Abstract
Cranial neural crest cells (CNCC) give rise to cranial mesenchyme (CM) that differentiates into the forebrain meningeal progenitors in the basolateral and apical regions of the head. This occurs in close proximity to the other CNCC-CM-derivatives such as calvarial bone and and dermal progenitors. We found active Wnt signaling transduction in the forebrain meningeal progenitors in basolateral and apical populations and in the non-meningeal CM preceding meningeal differentiation. Here, we dissect the source of Wnt ligand secretion and requirement of Wnt/β-catenin signaling for the lineage selection and early differentiation of the forebrain meninges. We find persistent canonical Wnt/β-catenin signal transduction in the meningeal progenitors in the absence of Wnt ligand secretion in the cranial mesenchyme or surface ectoderm, suggesting additional sources of Wnts. Conditional mutants for Wntless and β-catenin in the cranial mesenchyme showed that Wnt ligand secretion and Wnt/β-catenin signaling were dispensable for specification and proliferation of early meningeal progenitors. In the absence β-catenin in the CM, we found diminished laminin matrix and meningeal hypoplasia, indicating a structural and trophic role of mesenchymal β-catenin signaling. This study shows that β-catenin signaling is required in the cranial mesenchyme for maintenance and organization of the differentiated meningeal layers in the basolateral and apical populations of embryonic meninges.
Keywords: cell fate selection, meningeal progenitors, skull bone
Introduction
The mammalian cranial lineages, such as the frontal bone of the skull, overlying dermis of the skin, and meninges that enclose the brain, differentiate from cranial mesenchyme (CM) that are derived from cranial neural crest cells (CNCC). (Serbedzija, Bronner-Fraser, and Fraser 1992; Jiang et al. 2002). Between E11.5–13.5, in mouse, these three lineages differentiate from CM in close proximity to one another between the neuroepithelium of the brain and the surface ectoderm in the basolateral mesenchyme of the supraorbital arch (Angelov and Vasilev 1989; McLone and Bondareff 1975; Tran et al. 2010). The meninges are a protective, fluid-filled membranous sac that cover the brain and serve as a reservoir of trophic factors, stem cells, and extracellular matrix that affect brain and skull bone development (Richtsmeier and Flaherty 2013; Bjornsson et al. 2015). Studies suggest there are two distinct pools of meningeal progenitors, with the basolateral population differentiating earlier than the population in the apex. The basolateral meningeal progenitors begin to differentiate to the distinct pia, arachnoid, and dura layers from E13.5 (Angelov and Vasilev 1989; McLone and Bondareff 1975). Concomitantly with the invagination of the dorsal brain at E10.5, there is a second residential population of dense CM in the dorsal midline/apex that appears to expand laterally by E14.5 and then begins to differentiate into the apical meningeal layers after E14.5 (Choe et al., 2014). Meninges complete their differentiation into the the three-layer structure only after E19.5 (Mercier, Kitasako, and Hatton 2002; Bjornsson et al. 2015; Bifari et al. 2015).
In the absence of lineage-specific genetic tools in the CM, several existing mutants have been used to understand the contribution of neighboring brain and calvarial bone to meningeal development (Ito et al. 2003; Kume et al. 1998; Rice et al. 2003).In the spontaneous mouse mutant, congenital hydrocephalus (ch), that results in a truncated Foxc1 protein, the apical expansion and differentiation of the basolateral meningeal mesenchyme is compromised and tracks the expansion of cranial bone anlagen, suggesting communication between the two mesenchymal populations during morphogenesis (Rice et al. 2003; Vivatbutsiri, Ichinose, Hytönen, et al. 2008). In addition, the Foxc1 null mutants lack closure and invagination of the dorsal telencephalon, and is also devoid of meninges and skull bone in the apex, suggesting a structural or signaling role of the dorsal brain (Kume et al. 1998). Reciprocally, the embryonic meninges have been implicated in producing inductive signaling to promote calvarial bone development in the deletion of TGF-β receptor 2 (TGFβIIR) in the premigratory CNCC (Ito et al. 2003). However, the cell-autonomous signals required for meningeal specification, differentiation, and apical expansion in vivo remains to be tested systematically.
Wnt signaling is one of the earliest pathways required in the induction, migration, and later in the differentiation of CNCC-derived CM lineages (Mani et al. 2010; Tran et al. 2010; Brault et al. 2001; Ikeya et al. 1997). Canonical Wnt signaling is transduced by β-catenin protein and has pleiotropic roles in embryonic development by regulating expression of context-specific downstream target genes (van Amerongen and Nusse 2009). Wnt signaling reporters are visible in the premigratory CNCC at E8.5–9.5, and then in the CM in the apex and in the newly invaginated cortical hem of the dorsal telencephalon at E10.5 (Choe, Zarbalis, and Pleasure 2014; Mani et al. 2010). Interestingly, mice with conditional deletion of β-catenin with Emx1Cre in the mouse forebrain have CNCC-derived structures, suggesting that Wnt signaling in the forebrain is dispensable for CM differentiation (Campos, Du, and Li 2004). Conditional deletion of β-catenin/Ctnbb1 in the premigratory CNCC with Wnt1Cre or Sox10 Cre leads to diminished forebrain and loss of craniofacial structures (Choe, Zarbalis, and Pleasure 2014; Brault et al. 2001; Ikeya et al. 1997). Furthermore, the β-catenin-mutant in premigratory CNCC fail to laterally expand at the apex and the cortical midline invagination is diminished, thereby confounding the analysis of meningeal development (Choe, Zarbalis, and Pleasure 2014).
Many questions remain about early differentiation of the meningeal progenitors and the role of canonical Wnt signaling in meningeal development in the apex and basolateral regions. For instance, what is the role of Wnt signaling in post-migratory CNCC for meningeal mesenchyme specification, differentiation to the distinct layers, expansion of the meningeal progenitors, proliferation, and survival? What is the source of Wnt ligands for Wnt signal transduction in the basolateral and apical meningeal progenitors? What is the requirement of the adjacent calvarial bone for the morphogenesis, differentiation and expansion of meningeal progenitors in the basal and apical region? We previously showed that conditional deletion of β-catenin in the post-migratory CNCC allows for the formation of CM in the supraorbital arch and closure of dorsal brain; therefore, making this model more amenable to study the role of Wnt signaling in forebrain meninges formation (Tran et al. 2010; Goodnough et al. 2012). Furthermore, deletion of β-catenin in the supraorbital arch CM leads to loss of calvarial bone and dermal fates, prompting us to investigate its role in meningeal specification and early differentiation.
In the continued absence of meningeal progenitor-restricted genetic tools, we performed this study to gain insights into these questions. We generated and examined various mouse models in vivo that lack Wnt ligand secretion specifically in either the ectoderm or the CM preceding specification of the various lineages. We directly tested the role of Wnt signal transduction in the meningeal vs non-meningeal mesenchyme and the requirement of cranial bone for meningeal differentiation in the basolateral population in vivo. Our results show that Wnt signaling in the meningeal mesenchymal progenitors is not required for the specification, but for the maintenance of the emerging meningeal layers, suggesting a trophic role.
RESULTS:
Meningeal progenitors transduce Wnt signaling
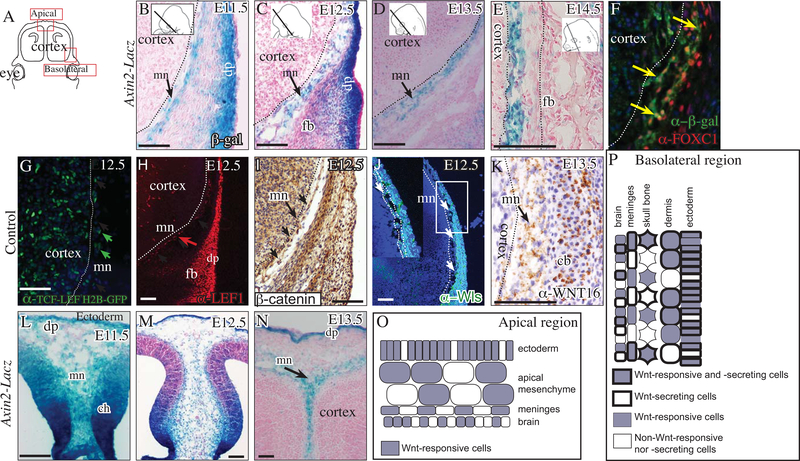
To investigate the spatiotemporal role of canonical Wnt signaling during the specification, differentiation, and expansion of meningeal progenitors, we first queried Wnt signaling activity in the cranial mesenchyme using two canonical Wnt signaling transgenic reporters: Axin2-LacZ/+ and TCF-LEF H2B-GFP/+, and target genes between E11.5–14.5 in the basolateral and apical regions (Figure 1A) (Lustig et al. 2002; Ferrer-Vaquer et al. 2010). Between E11.5–14.5, expression of Wnt signaling reporters was visible in the meningeal progenitors in the basolateral (Figure 1B-G) and apical sites (Figure 1L-N, S1). A subset of FOXC1+ meningeal progenitors were Axin2LacZ expressing cells (Figure 1F). At E12.5, Axin2-LacZ was clearly visible in the neighboring dermal and cranial bone progenitors in the basolateral site (Figure 1C). Between E11.5–12.5, Axin2-LacZ expression was visible in the cortical hem, the CM, and the surface ectoderm in the apex (Figure 1 L, M). At E13.5, Axin2-LacZ expression in the apical site became more restricted and remained visible in the meningeal progenitors and the dermal progenitors (Figure 1N). Lef1 mRNA expression is dependent on high levels of Wnt signaling(Rudloff and Kemler 2012). At E12.5, LEF1 protein expression was visible in a subset of the meninges closest to the brain, in the frontal bone progenitors, dermal progenitors, and in a few cells in the cortex (Figure 1H). As a direct readout of Wnt signaling activation, we detected nuclear β-catenin expression at E12.5 in the meningeal progenitors and the neighboring non-meningeal mesenchyme (Figure 1I).
