Abstract
We have generated a high-density, high-throughput genotyping array for characterizing genome-wide variation in Arctic charr (Salvelinus alpinus). Novel single nucleotide polymorphisms (SNPs) were identified in charr from the Fraser, Nauyuk and Tree River aquaculture strains, which originated from northern Canada and fish from Iceland using high coverage sequencing, reduced representation sequencing and RNA-seq datasets. The array was designed to capture genome-wide variation from a diverse suite of Arctic charr populations. Cross validation of SNPs from various sources and comparison with previously published Arctic charr SNP data provided a set of candidate SNPs that generalize across populations. Further candidate SNPs were identified based on minor allele frequency, association with RNA transcripts, even spacing across intergenic regions and association with the sex determining (sdY) gene. The performance of the 86,503 SNP array was assessed by genotyping Fraser, Nauyuk and Tree River strain individuals, as well as wild Icelandic Arctic charr. Overall, 63,060 of the SNPs were polymorphic within at least one group and 36.8% were unique to one of the four groups, suggesting that the array design allows for characterization of both within and across population genetic diversity. The concordance between sdY markers and known phenotypic sex indicated that the array can accurately determine the sex of individuals based on genotype alone. The Salp87k genotyping array provides researchers and breeders the opportunity to analyze genetic variation in Arctic charr at a more detailed level than previously possible.
Introduction
Arctic charr (Salvelinus alpinus) has a Holarctic distribution spanning marine and freshwater ecosystems and is one of the most morphologically and ecologically diverse vertebrates [1,2]. The species is subdivided into several genetically differentiated phylogeographic groups, which are thought to have diverged in refugia during the early to mid-Pleistocene [1,3]. Arctic charr are of economic importance and are an attractive option for the expansion of aquaculture production at northern latitudes [4]. Characteristics such as early maturation, poor salinity tolerance and uneven growth limit current Arctic charr aquaculture production [5,6]. Improving the characterization of the Arctic charr genome will allow for detailed study of the genetic basis of these important traits and provide a starting point for selective breeding programs that aim to improve economically important aspects of the Arctic charr phenotype using genomic information.
Studies of the genetic architecture of traits and the discovery of quantitative trait loci (QTL) in Arctic charr have been limited by the relatively small numbers of available genetic markers [7–12]. Low cost methods for massively parallel genetic marker discovery through reduced representation sequencing [13,14] have resulted in the discovery of thousands of novel single nucleotide polymorphisms (SNPs) in Arctic charr and led to the creation of a 4,508 marker genetic linkage map for the Canadian Fraser strain [15]. The linkage map has been used to characterize the evolutionary history of Arctic charr chromosomes and identify homologous chromosomal regions in closely related salmonid species. A large suite of SNPs has also been identified through a transcriptomic analysis of salinity tolerance [16]. In addition to these genomic resources, a recently developed Arctic charr reference genome assembly and transcriptome annotation [17] have allowed for the identification of orthologous genes between Arctic charr, other salmonids and northern pike (Esox lucius) that might provide insight on the adaptive divergence of salmonid species.
Further insights into the genetics and evolution of Arctic charr require a high-throughput, high-density genotyping array so that fish can be genotyped for a large number of markers in a cost-effective manner. High-density SNP genotyping assays (6K to 285K) for other salmonids such as rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar) [18–22] have been used successfully to determine the genetic basis of growth, maturation and disease resistance traits [23–28] and to characterize population structure [29,30]. Genotyping arrays have also been designed for other aquaculture species such as carp (Cyprinus carpio) [31] and some have also been designed to work on multiple species, such as those for Pacific and European oysters (Crassostrea gigas and Ostrea edulis) [32] and blue catfish and channel catfish (Ictalurus furcatus and I. punctatus) [33].
The creation of SNP arrays for aquaculture species follow previous developments in terrestrial livestock (such as poultry and cattle) and data from these arrays are now being successfully applied in genomic selection programs that improve the performance of aquaculture populations for important traits such as disease resistance [34–40]. Within Atlantic salmon and rainbow trout, GWAS based on genotyping array data have successfully identified QTL for important aquaculture traits such as fillet yield, growth and body mass and for Atlantic salmon also identified a single locus (vgll3) that controls variation in age at maturity [24–26,41–43]. Characterizing genome-wide variation within and across populations of Arctic charr using a genotyping array would pave the way for genome-wide association analyses (GWAS) and identification of the genetic basis of important aquaculture traits. Pairing accurate genotype information obtained from an array with knowledge of the Arctic charr genome [17] could also provide fundamental information about the distribution and evolution of functional genes as well as insights into differences in genomic architecture between Arctic charr and its close taxonomic relatives.
Our aims were to: (1) Expand the number of SNPs identified in Arctic charr; (2) Determine the position of SNPs within the genome identified through different molecular approaches for comparative analysis; (3) Design a SNP genotyping array that captures the diversity of Arctic charr by incorporating SNPs identified in a diverse suite of populations and (4) Design an array that contains SNPs located in functional genes and coverage of intergenic regions through the inclusion of markers that are evenly spaced throughout the genome. Putative SNPs were identified in fish from the three major Canadian aquaculture strains (Fraser, Nauyuk and Tree River) that were founded from populations in northern Canada [44] as well as Icelandic fish originating from two lakes (Þingvallavatn and Vatnshlíðarvatn) and populations in or near Lake Mývatn. Following the creation of the genotyping array, we tested its performance with samples from the same populations/strains used for SNP discovery as well as fish from additional Icelandic populations (lakes Galtaból, Mjóavatn, Mývatn, and Svínavatn; Fljótaá River) to discover the number of polymorphic array markers in the different groups. By designing the array using markers identified in different groups, we hoped to create a tool that could characterize genetic variation across the range of the species.
Materials and methods
Ethics statement
Animals were reared and sampled in compliance with the animal utilization protocols (AUP) #3174 and #2431, which were approved by the University of Guelph Animal Care Committee.
