Abstract
Atherosclerotic plaques are characterized by an accumulation of macrophages, lipids, smooth muscle cells, and fibroblasts, and, in advanced stages, necrotic debris within the arterial walls. Dietary habits such as high fat and high cholesterol (HFHC) consumption are known risk factors for atherosclerosis. However, the key metabolic contributors to diet-induced atherosclerosis are far from established. Herein, we investigate the role of a 2-year HFHC diet challenge in the metabolic changes of development and progression of atherosclerosis. We used a non-human primate (NHP) model (baboons, n = 60) fed a HFHC diet for two years and compared metabolomic profiles in serum from animals on baseline chow with serum collected after the challenge diet using two-dimensional gas chromatography time-of-flight mass-spectrometry (2D GC-ToF-MS) for untargeted metabolomic analysis, to quantify metabolites that contribute to atherosclerotic lesion formation. Further, clinical biomarkers associated with atherosclerosis, lipoprotein measures, fat indices, and arterial plaque formation (lesions) were quantified. Using two chemical derivatization (i.e., silylation) approaches, we quantified 321 metabolites belonging to 66 different metabolic pathways, which revealed significantly different metabolic profiles of HFHC diet and chow diet fed baboon sera. We found heritability of two important metabolites, lactic acid and asparagine, in the context of diet-induced metabolic changes. In addition, abundance of cholesterol, lactic acid, and asparagine were sex-dependent. Finally, 35 metabolites correlated (R2, 0.068–0.271, P < 0.05) with total lesion burden assessed in three arteries (aortic arch, common iliac artery, and descending aorta) which could serve as potential biomarkers pending further validation. This study demonstrates the feasibility of detecting sex-specific and heritable metabolites in NHPs with diet-induced atherosclerosis using untargeted metabolomics allowing understanding of atherosclerotic disease progression in humans.
Introduction
About 17.7 million people died from cardiovascular diseases (CVDs) in 2015, representing 31% of all global deaths. Of these, 7.4 and 6.7 million were due to coronary heart disease (CHD) and stroke, respectively [1]. The National Center for Health Statistics (NCHS) data suggested 787,650 deaths (32% of all mortalities) from CVDs with an estimated 83.6 million affected in USA in 2010 alone [2]. CVDs are associated with a wide range of genetic, dietary, environmental, and lifestyle factors; and other risk factors such as hypertension, diabetes, hyperlipidemia, but the majority of these have not been fully characterized.
Atherosclerosis is a chronic inflammatory disorder in arteries which causes major clinical problems such as acute myocardial infarction and stroke. Atherosclerotic plaques are characterized by an accumulation of macrophages, lipids, smooth muscle cells, and fibroblasts, and, in advanced stages, necrotic debris within the arterial wall. Among cardiometabolic risk factors, elevated serum cholesterol can induce development of atherosclerosis in the absence of other risk factors [3]. To assess the impact of diet on atherosclerosis, the Lifestyle Heart Study randomized patients with coronary atherosclerotic heart disease to a low-fat vegetarian diet or a standard diet for one year. Patients on the low-fat vegetarian diet showed regression of angiographically detected coronary atherosclerosis and a 91% reduction of frequency of angina, while patients on the standard diet showed a 186% increase in the frequency of angina [4, 5]. Similarly, long-term caloric restriction in humans has been shown to have a powerful protective effect against atherosclerosis when compared to subjects on a normal American diet [6]. Many diets and dietary components have been shown to impact coronary artery disease, including the protective effect of the Mediterranean diet, the “prudent” diet, vegetables, and nuts, or the harmful effect of a “Western” diet (WD), trans-fatty acids, and a diet with a high glycemic index or load [7]. The WD is also known to exacerbate the metabolic syndrome, obesity, and diabetes, in addition to atherosclerosis.
The plasma levels of apoB, apoAI, remnant-like particle (RLP)-C, lipoprotein (a) [Lp(a)], and hs-CRP, in addition to plasma levels of lipids, are typically used as predictors of atherosclerosis in clinical practice [8], whereas non- high-density lipoprotein cholesterol (HDL-C) is a better predictor of severity of coronary atherosclerosis than LDL-C [9]. Unfortunately, the visit-to-visit LDL-C and HDL-C in a 5-year follow-up study (n = 130 patients) presented with ST-segment elevation myocardial infarction (STEMI) [10] was shown to be highly variable. Moreover, a large meta-regression analysis indicated that an increased amount of circulating HDL-C alone does not reduce the risk of CHD events, CHD deaths, or total deaths [11]. HDL-C does not necessarily reflect HDL function, and HDL function may be a better biomarker of cardiovascular risk [12]. Thus, the underlying complex metabolic changes in serum correlated with diet-induced atherosclerosis remain unknown. Additional serum-based biomarkers with higher accuracy in reflecting these complex changes potentially would be useful for human diagnostic testing of atherosclerosis.
The diversity of environmental and genetic factors that contribute to atherosclerosis results in extensive phenotypic variation and consequently in individual serum metabolomes. A typical GC-TOF-MS -based metabolomics study can quantify nearly 80 metabolites in serum [13]. Studies have revealed significant changes in the human serum metabolome one minute after a cardiac ischemic event [14]. However, no metabolites have been associated with atherosclerosis that could provide more accurate biomarkers than current serum-based markers such as LDL-C. A pedigreed cohort of baboons provide a unique opportunity to explore the heritability of these circulatory serum metabolites with the ability to control environment and genetics.
Interestingly, atherosclerosis risk, and the risk for CVD complications, is different in men and women. In a study involving 142 coronary artery disease patients, men had more severe structural and functional abnormalities in epicardial coronary arteries than women, even in patients with early atherosclerosis [15]. Another study spanning 12 years, where 495 cases of first myocardial infarction among men and 103 cases among women revealed that myocardial infarction incidence was 4.6 times higher among men [16]. Finally, another study involving 1782 subjects at risk of CVD including 926 cases with hypercholesterolemia, revealed that important sex differences exist in the current clinical definitions of the metabolic syndrome with regard to predicting early atherosclerotic lesions [17]. Moreover, the sex differences in lipid and lipoprotein metabolism have been attributed to a variety of factors, not just sex hormones [18], but sex-specific differences affecting metabolite levels, such as sex dimorphism in cholesterol kinetics, remain to be elucidated. Women exhibit more favorable adipokine, lipid, and immune profiles compared to men, which may explain the lower instability grade in their carotid atherosclerotic plaques [19].
For over 50 years, baboons have served as an experimental non-human primate (NHP) model for cardiometabolic disorders, owing to their commonalities in genome, pathophysiology (in cardiovascular diseases, obesity, type 2 diabetes), and development to humans [20, 21]. In human biomedical research, where controlled diet-challenge studies suffer from logistic drawbacks and other confounding factors (diet dysregulation, life styles, ethnicity-genotype issues etc.), and obtaining tissue biopsies from healthy individuals, is challenging, studies with NHPs circumvent these limitations. In captivity, controlled and long-term diet-challenge studies in baboons are feasible. The baboon is a well-characterized model for dyslipidemia and atherosclerosis [22, 23], and is very similar to humans both physiologically and genetically. More recently, research efforts have focused on understanding atherosclerosis using epigenetics [24], gut microbiome [25], miRNA [26], DNA methylation, and mitochondrial roles [27] among other approaches. Baboons on a HFHC diet develop typical cardiometabolic diseases, such as obesity, insulin resistance, dyslipidemia, atherosclerosis, and CVD, essentially identical to those of humans. In a recent analysis, we have demonstrated characteristic transcriptomic (mRNA and miRNA) changes in HFHC diet-challenged baboons [28, 29].
Previously, Barba and colleagues [30] used nuclear magnetic resonance spectroscopy (1H NMR) based metabolomics analysis of blood serum to discriminate patients with myocardial ischemia by changes in lactate, glucose, and saturated lipid resonances following exercise. Similarly, using untargeted LC-MS based metabolomics in venous blood collected from human subjects with coronary atherosclerosis, Gao et al [31] suggested significant metabolic dysfunction in phospholipid, sphingolipid, and fatty acid metabolism in the patients. In another study, using ultra-high performance liquid chromatography coupled to quadruple time-of-flight mass spectrometry (UHPLC-QTOF/MS), Li et al [32] explored the global metabolic perturbation profile for CHD in 300 human subjects and identified 3 novel metabolites (4-pyridoxic acid, PG (20:3/2:0) and lithocholic acid) that exhibited strong correlations with CHD. Moreover, the above studies captured many fatty acids and lipid metabolites, but not polar metabolites which are only captured in GC-MS-based analyses. In addition, none of the above studies was conducted under controlled dietary conditions, which are difficult in research with human subjects.
Here, we report on characterization of circulatory serum metabolite changes in HFHC diet-challenged baboons that are associated with atherosclerotic disease status. The identification of metabolite-based biomarkers will strengthen our understanding of atherosclerosis development and progression. Studies have revealed significant changes in the human serum metabolome one minute after a cardiac ischemic event [14]. However, no metabolites have been associated with atherosclerosis that could provide more accurate biomarkers than current serum-based markers such as LDL-C. Analysis of our pedigreed cohort of baboons provides a unique opportunity to understand the heritability of these circulatory serum metabolites in the context of HFHC diet challenge. In addition, identification of individual metabolites and pathway biomarkers of atherosclerosis will help improve diagnosis of atherosclerosis beyond current clinical practice.
Materials and methods
Chemicals
Acetonitrile, isopropanol, methanol, and pyridine (all HPLC grade solvents), and methoxyamine hydrochloride (MeOX), 1% TMCS in N-methyl-N-trimethylsilyl-trifluoroacetamide (MSTFA), 1% TMCS containing N-(t-butyldimethylsilyl)-N-methyltrifluoroacetamide (MTBSTFA), and adonitol (internal standard) were obtained from Sigma-Aldrich, St. Louis, USA.
Animals and serum samples
All procedures involving animals were reviewed and approved by the Texas Biomedical Research Institute’s Institutional Animal Care and Use Committee (IACUC). Southwest National Primate Research Center (SNPRC) facilities at the Texas Biomedical Research Institute and the animal use programs are accredited by Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC), operate according to all National Institutes of Health (NIH) and U.S. Department of Agriculture guidelines, and are directed by doctors of veterinary medicine. All animal care decisions are made by the SNPRC veterinarians. All animals were housed in group cages allowing them to live in their normal social groups with ad lib access to food and water. Enrichment was provided on a daily basis by the SNPRC veterinary and behavioral staff in accordance with AAALAC, NIH, and U.S. Department of Agriculture guidelines. For this study, we utilized a cohort of olive baboons (Papio hamadryas; Taxonomy ID 9557) maintained as part of the baboon colony at the Southwest National Primate Research Center (SNPRC), located on the campus of the Texas Biomedical Research Institute, San Antonio, Texas. The baboons were raised and maintained on a standard monkey chow diet [(high complex carbohydrates; low fat (“Monkey Diet 15%/5LEO,” LabDiet, PMI Nutrition International, St. Louis, MO)] prior to the study initiation. Freshly collected serum samples were stored in aliquots at -80°C until analysis. After study completion animals were euthanized by an SNPRC veterinarian using ketamine and pentobarbital followed by exsanguination in accordance with American Veterinary Medical Association (AVMA) guidelines [33]. Humane endpoint was confirmed by lack of pulse, breathing, corneal reflex, and response to firm toe pinch, and inability of the veterinarian to hear respiratory sounds and heartbeat by stethoscope.
