Abstract
Progranulin (PGRN), a widely expressed glycoprotein with pleiotropic function, has been linked to a host of physiological processes and diverse pathological states. Currently, various therapeutic strategies targeting PGRN have been developed in a series of preclinical models of diseases and clinical trials, highlighting PGRN as a promising target in diseases treatment. Herein we summarize available knowledge of PGRN targeting in various kinds of diseases, including common neurological diseases, inflammatory autoimmune diseases, cancer, tissue repair, and rare lysosomal storage diseases, with the focus on the functional domain-oriented drug development strategies. In particular, we emphasize the potential role of PGRN as a non-conventional, extracellular matrix bound, growth factor-like conductor orchestrating multiple membrane receptors and simultaneously as an intracellular co-chaperone.
Keywords: Progranulin, Membrane Receptors, Inflammation, Autoimmune Diseases, Neurodegenerative Diseases, Cancer, Lysosomal Storage Diseases
1. Introduction
Progranulin (PGRN), also known as granulin-epithelin precursor (GEP), proepithelin (PEPI), acrogranin, GP88 and PC-cell-derived growth factor (PCDGF), was first identified as a 593-aa secreted glycoprotein involved in the regulation of cancer progression and wound healing [1–5]. As a broadly expressed and functionally pleiotropic protein, PGRN has been considered a growth factor-like molecule implicated in additional biological and pathological processes including early embryogenesis [6], inflammation [7], host defense [8], cartilage development and degradation [9]. PGRN also functions as a neurotrophic factor [10, 11], and the heterozygous and homozygous mutations of its encoding gene Grn are associated with frontotemporal dementia (FTD) and lysosomal storage diseases (LSDs) [12–18].
Currently, various PGRN-targeted approaches are emerging as attractive therapeutic interventions in a broad spectrum of diseases including cancer [11], inflammatory diseases [19–24], neurological disorders [25], injury [26], tissue regeneration [27–29], and some rare diseases such as lysosomal diseases [30]. Multiple PGRN targeting strategies such as small molecule compounds that boost PGRN expression, viral vectors or mesenchymal stem cells (MSCs) delivering Grn genes, engineered full length proteins or domains, monoclonal neutralizing antibodies, and 3D-printed scaffold-incorporated recombinant proteins have been developed, and over 30 preclinical and clinical trials in this field have been undertaken (Table 1).
Table 1.
Targeting PGRN Strategies in Various Diseases
| Diseases | Subjects | Targeting strategy | Outcome | References |
|---|---|---|---|---|
| Frontotemporal dementia (FTD) | Lymphoblasts from patients; PGRN deficient SH-SY5Y neuroblastoma cells | Compound enhancing PGRN expression, SAHA | Inhibit the cytosolic TDP-43 accumulation | [56] |
| Frontotemporal dementia | Patients in a phase 2 clinical trial, (NCT02149160) | Compound enhancing PGRN expression, FRM-0334 | Unavailable | [57] |
| Frontotemporal dementia | Patients in a phase 1 clinical trial, (NCT01835665) | Compound enhancing PGRN expression, nimodipine | Unavailable | [57] |
| Frontotemporal dementia | Patients in a pilot study | Compound enhancing PGRN expression, amiodarone | No effect | [59] |
| Frontotemporal dementia | Organotypic cortical slice cultures from Grn deficient mice; Primary cells derived from human patients | Compound enhancing PGRN expression, clinically used alkalizing drugs reagents (chloroquine, bepridil, and amiodarone) | Rescue Grn deficiency | [58] |
| Frontotemporal dementia | Grn +/− mice | Adeno-associated virus vector delivering Grn gene | Correct restored social behavior deficits and normalized lysosomal abnormalities | [60] |
| Frontotemporal dementia; Neuronal ceroid lipofuscinosis (NCL) | Grn +/− mice | Adeno-associated virus vector delivering Grn gene | Reduced lipofuscinosis, microgliosis, and improved lysosomal function | [61] |
| Parkinson’s disease | MPTP induced mice model | Lentiviral delivery of the Grn gene | Reduced inflammation and apoptosis status; Preserved both dopamine content and locomotor function | [62] |
| Alzheimer’s disease | Alzheimer’s disease mice | Lentiviral delivery of the Grn gene | Lowered plaque load and prevent spatial memory deficits and hippocampal neuronal loss | [64] |
| Huntington’s disease | Caenorhabditis elegans model | Plasmid expressing human PGRN | Reduced polyglutamine toxicity by TDP-43 | [65] |
| Subarachnoid hemorrhage (SAH) | Experimental SAH in rats | rPGRN | Alleviates early brain injury after SAH | [66] |
| Traumatic brain injury (TBI) | Mice model of controlled cortical impact (CCI) | rPGRN | Intracerebroventricular administration prevented brain damage and neurological deficits | [23] |
| Stroke | Mice following middle cerebral artery occlusion (MCAo) | Lentiviral mediated Grn gene delivery | Decreased infarcted tissue damage and improved post-ischemic neurological functions | [67] |
| Stroke | MCAO (middle cerebral artery occlusion) | rPGRN | Intra-cerebroventricular administered reduced the infarct volume, decreased brain swelling, and improved neurological scores and survival rare | [68] |
| Stroke | Rat autologous thrombo- embolic model | rPGRN | Intravenously administered recombinant progranulin reduced cerebral infarct and oedema, suppressed haemorrhagic transformation, and improved motor outcomes. | [69] |
| Inflammatory arthritis | Collagen antibody-induced, collaben-induced arthritis and TNF-α transgenic mouse models | rPGRN; Attstrin | Both agents effectively inhibited the progression of inflammatory arthritis | [7] |
| Osteoarthritis | Surgically induced OA models | rPGRN | Significantly attenuated OA-like phenotypes and protected against its progression | [73] |
| Osteoarthritis | Non-surgically induced rat; surgically induced murine OA models | Atsttrin | Exhibited a preventative effect | [74] |
| Osteoarthritis | Surgically induced OA mouse model | Atsttrin-transduced mesenchymal stem cells (MSCs) articular treatment | Preventive effect on the progression of degenerative changes | [75] |
| Inflammatory bowel disease (IBD) | DSS and TNBS colitis models | rPGRN | Reduced the histological score, colonic hyperplasia and leukocyte infiltration | [76] |
| Myocarditis | coxsackievirus-B3-induced myocarditis in mice | rPGRN | Attenuated phenotypes by downregulating Th1 and Th17 cells, but no effect on Treg cells. | [77] |
| Allergic asthma | Antigen-challenged mouse allergic asthma | rPGRN | Intranasal pretreatments inhibited bronchial smooth muscle hyperresponsiveness | [78] |
| Dermatitis | oxazolone-induced mice model | Atsttrin | Effectively attenuated inflammation | [79] |
| Hyperhomocysteinemia (hHcys) | Uninephrectomy and folate-free diet induced hHcys mice model | Recombinant PGRN | Protected against cardiorenal dysfunction | [80] |
| Immune thrombocytopenia (ITP) | Anti-CD41 platelet antibody-induced mice ITP model Antibody- and CD8+ T cell-mediated mice ITP model |
Recombinant PGRN | Increased platelet count; Promoted Treg cells Proliferation |
[81] |
| Endo-toxic shock | Lipopolysaccharide induced model | rPGRN | Pretreatments ameliorated the survival and abnormalities | [82] |
| Lung injury | LPS-induced severe acute lung injury in mice. | rPGRN | Effectively reduced lung injury | [83] |
| Gaucher disease | OVA-challenged, PGRN-deficient animal models; D409V/- GD mice; Human fibroblasts from GD patients | rPGRN; Pcgin | Stabilized and increased the levels of GCase, reduced the pathological severity of GD models, and inhibited the accumulation of glycolipids, including β-GlcCer. | [25] |
| Tay-Sachs disease (TSD) | Aged or ovalbumin-challenged adult PGRN-deficient mice models with typical TSD phenotypes | rPGRN; Pcgin | Reduced GM2 accumulation and lysosomal storage | [18] |
| Hepatocellular carcinoma (HCC) | A nude mice model transplanted with human HCC | PGRN monoclonal antibody | Inhibited the growth of established tumors in a dose-dependent manner but without inhibitory effect on normal liver cells | [100] |
| Hepatocellular carcinoma (HCC) | A nude mice model transplanted with human HCC | PGRN monoclonal antibody | Sensitize chemotherapeutic agents-induced apoptosis | [102] |
| Bone defect | Segmental femoral bone defect model; Femoral drill-hole model; Nonunion segmental radial defect model; BMP-2-induced ectopic bone formation model | rPGRN | Enhanced bone regeneration | [104] |
| Inflammatory periodontal bone defect | Periodontal bone defects in periodontitis rats | rPGRN | Had significantly superior quantity and quality of newly formed bone, inhibited osteoclastogenesis and inflammation | [105] |
| Osteolysis | Titanium particles stimulated the mouse air pouch model; Two mouse osteoysis models | rPGRN | Inhibited inflammation and prevented the pathological progression | [106] |
| Bone defects | Mice calvarial bone defects model | 3D-printed Atsttrin scaffold incorporated to alginate/hydroxyapatite | Enhanced the regeneration of bone defects | [24] |
In this review, we will summarize PGRN-centered therapeutic approaches in all diseases and conditions reported, along with an illustration of its multifaceted roles in related pathological processes. It is noted that the role of PGRN and the underlying mechanisms in neurological diseases, cancer, inflammatory autoimmune diseases, and lysosomal storage diseases have been well reviewed, usually with a focus on a certain disease type [25, 31–36]. Therefore, the present review specifically focuses on contemporary efforts to develop potential PGRN-targeting methods and evaluation of drug candidates in preclinical models or clinical trials for potential application in treatment of an array of common and rare diseases.