Sample information
The fish used for SNP discovery and testing of the array originated from aquaculture strains in Canada and natural populations in Iceland (Table 1). The Nauyuk and Tree River aquaculture strains were founded from adults obtained in the 1970’s and 1980’s from locations of the same name in Nunavut, Canada while the founders of the Fraser strain were collected from the Fraser River, Labrador Canada between 1980 and 1984 [44,45]. The Tree River and Nauyuk adults and families (pure strain and hybrids) used in the current study were obtained from Icy Waters, Ltd (Whitehorse, Yukon, Canada) while those from the Fraser strain were obtained from the Alma Aquaculture Research Station (Alma, Ontario, Canada) and the Coastal Zones Research Institute (CZRI) (Shippagan, New Brunswick, Canada). SNP discovery in the Icelandic fish was based on eight full-sib families produced from adults collected from the lakes Þingvallavatn and Vatnshlíðarvatn (see Parsons et al. 2011 [46] for details) and fish sampled from Lake Mývatn and 11 nearby lava caves. The array was tested on Icelandic fish sampled from six lakes (Galtaból, Mývatn, Mjóavatn, Svínavatn, Þingvallavatn and Vatnshlíðarvatn), a river (Fljótaá) and lava caves near Lake Mývatn. The Nauyuk and Tree River populations are part of the Arctic phylogeographic group, while the Fraser strain and Icelandic charr are part of the Atlantic phylogeographic group [1].
Table 1. Sources of fish, sequence data types used in the design and testing of the Arctic charr genotyping array.
| Source of fish | Methodology | Individuals | Structure | Publication |
|---|---|---|---|---|
| Fraser aquaculture strain (Coastal Zones Research Institute, New Brunswick, Canada) | GBS | 91 | Full sib family | Nugent et al. 2017 |
| mRNA-seq | 18 | Two full sib families | Norman et al. 2014 | |
| Fraser aquaculture strain (Alma Aquaculture Research Station) | GBS | 108 | Two half sib families | |
| Array testing | 33* | Population | ||
| Nauyuk aquaculture strain (Icy Waters, Yukon, Canada) | GBS | 24 | Population | |
| Array testing | 42† | Population | ||
| Nauyuk–Tree River aquaculture strain hybrids (Icy Waters) | RAD-seq | 238 | Nine hybrid Families | Christensen et al. 2018 |
| Fraser–Nauyuk aquaculture strain hybrids | RAD-seq | 67 | Two hybrid families | Christensen et al. 2018 |
| Nauyuk–Tree River aquaculture strain hybrids | High coverage sequencing | 8 | Population | Christensen et al. 2018 |
| Tree River aquaculture strain (Icy Waters) | Array testing | 18 | Population | |
| Lake Þingvallavatn Iceland | GBS | 320 | Four full sib families | Parsons et al. 2011 |
| Array testing | 95 | Population | ||
| Lake Vatnshlíðarvatn, Iceland | GBS | 362 | Four full sib families | Parsons et al. 2011 |
| Array testing | 64 | Population | ||
| Lake Mývatn, Iceland | GBS | 9 | Population | |
| Mývatn lava caves, Iceland | GBS | 39 | Population | |
| Array testing | 20⧧ | Population | ||
| Lake Galtaból, Iceland | Array testing | 57 | Population | |
| Lake Mjóavatn, Iceland | Array testing | 31 | Population | |
| Lake Svínavatn, Iceland | Array testing | 90 | Population | |
| River Fljótaá, Iceland | Array testing | 32 | Population |
* Three of the Fraser strain individuals in the test set were also parents of the two half sib families utilized in GBS in SNP discovery.
† Twenty-four of the Nauyuk strain individuals in the test set were also used in SNP discovery.
⧧All 20 of the Mývatn lava cave fish in the test set were also used for SNP discovery
SNP discovery
Candidate SNPs for the array were detected using a variety of sequencing methodologies. First, genotype-by-sequencing (GBS) [14] was performed on 951 individuals from multiple sources (Table 1). DNA was extracted from tissue using a commercial kit (Qiagen DNeasy Blood & Tissue) as per the manufacturer’s instructions. Samples were quantified using a Qubit Fluorometer and diluted to a concentration of 75ng/uL. For each individual, 30μl of sample was digested with the restriction enzyme EcoT22I and unique barcode adapters were ligated to the restriction cut sites. After unique barcodes were added, sequencing primers and the DNA samples from all individuals were pooled and amplified through the polymerase chain reaction (PCR) and sequenced (see Nugent et al. 2017 [15] for details).
After sequencing, raw fastq files were filtered for quality control in Trimmomatic using default parameters [47] (Version: Trimmomatic-0.36). Following quality control, data were analyzed using the software package Stacks for de novo SNP identification [48] (Version: 1.44). The subprograms of Stacks were implemented sequentially (process_radtags, ustacks, cstacks, sstacks using default parameters). For the Fraser and Icelandic families (Table 1), the inheritance of alleles could be tracked, so the Stacks ‘genotypes’ module was used to generate output information on SNP variation. The Stacks ‘populations’ module was used to generate genotype output data for the population samples (Nauyuk and Mývatn area), where the relationships of individuals were unknown.
The GBS dataset was processed with Stacks twice, the first time using a process_radtags trim parameter (-t) of 85 and the second time using a trim parameter of 40 (-t 40). This dual approach was used because a trim parameter of 85 caused stacks to eliminate any reads shorter than 85 bp in length. Previous analysis of GBS data in the production of the first generation Arctic charr SNP linkage map (NCBI sequence read archive (www.ncbi.nlm.nih.gov/sra) BioProject accession number #SRP026259 and BioSample accession numbers #SAMN06165956 and #SAMN06165957) [15] identified SNPs on sequences shorter than 85bp in length. Therefore, the lower cutoff threshold (40bp) was used to retain shorter reads in an attempt to observe the short read SNPs in newly sequenced individuals. To prevent redundancy, SNPs with identical polymorphisms and base pair sequences from the two Stacks analyses and the first generation linkage map [15] were identified and a single copy was retained.
