Abstract
The removal of afferent input to the olfactory bulb by both cautery and chemical olfactory organ ablation in adult zebrafish results in a significant decrease in volume of the ipsilateral olfactory bulb. To examine the effects of deafferentation at a cellular level, primary output neurons of the olfactory bulb, the mitral cells, were investigated using retrograde tract tracing with fluorescent dextran using ex vivo brain cultures. Morphological characteristics including the number of major dendritic branches, total length of dendritic branches, area of the dendritic arbor, overall dendritic complexity, and optical density of the arbor were used to determine the effects of deafferentation on mitral cell dendrites. Following 8 weeks of permanent deafferentation there were significant reductions in the total length of dendritic branches, the area of the dendritic arbor, and the density of fine processes in the dendritic tuft. With 8 weeks of chronic, partial deafferentation there were significant reductions in all parameters examined, including a modified Sholl analysis that showed significant decreases in overall dendritic complexity. These results show the plasticity of mitral cell dendritic structures in the adult brain and provide information about the response of these output neurons following the loss of sensory input in this key model system.
Keywords: Output neurons, retrograde labeling, dextran, teleosts, modified Sholl analysis
Introduction
Neurons possess distinct, complex structures, especially with regard to their dendritic processes. The morphology of neurons is essential for their proper functioning, and proper synaptic connections are critical for maintenance of neuronal structure (Parrish et al. 2007). The specific mechanisms involved in the afferent-target interactions that maintain dendritic morphology in the adult brain are not well understood, and removal of sensory input is commonly used to examine the effects of deafferentation in a variety of adult brain regions. The current study explores mitral cell dendritic structure following removal of afferent innervation in the adult zebrafish olfactory bulb.
The importance of afferent-target interactions during development has been well studied (Wong and Ghosh 2002), and within the olfactory system the effects of deafferentation have been well examined in both the developing and mature brain. In rats, neonatal naris occlusion has a profound effect on the development of the olfactory bulb, including a reduction of bulb volume and protein synthesis and an increase in cell death (Brunjes 1994; Coppola 2012). Within mammalian systems, loss of olfactory bulb volume occurs following removal of olfactory innervation (Matthews and Powell 1962; Margolis et al. 1974; Harding et al. 1978; Brunjes, 1985), and olfactory deprivation results in reduced cell number (Henegar and Maruniak 1991; Meisami and Safari 1991; Corotto et al. 1994) and decreased neurogenesis (Corotto et al. 1994). Loss of innervation causes changes to olfactory bulb structure (Wilson and Wood, 1992), enzymatic activity (Nadi et al. 1981; Baker et al. 1984; Baker et al. 1993) and mRNA protein expression (Ehrlich et al. 1990; Stone et al. 1991; Ferraris et al. 1997; Casabona et al. 1998; Oberto et al. 2001) in the deprived bulb. Thus the olfactory system is renowned for its ability to respond to changes in innervation.
Neural plasticity is well documented in teleosts, with adult animals capable of regenerating portions of the peripheral and central nervous systems after injury and experimental insult (Zippel and von Rekowski 1993; Sirbulescu and Zupanc 2009; Gemberling et al. 2013), including regeneration of the olfactory bulb (Paskin et al. 2011). Thus, a teleost model has been selected for the current analysis of potential plasticity of dendrites following the removal of afferent axons, and zebrafish are a particularly tractable model for these studies since their olfactory system is very similar to that of other animals (Byrd and Brunjes 2001; Hansen and Zielinski, 2005; Fuller et al., 2006). Previous studies in adult zebrafish show that removal of afferent input profoundly affects the olfactory bulb (Byrd, 2000; Poling and Brunjes, 2000). Effects include a reduction of bulb volume (Byrd 2000), increased apoptosis (Vankirk and Byrd 2003), decreased immunoreactivity of various proteins (Byrd 2000; Fuller et al. 2005), alterations in cell genesis and neuronal fate (Villanueva and Byrd-Jacobs 2009; Trimpe and Byrd-Jacobs 2016), and deficits in olfactory-mediated behavior (Paskin and Byrd-Jacobs 2012; White et al. 2015). Thus, interactions between the olfactory organ and olfactory bulb influence many aspects of bulb structure and function.
Mitral cells are large, aspinous neurons (Kosaka et al. 2001) that serve as the principle relay neurons of the olfactory bulb and are a primary component of bulbar organization. In the mammalian system, mitral cells are located generally in the mitral cell layer of the olfactory bulb, with a single primary dendrite projecting vertically from the soma and branching to form a distinct arbor within a single glomerulus, while secondary dendrites extend horizontally, contacting interneurons (Lledo et al. 2005). In zebrafish, mitral cells are found scattered throughout the glomerular layer of the olfactory bulb (Byrd and Brunjes 2001; Edwards and Michel 2002; Fuller et al. 2006), and their morphologies differ slightly from mammalian systems. Dendritic morphologies of zebrafish mitral cells consist of unidendritic cells that have a single, primary dendrite with one or more tufts and multidendritic cells that have several dendritic projections with tufts. Unidendritic mitral cells are the predominant type and, regardless of shape, the majority of mitral cells, including multidendritic cells, innervate a single glomerulus (Fuller et al. 2006).
While much is known about the formation of dendritic arbors, studies addressing the maintenance of dendritic structures in the adult zebrafish are limited. Even less is known about the effects of denervation on the dendritic structure of aspinous neurons such as mitral cells. This work further clarifies mitral cell morphology by describing the effects of deafferentation on dendritic arbor structures. A better understanding of the cellular changes that occur in mitral cell dendritic structures following deafferentation can lead to exploration of factors involved in stabilization of adult brain organization. Here, we used retrograde tract tracing with a fluorescent dextran to investigate the potential effects of deafferentation on mitral cell dendritic morphology. We hypothesized that there would be a reduction in mitral cell dendritic arbors due to loss of afferent axonal innervation.
Materials and Methods
Animals
Adult male and female zebrafish, Danio rerio, over 6 months of age, were obtained from local commercial sources. The fish were maintained in 15-gallon aquaria filled with aerated, conditioned fish water at 28.5°C, and they were fed commercial flake food (Tetra) twice daily, each morning and afternoon. Twenty-one animals were used over the course of this experiment. All protocols on animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee.
Deafferentation
Permanent deafferentation:
Complete and permanent removal of afferent input to the olfactory bulb was achieved by olfactory organ ablation as described previously (Byrd 2000). Briefly, zebrafish were anesthetized with 0.03% MS222 (3-amino benzoic acid ethyl ester, Sigma) and the right olfactory organ was removed using a small-vessel cautery iron; the left olfactory organ remained intact for use as an internal control for comparison. Deafferented fish were allowed to survive for 3 and 8 weeks. Unlesioned control fish were processed on the same date that experimental fish underwent olfactory organ ablation (day 0) and at each subsequent time point. After the appropriate survival period, the length, weight, and brain weight of deafferented fish and unlesioned control fish were measured, and then fish were processed as described below.
Chronic, partial deafferentation:
Partial and temporary removal of afferent input to the olfactory bulb was achieved through repeated chemical ablation of the olfactory organ with the detergent Triton X-100 as previously described (Paskin et al. 2011). Briefly, zebrafish were anesthetized with 0.03% MS222 and approximately 1μl of a 0.7% Triton X-100 and 0.005% methylene blue in 0.1M phosphate-buffered saline (PBS) solution was infused into the right nasal cavity. The olfactory organ remained exposed to the detergent solution for 2 minutes before the fish was returned to its housing tank. This procedure was repeated every 3 days for 8 weeks. A group of unlesioned control fish was processed on the same date that the experimental fish underwent their first detergent treatment and at 8 weeks. After 8 weeks, the length, weight, and brain weight of deafferented fish and unlesioned control fish were measured, and then fish were processed as described below.