Fig. 1. Meningeal mesenchyme transduces Wnt signaling preceding differentiation of meningeal layers.
Schematic illustration of coronal embryonic mouse head sections at the eye level along the dorsoventral axis in the forebrain (A). β-galactosidase staining and counterstain with eosin Y showing canonical Wnt signaling in meningeal mesenchyme progenitors (mn) between E11.5–14.5 (basolateral, B-E; apical L-N). Indirect immunofluorescence with DAPI counterstain (F, G, H, J) and immunohistochemistry with hematoxylin counterstain (I, K) showing canonical Wnt signaling (G, H, I) and Wnt ligand secretion capacity (J, K) in the meningeal mesenchyme (F) and neighboring cranial lineages. Summary schematic illustration of the cranial mesenchyme lineages and meninges in the apical region (O) and basolateral (P) regions at E12.5. Other abbreviations: dermal progenitors (dp) mesenchyme, and frontal bone (fb) primordia, cartilage base (cb), cortical hem (ch). All arrows point to the meningeal mesenchyme. Dotted line demarcates the frontal cortex of the brain from the meningeal mesenchyme. Scale bars represent 100μm.
Next, we examined the source of Wnt ligand-expressing cells. Wntless (Wls) is required for the secretion of all Wnt ligands and we found WNTLESS protein expression in all layers of the cranial mesenchyme, including meningeal progenitors and the overlying surface ectoderm at E12.5 (Figure 1J) (Bänziger et al. 2006; Bartscherer et al. 2006; Goodman et al. 2006). We previously identified numerous Wnt ligands expressed in a spatially-restricted pattern in the cranial ectoderm, mesenchyme, and cortex at E12.5 (Goodnough et al., 2014). Of the canonical Wnt ligands detectable in the cranial mesenchyme, we reliably detected protein expression of the canonical Wnt16 ligand in the meningeal mesenchyme, calvarial bones, and in the cartilage base at E13.5, showing that meningeal fibroblast progenitors can produce a canonical Wnt ligand ( Figure 1K). Together, these results show that canonical Wnt signaling transduction and Wnt ligand secretion occurs in the meningeal progenitors and neighboring cranial lineages in the basal and apical sites during early meningeal progenitor specification and differentiation (Figure 1O, P).
Cranial mesenchyme Wnt ligand secretion is dispensable for meningeal specification
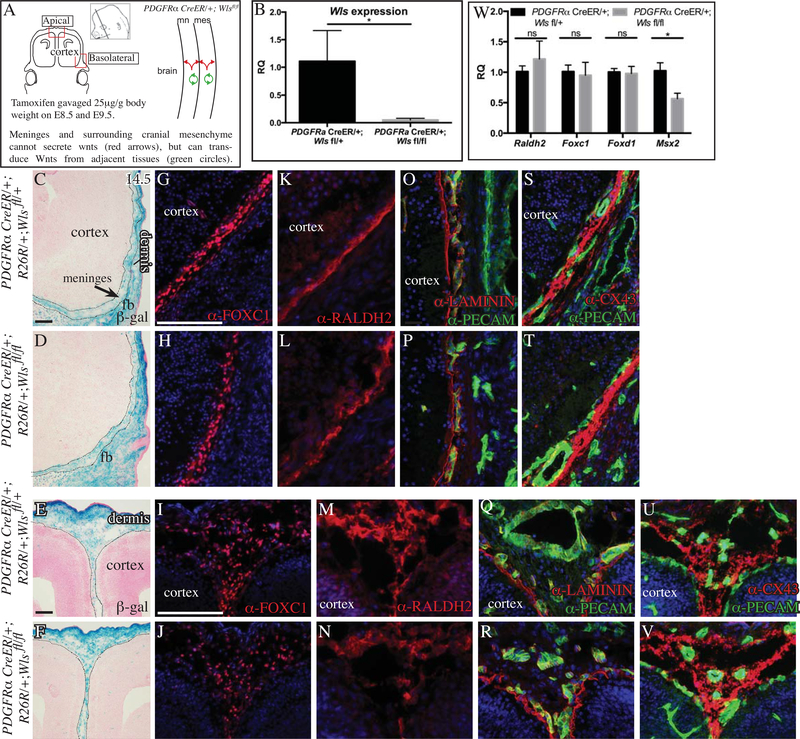
Preceding the differentiation of meninges into distinct layers, numerous Wnt ligands are expressed in the CM and surface ectoderm between E11.5–12.5 (Goodnough et al., 2014). First, we examined the role of Wnt ligand secretion by CM in meninges development by conditionally deleting Wntless (Wls) with the tamoxifen-inducible PDGFRαCreERT2/+ and Dermo1Cre/+ line by E9.5 (Figure 2A, S2) (Rivers et al. 2008; Carpenter et al. 2010). Relative quantity of Wls mRNA was markedly reduced in the CM of conditional Wls mutants, showing robust deletion of Wls (Figure 2B). The distribution of PDGFRαCreER/+;Rosa26-β-gal lineage-marked descendants was comparable between controls and conditional Wls mutants in the basolateral region (Figure 2C,D compare E,F). At E14.5, we found comparable domains of protein expression of meningeal fibroblast markers for FOXC1 (pan-meningeal), RALDH2 (subset of meningeal fibroblasts), in the basolateral domains of control and PDGFRαCreER/+;Wlsfl/fl mutants (Figure 2G, H, K, L) (Vivatbutsiri, Ichinose, Hytonen, et al. 2008; Spector et al. 2002; Zarbalis et al. 2007). Using qRT-PCR on E14.5 cranial mesenchyme, we found that relative quantity of Raldh2, Foxc1, and Foxd1 mRNA levels were comparable in the CM of control and Wls mutants at E14.5 (Figure 2W). The relative quantity of Msx2, a marker of the dura mater layer and calvarial bone primordia (Vivatbutsiri, Ichinose, Hytönen, et al. 2008) was significantly lower in the PDGFRαCreER/+;Wlsfl/fl mutant at E14.5. In the apical meningeal progenitors at E14.5, the distribution of FOXC1+ and RALDH2 expresssing cells was comparable between controls and PDGFRαCreER/+;Wlsfl/fl mutants (Figure 2I, J, M, N). Previously, we showed pan-mesenchyme Wls mutants have diminished calvarial bone differentiation, thereby contributing to decrease in relative quantity of Msx2 in our cranial mesenchyme preparation (Goodnough, et al., 2014). The number and volume occupied by FOXC1+ cells were not significantly different between control and PDGFRαCreER/+;Wlsfl/fl mutant mutants in the basolateral and apical sites (Table1 n=4–6 embryos per genotype)
Fig.2. Conditional deletion of Wntless (Wls) in cranial mesenchyme.
Schematic illustration of the coronal plane, tamoxifen induction regimen, lateral view of the embryonic head in the region of interest, and simplified schematic of genetic model (A). Relative quantity (RQ) of mRNA in E14.5 cranial mesenchyme of control and conditional Wls mutant cranial mesenchyme (n=4) (B, W). β-galactosidase staining with eosin counterstaining and black dotted lines outline meningeal mesenchyme (C-F). Indirect immunofluorescence for markers of meninges (G-N), basement membrane LAMININ (red, O-R), CD31/PECAM+ endothelial cells (green, O-V), Connexin43 in gap junctions (red, S-V) with DAPI-stained (blue) nuclei (G-V). Scale bars represent 100μM. * indicates statistical significance with p value between 0.01 and 0.05 (B, W). White and black dotted line demarcate the meningeal mesenchyme from adjacent cranial mesenchyme and cortex.
Table 1: Quantification of FOXC1+ cell number, volume and density of basolateral and apical meninges.