2. Domain-dependent interactions of PGRN with its partners
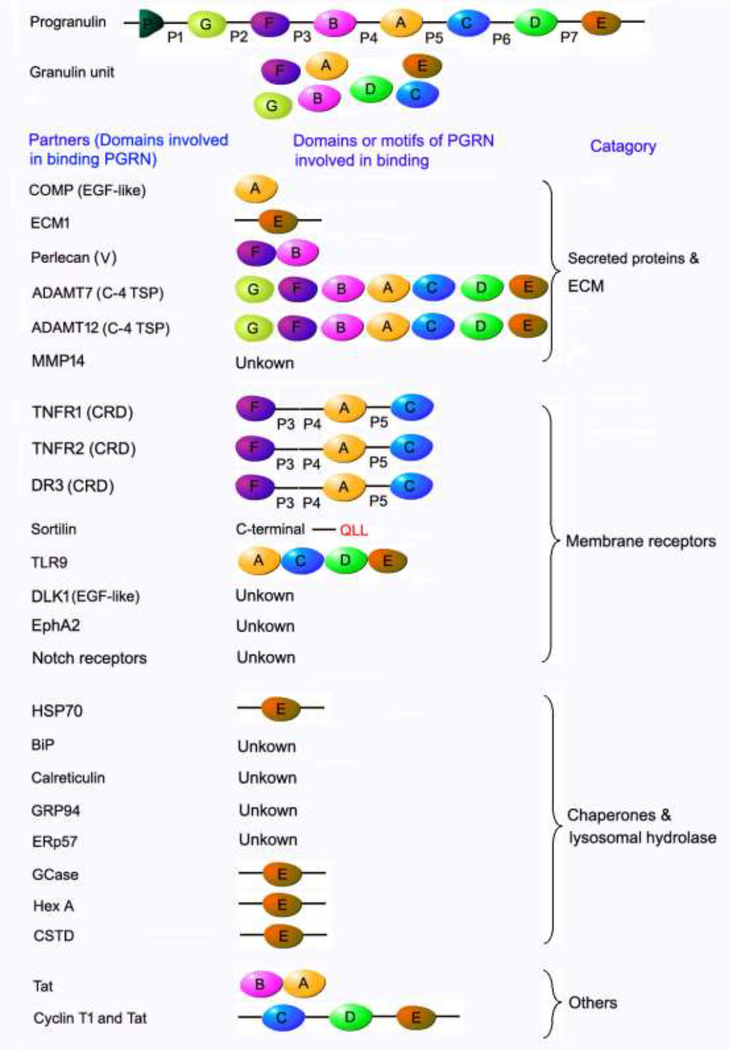
The functional pluripotency of PGRN lies in its domain or motif-dependent interactions with different partner proteins (Figure 1). Full length PGRN contains 7½ tandem, non-identical repeats of a cysteine-rich motif (CX5–6CX5CCX8CCX6C CXDX2HCCPX4CX5–6C, X: any amino acid) separated by 7 linker regions (P1-P7) in the order P-G-F-B-A-C-D-E, where A-G are full repeats, and P is the 1/2 motif [2, 37–39].
Figure 1. Summary of the domains of PGRN known to be involved in the interactions of PGRN with its binding partners.
PGRN possesses a high plasticity to bind to a wide spectrum of ECM proteins, membranous receptors and cytoplasmic chaperone and lysosomal hydrolases due to its unique beads-on-a-string structure and multiple binding domains. The domains known to be involved in PGRN and binding partner interactions are indicated. The cysteine rich domain (CRD) of TNFR and DR3 has been experimentally demonstrated to be required for interactions of these receptors with PGRN. Interestingly, a CRD is also present in the extracellular domains of Sortlin, EphA2, Notch receptors and TLR9. In addition, the extracellular domains of both Dlk1 and Notch receptors contain EGF-like domains, which is also known to bind to PGRN. It is expected that CRD and EGF-like domains are probably involved in the interactions of these aforementioned receptors with PGRN, although these associations need to be experimentally validated. “unknown” indicates that the binding domain(s) in PGRN remains to be determined.
PGRN can be secreted, cytoplasmic, and is also abundant in extracellular matrix (ECM). PGRN directly binds to some other ECM components such as cartilage oligomeric matrix protein (COMP) (with A domain, involved in regulating PGRN-induced proliferation) [9], extracellular matrix protein 1 (ECM1) [40], perlecan (with granluin FB, involved in the promotion of tumor growth [41]), and several ECM proteases ADAM metallopeptidase with thrombospondin type 1 motif 7 (ADAMTS7) and ADAM metallopeptidase with thrombospondin type 1 motif 12 (ADAMTS12) with each granulin unit [42–45]. PGRN can be subject to degradation into individual, approximately 6-kDa GRN fragments by these binding enzymes and other various proteinases, such as elastease and protease 3 [46]. Importantly, these degraded GRN fragments are also biologically active, but often demonstrate opposite actions to the full-length protein; for example, GRN fragments act as pro-inflammatory factors that can neutralize the intact PGRN’s anti-inflammatory activities [4]. The secretory leucocyte protease inhibitor (SLPI) can bind at sequences between granulin modules to protect full length PGRN from proteolysis by elastase and other proteases [4].
Half units of granulins A, C, and F plus linkers P3, P4, and P5 are responsible for the interaction between PGRN and the CRD2 and CRD3 domains of tumor necrosis factor receptor 1 (TNFR1) and tumor necrosis factor receptor 2 (TNFR2) [7, 47–49]. An engineered recombinant protein was created from this “minimal” FAC domain, referred to as Atsttrin (antagonist of TNF/TNFR signaling via targeting to TNF receptors), which exhibits higher binding affinity for TNFR2, but lower affinity for TNFR1 than TNF-α [7, 47, 49, 50]. In addition to TNFR, through FAC domains, PGRN and Atsttrin also bind to death receptor 3 (DR3), the highest homolog of TNFR1, and effectively inhibit the interaction of DR3 to its only known ligand TNF-like ligand 1A (TL1A) [48, 51]. Other domain-dependent PGRN binding membrane receptors include Toll-like receptor 9 (TLR9), interaction with granulin ACDE assists CpG binding to TLR9 [52], and sortilin, which associates with the last three amino acids QLL at C-terminal of PGRN to deliver PGRN to lysosome [53]. Notch receptors and EPH receptor A2 (EphA2) also demonstrated to be PGRN binding partners [54, 55]; however, whether the interaction is direct or indirect and the binding domains involved remain to be determined.
PGRN has been shown to function as a chaperone of lysosomal enzymes together with heat-shock protein 70 (HSP 70), and its granulin G and E domains are required for the binding to lysosomal hydrolase glucocerebrosidase (GCase) [17, 56], β-hexosaminidase A (HexA) [18], cathepsin D (CSTD) [57] and chaperone HSP70 [56]. Pcgin, a 98 amino acid engineered PGRN derivative, bears the PGRN C-terminal granulin E domain and is sufficient for effective binding to lysosomal enzymes and Hsp70, but lacks PGRN’s oncogenic activity [56].
Meanwhile, there are several domain-dependent interactions, such as HIV-1 Tat (trans-activator protein) with granulin BA or CDE, nuclear Cyclin T with granluin CDE [58–61], although the binding has been confirmed, the significance of these interactions is still unclear.
Although various PGRN-binding partners have been reported, the majority of PGRN associated proteins can be classed into three categories (Figure 1): secreted and ECM molecules (e.g. COMP [9], ECM1 [40], perlecan [41], ADAMTS-7 [42], ADAMTS-12[42] and matrix metalloproteinase 14 [MMP14] [62]), cell transmembrane receptors (e.g. TNFR1 [7, 47–49, 63], TNFR2 [7, 47–49, 63], DR3 [47–49], sortilin[53], Toll-like receptor 9 [TLR9][52], Notch receptors[54], and EphA2 [55]), and intracellular chaperones (e.g. BiP [64], calreticulin[64], GRP94 [64], ERp57[64], HSP70 [56]) and lysosomal hydrolases through which PGRN acts as a co-chaperone of HSP70 (e.g. lysosomal hydrolase GCase [17, 56], HexA [18], CSTD [57]).
In terms of the interactions between PGRN and cell membrane receptors, a structural comparison of extracellular domains revealed that the extracellular domains of all of these reported PGRN-binding receptors contain either cysteine rich domain (CRD) or EGF-like domain or both, which are known PGRN-binding domains [7, 9, 47, 48, 65]. Specifically, the extracellular domains of TNFR1, TNFR2, DR3, Sortlin, EphA2, TLR9 have CRD, the extracellular domain of Dlk1 has EGF-like domain, whereas Notch receptors posses both CRD and EGF-like domains. So far, only the CRDs of TNFR and DR3 have been confirmed to be required for interactions with PGRN [47–49]. We expect that the CRD in Sortlin, EphA2, TLR9 and Notch receptors and the EGF-like domain in Dlk1 and Notch receptors are likely also involved in their interactions with PGRN, although these associations need to be experimentally validated. In brief, the extracellular domains of currently reported PGRN receptors share domain/structure similarities, although these receptors are functionally different and belong to various receptors families.
3. PGRN targeting in neurological diseases
3.1. Neurodegenerative diseases
Decreased PGRN level due to heterozygous mutation of GRN gene mutations is the major cause of FTD-TDP, a subtype of FTD characterized by ubiquitinated and fragmented TDP-43 proteinopathy. Developing small molecule drug modifiers to restore the reduced expression of PGRN to its normal levels is becoming a promising avenue for the treatment of FTD-TDP [25, 66]. Suberoylanilide hydroxamic acid (vorinostat, SAHA), a histone deacetylase (HDAC) inhibitor approved for use in cancer treatment, was identified as the first potent inducer of PGRN expression in a screen of FDA-approved compounds [67]. SAHA enhanced PGRN levels at both mRNA and protein levels in haploinsufficient cells, and combined with ERK1/2 blocker selumetinib, SAHA could significantly inhibit cytosolic TDP-43 accumulation [67]. Currently, another HDAC inhibitor - FRM-0334, is in phase 2 clinical trials for amelioration of PGRN insufficiency resultant of GRN gene mutations [68]. Compared with SAHA, FRM-0334 can more easily cross the blood-brain barrier [68]. Except for HDAC inhibitors, channel blocker nimodipine, vacuolar ATPase inhibitors (bafilomycin A1, concanamycin A, archazolid B, and apicularen A), clinically used alkalizing drugs (chloroquine, bepridil, and amiodarone), and an mTOR-independent autophagy activator trehalose are also potential pharmacological stimulators of PGRN production [69]. Among them, nimodipine is in a phase 1 trial as a PGRN-elevating drug, however, a pilot study evaluating the effect of amiodarone on PGRN rescue in FTD-Grn patients failed to demonstrate a definite therapeutic result [70].
PGRN gene therapy is an alternate rational therapeutic strategy for neurodegenerative disorders due to Grn mutations. Arrant et al. observed that adeno-associated virus (AAV) vector delivery of Grn gene to the medial prefrontal cortex could correct social behavior deficits and normalize lysosomal abnormalities in Grn +/− mice [71]. They further extended the gene therapy approach into Grn−/− mice, which model aspects of neuronal ceroid lipofuscinosis (NCL) and FTD, and found that PGRN replacement specifically targeted neurons, reduced lipofuscinosis, microgliosis, and improved lysosomal function even at low doses [72].