Olfactory Tract Tracing
Mitral cells were labeled using retrograde tract tracing from their axons in the telencephalon as described previously (Fuller et al. 2006). Fish were over-anesthetized with 0.03% MS222 and perfused transcardially with PBS. The brains were dissected, and approximately 0.05μl−0.1μl of Texas Red dextran (10,000 MW, 5mg/ml in PBS, ThermoFisher Scientific) was injected into the medial and lateral olfactory tracts using a PV800 Pneumatic Picopump (World Precision Instruments). To allow active transport of the tracer molecule throughout the length of the neurons brains were cultured using a method modified from Tomizawa et al. (2001) and Fuller et al. (2006). The brains were placed on a 3μm polycarbonate filter in a sterilized six-well culture dish (Costar) containing artificial fish cerebrospinal fluid (100mM NaCl, 2.46mM KCl, 1mM MgCl2H2O, 0.44mM NaH2PO4H2O, 1.13mM CaCl2H2O, 5mM NaHCO3) and incubated at 28.5°C with 3–5% CO2 for approximately 4 hours. Following fixation in 4% paraformaldehyde for 24 hours at 4°C, the tissue was rinsed in PBS and mounted between two coverslips in a polyvinyl alcohol (PVA)-based aqueous hardening mounting medium (13% polyvinyl alcohol, 1.7% 1,4-diazobicyclo[2,2,2]octane, 33% glycerol in 0.2M Tris-HCl) and viewed using confocal microscopy.
Dendritic Analysis
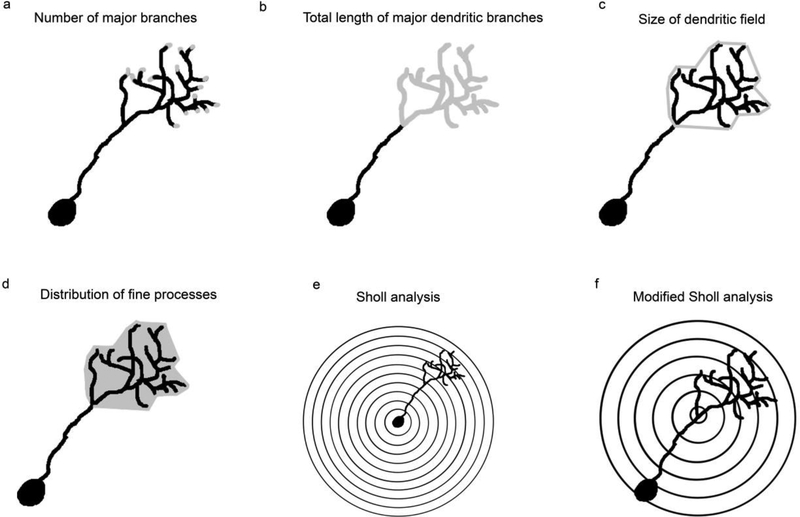
To obtain dendritic reconstructions of the dextran-labeled cells, whole mounted tissue was examined using confocal microscopy. Z-stack imaging was used to gather fine optical sections of the tissue throughout the olfactory bulb at 0.25μm intervals. Not all labeled mitral cells were chosen for a detailed analysis: individual, unidendritic cells were selected at random throughout the entire olfactory bulb. Only unidendritic cells were examined because they are the most predominant and uniform mitral cell type, and their localized projections increased the likelihood that the entire dendritic arbor could be viewed. All attempts were made to select cells that were fully labeled, with unobscured and complete branches. A minimum of 5 cells from each right and left olfactory bulb in at least 4 fish from each group was used for dendritic analyses. Once a cellular profile was identified, a 2 dimensional (D) projection image was created and the image was analyzed within Fiji (Schindelin et al. 2012). The Simple Neurite Tracer Plugin was used to create a 2D outline of the arbor by tracing the major dendritic branches, which were considered to be primary, secondary, tertiary, and, on occasion, quaternary branches that had clear, defined borders. The trace was analyzed for complexity characteristics by counting the number of major branches in the dendritic arbor (Fig. 1a) and measuring the total length of the major branches within the dendritic arbor (Fig. 1b). The relative size of the dendritic field was estimated by connecting the distal tips of the major branches of the dendritic arbor in z-stack projection images and measuring the 2D area (Fig. 1c). While this is a 2D representation of a 3D arbor, it is an estimate used for comparison only. Optical density (OD) of dextran labeling of the dendritic arbor was used to estimate the effects of deafferentation on the fine processes forming the tuft and was calculated from the same region of the tissue measured for the size of the dendritic field (Fig. 1d). Within Fiji the mean gray level from the area of the arbor was determined for each cell and converted to OD using the formula: OD = -log (intensity of background/intensity of area of interest). Background intensity was determined by measuring the mean gray level from a non-stained tissue area near the cell, within the same projection image and with similar size to the area of interest. A modified Sholl analysis was used to examine the overall complexity of the dendritic arbors. Traditional Sholl analyses determine dendritic complexity based on the number of intersections arbor branches make with concentric rings originating at the cell soma (Fig. 1e; Sholl 1953). However, due to the variability in dendritic arbor distance from the soma in unidendritic zebrafish mitral cells, concentric rings 2μm apart originating at the base of the dendritic arbor, rather than the soma, were analyzed to determine overall complexity (Fig. 1f).
Fig. 1.
Parameters used to quantify the effects of deafferentation on digital reconstructions of mitral cell dendritic arbors were (a) number of major branches (gray); (b) total length of the major dendritic branches, calculated by adding the lengths of the traced branches (gray); (c) estimate of the relative size of the dendritic arbor, by connecting the distal tips (gray) of the traced branches within the dendritic arbor; and (d) distribution of fine processes of the dendritic arbor (gray), calculated from the optical density of dextran labeling of the dendritic field projection images A modified Sholl analysis was also used. (e) In a traditional Sholl analysis, concentric rings with a set distance between them are centered over the soma and the overall complexity of a dendritic arbor is evaluated. (f) A modified Sholl analysis was developed in which concentric rings with a set distance between them are placed at the base of the dendritic arbor. Using this modified Sholl analysis, mitral cells with dendritic tufts located at various distances from the soma can be directly compared
Data from cells from right and left olfactory bulbs were compared within groups using paired, two-tailed t-tests. Data was analyzed between groups using an ANOVA with Tukey’s test for multiple comparisons. Data from the Sholl analysis was analyzed across data points at each distance using paired t-tests, and a Kolmogorov-Smirnov test was performed to determine significant differences in the lines formed by deafferented and control dendritic arbors. There was no reason to believe the data was not normally distributed. P values less than 0.05 were considered significant.
Immunohistochemistry
To show the relationship between mitral cells and olfactory sensory neurons, control samples underwent double labeling with the olfactory tract tracing procedure described above followed by an antibody to keyhole limpet hemocyanin (anti-KLH). After fixation in 4% paraformaldehyde for 24 hours at 4°C, the tissue was rinsed in PBS and immersed in a blocking solution of 3% normal goat serum and 0.4% Triton X-100 in PBS for 2 hours at room temperature. Samples were incubated for 2 days at room temperature with anti-KLH (Sigma; 1:200 in 0.25% Triton X-100 in PBS [PBS-T]), rinsed in PBS-T, and incubated in Alexa Flour 488-conjugated anti-rabbit secondary antibody (Invitrogen; 1:200 in PBS-T) for 1 day at room temperature. Brains were then rinsed in PBS-T followed by PBS and mounted between two coverslips in a PVA-based aqueous hardening mounting medium and viewed using confocal microscopy.