Statistical significance was calculated by comparing Cre positive heterozygotes controls with Wlsfl/fl or β-cateninfl/Δ mutants using two-tailed Student t-test.
| Genotype | Site | Average Cell Number per field | P Value | Average Meningeal Volume per field (pixels) | P Value | Average Meningeal Cell Density (cells per pixel) | P Value |
|---|---|---|---|---|---|---|---|
| PDGFRα Cre/+; Wlsfl/+ control | Basolateral | 85 ± 19.16 | 0.071 | 115368.67 ± 8854.83 | 0.48 | 7.3×10−4 ± 1.3×10−4 | 0.104 |
| PDGFRα Cre/+; Wlsfl/fl mutant | Basolateral | 118.1 ± 17.1 | 128030.48 ± 35153.08 | 9.6×10−4 ± 2.05×10−5 | |||
| PDGFRα Cre/+; Wlsfl/+ | Apical | 130.44 ± 35.77 | 0.95 | 324072.11 ± 69119.16 | 0.81 | 4.02×10–4 ± ×10−4 | 0.43 |
| PDGFRα Cre/+; Wlsfl/fl | Apical | 131.75 ± 35.70 | 311985.5 ± 81737.22 | 4.23×10–4 ± ×10−5 | |||
| Dermo1 Cre/+; β-cateninfl/+ control | Basolateral | 119.59 ± 28.28 | 0.081 | 190945.9 ± 13511.96 | 0.0017** | 6.607×10−4 ± 1.4×10−4 | 0.0063** |
| Dermo1 Cre/+; β-cateninfl/Δ mutant | Basolateral | 92.66 ± 12.06 | 111161.2 ± 35280.88 | 8.423×10−4 ± 8.73×10−5 | |||
| Dermo1 Cre/+; β-cateninfl/+ | Apical | 160.53 ± 41 | 0.002** | 270108.6 ± 39129.62 | 0.0024** | 6.0×10−4 ± 1.46×10−4 | 0.059 |
| Dermo1 Cre/+; β-cateninfl/Δ | Apical | 72.47 ± 17.94 | 168065.78 ± 47057.22 | 4.45×10−4 ± 9.62×10−5 |
Meninges secrete matrix molecules such as laminin and heparin sulfate proteoglycans and contribute to the basement membrane (BM) of the meninges (Bifari et al. 2015; Richtsmeier and Flaherty 2013; Mercier, Kitasako, and Hatton 2002). The vasculature of the meninges with PECAM/CD31 expression in endothelial cells and gap junctions expressing CONNEXIN(CX43) in the arachnoid layer. Expression of these proteins have been used as an indicator of meningeal development (Baldwin et al. 1994; Yancey, Biswal, and Revel 1992; Vivatbutsiri, Ichinose, Hytönen, et al. 2008; Zarbalis et al. 2007). We found continuous expression of LAMININ protein medial to the layer of PECAM+ endothelial cells in control and PDGFRαCreER/+;Wlsfl/fl mutants at E14.5 in the basolateral and apical regions (Fig 2 O-R). Similarly, we found comparable distribution of CX43 expression in the basolateral and apical sites in the control and the PDGFRαCreER/+;Wlsfl/fl mutants (Figure 2S, T, U, V). We examined the presence of Wnt signal transduction in the meningeal mesenchyme by LEF1 protein expression and TCF-LEF H2B-GFP protein expression and found that PDGFRαCreER/+;Wlsfl/fl mutants transduced canonical Wnt signaling in the meningeal progenitors in the basolateral and apical sites, suggesting other sources of Wnts (Figure S1). We obtained similar results in the conditional Wls mutants with another pan-CM Cre line, such as the Dermo1Cre/+; Axin2LacZ/+; Wlsfl/fl mutants (Figure S2). Together, these studies suggest that Wnt ligand secretion in the cranial mesenchyme is dispensable for the specification and early differentiation of meningeal progenitors.
The ectoderm is a rich source of numerous Wnt ligands and is required for canonical Wnt signaling transduction in the underlying CM (Goodnough, et al., 2014). This led us to hypothesize that ectoderm Wnt ligands could be required for meningeal development. We used the Crect/+ line, which is active in the cranial ectoderm by E9.5, to delete Wls in the the surface ectoderm (Reid et al. 2011; Goodnough, et al., 2014). Crect/+; Wlsfl/fl mutants lack dermal and calvarial bone progenitors specification and apical expansion, and instead have ectopic cartilage at the basolateral site (Goodnough, et al., 2014). At E13.5, FOXC1+ meninges and RALDH2 expression in a subset of meningeal fibroblast progenitors was present in the control and Crect/+; Wlsfl/fl mutants (Figure S3). In the basolateral region, distribution of FOXC1+ cells was shifted laterally towards the ectoderm and appeared beneath the ectoderm in the medial regions (Figure S3). We found meningeal progenitors were also able to transduce canonical Wnt signaling in the Crect/+; Wlsfl/fl mutants at E13.5, suggesting the source of Wnt ligands for meningeal Wnt signaling excludes the surface ectoderm and CM (Figure S3). Given the absence of cranial bone and atrophy of the cranial mesenchyme, we could not analyze these mutants at later time points. Thus, by E13.5, ectoderm Wnts are not required for the specification of FOXC1+ meninges in both the basolateral and the apical regions, but our analysis does not exclude a role for ectodermal Wnts later in meningeal differentiation.
Basolateral meningeal layers are supported by Wnt/β-catenin-transducing non-meningeal mesenchyme
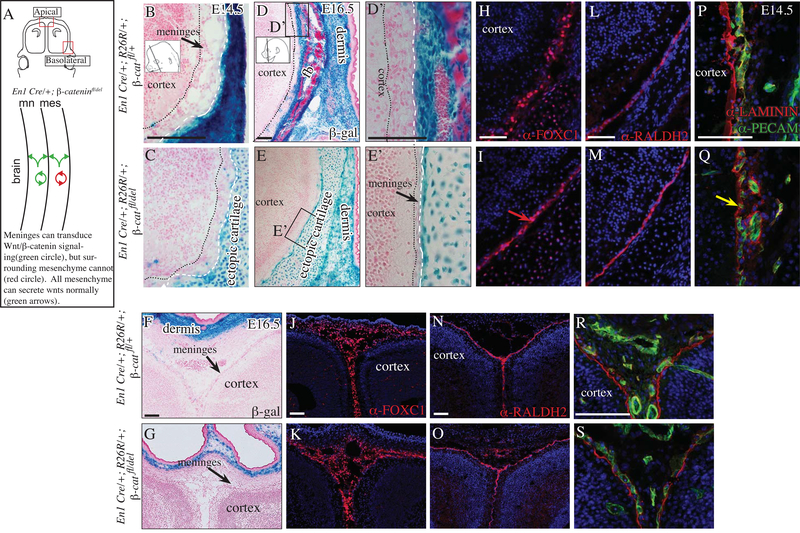
Next, we tested the requirement of β-catenin in CM for meningeal differentiation between E14.5–16.5. We used two different Cre lines to dissect the requirement of canonical Wnt/β-catenin signaling in meningeal (Dermo1Cre) and non-meningeal mesenchyme (Engrailed1Cre, En1Cre) during meningeal development in the basolateral and apical sites (Figure 3A, 4A). We use the En1Cre line to efficiently delete β-catenin in non-meningeal cranial mesenchyme between E10.5–11.5 as previously shown (Tran et al. 2010; Goodnough et al. 2012). The En1Cre lineage-marked cells do not contribute to meningeal fibroblast progenitors basolaterally and apically beneath the frontal bone (Figure 3B-G). Therefore, the En1Cre/+; β-cateninfl/del mutants allow us to test the hypothesis that β-catenin and canonical Wnt signal transduction in the adjacent non-meningeal cranial mesenchyme is required for meningeal differentiation. At E14.5 we found the meninges appeared thinner in the basolateral region of En1Cre/+; β-cateninfl/del mutants, and by E16.5 we observed marked hypoplasia of the meninges in the basolateral region (Figure 3B-E). Compared to controls at E16.5, the volume of pan-meningeal markers, such as FOXC1+, was thinner in the mutants. However, RALDH2 expression domains was comparable in the control and mutant in the basolateral region (Figure 3H, I, L, M). In order to determine the source of the hypoplasia, we examined expression of of the LAMININ and PECAM at E14.5. Compared to the control, the expression of BM-LAMININ adjacent to the PECAM+ cells was markedly diminished in the mutant. The PECAM cell layer was disorganized and appeared consistently overlapping the LAMININ layer in the mutant, suggesting changes in the matrix and vasculature in basolateral region. Thus, at the basolateral site by E16.5, Wnt/β-catenin signal transduction in the non-meningeal cranial mesenchyme is required for maintenance and organization of meningeal volume, BM, and vasculature. In the apex, we found the morphology and expression of meningeal markers, and LAMININ in the En1Cre/+;R26R/+; β-cateninfl/del mutants were comparable to controls, showing that Wnt/β-catenin signaling in the non-meningeal cranial mesenchyme is dispensable for meningeal development in the apical site (3J, K, N, O, R, S).
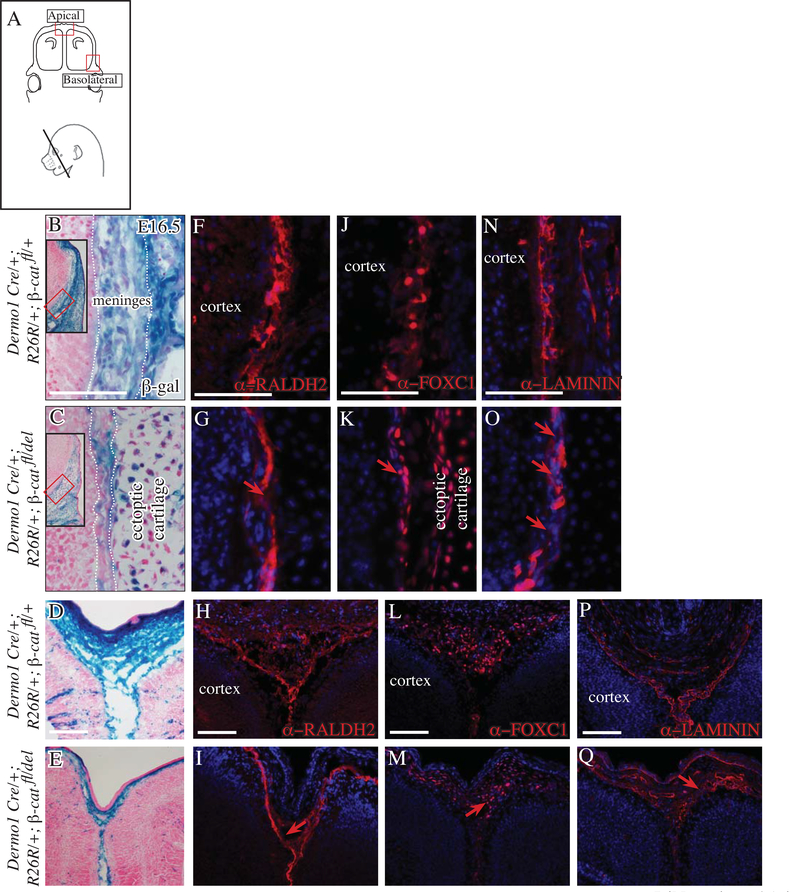
Fig 3. Basolateral meninges are diminished in the absence of β-Catenin in the non-meningeal cranial mesenchyme.
