for analysis. Triple-positive IdU/CldU/GFAP cells were observed in the SGZ and SVZ, along with IdU and CldU single-positive cells (Figures 2B–2C′ and 3A–3D′) but it was difficult to establish whether a cell was definitively GFAP+ and thymidine analog positive; therefore, GFAP expression was not included in the stereology analyses. The numbers of single- and double-positive cells for the analogs were counted using unbiased stereology (Figures 2D and 3E). Significantly fewer IdU+/CldU+ cells were observed in both the SGZ (Figure 2D) and SVZ (Figure 3E) in the iKO mice compared with WT mice, consistent with an essential function for IGF-II in NSC homeostasis. Concomitant with the loss of IdU+/CldU+ cells, there was an increase in progenitors that were only CldU+ in the iKOs attributable to maturation of NSCs into progenitor cells (Figures 2D and 3E). Using a four-color flow-cytometry panel established in the Levison lab (Buono et al., 2012, Buono et al., 2015a), we evaluated the composition of the SVZ 12 months after Cre induction. Compared with the controls there was a statistically significant reduction in the NSCs (1.27% ± 0.09% NSCs in WT versus 1.01% ± 0.07% in iKOs) with modest increases in the proportions of two progenitor cell populations (Table 1). We evaluated the extent of apoptotic death within the SVZ and SGZ using in situ end labeling but detected no difference between the WT and iKO mice, indicating it was unlikely that the decrease in IdU/CldU-labeled cells in the iKO mice was due to apoptotic cell death (Figure S3).
Figure 2.
IGF-II Maintains Label-Retaining Cells in the SGZ of the Hippocampus and Is Necessary for Learning and Memory
Schematic of timeline for tamoxifen administration and labeling with IdU and CldU (A). Representative images of the hippocampi of WT (B) and iKO mice (C). Scale bars, 100 μm. IdU red, CldU green, GFAP white, and DAPI blue. (B′) and (C′) are insets from the regions marked in (B) and (C), respectively (scale bars, 20 μm). Counts of single- and double-positive cells for CldU and IdU in the SGZ (D), n = 6 WT and n = 6 iKO mice. See also Figures S2 and S3. Morris water maze latency to platform in seconds (E), and probe trial (F), n = 13 WT and n = 16 iKO mice. Open field test percent time spent in center across each 5-min interval (G), and maps of typical path taken (H), n = 10 WT and n = 15 iKO mice. WT black, iKO red. Statistical significance in (D) and (F) by Student’s t test (∗p < 0.05) and repeated-measures ANOVA in (E) and (G) (∗p < 0.05). In (E), a session effect was observed in WT mice but not in iKO mice. Error bars represent SEM.
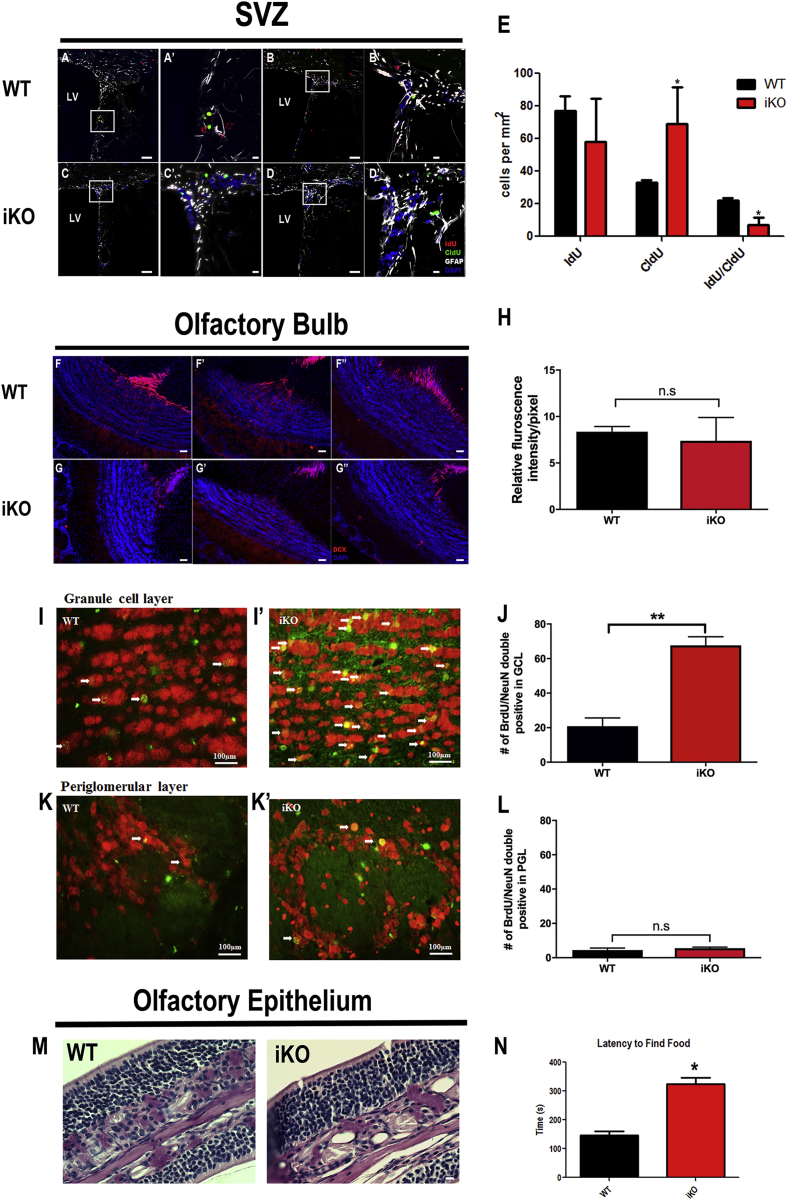
Figure 3.
IGF-II Is Required for SVZ NSC Maintenance, OB Neurogenesis, and Olfaction
WT (A and B) and iKO (C and D) mouse SVZs stained for IdU, CldU, and GFAP; insets (with primes) are enlargements. Scale bars, 100 μm (A–D) and 20 μm (A′–D′). IdU red, CldU green, GFAP white, and DAPI blue. Counts of single- and double-positive cells for CldU and IdU within 20 μm of the lateral ventricle wall (E), n = 6 WT and n = 6 iKO mice. See also Figures S2 and S3. Olfactory bulb was examined for immature neurons using doublecortin (DCX, red) and DAPI (blue) in WT (F) and iKO (G). Scale bars, = 100 μm. Fluorescence intensity was measured for DCX+ cells (H), n = 3 WT and n = 3 iKO mice. WT (I and K) and iKO (I′ and K′) for granule cell layer (I and I′) and periglomerular layer (K and K′) stained for BrdU and NeuN, 5 weeks after administering BrdU. Arrows indicate double-positive, newly generated neurons. Scale bars, 100 μm. Quantification of newly formed cells in the granule cell layer (J) and periglomerular cell layer (L), n = 3 WT and n = 3 iKO mice. See also Figure S4. Paraformaldehyde fixed and decalcified preparations of mouse nasal pharynxes were embedded in paraffin and sectioned at 8 μm. Sections from WT and iKO mice were stained using PAS and visualized by light microscopy; representative sections of WT and iKO mice are provided. Scale bar, 20 μm (M). Buried food test showing latency to find food versus genotype (N), WT (black) and iKO (red), n = 5 WT and n = 6 iKO mice. Statistical significance: ∗p < 0.05 and ∗∗p < 0.005 using Student's t test, iKO versus WT. Error bars represent SEM.
Table 1.
