Abstract
Threat processing is central to understanding debilitating fear- and trauma-related disorders such as Posttraumatic Stress Disorder (PTSD). Progress has been made in understanding the neural circuits underlying the ‘engram’ of threat- or fear-memory formation that complements a decades-old appreciation of the neurobiology of fear and threat involving hub structures such as the amygdala. In this review, we examine key recent findings as well as integrate the importance of hormonal and physiological approaches to provide a broader perspective of how bodily systems engaged in threat responses may interact with amygdala-based circuits in the encoding and updating of threat-related memory. Understanding how trauma-related memories are encoded and updated throughout the brain and the body will ultimately lead to novel biologically-driven approaches for treatment and prevention.
Keywords: Memory, Fear, Threat, PTSD, Amygdala, Stress, Circuit, Physiology
Introduction
Fear is an emotional experience deeply rooted in evolution to facilitate survival and to aid in the avoidance or escape from potentially harmful situations. However, unlike our evolutionary ancestors, the contexts, circumstances and stimuli which have come to engage evolutionarily-based fear circuitry have expanded well beyond avoidance of potential predators to promote survival (LeDoux, 1993). This involvement of evolutionarily-based fear circuitry is largely attributed to the expanding types of stressors, including increased rates of trauma, violence, emotional, physical and sexual abuse that, unfortunately, have become more pervasive and common place. As a result of the brain’s plasticity, or adaptability, prior exposure to traumatic and threatening experiences enables more rapid responses to threats in the future. Such threat-related responses may become intrusive and maladaptive, and these maladaptive responses to threat are central to anxiety and trauma-related ailments such as Posttraumatic Stress Disorder (PTSD). PTSD and other trauma and stressor related disorders have been distinguished from anxiety disorders in the recent iteration of the Diagnostic and Statistical Manual of Mental Disorders because there is often a singular event, or limited number of events, that are foundational to the development of PTSD (American Psychiatric Association and American Psychiatric Association DSM-5 Task Force., 2013).
The truly subjective and phenomenological nature of the fear experience in humans is not one that easily avails itself to direct study using laboratory animals (LeDoux, 2014), but the use of more precise terminology such as ‘threat-related’ or ‘trauma-related’ behaviors is particularly important in translational work. In fact, the circumstances and frameworks that do mediate physiological and physical responses, largely mediated by activation of the autonomic nervous system to threatening stimuli, can be thought of as integrated and are tractable from an evolutionary perspective. Studying model organisms greatly aides our progress towards uncovering the mechanisms that are necessary for the persistence of threat and trauma-relevant memories (Fanselow and Pennington, 2018).
Pavlovian threat conditioning involves the introduction of a rodent to a behavioral testing chamber (context) and the subsequent presentation of novel cues to serve as conditioned stimuli (CSs) that are presented contingent with an unconditioned stimulus (US) such as a brief electric footshock. The use of Pavlovian (or Classical) conditioning has enabled incredible progress in uncovering the conserved neuroanatomical and molecular substrates that are required for long-lasting behavioral alterations due to threat and trauma exposure in mammals (Davis, 1992; Fanselow, 1980). Threat encoding is the initiation of cellular and molecular processes within neuronal synapses which occur in response to stimuli present at the time of threat exposure, resulting in alterations in synaptic plasticity within and across relevant neural circuits. The subsequent stabilization of these changes in synaptic plasticity at the neuronal and circuit level following memory encoding is referred to as the “engram” and incorporates aspects that can be distinguished as discreet temporal, molecular, and neural circuit events which support the persistence of memory evident by the emergent behavioral, molecular, and physiological manifestations that result from threat and traumatic experiences (Figure 1; Fanselow and LeDoux, 1999; Schafe et al., 2005).
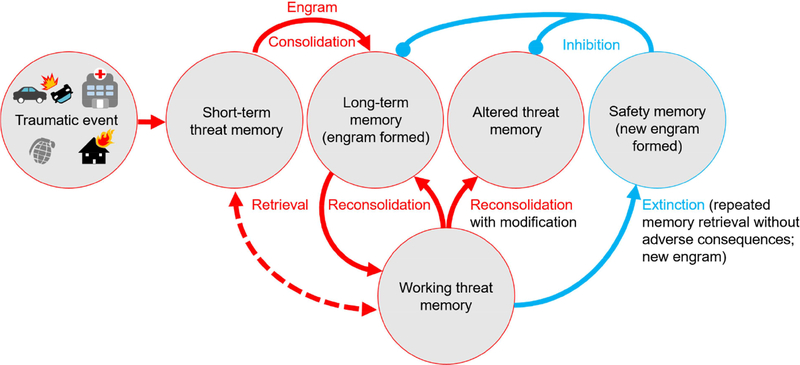
Figure 1. Schematic illustration of the main stages of memory encoding following a traumatic event consisting of consolidation, reconsolidation, and extinction.
Soon after experiencing trauma, the memory is in an active state in short-term memory until it gets consolidated and stabilized into long-term memory. Since short-term memories are instantly available to conscious awareness, they are also temporarily available to working memory while being consolidated. The retrieval of a consolidated memory at later time points returns the memory from an inactive state in long-term memory to an active state in working memory. From there, the reactivated memories are stabilized again during a process called reconsolidation. Reconsolidation most readily takes place after brief reactivation, thereby strengthening the long-term memory. During reconsolidation events, the active memory traces are potentially susceptible to modification. Extinction (safety memory which inhibits the original threat memory) is induced upon recurrent reactivation of a memory without adverse consequences. Reconsolidation and extinction represent contrasting processes that work in concert to either strengthen or inhibit threat memory expression over time.
Decades’ old work has revealed that the amygdala, and its now increasingly better understood subnuclei, is a master coordinator of threat-response and trauma-related behavioral alterations (Fox et al., 2015; Liberzon et al., 2003; Morris et al., 1998; Phelps and Anderson, 1997; Vuilleumier et al., 2001). Furthermore, work in recent years suggests that a much wider pool of brain regions and circuits also participate in threat- and trauma-relevant encoding. This circuitry now includes the amygdala, hippocampus, cortical and thalamic regions, and downstream regions in the brainstem, to regulate the constellation of behavioral and physiological consequences resulting from exposure to traumatic or threatening circumstances and later encounters with cues associated with the treat or trauma (Figure 2).
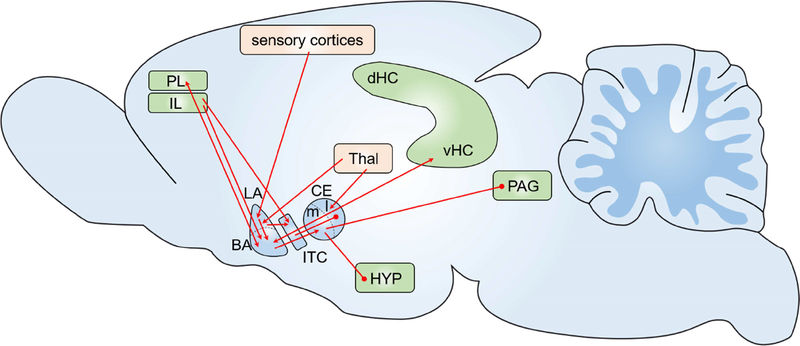
Figure 2. A simplified schematic of the neural threat circuitry.
The amygdala and its subnuclei are key nodes of threat and trauma-relevant encoding. The lateral amygdala (LA) and the lateral central amygdala (CEA-l) receive input from sensory cortices and thalamic structures (Thal) and represent a major site of threat-related neuronal plasticity. This plasticity is controlled by reciprocal connections between the basal amygdala (BA) and the prelimbic cortex (PL) as well as between the BA and the ventral hippocampus (vHC). Subsequently, the medial part of the central amygdala (CEA-m) projects to the hypothalamus (HYP) and other subcortical and brainstem regions to promote threat. Threat extinction is controlled by different circuits within the same structures. Input from the infralimbic cortex (IL) to the BA and to the intercalated (ITC) cells of the amygdala is crucial in reducing threat output from central amygdala nuclei (CE) to the hypothalamus (HYP) and the periaqueductal grey (PAG).
While we have gained immense insight into the underpinnings of most elementary emotional memory processes by examining the mechanisms that mediate encoding of CS-US associations, threat-related freezing is just one outcome of the many associations that is acquired and encoded at the time of threat and trauma exposure. Attention paid to other variables and associations that alter the entraining of emotional memories, or are themselves encoded into their own distinct or over-lapping engrams at the time of threat or trauma, is critical. Understanding these variables will help to prevent the initial encoding or alleviate the persistence of previously encoded trauma-related memories, such as those seen in PTSD. Therefore, this review aims to address how many of the neurobiological underpinnings, stimulus and contextual factors that are critical for the formation of enduring trauma-relevant memory engrams is relevant to the shortcomings of current therapeutic interventions for trauma-relevant memories (Figure 3).
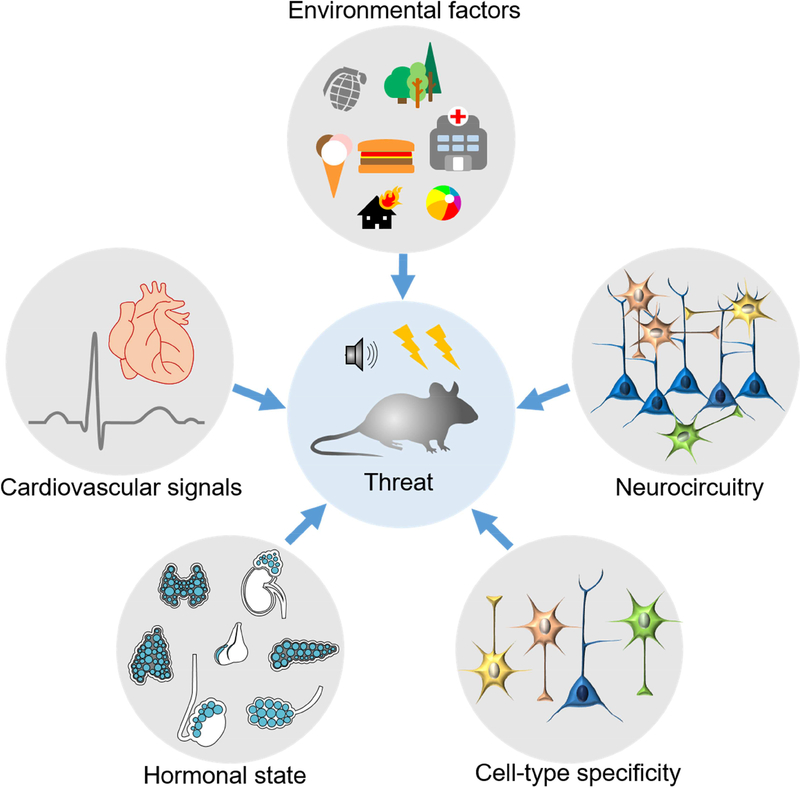
Figure 3. Factors that can influence threat memories.