SNPs were filtered in different ways depending on the source. Those derived from families were analyzed manually to remove SNPs that met one of the following criteria in all families: 1. >50% of progeny with missing genotypes; 2. detection of erroneous genotypes (e.g., presence of bb genotypes when parents had aa and ab genotypes); and 3. significant segregation distortion (analyzed in the linkmfex_V3 program ‘OneMap_Segregation_Distortion_Check’) [49]. Markers derived from population samples (Nauyuk and Mývatn area) were filtered to retain SNPs with observed minor allele frequencies > = 0.05. Finally, SNPs meeting the above criteria were retained only if the short DNA sequences [40–85 bp in length) containing the SNP aligned to a single location in the Arctic charr draft genome, as determined through a Burrows-Wheeler alignment (NextGene, SoftGenetics LLC). SNPs sequences that did not align or aligned to two or more locations were omitted.
Second, 11 families (nine Nauyuk x Tree River and two Fraser x Nauyuk hybrid families, Table 1 of Christensen et al. 2018 [17]) were RAD-sequenced (Methods section: ‘Data processing and genetic map construction’ in Christensen et al. 2018 [17]). SNPs that passed all quality control steps were used to construct a genetic linkage map and were added to the list of candidate markers.
Third, eight hybrid fish derived from crosses between the Tree River and Nauyuk strains were each sequenced on one lane of an Illumina HiSeq2500 (~40x coverage, paired-end sequencing) [17]. A Burrows-Wheeler alignment was performed to align raw paired-end reads (no filtering or trimming applied) to the Arctic charr draft genome. Within the program SAMtools, the Mpileup function was used with Bcftools to generate SNPs from the alignment data. SNPs were filtered based on the following criteria: filter = ‘.’, quality score for alternate assertion ≥ 20, RMS mapping quality ≥ 30, genotype quality ≥ 20, 1 ≤ depth ≤ 100. SNPs remaining after filtering (Table 6 in Christensen et al. 2018 [17]) were retained for the current analysis.
Fourth, SNPs were identified from a previous transcriptomic analysis of Fraser strain Arctic charr [16]. These SNPs were initially characterized during a de novo assembly that was performed using mRNA sequence libraries from 18 individuals. Briefly transcriptome assemblies were constructed in the Velvet-Oases software package using eight different k-mer lengths (33, 41, 49, 57, 65, 73, 81, 89) [50,51]. Contigs less than 300bp in length were removed and the assemblies were merged using the Oases-M module and a k-mer length of 105. CD-HT-EST [52] was used to cluster contigs where shorter sequences shared 95% identity within local alignments to larger sequences. SNPs were then retained only if the contig containing the SNP aligned to a single location in the Arctic charr draft genome, as determined through a Burrows-Wheeler alignment (NextGene, SoftGenetics LLC).
Selection of SNPs for the genotyping array
We first selected SNPs that had been detected by more than one sequencing platform (i.e., high coverage, GBS, RAD-seq, RNA-seq). These were considered as cross validated if SNPs in the two datasets were found at the same base pair position in the Arctic charr draft genome (precursor to the newest Arctic charr genome build, GenBank accession: GCA_002910315.2) [17] and if they had matching alleles. We used the draft genome as a reference during array design as the genome build was incomplete at the time. We next prioritized SNPs identified through GBS that met one of the following criteria: (a) SNP was detected in two populations, (b) SNP had a minor allele frequency >0.05 in a population, (c) SNP was segregating in two or more families. We filtered out most SNPs with G/C and A/T polymorphisms because these require twice as many assays on Affymetrix arrays and are therefore inefficient. However, we retained those in the Icelandic samples to maximize the number of polymorphisms observed in these individuals.
We next included markers that could be used to determine the genotypic sex of individuals. The eight libraries from the high coverage sequencing SNP data were compared to a partial transcript for the Arctic charr sex-determining gene, sdY (GenBank accession: JF826022.1), using Burrows-Wheeler Aligner (BWA) [53]. SAMtools Mpileup was used to call SNPs using the results from these BWA alignments [54].
We next focused on SNPs identified in the eight Nauyuk x Tree River hybrid individuals subjected to high coverage sequencing that had not been selected through cross validation. These SNPs were placed in a MySQL database and filtered based on the following initial parameters: depth (5 ≤ DP ≤ 45), quality score (QUAL ≥ 20), genotype quality (GQ ≥ 20) and mapping quality (MQ ≥ 30). In order to identify which SNPs fell within transcripts, Blastn was used to compare 101bp probes (SNP at bp 51) to the transcriptome from Christensen et al. [17] and SNPs were labeled based on their presence or absence within transcripts. The SNPs were compared to the transcriptome and not directly to the reference genome because the reference genome had not been finalized at the time of this analysis. SNPs from different contigs aligning to the same transcript were excluded due to the potential ambiguity. SNPs were then excluded if they had less than 35bp of flanking sequence on either side. A/T and G/C variants were filtered from the dataset and SNPs were split into rare (0.05 ≤ AF < 0.15) and common SNPs (0.15 ≤ AF ≤ 0.85). A set of rare intergenic and intragenic SNPs were selected to produce ~900Kb intervals between markers. Additionally, common SNPs not found in transcripts were selected to produce a set of common intergenic markers spaced at ~62Kb intervals. Finally, to fill the remaining room on the array, SNPs from the GBS, and RNA-seq datasets that had been successfully aligned to the draft genome but that were rejected in previous filtering steps due to a lack of available information (no minor allele frequencies or segregation data) or unsuccessful cross validation were included in the initial selection of SNPs. These SNPs were given the lowest priority in array design due to their lack of validation and putative nature.