As a potent regulator of neuro-inflammation and an autocrine neurotrophic factor, PGRN is also important for the long-term neuronal survival, and increased availability in the brain may have therapeutic benefits in neurodegenerative diseases other than FTD and NCL. Van Kampen et al. found that lentiviral delivery of the Grn gene could increase the PGRN expression level in nigrostriatal neurons accompanied by reduced inflammation and apoptosis in a 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced murine model of Parkinson’s disease [73]. Meanwhile, PGRN gene therapy also preserved both dopamine content and locomotor function [73]. Grn polymorphism may be associated with the late-onset Alzheimer’s disease [74] and, functionally, PGRN inhibits amyloid β (Aβ) deposition and protects against Aβ toxicity [75]. Lentivirus-mediated PGRN overexpression lowered plaque load and prevented spatial memory deficits and hippocampal neuronal loss in Alzheimer’s disease mice [75]. Therefore, these data supports the efficacy of PGRN-expressing gene therapy for neurodegenerative pathologies. A further study suggests that PGRN may have therapeutic benefits in a Caenorhabditis elegans model of Huntington’s disease by interacting with TDP-43 to regulate polyglutamine toxicity [76].
3.2. Nervous system injuries
In addition to neurodegenerative diseases, PGRN may also act as a treatment target for neurological injury. The PGRN level is significantly decreased in cerebrospinal fluid from subarachnoid hemorrhage (SAH) patients and cerebral cortex tissues after experimental SAH in rats. Recombinant PGRN (rPGRN) administration alleviated early brain injury in SAH rats, possibly by inhibiting neutrophil recruitment and the activity of inflammatory cytokines [77]. Another study indicated that intra-cerebroventricular administration of rPGRN could prevent traumatic injury induced brain damage and neurological deficits, partly due to its inhibitory effect on inflammatory factors such as TNF-α and iNOS [26]. The data from transgenic Grn mice or Grn−/− mice also support a protective benefit of PGRN on peripheral nerve regeneration and re-innervation [54]; however, the therapeutic effect of rPGRN strategies in peripheral nerve injury has not been reported.
3.3. Stroke
Several lines of evidences support therapeutic potential for PGRN in experimental acute ischemic stroke by multiple mechanisms including suppression of neuro-inflammation and neuro-protection, attenuation of ischemia-reperfusion and reduction of blood-brain barrier disruption. Several PGRN targeting strategies, including lentiviral mediated Grn gene delivery, intra-cerebroventricular and intravenously administered rPGRN protein with and without tissue plasminogen activator, have revealed the consistent and significant therapeutic effects of PGRN on infarcted tissue damage reduction and improvements in post-ischemic neurological functions [78–80].
4. PGRN targeting in autoimmune and inflammatory diseases
4.1. Inflammatory arthritis
Tang et al. demonstrated that rPGRN and Atsttrin, an engineered PGRN derivative containing the minimal TNFR binding motifs, significantly alleviated the disease severity in collagen antibody-induced, collagen-induced and TNF-α transgenic inflammatory arthritis mouse models [7, 48, 49, 81]. Both agents effectively inhibited the progression of inflammatory arthritis, and inflammatory phenotypes returned following the cessation of Atsttrin treatment [7]. Additionally, PGRN and Atsttrin treatment also have decreased circulating levels of fragmentary COMP, a marker for cartilage destruction [7].
Generally, Atsttrin exhibits potent anti-inflammatory activity, which surpasses PGRN in vivo [7]. Compared to TNFα, Atsttrin exhibited a higher (10-fold) binding affinity for TNFR2, but lower (18-fold) affinity for TNFR1, and reversed the clinical scores of inflammatory arthritis mouse models to normal baseline level [7]. Pharmacokinetically, Atsttrin was well absorbed following intraperitoneal administration and demonstrated high stability with a significantly longer half-life (~120 h) when compared to PGRN (~40 h) [7]. More importantly, Atsttrin lacks other multiple functions of the source protein, particularly PGRN’s oncogenic activity. Additionally, the composition of Atsttrin allows it to escape digestion into individual granulin units. No Atsttrin-related cytotoxic effects or lethality was observed even at exceedingly high dosages [7].
Compared with current TNF-α inhibitors, Atsttrin has a unique mechanism of action targeting TNFR, but not TNF-α [19, 20]. Mechanistically, the anti-inflammatory effects of Atsttrin mostly depend on its direct activation of TNFR2 protective anti-inflammatory pathway, for example, through regulating the functions and differentiation of Treg cells in a TNFR2-dependent manner [82], therefore, the patients who fail to respond to current TNFα blockers may benefit from Atsttrin treatment. In several inflammatory arthritis mice models, Atsttrin also demonstrated more efficacious results than the current TNF-α inhibitors etanercept and adalimumab [7]. In addition, unlike current TNF-α inhibitors, Atsttrin does not increase cancer incidence. Moreover, Attstrin may act has a tumor suppressor and has potential for treating cancers that feature high PGRN expression [19].
4.2. Osteoarthritis (OA)
Osteoarthritis (OA) is the most common type of degenerative arthritis, and is also currently accepted as a chronic mild inflammatory joint disease [83–85]. It is believed that inflammatory cytokines, including TNF-α and IL-1β, play important roles in the pathogenesis of OA. PGRN has also been shown to be a potential target for the treatment of OA. Zhao et al. found that intra-articular injection of rPGRN protein significantly attenuated OA-like phenotypes and protected against its progression in surgically induced OA models [86]. This therapeutic effect is primarily reliant upon the stimulation of TNFR2-Akt-Erk1/2-dependent chondrocyte anabolism and inhibition of TNF-α/TNFR1-mediated inflammatory catabolism [86]. Wei et al. further extended the investigation to Atsttrin, revealing a preventative effect of the PGRN-derivative in OA in both non-surgically induced rat and surgically induced mouse OA models [87]. In addition, Xia et al. applied Atsttrin-transduced mesenchymal stem cells (MSCs) to deliver Atsttrin to the articular joint and demonstrated a similar preventive effect on the progression of degenerative changes in the surgically induced OA mouse model, providing an alternative cell-based delivery strategy to supply PGRN or Atsttrin into disease sites. [88].
4.3. Inflammatory bowel disease (IBD)
Wei et al. revealed that rPGRN ameliorated the pathology and reduced the histological score in both Dextran sulfate sodium (DSS)- and picrylsulfonic acid (TNBS)-induced colitis models [89]. Similar to results from models of inflammatory arthritis and OA, the PGRN-mediated protective action in IBD is also TNFR2-dependent and anti-inflammatory IL-10 signaling is required as well [89]. PGRN treatment significantly increased the IL-10 release in colonic explants from DSS colitis mice; colonic hyperplasia and leukocyte infiltration were also reduced [89].
4.4. Other inflammatory diseases
The therapeutic benefits of rPGRN and its derived Atsttrin were also reported in other inflammatory disease models. For example, rPGRN treatment attenuated coxsackievirus-B3-induced myocarditis in mice by downregulating Th1 and Th17 cells, but had no effect on Treg cells [90]. Intranasal pretreatments of rPGRN could inhibit bronchial smooth muscle hyper-responsiveness in antigen-challenged mouse allergic asthma [91]. Zhao et al. reported that Atsttrin effectively attenuated inflammation in a murine oxazolone-induced dermatitis model [92]. Fu et al. demonstrated that pretreatment with rPGRN protected against cardiorenal dysfunction in mice with hyperhomocysteinemia by negatively regulating Wnt/β-catenin signaling [93]. Yu et al. showed that administration of rPGRN increased platelet count in immune thrombocytopenia model mice by promoting Treg cell proliferation [94].
Not limited to chronic inflammatory diseases, acute inflammatory diseases may be also good candidates for PGRN treatment. Yu et al. reported that pretreatment with rPGRN significantly ameliorated the survival and abnormalities observed in mice subjected to endotoxic shock by lipopolysaccharide (LPS) [95]. Guo et al. also revealed that administration of rPGRN effectively reduced LPS-induced severe acute lung injury in mice [96].
5. PGRN targeting in lysosomal storage diseases
The therapeutic potential of PGRN for LSDs such as Gaucher disease (GD) mostly relies on its intracellular activity as a shared co-chaperone required for lysosomal delivery of lysosomal enzyme GCase, whose mutations cause GD [30]. Jian et al. found that rPGRN is therapeutic in various animal models of GD and human fibroblasts from GD patients, and more significantly, Pcgin, a 98 amino acid derivative of the PGRN C-terminal granulin E domain required for the binding to GCase and HSP70, can recapitulate this therapeutic effect [17, 56]. In addition, another study also identified a PGRN downstream molecule chitinase-3-like Protein 1 (CHI3L1) as a novel biomarker for diagnosis and treatment efficacy surveillance of GD [97].
In addition to GCase, PGRN was shown to be therapeutic against aberrant accumulation of other lysosomal enzymes. Using fibroblasts from various LSDs patients, Chen et al. demonstrated that rPGRN was effective in reducing lysosomal storage in Tay-Sachs disease (TSD) cells [18]. Mechanically, PGRN significantly increased the enzymatic activity and lysosomal delivery of TSD-associated enzyme Hex A, and PGRN directly bound to Hex A through granulins G and E [18]. Chen et al. also found that aged or ovalbumin-challenged adult PGRN-deficient mice showed typical TSD phenotypes including significant GM2 accumulation and the existence of typical TSD cells containing zebra bodies. Both rPGRN and PGRN derivative Pcgin significantly reduced GM2 accumulation and lysosomal storage in these animal models [18].
Actually, PGRN has a more universal function as a lysosomal protein chaperone, beyond its associations with GCase and Hex A. Beel, et al. showed that cathepsin D (CSTD) is also a PGRN-binding lysosomal enzyme; their association is also mediated by PGRN’s C-terminal granulin E domain [57]. Chen et al. observed that rPGRN also effectively reverted the altered lysosomes in fibroblasts from Farber’s disease, mucolipidosis III, and mucopolysacharidosis III/VI [18].
6. PGRN targeting in cancers
PGRN is frequently overexpressed by many types of cancer and contributes to their progression [11, 33, 98–103]. It has been well-established that PGRN exerts complex and multifaceted actions on tumor development by regulating cancer cell proliferation, invasion, stem cell properties, and angiogenesis [3, 103–106], and PGRN is also involved in stroma formation, resistance to anticancer drugs and immune evasion [107–112].
Currently, the most investigated and applicable PGRN-targeting strategy in cancer treatment is developing monoclonal neutralizing antibodies against PGRN. The feasibility of this strategy in the preclinical hepatocellular carcinoma (HCC) model has been demonstrated [113, 114]. Ho et al. developed an anti-PGRN monoclonal antibody that significantly inhibited the growth of established tumors but not normal liver cells in a dose-dependent manner [113]. The in vivo therapeutic effect of anti-PGRN monoclonal antibody was accompanied by decreased serum PGRN levels, suggesting its circulating level can be used to identify candidate HCC patients susceptible to anti-PGRN treatment and to monitor treatment response [113]. Wong et al. further showed that monoclonal antibodies against PGRN can sensitize chemotherapeutic agents-induced apoptosis in HCC cells and human HCC orthotopic xenograft models, indicating that blocking PGRN might be an alternative strategy to provoke cancer cell death [115].