Diagram of coronal embryonic mouse head sections at the eye level in the forebrain showing the regions of interest and simplified schematic of genetic model (A). β-galactosidase staining counterstained with eosin Y (basolateral B-E; apical F, G. Higher mag basolateral D’, E’; apical F’, G’). Indirect immunofluorescence for markers of meninges (H-O), basement membrane (BM) LAMININ (red, P-S), CD31/PECAM+ endothelial cells (green, P-S) with DAPI-stained (blue) nuclei (H-S). Black arrow points to meningeal hypoplasia (E, E’) and red arrow shows compaction of FOXC1+ expressing cells in the basolateral site of the mutant at E16.5 (I). Yellow arrow points to diminished BM-LAMININ in the mutant (Q). Scale bars represent 100μm. Abbreviations: fb: frontal bone, hf: hair follicle. Dotted black line demarcates the cortex and white hatched line demarcates the frontal bone from the meninges (B-E).
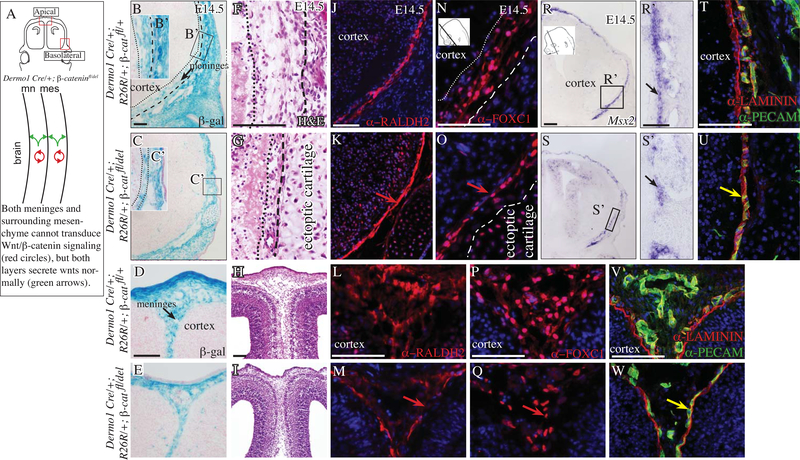
Fig 4. Conditional deletion of cranial and meningeal mesenchyme β-catenin results in thinner meninges.
Diagram of coronal embryonic mouse head sections at the eye level in the forebrain showing the regions of interest and simplified schematic of genetic model (A) Coronal mouse embryonic sections at eye level (B-W) in the basolateral region (top two rows) and apical region (bottom two rows). β-galactosidase expression counterstained with eosin Y (B-E), hematoxylin and eosin stain (F-I). Indirect immunofluorescence for meningeal markers (J-Q), basement membrane (BM) LAMININ (red, T-W), CD31/PECAM+ endothelial cells (green, T-W). Red arrow points to compaction of meninges marker expressing cells (K, O) and diminished domain of expression in the apical site in the mutant (M, Q). Yellow arrow points to overlap of BM LAMININ and PECAM layers in the mutant (red and green, U, W). Scale bars represent 100μm.
The En1Cre/+;R26R/+; β-cateninfl/del mutants have ectopic cartilage nodules in place of calvarial bone and dermis. To test the requirement of calvarial bone primordia for specification of meningeal progenitors, we analyzed Dermo1Cre/+; R26R/+; Twist1fl/fl mutants, which lack calvarial osteoprogenitors and Twist1 throughout the cranial mesenchyme (Goodnough, DiNuoscio, and Atit 2016). At E12.5, we found comparable expression domains of FOXC1 expression in the controls and conditional Twist1 mutants, suggesting the calvarial bone primordia and Twist1 are not required to specify FOXC1+ meningeal progenitors (Figure S4). Due to extensive agenesis of cranial mesenchyme in the apical regions, we could not analyze the apex and meninges differentiation in later stages of development in the Twist1 mutants.
Deletion of Wnt signal transduction in cranial and meningeal mesenchyme leads to structural changes and hypoplasia of the meninges
Finally, we queried if transduction of canonical Wnt/β-catenin signaling in the meningeal layers is required for meningeal differentiation in the basolateral and apical regions. In the absence of a meningeal progenitor-specific Cre line, we conditionally deleted β-catenin broadly in the CM by E10.5 with Dermo1Cre (Figure 4A)(Goodnough et al. 2012). In the control and mutant, Dermo1Cre lineage-marked cells were present in the meningeal fibroblast progenitors at E14.5 in both the basolateral and apical regions (Figure 4B-E). Histology of the Dermo1Cre/+; R26R/+; β-cateninfl/del mutants showed marked hypoplasia of the meningeal layers at E14.5 in the basolateral and apical sites (Figure 4F-I). Marker analysis at E14.5 revealed the distribution of expression domains of RALDH2 in a subset of meningeal fibroblasts appeared compressed in the basolateral region and had breeches in expression in the apical region (Figure 4J-M). FOXC1 was also expressed in controls and mutants but the domain of expression appeared narrower (Figure 4N-Q). Msx2 expressing meningeal fibroblasts form in close proximity to the calvarial bone primordia (Vivatbutsiri, Ichinose, Hytonen, et al. 2008). We found Msx2 mRNA expression domain at E14.5 was continuous in the control and had gaps in expression between the ectopic cartilage nodules in the basolateral region of the Dermo1Cre/+; R26R/+; β-cateninfl/del (Figure 4R, S, R’ S’).
Quantifying the average number of FOXC1+ meningeal fibroblast progenitors in a fixed field revealed that the number of cells were not significantly different between controls and Dermo1Cre/+; R26R/+; β-cateninfl/del mutants in the basolateral region (Figure 4N-Q, Table 1). However, in the apical region, the number of FOXC1+ were significantly lower in the mutants. Next, we analyzed the volume of FOXC1+ cells in a fixed area in control and in the Dermo1Cre/+; R26R/+; β-cateninfl/del mutant. We found significantly decreased volume in the mutant and conversely the density was increased in the basolateral region; thereby, accounting for the meningeal hypoplasia in the basolateral site. In the apical site, the FOXC1+ cell number and volume were significantly lower in the mutant and the density did not approach statistical significance (Table1). Finally, we examined if meningeal hypoplasia was due to changes the meningeal-derived LAMININ or vasculature. We found continuity in the BM-LAMININ in the basolateral and apical regions at E14.5 in the controls and Dermo1Cre/+; R26R/+; β-cateninfl/del mutant. However, the distribution of PECAM+ endothelial cells in the meninges was different. The PECAM+ layer was not juxtaposed to the BM-LAMININ, but was overlapping with the BM in the basolateral and apical regions. These results suggest there is a collapse of the matrix and vasculature layers on each other.
Next we examined other causative mechanisms that can contribute to the observed meningeal hypoplasia in the Dermo1Cre/+; R26R/+; β-cateninfl/del by E14.5 First, we examined cell survival by activated-CASPASE 3 protein expression at E12.5 and E14.5. We did not detect an appreciable increase in cell death preceding the hypoplasia of the meninges in the basolateral region of the mutants (Figure S5). Second, the proliferation index of meningeal progenitors was comparable in controls and mutants at E12.5 (51% ±6% in controls and 47.4% ±1.8 in the mutants) and E14.5 (49.4% ±5.3% in controls and 43% ±7.2 in the mutants). Our proliferation index in E14.5 controls is consistent with a published report in embryonic rat meninges (Bifari et al. 2015). Third, we investigated the possibility of some of the meningeal progenitors convert to ectopic cartilage phenotype in the conditional β-cateninfl/del mutant; thereby, leading to less meningeal tissue. The non-meningeal mesenchyme, containing cranial bone and dermal progenitors, ectopically express the key cartilage determinant marker, SOX9, by E12.5 (Tran et al. 2010; Goodnough et al. 2012). We did not find ectopic expression of SOX9 in the basolateral or apical meningeal sites (Figure S6). SOX5, a downstream target of SOX9 in chondrocytes was excluded in the meningeal mesenchyme in the control and in the mutant, showing the meningeal progenitors do not convert to the ectopic cartilage in the conditional β-cateninfl/del mutant (Figure S6) (Lefebvre et al.,1998). Fourth, meninges can also produce several growth factors that can signal through mitogen activated protein kinase/extracellular regulated kinase (MAPK/ERK) (Bifari et al. 2015; Richtsmeier and Flaherty 2013; Mercier et al., 2002). Compared to the control, we could not detect an appreciable loss of activated pERK at E12.5(data not shown), suggesting the meningeal hypoplasia is due to changes in the BM and vasculature.