Study design
In our previously published study [34] 112 baboons from the SNPRC pedigreed colony were fed a HFHC diet for 2 years. Age- and sex- matched young adult baboons (8–12 years) (Papio hamadryas) were fed monkey chow ad libitum from weaning until the diet challenge study began (Chow diet: 12% energy from fat, 18% from protein, and 69% from carbohydrate consisting of 0.29% glucose and 0.32% fructose; “Monkey Diet 25/50456,” LabDiet, PMI Nutrition International, St. Louis, MO), and were then challenged for 2 years with an atherogenic diet (40% of calories as fat and 1.7 mg of cholesterol per kcal) prepared from a mix of lard, cholesterol, sodium chloride, vitamins [ascorbic acid and vitamin A (retinyl acetate)], and water to a base diet (“Monkey Diet 25/50456,” LabDiet, PMI Nutrition International, St. Louis, MO). The approximate mean per animal daily intake was 400 g (~1200 kcal) for the HFHC diet. Clinical measures were obtained from plasma samples for the described cohort of baboons at the baseline (chow diet) and at the end of the 2-year HFHC diet challenge. These included body weight, lipoprotein measures, lesion data, inflammation and oxidative stress markers, and arterial compliance. For this particular study, we selected a subset of those 112 animals (males = 34, females = 26) based on the values for blood concentrations of LDL-C, HDL-C, total cholesterol, and glucose, and atherosclerotic lesion burden, ectopic fat scores for heart, and body weight as the end-point measures to select the most extreme HFHC diet “responders” and “non-responders”, i.e., to enrich the extreme metabolic phenotypes and thus to enhance the ability to discern signal (where large scale changes in clinical measures were observed) from noise (where no significant changes in clinical measures were recorded).
Arterial lesions, CVD-related biomarkers, lipoprotein and other clinical measures
Lesion formation in three major arteries: the aortic arch, thoracic section of the descending aorta, and the common iliac artery were assessed in baboons after 2 years of HFHC diet challenge that were humanely euthanized and were subjected to standard necropsy procedures as described [34]. Total serum cholesterol and triglyceride (TG) concentrations were determined enzymatically using commercial reagents in a clinical chemistry analyzer. HDL-C was measured in the supernatant after heparin- Mn+2 precipitation. Blood glucose was measured using the Alfa Wasserman ACE clinical chemistry instrument (West Caldwell., NJ). Summaries of the raw data are presented as means, and standard deviations.
Serum sample extraction and derivatization for GC-MS analysis
Aliquots (30 μL) of serum samples were subjected to sequential solvent extraction, once each with 1 mL of acetonitrile: isopropanol: water (3:3:2) and 500 μL of acetonitrile: water (1:1) mixtures at 4°C [35]. Adonitol (5 μL from 10 mg/ml stock) was added to each aliquot as an internal standard prior to the extraction. The pooled extracts (~ 1500 μL) from the two steps were dried under vacuum at 4°C prior to chemical derivatization (silylation reactions). Dummy extractions performed in microcentrifuge blank tubes served as extraction blanks to account for background noise and other sources of contamination. Serum samples were then sequentially derivatized with methoxyamine hydrochloride (MeOX) and 1% TMCS in N-methyl-N-trimethylsilyl-trifluoroacetamide (MSTFA) or 1% TMCS containing N-(t-butyldimethylsilyl)-N-methyltrifluoroacetamide (MTBSTFA) as described elsewhere [36–39]. Briefly, the steps involved addition of 10 μL of MeOX (20 mg mL-1) in pyridine incubated under shaking at 55°C for 60 min followed by trimethylsilylation at 60°C for 60 min after adding 90 μL MSTFA or MTBSTFA [38–40].
Metabolomic analysis by 2D GC-ToF-MS
Two dimensional gas chromatography-mass spectrometry (2D GC-ToF-MS) was performed as described [36]. Derivatized samples were injected in splitless mode using an autosampler (VCTS, Gerstel™, Linthicum, MD, USA) that consisted of an Agilent© 7890 B gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) in line with a Pegasus ® 4D ToF-MS instrument (Leco Corp., San Jose, CA, USA) with an electron ionization (EI) source. Injection temperature was set at 250°C (front inlet) and helium at 1 mL min-1 was used as a carrier gas. Separation on the GC was achieved using two columns in line, a primary Rxi®-5Sil MS capillary column (Cat. No. 13623–6850, Restek, Bellefonte, PA, USA) (30 m × 0.25 mm × 0.25 μm) and a secondary Rxi®-17Sil capillary column (Cat. No. 40201–6850, Restek, Bellefonte, PA, USA) (2 m × 0.15 mm × 0.15 μm). The temperature program for the primary column started isothermal at 70°C for 1 min followed by a 6°C min-1 ramp to 310°C and a final 11 min hold at 310°C. The secondary oven temperature was programmed with an offset of 5°C; the modulator temperature offset was 15° C relative to the first oven temperature. The modulation temperature (second-dimension separation time) was set at 4 s divided into a hot and cold pulse times of 0.60 s and 1.4 s, respectively between the two stages. Transfer line temperature was maintained at 250°C and the ion source temperature was 250°C. All samples were run with an offset time of 470 s to allow the solvents and derivatizing reagents to pass through without reaching the detector. The system was then temperature-equilibrated at 70°C for 5 min before the next sample. The acquisition sequence started with blank solvent (pyridine) injections, followed by a randomized list of the following: extraction blanks (B), reagent blanks (R), and samples (S). Further, pooled QC samples were injected at scheduled intervals for tentative identification and monitoring shifts in retention times for quality control (QC) checks. Mass spectra were collected at 200 scans/s with a range of m/z 40–600.
Processing of 2D GC-MS data
The 2D GC-MS data sets were processed (cleaned, aligned, and annotated) using ChromaToF version 4.71.0.0 (LECO Corp., Michigan, USA) as described [36, 38, 39] with settings such as S/N: 25; peak width: 0.15, base line offset: 1; m/z range: 40–600, library matching score cut off at 600 as initial cut-off). Spectral library matching for compound identification was performed essentially following settings as described earlier [38, 39]. The filtered raw GC-MS data included ~600 manually curated metabolites. Base peak areas (BPCs) of the mass fragments (m/z) were normalized using median normalization followed by log2 transformation. Peak areas were further normalized by dividing each peak area value by the area of the internal standard (adonitol) for a given sample. For both platforms, metabolite annotation and assignment followed the metabolomics standards initiative (MSI) guidelines for metabolite identification [41], i.e., Level 2: identification was based on spectral database (match factor >80%) which is accepted for EI-MS mass spectra generated at 70 eV and Level 3: only compound groups were known, e.g. specific ions and RT regions of metabolites leading to putative identification.
Statistical analysis
Statistical processing of both the GC-MS data sets was performed using statistical software R (Version 3.5.1) [42, 43]. Normalized, transformed, imputed, outlier removed, and scaled peak area representative of relative metabolite amounts obtained from using DeviumWeb [44] are presented. We used fold change (FC) cut offs of FC < 0.8 as low and FC > 1.2 as high as arbitrary values to find directionality of change for diet (chow vs HFHC) and sex (males vs females). All P-values reported were nominal with a cut-off for significance (P, < 0.05) unless specified otherwise.
Univariate and multivariate analysis
Hierarchical clustering analysis (HCA) was performed on Pearson distances using the standalone tool PermutMatrix [45], where the data was normalized using z-scores of the relative abundance of the metabolites for heat map display. Correlations were performed using R package ‘corrplot’ and reported are Pearson and Spearman rank correlations. Principal components analysis (PCA) was performed using DeviumWeb [44] where the output displayed score plots to visualize the sample groups. The data were scaled with unit variance without transformation.
Pathway enrichment and clustering analysis
Pathway enrichment analysis was performed using the webserver MetaboAnalyst 3.0 (www.metaboanalyst.ca) [46]. Pathways were connected as networks and visualized using Cytoscape (version 3.6.1) for visualization purposes. For ID conversions, the Chemical Translation Service (CTS: http://cts.fiehnlab.ucdavis.edu/conversion/batch) was used to convert the common chemical names into their Kyoto Encyclopedia of Genes and Genomes (KEGG), Human Metabolome Database (HMDB), METLIN, PubChem Compound ID (PubChem CID), and Chemical Entities of Biological Interest (ChEBI) identifiers.
2D GC-ToF- MS–based raw metabolomics datasets availability
The raw datasets and the metadata obtained from mass-spectrometry based 2D GC-ToF- MS metabolomics efforts are made publicly available at the deposited at the Global Natural Product Social Molecular Networking (GNPS) database (Study ID: MassIVE MSV000083256) and is available for download at this link: ftp://massive.ucsd.edu/MSV000083256 (doi:10.25345/C5Z608) as .cdf (netCDF) files.
Heritability estimates
We used a maximum-likelihood-based variance decomposition approach implemented in Sequential Oligogenic Linkage Analysis Routines (SOLAR) [47] to estimate heritability for each metabolite in the 60 pedigreed baboons maintained on a chow diet and HFHC diet. This approach uses information from possible relationships (i.e., kinship) simultaneously to disentangle the genetic architecture of the metabolites. In a simple model, variances or covariances between relatives as a function of the genetic relationships can be specified, and the proportion of phenotypic variance that is attributed to (additive) genetic effects [i.e., heritability (h2)] can be estimated from the components of variance [47]. The P values for the heritability estimates are obtained by likelihood ratio tests, where the likelihood of a model is estimated and compared with the likelihood of a model in which the heritability is constrained to zero. Two times the difference in the natural logarithmic likelihood is distributed asymptotically as a 1/2:1/2 mixture of a χ2 variable with one degree of freedom and a point mass at zero [48].
Results and discussion
A recent study in a cohort of 112 baboons showed that lipoprotein measures are associated with atherosclerotic lesion burden in this NHP [34]. We used an untargeted MS-based metabolomics approach in in a subset of this cohort to identify circulating serum metabolites in baboons before (baseline, 0 d) and after a 2-year HFHC diet challenge in this model of atherosclerosis. This long-term diet-challenge has the potential to allow identification of heritable metabolites predictive of atherosclerosis, yield serum-based metabolite biomarkers of atherosclerotic lesion burden, and provide novel biomarkers for early detection/diagnosis of atherosclerosis.