A broad spectrum of factors can have a crucial impact on the encoding of features of enduring trauma and threat-relevant memories. Amongst others these are: environmental factors (e.g. early life adversity, diet), neurocircuitry (e.g. alterations within GABAergic, glutamatergic, serotonergic or dopaminergic circuits), cell-type specificity (e.g. Dkk3, Tac2 in BLA/CeA), hormonal state (.e.g. CRH, glucocorticoid and estrogen levels) and cardiovascular signals (e.g. heart rate). Most often it is an interaction of several of these factors that influence threat memories.
Forming the Engram: What is encoded into an engram?
Studies employing rodent models of threat learning have often examined the cellular and molecular mechanisms that underlie the initial associations of discrete sensory cues, such as an auditory tone or olfactory cue, paired with a noxious unconditioned stimulus such as a brief electric footshock. It is now well known that principal neurons within the lateral nucleus of the amygdala (LA) receive projections directly from the sensory thalamus, positioning it to receive stimulus information at the time of threat conditioning (LeDoux et al., 1990; Romanski et al., 1993). Simultaneously, the LA receives synaptic input concerning the unconditioned stimulus from somatosensory cortex and much work has revealed that as a direct consequence of these co-occurring inputs onto LA neurons, plasticity occurs at synapses following CS-US pairings within the LA and can thus be considered the most elementary memory engram for CS-US associations. (Romanski et al., 1993). Studies - now considered classic in the field - utilized field potential recordings in the LA to examine the existence of stimulus-preference for LA neurons in response to auditory tone-CS presentations and shock-US presentations (Rogan and LeDoux, 1995; Rogan et al., 1997). This early work revealed cell populations that were preferentially active in response to auditory information alone vs. those responding to US information alone, and a final population that responded to both CS and US information. This work revealed for the first time the cells poised for undergoing robust plasticity following CS-US pairings, and thus able to be encoded into the engram supporting this CS-US association. More recent work utilizing optogenetics has suggested that a combination of Hebbian plasticity and modulation by neurotransmitter systems, such as adrenergic signaling, must occur synergistically to support plasticity and encoding of CS-US associations within the LA (Johansen et al., 2014), thus the engram must encode both fast neuronal input and slower physiologic contextual information.
Stimulus specificity of the engram
The mechanisms which regulate the encoding of discrete sensory stimulus memories to permit discrimination and prevent generalization have yet to be fully established, however a number of interesting hypotheses have emerged. These include methods for increasing signal to noise processing within the amygdala, hippocampus, and insula, in addition to much plasticity that occurs in sensory thalamic and cortical areas, potentially shaping the sensitivity to sensory information prior to its processing in limbic structures (Banerjee et al., 2017; Jones et al., 2008; Morrison et al., 2015; Weinberger, 2011). Successful discrimination of sensory cues that are relevant to emotionally salient events, such as threat conditioning, requires reciprocal changes in LA principal neurons and in sensory systems projecting there. Thus, while there is a strengthening of synapses relevant to the US, there is a depotentiation of those that are irrelevant (Collins and Pare, 2000).
The concept of stimulus-specific plasticity in memory encoding has also recently been supported with a study revealing a selective increase in AMPA/NMDA ratios for auditory CS cues associated with a US, but no change in familiar auditory cues which remained unassociated with a US (Kim and Cho, 2017). These data suggest that the use of discrete and discriminative cues alters synaptic plasticity to support encoding of engrams that are restricted to threat-related cues and suggest that distinct subsets of heterogeneous cells encode different aspects of information within the threat-related neuronal ensemble, or engram.
The molecular state of a neuron at the time of conditioning impacts the likelihood that it will participate in the encoding of a subsequent memory engram (Josselyn et al., 2001). Studies examining the activity of the transcription factor cyclic-AMP response element binding protein (CREB) have established that neurons that have high CREB activation states, either naturalistically or experimentally manipulated, are more likely to be recruited into a conditioning-related engram (Han et al., 2007). More recently, it has been shown that the state of neuronal excitability, related to neuronal or hormonal signal at the time of conditioning, impacts neurons which are most likely to be allocated into the engram. In addition, principal neurons in the LA are poised to encode stimulus memories that closely occur in time into overlapping neuronal ensembles, while those stimulus presentations occurring at more distal times are more likely to be encoded into distinct non-overlapping neuronal ensembles, which may involve gating of newly engram-allocated LA principal neurons by GABA-ergic parvalbumin interneurons (Morrison et al., 2016).
The above studies suggest that the stimulus specificity of individual cell types will be critical to understand encoding and the engram. While much current work aims to identify cell-types based on molecular identity which then may play differential roles in threat learning and memory encoding, understanding cell’s individual synaptic connectivity, and thus stimulus preference, will be another important molecular indicator of function that may not be directly encoded in the transcriptome.
Cell-type and circuit-specific encoding of threat memory engrams
Recent approaches in neurobiology have similarly aimed to identify discrete cell populations and their respective circuits, which may be relevant for the encoding of specific aspects of threat-relevant memories (e.g. Haubensak et al., 2010). For example, cell-type specific excitatory and inhibitory optogenetic and chemogenic approaches have revealed a subset of pyramidal neurons within the BLA that preferentially projects to nucleus accumbens (NAc) over central amygdala (CeA). These cells are marked by Dkk3 and other cell-type specific gene expression, and appear to support the inhibition of threat memories (Jasnow et al., 2013; McCullough et al., 2016). Separately, a set of neurons within the CeA marked by the Tachykinin 2 gene (Tac2) projects preferentially to distinct brainstem regions and supports threat encoding and expression over threat inhibition (Andero et al., 2014).
Another recent approach utilized a genetic strategy to identify the representations of rewarding and aversive USs in the BLA, demonstrating that activation of an ensemble of US-responsive BLA cells elicits innate physiological and behavioral responses of different valence (Gore et al., 2015). Additionally, they found that US-responsive BLA cells are necessary for the expression of a conditioned response. Such data suggest that neural representations of US and CS connect to US-responsive cells in the BLA to elicit both unconditioned and learned responses. Together these and other recent papers utilizing optogenetic and chemogenetic causal ‘circuit-busting’ approaches to understanding genetically defined amygdala populations offer great promise to better understand the cellular specificity of threat and trauma-related memory encoding.
Although much focus has been spent on the discrete sensory cues which mediate the behavioral concomitants of encoded threat and trauma-relevant memories, the emergence of behavioral and autonomic consequences following conditioning suggests enduring plasticity and the establishment of engrams within individual circuits mediating each outcome. For example, the emergence of conditioned freezing responses is known to be mediated by the induction of AMPA and NMDA receptor mechanisms within the periaqueductal gray (PAG; Kim et al., 2013; Reimer et al., 2012). Conversely, while neurons within the lateral hypothalamus have been identified to have no direct impact on the emergence of freezing behaviors but are vital for conditioned changes in arterial pressure (Iwata et al., 1986), more recent studies have suggested that intermingled cell types within the hypothalamus differentially regulate different threat-response behaviors (Santos et al., 2008). These studies reveal some progress in understanding circuit-based alterations of conditioned response components, and multiple circuit-based engrams may interact to form the complete threat response, with its multiple concomitants. With relevance to the observation of cellular heterogeneity in the hypothalamus mediating discrete physiological outcomes to threat, and studies identifying amygdala cell-type preference in the behavioral responses to threat, more concerted efforts aimed at delineating the cell-type makeup and specificity of trauma-relevant engram formation will be vital towards progress in understanding the neuroanatomical basis of threat and trauma-relevant memory.
Encoding of Interoceptive & Physiologic Information
While much work has aimed to examine the mechanisms which underlie engram formation for elementary trauma-relevant CS-US associations, it has long been appreciated that physiologic state and hormonal processes present at the time of traumatic memory acquisition, as well as those altered as a result of exposure to stressor and threat, also impact the encoding and later expression of memory. The late 19th century James-Lange theory of emotion emphasized physiological state in determining the immediate consequences, both physically and physiologically, to emotional stimuli as antecedents to conscious appraisal, or subjective perceptual labelling, of stimuli as emotionally arousing. While the specifics of this model have largely been set aside, as no concrete evidence has emerged for the existence of specific autonomic indices that are relevant for discrete emotional states (Friedman, 2010), the theory did nonetheless garner an appreciation for nonconscious and interoceptive processes in emotional learning and memory. Given the widespread impacts that hormonal and physiological processes can exert throughout the central and peripheral nervous systems, coupled with the known physiological manifestations of threat and trauma-relevant memories, the logical next step is to consider the mechanisms through which physiological response to trauma affects threat encoding to establish one or more engrams. Additionally, the physiological response itself may be altered in response to trauma, serving as a powerful interoceptive trauma-related cue impacting expression of threat-relevant memories. While a full review of all potential interoceptive cues and physiological factors that may influence the encoding of trauma and threat-relevant memories is outside of the scope of this review, we highlight a few examples of hormones and neurochemicals to demonstrate the idea that attention to these interoceptive cues, is vital towards uncovering the mechanisms underlying initial engram encoding and later alleviating trauma memories.
Hormonal and Neurochemical Contributions to Memory Encoding
Glucocorticoids, the classic steroid stress hormones that include cortisol and corticosterone, have long been appreciated to regulate stress response through feedback mechanisms modulating the release of corticotropin-releasing hormone (CRH). This occurs in brain regions known to mediate anxiety and stress- related behaviors including the hypothalamus, amygdala, cortex and the bed nucleus of the stria terminalis (BNST; McEwen et al., 2015). In the setting of an acute stressor in a naïve animal, brief elevation in glucocorticoid signal results in stress-related neuronal and behavioral alterations which are largely associated with the co-engagement of noradrenergic signaling to facilitate memory consolidation for both hippocampal-dependent contextual and amygdala-mediated auditory memory (Roozendaal et al., 2006). However, the role of glucocorticoid signaling in trauma-relevant memory processes following periods of chronic stress exposure is less conclusive. It is well established that chronic exposure to stress hormones such as glucocorticoids results in reduced dendritic complexity in both cortical regions and hippocampus (Conrad et al., 1999; Liston et al., 2006; Vyas et al., 2002). However, studies suggest that two distinct sites of glucocorticoid-modulating CRH pathways may exist, one constrained through negative feedback to the hypothalamic system, and one non-constrained system, which includes the CeA and BNST, and may be relevant to the reported dendritic hypertrophy observed in these regions with chronic exposure (Schulkin et al., 1998).