Following the initial selection, 103,932 candidate SNPs were submitted to Affymetrix for review in 71-mer format, with both alleles for the SNP on the forward strand provided at base pair position 36. In silico analysis produced a probability of conversion to a reliable assay for each SNP (p-convert score). This returned a set of 80,786 SNPs (77.7%) from the initial submission with a ‘recommended’ or ‘neutral’ designation. To fill the remaining spots on the array, 13,912 additional intergenic common SNPs from the high coverage sequencing dataset were added and the revised set of candidate markers was resubmitted. After resubmission to Affymetrix for array tiling, the Salp87k array design with 86,503 SNPs was finalized (S1 File).
Following design and construction of the Arctic charr genotyping array, an additional Blast alignment was conducted to align the array SNPs to the final Arctic charr reference genome assembly (GenBank assembly accession: GCA_002910315.2) [17]. We also determined how well the Salp87k array was representing the genes within the genome. The positions of SNPs in the Arctic charr reference genome were compared to location of the 42,439 genes reported in genome annotation file (GenBank assembly accession: GCA_002910315.2) in order to count the number of genes that contained an array marker between their base pair start and end positions.
Testing of the genotyping array
To investigate the ability of the array to characterize the genetic diversity of divergent populations, SNP variation in a test set of 482 individuals including fish from the four groups (three aquaculture strains and wild fish from Iceland) was evaluated (Table 1). Three of the Fraser fish, 24 of the Nauyuk fish and 20 fish from the caves near Lake Mývatn in the test set were previously used for SNP discovery with GBS.
Aliquots of DNA were sent to the Clinical Genomics Centre at Mt. Sinai Hospital, Toronto, Canada and genotyped as per the manufacturer’s instructions. Genotypic data were imported into the Axiom Analysis Suite (Version 3.1.51) and filtered following the manufacturer’s ‘best practices workflow’ (diploid genome, filtered for dish quality control values >0.82, quality control call rate > 0.97 and average call rate for passing samples > 0.98). Genotypic data for the four groups were generated in separate Axiom Analysis Suite sessions, following the manufacturer’s ‘best practices workflow’. A recommended SNP was one whose genotype data met all quality control thresholds (Axiom™ Analysis Suite User Manual version 3.1). Recommended SNPs for each group were obtained and compared to one another to assess the number of assays that were polymorphic (and therefore informative) within the different groups. Finally, we validated the ability of the sdY associated markers to identify sex by comparing genotypes to phenotypic sex based on visual examination of the gonads in 446 of the test fish.
Results and discussion
SNP discovery and selection
Cross validation of SNPs between sequencing platforms and the filtering of GBS data produced a set of 19,587 SNPs that were given the highest priority in array design. Of these, 14,768 SNPs were cross validated between the high coverage sequencing and one of the smaller data sets (GBS, RAD-seq, RNA-seq) (Table 2). We detected no overlap in SNP identity among the smaller data sets. This is partially due to lower genome coverage and the use of different restriction enzymes in the two reduced representation sequencing data sets. Of the GBS-derived SNPs, 4,276 were cross validated between families from two or more populations but 1,733 of these had already been identified through cross platform validation leaving 2,443 for addition to the high priority list. The population samples subjected to GBS (Table 1) yielded 1,171 additional SNPs based on observed minor allele frequencies (>0.05 in at least one population). The remaining 1,205 SNPs were selected because they were observed in at least two Fraser strain families. Of the GBS-derived SNPs, 1,741 were omitted due to being A/T or G/C variants, leaving a final set of 17,846 high priority markers for array design.
Table 2. Summary of the number of candidate SNPs derived from each data source and the number of SNPs from each data source that were included in the final array design.
| Data Source | Number included on array | Candidates for array design | Conversion rate |
|---|---|---|---|
| High coverage sequencing & GBS cross validation | 3,149 | 5,451 | 57.8% |
| High coverage sequencing & RNA-seq cross validation | 368 | 583 | 63.1% |
| High coverage sequencing & RAD-seq cross validation | 3,875 | 8,734 | 44.36% |
| GBS | 6,046 | 14,959 | 40.5% |
| RNA-seq | 10,491 | 14,922 | 70.3% |
| High coverage sequencing | 62,568 | 59,277 + 13,912* | 85.5% |
| sdY markers | 6 | 6 | 100% |
| Totals | 86,503 | 117,844* | 73.4% |
*The initial candidate list was 103,932 SNPs. When this initial set of candidates failed to yield enough SNPs recommended for use on the array 13,912 high coverage SNPs were added to the list of candidates
In addition to the 17,846 high priority markers, the initial candidate marker set included SNPs from the high coverage sequencing data (59,277), the sex associated markers (6) and non-cross validated markers from the GBS and RNA-seq datasets (26,803) for a total of 103,932 markers. The high coverage sequencing dataset yielded the largest set of SNPs in the initial submission but was constructed using sequence data from only eight individuals. This meant that there was relatively low-resolution allele frequency information available to inform decisions about which markers to include. Care was taken to assess the genomic location of the SNPs from these eight individuals and to select SNPs that represented as many genes as possible and also provide even coverage of intergenic regions. We aimed to directly represent as many genes as possible on the array so that future analyses utilizing the array, such as genome-wide association studies, could accurately identify potential causative genes associated with important SNPs. The lack of validation of most of the SNPs from the eight individuals means that we cannot rule out the possibility that the observed polymorphisms could be the result of sequencing error or other non-biological causes. Thus, these were considered putative in nature prior to validation through assessment of array performance.
The different data sources utilized in SNP discovery were complimentary, providing detail on marker frequency and segregation in populations (GBS, RAD-seq) or high depth of coverage and genomic context (RNA-seq, high coverage sequencing) (Table 2). SNPs from the reduced representation sequencing methods had the highest quality supporting information (allele frequencies, observed segregation) but were the least abundant data source. Alternatively, the high coverage sequencing data had a large library of SNPs to select from, but the supporting information was scant (allele frequencies based on just 8 individuals). By using SNPs from these different data sources, we were able to select the best candidates for array design and give them the highest priority for inclusion on the array.