Furthermore, Atsttrin was able to inhibit PGRN-stimulated cell proliferation of several cancer cell lines in vitro [7], suggesting Atsttrin may also be applicable for PGRN highly active cancers like breast cancer, ovarian carcinoma, and multiple myeloma, however further in vivo validation study is still needed.
Considering the utility of boosting PGRN strategies in neurological diseases, it is conceivable that small molecule compounds inhibiting PGRN expression or its activity (antagonists) should be an alternative approach to treat PGRN-associated cancers.
7. PGRN targeting in tissue repair and engineering
Considering the role of PGRN in stimulating chondrocyte differentiation and endochondral ossification [116], it is reasonable to speculate that PGRN would be also required for normal cartilage callus formation during bone regeneration. Indeed, Zhao et al. revealed that rPGRN enhanced bone regeneration in three surgically-induced bone defect models (segmental femoral bone defect model, femoral drill-hole model and nonunion segmental radial defect model) and one bone morphogenetic protein-2 (BMP-2)-induced ectopic bone formation model [117]. This effect remained in TNFR1 knockout mice but was lost in TNFR2 deficient mice, therefore, PGRN-stimulated bone regeneration primarily depends on TNFR2 [117]. Chen et al. demonstrated that local administration of rPGRN promotes regeneration of inflammatory periodontal bone defect in rats [118].
Bone-implant interface inflammatory osteolysis may result in aseptic loosening and subsequent failure in total joint arthroplasty. Zhao et al. revealed that rPGRN effectively inhibited inflammation in the titanium particle stimulated air pouch model, and prevented the pathological progression in two mouse osteoysis models through inhibiting TNF-α/NF-κB signaling pathway [119].
Wang et al. incorporated Atsttrin into alginate/hydroxyapatite to produce a 3D-printed scaffold that sustained Atsttrin release for at least 5 days with negligible cytotoxicity, and high cell adhesion ability and the 3D-printed Atsttrin scaffold significantly enhanced the regeneration of murine calvarial bone defects [27].
8. Targeting PGRN associated signaling mechanisms
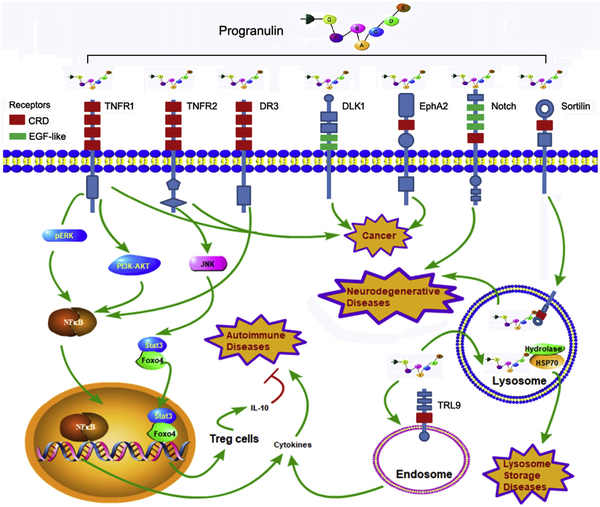
The therapeutic effects of targeting PGRN in various diseases depend on PGRN’s mediation of various signaling pathways by associating with individual receptors or binding proteins (Figure 2). PGRN binds to TNFR1 and activates ERK and PI3K/AKT pathways to competitively inhibit TNF-α activated NF-κB inflammatory pathway [7, 47, 49, 116, 120]. In contrast, the binding of PGRN to TNFR2 triggers the JNK-Stat3/Foxo4 protective signaling cascade and promotes the differentiation and function of regulatory T cells (Tregs) in inflammation [82, 121]. In addition to cell transmembrane receptors, PGRN also binds to TLR9 in the endosome to assist the recruitment of CpG-ODNs in macrophages, leading to the enhancement of innate immunity against bacterial infection [52].
Figure 2. Systematic illustration of PGRN-mediated signaling pathways.
The functional pluripotency of PGRN and its associated targeting strategies lie on its motif-dependent activation of various signaling pathways. Associations with different membrane receptors from different families may account for PGRN’s multiple functions under different pathophysiological conditions. For instance, in the course of inflammation, PGRN binds to TNFR1 and activates ERK and PI3K/AKT pathways, leading to the inhibition of TNF-α induced NF-κB inflammatory pathway, whereas PGRN also directly binds to TNFR2 with high affinity and promotes Treg cell differentiation by activating JNK-Stat3/Foxo4 signal cascade. PGRN binding to TLR9 in the endosome plays key roles in innate immunity against bacterial infection. Interactions with sortilin and Notch receptors play important roles in PGRN’s protective role in neurons, particularly in preventing neuronal degeneration. EphA2 may be also involved in PGRN-mediated cell proliferation during carcinogenesis. In addition to functioning as a growth factor-like molecule extracellularly, PGRN also acts as a cytoplasmic co-chaperone intracellularly to assist lysosomal enzyme trafficking, and absence of this function leads to various lysosome storage diseases. The CRD and EGF-like domains in various receptors, which are probably involved in their interactions with PGRN, are indicated.
Other PGRN-binding receptors include sortilin, Notch receptors, and EphA2 [53–55, 122, 123]. Interaction between PGRN and sortilin was reported to be critical for their trafficking into lysosomes, particularly in neurons [122, 124]. Altmann et al. suggested that PGRN may also bind to the extracellular domain of Notch receptors, and enhance the peripheral nerve regeneration and re-innervation [54]. Interaction between PGRN and EphA2 may be involved in the oncogenic role of PGRN [55]. The anti-proliferation effect on tumor cells of anti-PGNP monoclonal antibodies was dependent on its modulation on the p44/42 MAPK and Akt pathways, whereas the chemotherapeutic effect may result from the suppression of cancer stem cells and Akt/Bcl-2 signaling [115].
The major mechanism underlying PGRN-mediated therapeutic effects in lysosomal storage diseases lies in the interaction between the granualin E domain of PGRN with chaperone HSP70 to facilitate the folding and trafficking of mutated lysosomal enzymes, and rescue, at least in part, their activity, in turn alleviating the phenotypes of LSDs [18, 30, 56, 72].
9. Perspectives
9.1. PGRN is a non-conventional, extracellular matrix bound, and multiple membrane receptors-associated growth factor-like molecule
PGRN has been long recognized as a growth factor for its binding with cell surface receptors. However, unlike conventional growth factors, which usually function at nanogram level, PGRN needs a relatively higher magnitude amount to activate the receptors, usually at microgram level. In addition, growth factors usually bind to the cell membrane receptors as the cognate and specific ligands. Increasing evidence indicate that PGRN binds to multiple receptors from several functionally and structurally different receptor families, and these associations are probably cell/tissue-specific and condition/disease-dependent. For example, the PGRN and sortilin interaction is more specific in neurological cells for delivering PGRN to lysosome. PGRN binding with TNFR has an important anti-inflammatory role in immune cells, particularly Tregs and macrophages. PGRN/EphA2 interaction is possibly involved in the proliferative influence of PGRN during carcinogenesis. Collectively, these emerging evidences support the conclusion that PGRN should be defined as an abundant, non-conventional, stress-induced, matrix-bound secreted growth factor-like molecule and cytoplasmic chaperone, that functions in a cellular and disease specific pattern.
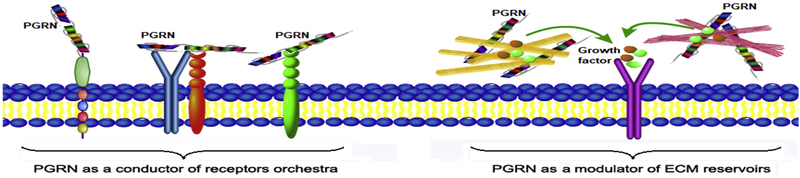
Considering the unique structure of PGRN and the ability to bind to multiple membrane receptors, it is speculated that PGRN may form a ternary complexes through associating with multiple receptors (Figure 3). In this case, PGRN may function as a “biological-glue” like conductor and mediates the signaling and activities of various receptors simultaneously and/or orchestrally. One or two receptors may play a major or dominant role and other receptors exert accompanist roles or are even not involved in a certain context, and vice versa in different conditions. This concept also provides new insights into the understanding of the perplexing phenomena as well as the controversy and inconsistency in the fields of PGRN researches and its involvements in diverse conditions. It is conceivable that additional PGRN-associated receptors may be identified in different cell and animal models using various unbiased screen approaches. It is also likely that rPGRN derivatives targeting two or more disease-associated receptors simultaneously may further enhance the therapeutic potential over the current one-receptor targeting strategies.
Figure 3. A proposed model to explain how PGRN acts as a conductor of a receptors orchestra and how PGRN regulates growth factors/cytokines indirectly through associating with extracellualr matrix “reservoirs”.
PGRN may bind to multiple receptors and mediate the signaling and activities of various receptors simultaneously and/or synergistically. Except for direct binding to membrane receptors, PGRN regulates various signaling pathways through its associated ECM molecules, which leads to the indirect modulation of various growth factors and/or cytokines implicated in diverse conditions.
ECM molecules are considered to act as “reservoirs” for certain growth factors and cytokines and regulate their signaling and activities through sequestering from or presenting to their cognate specific receptors. For instance, PGRN-interacting COMP is known to associate with numerous growth factors, including BMP2 [125] and TGFβ1 [126]. Therefore, it is speculated that PGRN’s regulation of various signaling pathways may be also attributed to its associations with the ECM molecules, particularly COMP, leading to indirect activations or inhibitions of various growth factors/cytokines in diverse conditions (Figure 3).