Work in rats using pharmacological targeting of amygdala nuclei has revealed that pre- and immediate post-training infusions of CRH in the LA resulted in reduced threat memory, while infusions prior to testing enhanced the expression of threat memory, and CRH infusions in CeA before or after training or testing had no effect on memory (Isogawa et al., 2013). In contrast, CeA-specific knockout of glucocorticoid receptors (GRs), the major receptor for corticosterone, results in impaired threat learning in mice, which can be overcome with replacement of CRH in the CeA (Kolber et al., 2008). Importantly, additional studies utilizing transgenic mouse approaches have supported the existence of heterogeneous CRH-expressing neurons contributing to divergent effects in response to CRH depending on the co-expression of other receptors. Deletion of GABA(A)a1 and Grin1 in CeACRH neurons impaired threat extinction thereby enhancing threat memory, but likely throug distinct mechanisms (Gafford et al., 2014; Gafford et al., 2012). Furthermore, deletion of GRs from LAGlut appear to attenuate threat-related behaviors (Hartmann et al., 2017). In line with these findings, further work to subdivide CRH expressing cells into more discrete neuronal populations based on co-expression of other receptors subtypes and/or projection specificity will lead to a more precise understanding of the potential acute role for CRH in the initial encoding of trauma-relevant memories as well as the role of this neuropeptide in the later expression of memories.
Sex hormones are also thought to bias response to threat, as women are at greater risk for developing PTSD following trauma exposure (Breslau et al., 1999; McLean et al., 2011). Given the cyclic nature of sex steroid hormones including estradiol and progesterone and periods of dramatic hormone change including puberty and menopause, their relationship to trauma and anxiety-related conditions is not as straight-forward or as well-understood as that of the glucocorticoid hormone system. Recent work from rodent studies has suggested that estrus status, the murine corollary to the human female menstrual cycle, directly impacts the strength of memory for traumatic events. Studies examining the acquisition of threat conditioning have revealed that naturally cycling rodents in low-estradiol phases and ovariectomized females have enhanced encoding of threat memories, which can be normalized upon estradiol replacement in ovariectomized rodents (Frye and Walf, 2004; Hiroi and Neumaier, 2006). These findings largely suggest the existence of a dynamic and phasic impact of estrogen on encoding of emotionally-salient memories, where low estrogen states are permissive for encoding and high estrogen states are protective against the encoding of these memories.
Studies using startle behavior paradigms have noted that elevated estrogen can impact sensorimotor gating, such that it reduces the emergence of prepulse inhibition (PPI) of the acoustic startle response. PPI is a behavior that is known to require the integration of both attentional and emotive processes and thus rely on amygdala and limbic circuitry (Koch, 1998; Swerdlow et al., 1999). High levels of estrogen have been found to promote a shift in the balance of excitation and inhibition in the amygdala towards greater inhibition, while low levels of estrogen have been found to result in dysregulation of GABAergic inhibition in the amygdala, suggesting a potential mechanism at the level of electrophysiology and neuronal excitability that is relevant to the cyclic nature of estrogen’s proposed protection against the effects of trauma (Blume et al., 2017; Yang et al., 2017). In the hippocampus, estrogen’s phasic effects impact known activity-dependent signaling cascades (Srivastava et al., 2013), but it is unknown if this is also the case in the amygdala, or if there is cell-type specificity as well.
Translation of these preclinical findings into human studies have replicated the same general rule for estrogen status in modulating threat and trauma memory, where low-levels of circulating estrogens are associated with enhanced encoding of trauma-relevant memories (Glover et al., 2012; Glover et al., 2013). Lower estrogen status has also been associated with other indices of traumatic memory encoding, including enhanced skin conductance responding to conditioned stimuli and increased rate of traumatic memory intrusions in daily life (Glover et al., 2012). Higher levels of estrogen have also been found to result in less distress in response to a psychosocial stressor, reduced activation of negative mood states and reduced limbic system activation (Albert et al., 2015). Neuroimaging studies have shown reduced activity in the amygdala during high estrogen states and increased activity during low estrogen states, suggesting a potential modulatory effect of estrogen on amygdala activity that may account for the differential effects on trauma memory (Goldstein et al., 2010). Intriguingly, one study has suggested that administration of Ogestral (combined ethinyl estradiol/norgestrel) or the emergency hormonal contraceptive Plan B (levonorgestrel) shortly following forensic examination within 48 hr following sexual assault was associated with reduced rates of PTSD symptoms during the follow-up assessment 6 months later (Ferree et al., 2012). These findings, in concert with preclinical rodent work revealing impaired encoding of threat memory during high estradiol states, suggest the potential utility of exploring estrogen-related pharmacological targets in the early hours following trauma as potential efficacious therapeutics in impairing deleterious effects of trauma via restraint on trauma-engram formation.
Arousal and Cardiovascular Signals as Interoceptive Stimuli
Exposure to noxious stimuli, such as those central to threat and trauma, is known to result in arousal via the release of norepinephrine (NE) from the locus coeruleus. This serves as a critical neuromodulator of trauma-relevant plasticity such that its inhibition results in the impairment of trauma-relevant encoding (Bush et al., 2010; McGaugh et al., 2002). Just as central nervous release of NE is vital for modulating threat and trauma-relevant plasticity, systemic release is similarly critical in establishing sympathetic nervous system concomitants including alterations in cardiovascular tone and increases in blood pressure. As such, NE plays a vital role not only in modulating the initial encoding of trauma-relevant plasticity but is also a critical interoceptive cue. Thus, this system may form its own engram which includes trauma-relevant associations; such that elevations of NE activate this engram modulating threat and trauma-relevant memory (see Rodrigues et al., 2009 for full review).
Likewise, much work has also revealed a role for cholinergic signaling in trauma-relevant plasticity as it is known to enhance threat memory encoding in the LA via acetylcholine receptors that not only enhance the firing rate of LA principle neurons but also enhance glutamatergic transmission. Furthermore, while reducing cholinergic input to LA principle neurons does not completely abolish threat encoding, it does reduce the later behavioral expression of cue-related freezing, suggesting that cholinergic tone at the time of conditioning can modulate the strength of initial memory encoding, and that other neurotransmitters and hormone systems are also involved (Jiang et al., 2016).
While a complete discussion of all potential interoceptive cues and stimuli that may participate and influence the formation of traumatic memory relevant engrams would be outside of the scope of this manuscript, there has been a steadily developing literature concerning the influence of heart rate on emotion perception. Changes in sympathetic and parasympathetic nervous system activity has long been appreciated in studies of classical conditioning, including rabbit studies noting the emergence of bradycardia alongside the conditioned eyeblink response (Powell et al., 2002). Similarly, studies of auditory threat conditioning have noted increases in arterial pressure directly attributable to classical conditioning processes, suggesting that autonomic processes can come under control of conditioned stimuli, thereby creating a separate yet integrated whole-body threat-encoded engram (Iwata and LeDoux, 1988; Iwata et al., 1986; Zhang et al., 2004).
As in preclinical studies, experiments using conditioned startle paradigms in humans have noted alterations in heart rate that emerge concomitantly with behavioral measures of startle, such that reinforced stimuli (CS+) are associated with the emergence of conditioned bradycardia not seen for non-reinforced (CS−) stimuli (Castegnetti et al., 2016). Additional indices of cardiovascular function, such as resting heart rate variability (HRV), considered a proxy for vagal tone, have been found to impact the extent of acquisition in acoustic startle tasks. In these studies, higher resting HRV was associated with impaired acquisition of conditioned startle (Pappens et al., 2014; Park et al., 2013), suggesting that arousal state via sympathetic and parasympathetic balance can impact reactivity to startle and ultimately bias conditioned behaviors.
Cardiac cycle is defined by two phases, systole - associated with baroreceptor signaling of the strength and timing of cardiac compression, and diastole - where baroreceptors are idle between heartbeats. Ratings of fearful faces presented during the systole phase were significantly higher than neutral faces or fearful faces presented during diastole. Furthermore, amygdala responses, as measured by evoked response obtained during fMRI task engagement, were higher for fearful faces presented at systole (Garfinkel et al., 2014; Pfeifer et al., 2017). These clinical behavioral and imaging studies suggest that while accurate classification of emotion does involve an interaction between the central and peripheral sympathetic nervous systems, cardiac signals can augment the perception of emotion, especially in the case of processing fear and threat-related information. While a complete understanding is needed of how even short-term fluctuations in interoceptive cues such as cardiac phase can impact the processing of emotional information, this intriguing hypothesis provides a tractable mechanism for intervention (Critchley and Garfinkel, 2016; Mather et al., 2016a, b). Further, an intriguing hypothesis extending from this work suggests that bodily arousal through NE impacts stimulus processing to enhance the saliency of stimulus perception (Mather et al., 2016b). While it is yet unclear whether this model, largely established from human neuroimaging studies, will hold up once tested mechanistically in preclinical animal models, it presents a novel and potentially plausible mechanism through which peripheral interoceptive cues can modulate sensory processing in a manner that can bias attention.
Manipulating the Established Engram
The most well-established approaches for interfering with a persistent emotionally salient memory engram, in both appetitive (e.g. addiction cues) and aversive (e.g. trauma cues) domains, includes repeated exposure to emotional cue reminders - clinically defined as exposure therapy. The underlying therapeutic mechanisms of exposure therapy are experimentally defined most often as extinction. However, more recently it is thought that interfering with the “reconsolidation” or re-storage and re-solidification of a memory’s engram following its brief retrieval may also underlie some of the important therapeutic mechanisms or clinical exposure.
Impairing Initial Consolidation of the Engram
Decades of work from preclinical rodent studies have suggested that there is a temporal gradient through which the encoding and subsequent stabilization of synaptic plasticity occurs in threat-related neuronal circuits following threat conditioning (Dudai, 2004; McGaugh, 2000; Rodrigues et al., 2004). A precise estimation of the width of this consolidation-mediated temporal gradient is not yet possible due to the numerous factors that appear to expedite this process, including hormonal state and the strength or severity of the threat. Nonetheless, the existence of such a temporal window of synaptic lability following threat and before stabilization of the engram has raised interest in developing methods to intervene with engram consolidation (Kearns et al., 2012).