At E16.5, we found progressive meningeal hypoplasia in Dermo1Cre/+; R26R/+; β-cateninfl/del mutants (Figure 5). First, we examined the distribution of lineage marked cells by β-gal staining. Compared to the control, the mutants had fewer lineage-marked cells in the hypoplastic meninges and replacement by wild-type cells in the basolateral region. In the apical site, substantial thinning was consistently observed (Figure 5 B-E). At E16.5, there were breaches in RALDH2 expression domain in the Dermo1Cre/+; R26R/+; β-cateninfl/del mutants at the basolateral site and apical sites (Figure 5F-I). Consistent with the visible hypoplasia at E14.5 and progressive hypoplasia by E16.5, the domain of FOXC1 expression pattern in the basolateral and apical sites at E16.5 was markedly diminished in the mutants (Figure 5 K-M). In the mutant, the periosteum of the ectopic cartilage nodules and cartilage expressed FOXC1 and was juxtaposed to the hypoplastic meninges in the basolateral site (Figure 5K). By E16.5 in the mutant, we found the BM became poorly organized with significant breeches in the domain of LAMININ protein expression in the basolateral and apical sites (Figure 5 P-S). These data show that in the absence of Wnt/β-catenin signaling in the meningeal and non-meningeal cranial mesenchyme, the number of FOXC1+ cells are affected at the apical site, and the whole meninges become progressively hypoplastic and less organized earlier in development, most likely due to BM defects.
Fig 5. Conditional deletion of cranial and meningeal mesenchyme β-catenin results in meningeal hypoplasia in the basolateral and apical regions.
Diagram of coronal embryonic mouse head sections at the eye level in the forebrain showing the regions of interest (A) in basolateral region (top two rows) and apical region (bottom two rows). β-galactosidase expression counterstained with eosin Y (B-E) showing replacement with non-lineage marked wild-type cells (pink). Indirect immunofluorescence for meningeal markers (F-M), basement membrane (BM) laminin (red, N-Q) with DAPI-stained nuclei. Red arrow points to breech in RALDH2 expression (G, I), diminished domain of FOXC1 expression (K, M), breech and disorganized BM (O, Q) in the mutants. Scale bars represent 100μm.
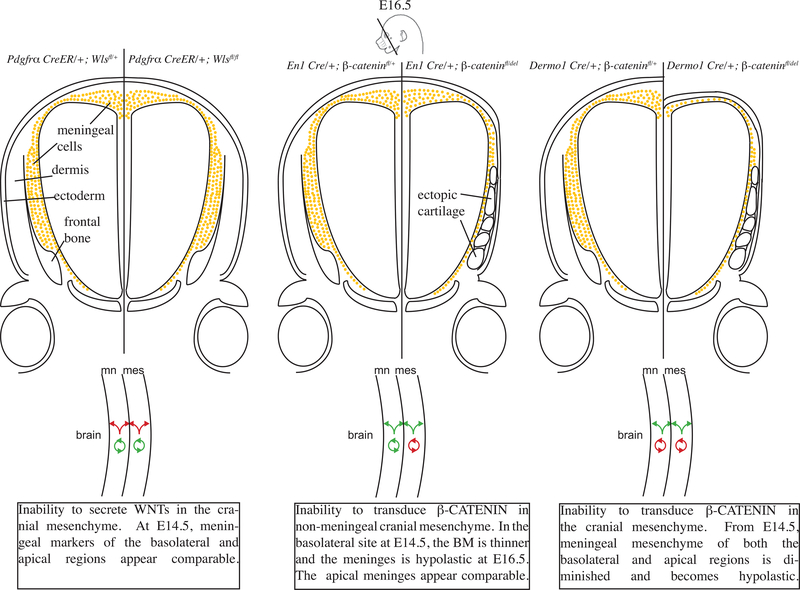
Taken together, these results demonstrate that the basolateral and apical regions of the meninges have differing requirements for Wnt/β-catenin signaling to prevent meningeal hypoplasia. Wnt/β-catenin transduction is required in the non-meningeal mesenchyme in the basolateral site and in the meningeal apical mesenchyme to sustain the emerging differentiated layers of the meninges and organization of BM-Laminin and vasculature (Figure 6).
Fig. 6. Summary schematic of the genetic mutants used throughout the study and their effects on meningeal development.
Wnt/β-catenin signaling is required in meningeal and non-meningeal cranial mesenchyme for proper development of meninges.
Discussion
In this study, we examined both loss of Wnt ligand secretion and Wnt signaling function in meningeal and non-meningeal populations during early meningeal development between E11.5–16.5. The salient findings are as follows: (1) The loss of Wnt ligand secretion in either the whole cranial mesenchyme or the surface ectoderm still allows for canonical Wnt signaling in the mesenchyme, and meningeal specification and differentiation proceeds. (2) β-catenin is dispensable or functionally redundant in meningeal mesenchyme for specification of FOXC1+ meningeal progenitors. (3) Wnt/β-catenin signaling in the non-meningeal cranial mesenchyme is required to prevent meningeal hypoplasia, likely through a paracrine mechanism in the basolateral site and cell-autonomously in the apical site. (4) Wnt/β-catenin signaling in the meningeal mesenchyme is required for supporting the cell number and volume of meninges and BM in basolateral and apical sites. These data suggest a mode where Wnt/β-catenin signaling in the cranial and meningeal mesenchyme supports the early differentiating meninges that produce organized and sufficient BM and prevents meningeal hypoplasia in the basolateral and apical regions.
The canonical Wnt/β-catenin signaling pathway is one of the earliest in the cranial meningeal and non-meningeal mesenchyme. The cranial surface ectoderm, CM, and the cortical hem are major sources of Wnt ligands for the basolateral and apical meninges preceding the specification and differentiation of the meninges, dermis, and calvarial bone (Goodnough, 2014a; Choe et al., 2012). The developmental program of the meninges coincides with the specification of calvarial bone and dermal progenitors in the CM, and Wnt/β-catenin signaling is required in the CM for the specification of those two tissues. Thus, the timing of these developmental programs prompted us to investigate the role of Wnt-mediated interactions within the meningeal and from the non-meningeal mesenchyme in post-migratory CM. Loss of Wnt ligand secretion from either the surface ectoderm or the cranial mesenchyme still allowed canonical Wnt signaling transduction in the meningeal progenitors in the basolateral and apical sites. We suspect that Wnt ligands from the brain and cortical hem or elsewhere may be transduced in the meningeal progenitors. Testing this hypothesis will be challenging because WNTLESS is required for brain development in Wnt 1 expressing cells (Fu et al. 2011). We found that conditional deletion of β-catenin in the meningeal and non-meningeal cranial mesenchyme allowed for the specification of FOXC1+ and RALDH2+ meningeal progenitors, suggesting a redundant role of another signaling pathway, or that Wnt/β-catenin signaling is dispensable for meningeal specification. Formally testing this hypothesis awaits the development of meningeal progenitor-specific genetic tools to demonstrate if other key signaling pathways such as retinoic acid signaling, TGFβ, and Bone morphogenetic protein (BMP) signaling converge with Wnt signaling to specify the meningeal progenitors.
As development proceeds, basolateral meningeal mesenchyme begins to differentiate into layers in close proximity to the ossifying cranial bone primordia. Previous studies implied that the dura and arachnoid layers fail to differentiate in the absence of calvarial bone (Kume et al. 1998; Zarbalis et al. 2007). Conditional deletion of transforming growth factor β receptor 2(TGFβR2) in the premigratory CNCC led to agenesis of calvarial bones and defects in the dura layer, suggesting interaction or inductive signaling events between paraxial mesoderm cranial bone and CNCC-derived meningeal progenitors (Ito et al. 2003). We investigated the requirement of cranial bone primordia or ossified cranial bone in the specification and differentiation of meningeal layers using three different mutants (Figure 3, 4, S4). We found that specification of FOXC1+ meningeal fibroblast progenitors occurs in the basolateral site, suggesting that cranial bone primordia and cranial bone are not required for specifying meningeal progenitors. But, we did find changes in Msx2 expression in the conditional Wls and Dermo1Cre; β-catenin mutants, suggesting that a subset of meningeal fibroblasts may be be dependent on cranial bone primordia. Similarly, in the En1Cre;β-catenin mutants, where we preserve Wnt signaling transduction in the meningeal layers and have cartilage replacement of cranial bone primordia, the basolateral meninges had changes in the organization of BM-LAMININ and vasculature and subsequently became hypoplastic in development, suggesting that Wnt signaling is required to sustain the emerging meningeal layers structurally after specification events. Future experiments will focus on elucidating how Wnt/β-catenin signaling in the non-meningeal mesenchyme sustains the basolateral meninges, BM, and vasculature layers.