Genotyping array performance
For each of the four groups (Fraser strain, Nauyuk strain, Tree River strain, Icelandic), more than 62,000 SNPs were recommended for use by the Axiom Analysis Suite and identified as either monomorphic or polymorphic (Table 3). It is important to note that different subsets of the markers on the array were recommended for use within the different groups. In total, 79,692 of the SNPs on the array were recommended for use within at least one of the four groups. Possible reasons for a SNP not being recommended for use in none of the groups include: the existence of off target SNP variants in the analyzed individuals, poor SNP call rates, or other sequence differences between the array probe set for the given SNP and the DNA sequence of the individuals being genotyped. These issues can be strain-specific, therefore causing certain markers to be recommended for use within Arctic charr derived from one strain and not recommended for individuals of a different strain.
Table 3. The number of polymorphic and monomorphic SNPs observed within the different test groups of Arctic charr.
The number of polymorphisms unique to each strain (Unique to strain) and the number shared with at least one other strain (Multiple strains) are shown. ‘Total recommended’ indicates markers classified as ‘recommended’ by the Axiom Analysis Suite’s best practices workflow. Polymorphic markers include the Axiom Analysis Suite, ‘polymorphic high resolution’ and ‘no minor homozygote’. Across the four groups, 79,692 unique SNPs were recommended for use.
| Fraser | Nauyuk | Tree River | Icelandic | ||
|---|---|---|---|---|---|
| Polymorphic | Unique to strain | 1,864 (9.4%) |
10,924 (24.2%) |
8,551 (22.3%) |
1,864 (13.1%) |
| Multiple strains | 17,898 | 34,250 | 29,865 | 12,329 | |
| Total observed | 19,762 | 45,174 | 38,416 | 14,193 | |
| Monomorphic | 42,898 | 25,151 | 25,531 | 54,602 | |
| Total Recommended | 62,660 | 70,325 | 63,947 | 68,795 |
For each group, between 14,000 and 46,000 polymorphic markers were identified. The highest number of polymorphic markers was observed in the Nauyuk strain (45,174; 64.2% of the recommended SNPs), while the lowest number of SNPs was seen in the Icelandic fish (14,193; 20.6%), despite the larger number of fish genotyped. This pattern was expected, as a large number of the SNPs included in the design of the array were identified from the Nauyuk and Tree River strains as the result of high coverage sequencing. The discovery and selection of SNPs for inclusion on the array could have been improved and the utility of the array maximized by analyzing all of the population samples with the high coverage sequencing method. However, it appears that the array is still able to characterize variation within Fraser and Icelandic fish, albeit to a lesser extent. Reduced representation sequencing, although yielding fewer markers in the Fraser and Icelandic fish, did provide more representative estimates of minor allele frequency and other metrics of SNP efficacy than the high coverage sequencing dataset.
Population specificity
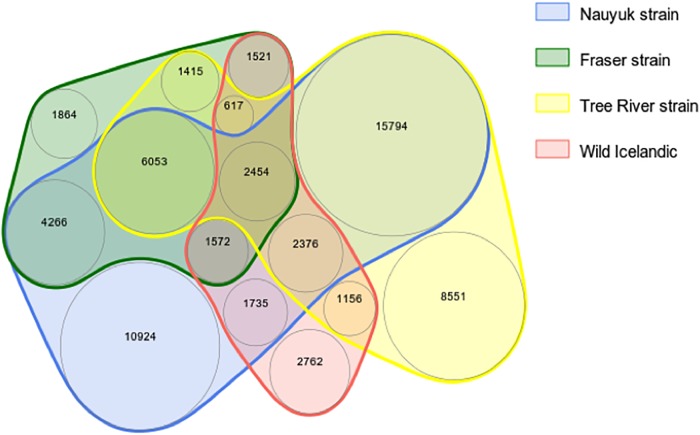
A total of 63,060 polymorphic markers were observed (72.9% of the markers on the array) across the four populations of Arctic charr in the test set (Fig 1, Table 3, S3 File). Of the total, 36.8% (22,203) were polymorphic within only one of the four groups, while 63.2% were polymorphic in multiple groups. This suggests that the Salp87k genotyping array is an effective tool for characterizing genetic variation within populations as well as for differentiation among populations. The 23,440 array SNPs that were not verified as informative within any of the four groups may include some SNPs that are not true biological polymorphisms. As more individuals are genotyped with the array, we will be able to better characterize the number of true SNPs on the array, as well as the number of putative SNPs that were included in the final design that fail to yield biologically relevant information in any circumstances.
Fig 1. A Venn diagram depicting the number of unique and shared polymorphic SNPs across the four groups of Arctic charr in the test set.
In total, 63,060 of the 86,503 SNPs on the Salp87k genotyping were identified as polymorphic while 21,785 were polymorphic within only one of the groups.
The percentage of SNPs shared between groups appeared to be a function of geographic separation rather than phylogeographic grouping (S3 File). The two populations in the closest proximity (from the same phylogeogaphic group) showed the greatest percentage of shared markers by far. Of the 56,913 total unique polymorphic markers from the Nauyuk and Tree River populations, 46.9% of SNPs (26,677) were polymorphic within both groups. The two groups derived from the Atlantic phylogeographic group (Fraser and Icelandic) had a lower percentage of shared SNPs (22.2%), which was similar to that between the Fraser and Tree River strains even though they belong to different phylogeographic groups.