Unfortunately, current therapeutic approaches which apply early post-trauma psychotherapy have yielded mixed results depending on the type of intervention. Cognitive behavioral therapy following trauma has been found to delay the onset of PTSD symptoms, but does not inhibit its development (Sijbrandij et al., 2007). Conversely, a modified prolonged-exposure intervention consisting of three sessions beginning in the emergency department an average of 12 hours following trauma yielded reductions in both PTSD and depression symptoms at both one and three-month post-trauma time points (Rothbaum et al., 2012). Interestingly, this same modified prolonged-exposure intervention, administered in an emergency department pilot study, has been suggested to also mitigate the presence of genetic risk which is associated with likelihood of PTSD diagnosis (Rothbaum et al., 2014). A full explanation for the discrepancy of these findings concerning the outcomes of early-psychotherapeutic interventions for trauma is not possible given the few studies with relatively small sample sizes that have been conducted in this area. However, it is likely that the psychotherapeutic modality employed is the largest contributor to therapeutic and preventative outcome. As the modified prolonged exposure therapy procedure involves a combination of imaginal exposure and processing of traumatic material, it is likely that these approaches yield activation and arousal of the nervous system. Thus, there may be a closer approximation of the interoceptive state present at the time of trauma to engage a bottom-up inhibitory process capable of desensitizing arousal systems, contributing to the noted efficacy using this approach. These results clearly suggest that further work examining early psychotherapeutic approaches, proximal to the time of trauma, is warranted (McNally et al., 2003).
Importantly, a large proportion of individuals fail to seek treatment for trauma until well after the initial encoding and memory stabilization has occurred. Therefore, extinction of traumatic memories with clinically administered exposure therapy and, possibly, interfering with the post-retrieval reconsolidation of traumatic memories are more relevant for therapeutic intervention in already established PTSD.
Extinguishing the Engram
The repeated exposure to a stimulus that was previously associated with an unconditioned stimulus is well known to induce a reduction in the behavioral response to that stimulus, a phenomenon known as extinction. The process of extinction is an active learning process known to result in the formation of a new memory engram, largely consisting of projections from the prefrontal cortex to the amygdala which come to inhibit the expression of the trauma-related memory (Myers and Davis, 2007). This new engram exists alongside, rather than overwriting, the previously formed engram(s) that were encoded at the time of trauma. It is suggested that this co-existence interferes with extinction-based interventions, preventing optimal long-lasting and complete inhibition or disruption of the threat memory (Figure 4). This top-down approach, which is thought to involve the application of cortical control to contextualize bottom-up processing, concerns the flow of information from sensory receptor to sensory perception. This leads, ultimately, to the expression of behaviors or memories relevant to those perceptions, and it is the current best practice for therapeutic intervention, and yet is limited in its success (Sussman et al., 2016). While many previous reviews have thoroughly discussed the short-comings of extinction in impairing trauma-relevant memories, we think it is important to briefly discuss these short-comings in relevance to inattention to the multiple discrete and overlapping engrams which may exist following a traumatic experience that might underlie the potential for reinstatement of the threat response.
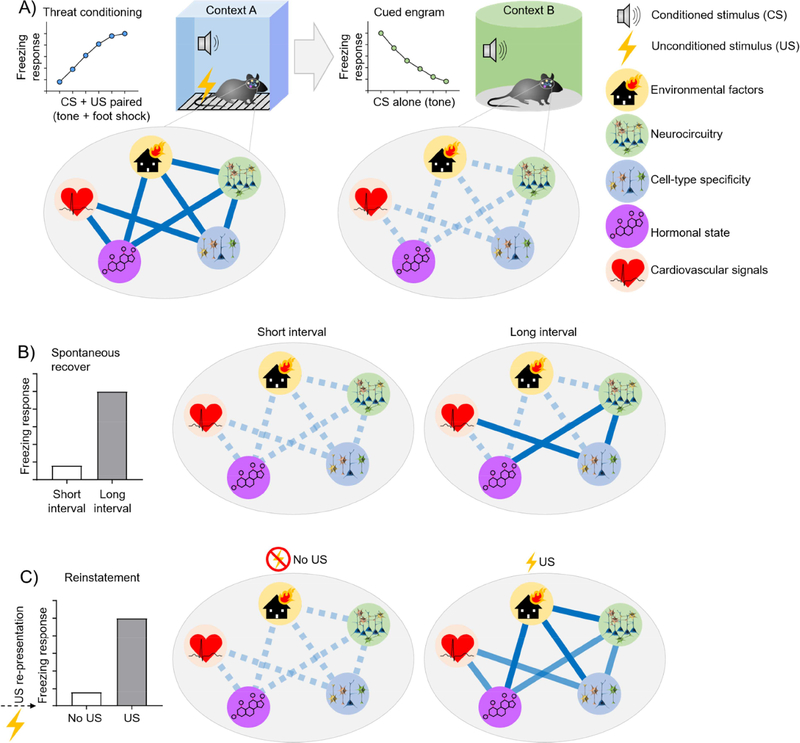
Figure 4. Failures of extinction conceptualized within an engram framework.
A) Encoding of threat-relevant engram and threat memory extinction in rodents. During extinction, repeated exposure to a CS loses its ability to evoke a conditioned response and weakens the CS engram, generally through context-dependent inhibition. (Notably, Impairment of reconsolidation may directly weaken the CS engram trace). B) Spontaneous recovery can occur when there is a long retention period between extinction and the exposure to a CS resulting in context-independent re-activation of a threat-relevant engram. C) Reinstatement occurs if the US is re-presented without the CS resulting in activation of the previously inhibited threat-relevant engram.
First, memories subjected to extinction can undergo spontaneous recovery over time (Quirk, 2002). Despite a long-standing appreciation for spontaneous recovery as a shortcoming of extinction, the conditions that mediate the emergence of spontaneous recovery largely remain unknown. In fact, some researchers speculate that spontaneous recovery supports the existence of co-existing threat and extinction, or safety memory engrams, such that it represents a failure to successfully retrieve an extinction memory rather than a loss of extinction memory itself and thus results from the presistence of threat-relevant engrams.
Second, memories subjected to extinction are may undergo reinstatement under circumstances where exposure to the unconditioned stimulus, or similarly noxious stimulus, result in the return of the threat memory (Bouton and Bolles, 1979; Harris and Westbrook, 1998a). While studies have revealed amygdala-mediated encoding of US (Debiec et al., 2010; Gore et al., 2015), and thus engram formation, other work has suggested that presentation of an entirely different noxious stimulus can result in expression of the originally conditioned response (Harris and Westbrook, 1998a). The mechanisms for a novel US resulting in reinstatement remain unknown, however it remains possible that engagement of the NE system either through re-exposure to the same US or one of enough saliency to engage adrenergic arousal may underlie reinstatement through mechanisms akin to state-dependent activation of arousal circuitry (Morris et al., 2005). If true, exposure to a US or stressor capable of engaging circuitry which would overlap with or recapitulate the initial threat memory engram will result in a resurgence of trauma-relevant memories. This phenomenon underscores the necessity of uncovering these mechanisms to allow for extension of extinction’s therapeutic value into real-world settings where stressors are not under experimental control.
Third, memories subjected to extinction frequently undergo renewal, whereby presentation of stimuli associated with the trauma in a context distinct from the one where extinction or exposure has occurred results in a return of threat expression. Some evidence suggests that the entorhinal cortex and fornix, both major interfaces of information integration in the hippocampus, likely contribute to renewal of threat behaviors under circumstances where integration of information fails to discriminate and differentiate between cues presented in non-extinction contexts which have no direct association with the US (Ji and Maren, 2008). Further, there is evidence to suggest that extinction learning results in the formation of neuronal ensembles within the LA that uniquely represent threat memories following extinction, and thus supports the notion of the coexistence of multiple and competing engrams within the LA, such that successful extinction requires appropriate pattern separation, or activation of relevant engrams vs irrelevant or less relevant ones (Orsini et al., 2013). Studies employing reversible inactivation of the dorsal hippocampus have revealed that this region is critical in successful pattern separation of contexts in which CS-US associations are likely versus those where CS-no-US are likely (Ji and Maren, 2005). Importantly, contexts in which the CS is presented come to acquire an association with the CS and in turn serve to provide predictive information about the likelihood of exposure to a reinforced or non-reinforced CS, to influence either existing engrams or create new yet-linked engrams due to a shared CS-association node. This is the main shortcoming of exposure-based therapies, as aversive stimuli outside of the therapeutic space re-enlisting renewal responses may interfere with the strength of a co-existing nascent engram. As many of the failures and short-comings of extinction-based interventions are a consequence of the co-existence of multiple memory engrams, identifying mechanisms through which potential integration of both engrams at the time of extinction learning or exposure therapy may be a powerful path forward. This idea is further conceptualized below as an integration of top-down and bottom-up processes for more efficacious therapeutic interventions.
Hormonal regulation of extinction
As discussed, the role of sex-hormone specific contributions to trauma-relevant disorders is an evolving area of investigation. Emerging evidence underscores a dynamic modulation of extinction processes by estrogen status, just as in the initial encoding of trauma-relevant memory. High-estrogen status has been repeatedly associated with enhanced extinction learning and thus greater success in adaptive threat inhibition processes (Glover et al., 2012; Glover et al., 2013; Hwang et al., 2015). Studies employing preclinical models suggest that high estrogen states are associated with enhanced cortical activity, specifically increased activity in the mPFC, and increased dendritic spine density of mPFC projections to the amygdala; suggesting enhancement of extinction via increased cortical inhibition of amygdala activity - a hypothesis which has not yet directly been tested (Maeng et al., 2017; Shansky et al., 2010; Zeidan et al., 2011). Therefore, attention to the status of individual hormonal state at the time of exposure-based approaches might allow therapeutic advantage on the beneficial modulation of extinction relevant PFC circuitry during high-estrogen phases (Antov and Stockhorst, 2014; Glover et al., 2015; Pineles et al., 2016).
Extinction and interoceptive states
As mentioned previously, the encoding and therefore contribution of interoceptive state at the time of trauma or threat can impact the initial consolidation and subsequent retrieval of memory. Studies have revealed that arousal state, largely mediated by beta-adrenergic receptor activation and impaired GABAergic inhibition, is likely responsible for much of the renewal observed in non-extinction contexts, suggesting that this mechanism which is shared with reinstatement of traumatic memories following extinction may warrant further exploration through which to overcome these shortcomings of extinction-mediated threat inhibition (Morris et al., 2005). In addition, studies showing impaired extinction learning and increased renewal suggest that GABAergic signaling may be critical for proper pattern separation, or engram activation and the successful learning of safety signals in novel, non-threatening contexts to allow for inhibition of the expression of competing CS-associated contextual memories (Chhatwal et al., 2005; Harris and Westbrook, 1998b; Heldt and Ressler, 2007). Temporary pharmacologic modulation, using beta-adrenergic antagonists (e.g. propranolol), or GABA-agonists (benzodiazepines) have been used in conjunction with extinction to boost context-independent extinction learning for this purpose, but these have undesirable side effects, and may also reduce new engram consolidation.