Clinical measures and fat scores as a result of diet-induced changes
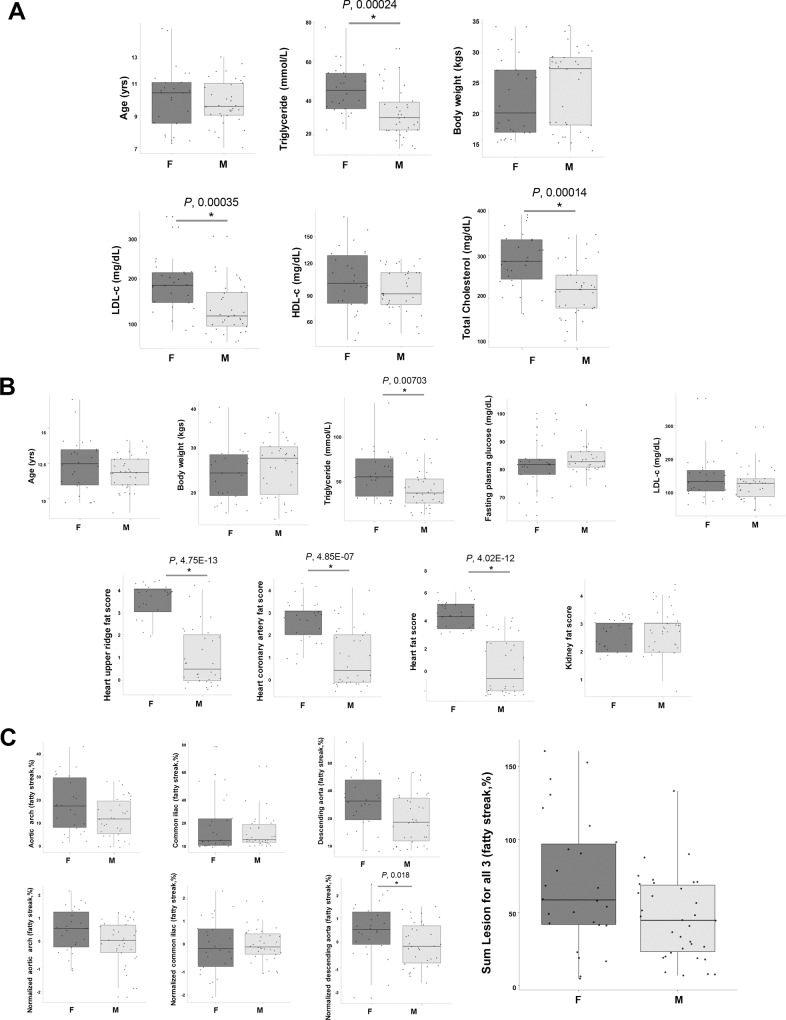
In the 60 baboons at the baseline (0 d), we observed no significant differences (P, >0.05) in age, body weight (BW), and HDL-C among male (n = 26) or female (n = 34) baboons. Further, we observed that BW (P, 6.044E-06), glucose (P, 0.0303), LDL-C (P, 3.113E-21), and TG (P, 0.00003) levels were all significantly different for baseline (0 d) by comparison with HFHC diet (2 yr) baboons (Table 1; S1 Table). In addition, significant differences between males and females were observed for TG (P, 0.00024), LDL-C (P, 0.00035), and total cholesterol (P, 0.00014) (Fig 1A).
Table 1. Comparisons of changes from baseline (0yr, Chow diet) to treatment (2 yrs, HFHC diet) in clinical measures between the two treatments (diet) groups.
| Factor | Chow Diet | HFHC Diet | T-test (P-value) |
|---|---|---|---|
| No of baboons | N = 60 | N = 60 | - |
|
BW (KGs)- Mean, 95% CI |
23.19 (21.50,24.88) | 25.02 (17.82,32.23) | 6.044E-06 |
|
Glucose (mg/dL)- Mean, 95% CI |
78 (75.25,80.74) | 82.18 (75.44,88.92) | 3.033E-02 |
|
LDL-C (mg/dL)- Mean, 95% CI |
43.41 (27.41,59.41) | 133.41 (117.26,149.56) | 3.113E-21 |
|
TG (mg/dL)- Mean, 95% CI |
37.33 (31.97,42.69) | 48.23 (41.86,54.60) | 3.067E-04 |
All P-values were significant, P, 0.05.
Fig 1.
Changes in clinical measures of 60 animals, and fat score indices for the males (n = 26) and females (n = 34) at [A] baseline and at [B] end of the 2 yr diet challenge. Also provided are the [C] lesion data for the 2 yr diet-intervention. Values are provided as mean +/- standard error of mean (SEM). Analysis performed was a nonparametric two-tailed t-test, comparing proportion normalizing with proportion between males and females and chow and HFHC diet intervention. P < 0.05. In the box-plots, the center lines show the medians; box limits indicate the 25th and 75th percentiles as determined by statistical software R; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles, outliers are represented by dots; crosses represent sample means; data points are plotted as black dots. Widths of boxes are proportional to square-roots of the number of observations.
Indeed, in the original study with 112 baboons, the concentrations (or activities) of the majority of circulating biomarkers of lipid/lipoprotein metabolism and inflammation showed mean changes in the animals fed the HFHC challenge diet for 2 years, but the values for HDL-C and LDL-C at 2 years (week 104) were not significantly different from those at the baseline (week 0) [34]. At the end of the 2-year diet challenge, fat scores for heart upper ridge, heart, coronary artery, and kidney were assessed (Fig 1B; S1 Table). We noted, significant changes in TG (P, 0.00703) and fat scores such as heart upper ridge fat score (P, 4.75E-13), heart coronary artery fat score (P, 4.85e-07), and heart total fat score (P, 4.02E-12), all of these which fat scores were significantly higher for females than for males.
Fatty streaks are typically characterized by enhanced accumulation of non-foamy and foamy macrophages with a small amount of extracellular lipids [49], that form even at relatively low-level hypercholesterolemia (200 to 400 mg/dl) in NHPs [50]. Thus, we analyzed the arterial lesion data obtained from the three major arteries, as fatty streak percentage (%). Our results revealed that the fatty streak percentage was significantly higher only in the descending aorta (P, 0.018) of females (Fig 1C). Indeed, epidemiological studies of human populations support experimental studies showing association between high blood cholesterol levels and fatty streaks [51]. Our observations of significantly higher fat scores, TG, total cholesterol, and LDL-C in female baboons were interesting—cholesterol levels at baseline and fatty streaks at the end of the challenge were significantly higher in females than males. In humans, a meta-analysis of 37 prospective cohort studies, the risk of fatal CHD is 50% higher in women with diabetes compared to males with diabetes [52]. Moderate or borderline hypertension (<140/90 mmHg) causes more endothelial dysfunction and cardiovascular complications in women than men [53]. Although women have often been under-represented in statin trials in the past, there is currently no doubt that in secondary prevention LDL-C reduction in women leads to equally lower coronary heart disease mortality as in men [54]. Microvascular dysfunction and diffuse coronary atherosclerosis without obstructive lesions is more prevalent in women than men and can be better visualized with positron emission tomography (PET) and cardiovascular magnetic resonance (CMR) techniques [55]. Beyond NHPs and human studies, the testicular feminized mouse (which has non-functional androgen receptor and low testosterone) develops fatty liver and aortic lipid streaks on a high-fat diet; whereas, androgen-replete XY littermate controls do not [56]. With fatty streak % results showing the varying degree of lesion formation in these HFHC diet fed baboons (S2 Table), we proceeded to quantify circulatory metabolites in the serum to correlate with the extent of atherosclerosis.
Metabolic pathways captured and individual metabolites changing with diet
In our study of the NHP model of atherosclerosis, we used the 2D GC-ToF-MS platform and dual derivatization strategies (MSTFA and MTBSTFA) to quantify 515 metabolites (of which 180 were assigned HMDB IDs) out of the total 3154 metabolite peaks that were found in at least in 2/3rd of the biological samples from the combined two time points (S1 Fig). The raw mass spectrometry abundance data (S3 Table) were normalized (S4 Table) and used for all statistical analysis. After removal of redundant metabolites and conversion to their biological identifiers (KEGG and HMDB), the 321 metabolites were mapped to 71 different KEGG-based pathways such as ammonia recycling, urea cycle, alanine metabolism, phenylalanine and tyrosine metabolism, aspartate metabolism, carnitine synthesis, malate-aspartate shuttle (all significant, P, <0.1), followed by glutamate metabolism, beta-alanine metabolism, propanoate metabolism, glucose-alanine cycle, fatty acid biosynthesis, vitamin K metabolism, arginine and proline metabolism, glycine and serine metabolism, valine, leucine and isoleucine degradation, cysteine metabolism, and oxidation of branched chain fatty acids (not-significant) (S2 Fig). Furthermore, a handful of metabolites tentatively identified could be of exposomal origin (i.e., diet, environment, drugs, hydrocarbons etc.) or are contaminants (S4 Table), for which we have yet to associate with disease status. More specifically, all quantified metabolites reported in this study remain to be validated (annotation level 4) to improve confidence in the data using other orthogonal technologies such as LC-MS and NMR [57].
Firstly, using two-way ANOVA analysis for sex and diet, we observed 35 and 34 metabolites that were significantly different for sex and diet, respectively (S5 Table). Important metabolites that varied with HFHC diet include N-formyl glycine (P, 0.000449, 0.5 fold), malonic acid (P, 0.009446944, 1.7 fold), aspartic acid (P, 0.0146, 0.6 fold), cholesterol (P, 0.0269, 1.7 fold), propionic acid (P, 0.0321, 1.3 fold), glyoxylic acid (P, 0.0332, 0.5 fold), 3-phenyllactic acid (P, 0.0371, 0.3 fold) among others. Significantly different metabolites for sex (males/females) were benzoic acid (P, 0.001945, 0.4 fold), methoxycitronellal (P, 0.0022063, 0.7 fold), decanedioic acid/ sebacic acid (P,0.00355, 1.9 fold), citramalic acid (P, 0.00465, 2.4 fold), 2-oxo-butanoic acid/alpha-ketobutyric acid (P, 0.00594, 4.6 fold), tyrosine (P, 0.00602, 2 fold), isobutyrate (P, 0.00686, 0.7 fold), hexanoic acid / caproic acid (P, 0.03953, 1.9 fold) among others (S5 Table). Further results from a repeated-measures ANOVA conducted for body weight, sex, age, clinical measures (glucose, HLD-C, LDL-C), and kinship suggested significant changes in seven metabolites. These were 3-cresotinic acid (a salicylic acid derivative), methyl malonate (involved in protein and fat metabolism), malonic acid (involved in malonyl-CoA decarboxylate metabolism), glycine (a non-essential amino acid), arsenous acid (arsenic derivative of possible dietary or environmental origin), 2-ketobutyric acid [involved in glycine, methionine, valine, leucine, serine, threonine, isoleucine, propanoate and C-5 branched dibasic acid (BCAA) metabolism], and 3-methyl-2-oxovaleric acid (an abnormal metabolite that arises from the incomplete breakdown of BCAA). Arsenic derivatives are known to promote cardiovascular toxicity of arsenic and eventually atherosclerosis [58]. Moreover, a deficiency of glycine to sarcosine converting enzyme glycine N-methyltransferase is known to aggravate atherosclerosis in apolipoprotein E-null mice [59]. Significant changes in methylmalonate and malonate are indicative of changes in aminomalonic acid pools that are found in atherosclerotic plaques [60]. Methylmalonate levels serve as an important biomarker in malonic and methylmalonic aciduria that are traced to genetic mutations [61]. Another study investigating changes in urinary metabolites from patients with ischemic heart failure found decreased excretion of TCA cycle intermediates [62], and the succinate precursor methyl malonate [63], indicating the involvement of malonate metabolism in cardiovascular ailments. These results indicate that the HFHC diet induced extensive changes in malonate metabolism (malonate and methylmalonate) and BCAA metabolism (2-ketobutyric acid, and 3-methyl-2-oxovaleric acid). In addition, the HFHC diet induced changes in other metabolites which have not previously been associated with atherosclerosis, such as glycine and 3-cresotinic acid. Validation in an independent cohort, i.e., in humans is required to validate the usefulness of these metabolites as biomarkers of atherosclerosis.
