Abstract
Objective:
To characterize complex multimorbidity among cancer survivors and evaluate the association between cancer survivorship, time since cancer diagnosis, and self-reported fair/poor health, self-rated worse health in 2 years, and 2-year mortality.
Methods:
We used the 2010–2012 Health and Retirement Study. Cancer survivors were individuals who reported a (nonskin) cancer diagnosis 2 years or more before the interview. We defined complex multimorbidity as the co-occurrence of chronic conditions, functional limitations, and/or geriatric syndromes. In addition to descriptive analyses, we used logistic regression to evaluate the independent association between cancer survivor status and health outcomes. We also examined whether cancer survivorship differed by the number of years since diagnosis.
Results:
Among 15,808 older adults (age ≥50 years), 11.8% were cancer survivors. Compared with cancer-free individuals, a greater percentage of cancer survivors had complex multimorbidity: co-occurring chronic conditions, functional limitations, and geriatric syndromes. Cancer survivorship was significantly associated with self-reported fair/poor health, self-rated worse health in 2 years, and 2-year mortality. These effects declined with the number of years since diagnosis for fair/ poor health and mortality but not for self-rated worse health.
Conclusion:
Cancer survivor status is independently associated with more complex multimorbidity, and with worse health outcomes. These effects attenuate with time, except for patient perception of being in worse health.
Keywords: Comorbidity, functional limitations, geriatric syndromes, multimorbidity, health status, health decline, mortality, cancer survivorship
Introduction
Cancer survivors constitute a growing segment of the population, reflecting, in great part, an aging population and the associated increase in the number new cancer cases combined with improvements in cancer treatment and survival. A recent study estimated that in 2016 there were 15.5 million cancer survivors in the United States, a number projected to grow to more than 20 million by January 2026 [1]. Overall, 56% of cancer survivors had received a diagnosis of cancer within the previous 10 years, and 74% were aged 60 years or older [1].
Previous studies have reported great clinical heterogeneity [2] and a higher chronic condition comorbidity burden among cancer survivors than among their cancer-free counterparts [3] – what Leach et al. [4] described as a “complex health profile.” Many of the comorbidities acquired after cancer may be associated with chemotoxicity [5–7], and effects of hormonal treatment [8–10] or radiation treatment [11–14]. In addition to chronic medical conditions, poor mental-health-related quality of life is more prevalent among cancer survivors than among cancer-free adults [15], and clinically meaningful levels of depression and anxiety have been reported across subgroups of cancer survivors [16–18].
A conference held jointly by the Cancer and Aging Research Group, the National Cancer Institute, and the National Institute on Aging provided a “framework for future research to improve the evidence base for the clinical care of older adults with cancer” [19]. In it, the group identified the first knowledge gap to be the lack of geriatric assessment-guided interventions to improve outcomes for older patients with cancer. Another report from a survey by the American Society of Clinical Oncology highlighted “relative lack of research” involving older cancer survivors (current age >65 years) [20]. To fill these gaps, however, a more comprehensive assessment is needed to evaluate not only the chronic condition comorbidity burden among older cancer survivors but also their complex multimorbidity burden to account for difficulty with physical functioning and geriatric syndromes, in addition to their chronic conditions. Such a comprehensive approach in studying the health profile of older cancer survivors is all the more important given the role of cancer in further functional decline and increased risk of depression [21].
In this study, we use data from the Health and Retirement Study (HRS), a longitudinal panel launched in 1992, through which a rich array of data are collected biennially on a US representative sample of adults aged 50 years or older. We take advantage of the rich HRS data to define complex multimorbidity as the occurrence or co-occurrence of chronic conditions, functional limitations, and/or geriatric syndromes (e.g., poor cognitive functioning, and incontinence). With this more expansive definition of multimorbidity, we move away from prior studies’ sole reliance on multiple chronic conditions. In addition, we acknowledge the cascading effects of chronic conditions that manifest themselves in the emergence or worsening of functional limitations and geriatric syndromes, as well as the previously documented cumulative effect of these conditions relative to health outcomes, including self-reported fair/poor health, self-reported worse heath in 2 years, and 2-year mortality [22].
This study has two objectives: (1) to characterize cancer survivors’ complex multimorbidity profile; and (2) to evaluate the independent association between cancer survivor status and short-term poor health outcomes, including fair/poor self-reported health, self-rated worse health in 2 years, and 2-year mortality. We hypothesize that after adjustment for the multimorbidity profile and potential confounders, cancer survivor status remains associated with worse health outcomes. Furthermore, we hypothesize that the association between cancer survivor status and health outcomes is attenuated as more time has elapsed since cancer was first diagnosed.