Glomerular F-actin Labeling
The glomerular distribution of actin filaments throughout the olfactory bulb was determined through the use of fluorescent phalloidin in control animals and those following 3 and 8 weeks of permanent deafferentation. Fish were over-anesthetized with 0.03% MS222 and perfused transcardially PBS. Following immersion in 4% paraformaldehyde for 24 hours at 4°C, whole brains were dissected, rinsed in PBS, and placed in a blocking solution of 3% normal goat serum and 0.4% Triton X-100 in PBS for 1 hour at room temperature. The brains were then placed in Alexa Fluor 594 Phalloidin (Molecular Probes; 1:100 in PBS) for 1 hour at room temperature, rinsed in PBS-T followed by PBS for 30 minutes each, and mounted between two coverslips in a PVA-based aqueous hardening mounting medium and viewed using confocal microscopy.
Results
Permanent Deafferentation
In untreated, control fish, retrogradely labeled mitral cells exhibited both unidendritic and multidendritic morphologies, as reported previously (Fuller et al. 2006). The unidendritic cells, which were the vast majority of dextran-labeled neurons (~70%, Fuller et al. 2006) and were the focus of this study, had pronounced dendritic arbors composed primarily of one main branch with several secondary branches and numerous fine processes that formed a dendritic tuft. These previously reported morphological characteristics were seen in labeled mitral cells throughout the glomerular layers of both the right and left olfactory bulbs of untreated, control fish (data not shown).
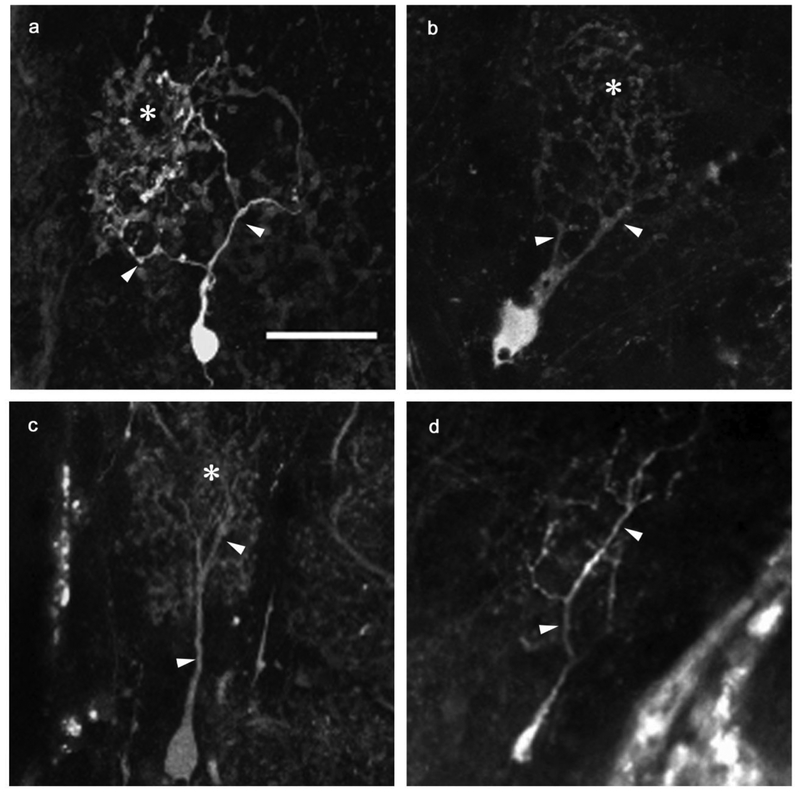
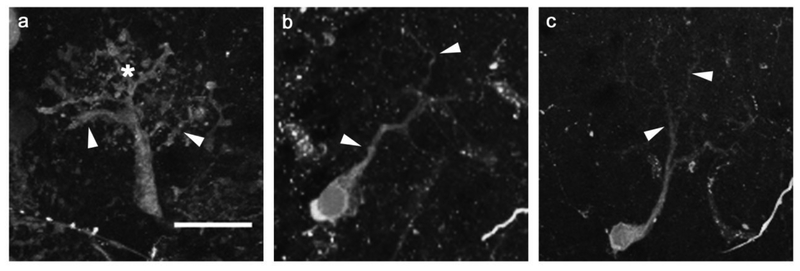
Permanent deafferentation of the olfactory bulb by cautery ablation of the olfactory organ resulted in noticeable alterations in mitral cell morphology. Both unidendritic and multidendritic cells were observed in the internal control and deafferented bulbs at all time points after ablation of the olfactory organ, although only unidendritic cells were analyzed for the purpose of this study. At 3 weeks following the cautery deafferentation procedure, mitral cells within the internal control olfactory bulb maintained the dendritic structures found in control cells of unlesioned fish (Fig. 2a). The mitral cells of the olfactory bulb on the deafferented side appeared to show no variation in overall gross structure: dendritic arbors contained a distinct primary dendrite and several secondary branches, with fine dendritic processes forming a dendritic tuft (Fig. 2b). After 8 weeks of deafferentation, internal control cells maintained their typical dendritic arbor structures (Fig. 2c). Deafferented cells at this time point appeared noticeably affected. They retained their major dendritic branches; however, the secondary and fine branches were greatly diminished (Fig. 2d).
Fig. 2.