Effect of diet, sex, and genotype on metabolites (trait/ phenotype) and biomarkers of atherosclerosis
The heritabilities (defined as the proportion of phenotypic variation of a trait that can be attributed to genetic variation) of small metabolites and amino acids have been reported to vary between 23% and 55%, whereas those of lipids and lipoproteins are, respectively, from 48% to 62% and 50% to 76% [64]. Previous studies demonstrated the heritability of metabolite concentrations from stored human red blood cells [65]. Use of this pedigreed baboon cohort provided the opportunity to probe the heritability of the quantified metabolites in serum samples for two diets. Our analysis revealed significant heritabilities on chow diet for lactic acid (83%, h2 = 0.83, P, 0.014; n = 58), L-asparagine (52%, h2 = 0.52, P, 0.008), and alpha-phenylpropanoic acid (31%, h2 = 0.31, P, 0.026) for chow diet (S6 Table). For the HFHC diet, we found significant heritabilities for lactic acid (67%, h2 = 0.66, P, 0.0056) and L-asparagine (48%, h2 = 0.48, P, 0.006) (S6 Table). Our findings are consistent with the human studies showing that serum metabolites are heritable and that changes from diet and environment can be quantified. On the basal diet, high heritability of lactate, i.e., 0.83 (residual kurtosis, 0.51) indicates that genetic factors (genotype) contribute 83% of the variation among animals when they are fed the basal diet; whereas, after HFHC-diet exposure, the lowered heritability to 0.67 (residual kurtosis, 0.36) is indicative that on HFHC diet, genes are responsible for 67% of the variation among individuals and environmental factors are responsible for 33% of the variation. An East Flanders Prospective Twin Study (of a Belgian population) involving 240 monozygotic and 138 dizygotic twin pairs aged 18 to 34 years revealed that heritability estimates of fasting glucose, fasting insulin, homeostasis model assessment of insulin resistance and beta cell function, as well as insulin-like growth factor binding protein levels, were 67, 49, 48, 62 and 47%, respectively [66]. For total cholesterol, LDL-C, HDL-C and triacylglycerol heritability estimates range between 0 and 98% [66].
In human carotid atherosclerotic plaques, metabolomic profiling revealed enrichment of lactate and taurine, indicative of increased anaerobic glycolytic activity and of inflammatory defense against free radicals, respectively, within the plaques [67]. Indeed, blood lactate is associated with carotid atherosclerosis, as attenuation of the association with adjustment for triglyceride/HDL ratio, a marker of insulin resistance, suggests that lactate's association with carotid atherosclerosis may be related to insulin resistance [68]. Lee et al., [69] performed metabolomic and lipidomic analysis of male C57BL/6J mice fed 1.25% (w/w) cholesterol and 0.5% cholate (w/w) using NMR and LC-MS and found that sulfur amino acid (SAA) and lipid metabolism were perturbed. This study captured amino acids (alanine, threonine, glutamate, tyrosine, phenylalanine, carnitine, glutamine, leucine, isoleucine, lysine, valine, methionine, N, N-dimethylglycine, cysteine, glycine, homocysteine, cystathionine, serine), sugars (glucose, lactose, mannose), organic acids (lactate, citrate, pyruvate, glycolate, fumarate, formate, acetate), glycerol, carnitine, ceramides, and free fatty acids among others using both NMR and LC-MS platforms as we also observed majority of these in our GC-MS results. Interestingly these mice fed an atherogenic diet (AD) were shown to demonstrate elevated levels of lactate in both serum and heart tissues as revealed from NMR and LC-MS analysis [69]. The enzymatic asparaginase treatment (to deplete systemic asparagine) is known to induce clots, strokes, and other thromboembolic events [70], indicating interference with lipid metabolism.
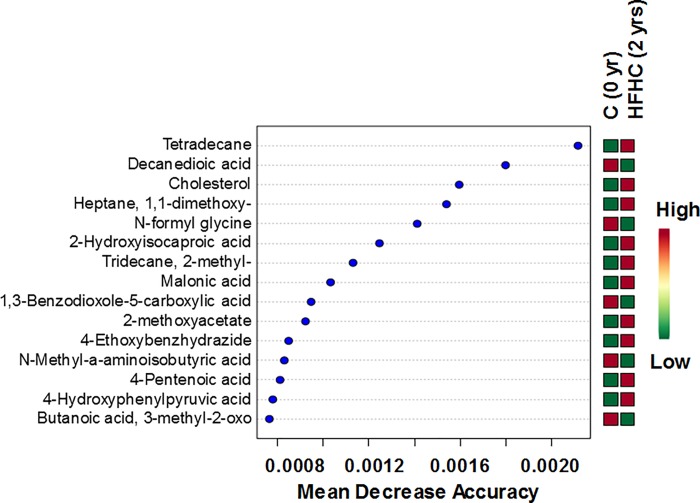
We further probed which metabolite abundance levels allowed discrimination of baseline (0 d) and HFHC diet-challenged (2 yr) serum samples. Random forest analysis is a data-driven method designed for prediction, and is conducted to identify metabolites that improved prediction of ASD and C groups. Contributions of individual predictors are measured by ‘variable importance’ using a conditional permutation scheme for correlated predictors. Variable importance greater (less) than 0 suggests an increase (decrease) in prediction accuracy. By relying on the ranges of values of each selected feature using our RF classifier, we can identify dependencies between features which result in separation for the two classes of interest to help identify the most important metabolites and exclude associations by chance. RF algorithms [71] have been extremely successful as a general purpose classification and regression method, shown to be useful for small sample sizes, and successfully implemented in the metabolomics literature [72–74]. We obtained larger mean decrease accuracy (MDA) values for metabolites involved and associated with fatty acid metabolism, such as cholesterol, decanedioic acid (involved in carnitine-acylcarnitine translocase deficiency and medium chain acyl-CoA dehydrogenase deficiency), 1,1,-dimethoxyheptane (an acetal, which is an energy source or storage molecule), 4-pentenoic acid (a straight chain fatty acid), and large-scale changes in amino acid metabolism, i.e., N-formylglycine (an alanine derivative), 2-hydroxyisocaproic acid (a BCAA amino acid leucine metabolite), N-methyl-alpha-aminoisobutyric acid, 4-hydroxyphenylpyruvic acid (involved in aromatic amino acid biosynthesis), 3-methyl-2-oxobutanoic acid (involved in valine, leucine, and isoleucine biosynthesis/degradation), and organic acids such as 2-methoxyacetate (a carboxylic acid nutrient), malonic acid (a dicarboxylic acid), 1,3-benzodioxole-5-carboxylic acid (an antioxidant), N-Methyl-a-aminoisobutyric acid (involved with IL-1beta and IL-6 transport) and other alkanes (2-methyl-tridecane, tetradecane) (Fig 2).
Fig 2. Random forest analysis showing discriminating biomarkers of diet-challenge in their decreasing orders of mean decrease accuracies (MDA).
Smaller MDAs tend to be better discriminatory markers than the higher values.
In atherosclerotic rats, higher decanedioic acid (sebacic acid) acid, a dicarboxylic acid known to produce acetyl-CoA and succinyl-CoA, which belong to important intermediates of the TCA cycle, due to their β-oxidation levels, was reported [75]. Dietary PUFAs reduce atherosclerosis by activating macrophage autophagy and attenuating NLRP3 activation (in mice) [76], and where macrophage metabolism undergoes changes in glycolysis pentose phosphate pathway, inflammation responses, and a defective oxidative phosphorylation among others [77]. These clearly indicate that the HFHC diet perturbed immunometabolism. Although single-gene defects in lipid metabolic pathways account only for a very small portion of familial and sporadic atherosclerosis, multiple genes and environmental factors are known to have a major role in determining elevated lipid levels and risk of atherosclerotic events [78]. Furthermore, in patients with stable atherosclerosis, plasma amino acids were significantly decreased, i.e., lower levels of alanine, aspartate, tyrosine, and serine [79]. Another study found significant associations between plasma branched chain amino acid metabolites and CAD and myocardial infarction [80]. A study comparing cardiac extraction of plasma substrates demonstrated that patients with CAD had decreased extraction of alanine and glutamate/glutamine, even when normalized to any differences in arterial amino acid concentrations [81]. These findings point to pervasive changes in amino acid metabolism, fatty acid metabolism, as well as immunometabolism in plasma of individuals with CAD compared to healthy individuals. Moreover, these individual discriminant metabolites can be used to monitor blood-based changes in metabolism in atherosclerotic individuals.
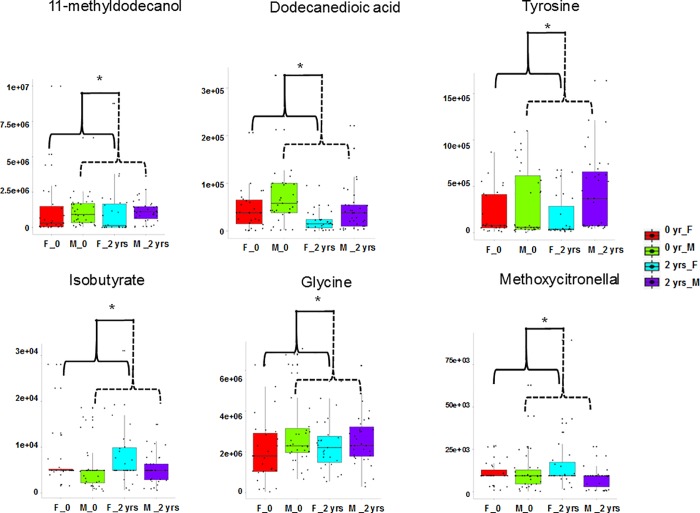
There is little information in the literature on sex differences in metabolite abundance related to CVD. Lipid metabolism is clearly sex-dimorphic [82]. CAD incidence in a cohort of 14,786 Finnish men and women was ~3 times higher in men compared with women, and mortality was ~5 times higher in men than women [83]. Female macaques, like women, are resistant to atherosclerosis [84]. Our study also revealed sex-dimorphism in abundance of metabolites in baboons (Fig 3, S5 Table). For instance, 11-methyldodecanol, decanedioic acid, tyrosine and glycine were significantly (P, <0.05) higher in males; whereas, methoxycitronellal and isobutyrate were significantly (P, <0.05) higher in females, independent of diet. Thus, we observed higher metabolite abundances of amino acids and lipids in females, than in males. Very little information is available on the sex-dimorphic metabolite abundances from human studies, which is starting to be explored and a very new area of active research in metabolomics [85].
Fig 3. Relative abundances of metabolites that are significantly (ANOVA, P < 0.05) sex-dimorphic.
In the box-plots, the center lines show the medians; box limits indicate the 25th and 75th percentiles; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles, outliers are represented by dots; crosses represent sample means; data points are plotted as black dots. Widths of boxes are proportional to square-roots of the number of observations.