A clear implication of our study is that an optimal level of Wnt signaling must be maintained in the cranial mesenchyme for the normal development of the meninges and production of organized and sufficient BM. Using our different genetic lines, we found that the basolateral and apical meninges have different signaling requirements to maintain meningeal layers. β-catenin signaling in the non-meningeal mesenchyme was required for the maintenance of meningeal layer volume and BM layer as demonstrated by both β-catenin mutant mouse lines. The En1Cre;β-catenin mutant indicates that Wnt/β-catenin signaling is required in the non-meningeal mesenchyme for meningeal mesenchyme development and organization of the basolateral site and not the apical site. However, as demonstrated by the Dermo1Cre;β-catenin mutant, cell autonomous β-catenin signaling in the meningeal progenitors in the apical site was required to support and maintain the volume of the meninges and cell number of the progenitors, suggesting a structural and trophic role. The lower number of FOXC1+ cells can disrupt the ability of the meninges to deposit laminin and maintain the BM which which leads to BM defects (Zarbalis et al., 2007). We previously found that β-catenin signaling transcriptionally regulates mRNA expression of ECM genes, such as Laminin, proteoglycans, and other ECM modulating proteins in embryonic and adult dermal fibroblasts (Budnick et al. 2016; Hamburg-Shields et al. 2015; Mullins et al., 2017) The trophic factors originating from the non-meningeal mesenchyme in the basolateral site appears to be β-catenin-dependent. The absence of β-catenin in meningeal mesenchyme accelerates the hypoplasia of the meningeal layers showing the cell autonomous and non-cell autonomous requirement for β-catenin in the meningeal layers. The overall picture that emerges is of a finely tuned system, in which the correct balance of β-catenin-dependent mesenchyme factors and subsequent response in the meningeal progenitors is needed to allow for promoting and maintaining meningeal layers and sufficient deposition of the BM matrix proteins.
This study provides new results related to early meningeal development. Meninges, with their trophic factors, ECM chemotactic factors, matrix molecules, and stem cells, serve as key players in brain and skull bone development. Further understanding of how meninges develop and interact with neighboring tissues will inform our understanding of the etiology of congenital defects arising in the CM-derived lineages and the interplay between them during disease and fracture healing.
Materials and Methods:
Mice and Genotyping
Mouse lines used for conditional functional studies included Dermo1/Twist2Cre (Yu 2003), En1Cre (Kimmel et al. 2000) (gift of Alexandra Joyner), PDGFRαCreER (Jax stock# 018280), Wls flox (Carpenter et al. 2010)(gift of Richard Lang), β-Catenin deleted (Haegel et al. 1995), β-Catenin conditional floxed (Brault et al. 2001). These mouse lines were maintained on a mixed genetic background. Mouse Cre lines were crossed with R62R (Soriano 1999) for genetic lineage tracing. Wnt signaling reporters Axin2 lacz (Jax stock 009120) Reference and TCFlefH2BGFP (Jax stock 013752) were also used. Mice were backcrossed to CD1 background at least 10 generations. Mice were time-mated to yield desired crosses with vaginal plug day assigned as E0.5. Dams carrying embryos between E8.5 and E9.5 were orally gavaged with tamoxifen (25μg/gm body weight) to activate CreER and conditionally delete Wls and activate R26R in the cranial mesenchyme. At desired time points, embryos were harvested and processed for frozen or paraffin sections as previously described (Atit et al. 2006). Each experiment was performed on a minimum of three mutants with litter-matched controls. A minimum of three litters were used for each functional analysis. All animal handling and experimental procedures were approved by the Case Western Reserve Institutional Animal Care and Use Committee.
Histology, β-Galactosidase, AP staining
Embryos were drop-fixed in 4% paraformaldehyde at 4°C, sucrose dehydrated, and embedded in O.C.T. Compound (Tissue-Tek Sakura) and sectioned at 10 microns as previously described (Atit et al. 2006; Ohtola et al. 2008). H&E staining was performed by standard protocol. For β-Galactosidase staining, embryos were prepared and sectioned as above. Slides were washed in X-gal wash buffer (0.1% deoxycholate, 0.2% NP40, 2 mM MgCl2, made up in 1X phosphate-buffered saline pH 7.4) and then incubated in X-gal staining solution (Amresco) at 1mg/mL in 5 mM ferricyanide, 5 mM ferrocyanide in wash buffer overnight at room temperature. The following day, slides were washed in PBS, counterstained with Eosin as described above, and mounted with Permount mounting medium (Fisherbrand). Alkaline phosphatase staining on sections was done as previously described (Goodnough, 2012). All microscopy was done on an Olympus BX60 and all microscopy photos were taken with a Leica DP72 camera using cellSens software.
In situ Hybridization, Immunohistochemistry
In situ hybridization on cryosections was performed as previously described (Holmes and Niswander 2001). Msx2 in situ probe was a gift from Dr. Jill Helms. For immunofluorescence, cryosections were post-fixed for 5 minutes in 4% PFA, and were blocked in 1% BSA. Species-specific serum with 0.01% Triton was used to block and incubate overnight in primary antibody at 4°C. Appropriate species-specific secondary antibodies were incubated for 1 hour the next day at room temperature, and then slides were mounted with Fluorshield (Sigma). For immunohistochemistry, heat-citrate-mediated antigen retrieval for 10 minutes and peroxidase block was performed before incubating with primary antibody overnight at 4°C. Secondary antibody incubation for 1 hour was performed the next day, followed by amplification with ABC reagent (Vector) and visualized with DAB (Amresco) and hematoxylin (Fisher) counterstain. Primary antibodies used include: anti- LEF1 (1:100)(Cell Signaling 2286), WNT16 (1:50)(Santa Cruz Bio 20268), β-CATENIN (1:100)(BD Biosciences 610153), non-phospho β-CATENIN (1:200)(Cell Signaling 8814), KI67 (1:100)(Abcam 15580), CONNEXIN43 (1:3200)(Sigma Aldrich c6219), PECAM (1:10)(BD Biosciences 550274), FOXC1 (1:100)(Cell Signaling 8758), RALDH2(1:200)(Sigma Aldrich HPA010022), active-CASPASE3 (1:250)(Abcam 13847), LAMININ (1:500)(Sigma Aldrich L9393), SOX9 (1:2000)(Chemicon ab5535), SOX5(1:1200)(Abcam ab94396), WNTLESS (1:2000)(gift of Richard Lang), β-Galactosidase (1:100; Abcam ab 9361).
Quantification of FOXC1+ cell number, volume, density was calculated in a fixed field. Total number of FOXC1+ cells were manually quantified and meningeal volume was quantified by outlining the domain occupied by FOXC1+ cells using FIJI Image J software. Cell density was calculated by dividing # of FOXC1+ cells/meningeal volume measurements. Significance was calculated using two-tail, type 3, Student t-test in Microsoft Excel.
Proliferation
Sections were collected and Ki67 immunohistochemistry was performed as above(Goodnough, DiNuoscio, Ferguson, Williams, Lang, and Atit 2014a). Total meningeal cells were counted in a fixed field of similar region on two to five sections per embryo a minimum of 50 microns apart (n = 6–9). Two-tail, type 1, Student’s t-test was used to determine statistical significance of Ki67 positive vs. negative cells.
Cell Death Assay
Sections were collected and active caspase3 immunohistochemistry was performed as above to assay for survival. Slides were mounted with Fluoroshield (Sigma). Photos were taken in aforementioned manner. Total meningeal cells were counted in a fixed field of similar region on two to five sections per embryo a minimum of 50 microns apart (n = 6–9). Two-tail, type 1, Student’s t-test was used to determine statistical significance of active Caspase3 positive vs. negative cells.
RNA Isolation and qPCR
Cranial mesenchyme, including the meningeal layers, was mechanically isolated from embryonic mouse heads at E14.5 and total RNA was isolated using Trizol (Invitrogen) per manufacturer’s instructions. cDNA was made using a Multiscribe Reverse Transcription kit (Life Technologies). qPCR was performed on a StepOne Plus (ABI) using Taqman chemistry. Probes used were Wls (Mm00509695_m1), Foxc1 (Mm01962704_m1), Raldh2 (Mm00501306_m1), Msx2 (Mm00442992_m1), and Foxd1 (Mm00843738_s1). Relative gene expression was calculated using the 2−ΔCt method (Livak and Schmittgen 2001).
Supplementary Material
Figure S1: Wnt signaling transduction is present in meningeal progenitors in the absence of cranial mesenchyme Wnt ligand secretion. Schematic illustration of the coronal plane, tamoxifen induction regimen, and inset depicts the lateral view of the embryonic head in the region of interest (A). Indirect immunofluorescence for LEF1 in the basolateral region (B,C), FOXC1 (pan-meningeal progenitor at E12.5) and GFP in the apical region (D,E). Red arrows point to LEF1+ cells, and yellow arrows point to TCF-LEF H2B-GFP and FOXC1+ cells. Scale bars represent 100μM. Meninges (mn), frontal bone (fb).
Figure S2. Conditional deletion of Wls in cranial mesenchyme does not appear to affect meningeal histology. Schematic illustration of the coronal plane (A). β−galactosidase staining, basolateral (B,C), and apical (D,E) sites. Indirect immunofluorescence for CONNEXIN43 (red) in gap junctions of the meninges and CD31/PECAM (green) in the endothelial cells of meningeal mesenchyme with DAPI-stained nuclei in the basolateral (F,G) and apical site (H,I). Scale bars represent 100μm.
Figure S3. Conditional deletion of Wls in cranial surface ectoderm. Schematic illustration of the coronal plane (A). Indirect immunofluorescence for FOXC1 (basolateral B, C; apical D, E), RALDH2 (basolateral F, G; apical H, I), and LEF1 (basolateral J, K; apical L, M) with DAPI-stained (blue) nuclei on coronal mouse embryonic head sections. Scale bars represent 100μM.