Dendritic morphologies of permanently deafferented mitral cells were compared with internal control cells. At the 3-week survival time, internal control cells (a) retained control morphology, with numerous major branches off the primary dendrite terminating in a dense tuft of fine processes. Scale bar = 20μm for all. Three-week deafferented cells (b) showed no obvious variation from control cells in overall gross structure of the dendritic arbor, although fine processes appeared to be less dense. At the 8-week time point, internal control cells (c) remained control-like in structure, while deafferented cells (d) exhibited reduced dendritic arbor morphology, with diminished major branches and an absence of the dense population of fine processes typically seen in control morphologies. arrowheads = major dendritic branches, * = fine processes
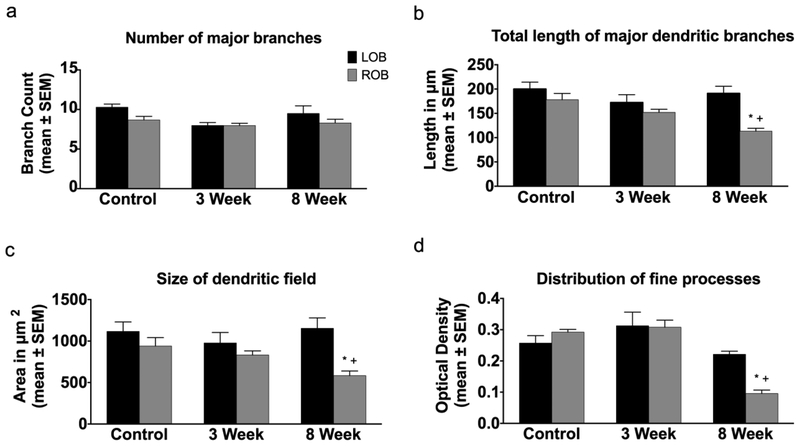
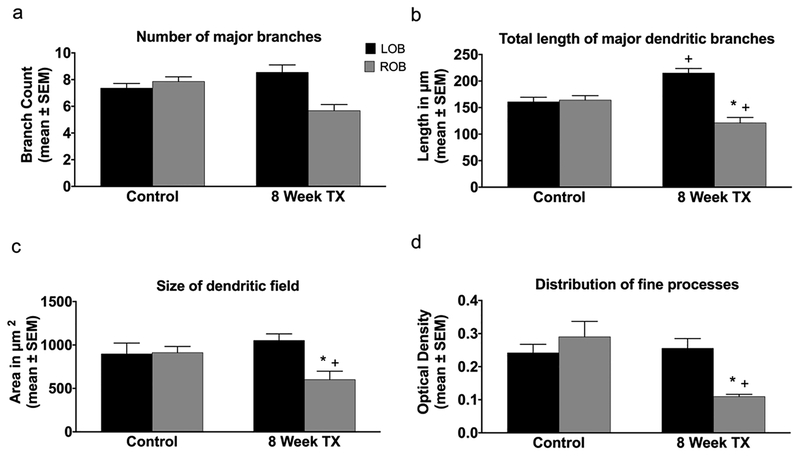
The effects of deafferentation on mitral cell dendritic arbors were quantified by measuring the total number of major branches, the lengths of those branches, the relative areas of the dendritic arbors, and the OD of dextran labeling in the dendritic tufts. First, control dendritic arbors were analyzed at day 0 and in unlesioned, cohort control fish at 3 weeks and 8 weeks to determine if there were any changes in control dendritic arbors over this time period. In general, there were no notable differences in dendritic arbor structures between right and left olfactory bulbs of control fish at any time point. There was no significant difference in the average number of major branches between day 0 control (9.48 ± 0.40) and 8-week cohort control (8.90 ± 0.55). The average total length of major dendritic branches in day 0 control animals was 189.10 ± 9.47μm and 216.30 ± 6.01μm in 8-week cohort control animals, with no significant difference between the two. There were no significant differences in the relative area of the dendritic arbor or the OD of dextran labeling in dendritic tufts in day 0 control animals (area = 1027.00 ± 77.97μm2; OD = 0.27 ± 0.01) compared to 8-week cohort control animals (area = 1263.00 ± 188.50μm2; OD = 0.25 ± 0.02). Therefore, cells from day 0 control animals were used throughout the dendritic analyses for comparisons with cells from deafferented animals.
In unlesioned control fish at day 0, there was no significant difference between the left and right olfactory bulbs in the number of major branches in mitral cell dendritic arbors (Fig. 3a). Following both 3 and 8 weeks of deafferentation there was no significant difference in the number of major dendritic branches in deafferented cells compared to internal control cells (Fig. 3a). There were no significant differences in the total length of major dendritic branches between cells in the right and left olfactory bulbs in unlesioned control fish at day 0 (Fig. 3b). With 3 weeks of deafferentation, there were no significant changes in the total length of the major dendritic branches of deafferented cells compared to internal control cells, but there was a significant reduction in the length of major branches of deafferented mitral cells at 8 weeks post-deafferentation compared to both internal control cells and unlesioned control cells (Fig. 3b).
Fig. 3.
Quantitative analysis of the effects of permanent deafferentation on mitral cell dendritic arbors. (a) There were no significant changes in the number of major dendritic branches of mitral cells following 3 and 8 weeks of cautery deafferentation compared to both internal control cells and day 0 unlesioned control fish. (b) While there were no significant changed in the length of dendritic branches 3 weeks post-deafferentation, at 8 weeks post-deafferentation there was a significant reduction in the length of dendritic branches in the deafferented bulb compared to both internal control cells and day 0 unlesioned control cells. (c) At 3 weeks post-deafferentation there was no significant effect on the size of the dendritic field, but with 8 weeks of deafferentation there was a significant decrease in the size of the dendritic field compared to both internal control arbors and day 0 unlesioned control animals. (d) There were no significant changes in the distribution of fine processes with 3 weeks of deafferentation, but with 8 weeks of deafferentation there was a significant decrease in the distribution of fine processes within the dendritic arbor compared to both internal control dendritic arbors and day 0 unlesioned control arbors. * = p<0.05 compared to internal control cells; + = p<0.05 compared to day 0 unlesioned control cells; LOB = left (internal control) olfactory bulb; ROB = right (deafferented) olfactory bulb
There were no significant differences in the size of the dendritic field between mitral cells in the left and right olfactory bulbs of unlesioned control animals at day 0 (Fig. 3c). At 3 weeks post-deafferentation, there were no changes to the size of the dendritic field of deafferented cells compared to internal control cells, but by 8 weeks post-deafferentation there was a significant reduction in dendritic field size compared to internal control cells, as well as when compared to unlesioned control fish (Fig. 3c). There was no significant difference in the OD of dextran labeling between mitral cells in the left and right olfactory bulbs of unlesioned control fish at day 0 or in dendritic arbors of cells at 3 weeks post-deafferentation compared to internal control cells (Fig. 3d). Following 8 weeks of deafferentation OD of dendritic arbor dextran labeling was significantly reduced in deafferented cells compared to internal control cells and unlesioned control cells (Fig. 3d).
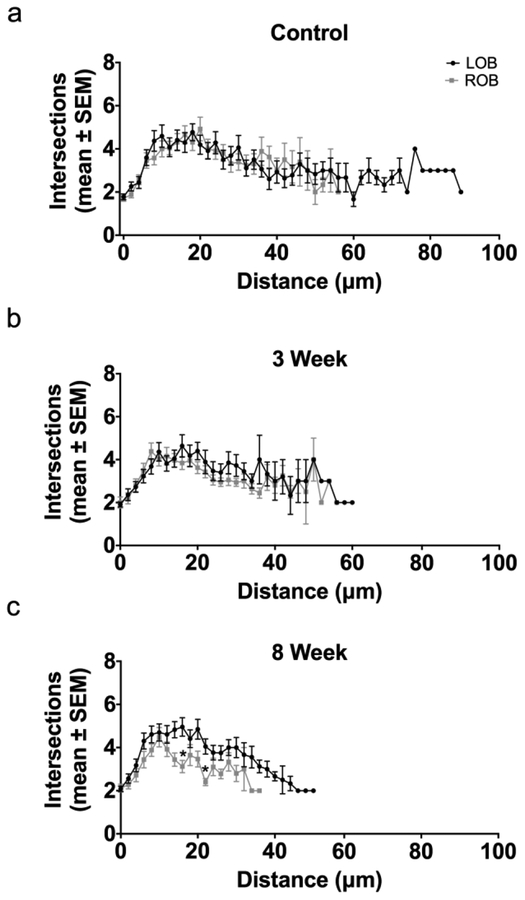
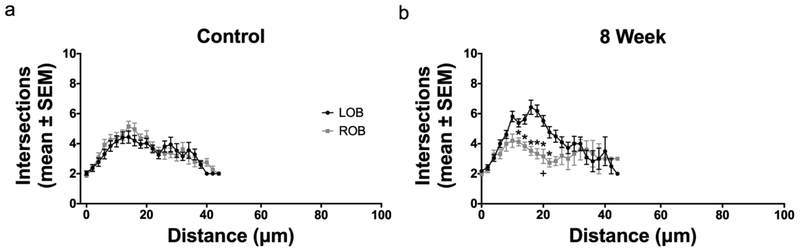
A modified Sholl analysis centered on the base of the dendritic arbor was used to quantify the effects of deafferentation on overall dendritic complexity. Sholl analyses showed no significant differences in the distribution of intersections in cells found in the right and left olfactory bulbs of unlesioned control animals, indicating no difference in overall dendritic complexity (Fig. 4a). Following 3 weeks of deafferentation, no significant changes to dendritic complexity were found; the overall shape, based on the number of dendritic intersections at various distances from the base of the tuft, of internal control and deafferented cells were closely matched (Fig. 4b). At 8 weeks post-deafferentation, the number of intersections at multiple distances from the base of the arbor was significantly reduced compared to the internal control cells (Fig. 4c). Kolmogorov-Smirnov tests further comparing the lines of the Sholl analyses indicated no significant difference in overall dendritic complexity following 3 and 8 weeks of deafferentation, although at 8 weeks post-deafferentation the trend towards a decrease in dendritic complexity approached significance (p=0.0832).