Metabolites associated with clinical measures of atherosclerosis
Increased dietary cholesterol intake is associated with atherosclerosis, and its development requires lipid and inflammatory components [86]. We observed interesting fatty streak lesion variation, including early stage (EGES), flat (F) and raised (R) lesions, corresponding, respectively, to AHA lesion types I, II and V as reported earlier in the original cohort of these baboons [34]. We observed evidence of atherosclerosis in all but one baboon fed the 2‐year challenge diet. CVD risk biomarkers, the prevalence, size, and complexity of arterial lesions, plus consequent arterial stiffness, were increased in comparison with dietary control animals [34]. The subset of 60 baboons used in this diet study were found to have at least one type of lesion.
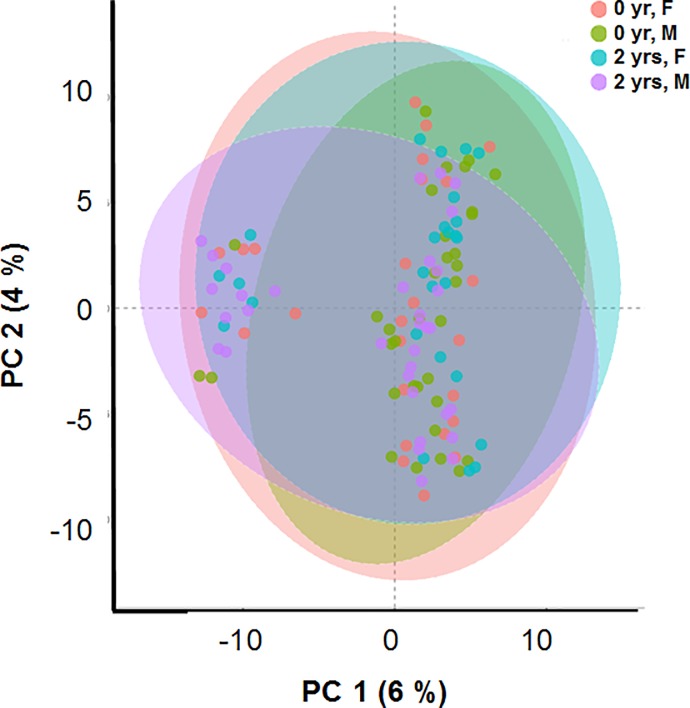
We further probed, whether metabolite abundances at base line (0 d) and post-diet challenge (2 yrs) demonstrated discrimination between these two time points. In the unsupervised PCA score plots, we observed that the two PCs contributed only 10% separation for the two diet-groups (chow and HFHC) (Fig 4).
Fig 4. Principal component analysis (PCA) displaying Chow (red) and HFHC diet (cyan) fed baboon samples clustered as well as appearance of a new smaller cluster comprised of 32 baboon samples.
No further improvement of clustering was observed in the partial least square- discriminant analysis (PLS-DA) (i.e., first two PCs contributed only 6%). These analyses point to the fact that the 2 yr diet-induced changes in metabolism are not strong or the metabolites captured were not truly representative of the plaques caused by atherosclerotic events. However, interestingly, a new smaller cluster of 32 serum samples (uniquely represented by 32 baboons) but belonging to both sexes and both diet groups, appeared in this analysis (S3 Fig). Upon further interrogation, we observed that these 32 baboons had higher plasma triglyceride (TG) concentrations than the rest of the baboons used in the study (S3 Table), which indicates that there must be metabolites, possibly not captured in a GC-MS analysis, in plasma, that are driven by or related to TG metabolism, biosynthesis, and/or accumulation and atherosclerosis. However, we did not detect any significant and notable correlations between plasma TG levels and other metabolites for these baboons or the group as a whole.
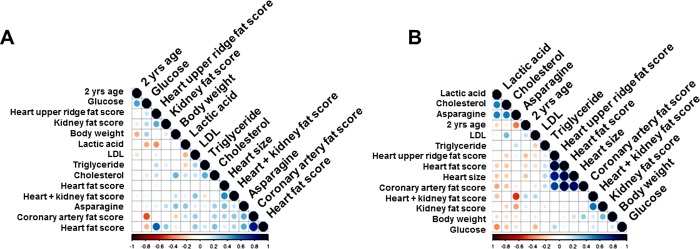
Further, we investigated whether clinical measures, heritable metabolites, lesion burden, and fat indices (scores) of various organs were correlated among each other and whether sex played an important role in this correlation. We performed a Pearson correlation analysis with a matrix for fat scores, clinical measures (body weight, LDL-C, TG) with selected metabolites (glucose, cholesterol, lactic acid, and asparagine) separately for males and females (S7 Table). Results demonstrated strong positive correlations among the fat scores from upper ridge, coronary artery, kidney, heart in the males that was weaker in females (Fig 5). Indeed, a study in >2100 older men and women in the Rotterdam cohort indicated that presence of carotid atherosclerosis (i.e., carotid intimal–medial thickness of >2.0 mm on carotid screening) was more common among men than women [87]. Location of ectopic fat in key target-organs of cardiovascular control (heart, blood vessels and kidneys) are known to play a role in the pathogenesis of CVD associated with obesity (induced by high-fat diet). Cardiac fat depots within and around the heart impair both systolic and diastolic functions and may in the long-term promote heart failure [88]. Moreover, metabolites lactic acid, cholesterol, and asparagine showed stronger positive correlations among themselves in males than females. However, asparagine correlated with fat scores in females. This shows that plaque-phenotype associated metabolites are well-represented in serum samples, and hence cold be better indicator of disease burden in males than in females. However, interestingly, in other studies metabolite abundance in carotid atherosclerotic plaques were not correlated with metabolite abundance in blood [89].
Fig 5.
Correlation between fat scores, clinical measures, and selected metabolites in (a) female (n = 26) and (B) male (n = 34) baboons. Correlations are in a scale of +1 (positive, blue) to -1 (negative, red).
We also assessed correlations of metabolite abundance with individual atherosclerotic phenotypes including lesion burden (percent covered by fatty streak or plaques, and the number of plaques) for aortic arch, common iliac artery, descending aorta, and total lesion burden, after adjustment for sex and age (S8 Table). We found that tridecane and 2-oxoglutarate showed strong correlation (R2, 0.6–0.8) with all four phenotypes, i.e., aortic arch, common iliac artery, descending aorta, and total lesion burden. Mahaney et al [34], also suggested significant correlations for the aortic arch and common iliac artery for lesion sizes. In addition, we found that asparagine, 2-oxo-3-phenylpropanoic acid, 2-oxoglutarate, and 1, 3-diaminopropane (a polyamine) strongly correlated among each other, but not with lesion burden (Fig 6). We found 35 metabolites significantly correlated (P, <0.05) with total lesion burden including metabolites in pathways involved in ammonia recycling (asparagine, and pyruvate), aspartate metabolism (asparagine, and malonic acid), fatty acid metabolism (palmitic acid, malonic acid), glycolic acid, isobutyric acid, tyramine, methymaleic acid, ethanol. Benzoylformate showed the highest correlation (R2, 0.27; P, 0.000035). However, it remains to be seen if the other metabolites identified tentatively as ‘unknowns’ that are correlated with the various lesion and fat score phenotypes are truly metabolites of exogenous origin, or are poorly annotated compounds requiring further structural validation (S9 Table).
Fig 6. Correlation of atherosclerotic phenotypes (lesion percentage distribution as fatty streak and/ plaque distribution and numbers) with the metabolite abundances.
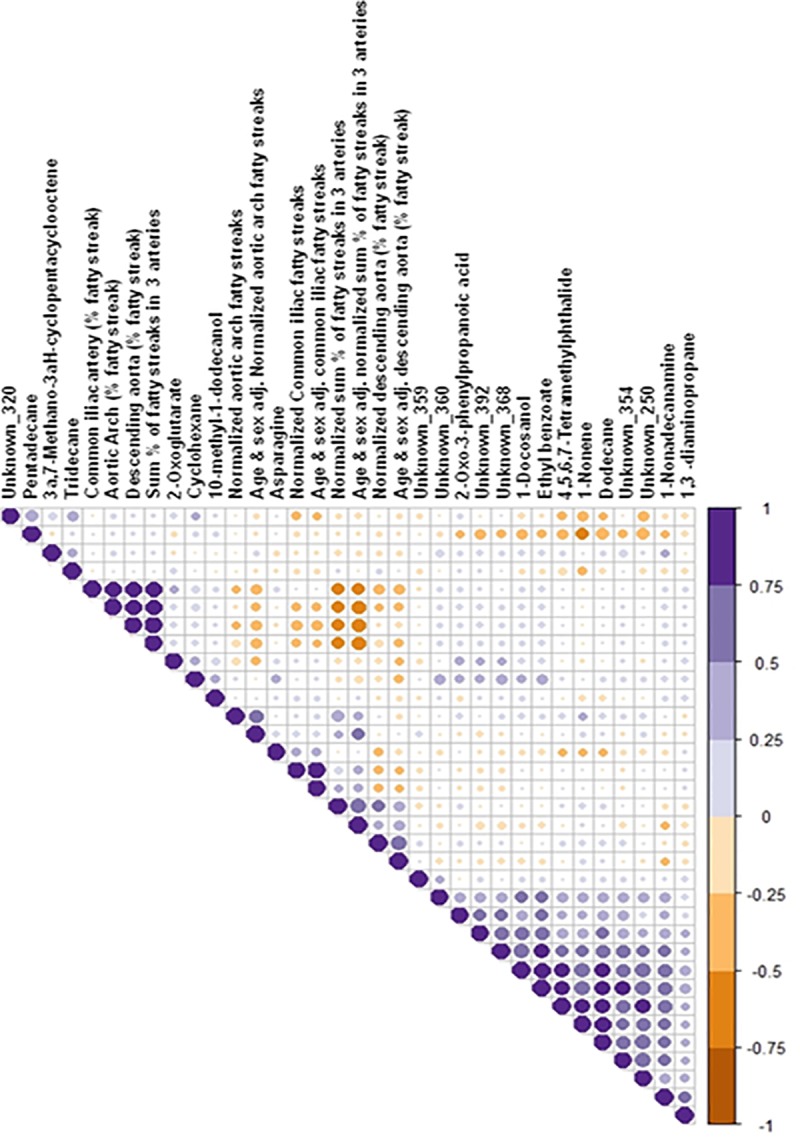
Correlations are in a scale of +1 (positive, purple) to -1 (negative, brown).
Comparability of the findings in NHP with human studies
Very few studies have been conducted in human atherosclerosis and CVD using metabolomics as a tool for biomarker discovery. Population based studies are laced with confounders such as life style, race, and dietary variations; nevertheless, we probed the overlapping biomarkers using qualitative analysis from recent studies. Vorkas et al [90] investigated the lipid metabolite profile in different areas of the same atherosclerotic tissues. They demonstrated clear lipid heterogeneity within these lesions and identified phosphatidylethanolamine-ceramide as a novel candidate biomarker for atherosclerosis development. Nine metabolites involved in lipid metabolism (i.e. lysophosphatidylcholine (LysoPC) (20:4), LysoPC(16:0), phosphatidylglycerol (18:0/0:0), elaidic acid, prasterone sulfate, l-fucose, monoglyceride (MG) (0:0/18:2(9Z,12Z)/0:0), diglyceride (DG) (20:2(11Z,14Z)/18:3(9Z,12Z,15Z)/0:0), and indoxylsulfuric acid) were selected as the best predictors for early coronary atherosclerosis discrimination [31] using LC-QToF-MS analysis, which are not captured in a GC-MS analysis. Other recent LC-MS and NMR–based studies based on atherosclerotic plaques or plasma samples in humans have shown [91–94] either lipids or other LC-MS amenable metabolites to be important atherosclerosis or CVD biomarkers, i.e., tryptophan, BCAA, gluconate, 2-hydroxycaproate, and trimethylamine n-oxide (TMAO)(a gut-microbiota derived established metabolite). Thus, our study is one the first in diet-induced atherosclerosis that has identified GC-MS amenable metabolites associated with CVD phenotypes.
