Abstract
This narrative review summarizes a decade of experience examining the cross-sectional and longitudinal relationships of arterial stiffness, as assessed by carotid-femoral pulse wave velocity, with outcomes in patients with chronic kidney disease enrolled in the Chronic Renal Insufficiency Cohort. Our goal here is to review the importance of the pulse wave contour and the pulse wave velocity and to present data on the reproducibility of pulse wave velocity measurements, determinants of pulse wave velocity, and the relationship velocity measurements have with longitudinal kidney and cardiovascular outcomes. Measures of arterial stiffness have contributed substantially to our understanding of mechanisms of cardiovascular disease, kidney disease progression and all-cause mortality. Given the independent relationship of arterial stiffness to a variety of outcomes it is our hope that future developments in behavioral, nutritional, and pharmacologic approaches to vascular destiffening will provide interventions that benefit patients with chronic kidney diseases.
Keywords: arterial stiffness, pulse wave velocity (PWV), chronic kidney disease (CKD), cardiovascular disease (CVD), heart failure (HF), hypertension, kidney disease progression, mortality, Chronic Renal Insufficiency Cohort (CRIC), review
Introduction
One of the most remarkable feats accomplished by the human circulation is the conversion of the pulsatile cannonades from the left ventricle in the smooth and continuous flow of blood at the tissue level. Achieving this feat occurs through a balance of transitioning both pressure and flow waves from pulsatile phenomenon into a steady procession of nutrients and oxygen at the level of the microcirculation1.
This marvelous journey starts in the proximal aorta and is characterized initially by an amplification of the systolic pressure wave as these wave moves more distally, followed by a successive dampening as arterial caliber rapidly diminishes until a steady pressure is achieved in the microcirculation. These pressure phenomena are mirrored by parallel changes in flow, whose character is also transformed from pulsatile to continuous by the unique adaptations of the vessels which constitute the arterial tree2,3. The result of these transformation is a stable, constant delivery of blood to each organ in the body.
The challenges of physical activity, gravity, and upright position, along with individual organ function (such as waste excretion, or neural activity control) create unique needs. In particular, the stable constant delivery blood occurs in basal conditions (like sleeping), as well us under a variety of physical alterations that mix increased tissue needs (like skeletal muscle nutrient needs when running) with issues like position—the brain, for example, is “uphill” of the rest of the body, yet requires vigilant blood flow no matter the physical activity level or body position. Moreover, the kidneys, whose weight is in the range of 1% of the body mass, receive more than 20% of the cardiac output. The ability of the circulation to maintain flow to the brain and the kidneys under a wide variety of physiologic circumstances comes at a price, namely a low vascular resistance4. This low resistance represents both a blessing and curse, particularly in chronic kidney diseases, as longitudinal studies have shown and as I review below.
What follows is a narrative review of the relationship between arterial stiffness as measured by carotid femoral pulse wave velocity and a variety of outcomes experienced by people with chronic kidney diseases. This narrative review began as an invited lecture (the Massry Lecture) at the 2017 National Kidney Foundation (NKF) Spring Clinical Meeting in Orlando FL. Dr. Massry is Professor (Emeritus) of Medicine, and Professor (Emeritus) of Biophysics at Keck School of Medicine at the University of Southern California in Los Angeles. He chaired the Nephrology Division at Keck from 1974 to 2000, serving as NKF President from 1992–1994 and contributed over 600 papers, 100 book chapters, and 30 books (as editor) during his career.
Background
Overview
Before blood pressure measurements became commonplace in clinical medical office encounters in the 20th century, a variety of ingenious devices were constructed to capture the arterial pulse profile as reviewed by O’Rourke5. Building on millennia of experience derived from palpation of the arterial pulse at the radial artery, or at the carotid artery, clinicians were aware of a panoply of pulse profiles that had prognostic significance, giving rise to a cornucopia of Latin phrases such as pulsus parvus, pulsus tardus, and pulsus bisferiens. Incorporating the Greek root word for pulse (σφυγμός – sphygmos), these machines were known as sphygmographs and became increasingly popular to use in patient evaluation until the advent of the sphygomanometer. They were somewhat cumbersome, had no means for calibration, and frequently the output was an etching into a waxed paper strip or cylinder (see5a), which made storage in a patient chart quite challenging.
Measurement of blood pressure proved to be easier, required less sophisticated equipment, and was reasonably comfortable to record through a simple notation of onset and disappearance of Korotkoff sounds6,7. This, coupled with the early recognition by actuaries of the prognostic significance of blood pressure (BP) as a predictor of death8,9, relegated sphygmographs into the realm of historical medical curiosities.
In the mid-1920s research was conducted on a different aspect of pulsology, which dealt with the velocity of pulse wave propagation in the circulation. Building on a wealth of physical sciences data dealing with fluids, flow, and viscosity, a series of human studies validated the ability to measure pulse wave velocity in humans10,11. These required invasive catheterization and remained suitable only for research until it became possible in the latter part of the 20th century to estimate arterial stiffness using non-invasive approaches12,13.