Extinction and exposure-based methods of impairing the expression of threat and trauma-relevant memories rely on cortical processing and the strengthening of cortical circuits which can in turn inhibit the expression of the coexisting trauma memories (Milad and Quirk, 2002; Sotres-Bayon et al., 2004). The involvement of cortical control over largely subcortical, amygdala-driven expression of traumatic memory is considered a top-down approach, whereby cortical control serves to inhibit and direct attentional processes away from amygdala response to the perception of stimuli or expression of sensory memories driven by presentation of threat-related cues (Quirk and Beer, 2006; Sotres-Bayon et al., 2004). Recall that chronic exposure to stress hormones results in reduced dendritic complexity within the cortex. Since extinction and exposure-based interventions rely on plasticity of cortical circuits, the impairments of plasticity suggest a potential mechanism which may explain the limited efficacy of exposure-based treatment in the clinic. Given these limitations, future work should examine the potential therapeutic approaches which target bottom-up mechanisms alone, or in concert with traditional top-down approaches, to account for subconscious experience (Capron et al., 2017; Grupe & Nitschke, 2013; Sussman et al., 2016).
Interestingly, studies of Patient SM, an informative neurological case in which bilateral calcification of the amygdala has occurred as a result of the rare genetic condition Urbach-Wiethe disease, have noted her inability to spontaneously direct her visual attention to the eyes of others. These data suggest that this may be the result of impaired amygdala-dependent bottom-up control of attentional processes which alters the balance of attention largely in favor of top-down processes (Adolphs et al., 2005; Kennedy and Adolphs, 2010). Other studies in amydgala-damaged humans note the dynamic interaction of top-down and bottom-up processes in emotional processing, and suggest that subjective ratings of emotional events via top-down processing impacts amygdala activity (Hsu and Pessoa, 2007; Pessoa, 2010; Taylor et al., 2003). Importantly, this line of investigation has highlighted top-down and bottom-up modulation of trauma-associated emotion and memory processes are not identical across anxiety and trauma disorder subtypes, indicating various engram inputs may lead to different expressions of disease that the field has yet to fully characterize (Nicholson et al., 2017).
Extinction and sensory representation of cues
Chronic stress has been found to impair hippocampal and cortical function and simultaneously enhance amygdala responsivity to sensory information, so interventions targeting sensory processing, relevant to both enduring trauma and threat-related memories may provide effective strategies for reducing the insult of these enduring memories (Vyas et al., 2002). Indeed, recent preclinical work demonstrates that acquisition of an odor-shock pairing is associated with an increase in the size of olfactory glomeruli specific for this odorant, while extinction training consisting of the repeated exposure to this odorant was associated with corresponding reductions in the size of these glomeruli. This suggests that exposure to discrete sensory stimuli may serve as a powerful bottom-up approach alteration at the level of the sensory receptor (Jones et al., 2008; Morrison et al., 2015). It is worth noting that the olfactory system is special in that it is the only sensory system that does not directly interface with the thalamus and instead sends axonal projections directly to the amygdala, so this bottom up approach may have utility to this sensory system only. Nonetheless, the demonstration of extinction reversing trauma-relevant increases in sensory receptor expression is encouraging, suggesting that there may be promise in targeting sensory-specific memory engrams for trauma memory interventions.
Similarly, clinical studies that combine bottom up with top down approaches also show improved efficacy and duration of effect. A recent clinical study using the combination of psychoeducation and interpretation bias modification suggested improved efficacy in reducing anxiety sensitivity over psychoeducation alone (Sussman et al., 2016). Similarly, vagus nerve stimulation (VNS), an FDA approved bottom-up intervention for the treatment of depression and seizure disorders, conducted in conjunction with extinction procedures enhances the acquisition of extinction (Burger et al., 2016). While a complete understanding of the mechanism through which VNS can enhance and expedite extinction processes has yet to emerge, one study has demonstrated that VNS is associated with an increase in plasticity of infralimbic cortex (IL) such that brief burst stimulation of IL was observed to result in a long-term depression-like phenomenon in the LA. This indicates VNS could induce greater cortical inhibition of the expression of threat-relevant behaviors mediated by LA (Pena et al., 2014), supporting a role for top-down within this bottom-up intervention. Additionally, extinction of threat memory has been found to result in reduction or reversal of conditioned increased mean arterial pressure and bradycardia, suggesting that attention paid to these interoceptive events as they are associated with traumatic memory, with partially overlapping circuitry or engrams with those others formed simultaneously at the time of trauma, may provide important readouts concerning the efficacy of extinction-based interventions in reversing physiological consequences that are associated with debilitating traumatic memory (Swiercz et al., 2018).
Destabilizing the Engram with Reconsolidation
Unlike extinction processes which are defined by repeated exposure to the same CS, retrieval of a memory via a single CS presentation has been found to allow for memory manipulations which can strengthen or weaken the memory prior to the restabilization or restorage of the memory engram through the process known as reconsolidation. As the strengthening of threat-relevant memory engrams via reconsolidation processes is not generally considered therapeutic we focus our discussion on engram destabilization. Presentation of a single CS to retrieve the memory results in a temporarily graded period of lability within the synapses which support the engram. Interference with any number of cellular and molecular processes required for the restabilization of those synapses can result in impairment of the memory upon subsequent retrieval trials (Nader et al., 2000). Work examining the effect of extinction upon opening of the window of post-retrieval lability is complicated, though some suggest that attention to the nature of stimulus contingencies at the time of initial encoding may provide important insights (Debiec et al., 2013). These include stimuli which co-occur and could reasonably be defined as compound stimuli, given their co-occurrence in time and space as discrete cues. While previous studies of reconsolidation have noted that there is specificity for reconsolidation-based destabilization of actively retrieved or reactivated sensory memories (Debiec et al., 2006; Diaz-Mataix et al., 2011; Doyere et al., 2007), recent work has suggested that the retrieval of one portion of a compound stimulus, such as a tone presented concurrently with a light cue, renders both memories susceptible to reconsolidation interference (Debiec et al., 2013). Thus, while this study demonstrated the impact of retrieval of one half of a compound association, and thus a portion of the engram, the retrieval of any portion of a compound cue that co-occurred within a shared time and space as another cue would likely also be subject to reconsolidation interference. Given the complexity of human memory and attention, there are many potentially salient compound associations and resulting engrams that may occur. Thus, collateral information of the contextual cues and factors from the time and relative space of a given trauma may reveal opportunities for implementing reconsolidation-based interventions.
Preclinical studies examining the specificity of reconsolidation-based interventions have revealed that re-exposure to discrete CS or US cues which were previously conditioned, combined with an intervention to interfere with the reconsolidation of the memory, such as application of the beta-adrenergic antagonist, propranolol, induce synapse lability and thus mediate the observed memory deficits, via engram destabilization. These are constrained by the cue used to retrieve the memory and also by the sensory cues which define the initial association (Debiec et al., 2010; Doyere et al., 2007). Given the role of NE as a strong interoceptive cue and in the regulation of sympathetic alterations that accompany trauma, such as changes in arousal and cardiac responses, the success of propranolol-assisted reconsolidation blockade may lay in the quieting of these interoceptive cues which provide bottom-up mediated direction of attention via sensory input into the amygdala. While the true complexity of associative memory engrams that exist in the human brain may be years from full comprehension, the demonstration of specificity for reconsolidation-based interventions provides some promise for future use in the treatment of discrete traumatic associations.
Reconsolidation disruption in humans
The first compelling translation of reconsolidation-based interventions into the clinical domain was informed by rodent studies demonstrating that infusions of the beta-adrenergic receptor antagonist propranolol following CS reactivation resulted in later memory impairment (Przybyslawski et al., 1999; Debiec and Ledoux, 2004). This work additionally revealed that propranolol administration following retrieval was effective in impairing the reconsolidation of longer-lasting memories and resulted in memory impairments that were also not susceptible to reinstatement or spontaneous recovery (Debiec and Ledoux, 2004). Propranolol-mediated reconsolidation impairments have now been replicated in human clinical studies several times (Brunet et al., 2018; Kindt et al., 2014; Schwabe et al., 2012). Impressively, these studies have noted the efficacy of propranolol in impairing the reconsolidation of trauma-relevant memories and noted reductions in PTSD symptomatology, reductions in activation of amygdala and thalamus to fearful faces, and reduction in skin conductance responses. However, there is also evidence that declarative memory for the traumatic event most associated with their clinical PTSD diagnosis has remained intact (Brunet et al., 2008; Brunet et al., 2014; Schiller et al., 2010). These findings suggest that the multiple imprints of a trauma, as left by the establishment of multiple engrams that support neurological, physiological and interoceptive alterations, may in fact become disentangled in the case of reconsolidation-based interventions. As such, the declarative memory of the traumatic event may be sustained, but its engagement of arousal and aversive emotional indices is impaired.
Interestingly, a series of studies has suggested that reactivating a threat engram and proceeding to administer extinction can result in long-lasting deficits in the later retrieval of that cued threat memory that may also be resistant to renewal or reinstatement, the classic shortcomings of extinction processes (Monfils et al., 2009; but see Luyten and Beckers, 2017). The biological underpinnings of the retrieval-extinction procedure remain largely unexplored, however a recent set of studies has suggested a stronger engagement of the amygdala and a different pattern of mPFC activity than what is typical from extinction alone, as revealed using immunohistochemistry and in situ hybridization methods to examine engagement of immediate early genes (Lee et al., 2015a; Tedesco et al., 2014). These preliminary observations suggest that retrieval-extinction may rely on combined bottom-up and top-down processes which converge within the amygdala to result in what appears to be an impairment or reversal of plasticity supporting the initial memory engram. These data also suggest that nonpharmacological approaches to engage reconsolidation-based impairment of emotional memory may be possible, additionally, given the role of memory retrieval as a putative memory updating mechanism which facilitates the modification of memories so that they remain relevant. Thus, clinical approaches utilizing retrieval-extinction procedures may serve to assist with directed updating of trauma-relevant memory. While there has been some encouraging success in translating the retrieval-extinction procedure into the clinical setting, there have been mixed results suggesting the existence of additional factors yet unknown that are critical for successful and reliable memory impairment using this procedure (Kindt and Soeter, 2013; Klucken et al., 2016; Schiller et al., 2010).