Methods
Overview
This is a short-term study using 2010–2012 data from the HRS to compare complex multimorbidity and health out-comes between cancer survivors and cancer-free individuals. Since the data originate from the public use files of the HRS, this study was deemed exempt by the Case Western Reserve University Institutional Review Board.
Data source
We used 2010–2012 data from the public use files of the HRS. The HRS is the largest longitudinal survey study in the United States, with data on nearly 30,000 US adults aged 50 years or older. Every 2 years, HRS participants are asked to report on chronic conditions, functional status, cognitive status, depressive symptoms, and a range of behavioral variables.
Study population
All HRS participants were eligible to be included in the study. Respondents with a new (incident) cancer diagnosis in 2010, as defined below, were excluded from the study. Respondents had to be self-reporting in 2010, and any proxy respondents were excluded. Our study population included 15,808 individuals (2025 cancer survivors, and 13,783 cancer-free individuals). The average time elapsed since diagnosis for cancer survivors was slightly more than 12 years.
Variables of interest
Outcome variables:
The following were our outcome variables:
Self-reported fair or poor health status in 2010 (versus excellent, very good, or good)
Self-rated 2-year decline, coded as ‘1’ if the participant rates his/her health as “worse” (versus “better” or “about the same”) in 2012 compared with when he or she was interviewed in 2010
Two-year mortality, based on deceased status in 2012, as reported by the spouse or another relative or through probabilistic matching with the National Death Index
Independent variables:
Our main independent variable was cancer survivor status, coded as ‘1’ if the respondent reported a (nonskin) cancer diagnosis at least 2 years before the interview. Otherwise participants were defined as cancerfree individuals. We coded the time elapsed since cancer diagnosis as 2–4 years, 5–9 years, and 10 years or more; alternative coding, including a linear specification, yielded similar results. Respondents who reported a new cancer diagnosis within the prior 2 years were excluded.
Complex multimorbidity was characterized by the presence and co-occurrence of chronic conditions, functional limitations, and/or geriatric syndromes in 2010, consistent with our previous work [22]. Self-reported chronic conditions included hypertension, arthritis, heart disease, lung disease, diabetes, stroke, and psychiatric disorders. These conditions were considered present if the respondent replied in the affirmative to the question: “Has the doctor ever told you that you have” one of the above conditions?
Functional limitations included self-reported “any difficulty” in upper body mobility (e.g., reaching or extending arms above shoulder level); lower body mobility (e.g., walking several blocks or difficulty climbing stairs); strength (e.g., lifting 10 lb); activities of daily living (e.g., eating, dressing, bathing); and instrumental activities of daily living (e.g., preparing meals or managing oral medications).
Geriatric syndromes included poor cognitive performance (rated in the bottom tertile on the Telephone Interview for Cognitive Status); depression (at least four depressive symptoms on the Center for Epidemiological Studies Depression Scale; incontinence; visual impairment; hearing impairment; persistent dizziness, and severe pain. For individuals aged 65 years or older, we also accounted for falls as part of geriatric syndromes; respondents younger than 65 years were not asked about falls.
On the basis of our previous work [22, 23] showing that the co-occurrence of chronic conditions, functional limitations, and geriatric syndromes had a cumulative effect on health outcomes, we derived a composite measure for complex multimorbidity, defined as follows: MM0 in the absence of any of the aforementioned conditions; MM1 in the presence (but without the co-occurrence) of chronic conditions, functional limitations, or geriatric syndromes; MM2 when conditions across two of the domains of chronic conditions, functional limitations, and geriatric syndromes co-occur; and MM3 when conditions across all three of these domains co-occur. This composite measure has been shown to be associated with the outcomes of interest in a dose–response fashion.
Other independent variables include age (grouped in 5-year increments), race/ethnicity (white non-Hispanic, black non-Hispanic, Hispanic, and other), sex (male and female), marital status (married, divorced, widowed, and never married), number of years of education (<9, 9–11, 12, 13–15, 16, and ≥17), and income as a percentage of the federal poverty level (<100%, 100%–199%, 200%–299%, ≥300%). Behavioral variables include smoking status (never smoked, former smoker, and current smoker), vigorous exercise (“sports or activities that are vigorous” at least once per week), alcohol use based on average number of drinks per day (none; moderate, one or two drinks; heavy, three or more drinks), and body mass index, defined as weight in kilograms divided by the square of height in meters (underweight, ≤18 kg/m2; normal/ overweight, 18–29 kg/m2; obese, ≥30 kg/m2).
Analytic strategy
In addition to descriptive analysis, we conducted multivariable logistic regression analysis to analyze the independent association between cancer survivorship and health outcomes, after adjustment for multimorbidity and other potential con-founders. We estimated two sets of multivariable models. The first examined the association between cancer survivor status and the health outcomes; the second examined the association between the time since cancer diagnosis and the health outcomes. Cancer-free individuals were the reference in both sets of models.