With the incorporation of measurements of arterial stiffness into a number of ongoing cohort studies two things have become clear. The first is that the velocity of pulse wave travel is a substantial component in the genesis of the systolic blood pressure14. The magnitude of the systolic blood pressure is dependent on the interaction of forward and backward traveling pressure waves, whose interaction varies depending on the location within the circulation where the measurement is obtained and the velocity of wave travel at that spot5,15. The pulse wave velocity typically increases as the pulse wave travels in the proximal aorta (3–4 m/s), descending aorta (5–6 m/s), ilial-femoral segment (7–8 m/s), and peripherally to the foot or the hand (9–10 m/s). The wall to lumen ratio and the amount of collagen relative to elastin are major contributors to this heterogeneity of pulse wave velocity (PWV) in the human circulation. Increasing wall to lumen ratios and increasing collagen to elastin ratios favor increased stiffness16.
Secondly, arterial stiffness, specifically aortic stiffness, independently predicts death (from all causes, and from cardiovascular causes in particular) and cardiovascular outcomes in healthy elderly people, diabetic patients, hypertensive patients, general adult populations like those sampled by the Framingham Study, and patients with end stage renal disease17–22. Thus measures of arterial stiffness appear to improve risk prediction, and countries such as Japan reimburse for yearly measurements of arterial stiffness in the office setting.
The history of the arterial stiffness field has been nicely summarized in several places1,23,24. The story of arterial stiffness measurements in participants enrolled in the Chronic Renal Insufficiency Cohort (CRIC) built further on the foundations outlined above. The CRIC Study was initiated in 2001 by the National Institute for Diabetes Digestive and Kidney Disorders (NIDDK), and enrolled a national sample of patients with chronic kidney disease (CKD) examining traditional and nontraditional risk factors for CKD progression25, and associated cardiovascular diseases26. When the first measurements of carotid-femoral PWV were undertaken in 2003 in CRIC participants there was virtually no longitudinal data on the role of arterial stiffness in non-dialysis CKD patients. In the sections that follow, a brief reviews of pulse wave analysis and PWV are presented, followed by findings from the CRIC Study that associate arterial stiffness with outcomes in people with CKD.
The pulse waveform and why it differs depending on where you measure it
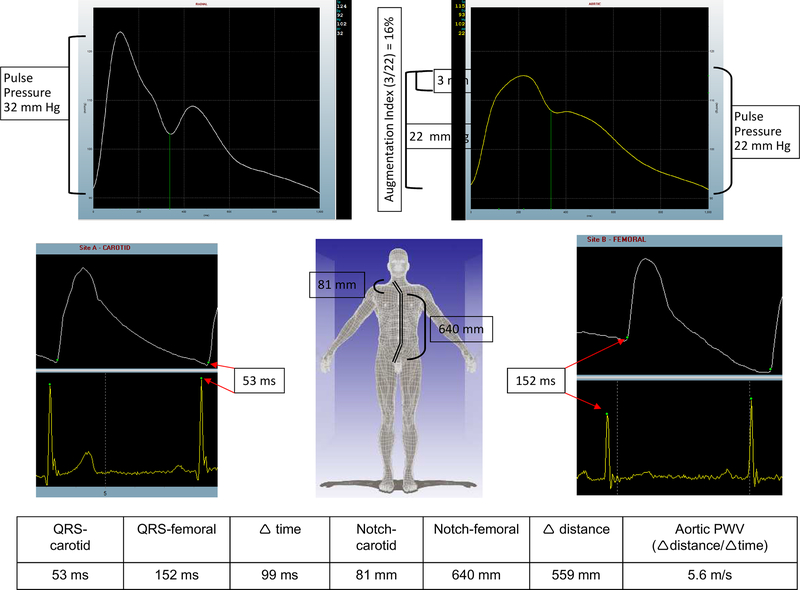
Figure 1 shows pulse waveforms from a CRIC participant obtained non-invasively at a clinical site. The upper portion consists of a radial pulse waveform obtained by using a Millar tonometer (AtcorMedical Sydney, AUSTR) in the supine position. The differences in pulse waveform morphology at the radial, proximal aorta, carotid, and femoral sites in this participant are evident. As the pressure wave enters the proximal aorta from the left ventricle, a number of forces shape its waveform as it travels throughout the circulation on a heartbeat by heartbeat basis. The major modulators are the heart rate, the inotropic state of the left ventricle, the velocity at which the pressure wave travels, changes in the transmission characteristics of vessels (which branch, bend, and change in their relative elastin to collagen concentration as well as their wall to lumen ratio), and the systemic vascular resistance. Several recent reviews provide in-depth physiology on how these forces shape the pressure wave in the human circulation. For our purposes it is enough to know that the pulse pressure changes as the pressure wave travels forward. In Figure 1 the pulse pressure is 22 mmHg at the level of the proximal aorta and 32 mmHg at the brachial artery level; the brachial value is used to calibrate the radial artery waveform by the software. The main characteristics incorporated into pulse wave analysis (PWA) are the proximal aortic pulse pressure, the magnitude of pulse pressure change determined by dividing the brachial pulse pressure by the aortic pulse pressure, and the augmentation index (see Figure 1 legend) that serves as a marker of the magnitude of the pressure wave reflected back into the proximal aorta. In health, there is a remarkable increase in the pulse pressure as the pressure wave travels peripherally in the circulation. In disease the proximal aortic pressures become more closely matched with the peripheral pulse pressure. Significant heterogeneity exists between people, and it is necessary to actually measure the shape of the radial (or brachial) pressure wave to get the best approximation of the central pressure profiles. A recent position paper from the American Society of Hypertension provides guidance on how to obtain and interpret pulse waveforms27.