Reliving the Engram(s) with Virtual Reality
In a strategy to activate as many aspects of the memory that were individually encoded at the time of the trauma, recent studies have begun to investigate the utility of virtual reality (VR)-based exposure interventions to impair the persistence and reduce the intensity and debilitating nature of these memories (Maples-Keller et al., 2017). VR integrated approaches may serve as a powerful option for integrating top-down and bottom-up information in the context of exposure therapy and thus can incorporate many more interoceptive cues from simulated environments than traditional psychotherapeutic approaches such as prolonged exposure. Still, there have been varied reports regarding the potential superiority of VR (Carl et al., 2018; Diemer et al., 2015). In line with the goals of VR allowing for greater integration of top-down and bottom-up processes, a recent study has revealed an association of NE during VR exposure and responses to combat-relevant stimulus presentations (Highland et al., 2015). Given the popularity of VR technology and the promise of these early data regarding efficacy of VR therapeutic approaches, the future of clinical interventions as an individualized immersive experience capable of seamlessly integrating numerous aspects of traumatic memory associated engrams may not be too far off.
Rewriting the Engram(s) with EMDR
Though pharmacologic treatment remains elusive, one therapy for PTSD, Eye movement desensitization and reprocessing (EMDR), seems to have clinically robust, evidence-based positive outcomes. First developed in the late 1990’s, it is to date the only therapy that has withstood randomized controlled trials (albeit with small n) to show an evidence-base for decreased traumatic memories and associated anxiety feelings (Bradley et al., 2005; Shapiro, 1989). Briefly, it involves a structured approach using standardized procedures including visualization of components of the trauma-associated memory which are rapidly associated with forced saccadic eye movements. (Shapiro, 2001). This is incrementally repeated to address past, present, and future aspects of a traumatic memory, as well as resulting physical and mental experiences. In general, a single memory is meant to be processed over one to three sessions, each lasting an hour. Disregarding the controversy surrounding this treatment or its theoretical utility, EMDR may, by associating a controlled physical act that engages multiple brain regions (Amano and Toichi, 2016; Levin et al., 1999) with re-processing of cognitive and emotional engrams, enforce the rewriting of multiple trauma-related engrams simultaneously. In fact, this eye-movement related circuit influence on fear extinction has been recently recapitulated through manipulation of a superior colliculus → mediodorsal thalamus circuit in mice, providing further opportunity for translation of this sensory-directed engram treatment (Baek et al., 2019).
Conclusions and Future Directions
Encoding of aversive memory is central to understanding debilitating threat- and trauma-related disorders such as PTSD. Here we have attempted a broad review of recent progress in understanding engrams underlying threat- and trauma-relevant memory formation. In addition to rapid and extremely exciting progress in understanding relevant neural circuits there is much work that remains to be done.
Ultimately, unique challenges to consider include: consideration of threat-memory encoding at synaptic and circuit levels, the simultaneous encoding of various aspects of the “fear” (stimuli, contexts, etc.), hormonal state of the animal at the time of threat/trauma, circadian status at the time of the trauma, and integration of these various interoceptive, physiological, and other stimuli into the ‘classic’ threat-circuit diagram. While it would be impossible to suggest that one could fully recapitulate the precise characteristics and circumstances that were present at the time of memory formation, fully targeting the trauma-related engram for clinical intervention likely depends on best approximating the ‘gestalt’ of the initial memory. Indeed, retrieving aspects of the memory at a later time and in a place distinct from where the memory was formed already imposes changes in the activation of the engram and may even support threat generalization in contrast to memory extinction. Given the wide-variety of cognitive, behavioral, and physiological alterations that follow trauma exposure, each with their own unique engram and circuitry, and the present short-comings of exposure-based approaches we propose that integration of current top-down, exposure-based approaches with bottom-up, sensory-experience based methods may extend the efficacy of current exposure therapies.
Recent work examining the role of distinct cell types and their contributions to emotional learning and memory will provide an important foundation for better understanding the mechanisms that underlie the discrete aspects of memory formation. Additional studies aimed at further classifying the interaction of interneurons and principal neurons that shape and support the memory engram will be critical to targeting generalization, sensitization, stabilization, and disruption of specific memory processes. Furthermore, circuit and molecular approaches to targeting synaptic plasticity and disruption of threat- and trauma-related memories will depend on a more complete basic understanding of the underlying neural circuitry and molecular specificity of these pathways.
More work also needs to examine how hormonal and neuromodulatory processes alter transcriptional and epigenetic processes within specific cell types and circuits to impact the subsequent encoding of a trauma experience. For example, it has been recently established, contrary to popular belief, that the impact of adrenergic tone has a dose-dependent differential impact on the subsequent encoding of threat-related memories – with lower levels enhancing neuronal activation to promote and enhance encoding processes, while higher levels on the other side of the ‘inverse U-curve’ serve to impair the encoding of information.
In summary, numerous spatial, contextual, discrete and interoceptive cues are all bound together to support the encoding of a threat-relevant memory, with each aspect of the encoded memory regulated by its own specific neuronal subtypes and circuitry. This ‘gestalt’ memory is critical to the development of threat and trauma-related disorders, and ineffective treatment is often the result of targeting only one aspect of the complex trauma engram. In the laboratory, even with immense control over the factors and stimuli present at the time of threat conditioning, it is extremely difficult to experimentally regulate all aspects of this gestalt. Nonetheless, despite the field’s rapid progress, an important frontier in the neuroscience of threat and trauma is to expand our understanding from the powerful reductionistic approaches in amygdala circuit biology to a broader systems biology encoding the full engram initiated by the traumatic experience.
Acknowledgements
Support was provided by NIH (R01 MH110441, R21 MH112956, R01MH108665 (KR) and KL2 UL1 TR001102 (RR), the Highland Street Foundation (RR), the McLean-Connor Kids and Communities Fund (KR), and the McLean Frazier Fund (KR). The authors would also like to acknowledge the members of the Ressler lab at McLean for their support and the animal care staff at McLean Hospital.
Footnotes
Declaration of Interests
Dr. Ressler provides fee-for-service consultation for Biogen, Alkermes and Resilience Therapeutics. He also holds patents for a number of targets related to improving extinction of fear, however, he has received no equity or income within the last 3 years related to these.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, and Damasio AR (2005). A mechanism for impaired fear recognition after amygdala damage. Nature 433, 68–72. [DOI] [PubMed] [Google Scholar]
- Albert K, Pruessner J, and Newhouse P (2015). Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology 59, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, and Toichi M (2016). Possible neural mechanisms of psychotherapy for trauma-related symptoms: cerebral responses to the neuropsychological treatment of post-traumatic stress disorder model individuals. Sci Rep 6, 34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association., and American Psychiatric Association. DSM-5 Task Force. (2013). Diagnostic and statistical manual of mental disorders : DSM-5, 5th edn (Washington, D.C.: American Psychiatric Association; ). [Google Scholar]
- Andero R, Dias BG, and Ressler KJ (2014). A role for Tac2, NkB, and Nk3 receptor in normal and dysregulated fear memory consolidation. Neuron 83, 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antov MI, and Stockhorst U (2014). Stress exposure prior to fear acquisition interacts with estradiol status to alter recall of fear extinction in humans. Psychoneuroendocrinology 49, 106–118. [DOI] [PubMed] [Google Scholar]
- Baek J, Sukchan L, Cho T, Kim SW, Kim M, Yoon Y, Kim KK, Byun J, Kim SJ, Jeong J, Shin H-S (2019). Neural circuits underlying a psychotherapeutic regimen for fear disorders. Nature 566, 339–343. [DOI] [PubMed] [Google Scholar]
- Banerjee SB, Gutzeit VA, Baman J, Aoued HS, Doshi NK, Liu RC, and Ressler KJ (2017). Perineuronal Nets in the Adult Sensory Cortex Are Necessary for Fear Learning. Neuron 95, 169–179 e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume SR, Freedberg M, Vantrease JE, Chan R, Padival M, Record MJ, DeJoseph MR, Urban JH, and Rosenkranz JA (2017). Sex- and Estrus-Dependent Differences in Rat Basolateral Amygdala. J Neurosci 37, 10567–10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, and Bolles RC (1979). Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process 5, 368–378. [DOI] [PubMed] [Google Scholar]
- Bradley R, Greene J, Russ E, Dutra L, and Westen D (2005). A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry 162, 214–227. [DOI] [PubMed] [Google Scholar]
- Breslau N, Chilcoat HD, Kessler RC, Peterson EL, and Lucia VC (1999). Vulnerability to assaultive violence: further specification of the sex difference in post-traumatic stress disorder. Psychol Med 29, 813–821. [DOI] [PubMed] [Google Scholar]
- Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, and Pitman RK (2008). Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res 42, 503–506. [DOI] [PubMed] [Google Scholar]
- Brunet A, Saumier D, Liu A, Streiner DL, Tremblay J, and Pitman RK (2018). Reduction of PTSD Symptoms With Pre-Reactivation Propranolol Therapy: A Randomized Controlled Trial. Am J Psychiatry 175, 427–433. [DOI] [PubMed] [Google Scholar]
- Brunet A, Thomas E, Saumier D, Ashbaugh AR, Azzoug A, Pitman RK, Orr SP, and Tremblay J (2014). Trauma reactivation plus propranolol is associated with durably low physiological responding during subsequent script-driven traumatic imagery. Can J Psychiatry 59, 228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger AM, Verkuil B, Van Diest I, Van der Does W, Thayer JF, and Brosschot JF (2016). The effects of transcutaneous vagus nerve stimulation on conditioned fear extinction in humans. Neurobiol Learn Mem 132, 49–56. [DOI] [PubMed] [Google Scholar]
- Bush DE, Caparosa EM, Gekker A, and Ledoux J (2010). Beta-adrenergic receptors in the lateral nucleus of the amygdala contribute to the acquisition but not the consolidation of auditory fear conditioning. Front Behav Neurosci 4, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron DW, Norr AM, Allan NP, and Schmidt NB (2017). Combined “top-down” and “bottom-up” intervention for anxiety sensitivity: Pilot randomized trial testing the additive effect of interpretation bias modification. J Psychiatr Res 85, 75–82. [DOI] [PubMed] [Google Scholar]
- Carl E, Stein AT, Levihn-Coon A, Pogue JR, Rothbaum B, Emmelkamp P, Asmundson GJG, Carlbring P, and Powers MB (2018). Virtual reality exposure therapy for anxiety and related disorders: A meta-analysis of randomized controlled trials. J Anxiety Disord. [DOI] [PubMed] [Google Scholar]