We applied survey weights to account for the complex survey design of the HRS. SAS® version 9.4 was used in all of our analyses.
Results
The distribution of the study population by sociodemographic variables is presented in Table 1. Cancer survivors accounted for 12.81% of participants. A greater percentage of cancer survivors than cancer-free individuals were aged 70 years or older (60.79% vs. 34.64%) and white non-Hispanic (76.69% vs. 63.56%); P<0.001 for both comparisons. No marked differences were observed by marital status, education, or income. Importantly, however, compared with cancer-free individuals, a significantly greater percentage of cancer survivors were former smokers (48.20% vs. 39.96%), and fewer engaged in vigorous exercise (20.74% vs. 25.59%).
Table 1.
Sociodemographic characteristics and health outcomes among cancer survivors and cancer-free individuals
| Variables of interest | Cancer survivors | Cancer-free individuals | Total |
|---|---|---|---|
| Age category | |||
| 50–54 | 138 (6.81%) | 2550 (18.50%) | 2688 (17.00%) |
| 55–59 | 180 (8.89%) | 2800 (20.31%) | 2980 (18.85%) |
| 60–64 | 227 (11.21%) | 2025 (14.69%) | 2252 (14.25%) |
| 65–69 | 249 (12.30%) | 1635 (11.86%) | 1884 (11.92%) |
| 70–74 | 385 (19.01%) | 1951 (14.16%) | 2336 (14.77%) |
| 75–79 | 359 (17.73%) | 1346 (9.77%) | 1705 (10.79%) |
| 80–84 | 275 (13.58%) | 817 (5.93%) | 1092 (6.91%) |
| ≥85 | 212 (10.47%) | 659 (4.78%) | 871 (5.51%) |
| Sexa | |||
| Male | 863 (42.62%) | 5751 (41.73%) | 6614 (41.84%) |
| Female | 1162 (57.38%) | 8032 (58.27%) | 9194 (58.16%) |
| Race/ethnicity | |||
| White non-Hispanic | 1553 (76.69%) | 8761 (63.56%) | 10314 (65.25%) |
| Black non-Hispanic | 286 (14.12%) | 2762 (20.04%) | 3048 (19.28%) |
| Hispanic | 148 (7.31%) | 1828 (13.26%) | 1976 (12.50%) |
| Other | 38 (1.88%) | 432 (3.13%) | 470 (2.97%) |
| Marital status | |||
| Married | 1213 (59.90%) | 8521 (61.82%) | 9734 (61.58%) |
| Divorced | 288 (14.22%) | 2785 (15.85%) | 2473 (15.64%) |
| Widowed | 437 (21.58%) | 2209 (16.03%) | 2646 (16.74%) |
| Never married | 87 (4.30%) | 868 (6.30%) | 955 (6.04%) |
| Education (years)b | |||
| <9 | 159 (7.85%) | 1216 (8.82%) | 1375 (8.70%) |
| 9–11 | 240 (11.85%) | 1564 (11.35%) | 1804 (11.41%) |
| 12 | 674 (33.28%) | 4392 (31.87%) | 5066 (32.05%) |
| 13–15 | 456 (22.52%) | 3361 (24.38%) | 3817 (24.15%) |
| 16 | 246 (12.15%) | 1683 (12.21%) | 1929 (12.20%) |
| ≥17 | 250 (12.35%) | 1567 (11.37%) | 1817 (11.49%) |
| Income as percentage of federal poverty levelc | |||
| <100% | 199 (9.83%) | 1716 (12.45%) | 1915 (12.11%) |
| 100%–199% | 417 (20.59%) | 2633 (19.11%) | 3050 (19.29%) |
| 200%–299% | 373 (18.42%) | 2269 (16.46%) | 2642 (16.72%) |
| ≥300% | 1036 (51.16%) | 7165 (51.98%) | 8201 (51.88%) |
| Smoking statusb | |||
| Never smoked | 829 (40.94%) | 6106 (44.30%) | 6935 (43.87%) |
| Former smoker | 976 (48.20%) | 5508 (39.96%) | 6484 (41.02%) |
| Current smoker | 220 (10.86%) | 2169 (15.74%) | 2389 (15.11%) |
| Alcohol use | |||
| None | 1298 (64.10%) | 8531 (61.90%) | 9829 (62.18%) |
| Moderate | 609 (30.07%) | 3857 (27.98%) | 4466 (28.25%) |
| Heavy | 118 (5.83%) | 1395 (10.12%) | 1513 (9.57%) |
| Body mass index (kg/m2)c | |||
| Missing | 18 (0.89%) | 259 (1.88%) | 277 (1.75%) |
| Underweight | 35 (1.73%) | 134 (0.97%) | 169 (1.07%) |
| Normal/overweight | 1322 (65.28%) | 8539 (61.95%) | 9862 (62.38%) |
| Obese | 650 (32.10%) | 4851 (35.20%) | 5501 (34.80%) |
| Vigorous exercisec | |||
| No | 1605 (79.26%) | 10,256 (74.41%) | 11,861 (75.03%) |
| Yes | 420 (20.74%) | 3527 (25.59%) | 3947 (24.97%) |
| Total | 2025 (100.00%) | 13,783 (100.00%) | 15,808 (100.00%) |
Unless indicated otherwise, (unadjusted) comparisons were significant at P<0.0001.