Fig. 4.
Modified Sholl analysis of dendritic arbor complexity following permanent deafferentation. (a) In unlesioned control fish there was no difference in dendritic complexity between right (ROB) and left (LOB) olfactory bulbs. (b) Following 3 weeks of deafferentation there was no significant decrease in mitral cells dendritic complexity (ROB) compared to the internal control side (LOB). (c) At 8 weeks post-deafferentation, there were significant differences (*) in the number of intersections at multiple distances from the base of the arbor in deafferented cells (ROB) compared to internal control cells (LOB), and there was a trend towards a significant decrease in overall dendritic complexity (p = 0.0832). * = p<0.05 differences at specific distance from the arbor base compared to internal control
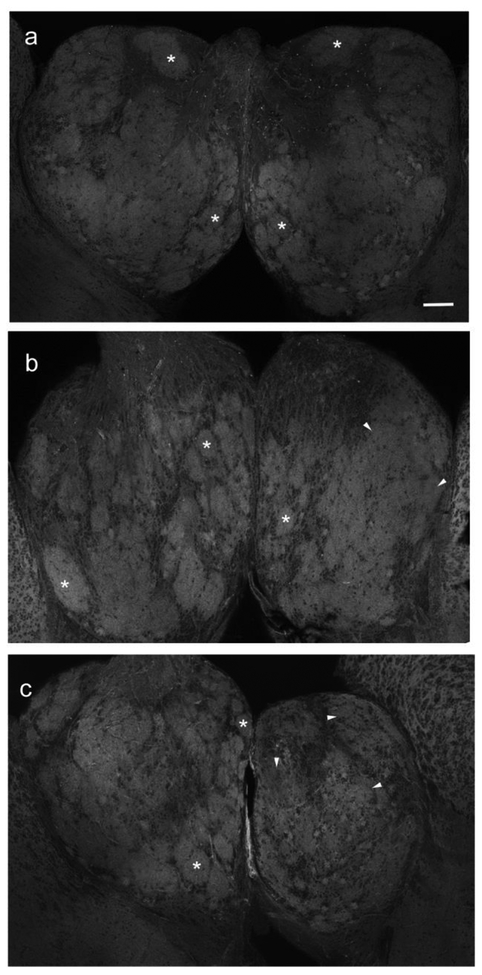
A fluorescently labeled phallotoxin revealed distinct glomerular structures throughout the olfactory bulb consisting of axonal and dendritic components and allowed confirmation of gross effects of dendritic arbor reduction with deafferentation. In unlesioned, control animals, labeling of actin filaments showed obvious glomerular features throughout the right and left olfactory bulbs (Fig. 5a) that appeared to follow the glomerular distribution previously reported with axonal labeling (Baier and Korsching 1994; Braubach et al. 2012; White et al. 2015). Following 3 weeks of permanent, cautery deafferentation there was a noticeable disruption of glomerular structure, with only a few distinct glomeruli still present (Fig. 5b). With 8 weeks of cautery deafferentation there was a noticeable reduction in bulb volume and in distinguishable glomeruli (Fig. 5c). The glomerular features that remained were smaller in size and more disorganized compared to those in the internal control bulb (Fig. 5c). This loss and disruption of actin filaments within the glomerular structures of the deafferented olfactory bulb corresponds to the significant decrease in OD observed in deafferented mitral cell dendritic arbors and likely indicates that the fine processes of mitral cell dendritic arbors that are composed primarily of actin filaments were lost following prolonged deafferentation.
Fig. 5.
Effects of permanent deafferentation on the distribution of actin filaments in olfactory bulb glomeruli. (a) Unlesioned control fish had distinct glomeruli (*) throughout both right and left olfactory bulbs. Scale bar = 100μm. (b) With 3 weeks of deafferentation, some glomeruli in the deafferented bulb (star) remained intact (*) but others appeared to lose their structural features (arrowheads). The internal control bulb continued to show typical glomerular structures (*). (c) At 8 weeks post-deafferentation, the internal control bulb had typical glomeruli (*), while the deafferented bulb (star) had lost distinct glomeruli (arrowheads)
Chronic, Partial Deafferentation
A method for chronic, partial deafferentation, involving repeated intranasal infusion with the detergent Triton X-100 every three days for eight weeks, was used to examine the potential effects of partial, temporary deafferentation since this treatment does not remove all afferent innervation to the bulb (Paskin et al. 2011). Mitral cells of internal control bulbs in these fish showed typical control morphology (Fig. 6a). Following 8 weeks of chronic, partial deafferentation, mitral cells retained their major dendritic branches; however, smaller secondary branches were noticeably diminished, and the fine processes that established the dendritic tuft of control cells were notably lacking in deafferented mitral cells (Fig. 6b,c).
Fig. 6.
The effects of chronic, partial deafferentation on mitral cell dendritic morphology were examined after 8 weeks of repeated detergent infusion. (a) Mitral cells in the internal control bulb had typical dendrite morphology, including numerous major branches (arrowheads) and a dense tuft of fine processes (*). Scale bar = 20μm for all. (b, c) With eight weeks of chronic detergent infusion, deafferented cells were greatly affected, with less elaborate overall dendritic arbor structure, reduced definition of major branches (arrowheads), and minimal fine processes
To better understand the effects of this method of chronic, partial deafferentation on mitral cell dendritic arbors, the total number of major dendritic branches, the lengths of those branches, the relative areas of the dendritic arbors, and the OD of dextran labeling of the dendritic tufts were measured. Unlesioned control fish exhibited no significant differences in the number of major branches in mitral cell dendritic arbors between the left and right olfactory bulbs (Fig. 7a). Following 8 weeks of chronic, partial deafferentation there was a significant decrease in the number of major branches in deafferented cells compared to both internal control cells and cells of unlesioned control fish (Fig. 7a). Unlesioned control fish showed no difference in the total length of major dendritic branches between the left and right olfactory bulbs (Fig. 7b). With 8 weeks of chronic, partial deafferentation there was a significant decrease in the length of major branches in deafferented cells compared to both internal control cells and unlesioned control cells (Fig. 7b). Additionally, there was a significant increase in the length of major branches in cells of the internal control bulb in the8-week post-deafferentation group compared to those in unlesioned control fish who were sampled at the start of the experiment (Fig. 7b). No significant differences in the size of the dendritic field between mitral cells in the left and right olfactory bulbs were found in unlesioned control animals (Fig. 7c). Following 8 weeks of chronic, partial deafferentation there was a significant decrease in the size of the dendritic field between deafferented mitral cells compared to both internal control cells and unlesioned control cells (Fig. 7c). The OD of dextran labeling did not differ between mitral cells in the left and right olfactory bulbs of unlesioned control fish (Fig. 7d). Eight weeks of chronic, partial deafferentation resulted in a significant decrease in the OD of dextran labeling in deafferented mitral cell dendritic arbors compared to internal control cells as well as unlesioned control cells (Fig. 7d).