9.2. Diversity of PGRN levels in diseases and conditions
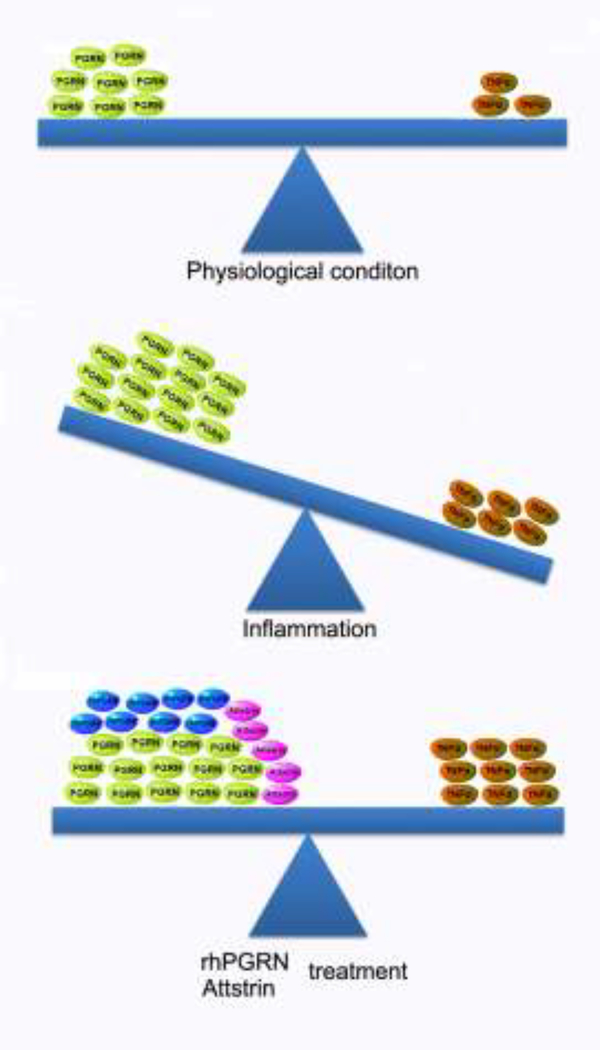
The levels of PGRN under different disease conditions are complicated. PGRN levels are usually elevated in multiple cancers [98–103], while decreased in most degenerative diseases such as FTD, NCL, and osteopenia [127] [128–130]. As an anti-inflammatory factor, the expression of PGRN in most inflammatory models and patients was paradoxically up-regulated [131–135]. It is noted that the balance between PGRN and TNF-α is critical to the initiation and progression of autoimmune and inflammatory disorders (Figure 4). PGRN and TNF-α reach a balance under physiological conditions at a low level, while inflammation may disturb the balance. Although the increase of PGRN has been frequently reported in inflammatory autoimmune diseases, probably due to the response of PGRN to inflammatory stress, its elevation is not sufficient to counteract elevated TNF-α under such conditions. When rPGRN and its derivatives are given, the imbalance would be restored and thus rPGRN and its derivatives demonstrate an anti-inflammatory effect in various inflammatory disease models. However, after PGRN supplementation, the level of TNF-α was reported to be unexpectedly increased, which suggests that a reciprocal feedback regulatory mechanism between PGRN and TNF-α may exist. Therefore, the regulatory pattern of PGRN expression in diseases is sophisticated and context-specific.
Figure 4. Diagram to explain the balance between anti-inflammatory PGRN and pro-inflammatory TNF-α in regulating inflammation.
The ratio of PGRN to TNF-α, but not their absolute concentrations, determines the trends of inflammation. Under physiological conditions, PGRN shows a higher baseline, and maintains an active balance of a pro-inflammation and anti-inflammation with TNF-α. In inflammatory autoimmune diseases, the level of PGRN is frequently found to be elevated, but increased PGRN is not sufficient to counteract the activity of simultaneously elevated TNF-α, leading to the disturbed balance between them and persistent inflammation. rPGRN and Attstrin exhibit therapeutic effects under such conditions, though, at least in part, restoring the balance between PGRN and TNF-α.
9.3. Consideration of side effects to targeting PGRN
The unique beads-on-a-string structure and sticky glycoprotein traits give PGRN high elasticity to bind to a wide spectrum of partners. PGRN, like other TNF blockers such as Humira (adalimumab) and Remicade (infliximab), exerts anti-inflammatory activities but has also been implicated in tumorigenesis, functioning as a double-edged sword. Long-term use of rPGRN is expected to cause excessive cell growth, thus increases the cancer risk; similar to the current clinically approved TNF-α blockers in treating TNF-associated autoimmune diseases. In addition to the cancer risk, direct use of rPGRN or boosting its expression using small compounds may have other side-effects due to its pluripotent features. Matsubara et al. indicated that administration of PGRN could induce insulin resistance [136]. Zhu et al. found that administration of rPGRN protein significantly promoted microglial activation in pilocarpine-induced status epilepticus [137]. Very recently, Amado et al. found that AAV delivering PGRN to the lateral ventricle or ependymal-targeting caused T cell-mediated hippocampal toxicity or ependymal hypertrophy despite a significant increase in PGRN expression [138]. Therefore, the adverse and the off-target effects of application of rPGRN protein or the gene encoding PGRN would be comprehensive and unpredictable; similar concerns may be also true for small molecule drugs to stimulate the expression of PGRN. Generally, full-length PGRN protein increasing strategies may only be considered for acute diseases and short-term treatment circumstances such as acute inflammation and bone fracture healing. In terms of chronic diseases, the pharmacological utility of PGRN must consider how to maximize diseases-specific targeting efficacy, and minimize the off-target results. Success in preclinical model indicates that engineered, diseases-specific PGRN-derivatives, such as Attstrin and Pcgin that maintain certain therapeutic effects of full length PGRN but possess minimal side effects, would lead to innovative therapeutics for various pathologies, in particular autoimmune diseases.
10. Conclusion
In summary, we present a timely update on the drug development efforts to target PGRN in multiple diseases, particularly in various preclinical disease models including neurological diseases, autoimmune diseases, lysosomal storage diseases, cancers and tissue repair and engineering. We emphasized that extracellular PGRN functions as a non-conventional, cellular and disease specific, matrix-bound, and multiple receptors-associated growth factor-like molecule, whereas intracellular PGRN acts as a co-chaperone involved in the folding and traffic of its associated proteins, particularly the lysosomal hydrolases. Associations of extracellular PGRN with cell membrane receptors mediate the uptake of PGRN and regulate the levels of intracellular PGRN (Jian and Liu, unpublished data); however, whether extracellular and intracellular PGRN mediated activities are functionally integrated or independent remains largely unknown. In addition, PGRN functional domain derivatives may provide innovative interventions to overcome the current bottlenecks in the efforts to develop PGRN targeting treatments.
Highlights.
PGRN interacts with its numerous binding partners in a highly domain- and context-dependent manner, which is critical for exerting its multiple functions. e.g. all reported PGRN-binding receptors contain at least one CRD or EGF-like domain or both.
PGRN is a promising therapeutic target in various kinds of diseases, including common neurodegenerative diseases, inflammatory autoimmune diseases, and rare lysosomal storage diseases.
Extracellular PGRN acts as a non-conventional, extracellular matrix bound, and multiple membrane receptors-associated growth factor-like molecule, i.e. a conductor of multiple membrane receptors.
Intracellular PGRN functions as a chaperone/co-chaperone that mediates the folding and traffic of its various binding partners, particularly the molecules associated with disaggregation, autophagy and lysosomal function.
Acknowledgments
CJ Liu is grateful to his gifted collaborators who made the explorations in his laboratory possible. We apologize to the colleagues whose publications are not included due to the space limitation. This work was supported partly by NIH research grants R01NS103931, R01AR062207, R01AR061484, and a DOD research grant W81XWH-16-1-0482 (CJ Liu). YZ Cui was funded by National Natural Science Foundation of China (81772300).
Biography
 Yazhou Cui received his M.D. from Shandong University. He is a Research Fellow in the Shandong Academy of Meducal Science. He works on disease related aberrant mineralization and functional genomics. He just completed his visiting scholar project in the Center for Translational Orthopaedic Research at NYU School of Medicine Department of Orthopaedic Surgery.
Yazhou Cui received his M.D. from Shandong University. He is a Research Fellow in the Shandong Academy of Meducal Science. He works on disease related aberrant mineralization and functional genomics. He just completed his visiting scholar project in the Center for Translational Orthopaedic Research at NYU School of Medicine Department of Orthopaedic Surgery.
 Aubryanna Hettinghouse received her Bachelor’s Degree from Indiana University Bloomington. She is a Research Assistant in the Center for Translational Orthopaedic Research at NYU Orthopedic Hospital. She works on immune and inflammatory pathologies with special attention aimed toward characterization of novel disease modifiers in Gaucher disease and development of comprehensive treatment strategies relevant to lysosomal storage diseases.
Aubryanna Hettinghouse received her Bachelor’s Degree from Indiana University Bloomington. She is a Research Assistant in the Center for Translational Orthopaedic Research at NYU Orthopedic Hospital. She works on immune and inflammatory pathologies with special attention aimed toward characterization of novel disease modifiers in Gaucher disease and development of comprehensive treatment strategies relevant to lysosomal storage diseases.
 Chuan-Ju Liu received his Ph.D. in Developmental Biology from Shandong University and Shanghai Institute of Cell Biology Chinese Academy of Science. He was a postdoctoral associate in the laboratory of Dr. Peter Lengyel at Yale University. He currently holds a dual appointment as Professor with tenure in the Department of Orthopaedic Surgery and the Department of Cell Biology at NYU School of Medicine. His research focuses on growth factors and cytokines in autoimmune diseases, musculoskeletal development and disorders/conditions, and lysosomal storage diseases.