P>0.05.
0.05≤P<0.01.
0.01≤P<0.001.
Table 2 presents the percentage of individuals presenting with the various chronic conditions, functional limitations, and geriatric syndromes by cancer survivor status. Regardless of the condition, the percentage of individuals presenting with the condition was significantly higher among cancer survivors than among cancer-free individuals. However, the greatest differentials between the two groups were for arthritis (67.65% vs. 52.98%) and heart disease (35.46% vs. 23.20%). Similarly, a greater percentage of cancer survivors than their cancer-free counterparts reported strength limitations, upper body mobility limitations, lower body mobility limitations, and limitations in independent activities of daily living. The differential between the two groups was the smallest for limitations in activities of daily living (4.59% vs. 3.58%, P=0.03; P<0.001 for all other comparisons). With regard to geriatric syndromes, we observed statistically significant differences between cancer survivors and cancer-free individuals with respect to incontinence (32.74% vs. 20.76%), severe pain (8.59% vs. 6.38%), hearing impairment (24.59% vs. 18.56%), and poor cognitive functioning (33.30% vs. 31.10%, P=0.047; all other comparisons significant at P<0.01).
Table 2.
Conditions constituting multimorbidity, by cancer survivor status
| Conditions of interest | Cancer survivors | Cancer-free individuals | Total |
|---|---|---|---|
| Chronic conditions | |||
| Hypertension | 1369 (67.60%) | 8204 (59.52%) | 9573 (60.56%) |
| Arthritis | 1370 (67.65%) | 7302 (52.98%) | 8672 (54.86%) |
| Heart disease | 718 (35.46%) | 3197 (23.20%) | 3915 (24.77%) |
| Lung disease | 280 (13.83%) | 1216 (8.82%) | 1496 (9.46%) |
| Stroke | 253 (12.49%) | 1102 (8.00%) | 1355 (8.57%) |
| Diabetes | 553 (27.31%) | 3116 (22.61%) | 3669 (23.21%) |
| Psychiatric conditions | 396 (19.56%) | 2197 (15.94%) | 2593 (16.40%) |
| Functional limitations | |||
| Strength limitations | 1302 (64.30%) | 7443 (54.00%) | 8745 (55.32%) |
| Upper body mobility limitations | 828 (40.89%) | 4379 (31.77%) | 5207 (32.94%) |
| Lower body mobility limitations | 1377 (68.00%) | 7723 (56.03%) | 9100 (57.57%) |
| Limitations in ADLsa | 93 (4.59%) | 494 (3.58%) | 587 (3.71%) |
| Limitations in IADLs | 373 (18.42%) | 1830 (13.28%) | 2203 (13.94%) |
| Geriatric syndromes | |||
| Low cognitive functioninga | 675 (33.33%) | 4287 (31.10%) | 4961 (31.38%) |
| Depressive symptomsb | 313 (15.46%) | 1994 (14.47%) | 2307 (14.59%) |
| Incontinence | 663 (32.74%) | 2862 (20.76%) | 3525 (22.30%) |
| Severe pain | 174 (8.59%) | 879 (6.38%) | 1053 (6.66%) |
| Visual impairmentd | 485 (23.95%) | 3105 (22.53%) | 3590 (22.71%) |
| Hearing Impairment | 498 (24.59%) | 2558 (18.56%) | 3056 (19.33%) |
| Total | 2025 (100.00%) | 13,783 (100.00%) | 15,808 (100.00%) |
Unless indicated otherwise, (unadjusted) comparisons were significant at P<0.0001.
ADLs, activity of daily living; IADL independent activities of daily living.
0.05≤P<0.01.
P>0.05.