Fig. 7.
Quantification of the effects of chronic, partial deafferentation on mitral cell dendritic structure. (a) At 8 weeks post-deafferentation there was a significant reduction in the number of major dendritic branches in deafferented mitral cells compared to internal control cells and day 0 unlesioned control animals. (b) The length of dendritic branches of 8-week deafferented cells was significantly reduced compared to internal control and day 0 unlesioned control cells. Additionally, there was a significant increase in the length of dendritic branches of internal control cells compared to day 0 unlesioned control cells. (c) Following 8 weeks of deafferentation, there was a significant decrease in the average size of dendritic arbors of deafferented mitral cells compared to internal control cells and day 0 unlesioned control cells. (d) There was a significant reduction in the distribution of fine processes within the dendritic arbor at 8 weeks post-deafferentation compared to internal control dendritic arbors and day 0 unlesioned control arbors. * = p<0.05 compared to internal control cells; + = p<0.05 compared to day 0 unlesioned control cells. LOB = left (internal control) olfactory bulb; ROB = right (deafferented) olfactory bulb
A modified Sholl analysis was used to estimate dendritic complexity and determine effects of deafferentation on the dendritic arbors of mitral cells. The complexity of mitral cell dendritic arbors did not differ between left and right olfactory bulbs of control animals (Fig. 8a). Following 8 weeks of chronic, partial deafferentation there were significant decreases in the number of dendritic intersections at various distances from the base of the arbor of deafferented cells compared to internal control dendritic arbors (Fig. 8b). Further, Kolmogorov-Smirnov tests comparing the measures of the Sholl analysis revealed a significant difference in the distributions of mitral cell dendritic arbors of chronically deafferented cells compared to the complexity of internal control cells, indicating a significant decrease in overall dendritic complexity due to chronic, partial deafferentation.
Fig. 8.
A modified Sholl analysis was used to determine the effects of chronic, partial deafferentation on overall dendritic arbor complexity. (a) In day 0 unlesioned control fish there were no significant differences in the dendritic complexity between right (ROB) and left (LOB) olfactory bulbs. (b) Following 8 weeks of chronic, partial deafferentation there were significant differences in the number of intersections at multiple distances from the base of the dendritic arbor (*), and there was a significant decrease in overall dendritic complexity (+), compared to internal control cells
Discussion
The purpose of this study was to examine afferent-target interactions contributing to the structural maintenance of an aspinous neuron, the mitral cell, in the adult brain. Our data shows that removal of afferent innervation to mitral cells in the olfactory bulb has a profound effect on their dendritic structure. Following the complete and permanent removal of sensory input to the olfactory bulb there were significant effects on mitral cell dendritic arbor structures and overall complexity. While 3 weeks of permanent deafferentation was not long enough to produce significant differences in dendritic arbor structures, 8 weeks of permanent deafferentation caused significant reductions in the total length of major dendritic branches, the relative size of the dendritic arbor, and the distribution of fine processes within the arbor. Modified Sholl analyses showed changes in dendritic arborization, with a near significant reduction in dendritic complexity. Although major branches are still present and not significantly different in number, it appears that they are retracting; this results in a significantly reduced total length of major dendritic branches within the arbor, and therefore an overall significant decrease in the size of the dendritic field. The time course of dendritic alterations corresponds to the loss of olfactory sensory axons, which takes longer than 3 weeks to achieve with permanent deafferentation by cautery of the olfactory organ (Byrd 2000).
Other studies have confirmed the influence of afferent input on mitral cell dendritic structure. Unilateral naris occlusion retards differentiation of primary and secondary dendrites in postnatal rats, resulting in an increased proportion of mitral cells with multiple primary dendrites compared to those with secondary dendrites, indicating that secondary dendrite development is activity dependent (Matsutani and Yamamoto 2000). Focal denervation of the olfactory bulb, through the prevention of new axon growth within portions of the olfactory nerve during development, causes a reduction in mitral cell number and density (Couper Leo et al. 2000), with loss of glomerular tufts (Couper Leo and Brunjes 2003). Additionally, the presence of the olfactory epithelium directly influences the final dendritic orientation of mitral cells in developing mice (Lopez-Mascaraque et al. 2004), and soluble trophic factors released by the olfactory epithelium promote extension and elaboration of mitral cell dendrites (Tran et al. 2008). Therefore it is likely that the significant loss of smaller, fine processes is a direct result of sensory neuron loss, while the relatively stable structure of major dendritic branches may be maintained by the continued presence of interactions with other cell types in the olfactory bulb, such as periglomerular, granule, and ruffed cells.
In adult mammalian systems dendritic arbors are relatively stable in structure. Using long-term imaging techniques, investigations found that dendritic arbors in pyramidal neurons (Holtmaat et al. 2005; Lee et al. 2006; Chow et al. 2009) and mitral/tufted cells in the olfactory bulb (Mizrahi and Katz 2003) are unchanged in their overall organization over time. These dendritic arbors also remain consistent in enriched environments (Holtmaat et al. 2005) and in response to learning (Mizrahi and Katz 2003). It is likely that dendritic stability is due in part to regulation of synaptic structures. Postsynaptic density protein 95 (PSD95) acts independent of neuronal activity and regulates microtubule organization at the synapse (Charych et al. 2006). It is thought that increases in dendritic complexity result in an increase in synaptic sites, further stabilizing dendritic structures. Conversely, decreases in dendritic complexity would result in a decrease in synaptic sites, leading to further destabilization of the dendritic arbors.
Following 8 weeks of chronic, partial deafferentation, there were significant decreases in all parameters examined compared to both internal control cells and day 0 unlesioned control cells. Additionally, a modified Sholl analysis showed a significant decrease in overall dendritic complexity. These results indicate that mitral cell dendritic arbors are slightly more susceptible to the effects of chronic, partial deafferentation than complete, permanent deafferentation. This technique utilizes regular disruption of the olfactory sensory epithelium every 3 days throughout the duration of the experiment, which does not allow for a potential stabilization period for olfactory bulb structures between each treatment. It is likely that this results in a greater disruption of mitral cell dendritic arbor structure compared to permanent, cautery deafferentation, which removes the olfactory organ with a single deafferentation incident. Previous research shows that olfactory sensory axons are reduced in the olfactory bulb with at least 3 weeks of chronic detergent treatment, with the majority of glomeruli affected (Paskin and Byrd-Jacobs 2012; White et al. 2014). Thus, the majority of mitral cells throughout the olfactory bulb are potentially affected at an earlier time point than with permanent deafferentation. An alternate possibility could be involvement of immature olfactory sensory neuron axons potentially present with chronic, partial deafferentation but eliminated with complete, permanent deafferentation. Since the repeated detergent infusions used to produce chronic, partial deafferentation target the superficial layers of the olfactory epithelium, some immature olfactory sensory neurons are spared until they mature and are removed with subsequent detergent infusions (Iqbal and Byrd-Jacobs 2010). These immature axons do not yet make mature synaptic contacts with mitral cell dendrites, but their interactions may influence dendritic arbor shape. Further study is needed to understand the factors influencing dendritic complexity.