Figure S4. Conditional mutant of Twist1 lacks bone primordia and ectopic cartilage, but FOXC1 meningeal progenitor are present. Schematic illustration of the regions of interest in the coronal plane (A). Hematoxylin and eosin staining of the basolateral region of E13.5 heads (B, C). Indirect immunofluorescence for FOXC1 in the meningeal fibroblasts with DAPI-stained nuclei on coronal mouse embryonic mouse sections showing they are expressed comparably in control and Twist1 mutant in the basolateral region (F, G) and apex (D, E). Scale bars represent 100μm. Frontal Bone (fb).
Figure S5. Cell survival is not compromised in Dermo1Cre/+ β-catenin fl/del mutants.
Immunohistochemistry for activated CASPASE3 present in apoptotic cells in the palate (A), basolateral meninges (B, C), and apical site (D, E) counterstained with hematoxylin. Scale bars represent 100μM.
Fig S6. Conditional β-catenin deletion does not result in ectopic expression of cartilage determinants or early reduction of laminin matrix protein. Indirect immunofluorescence on mouse embryonic coronal sections at eye level for chondrocyte markers SOX5 (A-D) or SOX9 (E-H) with DAPI (blue) counterstained nuclei. Scale bars represent 100μm.
Acknowledgments:
Thanks to previous and current members of the Atit laboratory for excellent discussion and advice. We thank the undergraduate students, Miarasa Steele, Samuel Pan and Vidhi Mendpara, for their assistance with sectioning tissues.
Funding: This work was supported by the following grants: NIH-NIDCR-R01DE01870 (R.P.A.)
Footnotes
Conflict of interest: The authors declare that they have no competing interests.
References:
- Angelov DN, and Vasilev VA. 1989. “Morphogenesis of Rat Cranial Meninges. a Light- and Electron-Microscopic Study.” Cell and Tissue Research 257 (1): 207–16. [DOI] [PubMed] [Google Scholar]
- Atit Radhika, Sgaier Sema K, Mohamed Othman A, Taketo Makoto M, Dufort Daniel, Joyner Alexandra L, Niswander Lee, and Conlon Ronald A. 2006. “Beta-Catenin Activation Is Necessary and Sufficient to Specify the Dorsal Dermal Fate in the Mouse.” Developmental Biology 296 (1): 164–76. doi: 10.1016/j.ydbio.2006.04.449. [DOI] [PubMed] [Google Scholar]
- Baldwin HS, Shen HM, Yan HC, DeLisser HM, Chung A, Mickanin C, Trask T, Kirschbaum NE, Newman PJ, and Albelda SM. 1994. “Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1/CD31): Alternatively Spliced, Functionally Distinct Isoforms Expressed During Mammalian Cardiovascular Development..” Development (Cambridge, England) 120 (9): 2539–53. [DOI] [PubMed] [Google Scholar]
- Bartscherer Kerstin, Pelte Nadège, Ingelfinger Dierk, and Boutros Michael. 2006. “Secretion of Wnt Ligands Requires Evi, a Conserved Transmembrane Protein.” Cell 125 (3): 523–33. [DOI] [PubMed] [Google Scholar]
- Bänziger Carla, Soldini Davide, Schütt Corina, Zipperlen Peder, Hausmann George, and Basler Konrad. 2006. “Wntless, a Conserved Membrane Protein Dedicated to the Secretion of Wnt Proteins from Signaling Cells.” Cell 125 (3): 509–22. [DOI] [PubMed] [Google Scholar]
- Bifari Francesco, Berton Valeria, Pino Annachiara, Kusalo Marijana, Malpeli Giorgio, Marzia Di Chio, Bersan Emanuela, et al. 2015. “Meninges Harbor Cells Expressing Neural Precursor Markers During Development and Adulthood.” Frontiers in Cellular Neuroscience 9 (October): 827. doi: 10.1016/0092-8674(91)90512-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsson, Christopher S, Apostolopoulou Maria, Tian Yangzi, and Temple Sally. 2015. “It Takes a Village: Constructing the Neurogenic Niche..” Developmental Cell 32 (4): 435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, Mcmahon AP, Sommer L, Boussadia O, and Kemler R. 2001. “Inactivation of the Beta-Catenin Gene by Wnt1-Cre-Mediated Deletion Results in Dramatic Brain Malformation and Failure of Craniofacial Development.” Development (Cambridge, England) 128: 1253–64. [DOI] [PubMed] [Google Scholar]
- Budnick Isadore, Hamburg-Shields Emily, Chen Demeng, Torre Eduardo, Jarrell Andrew, Akhtar-Zaidi Batool, Cordovan Olivia, Spitale Rob C, Scacheri Peter, and Atit Radhika P. 2016. “Defining the Identity of Mouse Embryonic Dermal Fibroblasts.” Genesis 54 (8). Wiley-Blackwell: 415–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos Victor E, Du Mengyuan, and Li Yuqing. 2004. “Increased Seizure Susceptibility and Cortical Malformation in Beta-Catenin Mutant Mice.” Biochemical and Biophysical Research Communications 320 (2): 606–14. [DOI] [PubMed] [Google Scholar]
- Carpenter April C, Rao Sujata, Wells James M, Campbell Kenneth, and Lang Richard A. 2010. “Generation of Mice with a Conditional Null Allele for Wntless..” Genesis 48 (9): 554–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe Youngshik, Siegenthaler Julie A, and Pleasure Samuel J. 2012. “A Cascade of Morphogenic Signaling Initiatedby the Meninges Controls Corpus Callosum Formation.” Neuron 73 (4). Elsevier Inc.: 698–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe Youngshik, Zarbalis Konstantinos S, and Pleasure Samuel J. 2014. “Neural Crest-Derived Mesenchymal Cells Require Wnt Signaling for Their Development and Drive Invagination of the Telencephalic Midline..” Edited by Bene Filippo Del. PloS One 9 (2): e86025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Vaquer Anna, Piliszek Anna, Tian Gianguan, Robert J Aho Daniel Dufort, and Hadjantonakis Anna-Katerina. 2010. “A Sensitive and Bright Single-Cell Resolution Live Imaging Reporter of Wnt/SS-Catenin Signaling in the Mouse.” BMC Developmental Biology 10 (1). BioMed Central Ltd: 121. doi: 10.1186/1471-213X-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Jiang, Yu Hsiao ÄêMan Ivy, Maruyama Takamitsu, Mirando Anthony J, and Hsu Wei. 2011. “Gpr177/Mouse Wntless Is Essential for Wnt-Mediated Craniofacial and Brain Development..” Developmental Dynamics: 240 (2): 365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RM, Thombre S, Firtina Z, Gray D, Betts D, Roebuck J, Spana EP, and Selva EM. 2006. “Sprinter: a Novel Transmembrane Protein Required for Wg Secretion and Signaling.” Development 133 (24): 4901–11. [DOI] [PubMed] [Google Scholar]
- Goodnough , L Henry, Chang Andrew T, Treloar Charles, Yang Jing, Scacheri Peter C, and Atit Radhika P. 2012. “Twist1 Mediates Repression of Chondrogenesis by Beta-Catenin to Promote Cranial Bone Progenitor Specification.” Development 139 (23): 4428–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnough L Henry, DiNuoscio Gregg J, and Atit Radhika P. 2016. “Twist1 Contributes to Cranial Bone Initiation and Dermal Condensation by Maintaining Wnt Signaling Responsiveness.” Developmental Dynamics : 245 (2): 144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry Goodnough, L, DiNuoscio Gregg J, Ferguson James W, Williams Trevor, Lang Richard A, and Atit Radhika P. 2014. “Distinct Requirements for Cranial Ectoderm and Mesenchyme-Derived Wnts in Specification and Differentiation of Osteoblast and Dermal Progenitors.” Edited by Gregory S Barsh. PLoS Genetics 10 (2): e1004152–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, and Kemler R. 1995. “Lack of Beta-Catenin Affects Mouse Development at Gastrulation.” Development 121 (11): 3529–37. [DOI] [PubMed] [Google Scholar]
- Hamburg-Shields Emily, DiNuoscio Gregg J, Mullin Nathaniel K, Lafayatis Robert, and Atit Radhika P. 2015. “Sustained Β-Catenin Activity in Dermal Fibroblasts Promotes Fibrosis by Up-Regulating Expression of Extracellular Matrix Protein-Coding Genes.” The Journal of Pathology 235 (5): 686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G, and Niswander L. 2001. “Expression of Slit-2 and Slit-3 During Chick Development..” Developmental Dynamics : 222 (2): 301–7. doi: 10.1002/dvdy.1182. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Lee SM, Johnson JE, Mcmahon AP, and Takada S. 1997. “Wnt Signalling Required for Expansion of Neural Crest and CNS Progenitors.” Nature 389 (6654): 966–70. [DOI] [PubMed] [Google Scholar]
- Ito Yoshihiro, Yeo Jae Yong, Chytil Anna, Han Jun, Bringas Pablo, Nakajima Akira, Shuler Charles F, Moses Harold L, and Chai Yang. 2003. “Conditional Inactivation of Tgfbr2 in Cranial Neural Crest Causes Cleft Palate and Calvaria Defects.” Development 130 (21): 5269–80. [DOI] [PubMed] [Google Scholar]
- Jiang Xiaobing, Iseki Sachiko, Maxson Robert E, Sucov Henry M, and Morriss-Kay Gillian M. 2002. “Tissue Origins and Interactions in the Mammalian Skull Vault..” Developmental Biology 241 (1): 106–16. [DOI] [PubMed] [Google Scholar]