Multicolor Flow Cytometry Reveals a Decrease in SVZ Neural Stem Cells after Igf2 Conditional Deletion
| Cell Type | NSC | MP1 | MP2 | GRP/MP3 | MP4 | PFMP | BNAP | GRP3 |
|---|---|---|---|---|---|---|---|---|
| WT | 1.27% ± 0.09%a | 18.73% ± 2.53% | 4.30% ± 1.30% | 10.33% ± 1.40% | 0.50% ± 0.31% | 0.90% ± 0.23% | 8.68% ± 1.90% | 0.34% ± 0.07% |
| iKO | 1.01% ± 0.07%∗b | 21.87% ± 2.02% | 4.91% ± 1.46% | 8.33% ± 1.30% | 0.33% ± 0.21% | 1.03% ± 0.19% | 13.46% ± 2.30% | 0.38% ± 0.06% |
See also Figure S3. NSC, neural stem cell; MP1, multipotential 1; MP2, multipotential 2; MP3/GRP2, multipotential 3/glial restricted progenitor 2; PFMP, PDGF- and FGF-responsive multipotential progenitors; BNAP/GRP1, bipotential neuronal-astrocytic precursor/glial restricted progenitor 1; GRP3, glial restricted progenitor 3; WT, wild-type; iKO, inducible KO.
Data are presented as mean values ± SEM, n = 17 WT and 19 iKO mice of both sexes.
∗p < 0.05 by one-way ANOVA and Tukey's post hoc test.
Several studies have correlated increased or decreased cell proliferation in the SGZ with improvements or deficits in learning and memory, respectively (for a review see Zhao et al., 2008). Since Igf2 deletion altered the numbers of dual nucleotide label-retaining cells and increased the number of CldU+ proliferating cells, we performed studies to determine whether the Igf2 iKO mice had deficits in hippocampal function using the Morris water maze to test spatial learning and memory and the open field test to evaluate exploratory activity and anxiety (Figures 2E–2H). WT mice initially required 50 s to find the hidden platform in the Morris water maze, but quickly learned the location of the platform, and their latency to find the platform decreased from 50 s to 10 s. By contrast, the iKO mice were impaired in learning and displayed longer latencies to find the platform (repeated-measures ANOVA, n = 16 iKO and n = 13 WT) (Figure 2E). To test memory retention, we tested mice in a probe trial. WT mice swam significantly longer (50%) in the target quadrant than the iKO mice (35%) (Figure 2F). There was no difference between genotypes in the ability to find a visible platform (data not shown).
We further assessed the effects of losing IGF-II on exploratory and anxiety behavior using an open field test. Mice were placed into an open field test apparatus and were evaluated across six 5-min intervals. Overall the total distance traveled was unaltered between WT and iKO mice (data not shown). However, the iKO mice traveled less distance than the WT mice in the first two intervals (iKO 1,683 ± 121 and 1,103 ± 104 versus WT 2,072 ± 129 and 1,426 ± 94), and the total time that the iKO mice were immobile was significantly increased compared with WT mice (iKO 955.4 ± 47.7 versus WT 761 ± 64.8). What emerged most clearly across trials was that the iKO mice avoided the center of the arena (Figures 2G and 2H) (n = 13 iKO and n = 10 WT), indicating increased anxiety in the iKO mice.
One function of the NSCs in the rodent SVZ is to give rise to neuroblasts that migrate through the rostral migratory stream (RMS) to the olfactory bulb, resulting in 10,000–30,000 new neurons per day (Lledo and Saghatelyan, 2005). As the iKO mice had fewer IdU+/CldU+ cells but increased CldU+ cells in the SVZ, we performed analyses to determine whether there were increased numbers of migrating neuroblasts or new neurons in the olfactory bulb. The relative numbers of doublecortin (DCX)-positive immature neurons in the RMS entering the olfactory bulb were equivalent between the WT and iKO mice (Figures 3F–3H). However, using bromodeoxyuridine (BrdU) to label dividing progenitors in the SVZ and BrdU+/NeuN+ immunostaining to trace their differentiation into new olfactory bulb neurons, we observed a dramatic increase in the number of double-positive BrdU/NeuN cells in the granule cell layer of the olfactory bulb in the iKO mice compared with the WT mice (Figures 3I and 3J). In contrast, the number of double-positive BrdU/NeuN cells was unchanged in the iKO periglomerular layer of the olfactory bulb (Figures 3K and 3L). It has been well established that the new olfactory bulb neurons produced by the SVZ are GABAergic inhibitory interneurons (Betarbet et al., 1996, Carleton et al., 2003, Kato et al., 2001, Petreanu and Alvarez-Buylla, 2002, Winner et al., 2002); however, we were unsuccessful in establishing which subset of interneurons were overproduced as neither calretinin, calbindin, nor tyrosine hydroxylase stained the BrdU+/NeuN+-labeled neurons (Figure S4).
Using the buried food test as an olfaction assay, we found that the iKO mice took twice as long as the WT mice (iKO 325 ± 24 s versus WT 146 ± 18 s) to find a buried Fruit Loop (Figure 3N). As there are stem cells and olfactory receptor cells in the olfactory epithelium that turn over, an involution of the olfactory epithelium could explain the olfaction deficit; however, the morphology and structure of the olfactory epithelium appeared unchanged in both H&E-stained and periodic acid-Schiff (PAS)-stained sections of the iKO mice (Figure 3M). Therefore, we conclude that the loss of IGF-II led to an increase in SVZ progenitors, which in turn led to the overproduction of new inhibitory neurons in the olfactory bulb, resulting in a deficit in olfaction.
Adult ISCs Require Sustained IGF-II Production
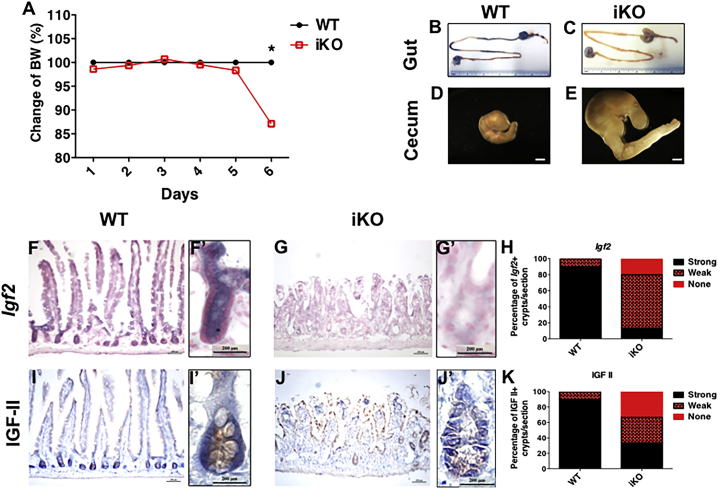
We next asked whether IGF-II has a broader function in maintaining other adult stem cells. We speculated that the lethality of Igf2 iKO mice receiving five consecutive daily doses of tamoxifen might be due to a rapid loss of ISCs, whose differentiated progeny normally live for 3–5 days after cell-cycle exit (van der Flier and Clevers, 2009). To test this hypothesis, we analyzed the intestines from tamoxifen-injected iKO mice and their WT littermate controls on day 6 after tamoxifen administration. At this time, there was an average of 15% body-weight loss in iKO mice when compared with the WT littermates (Figure 4A). Analyses of the chemical composition of the plasma failed to detect consistent differences between the iKOs and WT mice; hematocrit was normal in iKOs, and no difference was detected in leukocyte frequencies (data not shown). However, iKO mice showed clear signs of declining health including hunched backs, lethargy, and reduced food/water intake (data not shown). All iKOs, if not sacrificed at this time, would die within a week. The intestinal tract of the iKO mice appeared translucently pale with dilated lumen, enlarged cecum, and no solid feces in the colonic lumen (Figures 4B–4E). These pathological effects were not due to tamoxifen toxicity, as intestines from the WT littermates subjected to the identical tamoxifen treatment appeared normal.