Figure 1:
Pulse waveforms from a CRIC participant. In the left upper panel is a radial waveform. The software uses an internal algorithm to estimate the proximal aortic profile (upper right) using this radial waveform. The pulse pressure rose from 22 mmHg at the proximal aorta to 32 mmHg from the brachial blood pressure entered by the operator to calibrate the radial waveform (amplification is 32/22 = 1.45). In the proximal aortic profile, the effect of the returning pressure wave generates a 3 mmHg augmentation shown in the blue box area within the upper right waveform. Since the proximal aortic pulse pressure is 22 mmHg, the 3 mmHg represents an augmentation index of 3/22 or 16%. In the lower part of figure 1, on the left is a sample of the carotid waveform showing an elapsed time of 53 milliseconds from the tip of the QRS complex to the onset, or ‘foot’, of the carotid pressure wave upstroke. Similarly, in the right lower panel, the elapsed femoral time was 152 milliseconds. The notch-carotid and notch-femoral capture site distances are shown (in millimeters), and the pulse wave velocity was calculated as the distance (notch-carotid subtracted from notch-femoral = 559 mm) divided by the elapsed time (99 millisecond) to generate the 5.6 meters/second as shown.
The pulse wave velocity measurement
To perform a PWV measurement the participant should be supine for at least 10 minutes with a blood pressure entered into the software to calibrate the waveforms, and three electrocardiogram (ECG) electrodes attached to generate a rhythm strip. The distance from the sternal notch to the carotid pulse is measured in millimeters. The distance from the sternal notch to the umbilicus and then to the femoral pulse is also measured in millimeters. The sternal notch-to-carotid distance is subtracted from the sternal notch-to-femoral distance to calculate the length of the pressure wave path. Once the data are entered into the computer the operator captures 10 seconds of carotid waveform with a Millar tonometer and moves to the groin to capture 10 seconds of femoral waveform with the tonometer. Using the R wave onset from the ECG tracing as a tether point, the time elapsed to the onset of the carotid waveform (in milliseconds) is calculated and the velocity is a simply division of distance by time. Even though the measurements are in millimeters and milliseconds, PWV is expressed in meters per second (see lower half of Figure 1). The American Heart Association (AHA) has published a set of recommendations to standardize PWV measurements28.
Observations regarding arterial stiffness in the CRIC participants
Caveat lector
The reader should keep in mind that PWV represents both target organ damage in the aorta, and also a mediator of damage to other target organs like the brain, heart, and kidney. Like the kidney in hypertension, it is both villain and victim29. A more thorough discussion can be found in the AHA Science Statement28.
An early goal in the CRIC Study was to demonstrate the reproducibility of the PWV measurement and this was undertaken in 31 CRIC participants30. The mean differences in PWV between two operators, who made independent measurements on the same CRIC participant were 0.05 ± 1.13 m/s. In that study there was 1 observation that fell outside of 2 standard deviations of the differences in the PWV between the operators, thus, the reproducibility of PWV in CRIC was deemed acceptable.
Validation of known associations with PWV and novel observations in CRIC
In the cross-sectional analysis of our data we confirmed the known relationships of increasing age and blood pressure with PWV31. We also noted that PWV in diabetic participants were consistently higher, in every decade, compared to decade-matched non-diabetic participants. We made two other novel observations in our cohort. The first was a clear relationship of increasing arterial stiffness with declining kidney function, whether or not diabetes was present. Secondly, we noted a small but definite association of serum glucose concentrations with PWV in our cohort, independent of diabetic status.
One of the consequences of increased arterial stiffness is a return of the pressure wave to the heart while the aortic valve is still open. This places an extra “load” of pressure on the left ventricle before it finishes contracting for an individual heartbeat, at a time when there is virtually no blood flow in the myocardium. During the same visit when PWV was measured, CRIC participants also underwent pulse wave analysis to evaluate the pressure wave profile at the level of the proximal aorta. A growing body of literature suggests that central pulse pressures ≥ 50 mmHg are associated with more cardiovascular disease (CVD)32. In our participants we observed that diabetic participants with CKD had central pulse pressures that were shifted to the right compared with non-diabetic participants. When we divided our participants by NKF stage of kidney disease we observed that the portion of participants with elevated central pulse pressures increased with each worsening stage of CKD33. In participants with stage 2 disease 19% had a central pulse pressure > 50 mmHg compared with 44% of those with CKD stage 4.