Table 3 shows the distribution of the study population by the complex multimorbidity composite score, as well as health out-comes, by cancer survivor status. Not surprisingly, compared with cancer-free individuals, a significantly greater percentage of cancer survivors presented with co-occurring chronic conditions, functional limitations, and geriatric syndromes, or MM3 (34.02% vs. 25.12%), reported fair/poor health status (34.79% vs. 25.55%), and rated their health as “worse” than that 2 years previously (29.34% vs. 21.42%, P<0.001 for all comparisons). Two-year mortality was more than double among cancer survivors compared with among cancer-free individuals (7.60% and 3.26% respectively, P<0.001).
Table 3.
Multimorbidity and health outcomes
| Variables of interest | Cancer survivors | Cancer-free individuals | Total |
|---|---|---|---|
| Multimorbidity/composite measure | |||
| MM0 | 148 (7.31%) | 1781 (12.92%) | 1929 (12.20%) |
| MM1 | 482 (23.81%) | 4196 (30.44%) | 4678 (29.59%) |
| MM2 MM3 |
706 (34.86%) 689 (34.02%) |
4344 (31.52%) 3462 (25.12%) |
5050 (31.95%) 4151 (26.26%) |
| Self-reported health statusa | |||
| Good/very good/excellent health | 1220 (65.21%) | 9927 (74.45%) | 11,147 (73.31%) |
| Fair/poor health | 651 (34.79%) | 3407 (25.55%) | 4058 (26.69%) |
| Two-year self-reported worse healtha | |||
| No | 1322 (70.66%) | 10,478 (78.58%) | 11,800 (77.61%) |
| Yes | 549 (29.34%) | 2856 (21.42%) | 3405 (22.39%) |
| Two-year mortality | |||
| No | 1871 (92.40%) | 13,334 (96.74%) | 15,205 (96.19%) |
| Yes | 154 (7.60%) | 449 (3.26%) | 603 (3.81%) |
| Total | 2025 (100.00%) | 13,783 (100.00%) | 15,808 (100.00) |
All comparisons significant at P<0.001.
MM0, absence of chronic conditions, functional limitations, or geriatric syndromes; MM1 presence (but without the co-occurrence) of chronic conditions, functional limitations, or geriatric syndromes; MM2, co-occurrence of conditions across two of the domains of chronic conditions, functional limitations, and geriatric syndromes; MM3, co-occurrence of chronic conditions, functional limitations, and geriatric syndromes.
Reports limited to respondents alive and interviewed in 2012.
The findings from our multivariable logistic regression analysis showed that the differences in health outcomes by cancer survivor status persisted after adjustment for age and complex multimorbidity status. Thus, as shown in Fig. 1, cancer survivors were significantly likelier to report fair/poor health [adjusted odds ratio 1.73, 95% confidence interval (CI) 1.49–2.01] or 2-year self-rated worse health (adjusted odds ratio 1.32, 95% CI 1.15–1.51), or to die within 2 years (adjusted odds ratio 1.51, 95% CI 1.16–1.96). Despite the higher levels of multimorbidity among cancer survivors, preliminary analyses (not shown) did not detect a statistically significant interaction between cancer survivorship and multimorbidity for any of the outcomes. Thus, the effect of multimorbidity on the health outcomes was not stronger (or weaker) for cancer survivors compared with cancer-free individuals.
Fig. 1.
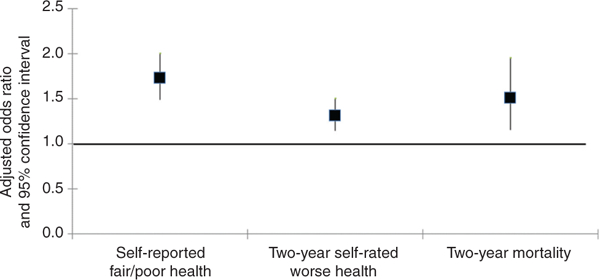
Results from the multivariable analysis showing the association between cancer survivor status and health outcomes, after adjustment for sociodemographic characteristics and multimorbidity.
The analyses examining the effect of time since diagnosis showed that the increased likelihood of self-reported fair/poor health and 2-year mortality was generally lower the more time that had elapsed (Fig. 2). Cancer survivors 10 years or more after diagnosis had odds of death that did not differ from that of cancer-free individuals. Cancer survivors 5–9 years and 10 years or more after diagnosis did not differ from one another in self-reported fair/poor health but remained different from cancer-free individuals. There were no differences in the perception of being in worse health by the time since diagnosis. Combined with the results from Fig. 1, this indicates that cancer survivors were slightly more likely to rate their health worse than that 2 years previously regardless of the time since diagnosis.
Fig. 2.