Figure 4.
Loss of IGF-II Abrogates Intestinal Homeostasis
Five consecutive injections of tamoxifen (75 mg/kg) were administered from day 1 to day 5 and tissues were analyzed on day 6. Body-weight curve of WT and iKO mice from day 1 to day 6 (A), n = 3 WT and n = 4 iKO mice. WT (B and D) and iKO (C and E) guts were surgically dissected (from the stomach to the colon) and the ceca were photographed at day 6. Scale bars, 6 inches for intestines and 200 μm for cecum. Igf2 mRNA (F–G′) and IGF-II protein (I–J′) levels were detected in WT (F, F′, I, and I′) and iKO (G, G′, J, and J′) intestine using in situ hybridization and immunohistochemistry, respectively. Scale bars, 200 μm. n = 3 WT and n = 3 iKO mice. ∗p < 0.05 by Student's t test. Error bars represent SEM.
In situ hybridization showed that Igf2 was highly expressed in WT crypts and at lower levels in villus epithelium as well as some mesenchymal cells (Figures 4F, 4F′, and 4H). Igf2 mRNA levels were significantly reduced in iKO intestines (Figures 4G–4H). Immunohistochemistry for IGF-II confirmed the mRNA pattern with IGF-II protein drastically reduced in iKO intestines, most notably in the crypts (Figures 4I–4K).
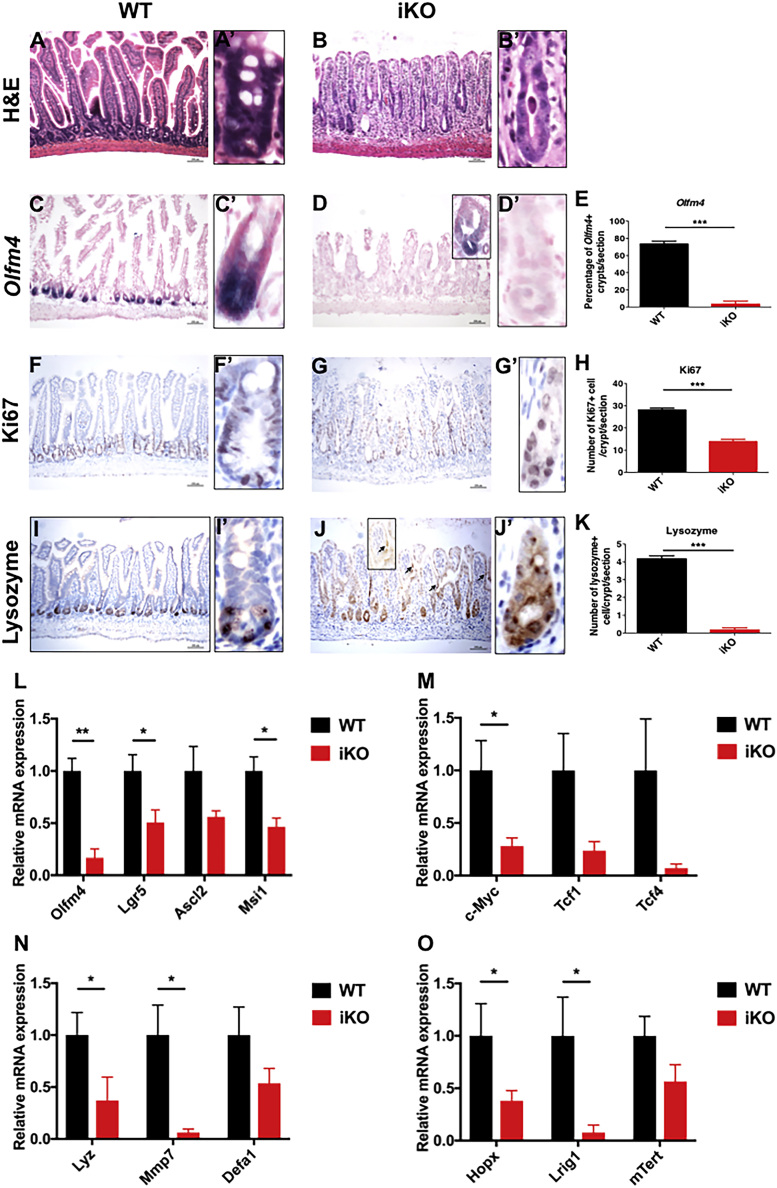
Histological examination suggested a clear loss of villus-crypt boundaries and a transit amplifying cell zone in iKO intestines, where the villi became severely blunted (Figures 5A–5B′). Approximately 80% of the WT crypts had robust expression of Olfm4, a marker of crypt-based columnar (CBC) stem cells (Barker et al., 2007, van der Flier and Clevers, 2009), whereas only 10% of the iKO crypts showed detectable Olfm4 expression (Figures 5C–5E). Even when Olfm4+ crypts were occasionally found, these crypts had an abnormal morphology (Figure 5D, inset). Ki67+ transit amplifying cells were abundant in WT crypts but were significantly decreased in iKO crypts (Figures 5F–5H), suggesting that loss of IGF-II caused a severe reduction of transit amplifying and potentially stem/progenitor cell populations.
Figure 5.
IGF-II Maintains the Morphology and Stemness of the Small Intestine
Small intestine of WT and iKO mice after five consecutive injections of tamoxifen (75 mg/kg) were stained and quantified for H&E (A–B′), Olfm4 (C–E), Ki67 (F–H), and lysozyme (I–K). qPCR analyses were performed in WT and iKO intestines to detect marker genes for stem cells (L), progenitors (M), Paneth cells (N), and quiescent stem cells (O). n = 3 (WT) and 3 (iKO) mice. See also Figure S5. Scale bars, 200 μm. ∗ p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 by Student's t test. Error bars represent SEM.
Lysozyme-positive Paneth cells were present at the bottom of the WT crypts (Figures 5I, 5I′, and 5K). In contrast, all Paneth cells were virtually eliminated from the iKO crypts (Figures 5J, 5J', and 5K). Some lysozyme+ cells were seen in the iKO villi (Figures 5J [arrowhead and inset], 5J′, and 5K). qRT-PCR verified an overall reduction of the genes in the iKO that are characteristic of Wnt/β-catenin signaling and fast-cycling stem cells (Figures 5L and 5M), as well as Paneth cells (Figure 5N) and alternative stem cell populations (Figure 5O). These data suggested that there was a severe impairment in the crypt compartment affecting both stem and Paneth cell populations in iKO mouse intestines.