Chuan-Ju Liu received his Ph.D. in Developmental Biology from Shandong University and Shanghai Institute of Cell Biology Chinese Academy of Science. He was a postdoctoral associate in the laboratory of Dr. Peter Lengyel at Yale University. He currently holds a dual appointment as Professor with tenure in the Department of Orthopaedic Surgery and the Department of Cell Biology at NYU School of Medicine. His research focuses on growth factors and cytokines in autoimmune diseases, musculoskeletal development and disorders/conditions, and lysosomal storage diseases.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bateman A, Belcourt D, Bennett H, Lazure C, Solomon S. Granulins, a novel class of peptide from leukocytes. Biochem Biophys Res Commun. 1990;173:1161–8. [DOI] [PubMed] [Google Scholar]
- [2].Bhandari V, Palfree RG, Bateman A. Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proc Natl Acad Sci U S A. 1992;89:1715–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].He Z, Ismail A, Kriazhev L, Sadvakassova G, Bateman A. Progranulin (PC-cell-derived growth factor/acrogranin) regulates invasion and cell survival. Cancer research. 2002;62:5590–6. [PubMed] [Google Scholar]
- [4].Zhu J, Nathan C, Jin W, Sim D, Ashcroft GS, Wahl SM, et al. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell. 2002;111:867–78. [DOI] [PubMed] [Google Scholar]
- [5].He Z, Ong CH, Halper J, Bateman A. Progranulin is a mediator of the wound response. Nature medicine. 2003;9:225–9. [DOI] [PubMed] [Google Scholar]
- [6].Diaz-Cueto L, Stein P, Jacobs A, Schultz RM, Gerton GL. Modulation of mouse preimplantation embryo development by acrogranin (epithelin/granulin precursor). Developmental biology. 2000;217:406–18. [DOI] [PubMed] [Google Scholar]
- [7].Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ, Liu GY, et al. The Growth Factor Progranulin Binds to TNF Receptors and Is Therapeutic Against Inflammatory Arthritis in Mice. Science. 2011;332:478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yin F, Banerjee R, Thomas B, Zhou P, Qian L, Jia T, et al. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J Exp Med. 2010;207:117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xu K, Zhang Y, Ilalov K, Carlson CS, Feng JQ, Di Cesare PE, et al. Cartilage oligomeric matrix protein associates with granulin-epithelin precursor (GEP) and potentiates GEP-stimulated chondrocyte proliferation. J Biol Chem. 2007;282:11347–55. [DOI] [PubMed] [Google Scholar]
- [10].Van Damme P, Van Hoecke A, Lambrechts D, Vanacker P, Bogaert E, van Swieten J, et al. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J Cell Biol. 2008;181:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bateman A, Bennett HP. The granulin gene family: from cancer to dementia. Bioessays. 2009;31:1245–54. [DOI] [PubMed] [Google Scholar]
- [12].Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–4. [DOI] [PubMed] [Google Scholar]
- [13].Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–9. [DOI] [PubMed] [Google Scholar]
- [14].Ward ME, Chen R, Huang HY, Ludwig C, Telpoukhovskaia M, Taubes A, et al. Individuals with progranulin haploinsufficiency exhibit features of neuronal ceroid lipofuscinosis. Science translational medicine. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gotzl JK, Mori K, Damme M, Fellerer K, Tahirovic S, Kleinberger G, et al. Common pathobiochemical hallmarks of progranulin-associated frontotemporal lobar degeneration and neuronal ceroid lipofuscinosis. Acta Neuropathol. 2014;127:845–60. [DOI] [PubMed] [Google Scholar]
- [16].Smith KR, Damiano J, Franceschetti S, Carpenter S, Canafoglia L, Morbin M, et al. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am J Hum Genet. 2012;90:1102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jian J, Zhao S, Tian QY, Liu H, Zhao Y, Chen WC, et al. Association Between Progranulin and Gaucher Disease. EBioMedicine. 2016;11:127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen Y, Jian J, Hettinghouse A, Zhao X, Setchell KDR, Sun Y, et al. Progranulin associates with hexosaminidase A and ameliorates GM2 ganglioside accumulation and lysosomal storage in Tay-Sachs disease. Journal of molecular medicine (Berlin, Germany). 2018;96:1359–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu CJ. Progranulin: a promising therapeutic target for rheumatoid arthritis. FEBS Lett. 2011;585:3675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu CJ, Bosch X. Progranulin: A growth factor, a novel TNFR ligand and a drug target. Pharmacology & therapeutics. 2012;133:124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wei J, Hettinghouse A, Liu C. The role of progranulin in arthritis. Annals of the New York Academy of Sciences. 2016;1383:5–20. [DOI] [PubMed] [Google Scholar]
- [22].Lata M, Hettinghouse AS, Liu CJ. Targeting tumor necrosis factor receptors in ankylosing spondylitis. Annals of the New York Academy of Sciences. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Williams A, Wang EC, Thurner L, Liu CJ. Review: Novel Insights Into Tumor Necrosis Factor Receptor, Death Receptor 3, and Progranulin Pathways in Arthritis and Bone Remodeling. Arthritis & rheumatology. 2016;68:2845–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Konopka J, Richbourgh B, Liu C. The role of PGRN in musculoskeletal development and disease. Front Biosci (Landmark Ed). 2014;19:662–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chitramuthu BP, Bennett HPJ, Bateman A. Progranulin: a new avenue towards the understanding and treatment of neurodegenerative disease. Brain : a journal of neurology. 2017;140:3081–104. [DOI] [PubMed] [Google Scholar]
- [26].Li B, He Y, Xu L, Hu Q, Tang J, Chen Y, et al. Progranulin Reduced Neuronal Cell Death by Activation of Sortilin 1 Signaling Pathways After Subarachnoid Hemorrhage in Rats. Critical care medicine. 2015;43:e304–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang Q, Xia Q, Wu Y, Zhang X, Wen F, Chen X, et al. 3D-Printed Atsttrin-Incorporated Alginate/Hydroxyapatite Scaffold Promotes Bone Defect Regeneration with TNF/TNFR Signaling Involvement. Advanced healthcare materials. 2015;4:1701–8. [DOI] [PubMed] [Google Scholar]
- [28].Zhao YP, Tian QY, Liu B, Cuellar J, Richbourgh B, Jia TH, et al. Progranulin knockout accelerates intervertebral disc degeneration in aging mice. Scientific reports. 2015;5:9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cao Z, Jiang B, Xie Y, Liu CJ, Feng JQ. GEP, a local growth factor, is critical for odontogenesis and amelogenesis. International journal of biological sciences. 2010;6:719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jian J, Hettinghouse A, Liu CJ. Progranulin acts as a shared chaperone and regulates multiple lysosomal enzymes. Genes & diseases. 2017;4:125–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Abella V, Pino J, Scotece M, Conde J, Lago F, Gonzalez-Gay MA, et al. Progranulin as a biomarker and potential therapeutic agent. Drug discovery today. 2017;22:1557–64. [DOI] [PubMed] [Google Scholar]
- [32].Jian J, Li G, Hettinghouse A, Liu C. Progranulin: A key player in autoimmune diseases. Cytokine. 2018;101:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bateman A, Cheung ST, Bennett HPJ. A Brief Overview of Progranulin in Health and Disease. Methods in molecular biology (Clifton, NJ). 2018;1806:3–15. [DOI] [PubMed] [Google Scholar]
- [34].Paushter DH, Du H, Feng T, Hu F. The lysosomal function of progranulin, a guardian against neurodegeneration. Acta neuropathologica. 2018;136:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kao AW, McKay A, Singh PP, Brunet A, Huang EJ. Progranulin, lysosomal regulation and neurodegenerative disease. Nature reviews Neuroscience. 2017;18:325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen Y, Sud N, Hettinghouse A, Liu CJ. Molecular regulations and therapeutic targets of Gaucher disease. Cytokine & growth factor reviews. 2018;41:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hrabal R, Chen Z, James S, Bennett HP, Ni F. The hairpin stack fold, a novel protein architecture for a new family of protein growth factors. Nat Struct Biol. 1996;3:747–52. [DOI] [PubMed] [Google Scholar]
- [38].Palfree RG, Bennett HP, Bateman A. The Evolution of the Secreted Regulatory Protein Progranulin. PloS one. 2015;10:e0133749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tolkatchev D, Malik S, Vinogradova A, Wang P, Chen Z, Xu P, et al. Structure dissection of human progranulin identifies well-folded granulin/epithelin modules with unique functional activities. Protein science : a publication of the Protein Society. 2008;17:711–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kong L, Zhao YP, Tian QY, Feng JQ, Kobayashi T, Merregaert J, et al. Extracellular matrix protein 1, a direct targeting molecule of parathyroid hormone-related peptide, negatively regulates chondrogenesis and endochondral ossification via associating with progranulin growth factor. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30:2741–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gonzalez EM, Mongiat M, Slater SJ, Baffa R, Iozzo RV. A novel interaction between perlecan protein core and progranulin: potential effects on tumor growth. J Biol Chem. 2003;278:38113–6. [DOI] [PubMed] [Google Scholar]
- [42].Guo F, Lai Y, Tian Q, Lin EA, Kong L, Liu C. Granulin-epithelin precursor binds directly to ADAMTS-7 and ADAMTS-12 and inhibits their degradation of cartilage oligomeric matrix protein. Arthritis Rheum. 2010;62:2023–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lin EA, Liu CJ. The role of ADAMTSs in arthritis. Protein & cell. 2010;1:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bai XH, Wang DW, Kong L, Zhang Y, Luan Y, Kobayashi T, et al. ADAMTS-7, a direct target of PTHrP, adversely regulates endochondral bone growth by associating with and inactivating GEP growth factor. Mol Cell Biol. 2009;29:4201–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Liu CJ. The role of ADAMTS-7 and ADAMTS-12 in the pathogenesis of arthritis. Nat Clin Pract Rheumatol. 2009;5:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kessenbrock K, Frohlich L, Sixt M, Lammermann T, Pfister H, Bateman A, et al. Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. The Journal of clinical investigation. 2008;118:2438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tian Q, Zhao S, Liu C. A solid-phase assay for studying direct binding of progranulin to TNFR and progranulin antagonism of TNF/TNFR interactions. Methods in molecular biology (Clifton, NJ). 2014;1155:163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jian J, Zhao S, Tian Q, Gonzalez-Gugel E, Mundra JJ, Uddin SM, et al. Progranulin directly binds to the CRD2 and CRD3 of TNFR extracellular domains. FEBS Lett. 2013;587:3428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tian Q, Zhao Y, Mundra JJ, Gonzalez-Gugel E, Jian J, Uddin SM, et al. Three TNFR-binding domains of PGRN act independently in inhibition of TNF-alpha binding and activity. Front Biosci (Landmark Ed). 2014;19:1176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Uddin SM, Mundra JJ, Jian J, Tian Q, Gonzalez-Gugel E, Richbourgh B, et al. Progranulin inhibition of TNFalpha. Immunology and cell biology. 2014;92:299–300. [DOI] [PubMed] [Google Scholar]