Strengths and limitations of the study
For the first time using a dual derivatization strategy and a 2D GC-MS platform for untargeted metabolomic analysis of serum, we showed diet-associated changes in atherosclerotic baboons at baseline and after a 2 yr HFHC diet challenge. Using a pedigreed cohort of NHP with controlled environment, analyses of metabolite abundance, and lesion and fat scores, we identified metabolites correlated with CVD signatures in serum. This model system also allowed us to investigate heritabilities of metabolites in the presence and absence of a HFHC diet challenge. Lastly, the obtained panel of metabolites have the potential to be confirmed in future human studies for replication and validation as biomarkers.
GC-MS as a technology is limited to analysis of polar metabolites and a handful of lipid metabolites. Although using a high throughput 2D platform and two derivatization reagents we analyzed more metabolites than are typically captured in conventional 1D GC-MS, an untargeted LC-MS based approach would have captured diverse metabolic intermediates and non-polar compounds not found in this study. Another interesting scope would be to analyze the metabolite composition of the diet itself using quantitative metabolomics to differentiate the diet-metabolites from those of the endogenous metabolites both qualitatively and quantitatively. Future work will improve confidence in our findings and provide additional biological context for quantified serum metabolites. In addition, it will be useful to determine whether serum metabolites correlate with tissue and lesion specific metabolites to better understand whether metabolic dysregulation is organ specific or systemic.
Conclusions
The goal of this study was to investigate metabolic changes associated with development and progression of atherosclerosis using untargeted metabolomic analysis of NHP where genetics and environment can be controlled. We identified two heritable metabolites and found that abundance of some metabolites is sex-dependent. In addition, we identified 35 metabolites that correlate with total lesion burden and may serve as biomarkers for atherosclerotic lesion burden. This study provides a first insight into diet-induced serum metabolomic changes associated with CVDs. Future studies to validate these biomarkers should take into account sex-differences and influence of diet.
Supporting information
A two-way Venn diagram displaying the unique and shared metabolites (180) between MTBSTFA (N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide) and MSTFA (N-methyl-N-(trimethylsilyl)trifluoroacetamide with 1% trimethylchlorosilane), the two derivatization regents.
(TIF)
KEGG-based metabolic pathways covered using the 2D GC-MS platform. The network view generated from MetaboAnalyst and modified using Cytoscape, displays the biological pathways covered using the metabolites quantified using 2D GC-ToF-MS used in this study. Nodes (yellow) are pathways and edges (lines) connect them for their relatedness.
(TIF)
Metabolite abundance differences (not significant, P, < 0.05) in the 32 baboon serum samples that were outliers, when comapred to itnernal standard (ribitol) used for the analytical runs. In the box-plots, the center lines show the medians; box limits indicate the 25th and 75th percentiles as determined; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles, outliers are represented by dots; crosses represent sample means; data points are plotted as black dots. Widths of boxes are proportional to square roots of the number of observations.
(PDF)
Columns represent: baboon number; sex, age at 2 yr experimental endpoint, triglyceride change (mg/dL), heart fat score, heart upper ridge fat score, heart coronary artery fat score, kidney fat score, change in body weight (kgs), change in glucose (mg/dL), LDL-c change (mg/dL), triglyceride at 2 yrs (mg/dL), body weight at 2 yrs (kgs), glucose at 2 yrs (mg/dL) and LDL-c at 2 yrs (mg/dL).
(XLSX)
(XLSX)
(XLSX)
Provided are 1st and 2nd dimension retention times (seconds), similarity matching scores against EI spectral libraries, signal/noise, unique (quantifier) mass, probability, retention time matrix.
(XLSX)
P-values reported are nominal (P, <0.05).
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Table is displaying R, slope, T-statistics, and P-values.
(XLSX)
Acknowledgments
We thank Vicki Mattern for conducting clinical assays, Shifra Burnham for providing serum samples.
Abbreviations
- 2D GC-ToF-MS
two-dimensional gas chromatography time-of-flight mass-spectrometry
- ANOVA
analysis of variance
- BCAA
branched chain amino acid
- CVD
cardiovascular disease
- HCA
hierarchical clustering analysis
- HDL-C
high density lipoprotein cholesterol
- HFHC
high fat and high cholesterol
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LDL-C
low density lipoprotein cholesterol
- m/z
mass-to-charge ratio
- MeOX
methoxyamine hydrochloride
- MSTFA
N-methyl-N-trimethylsilyl-trifluoroacetamide
- MTBSTFA
N-(t-butyldimethylsilyl)-N-methyltrifluoroacetamide
- NHP
non-human primate
- PCA
principal component analysis
- PLS-DA
partial least square-discriminant analysis
- QC
quality control
Data Availability
The raw datasets and the metadata obtained from mass-spectrometry based 2D GC-ToF- MS metabolomics efforts are made publicly available at the deposited at the Global Natural Product Social Molecular Networking (GNPS) database (Study ID: MassIVE MSV000083256) and is available for download at this link: https://massive.ucsd.edu/ProteoSAFe/dataset.jsp?task=dd88e038114043f8830fd8b3e5ee77a6 (doi:10.25345/C5Z608).
Funding Statement
BBM received a Texas Biomedical Forum Grant (BM-17-04629) awarded by the Texas Biomedical Forum, San Antonio, Texas. This work was supported in part by grants from the Texas Biomedical Forum and the National Institutes of Health (NIH) (https://www.nih.gov/) (P01 HL028972). This investigation used resources, which were supported by the Southwest National Primate Research Center grant P51 RR013986 from the National Center for Research Resources, National Institutes of Health, USA (https://www.nih.gov/), currently supported by the Office of Research Infrastructure Programs (ORIP), NIH through grant P51 OD011133. This investigation was conducted in facilities constructed with support from ORIP through grant numbers C06 RR14578, C06 RR15456, C06 RR013556, and C06 RR017515. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Organization WH. The world health report 2002: reducing risks, promoting healthy life: World Health Organization; 2002. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012;(82):1–8. Epub 2012/05/24. . [PubMed] [Google Scholar]
- 3.Reardon CA, Getz GS. Mouse models of atherosclerosis. Curr Opin Lipidol. 2001;12(2):167–73. Epub 2001/03/27. . [DOI] [PubMed] [Google Scholar]
- 4.Ornish D. Can lifestyle changes reverse coronary heart disease? Nutrition and Fitness in Health and Disease. 72: Karger Publishers; 1993. p. 38–48. [DOI] [PubMed] [Google Scholar]
- 5.Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280(23):2001–7. Epub 1998/12/24. . [DOI] [PubMed] [Google Scholar]
- 6.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101(17):6659–63. Epub 2004/04/21. 10.1073/pnas.0308291101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169(7):659–69. Epub 2009/04/15. 10.1001/archinternmed.2009.38 . [DOI] [PubMed] [Google Scholar]
- 8.Homma Y. Predictors of atherosclerosis. J Atheroscler Thromb. 2004;11(5):265–70. Epub 2004/11/24. . [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Wu NQ, Li S, Zhu CG, Guo YL, Qing P, et al. Non-HDL-C is a Better Predictor for the Severity of Coronary Atherosclerosis Compared with LDL-C. Heart Lung Circ. 2016;25(10):975–81. Epub 2016/09/17. 10.1016/j.hlc.2016.04.025 . [DOI] [PubMed] [Google Scholar]
- 10.Boey E, Gay GMW, Poh K-K, Yeo T-C, Tan H-C, Lee C-HJA. Visit-to-visit variability in LDL-and HDL-cholesterol is associated with adverse events after ST-segment elevation myocardial infarction: A 5-year follow-up study. Atherosclerosis. 2016;244:86–92. 10.1016/j.atherosclerosis.2015.10.110 WOS:000367375100051. [DOI] [PubMed] [Google Scholar]
- 11.Briel M, Ferreira-Gonzalez I, You JJ, Karanicolas PJ, Akl EA, Wu P, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. Bmj-Brit Med J. 2009;338:b92 Epub 2009/02/18. 10.1136/bmj.b92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hafiane A, Genest J. High density lipoproteins: Measurement techniques and potential biomarkers of cardiovascular risk. BBA Clin. 2015;3:175–88. Epub 2015/12/18. 10.1016/j.bbacli.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiye A, Trygg J, Gullberg J, Johansson AI, Jonsson Pr, Antti H, et al. Extraction and GC/MS analysis of the human blood plasma metabolome. Anal Chem. 2005;77(24):8086–94. 10.1021/ac051211v [DOI] [PubMed] [Google Scholar]
- 14.Mamas M, Dunn WB, Neyses L, Goodacre R. The role of metabolites and metabolomics in clinically applicable biomarkers of disease. Arch Toxicol. 2011;85(1):5–17. Epub 2010/10/19. 10.1007/s00204-010-0609-6 . [DOI] [PubMed] [Google Scholar]
- 15.Han S-H, Bae J-H, Holmes DR Jr, Lennon RJ, Barsness GW, Rihal CS, et al. Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Am Heart Assoc; 2007. [DOI] [PubMed] [Google Scholar]
- 16.Njølstad I, Arnesen E, Lund-Larsen PG. Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction: a 12-year follow-up of the Finnmark Study. Circulation. 1996;93(3):450–6. [DOI] [PubMed] [Google Scholar]
- 17.Skilton MR, Moulin P, Serusclat A, Nony P, Bonnet F. A comparison of the NCEP-ATPIII, IDF and AHA/NHLBI metabolic syndrome definitions with relation to early carotid atherosclerosis in subjects with hypercholesterolemia or at risk of CVD: evidence for sex-specific differences. Atherosclerosis. 2007;190(2):416–22. Epub 2006/04/18. 10.1016/j.atherosclerosis.2006.02.019 . [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Magkos F, Mittendorfer B. Sex differences in lipid and lipoprotein metabolism: it's not just about sex hormones. The Journal of Clinical Endocrinology Metabolism. 2011;96(4):885–93. 10.1210/jc.2010-2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasbarrino K, Zheng HA, Veinot J, Daskalopoulou S. Sex Differences in the Adipokine, Lipid, and Immune Profiles of Men and Women with Severe Carotid Atherosclerosis. Atherosclerosis Supplements. 2018;32:13–4. 10.1016/j.atherosclerosissup.2018.04.039 WOS:000434628100038. [DOI] [Google Scholar]