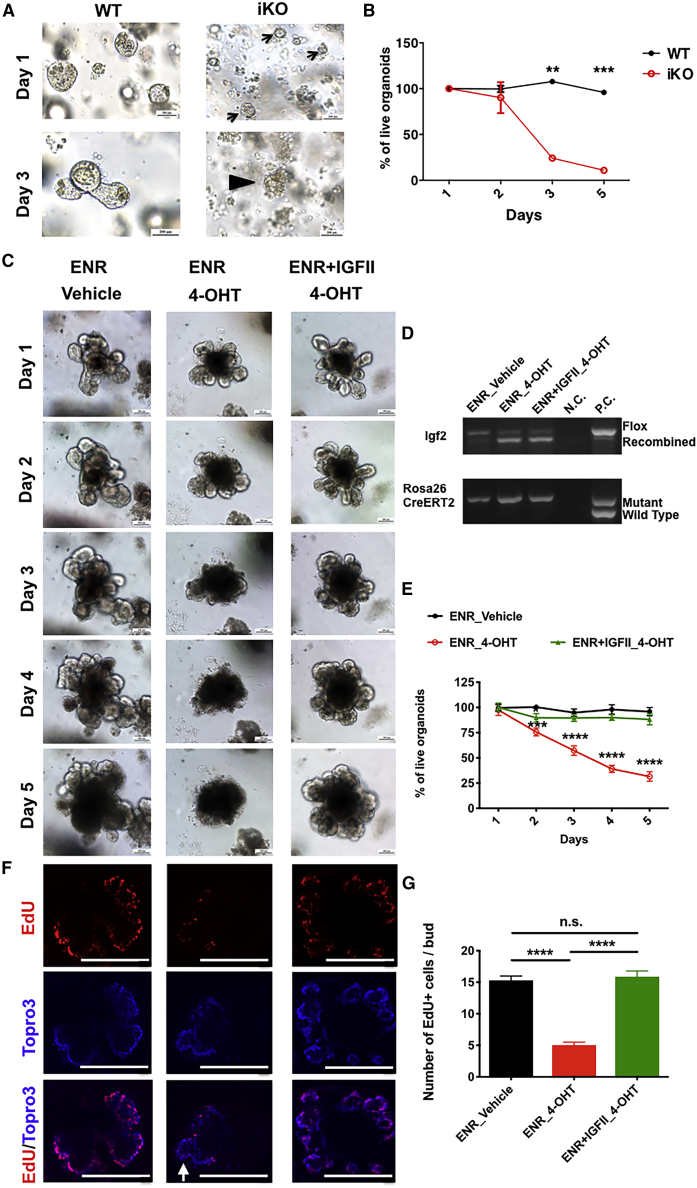
To determine whether ISCs in iKO crypts indeed lost self-renewal capacity, we isolated intestinal crypts from WT and iKO mice 5 days after tamoxifen administration in vivo, and cultured them in EGF, Noggin, and R-spondin-1 (ENR) medium. WT crypts underwent continuous budding (Figure 6A), whereas few iKO crypts initiated budding (arrows in Figure 6A). The percentage of viable iKO organoids was significantly reduced (Figure 6B). We then performed inducible Igf2 deletion in culture, by adding 4-hydroxytamoxifen (4-OHT) to the iKO organoids that already formed in vitro. Addition of 4-OHT, but not vehicle, led to clear regression of buds in iKO organoids at day 3 (compare left and middle columns in Figure 6C). Tamoxifen-induced genomic recombination at Igf2 locus was verified by PCR (Figure 6D). When we supplemented the ENR medium with IGF-II, such bud regression was prevented (right column in Figure 6C), even though similar recombination occurred in the organoid (Figure 6D). Counting of normal-appearing budding organoids from these cultures indicated a rescuing effect by the exogenous IGF-II protein (Figure 6E). 5-Ethynyl-2′-deoxyuridine (EdU) incorporation further suggested that 4-OHT treatment decreased numbers of the proliferative cells in epithelial buds, which was restored by IGF-II addition (Figures 6F and 6G). These data collectively demonstrate that IGF-II loss reduces the clonogenic activity of ISCs, supporting the idea that IGF-II is essential for adult ISC maintenance.
Figure 6.
Igf2 Knockout Impairs the Growth of Intestinal Organoids
WT and iKO (Igf2fl/flCre+/−) organoids were cultured in medium containing EGF, Noggin, and R-spondin1 (ENR) for 5 days. Arrows delineate small organoids with few Paneth cells. Arrowhead indicates dying organoid on day 3, which was confirmed to be dead on day 5 (A). Survival curve of organoids from WT and iKO mice (B). 4-OHT or vehicle was added to iKO (Igf2fl/flCre+/+) organoid cultures at day 7. The organoids were imaged daily (C), harvested for genotyping (D), and scored daily (E). The positive control (p.c.) used in (D) is the genomic DNA sample from a Rosa26-CreER heterozygous mouse sample to show the WT (bottom) and mutant (upper) PCR products. EdU staining was performed at day 4 after 4-OHT treatment (F and G). Scale bars, 200 μm. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 versus controls by Student's t test or one-way ANOVA. Data were analyzed from n = 3 independent mice. Error bars represent SEM.
Discussion
Taken altogether, our studies demonstrate that IGF-II is necessary for the maintenance of distinct adult epithelial stem cells in both the SVZ and SGZ of the brain and in the intestine, thus making it an essential factor for these stem cell niches. In these studies we removed Igf2 from adult mice using two different tamoxifen dosing schemes, one time a day for 5 days or one time every 3 days across 15 days. Both dosing schemes significantly reduced levels of IGF-II; however, acutely deleting Igf2 resulted in death of the mice and, therefore, could only be used for short-term studies and not for long-term neurogenesis studies. The stem cells and proliferating progenitors in the intestinal crypt failed to self-renew, lethally compromising the functions of the gastrointestinal tract. Within a crypt, two stem cell populations have been described: fast-cycling and quiescent stem cells, and specific markers are available for their identification. Lgr5 and Olfm4 mark the fast-cycling or CBC stem cells, whereas Hopx, Bmi1, and Lrig1 mark the quiescent stem cells (Barker et al., 2007, Sangiorgi and Capecchi, 2008, Takeda et al., 2011, Yin et al., 2014). Under certain conditions (e.g., injury, radiation), interconversion between the two stem cell populations has been reported, although the functional significance of the quiescent stem cells remains under debate given the robust expression of all quiescent stem cell markers by Lgr5+ cells (Porter et al., 2002, Sato et al., 2011). When ISCs were examined using the same tamoxifen administration protocol as used to study the NSCs (five doses of tamoxifen administered every 3 days), little effect was observed. The small intestines in the iKO mice appeared normal upon gross inspection. However, there was a significant increase in the Ki67+ transit amplifying cell population (p < 0.001), and a slight but nonsignificant increase in crypts with multiple Hopx+ cells (Figure S5). These results suggest that slow deletion of Igf2 allowed alternative intestinal epithelial cell populations, as we recently reported (Yu et al., 2018), to compensate for the loss of fast-cycling stem cells, which appear to be more sensitive to IGF-II loss.
Igf2 deletion reduced the numbers of dual nucleotide label-retaining cells and increased newly dividing cells labeled for the second nucleotide (CldU) in both the SGZ and SVZ, which correlated with behavioral deficits in the Morris water maze, open field test, and the buried food test. Our findings taken together with the prior studies by Bracko et al. (2012) and Ferron et al. (2015) now reveal that IGF-II regulates adult NSCs in both the SVZ and SGZ, but that distinct sources of IGF-II support these two neurogenic niches. In the SGZ/DG, Igf2 is expressed by the NSCs themselves, and this autocrine production is likely the main source of IGF-II for the NSCs. Ferron et al. (2015) demonstrated that Igf2 deletion from the endothelial compartment has little impact on the SGZ/DG NSCs. In the SVZ, the main sources of IGF-II that regulate the NSCs likely are from both the choroid plexus and endothelial cells (Ferron et al., 2015, Lehtinen et al., 2011). The complete deletion of Igf2 in the adult brain in our study resulted in a reduction in the SGZ/DG and SVZ NSCs and likely contributed to the greater effect we saw on the neurogenic niches that resulted not only in a reduction in NSC numbers but also in an increase in rapidly dividing progenitors. Moreover, we observed a burst of newly generated neurons in the olfactory bulb. Whereas embryonic deletion of Igf2 caused a reduction in NSCs in both adult niches, Ferron et al. (2015) saw no concomitant increase in rapidly dividing progenitors or in newly generated neurons in the olfactory bulb in these mice or in mice with endothelial Igf2 deletion. It is possible that the embryonic deletion of Igf2 significantly altered the initial establishment of NSCs present in the adult SVZ, consistent with the significant reduction in brain size in these mice.