The morphology of mitral cells of the internal control bulbs, contralateral to the deafferentation wound, remained similar to those observed in unlesioned control fish mitral cells, suggesting a direct effect of the removal of afferent axons. Following 8 weeks of permanent deafferentation there were no differences in the measurements of internal control bulb mitral cell dendritic structures compared to those in unlesioned fish. However, following 8 weeks of chronic, partial deafferentation there was a significant increase in the length of major dendritic branches of internal control mitral cells compared to unlesioned control mitral cells, which could indicate growth-related changes to mitral cell dendritic arbors. Zebrafish maintained in a constant, optimal temperature environment have been shown to continue growth over time (Schaefer and Ryan 2006). It has also been shown that with continued growth, the dendritic arbors of retinal ganglion cells in goldfish increase in size (Bloomfield and Hitchcock 1991; Lee and Stevens 2007). Therefore, future studies may be necessary to determine potential growth-related changes occurring to structures within the adult zebrafish brain.
In this study we show that while there are significant changes to the distribution of fine processes within mitral cell dendritic arbors following both permanent and partial, chronic deafferentation, the effects on the major dendritic branches are variable. This study shows the importance of activity on the maintenance of adult brain structures in the zebrafish olfactory system through detailing the response to deafferentation of the major output neurons of the zebrafish olfactory bulb by describing the changes to dendritic arbor morphology and complexity over time. Future studies examining the specific cellular and molecular changes that occur within dendritic arbors following deafferentation in mitral cells can begin to explore the mechanisms involved in afferent-target interactions in the adult brain.
Acknowledgements:
We are grateful to the Western Michigan University Biological Imaging Center for the use of the confocal microscope and Natalie K. Hamilton for her technical assistance.
Funding
Supported by National Institutes of Health-NIDCD grants #04262 and #011137 (CBJ)
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Baier H, Korsching S. 1994. Olfactory glomeruli in the zebrafish form an invariant pattern and are identifiable across animals. J Neurosci. 14: 219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H, Kawano T, Albert V, Joh TH, Reis DJ, Margolis FL. 1984. Olfactory bulb dopamine neurons survive deafferentation-induced loss of tyrosine hydroxylase. Neurosci. 11: 605–615 [DOI] [PubMed] [Google Scholar]
- Baker H, Morel K, Stone DM, Maruniak JA. 1993. Adult naris closure profoundly reduces tyrosine hydroxylase expression in mouse olfactory bulb. Brain Res. 614: 109–116 [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Hitchcock PF. 1991. Dendritic arbors of large-field ganglion cells show scaled growth during expansion of the goldfish retina: a study of morphometric and electronic properties. J Neurosci. 11: 910–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braubach OR, Fine A, Croll RP. 2012. Distribution and functional organization of glomeruli in the olfactory bulbs of zebrafish (Danio rerio). J Comp Neuro. 520:2317–2339 [DOI] [PubMed] [Google Scholar]
- Brunjes PC. 1985. Unilateral odor deprivation: time course of changes in laminar volume. Brain Res Bull. 14: 233–7 [DOI] [PubMed] [Google Scholar]
- Brunjes PC. 1994. Unilateral naris closure and olfactory system development. Brain Res Rev. 19: 146–160 [DOI] [PubMed] [Google Scholar]
- Byrd CA. 2000. Deafferentation-induced changes in the olfactory bulb of adult zebrafish. Brain Res. 866: 92–100 [DOI] [PubMed] [Google Scholar]
- Byrd CA, Brunjes PC. 2001. Neurogenesis in the olfactory bulb of adult zebrafish. Neuroscience. 105: 793–801 [DOI] [PubMed] [Google Scholar]
- Casabona G, Catania MV, Sorto M, Ferraris N, Perroteau I, Fasolo A, Nicoletti F, Bovolin P. 1998. Deafferentation Up-Regulates the Expression of the mGluR1a Metabotropic Glutamate Receptor Protein in the Olfactory Bulb. Eur J Neurosci. 10: 771–776 [DOI] [PubMed] [Google Scholar]
- Charych EI, Akum BF, Goldberg JS, Jornsten RJ, Rongo C, Zheng JQ, Firestein BL. 2006. Activity-independent regulation of dendrite patterning by postsynaptic density protein PSD-95. The Journal of Neuroscience. 26: 10164–10176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow DK, Groszer M, Pribadi M, Machniki M, Carmichael ST, Liu X, Trachtenberg JT. 2009. Laminar and compartmental regulation of dendritic growth in mature cortex. Nat Neurosci. 12: 116–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde C, Caceres A. 2009. Microtubule assembly, organization and dynamics in axons and dendrites. Nature Rev Neurosci. 10: 319–332 [DOI] [PubMed] [Google Scholar]
- Coppola DM. 2012. Studies of olfactory system neural plasticity: the contribution of the unilateral naris occlusion technique. Neural Plast. 2012: 351752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corotto FS, Henegar JR, Maruniak JA. 1994. Odor deprivation leads to reduced neurogenesis and reduced neuronal survival in the olfactory bulb of the adult mouse. Neuroscience. 61: 739–744 [DOI] [PubMed] [Google Scholar]
- Couper Leo JM, Devine AH, Brunjes PC. 2000Focal denervation alters cellular phenotypes and survival in the developing rat olfactory bulb. J Comp Neurol. 417: 325–336 [DOI] [PubMed] [Google Scholar]
- Couper Leo JM, Brunjes PC. 2003. Neonatal focal denervation of the rat olfactory bulb alters cell structure and survival: a Golgi, Nissl, and confocal study. Dev Brain Res. 140: 277–286 [DOI] [PubMed] [Google Scholar]
- Drechsel DN, Hyman AA, Cobb MH, Kirschner MW. 2009. Modulation of the dynamic instability of tubulin assembly by the microtubule associated protein tau. Mol Bio Cell. 3: 1141–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JG, Michel WC. 2002. Odor-stimulated glutamatergic neurotransmission in the zebrafish olfactory bulb. J Comp Neurol. 454: 294–309 [DOI] [PubMed] [Google Scholar]
- Ehrlich M, Grillo M, Joh TH, Margolis FL, Baker H. 1990. Transneuronal regulation of neuronal specific gene expression in the mouse olfactory Bulb. Mol Brain Res. 7: 115–122 [DOI] [PubMed] [Google Scholar]
- Ferraris N, Perroteau I, De Marchis S, Fasolo A, Bovolin P. 1997. Glutamatergic deafferentation of olfactory bulb modulates the expression of mGluR1a mRNA. Neuroreport. 8: 1949–1953 [DOI] [PubMed] [Google Scholar]
- Fuller CL, Yettaw HK, Byrd CA. 2006. Mitral cells in the olfactory bulb of adult zebrafish Danio rerio: Morphology and distribution. J Comp Neurol. 499: 218–230 [DOI] [PubMed] [Google Scholar]
- Gemberling M, Bailey TJ, Hyde DR, Poss KD. 2013. The zebrafish as a model for complex tissue regeneration. Trends in Genet. 29: 611–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding JW, Getchell TV, Margolis FL. 1978. Denervation of the primary olfactory pathway in mice. V. Long-term effect of intranasal ZnSO4 irrigation on behavior, biochemistry and morphology. Brain Res. 14: 271–285 [DOI] [PubMed] [Google Scholar]
- Henegar JR, Maruniak JA. 1991. Quantification of the effects of long-term unilateral naris closure on the olfactory bulbs of adult mice. Brain Res. 568: 230–234 [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L. 2005. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 45: 279–91 [DOI] [PubMed] [Google Scholar]