- Kimmel Robin A, Turnbull Daniel H, Blanquet Veronique, Wurst Wolfgang, Loomis Cynthia A, and Joyner Alexandra L. 2000. “Two Lineage Boundaries Coordinate Vertebrate Apical Ectodermal Ridge Formation.” Genes & Development 14 (11): 1377–89. [PMC free article] [PubMed] [Google Scholar]
- Kume T, Deng KY, Winfrey V, Gould DB, Walter MA, and Hogan BL. 1998. “The Forkhead/Winged Helix Gene Mf1 Is Disrupted in the Pleiotropic Mouse Mutation Congenital Hydrocephalus.” Cell 93 (6): 985–96. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Li P, and de Crombrugghe B. 1998. “A New Long Form of Sox5 (L-Sox5), Sox6 and Sox9 Are Coexpressed in Chondrogenesis and Cooperatively Activate the Type II Collagen Gene.” The EMBO Journal 17 (19). EMBO Press: 5718–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, and Schmittgen TD. 2001. “Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method.” Methods (San Diego, Calif.) 25 (4): 402–8. [DOI] [PubMed] [Google Scholar]
- Lustig Barbara, Jerchow Boris, Sachs Martin, Weiler Sigrid, Pietsch Torsten, Karsten Uwe, van de Wetering Marc, et al. 2002. “Negative Feedback Loop of Wnt Signaling Through Upregulation of Conductin/Axin2 in Colorectal and Liver Tumors.” Molecular and Cellular Biology 22 (4): 1184–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani Preethi, Jarrell Andrew, Myers John, and Atit Radhika. 2010. “Visualizing Canonical Wnt Signaling During Mouse Craniofacial Development.” Developmental Dynamics: 239 (1): 354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLone DG, and Bondareff W. 1975. “Developmental Morphology of the Subarachnoid Space and Contiguous Structures in the Mouse.” The American Journal of Anatomy 142 (3): 273–93. [DOI] [PubMed] [Google Scholar]
- Mercier Frederic, Kitasako John T, and Hatton Glenn I. 2002. “Anatomy of the Brain Neurogenic Zones Revisited: Fractones and the Fibroblast/Macrophage Network.” J Comp Neurol 451 (2): 170–88. [DOI] [PubMed] [Google Scholar]
- Ohtola Jennifer, Myers John, Akhtar-Zaidi Batool, Zuzindlak Diana, Sandesara Pooja, Yeh Karen, Mackem Susan, and Atit Radhika. 2008. “Beta-Catenin Has Sequential Roles in the Survival and Specification of Ventral Dermis.” Development 135 (13). The Company of Biologists Ltd: 2321–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid Bethany S, Yang Hui, Melvin Vida Senkus, Taketo Makoto M, and Williams Trevor. 2011. “Ectodermal Wnt/Beta-Catenin Signaling Shapes the Mouse Face.” Developmental Biology 349 (2). Elsevier Inc.: 261–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice Ritva, Rice David P C, Olsen Bjorn R, and Thesleff Irma. 2003. “Progression of Calvarial Bone Development Requires Foxc1 Regulation of Msx2 and Alx4.” Developmental Biology 262 (1): 75–87. [DOI] [PubMed] [Google Scholar]
- Richtsmeier Joan T, and Flaherty Kevin. 2013. “Hand in Glove: Brain and Skull in Development and Dysmorphogenesis..” Acta Neuropathologica 125 (4): 469–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers Leanne E, Young Kaylene M, Rizzi Matteo, Jamen Françoise, Psachoulia Konstantina, Wade Anna, Kessaris Nicoletta, and Richardson William D. 2008. “PDGFRA/NG2 Glia Generate Myelinating Oligodendrocytes and Piriform Projection Neurons in Adult Mice.” Nature Neuroscience 11 (12): 1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudloff S, and Kemler R. 2012. “Differential Requirements for Beta-Catenin During Mouse Development.” Development 139 (20): 3711–21. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, and Fraser SE. 1992. “Vital Dye Analysis of Cranial Neural Crest Cell Migration in the Mouse Embryo..” Development (Cambridge, England) 116 (2): 297–307. [DOI] [PubMed] [Google Scholar]
- Spector Jason A, Greenwald Joshua A, Warren Stephen M, Bouletreau Pierre J, Detch Robert C, Fagenholz Peter J, Crisera Francesca E, and Longaker Michael T. 2002. “DuraMater Biology: Autocrine and Paracrine Effects of Fibroblast Growth Factor 2.” Plastic and Reconstructive Surgery 109 (2): 645–54. [DOI] [PubMed] [Google Scholar]
- Tran Thu H, Jarrell Andrew, Zentner Gabriel E, Welsh Adrienne, Brownell Isaac, Scacheri Peter C, and Atit Radhika. 2010. “Role of Canonical Wnt Signaling/Beta-Catenin via Dermo1 in Cranial Dermal Cell Development.” Development 137 (23): 3973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen, Ren e e, and Nusse Roel. 2009. “Towards an Integrated View of Wnt Signaling in Development.” Development 136 (19): 3205–14. [DOI] [PubMed] [Google Scholar]
- Vivatbutsiri P, Ichinose S, Hytonen M, Sainio K, Eto K, and Iseki S. 2008. “Impaired Meningeal Development in Association with Apical Expansion of Calvarial Bone Osteogenesis in the Foxc1 Mutant.” Journal of Anatomy 212 (5): 603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivatbutsiri Philaiporn, Ichinose Shizuko, Marjo Hytönen Kirsi Sainio, Eto Kazuhiro, and Iseki Sachiko. 2008. “Impaired Meningeal Development in Association with Apical Expansion of Calvarial Bone Osteogenesis in the Foxc1 Mutant..” Journal of Anatomy 212 (5): 603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey SB, Biswal S, and Revel JP. 1992. “Spatial and Temporal Patterns of Distribution of the Gap Junction Protein Connexin43 During Mouse Gastrulation and Organogenesis.” Development (Cambridge, England) 114 (1): 203–12. [DOI] [PubMed] [Google Scholar]
- Yu K 2003. “Conditional Inactivation of FGF Receptor 2 Reveals an Essential Role for FGF Signaling in the Regulation of Osteoblast Function and Bone Growth.” Development 130 (13): 3063–74. [DOI] [PubMed] [Google Scholar]
- Zarbalis Konstantinos, Siegenthaler Julie A, Choe Youngshik, May Scott R, Peterson Andrew S, and Pleasure Samuel J. 2007. “Cortical Dysplasia and Skull Defects in Mice with a Foxc1 Allele Reveal the Role of Meningeal Differentiation in Regulating Cortical Development.” Proceedings of the National Academy of Sciences of the United States of America 104 (35). National Academy of Sciences: 14002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Wnt signaling transduction is present in meningeal progenitors in the absence of cranial mesenchyme Wnt ligand secretion. Schematic illustration of the coronal plane, tamoxifen induction regimen, and inset depicts the lateral view of the embryonic head in the region of interest (A). Indirect immunofluorescence for LEF1 in the basolateral region (B,C), FOXC1 (pan-meningeal progenitor at E12.5) and GFP in the apical region (D,E). Red arrows point to LEF1+ cells, and yellow arrows point to TCF-LEF H2B-GFP and FOXC1+ cells. Scale bars represent 100μM. Meninges (mn), frontal bone (fb).
Figure S2. Conditional deletion of Wls in cranial mesenchyme does not appear to affect meningeal histology. Schematic illustration of the coronal plane (A). β−galactosidase staining, basolateral (B,C), and apical (D,E) sites. Indirect immunofluorescence for CONNEXIN43 (red) in gap junctions of the meninges and CD31/PECAM (green) in the endothelial cells of meningeal mesenchyme with DAPI-stained nuclei in the basolateral (F,G) and apical site (H,I). Scale bars represent 100μm.
Figure S3. Conditional deletion of Wls in cranial surface ectoderm. Schematic illustration of the coronal plane (A). Indirect immunofluorescence for FOXC1 (basolateral B, C; apical D, E), RALDH2 (basolateral F, G; apical H, I), and LEF1 (basolateral J, K; apical L, M) with DAPI-stained (blue) nuclei on coronal mouse embryonic head sections. Scale bars represent 100μM.
Figure S4. Conditional mutant of Twist1 lacks bone primordia and ectopic cartilage, but FOXC1 meningeal progenitor are present. Schematic illustration of the regions of interest in the coronal plane (A). Hematoxylin and eosin staining of the basolateral region of E13.5 heads (B, C). Indirect immunofluorescence for FOXC1 in the meningeal fibroblasts with DAPI-stained nuclei on coronal mouse embryonic mouse sections showing they are expressed comparably in control and Twist1 mutant in the basolateral region (F, G) and apex (D, E). Scale bars represent 100μm. Frontal Bone (fb).
Figure S5. Cell survival is not compromised in Dermo1Cre/+ β-catenin fl/del mutants.
Immunohistochemistry for activated CASPASE3 present in apoptotic cells in the palate (A), basolateral meninges (B, C), and apical site (D, E) counterstained with hematoxylin. Scale bars represent 100μM.
Fig S6. Conditional β-catenin deletion does not result in ectopic expression of cartilage determinants or early reduction of laminin matrix protein. Indirect immunofluorescence on mouse embryonic coronal sections at eye level for chondrocyte markers SOX5 (A-D) or SOX9 (E-H) with DAPI (blue) counterstained nuclei. Scale bars represent 100μm.