- Castegnetti G, Tzovara A, Staib M, Paulus PC, Hofer N, and Bach DR (2016). Modeling fear-conditioned bradycardia in humans. Psychophysiology 53, 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, and Davis M (2005). Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci 25, 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DR, and Pare D (2000). Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS(+) and CS(−). Learn Mem 7, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, and McEwen BS (1999). Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci 113, 902–913. [DOI] [PubMed] [Google Scholar]
- Critchley HD, and Garfinkel SN (2016). Bodily arousal differentially impacts stimulus processing and memory: Norepinephrine in interoception. Behav Brain Sci 39, e205. [DOI] [PubMed] [Google Scholar]
- Davis M (1992). The role of the amygdala in fear and anxiety. Annu Rev Neurosci 15, 353–375. [DOI] [PubMed] [Google Scholar]
- Debiec J, Diaz-Mataix L, Bush DE, Doyere V, and Ledoux JE (2010). The amygdala encodes specific sensory features of an aversive reinforcer. Nat Neurosci 13, 536–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Diaz-Mataix L, Bush DE, Doyere V, and LeDoux JE (2013). The selectivity of aversive memory reconsolidation and extinction processes depends on the initial encoding of the Pavlovian association. Learn Mem 20, 695–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Doyere V, Nader K, and Ledoux JE (2006). Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc Natl Acad Sci U S A 103, 3428–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, and Ledoux JE (2004). Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience 129, 267–272. [DOI] [PubMed] [Google Scholar]
- Diaz-Mataix L, Debiec J, LeDoux JE, and Doyere V (2011). Sensory-specific associations stored in the lateral amygdala allow for selective alteration of fear memories. J Neurosci 31, 9538–9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diemer J, Alpers GW, Peperkorn HM, Shiban Y, and Muhlberger A (2015). The impact of perception and presence on emotional reactions: a review of research in virtual reality. Front Psychol 6, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyere V, Debiec J, Monfils MH, Schafe GE, and LeDoux JE (2007). Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci 10, 414–416. [DOI] [PubMed] [Google Scholar]
- Dudai Y (2004). The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol 55, 51–86. [DOI] [PubMed] [Google Scholar]
- Fanselow MS (1980). Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci 15, 177–182. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, and LeDoux JE (1999). Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23, 229–232. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, and Pennington ZT (2018). A return to the psychiatric dark ages with a two-system framework for fear. Behav Res Ther 100, 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferree NK, Wheeler M, and Cahill L (2012). The influence of emergency contraception on post-traumatic stress symptoms following sexual assault. J Forensic Nurs 8, 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Tromp do PM, Fudge JL, and Kalin NH (2015). Extending the amygdala in theories of threat processing. Trends Neurosci 38, 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman BH (2010). Feelings and the body: the Jamesian perspective on autonomic specificity of emotion. Biol Psychol 84, 383–393. [DOI] [PubMed] [Google Scholar]
- Frye CA, and Walf AA (2004). Estrogen and/or progesterone administered systemically or to the amygdala can have anxiety-, fear-, and pain-reducing effects in ovariectomized rats. Behav Neurosci 118, 306–313. [DOI] [PubMed] [Google Scholar]
- Gafford G, Jasnow AM, and Ressler KJ (2014). Grin1 receptor deletion within CRF neurons enhances fear memory. PLoS One 9, e111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafford GM, Guo JD, Flandreau EI, Hazra R, Rainnie DG, and Ressler KJ (2012). Cell-type specific deletion of GABA(A)alpha1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proc Natl Acad Sci U S A 109, 16330–16335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Minati L, Gray MA, Seth AK, Dolan RJ, and Critchley HD (2014). Fear from the heart: sensitivity to fear stimuli depends on individual heartbeats. J Neurosci 34, 6573–6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, and Norrholm SD (2012). Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry 72, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, and Norrholm SD (2015). Estrogen and extinction of fear memories: implications for posttraumatic stress disorder treatment. Biol Psychiatry 78, 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Mercer KB, Norrholm SD, Davis M, Duncan E, Bradley B, Ressler KJ, and Jovanovic T (2013). Inhibition of fear is differentially associated with cycling estrogen levels in women. J Psychiatry Neurosci 38, 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, and Makris N (2010). Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci 30, 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore F, Schwartz EC, Brangers BC, Aladi S, Stujenske JM, Likhtik E, Russo MJ, Gordon JA, Salzman CD, and Axel R (2015). Neural Representations of Unconditioned Stimuli in Basolateral Amygdala Mediate Innate and Learned Responses. Cell 162, 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB. (2011) Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci. 14(7):488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, and Westbrook RF (1998a). Benzodiazepine-induced amnesia in rats: reinstatement of conditioned performance by noxious stimulation on test. Behav Neurosci 112, 183–192. [DOI] [PubMed] [Google Scholar]
- Harris JA, and Westbrook RF (1998b). Evidence that GABA transmission mediates context-specific extinction of learned fear. Psychopharmacology (Berl) 140, 105–115. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Dedic N, Pohlmann ML, Hausl A, Karst H, Engelhardt C, Westerholz S, Wagner KV, Labermaier C, Hoeijmakers L, et al. (2017). Forebrain glutamatergic, but not GABAergic, neurons mediate anxiogenic effects of the glucocorticoid receptor. Mol Psychiatry 22, 466–475. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Lüthi A, Anderson DJ. (2010) Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 468(7321):270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, and Ressler KJ (2007). Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci 26, 3631–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highland KB, Costanzo ME, Jovanovic T, Norrholm SD, Ndiongue RB, Reinhardt BJ, Rothbaum B, Rizzo AA, and Roy MJ (2015). Catecholamine responses to virtual combat: implications for post-traumatic stress and dimensions of functioning. Front Psychol 6, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi R, and Neumaier JF (2006). Differential effects of ovarian steroids on anxiety versus fear as measured by open field test and fear-potentiated startle. Behav Brain Res 166, 93–100. [DOI] [PubMed] [Google Scholar]
- Hsu SM, and Pessoa L (2007). Dissociable effects of bottom-up and top-down factors on the processing of unattended fearful faces. Neuropsychologia 45, 3075–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang MJ, Zsido RG, Song H, Pace-Schott EF, Miller KK, Lebron-Milad K, Marin MF, and Milad MR (2015). Contribution of estradiol levels and hormonal contraceptives to sex differences within the fear network during fear conditioning and extinction. BMC Psychiatry 15, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogawa K, Bush DE, and LeDoux JE (2013). Contrasting effects of pretraining, posttraining, and pretesting infusions of corticotropin-releasing factor into the lateral amygdala: attenuation of fear memory formation but facilitation of its expression. Biol Psychiatry 73, 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata J, and LeDoux JE (1988). Dissociation of associative and nonassociative concomitants of classical fear conditioning in the freely behaving rat. Behav Neurosci 102, 66–76. [DOI] [PubMed] [Google Scholar]
- Iwata J, LeDoux JE, and Reis DJ (1986). Destruction of intrinsic neurons in the lateral hypothalamus disrupts the classical conditioning of autonomic but not behavioral emotional responses in the rat. Brain Res 368, 161–166. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Ehrlich DE, Choi DC, Dabrowska J, Bowers ME, McCullough KM, Rainnie DG, and Ressler KJ (2013). Thy1-expressing neurons in the basolateral amygdala may mediate fear inhibition. J Neurosci 33, 10396–10404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Kundu S, Lederman JD, Lopez-Hernandez GY, Ballinger EC, Wang S, Talmage DA, and Role LW (2016). Cholinergic Signaling Controls Conditioned Fear Behaviors and Enhances Plasticity of Cortical-Amygdala Circuits. Neuron 90, 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Diaz-Mataix L, Hamanaka H, Ozawa T, Ycu E, Koivumaa J, Kumar A, Hou M, Deisseroth K, Boyden ES, et al. (2014). Hebbian and neuromodulatory mechanisms interact to trigger associative memory formation. Proc Natl Acad Sci U S A 111, E5584–5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SV, Choi DC, Davis M, and Ressler KJ (2008). Learning-dependent structural plasticity in the adult olfactory pathway. J Neurosci 28, 13106–13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Shi C, Carlezon WA Jr., Neve RL, Nestler EJ, and Davis M (2001). Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J Neurosci 21, 2404–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns MC, Ressler KJ, Zatzick D, and Rothbaum BO (2012). Early interventions for PTSD: a review. Depress Anxiety 29, 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, and Adolphs R (2010). Impaired fixation to eyes following amygdala damage arises from abnormal bottom-up attention. Neuropsychologia 48, 3392–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, and Soeter M (2013). Reconsolidation in a human fear conditioning study: a test of extinction as updating mechanism. Biol Psychol 92, 43–50. [DOI] [PubMed] [Google Scholar]
- Kindt M, Soeter M, and Sevenster D (2014). Disrupting reconsolidation of fear memory in humans by a noradrenergic beta-blocker. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken T, Kruse O, Schweckendiek J, Kuepper Y, Mueller EM, Hennig J, and Stark R (2016). No evidence for blocking the return of fear by disrupting reconsolidation prior to extinction learning. Cortex 79, 112–122. [DOI] [PubMed] [Google Scholar]
- Koch M (1998). Sensorimotor gating changes across the estrous cycle in female rats. Physiol Behav 64, 625–628. [DOI] [PubMed] [Google Scholar]
- Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, and Muglia LJ (2008). Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc Natl Acad Sci U S A 105, 12004–12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE (1993). Emotional memory: in search of systems and synapses. Ann N Y Acad Sci 702, 149–157. [DOI] [PubMed] [Google Scholar]