- 20.Cox LA, Comuzzie AG, Havill LM, Karere GM, Spradling KD, Mahaney MC, et al. Baboons as a model to study genetics and epigenetics of human disease. ILAR J. 2013;54(2):106–21. Epub 2013/11/01. 10.1093/ilar/ilt038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox LA, Olivier M, Spradling-Reeves K, Karere GM, Comuzzie AG, VandeBerg JL. Nonhuman primates and translational research—cardiovascular disease. ILAR journal. 2017;58(2):235–50. 10.1093/ilar/ilx025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGill Jr HC, McMahan CA, Kruski AW, Kelley JL, Mott GE. Responses of serum lipoproteins to dietary cholesterol and type of fat in the baboon. Arteriosclerosis: An Official Journal of the American Heart Association, Inc. 1981;1(5):337–44. [DOI] [PubMed] [Google Scholar]
- 23.McGill Jr HC, McMahan CA, Zieske AW, Tracy RE, Malcom GT, Herderick EE, et al. Association of coronary heart disease risk factors with microscopic qualities of coronary atherosclerosis in youth. Circulation. 2000;102(4):374–9. [DOI] [PubMed] [Google Scholar]
- 24.Khyzha N, Alizada A, Wilson MD, Fish JE. Epigenetics of Atherosclerosis: Emerging Mechanisms and Methods. Trends Mol Med. 2017;23(4):332–47. Epub 2017/03/16. 10.1016/j.molmed.2017.02.004 . [DOI] [PubMed] [Google Scholar]
- 25.Li DY, Tang WHW. Gut Microbiota and Atherosclerosis. Curr Atheroscler Rep. 2017;19(10):39 Epub 2017/08/27. 10.1007/s11883-017-0675-9 . [DOI] [PubMed] [Google Scholar]
- 26.Nguyen MA, Karunakaran D, Geoffrion M, Cheng HS, Tandoc K, Perisic Matic L, et al. Extracellular Vesicles Secreted by Atherogenic Macrophages Transfer MicroRNA to Inhibit Cell Migration. Arterioscler Thromb Vasc Biol. 2018;38(1):49–63. Epub 2017/09/09. 10.1161/ATVBAHA.117.309795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaina S, Lund G. Connecting the Dots Between Fatty Acids, Mitochondrial Function, and DNA Methylation in Atherosclerosis. Curr Atheroscler Rep. 2017;19(9):36 Epub 2017/07/25. 10.1007/s11883-017-0673-y . [DOI] [PubMed] [Google Scholar]
- 28.Karere GM, Glenn JP, VandeBerg JL, Cox LA. Differential microRNA response to a high-cholesterol, high-fat diet in livers of low and high LDL-C baboons. BMC Genomics. 2012;13(1):320 Epub 2012/07/20. 10.1186/1471-2164-13-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox LA, Li C, Glenn JP, Lange K, Spradling KD, Nathanielsz PW, et al. Expression of the placental transcriptome in maternal nutrient reduction in baboons is dependent on fetal Sex. The Journal of nutrition. 2013;143(11):1698–708. 10.3945/jn.112.172148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barba I, de León G, Martín E, Cuevas A, Aguade S, Candell‐Riera J, et al. Nuclear magnetic resonance‐based metabolomics predicts exercise‐induced ischemia in patients with suspected coronary artery disease. Magn Reson Med. 2008;60(1):27–32. Epub 2008/06/27. 10.1002/mrm.21632 . [DOI] [PubMed] [Google Scholar]
- 31.Gao X, Ke C, Liu H, Liu W, Li K, Yu B, et al. Large-scale Metabolomic Analysis Reveals Potential Biomarkers for Early Stage Coronary Atherosclerosis. Sci Rep. 2017;7(1):11817 Epub 2017/09/20. 10.1038/s41598-017-12254-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Zhang D, He Y, Chen C, Song C, Zhao Y, et al. Investigation of novel metabolites potentially involved in the pathogenesis of coronary heart disease using a UHPLC-QTOF/MS-based metabolomics approach. Sci Rep. 2017;7(1):15357 Epub 2017/11/12. 10.1038/s41598-017-15737-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leary SL, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, et al. , editors. AVMA guidelines for the euthanasia of animals: 2013 edition2013: American Veterinary Medical Association Schaumburg, IL. [Google Scholar]
- 34.Mahaney MC, Karere GM, Rainwater DL, Voruganti VS, Dick EJ Jr., Owston MA, et al. Diet-induced early-stage atherosclerosis in baboons: Lipoproteins, atherogenesis, and arterial compliance. J Med Primatol. 2018;47(1):3–17. Epub 2017/06/18. 10.1111/jmp.12283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiehn O, Wohlgemuth G, Scholz M, Kind T, Lee DY, Lu Y, et al. Quality control for plant metabolomics: reporting MSI-compliant studies. The Plant journal: for cell and molecular biology. 2008;53(4):691–704. [DOI] [PubMed] [Google Scholar]
- 36.Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nature protocols. 2006;1(1):387–96. 10.1038/nprot.2006.59 [DOI] [PubMed] [Google Scholar]
- 37.Wachsmuth CJ, Vogl FC, Oefner PJ, Dettmer K. Gas chromatographic techniques in metabolomics. Chromatographic Methods in Metabolomics. 2013;Hyotylainen, T., Wiedmer, S., Eds:87–105. [Google Scholar]
- 38.Misra BB, Bassey E, Bishop AC, Kusel DT, Cox LA, Olivier M. High Resolution GC/MS Metabolomics of Non-Human Primate Serum. Rapid Commun Mass Spectrom. 2018. Epub 2018/06/07. 10.1002/rcm.8197 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Misra BB, Upadhayay RP, Cox LA, Olivier M. Optimized GC–MS metabolomics for the analysis of kidney tissue metabolites. Metabolomics. 2018;14(6):75 10.1007/s11306-018-1373-5 [DOI] [PubMed] [Google Scholar]
- 40.Developmental variations in sesquiterpenoid biosynthesis in East Indian sandalwood tree (Santalum album L.).
- 41.Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics: Official journal of the Metabolomic Society. 2007;3(3):211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Team RC. R: A language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2015. 2015. [Google Scholar]
- 43.Sokal RR, Rohlf FJ. The principles and practice of statistics in biological research: WH Freeman and company; San Francisco:; 1969. [Google Scholar]
- 44.Grapov D. DeviumWeb: Dynamic Multivariate Data Analysis and Visualization Platform. 2014. [Google Scholar]
- 45.Caraux G, Pinloche S. PermutMatrix: a graphical environment to arrange gene expression profiles in optimal linear order. Bioinformatics. 2005;21(7):1280–1. Epub 2004/11/18. 10.1093/bioinformatics/bti141 . [DOI] [PubMed] [Google Scholar]
- 46.Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic acids research. 2015;43(W1):W251–W7. 10.1093/nar/gkv380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–211. Epub 1998/05/23. 10.1086/301844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Self SG, Liang KY. Asymptotic Properties of Maximum-Likelihood Estimators and Likelihood Ratio Tests under Nonstandard Conditions. Journal of the American Statistical Association. 1987;82(398):605–10. 10.2307/2289471 WOS:A1987J105700033. [DOI] [Google Scholar]
- 49.Nakagawa K, Nakashima Y. Pathologic intimal thickening in human atherosclerosis is formed by extracellular accumulation of plasma-derived lipids and dispersion of intimal smooth muscle cells. Atherosclerosis. 2018;274:235–42. Epub 2018/04/07. 10.1016/j.atherosclerosis.2018.03.039 . [DOI] [PubMed] [Google Scholar]
- 50.Masuda J, Ross R. Atherogenesis during low level hypercholesterolemia in the nonhuman primate. I. Fatty streak formation. Arteriosclerosis. 1990;10(2):164–77. Epub 1990/03/01. . [DOI] [PubMed] [Google Scholar]
- 51.Newman WP III, Freedman DS, Voors AW, Gard PD, Srinivasan SR, Cresanta JL, et al. Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis. J New England Journal of Medicine. 1986;314(3):138–44. [DOI] [PubMed] [Google Scholar]
- 52.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. Bmj-British Medical Journal. 2006;332(7533):73–6. 10.1136/bmj.38678.389583.7C WOS:000234777000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. New Engl J Med. 2001;345(18):1291–7. 10.1056/NEJMoa003417 WOS:000171896300001. [DOI] [PubMed] [Google Scholar]
- 54.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–78. 10.1016/S0140-6736(05)67394-1 WOS:000232405700025. [DOI] [PubMed] [Google Scholar]
- 55.Shaw LJ, Bugiardini R, Merz CNB. Women and Ischemic Heart Disease Evolving Knowledge. Journal of the American College of Cardiology. 2009;54(17):1561–75. 10.1016/j.jacc.2009.04.098 WOS:000271089900001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelly DM, Akhtar S, Sellers DJ, Muraleedharan V, Channer KS, Jones TH. Testosterone differentially regulates targets of lipid and glucose metabolism in liver, muscle and adipose tissues of the testicular feminised mouse. Endocrine. 2016;54(2):504–15. Epub 2016/10/28. 10.1007/s12020-016-1019-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruiz-Canela M, Hruby A, Clish CB, Liang L, Martinez-Gonzalez MA, Hu FB. Comprehensive Metabolomic Profiling and Incident Cardiovascular Disease: A Systematic Review. J Am Heart Assoc. 2017;6(10). Epub 2017/10/01. 10.1161/JAHA.117.005705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silva LFN, Lemaire M, Lemarié CA, Plourde D, Bolt AM, Chiavatti C, et al. Effects of Inorganic Arsenic, Methylated Arsenicals, and Arsenobetaine on Atherosclerosis in the [… formula …] Mouse Model and the Role of As3mt-Mediated Methylation. Environmental Health Perspectives. 2017;125(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen C-Y, Ching L-C, Liao Y-J, Yu Y-B, Tsou C-Y, Shyue S-K, et al. Deficiency of glycine N-methyltransferase aggravates atherosclerosis in apolipoprotein E–null mice. Molecular Medicine. 2012;18(1):744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Buskirk JJ, Kirsch WM, Kleyer DL, Barkley RM, Koch TH. Aminomalonic acid: identification in Escherichia coli and atherosclerotic plaque. Proc Natl Acad Sci U S A. 1984;81(3):722–5. Epub 1984/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levtova A, Waters PJ, Buhas D, Lévesque S, Auray-Blais C, Clarke JT, et al. Combined malonic and methylmalonic aciduria due to ACSF3 mutations: benign clinical course in an unselected cohort. Journal of inherited metabolic disease. 2018:1–10. 10.1007/s10545-017-0121-8 [DOI] [PubMed] [Google Scholar]
- 62.Heather LC, Wang XZ, West JA, Griffin JL. A practical guide to metabolomic profiling as a discovery tool for human heart disease. J Mol Cell Cardiol. 2013;55:2–11. 10.1016/j.yjmcc.2012.12.001 WOS:000314626200002. [DOI] [PubMed] [Google Scholar]
- 63.Kang S-M, Park J-C, Shin M-J, Lee H, Oh J, Hwang G-S, et al. 1H nuclear magnetic resonance based metabolic urinary profiling of patients with ischemic heart failure. J Clinical biochemistry. 2011;44(4):293–9. [DOI] [PubMed] [Google Scholar]