Several studies have begun to establish a link between IGF-II, memory, and cognitive impairment. For example, IGF-II in the hippocampus is necessary for memory consolidation and promotes memory enhancement (Alberini and Chen, 2012, Chen et al., 2011). Moreover, IGF-II is being considered as a therapeutic target for Alzheimer's disease (Mellott et al., 2014). Our studies demonstrating that IGF-II is required for maintenance of a subset of adult NSCs suggest that IGF-II may be important for healthy aging and that reduced levels of IGF-II that occur with aging (Bartke et al., 2003, Chen et al., 2008, Kelijman, 1991) may contribute to neurodegenerative or psychiatric diseases.
Our studies demonstrating that IGF-II also is essential for the rapidly dividing ISCs has important implications for understanding the regulation of epithelial stem cell renewal in the intestines as well as for understanding and treating colorectal cancers. The rapid deletion of Igf2 impaired ISC homeostasis, as manifested by loss of CBCs and transit amplifying cells. This phenotype was strong in all iKO mice that received five consecutive doses of tamoxifen. The lethality and the histopathology are reminiscent of Tcf4 deletion (Korinek et al., 1998, van Es et al., 2012), conditional deletion of β-catenin (Fevr et al., 2007, Ireland et al., 2004), or overexpression of secreted Dickkopf-1, a Wnt inhibitor (Kuhnert et al., 2004, Pinto et al., 2003). Redundancy exists among various Wnt proteins, and there are multiple sources of Wnts in the intestinal stem cell niche (Aoki et al., 2016, Farin et al., 2012, Kabiri et al., 2014). In contrast, the phenotype presented here that resulted from the loss of a single growth factor, IGF-II, was striking.
LOI of Igf2 is found commonly in many types of cancer, including colorectal cancer (CRC) (Gelato and Vassalotti, 1990, Lamonerie et al., 1995, Yee et al., 1988), and Igf2 LOI is a potential marker for CRC risk (Cui et al., 2003). Increased allelic Igf2 expression promotes intestinal tumor formation and hyperproliferation of crypt epithelium with longer intestinal crypts (Bennett et al., 2003, Harper et al., 2006). Overexpression of Igf2 shifts cells to a less differentiated state, resulting in increased tumor initiation rather than proliferation (Sakatani et al., 2005). Insulin receptor substrate 1 (IRS-1), which is an intracellular mediator of both insulin and the IGF actions, has been implicated as an important determinant for ISC survival after injury, and partial or absolute IRS-1 deficiency in mice carrying the Apcmin/+ mutation led to reduced tumor number relative to IRS-1+/+/Apcmin/+ mice. Furthermore, elevated levels of insulin correlate with both low intestinal epithelial cell apoptosis and increased adenoma risk (Keku et al., 2005, Ramocki et al., 2008).
Our prior published studies indicate that IGF-II promotes SVZ NSC stemness in vitro through the A splice isoform of the insulin receptor and independently of either the IGF type 1 receptor (IGF-1R) or IGF-2R (Ziegler et al., 2012, Ziegler et al., 2014). Interestingly, the recent study by Bracko et al. (2012) showed that IGF-II acted through the IGF-1R to support DG/SGZ NSCs. Thus, IGF-II may have specific effects on the two neurogenic niches through distinct receptors to promote cell regeneration and healthy aging. While structurally similar, the differences in the biological effects of IGF-I and IGF-II are significant, and our studies demonstrate that much more needs to be learned about IGF-II and how it signals through its cognate receptors to affect specific adult stem cell populations.
Experimental Procedures
Animals
All experiments were performed in accordance with protocols approved by the institutional animal care and use committee of Rutgers-New Jersey Medical School and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised in 1996. Mouse strains included CreER driven off a Gt(ROSA)26Sor promoter (Gt(ROSA)26Sortm1(cre/Esr1)Nat/J) (Jax mouse stock #004847) and floxed Igf2 mice (Haley et al., 2012). Mice were obtained as C57BL/6 mixed strain and were backcrossed for five generations onto C57BL/6 (Jackson Laboratory) prior to use. Male and female mice, heterozygous for Cre, Gt(ROSA)26Sortm1(cre/Esr1)Nat/J and homozygous for floxed Igf2 were bred to generate mice used for the experiments. Progeny from the cross consisted of either heterozygous or homozygous Cre with homozygous floxed Igf2 (iKO) and homozygous floxed Igf2 mouse littermates that did not carry the Cre (WT).
Tamoxifen and Nucleotide Administration
Both Cre+ (iKO) and Cre− (WT) mice were administered tamoxifen (Sigma T5648; 75 mg/kg) intraperitoneally beginning at 4–5 weeks of age according to the schedule in Figure 1 for the NSC analyses or over 5 consecutive days for the intestinal analyses. For the NSC analysis, mice were 54 days old (±5 days) at the start of dual analog labeling and approximately 6 months old at tissue collection, and were the same across genotypes. A distinct cohort of mice between the ages of 2.5 and 6 months were used for behavioral studies. For the ISC analyses, mice were used 5–6 days following administration of tamoxifen.
Thymidine Analog Detection
IdU (Sigma I7125) or BrdU (Sigma, B5002) were provided at 1 mg/mL in 1% sucrose water. CldU (Sigma C-6891), dissolved in 0.9% saline, was administered intraperitoneally at 42.5 mg/kg. IdU and CldU detection was performed as described by Tuttle et al. (2010). GFAP (DAKO 1:500) was also included for analysis. IdU was detected with Dylight 549 conjugated secondary antibody, CldU with Dylight 488 conjugated secondary antibody, and GFAP with an Alexa 647 conjugated secondary antibody (all from Jackson ImmunoResearch) along with 4′,6′-diamidino-2-phenylindole (DAPI) at 1:1,000 in PBS for 15 min. Slides were coverslipped using Fluorogel (Electron Microscopy Sciences, #19440).
Stereology
For stereology analysis, 8-μm adjacent hippocampal or 5-μm adjacent SVZ tissue sections stained with CldU/IdU/GFAP/DAPI were used. Cell counts were performed using the physical fractionator (StereoInvestigator; MBF Bioscience). A systematic random sampling of cells within the regions of interest in adjacent sections was performed bidirectionally to obtain an unbiased estimate of the number of single-, double-, and triple-labeled cells. Sections were coded to ensure blinding during image acquisition and counting.
DCX, NeuN, and In Situ End Labeling Staining
DCX staining was performed using a goat polyclonal anti-DCX (1:100, Santa Cruz Biotechnology SC 8066) as previously described (Yang et al., 2008). The relative fluorescence intensity of DCX-positive cells was quantified using ImageJ software. Sections were stained for BrdU and NeuN as described previously (Goodus et al., 2015). In situ end labeling was performed as described previously (Romanko et al., 2004). Images were taken using a Q-imaging mono 12-bit camera interfaced with iVision 4 scientific imaging software (Scanalytics).
For multicolor flow cytometry, WT and iKO mice were administered tamoxifen (75 mg/kg) every 3 days beginning at 4–5 weeks of age and were euthanized at 12 months of age. SVZ cells were dissociated and stained using fluorescently labeled probes to the following cell-surface antigens: CD133/LeX/NG2/CD140a. A total of 50,000 cell events were analyzed per animal after excluding debris and dead cells by DAPI. A two-gate strategy was used to group cells into one of eight categories.