The array is likely to be of value for the study of cultured and wild populations of Arctic charr in Canada and Iceland. Results of the test set showcase the ability of the array to characterize genetic variation in the three major Canadian aquaculture strains for use in selective breeding programs. Moreover, since these strains were founded relatively recently (1974–1988), it is possible that the array could be effective at characterizing genetic variation in wild Canadian populations. However, array performance in wild fish would need to be tested given that the aquaculture strains were created with small numbers of founders [44,45] and therefore may not be genetically representative of wild populations. The array was also able to capture genetic variation in the Icelandic populations studied but less optimally. Given that less genetic information was available from Icelandic individuals during the design of the array, SNPs from Icelandic individuals were prioritized in an effort to optimize performance in the genotyping of these fish. Even though the numbers of Icelandic test individuals far outnumbered those from the three Canadian aquaculture strains, they had the lowest number of observed polymorphic SNPs (~15 K). However, this number is suitable for many population genetic/genomic applications but would be less optimal for fine scale genomic analyses. Thus, it may be necessary to develop a location specific array, similar to what has occurred in Atlantic salmon [18–20] for certain applications such as genomic selection.
Genome coverage
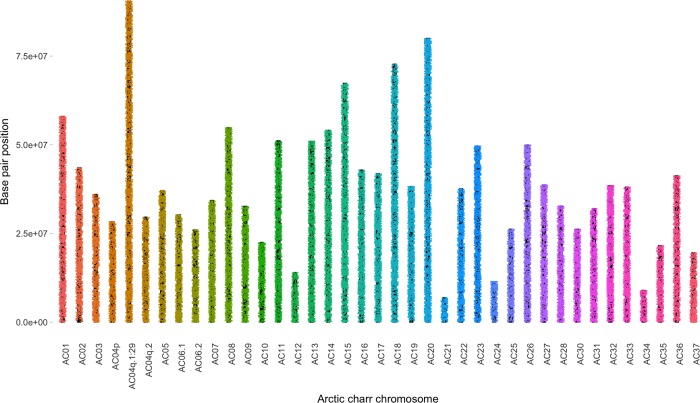
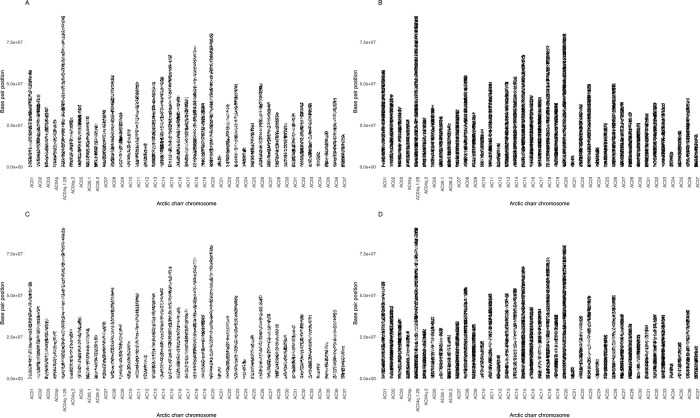
Of the 86,503 markers on the array, 84,920 (98.2%) were successfully positioned to a single location on the new Arctic charr reference genome assembly (GenBank assembly accession: GCA_002910315.2, S2 File). 58,495 of these were distributed across the 39 chromosomes (Table 4), for an average of 38.5 markers per megabase of chromosome sequence (Fig 2, Table 4). When chromosomes were partitioned into 1Mb segments for subsequent analyses, only 3 segments on the entire genome did not contain a marker on the array. The three 1Mb segments of chromosome with no SNP were: AC01 between 58-59Mb, AC03 between 36–37 Mb, and AC06.2 between 26-27Mb. Across the whole test set, a polymorphic marker was observed every 34Kb of chromosome sequence. The average interval between polymorphic markers was lowest in the Nauyuk strain (48Kb) and higher in the Tree River (59Kb), Fraser (109Kb) and Icelandic groups (157Kb) (Fig 3). This indicates that the array provides a genome-wide characterization of genetic variation with only a few regions on the chromosomes being underrepresented.
Table 4. Summary table of Arctic charr reference genome coverage (GCA_002910315.2) by markers included on the 87k Arctic charr SNP genotyping array.
| Chromosome name | NCBI Accession number |
Number of markers | Average base pair gap between markers (kilobase pairs) |
Total chromosome length (kilobase pairs) |
Average markers per megabase |
|---|---|---|---|---|---|
| AC1 | NC_036838.1 | 2275 | 25.5 | 58017 | 39.2 |
| AC2 | NC_036839.1 | 1641 | 26.5 | 43539 | 37.7 |
| AC3 | NC_036840.1 | 1525 | 23.6 | 36001 | 42.4 |
| AC4p | NC_036841.1 | 1035 | 27.3 | 28293 | 36.6 |
| AC4q.1:29 | NC_036842.1 | 3230 | 28.0 | 90519 | 35.7 |
| AC4q.2 | NC_036843.1 | 1082 | 27.3 | 29596 | 36.6 |
| AC5 | NC_036844.1 | 1414 | 26.2 | 37081 | 38.1 |
| AC6.1 | NC_036845.1 | 1351 | 22.4 | 30249 | 44.7 |
| AC6.2 | NC_036846.1 | 937 | 27.7 | 26025 | 36.0 |
| AC7 | NC_036847.1 | 1449 | 23.7 | 34303 | 42.2 |
| AC8 | NC_036848.1 | 2117 | 25.9 | 54842 | 38.6 |
| AC9 | NC_036849.1 | 1285 | 25.4 | 32654 | 39.4 |
| AC10 | NC_036850.1 | 924 | 24.3 | 22457 | 41.1 |
| AC11 | NC_036851.1 | 1768 | 28.9 | 51124 | 34.6 |
| AC12 | NC_036852.1 | 468 | 29.8 | 13981 | 33.5 |
| AC13 | NC_036853.1 | 1923 | 26.5 | 50975 | 37.7 |
| AC14 | NC_036854.1 | 1961 | 27.6 | 54096 | 36.3 |
| AC15 | NC_036855.1 | 2682 | 25.1 | 67329 | 39.8 |
| AC16 | NC_036856.1 | 1623 | 26.4 | 42871 | 37.9 |
| AC17 | NC_036857.1 | 1721 | 24.3 | 41841 | 41.1 |
| AC18 | NC_036858.1 | 2617 | 27.8 | 72741 | 36.0 |
| AC19 | NC_036859.1 | 1692 | 22.6 | 38229 | 44.3 |
| AC20 | NC_036860.1 | 3350 | 23.9 | 79996 | 41.9 |
| AC21 | NC_036861.1 | 292 | 23.6 | 6905 | 42.3 |
| AC22 | NC_036862.1 | 1445 | 26.0 | 37604 | 38.4 |
| AC23 | NC_036863.1 | 1814 | 27.3 | 49633 | 36.5 |
| AC24 | NC_036864.1 | 443 | 25.7 | 11433 | 38.7 |
| AC25 | NC_036865.1 | 962 | 27.2 | 26198 | 36.7 |
| AC26 | NC_036866.1 | 1943 | 25.7 | 49931 | 38.9 |
| AC27 | NC_036867.1 | 1491 | 26.0 | 38733 | 38.5 |
| AC28 | NC_036868.1 | 1213 | 27.0 | 32734 | 37.1 |
| AC30 | NC_036869.1 | 1029 | 25.4 | 26194 | 39.3 |
| AC31 | NC_036870.1 | 1351 | 23.7 | 32007 | 42.2 |
| AC32 | NC_036871.1 | 1594 | 24.1 | 38481 | 41.4 |
| AC33 | NC_036872.1 | 1503 | 25.3 | 38085 | 39.5 |
| AC34 | NC_036873.1 | 311 | 28.7 | 8959 | 34.7 |
| AC35 | NC_036874.1 | 909 | 23.7 | 21596 | 42.1 |
| AC36 | NC_036875.1 | 1327 | 31.0 | 41233 | 32.2 |
| AC37 | NC_036876.1 | 798 | 24.5 | 19547 | 40.8 |