- Iqbal T, Byrd-Jacobs C. 2010. Rapid degeneration and regeneration of the zebrafish olfactory epithelium after Triton X-100 application. Chem Senses. 35:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izeddin I, Specht CG, Lelek M, Drazacq X, Triller A, Zimmer C, Dahan M. 2011. Super-resolution dynamic imaging of dendritic spines using a low-affinity photoconvertible actin probe. PLoS ONE. 6: e15611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka K, Aika Y, Toida K, Koska T. 2001. Structure of intraglomerular dendritic tufts of mitral cells and their contacts with olfactory nerve terminals and calbindin-immunoreactive type 2 periglomerular neurons. J Comp Neurol. 440: 219–235 [DOI] [PubMed] [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Naim AC, Greengard P. 2006. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. PNAS. 103: 3399–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Stevens CF. 2007. General design principle for scalable neural circuits in a vertebrate retina. Proc Natl Acad Sci USA. 104: 12931–12935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Gheusi G, Vincent JD 2005. Information processing in the mammalian olfactory system. Physiol Rev. 85: 281–317 [DOI] [PubMed] [Google Scholar]
- Lopez-Mascaraque L, Garcia C, Blanchart A, De Carlos JA. 2004. Olfactory epithelium influences the orientation of mitral cell dendrites during development. Dev Dyn. 232: 325–335 [DOI] [PubMed] [Google Scholar]
- Luo L 2002. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Ann Rev Cell Dev Biol. 18: 601–635 [DOI] [PubMed] [Google Scholar]
- Margolis FL, Roberts N, Ferriero D, Feldman J. 1974. Denervation in the primary olfactory pathway of mice: biochemical and morphological effects. Brain Res. 81: 469–483 [DOI] [PubMed] [Google Scholar]
- Matsutani S, Yamamoto N. 2000. Differentiation of mitral cell dendrites in the developing main olfactory bulbs of normal and naris-occluded rats. J Comp Neurol. 418: 402–410 [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. 2004. Structural basis of long-term potentiation in single dendritic spines. Nature. 429: 761–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews MR, Powell TPS. 1962. Some observations on transneuronal cell degeneration in the olfactory bulb of the rabbit. Journal of Anat. 96: 89–102 [PMC free article] [PubMed] [Google Scholar]
- Matus A 2000. Actin-based plasticity in dendritic spines. Science. 290: 754–758 [DOI] [PubMed] [Google Scholar]
- Meisami E, Safari L. 1991. A quantitative study of the effects of early unilateral olfactory deprivation on the number and distribution of mitral and tufted cells and of glomeruli in the rat olfactory bulb. Brain Res. 221: 81–107 [DOI] [PubMed] [Google Scholar]
- Mizrahi A, Katz LC. 2003. Dendritic stability in the adult olfactory bulb. Nat Neurosci. 11: 1201–1207 [DOI] [PubMed] [Google Scholar]
- Nadi NS, Head R, Grillo M, Hempstead J, Grannot-Reisfeld N, Margolis FL. 1981. Chemical deafferentation of the olfactory bulb: plasticity of the levels of tyrosine hydroxylase, dopamine and norepinephrine. Brain Res. 213: 365–377 [DOI] [PubMed] [Google Scholar]
- Oberto M, Soncin I, Bovolin P, Voyron S, De Bortoli M, Dati C, Fasolo A. 2001. I. Perroteau, ErbB-4 and neuregulin expression in the adult mouse olfactory bulb after peripheral deafferentation. Eur J Neurosci. 14: 513–521 [DOI] [PubMed] [Google Scholar]
- Okamoto K, Nagai T, Miyawaki A, Hayashi Y. 2004. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nature Neurosci. 7: 1104–1112 [DOI] [PubMed] [Google Scholar]
- Parrish JZ, Emoto K, Kim MD, Jan YN. 2007. Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Annu Rev Neurosci. 30: 399–423 [DOI] [PubMed] [Google Scholar]
- Paskin TR, Iqbal TR, Byrd-Jacobs CA. 2011. Olfactory bulb recovery following reversible deafferentation with repeated detergent application in the adult zebrafish. Neuroscience. 196: 276–284 [DOI] [PubMed] [Google Scholar]
- Paskin TR, Byrd-Jacobs CA. 2012. Reversible deafferentation of the adult zebrafish olfactory bulb affects glomerular distribution and olfactory–mediated behavior. Behav Brain Res. 235: 293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer J, Ryan A. 2006. Developmental plasticity in the thermal tolerance of zebrafish Dani rerio. Fish Biol. 69: 722–734 [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E 2012. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA. 1953. Dendritic organization in the neurons of the visual and motor cortices of the cat. Journal of Anat. 87: 387–406 [PMC free article] [PubMed] [Google Scholar]
- Sirbulescu RF, Zupanc GKH. 2009. Dynamics of caspase-3-mediated apoptosis during spinal cord regeneration in the teleost fish, apteronotus leptorhynchus. Brain Res. 1304: 14–25 [DOI] [PubMed] [Google Scholar]
- Stone DM, Grillo M, Margolis FL, Joh TH, Baker H. 1991. Differential effect of functional olfactory bulb deafferentation on tyrosine hydroxylase and glutamic acid decarboxylase messenger RNA levels in rodent juxtaglomerular neurons. J Comp Neurol. 311: 223–233 [DOI] [PubMed] [Google Scholar]
- Szebenyi G, Bollati F, Bisbal M, Sheridan S, Fass L, Wray R, Haferkamp S, Nguyen S, Caceres A, Brady ST. 2005. Activity-driven dendritic remodeling requires microtubule-associated protein 1A. Curr Biol. 15: 1820–1826 [DOI] [PubMed] [Google Scholar]
- Teng J, Takei Y, Harada A, Nakata T, Chen J, Hirokawa N. 2001. Synergistic effects of MAP2 and MAP1B knockout in neuronal migration, dendritic outgrowth, and microtubule organization. Cell Biol. 155: 165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa K, Kunieda J, Nakayasu H. 2001. Ex vivo culture of isolated zebrafish whole brain. J Neurosci Methods. 107: 31–38 [DOI] [PubMed] [Google Scholar]
- Tran H, Chen H, Walz A, Posthumus JC, Gong Q. 2008. Influence of olfactory epithelium on mitral/tufted cell dendritic outgrowth. PLoS ONE. 3: e3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimpe DM, Byrd-Jacobs CA. 2016. Patterns of olfactory bulb neurogenesis in the adult zebrafish are altered following reversible deafferentation. Neuroscience. 331: 134–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant AR, Zanassi P, Walsh GS, Aumont A, Alonso A, Miller FD. 2002. Signal mechanisms underlying reversible, activity-dependent dendrite formation. Neuron. 34: 985–998 [DOI] [PubMed] [Google Scholar]
- Vankirk AM, Byrd CA. 2003. Apoptosis following peripheral sensory deafferentation in the olfactory bulb of adult zebrafish. J Comp Neurol. 455: 488–498 [DOI] [PubMed] [Google Scholar]
- Villanueva R, Byrd-Jacobs CA. 2009. Peripheral sensory deafferentation affects olfactory bulb neurogenesis in zebrafish. Brain Res. 1269:31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EJ, Kounelis SK, Byrd-Jacobs CA. 2015. Plasticity of glomeruli and olfactory-mediated behavior in zebrafish following detergent lesioning of the olfactory epithelium. Neuroscience. 284: 622–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ROL, Gosh A. 2002. Activity-dependent regulation of dendritic growth and patterning. Nature Rev. 3: 803–812 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. 2004. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 44: 749–757 [DOI] [PubMed] [Google Scholar]
- Zippel HP, von Rekowski C. 1993. In goldfish the qualitative discriminative ability for odors rapidly returns after bilateral nerve axotomy and lateral olfactory tract transection. Brain Res. 618: 338–340 [DOI] [PubMed] [Google Scholar]