PWV and proteinuria in CRIC
Early in the CRIC study we noted a positive correlation between level of 24h protein excretion and arterial stiffness. In a sample of 2144 diabetic CRIC participants with proteinuria data around the time of PWV measurement we observed that PWV independently associated with 24h urine protein excretion; this association was not seen in the non-diabetic participants34. In CRIC we have noted that the magnitude of the 24h urine protein excretion is the most significant predictor of future kidney function loss, thus, the additional predictive contribution of PWV in models already incorporating systolic blood pressure in diabetic CKD participants in CRIC further highlights the potential role of stiff vessels in CKD progression.
PWV and Mineral & Bone Disorder in CRIC
A growing body of evidence points to an increased CVD occurrence in patients with vascular calcification. A number of peptides reflect increased bone turnover and among these is osteoprotegerin, a protein in the tumor necrosis factor family that interferes with osteoclast function that appears to be important in the prediction of cardiovascular (CV) risk35. An ancillary study using CRIC PWV data initiated by the Hopkins site evaluated the relationship between osteoprotegerin and PWV in a subset of 226 CRIC participants who had measurements of both36. Increasing tertiles of osteoprotegerin levels were positively related to increasing PWV, even after multiple adjustments for demographics and other bone mineral density considerations. The mean PWV for the group was 9.3 m/s, and those in the highest osteoprotogerin tertile had a 30% higher PWV compared with those in the lowest tertile.
PWV and CKD progression in CRIC
The original mission of the CRIC study was to gather together a cohort of CKD patients from across the US large enough, and diverse enough, to evaluate factors important in further loss of kidney function, and to simultaneously identify mechanisms of CVD incidence and/or progression in CKD. When CRIC began, diabetes, hypertension, black race and proteinuria were the commonly recognized risk factors for CKD progression. The PWV ancillary study in CRIC hypothesized that arterial stiffness, independently of blood pressure, also contributed to further kidney function loss.
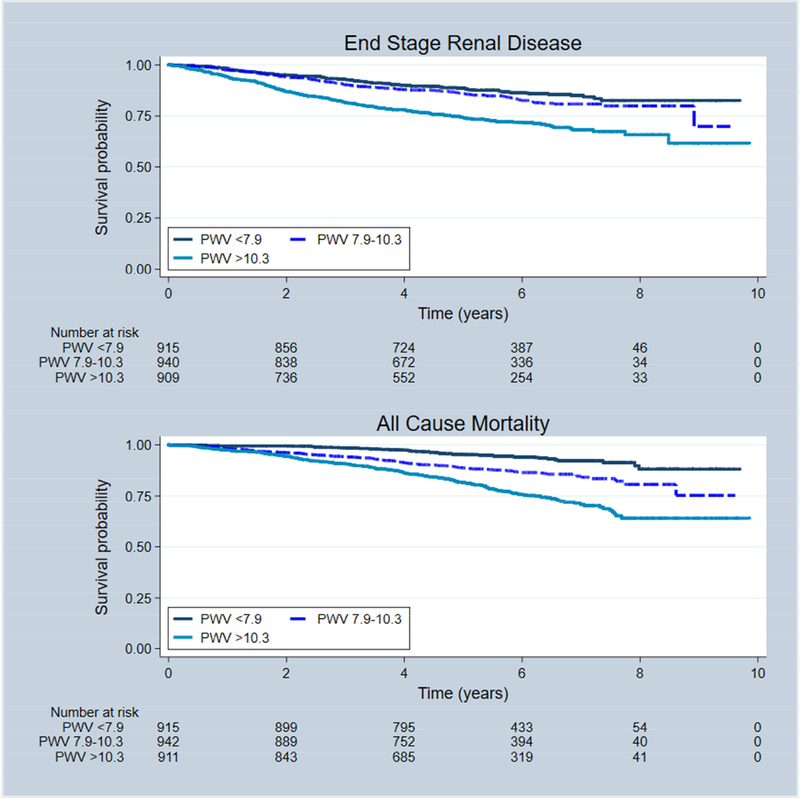
In CRIC we evaluate kidney function progression in two ways: either the development of end-stage renal disease (ESRD), or the time to halving of the initial estimated glomerular filtration rate (eGFR). We performed PWV measurements on 2795 CRIC participants and followed them for about 5 years to determine the relationship between PWV and CKD progression37. Using tertiles of PWV, where the lowest tertile was characterized by PWV < 7.7 m/s, and the highest tertile by values of 10.3 m/s, we observed a 37% increase in the hazard ratio for ESRD in the highest compared with the lowest tertile of PWV after adjustment for demographics, mean arterial blood pressure, diabetes, 24h urine protein excretion, and baseline eGFR. The hazard for all-cause death was 72% higher in the highest tertile compared with the lowest. Figure 2 depicts an unadjusted Kaplan-Meier plot of the outcomes of the outcomes of ESRD, halving of eGFR or ESRD, and death by PWV tertiles. The mechanism for these associations is likely related to the penetration of the energy within the pulse wave deeply into a low-resistance tissue like the kidney where the excess energy may be transmitted into pressure-sensitive tissue like the glomerulus, resulting in damage and loss of function4.