- LeDoux JE (2014). Coming to terms with fear. Proc Natl Acad Sci U S A 111, 2871–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Farb C, and Ruggiero DA (1990). Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci 10, 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Haberman RP, Roquet RF, and Monfils MH (2015a). Extinction and Retrieval + Extinction of Conditioned Fear Differentially Activate Medial Prefrontal Cortex and Amygdala in Rats. Front Behav Neurosci 9, 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin P, Lazrove S, and van der Kolk B (1999). What psychological testing and neuroimaging tell us about the treatment of Posttraumatic Stress Disorder by Eye Movement Desensitization and Reprocessing. J Anxiety Disord 13, 159–172. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Phan KL, Decker LR, and Taylor SF (2003). Extended amygdala and emotional salience: a PET activation study of positive and negative affect. Neuropsychopharmacology 28, 726–733. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, and McEwen BS (2006). Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 26, 7870–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten L, and Beckers T (2017). A preregistered, direct replication attempt of the retrieval-extinction effect in cued fear conditioning in rats. Neurobiol Learn Mem 144, 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng LY, Cover KK, Taha MB, Landau AJ, Milad MR, and Lebron-Milad K (2017). Estradiol shifts interactions between the infralimbic cortex and central amygdala to enhance fear extinction memory in female rats. J Neurosci Res 95, 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maples-Keller JL, Bunnell BE, Kim SJ, and Rothbaum BO (2017). The Use of Virtual Reality Technology in the Treatment of Anxiety and Other Psychiatric Disorders. Harv Rev Psychiatry 25, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Clewett D, Sakaki M, and Harley CW (2016a). GANEing traction: The broad applicability of NE hotspots to diverse cognitive and arousal phenomena. Behav Brain Sci 39, e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Clewett D, Sakaki M, and Harley CW (2016b). Norepinephrine ignites local hotspots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behav Brain Sci 39, e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough KM, Choi D, Guo J, Zimmerman K, Walton J, Rainnie DG, and Ressler KJ (2016). Molecular characterization of Thy1 expressing fear-inhibiting neurons within the basolateral amygdala. Nat Commun 7, 13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gray J, and Nasca C (2015). Recognizing Resilience: Learning from the Effects of Stress on the Brain. Neurobiol Stress 1, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL (2000). Memory--a century of consolidation. Science 287, 248–251. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, McIntyre CK, and Power AE (2002). Amygdala modulation of memory consolidation: interaction with other brain systems. Neurobiol Learn Mem 78, 539–552. [DOI] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, and Hofmann SG (2011). Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res 45, 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ, Bryant RA, and Ehlers A (2003). Does Early Psychological Intervention Promote Recovery From Posttraumatic Stress? Psychol Sci Public Interest 4, 45–79. [DOI] [PubMed] [Google Scholar]
- Milad MR, and Quirk GJ (2002). Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420, 70–74. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, and LeDoux JE (2009). Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science 324, 951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Ohman A, and Dolan RJ (1998). Conscious and unconscious emotional learning in the human amygdala. Nature 393, 467–470. [DOI] [PubMed] [Google Scholar]
- Morris RW, Westbrook RF, and Killcross AS (2005). Reinstatement of extinguished fear by beta-adrenergic arousal elicited by a conditioned context. Behav Neurosci 119, 1662–1671. [DOI] [PubMed] [Google Scholar]
- Morrison DJ, Rashid AJ, Yiu AP, Yan C, Frankland PW, and Josselyn SA (2016). Parvalbumin interneurons constrain the size of the lateral amygdala engram. Neurobiol Learn Mem 135, 91–99. [DOI] [PubMed] [Google Scholar]
- Morrison FG, Dias BG, and Ressler KJ (2015). Extinction reverses olfactory fear-conditioned increases in neuron number and glomerular size. Proc Natl Acad Sci U S A 112, 12846–12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, and Davis M (2007). Mechanisms of fear extinction. Mol Psychiatry 12, 120–150. [DOI] [PubMed] [Google Scholar]
- Myers KM, Ressler KJ, and Davis M (2006). Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learn Mem 13, 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Schafe GE, and Le Doux JE (2000). Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406, 722–726. [DOI] [PubMed] [Google Scholar]
- Nicholson AA, Friston KJ, Zeidman P, Harricharan S, McKinnon MC, Densmore M, Neufeld RWJ, Theberge J, Corrigan F, Jetly R, et al. (2017). Dynamic causal modeling in PTSD and its dissociative subtype: Bottom-up versus top-down processing within fear and emotion regulation circuitry. Hum Brain Mapp 38, 5551–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Yan C, and Maren S (2013). Ensemble coding of context-dependent fear memory in the amygdala. Front Behav Neurosci 7, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappens M, Schroijen M, Sutterlin S, Smets E, Van den Bergh O, Thayer JF, and Van Diest I (2014). Resting heart rate variability predicts safety learning and fear extinction in an interoceptive fear conditioning paradigm. PLoS One 9, e105054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G, Van Bavel JJ, Vasey MW, and Thayer JF (2013). Cardiac vagal tone predicts attentional engagement to and disengagement from fearful faces. Emotion 13, 645–656. [DOI] [PubMed] [Google Scholar]
- Pena DF, Childs JE, Willett S, Vital A, McIntyre CK, and Kroener S (2014). Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front Behav Neurosci 8, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L (2010). Emotion and cognition and the amygdala: from “what is it?” to “what’s to be done?”. Neuropsychologia 48, 3416–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer G, Garfinkel SN, Gould van Praag CD, Sahota K, Betka S, and Critchley HD (2017). Feedback from the heart: Emotional learning and memory is controlled by cardiac cycle, interoceptive accuracy and personality. Biol Psychol 126, 19–29. [DOI] [PubMed] [Google Scholar]
- Phelps EA, and Anderson AK (1997). Emotional memory: what does the amygdala do? Curr Biol 7, R311–314. [DOI] [PubMed] [Google Scholar]
- Pineles SL, Nillni YI, King MW, Patton SC, Bauer MR, Mostoufi SM, Gerber MR, Hauger R, Resick PA, Rasmusson AM, et al. (2016). Extinction retention and the menstrual cycle: Different associations for women with posttraumatic stress disorder. J Abnorm Psychol 125, 349–355. [DOI] [PubMed] [Google Scholar]
- Powell DA, McLaughlin J, Churchwell J, Elgarico T, and Parker A (2002). Heart rate changes accompanying jaw movement Pavlovian conditioning in rabbits: concomitant blood pressure adjustments and effects of peripheral autonomic blockade. Integr Physiol Behav Sci 37, 215–227. [DOI] [PubMed] [Google Scholar]
- Przybyslawski J, Roullet P, Sara SJ. (1999) Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J Neurosci. 19(15):6623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ (2002). Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem 9, 402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, and Beer JS (2006). Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol 16, 723–727. [DOI] [PubMed] [Google Scholar]
- Reimer AE, de Oliveira AR, and Brandao ML (2012). Glutamatergic mechanisms of the dorsal periaqueductal gray matter modulate the expression of conditioned freezing and fear-potentiated startle. Neuroscience 219, 72–81. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, and Sapolsky RM (2009). The influence of stress hormones on fear circuitry. Annu Rev Neurosci 32, 289–313. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, and LeDoux JE (2004). Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron 44, 75–91. [DOI] [PubMed] [Google Scholar]
- Rogan MT, and LeDoux JE (1995). LTP is accompanied by commensurate enhancement of auditory-evoked responses in a fear conditioning circuit. Neuron 15, 127–136. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, and LeDoux JE (1997). Fear conditioning induces associative long-term potentiation in the amygdala. Nature 390, 604–607. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Clugnet MC, Bordi F, and LeDoux JE (1993). Somatosensory and auditory convergence in the lateral nucleus of the amygdala. Behav Neurosci 107, 444–450. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hui GK, Hui IR, Berlau DJ, McGaugh JL, and Weinberger NM (2006). Basolateral amygdala noradrenergic activity mediates corticosterone-induced enhancement of auditory fear conditioning. Neurobiol Learn Mem 86, 249–255. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Kearns MC, Price M, Malcoun E, Davis M, Ressler KJ, Lang D, and Houry D (2012). Early intervention may prevent the development of posttraumatic stress disorder: a randomized pilot civilian study with modified prolonged exposure. Biol Psychiatry 72, 957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Kearns MC, Reiser E, Davis JS, Kerley KA, Rothbaum AO, Mercer KB, Price M, Houry D, and Ressler KJ (2014). Early intervention following trauma may mitigate genetic risk for PTSD in civilians: a pilot prospective emergency department study. J Clin Psychiatry 75, 1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JM, Macedo CE, and Brandao ML (2008). Gabaergic mechanisms of hypothalamic nuclei in the expression of conditioned fear. Neurobiol Learn Mem 90, 560–568. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Doyere V, and LeDoux JE (2005). Tracking the fear engram: the lateral amygdala is an essential locus of fear memory storage. J Neurosci 25, 10010–10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, and Phelps EA (2010). Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463, 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulkin J, Gold PW, and McEwen BS (1998). Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology 23, 219–243. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Nader K, Wolf OT, Beaudry T, and Pruessner JC (2012). Neural signature of reconsolidation impairments by propranolol in humans. Biol Psychiatry 71, 380–386. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, and Morrison JH (2010). Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex 20, 2560–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro F (1989). Eye movement desensitization: a new treatment for post-traumatic stress disorder. J Behav Ther Exp Psychiatry 20, 211–217. [DOI] [PubMed] [Google Scholar]
- Shapiro F (2001). Eye movement desensitization and reprocessing (EMDR) : basic principles, protocols, and procedures, 2nd edn (New York: Guilford Press; ). [Google Scholar]
- Sijbrandij M, Olff M, Reitsma JB, Carlier IV, de Vries MH, and Gersons BP (2007). Treatment of acute posttraumatic stress disorder with brief cognitive behavioral therapy: a randomized controlled trial. Am J Psychiatry 164, 82–90. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, and LeDoux JE (2004). Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem 11, 525–535. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, and Penzes P (2013). Insights into rapid modulation of neuroplasticity by brain estrogens. Pharmacol Rev 65, 1318–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman TJ, Jin J, and Mohanty A (2016). Top-down and bottom-up factors in threat-related perception and attention in anxiety. Biol Psychol 121, 160–172. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, and Geyer MA (1999). Cross-species studies of sensorimotor gating of the startle reflex. Ann N Y Acad Sci 877, 202–216. [DOI] [PubMed] [Google Scholar]
- Swiercz AP, Seligowski AV, Park J, and Marvar PJ (2018). Extinction of Fear Memory Attenuates Conditioned Cardiovascular Fear Reactivity. Front Behav Neurosci 12, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, and Liberzon I (2003). Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage 18, 650–659. [DOI] [PubMed] [Google Scholar]
- Tedesco V, Roquet RF, DeMis J, Chiamulera C, and Monfils MH (2014). Extinction, applied after retrieval of auditory fear memory, selectively increases zinc-finger protein 268 and phosphorylated ribosomal protein S6 expression in prefrontal cortex and lateral amygdala. Neurobiol Learn Mem 115, 78–85. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, and Dolan RJ (2001). Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron 30, 829–841. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, and Chattarji S (2002). Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22, 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM (2011). The medial geniculate, not the amygdala, as the root of auditory fear conditioning. Hear Res 274, 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook RF, Iordanova M, McNally G, Richardson R, and Harris JA (2002). Reinstatement of fear to an extinguished conditioned stimulus: two roles for context. J Exp Psychol Anim Behav Process 28, 97–110. [PubMed] [Google Scholar]
- Yang R, Zhang B, Chen T, Zhang S, and Chen L (2017). Postpartum estrogen withdrawal impairs GABAergic inhibition and LTD induction in basolateral amygdala complex via down-regulation of GPR30. Eur Neuropsychopharmacol 27, 759–772. [DOI] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, and Milad MR (2011). Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry 70, 920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WN, Murphy CA, and Feldon J (2004). Behavioural and cardiovascular responses during latent inhibition of conditioned fear: measurement by telemetry and conditioned freezing. Behav Brain Res 154, 199–209. [DOI] [PubMed] [Google Scholar]