Fig 2. Visual representation of the distribution of the array markers among the 39 chromosomes of the Arctic charr genome (GCA_002910315.2).
The black bars in the background represent the chromosome sequence, and each dot represents the location of SNP on the Arctic charr genotyping array. A total of 58,495 SNPs from the array are located along the chromosomes, while 26,425 additional SNPs are found on the unplaced contigs.
Fig 3. The distribution of polymorphic markers across the Arctic charr genome identified in four test groups.
A: Fraser strain, Panel B: Nauyuk strain, Panel C: Tree River strain, Panel D: Icelandic.
The 26,425 markers from the array not located on the chromosomes were distributed across 15,216 unplaced contigs. Of these unplaced contigs, 55.7% (8,471) contained one or more array SNPs, while 44.3% (6,744) were not represented by any SNPs on the array. The 55.7% of unplaced contigs represented by one or more SNP on the genotyping array comprise 91.6% of the sequence data within the unplaced contigs (598.5Mb out of 653.5Mb total) indicating that the smallest unplaced contigs were not well represented (S2 File).
The number of polymorphisms observed in the genome’s 15,216 unplaced contigs was sparser than within the 39 chromosomes. The percentage of contigs that had one or more polymorphic loci varied among strains (Icelandic—2,721 contigs, 17.9%; Fraser– 3,090 contigs, 20.3%; Tree River– 4,975 contigs, 32.7% and Nauyuk– 5,468 contigs, 35.9%). Thus, genetic diversity across these unplaced regions was not as well represented as across the chromosomes. This is likely in part due to the small size of these contigs relative to the chromosomes (chromosome N50: 1.02Mb, contig N50: 55.6Kb) [17]. Future efforts should focus on incorporating these contigs into the chromosomes so that they can be placed in the proper genomic context and better represented in future analyses of the genome.
Distribution of SNPs within genes
Of the 42,439 gene entries, 22,433 genes had one or more array SNP present between their start and end positions. This indicates that 52.8% of the genes in the genome were directly represented by a SNP on the array, with between 15% and 47% of these genes possessing a polymorphic SNP among the four test groups (S3 File). This relatively sparse coverage of the genes is partially the result of the annotated genome not being available at the time of array design. The Blastn alignment of the SNP sequences to the transcriptome provided some information on which SNPs could be used to represent genes, but a SNP representative for each gene (which also passed all Affymetrix quality control metrics) could not always be identified. Even though not all genes are directly represented by a SNP on the array, the overall coverage of the genome (average of 38.5 markers per megabase of chromosome sequence) and known locations of SNPs does provide a means of associating genes of interest with nearby segregating markers.
Sex determination
The genotypes for the 6 SNPs present in the sdY gene accurately predicted sex for all 463 individuals with known phenotypic sex (S3 File). The Salp87k array can therefore be used to accurately determine the sex of individuals without the need for conducting a separate analysis to genotype individuals for the sdY gene [55]. Sexing fish with the new array is not intended to be a direct replacement for the established method [55], which costs considerably less and is much faster. The major benefit of including the sdY markers on the array is that sex can be determined routinely while performing other analyses. Importantly, the sdY markers accurately determined sex in both North American and Icelandic Arctic charr, even though the location of the sdY gene is not conserved across these populations [9,15,56]. Since the sdY markers are associated with the sdY gene transcript, their performance was not influenced by the translocation position of the sdY gene in the Arctic charr genome.
Conclusions
We have produced a new 87k Affymetrix Axiom genotyping array for Arctic charr and demonstrated the effective characterization of genetic variation across three Canadian aquaculture strains and several wild Icelandic populations. The array yields 14-46k polymorphic markers in each population, which is similar to documented performance of other generalist arrays that accommodate multiple species or divergent populations (range: 5–48% polymorphic array assays) [32,33]. This indicates that the Salp87k genotyping array is a generalist that provides lower amounts of information than specialized arrays (range: 83–93% polymorphic array assays), but information can be provided for wider variety of populations through the mixture of population specific and general SNPs [22]. Overall the array provides the ability to characterize both within and across population genetic diversity as well as genetic sex and it can be employed in analysis of the genetic basis of quantitative traits, the structure and pedigree of wild populations and the study of the evolutionary divergence of wild populations.