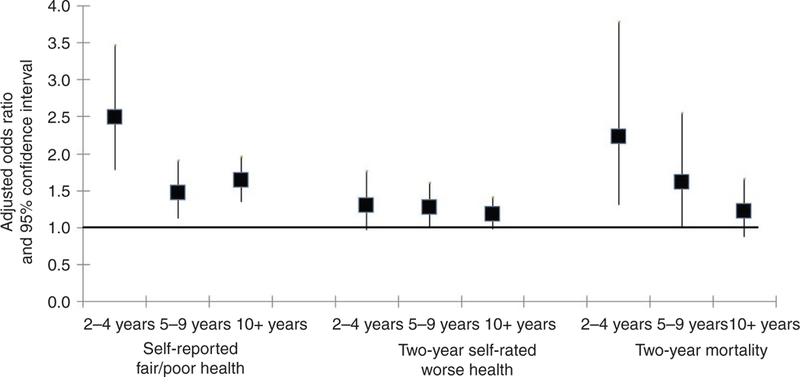
Results from the multivariable analysis showing the association between time elapsed since cancer diagnosis and health outcomes, after adjustment for sociodemographic characteristics and multimorbidity.
Discussion
Consistent with previous studies [4], we documented a ‘complex health profile’ and a greater multimorbidity burden among cancer survivors compared with their cancer-free counterparts. With the exception of limitations in activities of daily living, depressive symptoms, and visual impairment, we observed a higher percentage of various conditions among cancer survivors. However, cancer survivors were significantly older, and a greater percentage of them were white non-Hispanic – perhaps a reflection, downstream, of well-documented disparities in cancer survival experienced by patients of minority descent.
Even after adjustment for age, other sociodemographic variables, and multimorbidity, cancer survivors were likelier to report fair/poor health and 2-year worse health, or to have died within 2 years. For health status, it is critically important to determine whether self-reported fair/poor health is perceived because of their past challenges or whether it reflects unmeasured conditions, such as fatigue. Here, the complex and some what paradoxical findings from Fig. 2 deserve some discussion. On one hand, with greater time elapsed since cancer diagnosis, older cancer survivors become more similar to cancer-free individuals in their reports of fair/poor health, possibly reflecting the ‘suppression’ of their cancer experience, as they face the emergence of aging-related physical, functional, psychological, and emotional challenges [24]. On the other hand, the fact that cancer survivors are likelier to rate their health as having become worse in the previous 2 years regardless of the amount of time since diagnosis, even as elevated levels of poor/fair health and mortality seem to recede, suggests that cancer survivorship fundamentally alters their perceptions of their current health relative to the past. This phenomenon, described by Kahana et al. [25] as posttraumatic transformations, reflects cancer survivors’ view that cancer is a “continuing and worrisome experience.”
For the most part, our findings are consistent with those of previous studies, indicating poorer health and greater functional impairment in cancer survivors. While we note that our study population excludes cancer survivors in whom with cancer had been diagnosed within the last 2 years, the lack of association between depressive symptoms and cancer survivor status in our study differs from prior studies. These studies show that cancer survivors continue to perceive cancer’s threats to their quality of life, even as they age into older adulthood [26].
Our study findings have important clinical implications. First, given cancer survivors’ complex multimorbidity profile, the role of primary care physicians (PCPs) in coordinating care among the various medical specialists is a critical one, given their responsibility to incorporate treatment summaries and surveillance regimens from their survivorship care plan [27]. While some prior studies have shown that PCPs can safely manage follow-up care in certain patient populations (e.g., early-stage breast cancer survivors), others have expressed preference for a survivorship care co-managed by PCPs and oncologists [28].
Second, given their poor mental and physical health outcomes, cancer survivors may benefit from cognitive behavioral therapy and physical activity interventions to improve their functioning and health self-assessments. Such programs have proven highly effective [15, 29].
Third, it is important that survivorship care plans be tailored to individuals’ physical and mental health, as well as psychosocial needs and preferences, and enhance their selfefficacy [30]. As noted by Halpern and Argenbright [30], survivorship programs should be evaluated on their long-term outcomes, including quality-adjusted life years and functional status; receipt of social support, as well as nutritional and rehabilitative services; and as judicious use of health services (i.e., avoidance of inappropriate use of resources).
Finally, given the increase in the number of cancer survivors and their improved life expectancy, it is critically important to determine whether self-assessed poor and worsening health reflects their past challenges or whether it signals unmeasured health conditions, such as fatigue. Remedial measures can be taken accordingly to improve their health status. As noted by Kahana et al. [25], “therapeutic exchanges” based on communications between patients, health care providers, and mental health professionals about patients’ appraisal of their illness, the life-changing nature of receiving a diagnosis of and being treated for cancer, and stigma can be highly beneficial to the patient.