Figure 2:
Kaplan-Meier survival curves depicting the relationship between tertiles of PWV and the CRIC outcomes of ESRD and all-cause Death. Data from Townsend et al37.
PWV and Heart Failure in CRIC
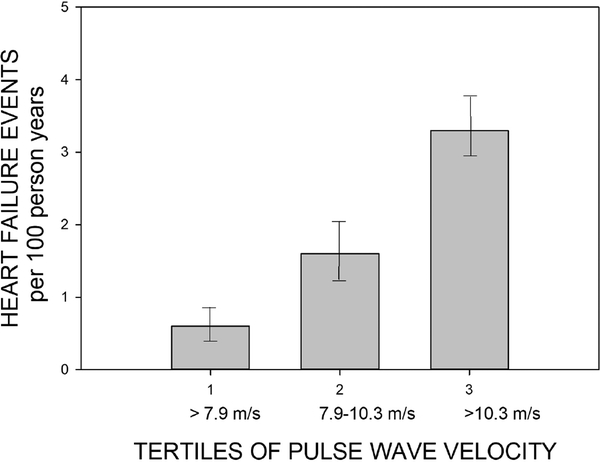
The most common non-fatal CV outcome in the CRIC study is hospitalized heart failure (HF). Enrollment criteria for CRIC excluded people with prior NYHA class III and IV heart failure25. We hypothesized that arterial stiffness would be a contributing factor to new-onset hospitalized HF in CRIC. We determined the effect of increasing arterial stiffness by evaluating 2602 participants in CRIC who were followed up for 3.5 years after their first PWV measurement, and we also determined the relationship of central pulse pressure on the HF outcome38. During that period 154 participants were hospitalized for their first occurrence of HF.
Using the lowest tertile of PWV (< 7.8 m/s) as referent, we observed a hazard ratio of 1.95 [95% CI, 0.92–4.13] for the middle tertile (7.8–10.3 m/s) and 3.01 [95% CI, 1.45–6.26] for the highest tertile (>10.3 m/s) in fully adjusted models (which included diabetes mellitus, proteinuria, the presence of chronic obstructive pulmonary disease, mean arterial pressure, heart rate, high-density lipoprotein-cholesterol, low-density lipoprotein-cholesterol, body mass index, triglycerides, history of myocardial infarction/revascularization, current smoking, hemoglobin, human recombinant erythropoietin use, angiotensin-converting enzyme inhibitor use, β-blocker use, calcium channel blocker use, history of hypertension, fasting glucose, and serum albumin). In that same fully adjusted model, using the lowest tertile of central pulse pressure (<35.4 mmHg) we observed a hazard ratio of 1.24 [95% CI, 0.58–2.65] for the middle tertile (35.4–50.8 mmHg) and 2.45 [95% CI, 1.14–5.27] for the highest tertile (>50.8 mmHg) of central pulse pressure. Figure 3 shows the event rate per 100 years of follow up stratified by PWV tertiles.
Figure 3:
Event rates per 100 person years for the outcome of hospitalized heart failure in the CRIC study by PWV Tertiles. Data from Chirinos et al38.
PWV and Masked Hypertension in CRIC
Masked hypertension describes a scenario in which blood pressure appears controlled in the office setting, yet exceeds thresholds considered normal on 24h ambulatory blood pressure monitoring (ABPM)39. This area of study is still somewhat nascent, and there is little guidance for how to manage patients with elevated outside office setting blood pressures. That said, there is clear evidence that masked hypertension confers almost as much risk as confirmed uncontrolled hypertension, where office and ABPM agree that blood pressure is above threshold levels of control40.
Using data on 1492 CRIC participants who underwent ABPM, we sought to determine what the prevalence of masked hypertension was in the CRIC population, and its relationship to arterial stiffness and left ventricular mass41. We used the definition of an office blood pressure <140/90 mmHg, measured carefully in triplicate by trained research coordinators using AHA standards at the CRIC visit where the ABPM was applied as controlled, and values > 130/80 mmHg on 24h ABPM as elevated. About half the CRIC population, 735 of 1492 participants, were controlled in the clinic setting, and by ABPM. We observed that 415 of 1492 participants, 28%, fit the criteria for masked hypertension (office <140/90 mmHg, 24h > 130/80 mmHg). Compared with the group controlled in the clinic and by 24h ABPM, the masked hypertension group had lower eGFR, more proteinuria, higher left ventricular mass, and a PWV that was ~1 m/s higher.