Behavioral Tests
Mice were tested for alterations in olfaction using a buried food test as described by Yang and Crawley (2009). Deficits in hippocampal function were determined using the Morris water maze to test spatial learning and memory and the open field test to evaluate exploratory activity and anxiety (for a review see Zhao et al., 2008). Details of these procedures can be found in Supplemental Experimental Procedures.
Intestinal Tissue Analyses
Small intestines were dissected and flushed with cold PBS. The proximal halves were fixed in DEPC-treated 4% paraformaldehyde for 1 h at room temperature, changed into 30% sucrose overnight at 4°C, frozen, and sectioned at 10 μm for in situ hybridization. The distal halves were fixed in 4% paraformaldehyde overnight, embedded in paraffin, and sectioned at 5 μm for immunohistochemistry. In situ hybridization analysis for Igf2 and Olfm4 was performed as previously described (Gregorieff and Clevers, 2010, Gregorieff et al., 2005). Paraffin sections were stained with H&E, for lysozyme (1:8, Biogenex), Ki67 (1:2000, Vector Laboratories), and Hopx (1:1,000, Santa Cruz). Frozen tissue sections were stained for IGF-II (1:1,000, Santa Cruz) as previously described (Gao et al., 2009). For Igf2 in situ hybridization and IGF-II immunohistochemistry, individual crypts were visually scored, based on staining intensities, into none, weak, and strong categories. Percentage of each category was presented in results. For Olfm4 in situ, crypts with positive Olfm4 staining were manually counted and reported from sections of intestinal tissues. For Ki67, lysozyme, and EdU staining, numbers of positive cells were counted for individual crypts and reported as the average number per crypt. For Hopx, percentages of crypts containing 1, 2, or 0 strongly Hopx-positive cells were reported. All values are presented as means ± SEM. Differences among groups were analyzed by Student’s t test with GraphPad Prism version 5.01. Significance was noted when p was less than 0.05.
Intestinal Organoid Culture
Organoids were derived from isolated crypts of the proximal small intestine of WT and iKO mice as described previously (Sato and Clevers, 2013, Sato et al., 2009). Organoids were maintained in an ENR-containing medium. Organoid growth was imaged and quantified by scoring the number of live organoids that developed during the 5 days after isolation or treatment. For in vitro induction of Igf2 deletion, organoids were derived and cultured as above from iKO (Igf2fl/flCre+/+) mice and were treated for 12 h with either 0.5 μM 4-OHT (iKO) or vehicle on day 7 as described previously (Farin et al., 2012). EdU (Life Technologies) staining was performed according to the manufacturer's instructions. Data were analyzed from organoids isolated from three independent mice.
Author Contributions
A.N.Z. collected and analyzed neural stem cell data; Q.F. collected intestinal stem cell data; J.M.T. performed and analyzed Morris water maze studies; M.C. and I.S. generated floxed Igf2 mice; S.C., E.K., and D.E.R. performed and analyzed the BrdU/NeuN immunostaining experiments; T.C. and K.P. contributed to behavioral study designs and data analysis; Q.F. and N.G. analyzed intestinal data; A.N.Z., S.W.L., and T.L.W. designed the studies of NSCs; A.N.Z., Q.F., N.G., S.W.L., and T.L.W. wrote the manuscript. All authors discussed the results, provided comments on drafts, and approved the final manuscript.
Acknowledgments
This work was supported by NIH National Institute on Neurological Disorders and Stroke R21NS076874 awarded to S.W.L and T.L.W., and F31NS065607 awarded to A.N.Z., NIH National Institute of Diabetes and Digestive and Kidney Diseases R01DK102934 awarded to N.G., NIH National Cancer Institute R21CA178599 awarded to N.G., American Cancer Society RSG-15-060-01-TBE awarded to N.G., and Medical Research Council (United Kingdom) MRC MC UU 12012/4 awarded to M.C.. Q.F. received a postdoctoral fellowship from the New Jersey Commission on Cancer Research (DFHS16PPC045). The authors would like to thank Dr. Robert Smith for sharing an important insight during a discussion at the IGF Gordon Conference. The authors also thank Dr. Eldo Kuzikandathil for use of his open field apparatus, and Dr. Soumya Das and Dr. Shiyan Yu for providing reagents.
Published: March 21, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2019.02.011.
Supplemental Information
References
- Alberini C.M., Chen D.Y. Memory enhancement: consolidation, reconsolidation and insulin-like growth factor 2. Trends Neurosci. 2012;35:274–283. doi: 10.1016/j.tins.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki R., Shoshkes-Carmel M., Gao N., Shin S., May C.L., Golson M.L., Zahm A.M., Ray M., Wiser C.L., Wright C.V. Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell. Mol. Gastroenterol. Hepatol. 2016;2:175–188. doi: 10.1016/j.jcmgh.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea T.C., Wang Y., Nathans J. A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J. Neurosci. 2003;23:2314–2322. doi: 10.1523/JNEUROSCI.23-06-02314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J., Liu J.P., Robertson E.J., Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Bartke A., Chandrashekar V., Dominici F., Turyn D., Kinney B., Steger R., Kopchick J.J. Insulin-like growth factor 1 (IGF-1) and aging: controversies and new insights. Biogerontology. 2003;4:1–8. doi: 10.1023/a:1022448532248. [DOI] [PubMed] [Google Scholar]
- Bennett W.R., Crew T.E., Slack J.M., Ward A. Structural-proliferative units and organ growth: effects of insulin-like growth factor 2 on the growth of colon and skin. Development. 2003;130:1079–1088. doi: 10.1242/dev.00333. [DOI] [PubMed] [Google Scholar]
- Betarbet R., Zigova T., Bakay R.A., Luskin M.B. Dopaminergic and GABAergic interneurons of the olfactory bulb are derived from the neonatal subventricular zone. Int. J. Dev. Neurosci. 1996;14:921–930. doi: 10.1016/s0736-5748(96)00066-4. [DOI] [PubMed] [Google Scholar]
- Bracko O., Singer T., Aigner S., Knobloch M., Winner B., Ray J., Clemenson G.D., Jr., Suh H., Couillard-Despres S., Aigner L. Gene expression profiling of neural stem cells and their neuronal progeny reveals IGF2 as a regulator of adult hippocampal neurogenesis. J. Neurosci. 2012;32:3376–3387. doi: 10.1523/JNEUROSCI.4248-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn R.C., King W.D., Winkler M.K., Fowlkes J.L. Early developmental changes in IGF-I, IGF-II, IGF binding protein-1, and IGF binding protein-3 concentration in the cerebrospinal fluid of children. Pediatr. Res. 2005;58:89–93. doi: 10.1203/01.PDR.0000156369.62787.96. [DOI] [PubMed] [Google Scholar]
- Buono K.D., Goodus M.T., Guardia Clausi M., Jiang Y., Loporchio D., Levison S.W. Mechanisms of mouse neural precursor expansion after neonatal hypoxia-ischemia. J. Neurosci. 2015;35:8855–8865. doi: 10.1523/JNEUROSCI.2868-12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buono K.D., Goodus M.T., Moore L., Ziegler A.N., levison S. Multimarker flow cytometric characterization, isolation and differentiation of neural stem cells and progenitors of the normal and injured mouse subventricular zone. In: Prusac J., editor. Neural Surface Antigens: From Basic Biology towards Biomedical Applications. Elsevier Press; 2015. pp. 175–185. [Google Scholar]