- [51].Liu C, Li XX, Gao W, Liu W, Liu DS. Progranulin-derived Atsttrin directly binds to TNFRSF25 (DR3) and inhibits TNF-like ligand 1A (TL1A) activity. PloS one. 2014;9:e92743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Park B, Buti L, Lee S, Matsuwaki T, Spooner E, Brinkmann MM, et al. Granulin Is a Soluble Cofactor for Toll-like Receptor 9 Signaling. Immunity. 2011;34:505–13. [DOI] [PubMed] [Google Scholar]
- [53].Hu F, Padukkavidana T, Vaegter CB, Brady OA, Zheng Y, Mackenzie IR, et al. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron. 2010;68:654–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Altmann C, Vasic V, Hardt S, Heidler J, Haussler A, Wittig I, et al. Progranulin promotes peripheral nerve regeneration and reinnervation: role of notch signaling. Molecular neurodegeneration. 2016;11:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Neill T, Buraschi S, Goyal A, Sharpe C, Natkanski E, Schaefer L, et al. EphA2 is a functional receptor for the growth factor progranulin. The Journal of cell biology. 2016;215:687–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Jian J, Tian QY, Hettinghouse A, Zhao S, Liu H, Wei J, et al. Progranulin Recruits HSP70 to beta-Glucocerebrosidase and Is Therapeutic Against Gaucher Disease. EBioMedicine. 2016;13:212–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Beel S, Moisse M, Damme M, De Muynck L, Robberecht W, Van Den Bosch L, et al. Progranulin functions as a cathepsin D chaperone to stimulate axonal outgrowth in vivo. Human molecular genetics. 2017;26:2850–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hoque M, Young TM, Lee CG, Serrero G, Mathews MB, Pe’ery T. The growth factor granulin interacts with cyclin T1 and modulates P-TEFb-dependent transcription. Mol Cell Biol. 2003;23:1688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Trinh DP, Brown KM, Jeang KT. Epithelin/granulin growth factors: extracellular cofactors for HIV-1 and HIV-2 Tat proteins. Biochem Biophys Res Commun. 1999;256:299–306. [DOI] [PubMed] [Google Scholar]
- [60].Shoham N, Cohen L, Gazit A, Yaniv A. The Tat protein of the caprine arthritis encephalitis virus interacts with the Notch2 EGF-like repeats and the epithelin/granulin precursor. Intervirology. 2003;46:239–44. [DOI] [PubMed] [Google Scholar]
- [61].Hoque M, Tian B, Mathews MB, Pe’ery T. Granulin and granulin repeats interact with the Tat.P-TEFb complex and inhibit Tat transactivation. J Biol Chem. 2005;280:13648–57. [DOI] [PubMed] [Google Scholar]
- [62].Butler GS, Dean RA, Tam EM, Overall CM. Pharmacoproteomics of a metalloproteinase hydroxamate inhibitor in breast cancer cells: dynamics of membrane type 1 matrix metalloproteinase-mediated membrane protein shedding. Mol Cell Biol. 2008;28:4896–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Tian QY, Zhao YP, Liu CJ. Modified yeast-two-hybrid system to identify proteins interacting with the growth factor progranulin. Journal of visualized experiments : JoVE. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Almeida S, Zhou L, Gao FB. Progranulin, a glycoprotein deficient in frontotemporal dementia, is a novel substrate of several protein disulfide isomerase family proteins. PloS one. 2011;6:e26454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Baladron V, Ruiz-Hidalgo MJ, Bonvini E, Gubina E, Notario V, Laborda J. The EGF-like homeotic protein dlk affects cell growth and interacts with growth-modulating molecules in the yeast two-hybrid system. Biochemical and biophysical research communications. 2002;291:193–204. [DOI] [PubMed] [Google Scholar]
- [66].Alquezar C, Esteras N, de la Encarnacion A, Moreno F, Lopez de Munain A, Martin-Requero A. Increasing progranulin levels and blockade of the ERK1/2 pathway: upstream and downstream strategies for the treatment of progranulin deficient frontotemporal dementia. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2015;25:386–403. [DOI] [PubMed] [Google Scholar]
- [67].Cenik B, Sephton CF, Dewey CM, Xian X, Wei S, Yu K, et al. Suberoylanilide hydroxamic acid (vorinostat) up-regulates progranulin transcription: rational therapeutic approach to frontotemporal dementia. J Biol Chem. 2011;286:16101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tsai RM, Boxer AL. Therapy and clinical trials in frontotemporal dementia: past, present, and future. Journal of neurochemistry. 2016;138 Suppl 1:211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Capell A, Liebscher S, Fellerer K, Brouwers N, Willem M, Lammich S, et al. Rescue of progranulin deficiency associated with frontotemporal lobar degeneration by alkalizing reagents and inhibition of vacuolar ATPase. J Neurosci. 2011;31:1885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sha SJ, Miller ZA, Min SW, Zhou Y, Brown J, Mitic LL, et al. An 8-week, open-label, dose-finding study of nimodipine for the treatment of progranulin insufficiency from GRN gene mutations. Alzheimer’s & dementia (New York, N Y). 2017;3:507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Arrant AE, Filiano AJ, Unger DE, Young AH, Roberson ED. Restoring neuronal progranulin reverses deficits in a mouse model of frontotemporal dementia. Brain : a journal of neurology. 2017;140:1447–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Arrant AE, Onyilo VC, Unger DE, Roberson ED. Progranulin Gene Therapy Improves Lysosomal Dysfunction and Microglial Pathology Associated with Frontotemporal Dementia and Neuronal Ceroid Lipofuscinosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2018;38:2341–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Van Kampen JM, Baranowski D, Kay DG. Progranulin gene delivery protects dopaminergic neurons in a mouse model of Parkinson’s disease. PloS one. 2014;9:e97032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Cortini F, Fenoglio C, Guidi I, Venturelli E, Pomati S, Marcone A, et al. Novel exon 1 progranulin gene variant in Alzheimer’s disease. Eur J Neurol. 2008;15:1111–7. [DOI] [PubMed] [Google Scholar]
- [75].Minami SS, Min SW, Krabbe G, Wang C, Zhou Y, Asgarov R, et al. Progranulin protects against amyloid beta deposition and toxicity in Alzheimer’s disease mouse models. Nature medicine. 2014;20:1157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Tauffenberger A, Chitramuthu BP, Bateman A, Bennett HP, Parker JA. Reduction of polyglutamine toxicity by TDP-43, FUS and progranulin in Huntington’s disease models. Human molecular genetics. 2013;22:782–94. [DOI] [PubMed] [Google Scholar]
- [77].Zhou C, Xie G, Wang C, Zhang Z, Chen Q, Zhang L, et al. Decreased progranulin levels in patients and rats with subarachnoid hemorrhage: a potential role in inhibiting inflammation by suppressing neutrophil recruitment. Journal of neuroinflammation. 2015;12:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Tao J, Ji F, Wang F, Liu B, Zhu Y. Neuroprotective effects of progranulin in ischemic mice. Brain research. 2012;1436:130–6. [DOI] [PubMed] [Google Scholar]
- [79].Egashira Y, Suzuki Y, Azuma Y, Takagi T, Mishiro K, Sugitani S, et al. The growth factor progranulin attenuates neuronal injury induced by cerebral ischemia-reperfusion through the suppression of neutrophil recruitment. J Neuroinflammation. 2013;10:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kanazawa M, Kawamura K, Takahashi T, Miura M, Tanaka Y, Koyama M, et al. Multiple therapeutic effects of progranulin on experimental acute ischaemic stroke. Brain : a journal of neurology. 2015;138:1932–48. [DOI] [PubMed] [Google Scholar]
- [81].Wei JL, Liu CJ. Establishment of a Modified Collagen-Induced Arthritis Mouse Model to Investigate the Anti-inflammatory Activity of Progranulin in Inflammatory Arthritis. Methods in molecular biology (Clifton, NJ). 2018;1806:305–13. [DOI] [PubMed] [Google Scholar]
- [82].Fu W, Hu W, Shi L, Mundra JJ, Xiao G, Dustin ML, et al. Foxo4- and Stat3-dependent IL-10 production by progranulin in regulatory T cells restrains inflammatory arthritis. FASEB J. 2017;31:1354–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Braddock M, Quinn A. Targeting IL-1 in inflammatory disease: new opportunities for therapeutic intervention. Nature reviews Drug discovery. 2004;3:330–9. [DOI] [PubMed] [Google Scholar]
- [84].Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis and rheumatism. 2001;44:1237–47. [DOI] [PubMed] [Google Scholar]
- [85].Wei JL, Buza J 3rd, Liu CJ. Does progranulin account for the opposite effects of etanercept and infliximab/adalimumab in osteoarthritis?: Comment on Olson et al.: “Therapeutic Opportunities to Prevent Post-Traumatic Arthritis: Lessons From the Natural History of Arthritis After Articular Fracture”. J Orthop Res. 2016;34:12–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zhao YP, Liu B, Tian QY, Wei JL, Richbourgh B, Liu CJ. Progranulin protects against osteoarthritis through interacting with TNF-alpha and beta-Catenin signalling. Ann Rheum Dis. 2015;74:2244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wei JL, Fu W, Ding YJ, Hettinghouse A, Lendhey M, Schwarzkopf R, et al. Progranulin derivative Atsttrin protects against early osteoarthritis in mouse and rat models. Arthritis research & therapy. 2017;19:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Xia Q, Zhu S, Wu Y, Wang J, Cai Y, Chen P, et al. Intra-articular transplantation of atsttrin-transduced mesenchymal stem cells ameliorate osteoarthritis development. Stem cells translational medicine. 2015;4:523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wei F, Zhang Y, Jian J, Mundra JJ, Tian Q, Lin J, et al. PGRN protects against colitis progression in mice in an IL-10 and TNFR2 dependent manner. Scientific reports. 2014;4:7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Li L, Li L, Xiao L, Shangguan J. Progranulin ameliorates coxsackievirus-B3-induced viral myocarditis by downregulating Th1 and Th17 cells. Experimental cell research. 2018;367:241–50. [DOI] [PubMed] [Google Scholar]
- [91].Chiba Y, Danno S, Suto R, Suto W, Yamane Y, Hanazaki M, et al. Intranasal administration of recombinant progranulin inhibits bronchial smooth muscle hyperresponsiveness in mouse allergic asthma. American journal of physiology Lung cellular and molecular physiology. 2018;314:L215–l23. [DOI] [PubMed] [Google Scholar]
- [92].Zhao YP, Tian QY, Liu CJ. Progranulin deficiency exaggerates, whereas progranulin-derived Atsttrin attenuates, severity of dermatitis in mice. FEBS Lett. 2013;587:1805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Fu Y, Sun Y, Zhou M, Wang X, Wang Z, Wei X, et al. Therapeutic Potential of Progranulin in Hyperhomocysteinemia-Induced Cardiorenal Dysfunction. Hypertension. 2017;69:259–66. [DOI] [PubMed] [Google Scholar]
- [94].Yu Y, Shi Y, Zuo X, Feng Q, Hou Y, Tang W, et al. Progranulin facilitates the increase of platelet count in immune thrombocytopenia. Thrombosis research. 2018;164:24–31. [DOI] [PubMed] [Google Scholar]