- 64.Dharuri H, Demirkan A, van Klinken JB, Mook-Kanamori DO, van Duijn CM, AC’t Hoen P, et al. Genetics of the human metabolome, what is next? Biochimica et Biophysica Acta -Molecular Basis of Disease. 2014;1842(10):1923–31. [DOI] [PubMed] [Google Scholar]
- 65.van't Erve TJ, Wagner BA, Martin SM, Knudson CM, Blendowski R, Keaton M, et al. The heritability of metabolite concentrations in stored human red blood cells. Transfusion. 2014;54(8):2055–63. 10.1111/trf.12605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Souren NY, Paulussen AD, Loos RJ, Gielen M, Beunen G, Fagard R, et al. Anthropometry, carbohydrate and lipid metabolism in the East Flanders Prospective Twin Survey: heritabilities. Diabetologia. 2007;50(10):2107–16. Epub 2007/08/19. 10.1007/s00125-007-0784-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mayr M, Grainger D, Mayr U, Leroyer AS, Leseche G, Sidibe A, et al. Proteomics, metabolomics, and immunomics on microparticles derived from human atherosclerotic plaques. Circ Cardiovasc Genet. 2009;2(4):379–88. Epub 2009/12/25. 10.1161/CIRCGENETICS.108.842849 . [DOI] [PubMed] [Google Scholar]
- 68.Shantha GP, Wasserman B, Astor BC, Coresh J, Brancati F, Sharrett AR, et al. Association of blood lactate with carotid atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Carotid MRI Study. Atherosclerosis. 2013;228(1):249–55. Epub 2013/03/21. 10.1016/j.atherosclerosis.2013.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee J, Jung S, Kim N, Shin MJ, Ryu DH, Hwang GS. Myocardial metabolic alterations in mice with diet-induced atherosclerosis: linking sulfur amino acid and lipid metabolism. Sci Rep. 2017;7(1):13597 Epub 2017/10/21. 10.1038/s41598-017-13991-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parsons SK, Skapek SX, Neufeld EJ, Kuhlman C, Young ML, Donnelly M, et al. Asparaginase-associated lipid abnormalities in children with acute lymphoblastic leukemia. Blood. 1997;89(6):1886–95. Epub 1997/03/15. . [PubMed] [Google Scholar]
- 71.Breiman L. Random forests. Mach Learn. 2001;45(1):5–32. 10.1023/A:1010933404324 WOS:000170489900001. [DOI] [Google Scholar]
- 72.Biau G, Scornet E. A random forest guided tour. Test-Spain. 2016;25(2):197–227. 10.1007/s11749-016-0481-7 WOS:000375792600001. [DOI] [Google Scholar]
- 73.Guo Y, Graber A, McBurney RN, Balasubramanian R. Sample size and statistical power considerations in high-dimensionality data settings: a comparative study of classification algorithms. Bmc Bioinformatics. 2010;11 Artn 447 10.1186/1471-2105-11-447 WOS:000282655600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gunduz N, Fokoué E. Robust classification of high dimension low sample size data. arXiv preprint 2015;arXiv:.00592. [Google Scholar]
- 75.Jia P, Wang S, Xiao C, Yang L, Chen Y, Jiang W, et al. The anti-atherosclerotic effect of tanshinol borneol ester using fecal metabolomics based on liquid chromatography-mass spectrometry. Analyst. 2016;141(3):1112–20. Epub 2015/12/23. 10.1039/c5an01970b . [DOI] [PubMed] [Google Scholar]
- 76.Shen L, Yang Y, Ou T, Key CC, Tong SH, Sequeira RC, et al. Dietary PUFAs attenuate NLRP3 inflammasome activation via enhancing macrophage autophagy. J Lipid Res. 2017;58(9):1808–21. Epub 2017/07/22. 10.1194/jlr.M075879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bories GFP, Leitinger N. Macrophage metabolism in atherosclerosis. FEBS Lett. 2017;591(19):3042–60. Epub 2017/08/11. 10.1002/1873-3468.12786 . [DOI] [PubMed] [Google Scholar]
- 78.Miller DT, Ridker PM, Libby P, Kwiatkowski DJ. Atherosclerosis: the path from genomics to therapeutics. J Am Coll Cardiol. 2007;49(15):1589–99. Epub 2007/04/17. 10.1016/j.jacc.2006.12.045 . [DOI] [PubMed] [Google Scholar]
- 79.Teul J, Ruperez FJ, Garcia A, Vaysse J, Balayssac S, Gilard V, et al. Improving metabolite knowledge in stable atherosclerosis patients by association and correlation of GC-MS and 1H NMR fingerprints. J Proteome Res. 2009;8(12):5580–9. Epub 2009/10/10. 10.1021/pr900668v . [DOI] [PubMed] [Google Scholar]
- 80.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. 2010;3(2):207–14. Epub 2010/02/23. 10.1161/CIRCGENETICS.109.852814 . [DOI] [PubMed] [Google Scholar]
- 81.Turer AT, Stevens RD, Bain JR, Muehlbauer MJ, van der Westhuizen J, Mathew JP, et al. Metabolomic profiling reveals distinct patterns of myocardial substrate use in humans with coronary artery disease or left ventricular dysfunction during surgical ischemia/reperfusion. Circulation. 2009;119(13):1736–46. Epub 2009/03/25. 10.1161/CIRCULATIONAHA.108.816116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Magkos F, Mittendorfer B. Gender differences in lipid metabolism and the effect of obesity. Obstet Gynecol Clin North Am. 2009;36(2):245–65, vii. Epub 2009/06/09. 10.1016/j.ogc.2009.03.001 . [DOI] [PubMed] [Google Scholar]
- 83.Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation. 1999;99(9):1165–72. Epub 1999/03/09. . [DOI] [PubMed] [Google Scholar]
- 84.Kaplan JR, Adams MR, Clarkson TB, Manuck SB, Shively CA, Williams JK. Psychosocial factors, sex differences, and atherosclerosis: lessons from animal models. Psychosom Med. 1996;58(6):598–611. Epub 1996/11/01. . [DOI] [PubMed] [Google Scholar]
- 85.Fan S, Yeon A, Shahid M, Anger JT, Eilber KS, Fiehn O, et al. Sex-associated differences in baseline urinary metabolites of healthy adults. Scientific reports. 2018;8(1):11883 10.1038/s41598-018-29592-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kleemann R, Verschuren L, van Erk MJ, Nikolsky Y, Cnubben NH, Verheij ER, et al. Atherosclerosis and liver inflammation induced by increased dietary cholesterol intake: a combined transcriptomics and metabolomics analysis. Genome Biol. 2007;8(9):R200 Epub 2007/09/26. 10.1186/gb-2007-8-9-r200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Glisic M, Mujaj B, Rueda-Ochoa OL, Asllanaj E, Laven JSE, Kavousi M, et al. Associations of Endogenous Estradiol and Testosterone Levels With Plaque Composition and Risk of Stroke in Subjects With Carotid Atherosclerosis. Circ Res. 2018;122(1):97–105. Epub 2017/11/04. 10.1161/CIRCRESAHA.117.311681 . [DOI] [PubMed] [Google Scholar]
- 88.Montani JP, Carroll JF, Dwyer TM, Antic V, Yang Z, Dulloo AG. Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. Int J Obes Relat Metab Disord. 2004;28 Suppl 4:S58–65. Epub 2004/12/14. 10.1038/sj.ijo.0802858 . [DOI] [PubMed] [Google Scholar]
- 89.Tomas L, Edsfeldt A, Mollet IG, Perisic Matic L, Prehn C, Adamski J, et al. Altered metabolism distinguishes high-risk from stable carotid atherosclerotic plaques. Eur Heart J. 2018;39(24):2301–10. Epub 2018/03/22. 10.1093/eurheartj/ehy124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vorkas PA, Shalhoub J, Isaac G, Want EJ, Nicholson JK, Holmes E, et al. Metabolic phenotyping of atherosclerotic plaques reveals latent associations between free cholesterol and ceramide metabolism in atherogenesis. Journal of proteome research. 2015;14(3):1389–99. 10.1021/pr5009898 [DOI] [PubMed] [Google Scholar]
- 91.Cardellini M, Ballanti M, Davato F, Cardolini I, Guglielmi V, Rizza S, et al. 2-hydroxycaproate predicts cardiovascular mortality in patients with atherosclerotic disease. Atherosclerosis. 2018. [DOI] [PubMed] [Google Scholar]
- 92.Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta‐analysis of prospective studies. Journal of the American Heart Association. 2017;6(7):e004947 10.1161/JAHA.116.004947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vojinovic D, van der Lee SJ, van Duijn CM, Vernooij MW, Kavousi M, Amin N, et al. Metabolic profiling of intra-and extracranial carotid artery atherosclerosis. Atherosclerosis. 2018;272:60–5. 10.1016/j.atherosclerosis.2018.03.015 [DOI] [PubMed] [Google Scholar]
- 94.Jung S, Song S-W, Lee S, Kim SH, Ann S-j, Cheon EJ, et al. Metabolic phenotyping of human atherosclerotic plaques: Metabolic alterations and their biological relevance in plaque-containing aorta. Atherosclerosis. 2018;269:21–8. 10.1016/j.atherosclerosis.2017.11.034 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A two-way Venn diagram displaying the unique and shared metabolites (180) between MTBSTFA (N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide) and MSTFA (N-methyl-N-(trimethylsilyl)trifluoroacetamide with 1% trimethylchlorosilane), the two derivatization regents.
(TIF)
KEGG-based metabolic pathways covered using the 2D GC-MS platform. The network view generated from MetaboAnalyst and modified using Cytoscape, displays the biological pathways covered using the metabolites quantified using 2D GC-ToF-MS used in this study. Nodes (yellow) are pathways and edges (lines) connect them for their relatedness.
(TIF)
Metabolite abundance differences (not significant, P, < 0.05) in the 32 baboon serum samples that were outliers, when comapred to itnernal standard (ribitol) used for the analytical runs. In the box-plots, the center lines show the medians; box limits indicate the 25th and 75th percentiles as determined; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles, outliers are represented by dots; crosses represent sample means; data points are plotted as black dots. Widths of boxes are proportional to square roots of the number of observations.
(PDF)
Columns represent: baboon number; sex, age at 2 yr experimental endpoint, triglyceride change (mg/dL), heart fat score, heart upper ridge fat score, heart coronary artery fat score, kidney fat score, change in body weight (kgs), change in glucose (mg/dL), LDL-c change (mg/dL), triglyceride at 2 yrs (mg/dL), body weight at 2 yrs (kgs), glucose at 2 yrs (mg/dL) and LDL-c at 2 yrs (mg/dL).
(XLSX)
(XLSX)
(XLSX)
Provided are 1st and 2nd dimension retention times (seconds), similarity matching scores against EI spectral libraries, signal/noise, unique (quantifier) mass, probability, retention time matrix.
(XLSX)
P-values reported are nominal (P, <0.05).
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Table is displaying R, slope, T-statistics, and P-values.
(XLSX)
Data Availability Statement
The raw datasets and the metadata obtained from mass-spectrometry based 2D GC-ToF- MS metabolomics efforts are made publicly available at the deposited at the Global Natural Product Social Molecular Networking (GNPS) database (Study ID: MassIVE MSV000083256) and is available for download at this link: https://massive.ucsd.edu/ProteoSAFe/dataset.jsp?task=dd88e038114043f8830fd8b3e5ee77a6 (doi:10.25345/C5Z608).