- Buono K.D., Vadlamuri D., Gan Q., Levison S.W. Leukemia inhibitory factor is essential for subventricular zone neural stem cell and progenitor homeostasis as revealed by a novel flow cytometric analysis. Dev. Neurosci. 2012;34:449–462. doi: 10.1159/000345155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton A., Petreanu L.T., Lansford R., Alvarez-Buylla A., Lledo P.M. Becoming a new neuron in the adult olfactory bulb. Nat. Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Charalambous M., Menheniott T.R., Bennett W.R., Kelly S.M., Dell G., Dandolo L., Ward A. An enhancer element at the Igf2/H19 locus drives gene expression in both imprinted and non-imprinted tissues. Dev. Biol. 2004;271:488–497. doi: 10.1016/j.ydbio.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Chen D.Y., Stern S.A., Garcia-Osta A., Saunier-Rebori B., Pollonini G., Bambah-Mukku D., Blitzer R.D., Alberini C.M. A critical role for IGF-II in memory consolidation and enhancement. Nature. 2011;469:491–497. doi: 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.L., Kassem N.A., Sadeghi M., Preston J.E. Insulin-like growth factor-ii uptake into choroid plexus and brain of young and old sheep. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:141–148. doi: 10.1093/gerona/63.2.141. [DOI] [PubMed] [Google Scholar]
- Chojnacki A., Cusulin C., Weiss S. Adult periventricular neural stem cells: outstanding progress and outstanding issues. Dev. Neurobiol. 2012;72:972–989. doi: 10.1002/dneu.22029. [DOI] [PubMed] [Google Scholar]
- Cui H., Cruz-Correa M., Giardiello F.M., Hutcheon D.F., Kafonek D.R., Brandenburg S., Wu Y., He X., Powe N.R., Feinberg A.P. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science. 2003;299:1753–1755. doi: 10.1126/science.1080902. [DOI] [PubMed] [Google Scholar]
- DeChiara T.M., Efstratiadis A., Robertson E.J. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345:78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- DeChiara T.M., Robertson E.J., Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991;64:849–859. doi: 10.1016/0092-8674(91)90513-x. [DOI] [PubMed] [Google Scholar]
- Farin H.F., Van Es J.H., Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143:1518–1529.e17. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- Feil R., Walter J., Allen N.D., Reik W. Developmental control of allelic methylation in the imprinted mouse Igf2 and H19 genes. Development. 1994;120:2933–2943. doi: 10.1242/dev.120.10.2933. [DOI] [PubMed] [Google Scholar]
- Ferguson-Smith A.C., Cattanach B.M., Barton S.C., Beechey C.V., Surani M.A. Embryological and molecular investigations of parental imprinting on mouse chromosome 7. Nature. 1991;351:667–670. doi: 10.1038/351667a0. [DOI] [PubMed] [Google Scholar]
- Ferron S.R., Radford E.J., Domingo-Muelas A., Kleine I., Ramme A., Gray D., Sandovici I., Constancia M., Ward A., Menheniott T.R. Differential genomic imprinting regulates paracrine and autocrine roles of IGF2 in mouse adult neurogenesis. Nat. Commun. 2015;6:8265. doi: 10.1038/ncomms9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevr T., Robine S., Louvard D., Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol. Cell. Biol. 2007;27:7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N., White P., Kaestner K.H. Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev. Cell. 2009;16:588–599. doi: 10.1016/j.devcel.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelato M.C., Vassalotti J. Insulin-like growth factor-II: possible local growth factor in pheochromocytoma. J. Clin. Endocrinol. Metab. 1990;71:1168–1174. doi: 10.1210/jcem-71-5-1168. [DOI] [PubMed] [Google Scholar]
- Giannoukakis N., Deal C., Paquette J., Goodyer C.G., Polychronakos C. Parental genomic imprinting of the human IGF2 gene. Nat. Genet. 1993;4:98–101. doi: 10.1038/ng0593-98. [DOI] [PubMed] [Google Scholar]
- Goodus M.T., Guzman A.M., Calderon F., Jiang Y., Levison S.W. Neural stem cells in the immature, but not the mature, subventricular zone respond robustly to traumatic brain injury. Dev. Neurosci. 2015;37:29–42. doi: 10.1159/000367784. [DOI] [PubMed] [Google Scholar]
- Gregorieff A., Clevers H. In situ hybridization to identify gut stem cells. Curr. Protoc. Stem Cell Biol. 2010 doi: 10.1002/9780470151808.sc02f01s12. Chapter 2, Unit 2F 1. [DOI] [PubMed] [Google Scholar]
- Gregorieff A., Pinto D., Begthel H., Destree O., Kielman M., Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Haley V.L., Barnes D.J., Sandovici I., Constancia M., Graham C.F., Pezzella F., Buhnemann C., Carter E.J., Hassan A.B. Igf2 pathway dependency of the Trp53 developmental and tumour phenotypes. EMBO Mol. Med. 2012;4:705–718. doi: 10.1002/emmm.201101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J., Burns J.L., Foulstone E.J., Pignatelli M., Zaina S., Hassan A.B. Soluble IGF2 receptor rescues Apc(Min/+) intestinal adenoma progression induced by Igf2 loss of imprinting. Cancer Res. 2006;66:1940–1948. doi: 10.1158/0008-5472.CAN-05-2036. [DOI] [PubMed] [Google Scholar]
- Ireland H., Kemp R., Houghton C., Howard L., Clarke A.R., Sansom O.J., Winton D.J. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta-catenin. Gastroenterology. 2004;126:1236–1246. doi: 10.1053/j.gastro.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Kabiri Z., Greicius G., Madan B., Biechele S., Zhong Z., Zaribafzadeh H., Edison, Aliyev J., Wu Y., Bunte R. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development. 2014;141:2206–2215. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- Kato T., Yokouchi K., Fukushima N., Kawagishi K., Li Z., Moriizumi T. Continual replacement of newly-generated olfactory neurons in adult rats. Neurosci. Lett. 2001;307:17–20. doi: 10.1016/s0304-3940(01)01914-0. [DOI] [PubMed] [Google Scholar]
- Keku T.O., Lund P.K., Galanko J., Simmons J.G., Woosley J.T., Sandler R.S. Insulin resistance, apoptosis, and colorectal adenoma risk. Cancer Epidemiol. Biomarkers Prev. 2005;14:2076–2081. doi: 10.1158/1055-9965.EPI-05-0239. [DOI] [PubMed] [Google Scholar]
- Kelijman M. Age-related alterations of the growth hormone/insulin-like-growth-factor I axis. J. Am. Geriatr. Soc. 1991;39:295–307. doi: 10.1111/j.1532-5415.1991.tb01654.x. [DOI] [PubMed] [Google Scholar]
- Korinek V., Barker N., Moerer P., van Donselaar E., Huls G., Peters P.J., Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- Kuhnert F., Davis C.R., Wang H.T., Chu P., Lee M., Yuan J., Nusse R., Kuo C.J. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc. Natl. Acad. Sci. U S A. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamonerie T., Lavialle C., Haddada H., Brison O. IGF-2 autocrine stimulation in tumorigenic clones of a human colon-carcinoma cell line. Int. J. Cancer. 1995;61:587–592. doi: 10.1002/ijc.2910610425. [DOI] [PubMed] [Google Scholar]