- [95].Yu Y, Xu X, Liu L, Mao S, Feng T, Lu Y, et al. Progranulin deficiency leads to severe inflammation, lung injury and cell death in a mouse model of endotoxic shock. Journal of cellular and molecular medicine. 2016;20:506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Guo Z, Li Q, Han Y, Liang Y, Xu Z, Ren T. Prevention of LPS-induced acute lung injury in mice by progranulin. Mediators of inflammation. 2012;2012:540794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Jian J, Chen Y, Liberti R, Fu W, Hu W, Saunders-Pullman R, et al. Chitinase-3-like Protein 1: A Progranulin Downstream Molecule and Potential Biomarker for Gaucher Disease. EBioMedicine. 2018;28:251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Carlson AM, Maurer MJ, Goergen KM, Kalli KR, Erskine CL, Behrens MD, et al. Utility of progranulin and serum leukocyte protease inhibitor as diagnostic and prognostic biomarkers in ovarian cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22:1730–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Serrero G, Hawkins DM, Yue B, Ioffe O, Bejarano P, Phillips JT, et al. Progranulin (GP88) tumor tissue expression is associated with increased risk of recurrence in breast cancer patients diagnosed with estrogen receptor positive invasive ductal carcinoma. Breast cancer research : BCR. 2012;14:R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Frampton G, Invernizzi P, Bernuzzi F, Pae HY, Quinn M, Horvat D, et al. Interleukin-6-driven progranulin expression increases cholangiocarcinoma growth by an Akt-dependent mechanism. Gut. 2012;61:268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Han JJ, Yu M, Houston N, Steinberg SM, Kohn EC. Progranulin is a potential prognostic biomarker in advanced epithelial ovarian cancers. Gynecol Oncol. 2011;120:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Pan CX, Kinch MS, Kiener PA, Langermann S, Serrero G, Sun L, et al. PC cell-derived growth factor expression in prostatic intraepithelial neoplasia and prostatic adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:1333–7. [DOI] [PubMed] [Google Scholar]
- [103].He Z, Bateman A. Progranulin gene expression regulates epithelial cell growth and promotes tumor growth in vivo. Cancer research. 1999;59:3222–9. [PubMed] [Google Scholar]
- [104].Eguchi R, Nakano T, Wakabayashi I. Progranulin and granulin-like protein as novel VEGF-independent angiogenic factors derived from human mesothelioma cells. Oncogene. 2017;36:714–22. [DOI] [PubMed] [Google Scholar]
- [105].Cheung ST, Wong SY, Leung KL, Chen X, So S, Ng IO, et al. Granulin-epithelin precursor overexpression promotes growth and invasion of hepatocellular carcinoma. Clin Cancer Res. 2004;10:7629–36. [DOI] [PubMed] [Google Scholar]
- [106].Monami G, Gonzalez EM, Hellman M, Gomella LG, Baffa R, Iozzo RV, et al. Proepithelin promotes migration and invasion of 5637 bladder cancer cells through the activation of ERK1/2 and the formation of a paxillin/FAK/ERK complex. Cancer Res. 2006;66:7103–10. [DOI] [PubMed] [Google Scholar]
- [107].Pizarro GO, Zhou XC, Koch A, Gharib M, Raval S, Bible K, et al. Prosurvival function of the granulin-epithelin precursor is important in tumor progression and chemoresponse. Int J Cancer. 2007;120:2339–43. [DOI] [PubMed] [Google Scholar]
- [108].Bandey I, Chiou SH, Huang AP, Tsai JC, Tu PH. Progranulin promotes Temozolomide resistance of glioblastoma by orchestrating DNA repair and tumor stemness. Oncogene. 2015;34:1853–64. [DOI] [PubMed] [Google Scholar]
- [109].Cheung PF, Cheng CK, Wong NC, Ho JC, Yip CW, Lui VC, et al. Granulin-epithelin precursor is an oncofetal protein defining hepatic cancer stem cells. PloS one. 2011;6:e28246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Elkabets M, Gifford AM, Scheel C, Nilsson B, Reinhardt F, Bray MA, et al. Human tumors instigate granulin-expressing hematopoietic cells that promote malignancy by activating stromal fibroblasts in mice. The Journal of clinical investigation. 2011;121:784–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Dong T, Yang D, Li R, Zhang L, Zhao H, Shen Y, et al. PGRN promotes migration and invasion of epithelial ovarian cancer cells through an epithelial mesenchymal transition program and the activation of cancer associated fibroblasts. Experimental and molecular pathology. 2016;100:17–25. [DOI] [PubMed] [Google Scholar]
- [112].Cheung PF, Yip CW, Wong NC, Fong DY, Ng LW, Wan AM, et al. Granulin-epithelin precursor renders hepatocellular carcinoma cells resistant to natural killer cytotoxicity. Cancer immunology research. 2014;2:1209–19. [DOI] [PubMed] [Google Scholar]
- [113].Ho JC, Ip YC, Cheung ST, Lee YT, Chan KF, Wong SY, et al. Granulin-epithelin precursor as a therapeutic target for hepatocellular carcinoma. Hepatology (Baltimore, Md. 2008;47:1524–32. [DOI] [PubMed] [Google Scholar]
- [114].Yip CW, Cheung PFY, Wong NCL, Fung SW, Cheung ST. Mouse Monoclonal Antibodies Against Progranulin (PGRN/GEP) as Therapeutics in Preclinical Cancer Models. Methods in molecular biology (Clifton, NJ). 2018;1806:131–44. [DOI] [PubMed] [Google Scholar]
- [115].Wong NC, Cheung PF, Yip CW, Chan KF, Ng IO, Fan ST, et al. Antibody against granulin-epithelin precursor sensitizes hepatocellular carcinoma to chemotherapeutic agents. Molecular cancer therapeutics. 2014;13:3001–12. [DOI] [PubMed] [Google Scholar]
- [116].Feng JQ, Guo FJ, Jiang BC, Zhang Y, Frenkel S, Wang DW, et al. Granulin epithelin precursor: a bone morphogenic protein 2-inducible growth factor that activates Erk1/2 signaling and JunB transcription factor in chondrogenesis. FASEB J. 2010;24:1879–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Zhao YP, Tian QY, Frenkel S, Liu CJ. The promotion of bone healing by progranulin, a downstream molecule of BMP-2, through interacting with TNF/TNFR signaling. Biomaterials. 2013;34:6412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Chen Q, Cai J, Li X, Song A, Guo H, Sun Q, et al. Progranulin Promotes Regeneration of Inflammatory Periodontal Bone Defect in Rats via Anti-inflammation, Osteoclastogenic Inhibition, and Osteogenic Promotion. Inflammation. 2018. [DOI] [PubMed] [Google Scholar]
- [119].Zhao YP, Wei JL, Tian QY, Liu AT, Yi YS, Einhorn TA, et al. Progranulin suppresses titanium particle induced inflammatory osteolysis by targeting TNFalpha signaling. Scientific reports. 2016;6:20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Mundra JJ, Jian J, Bhagat P, Liu CJ. Progranulin inhibits expression and release of chemokines CXCL9 and CXCL10 in a TNFR1 dependent manner. Scientific reports. 2016;6:21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Wei F, Zhang Y, Zhao W, Yu X, Liu CJ. Progranulin Facilitates Conversion and Function of Regulatory T Cells under Inflammatory Conditions. PloS one. 2014;9:e112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zhou X, Sun L, Bastos de Oliveira F, Qi X, Brown WJ, Smolka MB, et al. Prosaposin facilitates sortilin-independent lysosomal trafficking of progranulin. J Cell Biol. 2015;210:991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Chitramuthu B, Bateman A. Progranulin and the receptor tyrosine kinase EphA2, partners in crime? J Cell Biol. 2016;215:603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Zhou X, Sun L, Bracko O, Choi JW, Jia Y, Nana AL, et al. Impaired prosaposin lysosomal trafficking in frontotemporal lobar degeneration due to progranulin mutations. Nature communications. 2017;8:15277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Du Y, Wang Y, Wang L, Liu B, Tian Q, Liu CJ, et al. Cartilage oligomeric matrix protein inhibits vascular smooth muscle calcification by interacting with bone morphogenetic protein-2. Circulation research. 2011;108:917–28. [DOI] [PubMed] [Google Scholar]
- [126].Haudenschild DR, Hong E, Yik JH, Chromy B, Morgelin M, Snow KD, et al. Enhanced activity of transforming growth factor beta1 (TGF-beta1) bound to cartilage oligomeric matrix protein. The Journal of biological chemistry. 2011;286:43250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Milajerdi A, Maghbooli Z, Mohammadi F, Hosseini B, Mirzaei K. Progranulin concentration in relation to bone mineral density among obese individuals. Archives of endocrinology and metabolism. 2018;62:179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Wilke C, Gillardon F, Deuschle C, Hobert MA, Jansen IE, Metzger FG, et al. Cerebrospinal Fluid Progranulin, but Not Serum Progranulin, Is Reduced in GRN-Negative Frontotemporal Dementia. Neuro-degenerative diseases. 2017;17:83–8. [DOI] [PubMed] [Google Scholar]
- [129].Schofield EC, Halliday GM, Kwok J, Loy C, Double KL, Hodges JR. Low serum progranulin predicts the presence of mutations: a prospective study. Journal of Alzheimer’s disease : JAD. 2010;22:981–4. [DOI] [PubMed] [Google Scholar]
- [130].Guven G, Bilgic B, Tufekcioglu Z, Erginel Unaltuna N, Hanagasi H, Gurvit H, et al. Peripheral GRN mRNA and Serum Progranulin Levels as a Potential Indicator for Both the Presence of Splice Site Mutations and Individuals at Risk for Frontotemporal Dementia. Journal of Alzheimer’s disease : JAD. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Yamamoto Y, Takemura M, Serrero G, Hayashi J, Yue B, Tsuboi A, et al. Increased serum GP88 (Progranulin) concentrations in rheumatoid arthritis. Inflammation. 2014;37:1806–13. [DOI] [PubMed] [Google Scholar]
- [132].Cerezo LA, Kuklova M, Hulejova H, Vernerova Z, Kasprikova N, Veigl D, et al. Progranulin Is Associated with Disease Activity in Patients with Rheumatoid Arthritis. Mediators Inflamm. 2015;2015:740357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Qiu F, Song L, Ding F, Liu H, Shu Q, Yang N, et al. Expression level of the growth factor progranulin is related with development of systemic lupus erythematosus. Diagnostic pathology. 2013;8:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Huang K, Chen A, Zhang X, Song Z, Xu H, Cao J, et al. Progranulin is preferentially expressed in patients with psoriasis vulgaris and protects mice from psoriasis-like skin inflammation. Immunology. 2015;145:279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Johnson J, Yeter K, Rajbhandary R, Neal R, Tian Q, Jian J, et al. Serum progranulin levels in Hispanic rheumatoid arthritis patients treated with TNF antagonists: a prospective, observational study. Clinical rheumatology. 2017;36:507–16. [DOI] [PubMed] [Google Scholar]
- [136].Matsubara T, Mita A, Minami K, Hosooka T, Kitazawa S, Takahashi K, et al. PGRN is a Key Adipokine Mediating High Fat Diet-Induced Insulin Resistance and Obesity through IL-6 in Adipose Tissue. Cell metabolism. 2012;15:38–50. [DOI] [PubMed] [Google Scholar]
- [137].Zhu S, Tai C, Petkau TL, Zhang S, Liao C, Dong Z, et al. Progranulin promotes activation of microglia/macrophage after pilocarpine-induced status epilepticus. Brain Res. 2013;1530:54–65. [DOI] [PubMed] [Google Scholar]
- [138].Amado DA, Rieders JM, Diatta F, Hernandez-Con P, Singer A, Mak JT, et al. AAV-Mediated Progranulin Delivery to a Mouse Model of Progranulin Deficiency Causes T Cell-Mediated Toxicity. Molecular therapy : the journal of the American Society of Gene Therapy. 2018. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]