We note several limitations to our study. First, our study does not account for the anatomic site of primary cancer, multiple primary cancers, recurrences, or cancer stage at diagnosis for the first or subsequent cancers, and while cancer survivorship was based on self-report, specificity, high negative predictive value, and general concordance between self-reported cancer and registry-confirmed diagnosis has been shown previously [31]. Second, a number of pertinent measures were not available in the HRS, including cancer treatment regimen, as well as fatigue, which may have helped with the interpretation of these findings. Regardless, consistent with a previous study in which we identified combinations of conditions that are closely associated with fair/poor health status and 2-year self-rated worse health [32], although basic prevalence measures are included in our models, the greater severity of functional limitations and pain experienced among cancer survivors may well explain poor(er) health outcomes in this group. With regard to the higher likelihood to die within 2 years up until 10 years or more after diagnosis, even after adjustment for age and multimorbidity, we note the lack of data on cancer recurrence or long-term complications of cancer treatment compromising survival.
These limitations notwithstanding, our study has a number of strengths. To our knowledge, this is the first study to use the HRS and take advantage of the richness of its data to characterize cancer survivors’ complex multimorbidity profile based on an expansive definition, incorporating not only co-occurring chronic conditions but also functional limitations and geriatric conditions. This approach is all the more important considering the expected increase in the number of cancer survivors, and their representation in older age groups. This study moves beyond traditional conceptualization and measurement of multimorbidity as only a list of diagnoses to included measures of functional health morbidity. This more fully reflects the complexity of multimorbidity and its effects on people’s lives.
Conclusion
Cancer survivors are likelier to experience complex multimorbidity and, although they eventually have risks of death similar to those without a history of cancer, cancer survivors continue to rate their health as fair/poor and worsening even a decade after diagnosis. These findings indicate the compelling need for continued integrated and personalized care for cancer survivors. While care should be focused on both the physical and the psychological needs in the near term, cancer survivors would greatly benefit from continued cognitive behavioral interventions to address persisting cancer-related stress.
Acknowledgments
Funding
This study was funded in part by Case Comprehensive Cancer Center support grant (P30 CA043703). The authors were also supported in part by grants from the National Institute on Minority Health and Health Disparities (R01 MD009699-01: Cynthia Owusu), the Clinical and Translational Science Collaborative of Cleveland (UL1TR000439: Siran M. Koroukian), the National Center for Advancing Translational Sciences (KL2TR000440: Nicholas Schiltz) component of the National Institutes of Health (NIH) and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors, and do not necessarily represent the official views of the NIH.
Footnotes
The results were presented in part at the annual meeting of the International Society of Geriatric Oncology (November 2016, Milan, Italy).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland J, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66(4):271–89. [DOI] [PubMed] [Google Scholar]
- 2.Sharp L, Deady S, Gallagher P, Molcho M, Pearce A, Thomas AA, et al. The magnitude and characteristics of the population of cancer survivors: using population-based estimates of cancer prevalence to inform service planning for survivorship care. BMC Cancer 2014;14:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “silver tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev 2016;25(7):1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leach CR, Weaver KE, Aziz NM, Alfano CM, Bellizzi KM, Kent E, et al. The complex health profile of long-term cancer survivors: prevalence and predictors of comorbid conditions. J Cancer Surviv 2015;9(2):239–51. [DOI] [PubMed] [Google Scholar]
- 5.Kalam K, Marwick TH. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. Eur J Cancer 2013;49(13):2900–9. [DOI] [PubMed] [Google Scholar]
- 6.Sioka C, Kyritsis AP. Central and peripheral nervous system toxicity of common chemotherapeutic agents. Cancer Chemother Pharmacol 2009;63(5):761–7. [DOI] [PubMed] [Google Scholar]
- 7.Newton HB. Neurological complications of chemotherapy to the central nervous system. Handb Clin Neurol 2012;105:903–16. [DOI] [PubMed] [Google Scholar]