Changes in PWV over time in CRIC
Cohorts which have included serial PWV measurements, which are often repeated at 2 year intervals, show that for every decade of life the PWV increases by about 1 m/s42,43. In normotensive individuals it is a little less, and in hypertensive people a little more, and the overall shape of the changes in PWV with aging are somewhat convex-upward in graphic representations, indicating that the changes with aging accelerate with each decade of survival. In recent years, besides blood pressure itself, levels of circulating factors which are associated with inflammation have been implicated both cross-sectionally with arterial stiffness, and with the rate at which arteries stiffen further over time44,45. It has been suggested that the healing phase of inflammation deposits collagen, which is relatively inelastic and therefor requires more pressure to distend an artery when the collagen deposition site is in the vessel wall, leads to a higher PWV. In the CRIC study we evaluated the relationship between a panel of cytokines and proteins associated with inflammation cross-sectionally with PWV, and with the changes in PWV over 3 visits occurring during 4 years of follow up (measurements taken at time 0, 2, and 4 years)46.
We observed in 2933 CRIC participants with at least two PWV measurements, that in the adjusted cross-sectional analyses, fibrinogen and IL-10 were independently associated with PWV. In the longitudinal analysis, only serum albumin concentration was predictive of changes in PWV over time.
Summary
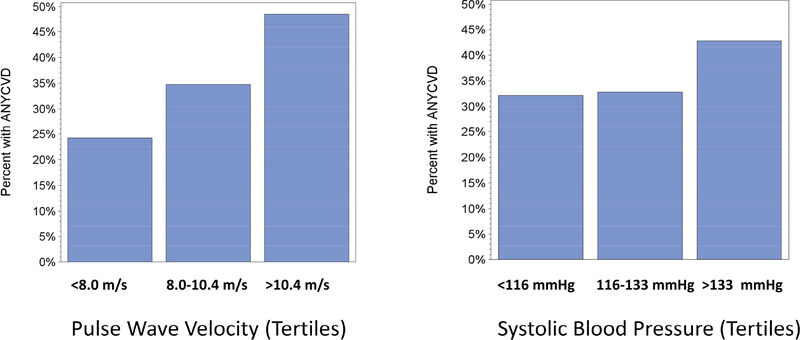
After more than a decade of studying arterial stiffness in a non-dialysis cohort of CKD patients several take home points are evident, as listed in Box 1. An emerging finding in both the CRIC Study, as well as other recent meta-analyses17,18, is that arterial stiffness measurements add predictive value to the office or clinic BP. Treatment of hypertension does not necessarily improve arterial stiffness. The vast majority of CRIC participants are on antihypertensive medications, and with reasonably good BP control47,48, yet the spectrum of arterial stiffness shows that about one-third of our cohort have PWV at or above 10 m/s, which is internationally considered to be the top limit of normal49. As depicted in Figure 4, from an early analysis of CRIC data, the relationship of systolic pressure at the time of CRIC enrollment was not as reflective of previous CVD as was the first PWV measurement. The degree of co-morbidity in patients with CKD, particular affecting the CV system, argues that there may be room for further improvement in CKD-related outcomes through interventions that de-stiffen the aorta. Discovering and testing agents that destiffen the aorta is a gap in our current knowledge. An additional gap is in the area of aortic calcium deposition. Calcified aortae are stiffer, but there is little we can do about decalcification, and we have no evidence to say that decalcification will actually be beneficial. One of the most important consequences of CKD, and ESRD, is the alarming incidence of sudden cardiac death50–52. Although not formally analyzed in the CRIC Study (yet), it may be that arterial stiffness is a predictor of sudden cardiac death, possibly because of either the long-term consequences of arterial stiffness predispose to left ventricular hypertrophy jeopardizing the endocardium to arrhythmia since it is vulnerable to the effects of increased afterload53 or the effects of arterial stiffness on baroreceptor dysfunction54. Future endeavors in the CRIC Study plan to focus more intensely on these aspects of CKD epidemiology. For now, PWV measures are still mainly used in research until clinical recommendations of what to do based on their measurement are formulated.
Box 1:
Take home messages regarding arterial stiffness in the CRIC Study
✓ Arterial stiffness is worse in diabetic compared with non-diabetic patients31
✓ Arterial stiffness worsens as kidney function declines irrespective of cause of CKD31
✓ Arterial stiffness is linked to proteinuria in diabetic patients with CKD34
✓ Arterial stiffness is associated with higher central pulse pressures in CKD33
✓ Arterial stiffness is linked to bone & mineral disorders36
✓ Arterial stiffness predicts death and CKD progression to ESRD37
✓ Arterial stiffness predicts new onset HF in CKD38
✓ Arterial stiffness is worse in CKD patients with masked hypertension41
CKD, chronic kidney disease, ESRD, end stage renal disease; HF, heart failure.
Figure 4:
Percentage of CRIC participants (Y Axis) who indicated the presence of ANY prior cardiovascular disease (heart failure, heart attack, stroke, peripheral arterial disease) broken down on the left panel by tertiles of PWV and broken down on the right panel by tertiles of systolic blood pressure in the CRIC study.