- Lander A.D., Kimble J., Clevers H., Fuchs E., Montarras D., Buckingham M., Calof A.L., Trumpp A., Oskarsson T. What does the concept of the stem cell niche really mean today? BMC Biol. 2012;10:19. doi: 10.1186/1741-7007-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen M.K., Zappaterra M.W., Chen X., Yang Y.J., Hill A.D., Lun M., Maynard T., Gonzalez D., Kim S., Ye P. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D.A., Alvarez-Buylla A. Adult neural stem cells stake their ground. Trends Neurosci. 2014;37:563–571. doi: 10.1016/j.tins.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.P., Baker J., Perkins A.S., Robertson E.J., Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Lledo P.M., Saghatelyan A. Integrating new neurons into the adult olfactory bulb: joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 2005;28:248–254. doi: 10.1016/j.tins.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Llorens-Martin M., Trejo J.L. Multiple birthdating analyses in adult neurogenesis: a line-up of the usual suspects. Front. Neurosci. 2011;5:76. doi: 10.3389/fnins.2011.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan A., Gonzalez A.M., Hill D.J., Berry M., Gregson N.A., Baird A. Coordinated pattern of expression and localization of insulin-like growth factor-II (IGF-II) and IGF-binding protein-2 in the adult rat brain. Endocrinology. 1994;135:2255–2264. doi: 10.1210/endo.135.5.7525264. [DOI] [PubMed] [Google Scholar]
- Logan A., Oliver J.J., Berry M. Growth factors in cns repair and regeneration. ProgGrowthFactorRes. 1994;5:379–405. doi: 10.1016/0955-2235(94)00008-9. [DOI] [PubMed] [Google Scholar]
- Mellott T.J., Pender S.M., Burke R.M., Langley E.A., Blusztajn J.K. IGF2 ameliorates amyloidosis, increases cholinergic marker expression and raises BMP9 and neurotrophin levels in the hippocampus of the APPswePS1dE9 Alzheimer's disease model mice. PLoS One. 2014;9:e94287. doi: 10.1371/journal.pone.0094287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L., Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J. Neurosci. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D., Gregorieff A., Begthel H., Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter E.M., Bevins C.L., Ghosh D., Ganz T. The multifaceted Paneth cell. Cell. Mol. Life Sci. 2002;59:156–170. doi: 10.1007/s00018-002-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramocki N.M., Wilkins H.R., Magness S.T., Simmons J.G., Scull B.P., Lee G.H., McNaughton K.K., Lund P.K. Insulin receptor substrate-1 deficiency promotes apoptosis in the putative intestinal crypt stem cell region, limits Apcmin/+ tumors, and regulates Sox9. Endocrinology. 2008;149:261–267. doi: 10.1210/en.2007-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H., Yin P., Duan C. IGFBP-5 regulates muscle cell differentiation by binding to IGF-II and switching on the IGF-II auto-regulation loop. J. Cell Biol. 2008;182:979–991. doi: 10.1083/jcb.200712110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanko M.J., Rothstein R.P., Levison S.W. Neural stem cells in the subventricular zone are resilient to hypoxia/ischemia whereas progenitors are vulnerable. J. Cereb. Blood Flow Metab. 2004;24:814–825. doi: 10.1097/01.WCB.0000123906.17746.00. [DOI] [PubMed] [Google Scholar]
- Sakatani T., Kaneda A., Iacobuzio-Donahue C.A., Carter M.G., de Boom Witzel S., Okano H., Ko M.S., Ohlsson R., Longo D.L., Feinberg A.P. Loss of imprinting of Igf2 alters intestinal maturation and tumorigenesis in mice. Science. 2005;307:1976–1978. doi: 10.1126/science.1108080. [DOI] [PubMed] [Google Scholar]
- Sangiorgi E., Capecchi M.R. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Clevers H. Primary mouse small intestinal epithelial cell cultures. Methods Mol. Biol. 2013;945:319–328. doi: 10.1007/978-1-62703-125-7_19. [DOI] [PubMed] [Google Scholar]
- Sato T., van Es J.H., Snippert H.J., Stange D.E., Vries R.G., van den Born M., Barker N., Shroyer N.F., van de Wetering M., Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Shinar D.M., Endo N., Halperin D., Rodan G.A., Weinreb M. Differential expression of insulin-like growth factor-I (IGF-I) and IGF-II messenger ribonucleic acid in growing rat bone. Endocrinology. 1993;132:1158–1167. doi: 10.1210/endo.132.3.8440176. [DOI] [PubMed] [Google Scholar]
- Silver D.P., Livingston D.M. Self-excising retroviral vectors encoding the Cre recombinase overcome Cre-mediated cellular toxicity. Mol. Cell. 2001;8:233–243. doi: 10.1016/s1097-2765(01)00295-7. [DOI] [PubMed] [Google Scholar]
- Stylianopoulou F., Herbert J., Soares M.B., Efstratiadis A. Expression of the insulin-like growth factor II gene in the choroid plexus and the leptomeninges of the adult rat central nervous system. Proc. Natl. Acad. Sci. U S A. 1988;85:141–145. doi: 10.1073/pnas.85.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N., Jain R., LeBoeuf M.R., Wang Q., Lu M.M., Epstein J.A. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteh P.W., Farin H.F., Clevers H. Plasticity within stem cell hierarchies in mammalian epithelia. Trends Cell Biol. 2014;25:100–108. doi: 10.1016/j.tcb.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Tuttle A.H., Rankin M.M., Teta M., Sartori D.J., Stein G.M., Kim G.J., Virgilio C., Granger A., Zhou D., Long S.H. Immunofluorescent detection of two thymidine analogues (CldU and IdU) in primary tissue. J. Vis. Exp. 2010:2166. doi: 10.3791/2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier L.G., Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- van Es J.H., Haegebarth A., Kujala P., Itzkovitz S., Koo B.K., Boj S.F., Korving J., van den Born M., van Oudenaarden A., Robine S. A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol. Cell. Biol. 2012;32:1918–1927. doi: 10.1128/MCB.06288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega C.J., Peterson D.A. Stem cell proliferative history in tissue revealed by temporal halogenated thymidine analog discrimination. Nat. Methods. 2005;2:167–169. doi: 10.1038/nmeth741. [DOI] [PubMed] [Google Scholar]
- Winner B., Cooper-Kuhn C.M., Aigner R., Winkler J., Kuhn H.G. Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. Eur. J. Neurosci. 2002;16:1681–1689. doi: 10.1046/j.1460-9568.2002.02238.x. [DOI] [PubMed] [Google Scholar]
- Yang M., Crawley J.N. Simple behavioral assessment of mouse olfaction. Curr. Protoc. Neurosci. 2009 doi: 10.1002/0471142301.ns0824s48. Chapter 8:Unit 8.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., You Y., Levison S.W. Neonatal hypoxic/ischemic brain injury induces production of calretinin-expressing interneurons in the striatum. J. Comp. Neurol. 2008;511:19–33. doi: 10.1002/cne.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee D., Cullen K.J., Paik S., Perdue J.F., Hampton B., Schwartz A., Lippman M.E., Rosen N. Insulin-like growth factor II mRNA expression in human breast cancer. Cancer Res. 1988;48:6691–6696. [PubMed] [Google Scholar]
- Yin X., Farin H.F., van Es J.H., Clevers H., Langer R., Karp J.M. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat. Methods. 2014;11:106–112. doi: 10.1038/nmeth.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Tong K., Zhao Y., Balasubramanian I., Yap G.S., Ferraris R.P., Bonder E.M., Verzi M.P., Gao N. Paneth cell multipotency induced by notch activation following injury. Cell Stem Cell. 2018;23:46–59.e45. doi: 10.1016/j.stem.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Deng W., Gage F.H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Ziegler A.N., Chidambaram S., Forbes B.E., Wood T.L., Levison S.W. Insulin-like growth factor-II (IGF-II) and IGF-II analogs with enhanced insulin receptor-a binding affinity promote neural stem cell expansion. J. Biol. Chem. 2014;289:4626–4633. doi: 10.1074/jbc.M113.537597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler A.N., Schneider J.S., Qin M., Tyler W.A., Pintar J.E., Fraidenraich D., Wood T.L., Levison S.W. IGF-II promotes stemness of neural restricted precursors. Stem Cells. 2012;30:1265–1276. doi: 10.1002/stem.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.