- 8.Keating NL, O’Malley A, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst 2012;104(19):1518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu JC, Williams SB, O’Malley AJ, Smith MR, Nguyen PL, Keating NL. Androgen-deprivation therapy for nonmetastatic prostate cancer is associated with an increased risk of peripheral arterial disease and venous thromboembolism. Eur Urol 2012;61(6):1119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun M, Choueiri TK, Hamnvik OP, Preston MA, De Velasco G, Jiang W, et al. Comparison of gonadotropin-releasing hormone agonists and orchiectomy: effects of androgen-deprivation therapy. JAMA Oncol 2016;2(4):500–7. [DOI] [PubMed] [Google Scholar]
- 11.Raj KA, Marks LB, Prosnitz RG. Late effects of breast radio therapy in young women. Breast Dis 2005;23:53–65. [DOI] [PubMed] [Google Scholar]
- 12.Steineck G, Skokic V, Sjoberg F, Bull C, Alevronta E, Dunberger G, et al. Identifying radiation-induced survivorship syndromes affecting bowel health in a cohort of gynecological cancer survivors. PLoS One 2017;12(2):e0171461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen KM, Offersen BV, Nielsen HM, Vaage-Nilsen M, Yusuf SW. Short and long term radiation induced cardiovascular disease in patients with cancer. Clin Cardiol 2017;40(4): 255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yusuf SW, Howell RM, Gomez D, Pinnix CC, Iliescu CA, Banchs J. Radiation-related heart and vascular disease. Future Oncol 2015;11(14):2067–76. [DOI] [PubMed] [Google Scholar]
- 15.Weaver KE, Forsythe LP, Reeve BB, Alfano CM, Rodriguez JL, Sabatino SA, et al. Mental and physical health-related quality of life among U.S. cancer survivors: population estimates from the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev 2012;21(11):2108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosher CE, Winger JG, Given BA, Helft PR, O’Neil BH. Mental health outcomes during colorectal cancer survivorship: a review of the literature. Psychooncology 2016;25(11): 1261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chongpison Y, Hornbrook MC, Harris RB, Herrinton LJ, Gerald JK, Grant M, et al. Self-reported depression and perceived financial burden among long-term rectal cancer survivors. Psychooncology 2016;25(11):1350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oancea SC, Cheruvu VK. Psychological distress among adult cancer survivors: importance of survivorship care plan. Support Care Cancer 2016;24(11):4523–31. [DOI] [PubMed] [Google Scholar]
- 19.Mohile SG, Hurria A, Cohen HJ, Rowland JH, Leach CR, Arora N, et al. Improving the quality of survivorship for older adults with cancer. Cancer 2016;122(16):2459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobsen PB, Rowland JH, Paskett ED, Van Leeuwen F, Moskowitz C, Katta S, et al. Identification of key gaps in cancer survivorship research: findings from the American Society of Clinical Oncology survey. J Oncol Pract 2016;12(3):190–3. [DOI] [PubMed] [Google Scholar]
- 21.Leach CR, Bellizzi KM, Hurria A, Reeve BB. Is it my cancer or am i just getting older?: Impact of cancer on age-related health conditions of older cancer survivors. Cancer 2016;122(12):1946–53. [DOI] [PubMed] [Google Scholar]
- 22.Koroukian SM, Warner DF, Owusu C, Given CW. Multimorbidity redefined: prospective health outcomes and the cumulative effect of co-occurring conditions. Prev Chronic Dis 2015;12:E55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koroukian SM, Schiltz N, Warner DF, Sun J, Bakaki PM, Smyth KA, et al. Combinations of chronic conditions, functional limitations, and geriatric syndromes that predict health outcomes. J Gen Intern Med 2016;31(6):630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowman KF, Deimling GT, Smerglia V, Sage P, Kahana B. Appraisal of the cancer experience by older long-term survivors. Psychooncology 2003;12(3):226–38. [DOI] [PubMed] [Google Scholar]
- 25.Kahana B, Kahana E, Deimling G, Sterns S, VanGunten M. Determinants of altered life perspectives among older-adult longterm cancer survivors. Cancer Nurs 2011;34(3):209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kypriotakis G, Deimling GT, Piccinin AM, Hofer SM. Correlated and coupled trajectories of cancer-related worries and depressive symptoms among long-term cancer survivors. Behav Med 2016;42(2):82–92. [DOI] [PubMed] [Google Scholar]
- 27.Ganz PA. Survivorship: adult cancer survivors. Prim Care 2009;36(4):721–41. [DOI] [PubMed] [Google Scholar]
- 28.Rowland JH, Bellizzi KM. Cancer survivorship issues: life after treatment and implications for an aging population. J Clin Oncol 2014;32(24):2662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nock NL, Owusu C, Flocke S, Krejci SA, Kullman EL, Austin K, et al. A community-based exercise and support group program improves quality of life in African-American breast cancer survivors: a quantitative and qualitative analysis. Int J Sports Exerc Med 2015;1(3):e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halpern MT, Argenbright KE. Evaluation of effectiveness of survivorship programmes: how to measure success? Lancet Oncol 2017;18(1):e51–9. [DOI] [PubMed] [Google Scholar]
- 31.Zeig-Owens R, Kablanian A, Webber MP, Liu Y, Mayerson E, Schwartz T, et al. Agreement between self-reported and confirmed cancer diagnoses in New York City firefighters and EMS workers, 2001–2011. Public Health Rep 2016;131(1):153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