Acknowledgements:
Special appreciation is noted for the assistance provided by the CRIC coordinators, the CRIC Principal Investigators, NIDDK Program Officers, and especially for the CRIC participants.
Support: The PWV studies were funded by NIH grant DK-067390.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The author declares that he has no other relevant financial interests.
Peer Review: Received December 1, 2017 in response to an invitation from the journal. Evaluated by three external peer reviewers, with direct editorial input from an Associate Editor, who served as Acting Editor-in-Chief. Accepted in revised form April 5, 2018. The involvement of an Acting Editor-in-Chief was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
References
- 1.O’Rourke MF. From theory into practice: arterial haemodynamics in clinical hypertension. Journal of Hypertension. 2002;20(10):1901–1915. [DOI] [PubMed] [Google Scholar]
- 2.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 1: pressure and flow measurements and basic principles of wave conduction and reflection. Hypertension. 2010;56(4):555–562. [DOI] [PubMed] [Google Scholar]
- 3.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 2: arterial pressure-flow and pressure-volume relations in humans. Hypertension. 2010;56(4):563–570. [DOI] [PubMed] [Google Scholar]
- 4.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46(1):200–204. [DOI] [PubMed] [Google Scholar]
- 5.O’Rourke MF, Gallagher DE. Pulse wave analysis. JHypertensSuppl. 1996;14(5):S147–S157. [PubMed] [Google Scholar]
- 6.Riva-Rocci S, Zanchetti A, Mancia G. A new sphygmomanometer. Sphygmomanometric technique. J Hypertens. 1996;14(1):1–12. [PubMed] [Google Scholar]
- 7.Babbs CF. The origin of Korotkoff sounds and the accuracy of auscultatory blood pressure measurements. J Am Soc Hypertens. 2015;9(12):935–950 e933. [DOI] [PubMed] [Google Scholar]
- 8.Fisher JW. The diagnostic value of the sphygmomanometer in examinations for life insurance. JAMA. 1914(63):1752–1754. [Google Scholar]
- 9.Society of A Blood Pressure: Report of the Joint Committee on Mortality of the Association of Life Insurance Medical Directors and the Actuarial Society of America. New York, Society of Actuaries; 1925. [Google Scholar]
- 10.Bramwell JC, Hill A. Velocity of transmission of the pulse wave and elasticity of arteries. Lancet. 1922;199(5149):891–892. [Google Scholar]
- 11.Bramwell JC, Hill AV. The velocity of the pulse wave in man. ProcSocLond(Biol). 1922;93(652):298–306. [Google Scholar]
- 12.Wright JS, Cruickshank JK, Kontis S, Dore C, Gosling RG. Aortic compliance measured by non-invasive Doppler ultrasound: description of a method and its reproducibility. ClinSci(Lond). 1990;78(5):463–468. [DOI] [PubMed] [Google Scholar]
- 13.Liang YL, Teede H, Kotsopoulos D, et al. Non-invasive measurements of arterial structure and function: repeatability, interrelationships and trial sample size. ClinSci(Lond). 1998;95(6):669–679. [DOI] [PubMed] [Google Scholar]
- 14.Izzo JL Jr. Arterial stiffness and the systolic hypertension syndrome. CurrOpinCardiol. 2004;19(4):341–352. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell GF. Arterial stiffness and wave reflection: Biomarkers of cardiovascular risk. Artery Research. 2009;3(2):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNulty M, Mahmud A, Spiers P, Feely J. Collagen type-I degradation is related to arterial stiffness in hypertensive and normotensive subjects. JHumHypertens. 2006;20(11):867–873. [DOI] [PubMed] [Google Scholar]
- 17.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. JAmCollCardiol. 2010;55(13):1318–1327. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am CollCardiol. 2014;63(7):636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–1241. [DOI] [PubMed] [Google Scholar]
- 20.Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111(25):3384–3390. [DOI] [PubMed] [Google Scholar]
- 21.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106(16):2085–2090. [DOI] [PubMed] [Google Scholar]
- 22.London GM, Marchais SJ, Guerin AP. Arterial stiffness and function in end-stage renal disease. AdvChronicKidney Dis. 2004;11(2):202–209. [DOI] [PubMed] [Google Scholar]
- 23.Chirinos JA. Arterial stiffness: basic concepts and measurement techniques. J CardiovascTranslRes. 2012;5(3):243–255. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell GF, Pfeffer JM, Pfeffer MA. The heart and conduit vessels in hypertension. Medical Clinics of North America. 1997;81(6):1247–1270. [DOI] [PubMed] [Google Scholar]
- 25.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. Journal of the American Society of Nephrology. 2003;14 (7 Supple 2):S148–S153. [DOI] [PubMed] [Google Scholar]
- 26.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. NEnglJMed. 2004;351(13):1296–1305. [DOI] [PubMed] [Google Scholar]
- 27.Townsend RR, Black HR, Chirinos JA, et al. Clinical Use of Pulse Wave Analysis: Proceedings From a Symposium Sponsored by North American Artery. J ClinHypertens(Greenwich). 2015;17(7):503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension. 2015;66(3):698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klahr S The kidney in hypertension--villain and victim. NEnglJMed. 1989;320(11):731–733. [DOI] [PubMed] [Google Scholar]
- 30.Wimmer NJ, Townsend RR, Joffee MM, Lash JP, Go AS, Investigators CS. Correlation between pulse wave velocity and other measures of arterial stiffness in chronic kidney disease. Clinical Nephrology. 2007;68(3):133–143. [PubMed] [Google Scholar]
- 31.Townsend RR, Wimmer NJ, Chirinos JA, et al. Aortic PWV in chronic kidney disease: a CRIC ancillary study. AmJHypertens. 2010;23(3):282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50(1):197–203. [DOI] [PubMed] [Google Scholar]
- 33.Townsend RR, Chirinos JA, Parsa A, et al. Central Pulse Pressure in Chronic Kidney Disease. A Chronic Renal Insufficiency Cohort Ancillary Study. Hypertension. 2010;56(3):518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weir MR, Townsend RR, Fink JC, et al. Hemodynamic correlates of proteinuria in chronic kidney disease. ClinJAmSocNephrol. 2011;6(10):2403–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blazquez-Medela AM, Garcia-Ortiz L, Gomez-Marcos MA, et al. Osteoprotegerin is associated with cardiovascular risk in hypertension and/or diabetes. Eur J Clin Invest. 2012;42(5):548–556. [DOI] [PubMed] [Google Scholar]
- 36.Scialla JJ, Leonard MB, Townsend RR, et al. Correlates of Osteoprotegerin and Association with Aortic Pulse Wave Velocity in Patients with Chronic Kidney Disease. ClinJAmSocNephrol. 2011;6(11):2612–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Townsend RR, Anderson AH, Chirinos JA, et al. Association of pulse wave velocity with chronic kidney disease progression and mortality: Findings from the CRIC study. Hypertension. Epublished April 30, 2018. 10.1161/HYPERTENSIONAHA.117.10648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chirinos JA, Khan AM, Bansal N, et al. Arterial Stiffness, Central Pressures and Incident Hospitalized Heart Failure in the Chronic Renal Insufficiency Cohort (CRIC) Study. CircHeart Fail. 2014;7(5):709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickering TG, Davidson K, Gerin W, Schwartz JE. Masked hypertension. Hypertension. 2002;40(6):795–796. [DOI] [PubMed] [Google Scholar]
- 40.Franklin SS, O’Brien E, Staessen JA. Masked hypertension: understanding its complexity. Eur Heart J. 2017;38(15):1112–1118. [DOI] [PubMed] [Google Scholar]
- 41.Drawz PE, Alper AB, Anderson AH, et al. Masked Hypertension and Elevated Nighttime Blood Pressure in CKD: Prevalence and Association with Target Organ Damage. Clin J Am Soc Nephrol. 2016;11(4):642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reference Values for Arterial Stiffness Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. EurHeartJ. 2010;31(19):2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alghatrif M, Strait JB, Morrell CH, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62(5):934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blacher J, Agnoletti D, Protogerou AD, et al. Aortic stiffness, inflammation, denutrition and prognosis in the oldest people. J HumHypertens. 2012;26(9):518–524. [DOI] [PubMed] [Google Scholar]
- 45.Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei MedJ. 2012;53(2):258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peyster E, Chen J, Feldman HI, et al. Inflammation and Arterial Stiffness in Chronic Kidney Disease: Findings From the CRIC Study. Am J Hypertens. 2017;30(4):400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muntner P, Anderson A, Charleston J, et al. Hypertension Awareness, Treatment, and Control in Adults with Chronic Kidney Disease: Results from the Chronic Renal Insufficiency Cohort (CRIC) Study. American Journal of Kidney Diseases. 2009;55(3):441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. ClinJAmSocNephrol. 2009;4(8):1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. Journal of Hypertension. 2012;30(3):445–448. [DOI] [PubMed] [Google Scholar]
- 50.Deo R, Lin F, Vittinghoff E, Tseng ZH, Hulley SB, Shlipak MG. Kidney dysfunction and sudden cardiac death among women with coronary heart disease. Hypertension. 2008;51(6):1578–1582. [DOI] [PubMed] [Google Scholar]
- 51.Makar MS, Pun PH. Sudden Cardiac Death Among Hemodialysis Patients. Am J Kidney Dis. 2017;69(5):684–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki T, Agarwal SK, Deo R, et al. Kidney function and sudden cardiac death in the community: The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2016;180:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim D, Shim CY, Hong GR, et al. Differences in left ventricular functional adaptation to arterial stiffness and neurohormonal activation in patients with hypertension: a study with two-dimensional layer-specific speckle tracking echocardiography. Clin Hypertens. 2017;23:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okada Y, Galbreath MM, Shibata S, et al. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension. 2012;59(1):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Wellcome Collection. Dudgeon’s Sphygmograph. https://wellcomecollection.org/works/ecu45qez Accessed April 29, 2018.