Supporting information
(TSV)
Base pairs given indicate the location of the SNP, and the INFO column contains the 71mer sequence (SNP at base pair position 36) utilized in the array design.
(VCF)
(DOCX)
Acknowledgments
We thank Andre Dumas, Claude Pelletier and Rodrigue Yossa (CZRI) with their help in obtaining the necessary fish and funding to build and test the array and the Alma Aquaculture Research Station, CZRI and Icy Waters Ltd. for supplying the fish used in this study. Rico Law, Amber Garber and Matthieu Renner provided invaluable assistance with obtaining and processing samples from Icy Water’s Ltd. Finally, we thank Amsale Belay at the Clinical Genomics Centre at Mt. Sinai Hospital, Toronto for valuable advice and excellent service and Affymetrix for assistance in the array design.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This project was funded by an Natural Sciences and Engineering Research Council of Canada (NSERC) Strategic Grant “Integration of Genomic Resources into an Arctic Charr Broodstock Program” to BK and WSD, an NSERC Collaborative Research and Development Grant “ The Development of Fast Growing, Late Maturing and Salinity Tolerant Strains of Arctic charr, with Icy Water’s Ltd., Valores (formally Coastal Zones Research Institute), Ridgeland Aqua Farms Ltd., Acadian Fish Farm Ltd., the Atlantic Innovation Fund/Atlantic Canada Opportunities Agency “Aquaculture Development and Profitable commercialization of Arctic Charr in Canada.”
References
- 1.Brunner PC, Douglas MR, Osinov A, Wilson CC, Bernatchez L. Holarctic phylogeography of Arctic charr (Salvelinus alpinus L.) inferred from mitochondrial DNA sequences. Evolution. 2001;55(3):573–86. [DOI] [PubMed] [Google Scholar]
- 2.Klemetsen A. The Charr Problem Revisited: Exceptional Phenotypic Plasticity Promotes Ecological Speciation in Postglacial Lakes. Freshwater Reviews. 2010;3(1):49–74. [Google Scholar]
- 3.Wilson CC, Hebert PDN, Reist JD, Dempsont JB, Dempson JB. Phylogeography and postglacial dispersal of arctic charr Salvelinus alpinus in North America. Molecular Ecology. 1996;5(2):187–97. [Google Scholar]
- 4.Sæther B-S, Siikavuopio SI, Thorarensen H, Brännäs E. Status of arctic charr (Salvelinus alpinus) farming in Norway, Sweden and Iceland. Journal of Ichthyology. 2013;53(10):833–9. [Google Scholar]
- 5.Jobling M, Jørgensen EH, Arnesen AM, Ringø E. Feeding, growth and environmental requirements of Arctic charr: a review of aquaculture potential. Aquacult Int. 1993. September 1;1(1):20–46. [Google Scholar]
- 6.François NRL, Jobling M, Carter C. Finfish Aquaculture Diversification. CABI; 2010. 703 p. [Google Scholar]
- 7.Somorjai IML, Danzmann RG, Ferguson MM. Distribution of temperature tolerance quantitative trait loci in Arctic Charr (Salvelinus alpinus) and inferred homologies in rainbow trout (Oncorhynchus mykiss). Genetics. 2003;165(November):1443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woram RA, McGowan C, Stout JA, Gharbi K, Ferguson MM, Hoyheim B, et al. A genetic linkage map for Arctic char (Salvelinus alpinus): evidence for higher recombination rates and segregation distortion in hybrid versus pure strain mapping parents. Genome / National Research Council Canada = Genome / Conseil national de recherches Canada. 2004;47(2):304–15. [DOI] [PubMed] [Google Scholar]
- 9.Moghadam HK, Poissant J, Fotherby H, Haidle L, Ferguson MM, Danzmann RG. Quantitative trait loci for body weight, condition factor and age at sexual maturation in Arctic charr (Salvelinus alpinus): Comparative analysis with rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar). Molecular Genetics and Genomics. 2007;277(6):647–61. 10.1007/s00438-007-0215-3 [DOI] [PubMed] [Google Scholar]
- 10.Quinn NL, McGowan CR, Cooper GA, Koop BF, Davidson WS. Identification of genes associated with heat tolerance in Arctic charr exposed to acute thermal stress. Physiological Genomics. 2011;43(11):685–96. 10.1152/physiolgenomics.00008.2011 [DOI] [PubMed] [Google Scholar]
- 11.Norman JD, Robinson M, Glebe B, Ferguson MM, Danzmann RG. Genomic arrangement of salinity tolerance QTLs in salmonids: A comparative analysis of Atlantic salmon (Salmo salar) with Arctic charr (Salvelinus alpinus) and rainbow trout (Oncorhynchus mykiss). BMC Genomics. 2012;13(1):420–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiasson MA, Quinton CD, Danzmann RG, Ferguson MM. Comparative analysis of genetic parameters and quantitative trait loci for growth traits in Fraser strain Arctic charr (Salvelinus alpinus) reared in freshwater and brackish water environments. Journal of Animal Science. 2013;91(5):2047–56. 10.2527/jas.2012-5656 [DOI] [PubMed] [Google Scholar]
- 13.Davey JW, Hohenlohe PA, Etter PD, Boone JQ, Catchen JM, Blaxter ML. Genome-wide genetic marker discovery and genotyping using next-generation sequencing. Nature Reviews Genetics. 2011;12(7):499–510. 10.1038/nrg3012 [DOI] [PubMed] [Google Scholar]
- 14.Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, et al. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE. 2011;6(5):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nugent CM, Easton AA, Norman JD, Ferguson MM, Danzmann RG. A SNP Based Linkage Map of the Arctic Charr (Salvelinus alpinus) Genome Provides Insights into the Diploidization Process After Whole Genome Duplication. G3: Genes, Genomes, Genetics. 2017;7(2):543–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norman JD, Ferguson MM, Danzmann RG. Transcriptomics of salinity tolerance capacity in Arctic charr (Salvelinus alpinus): a comparison of gene expression profiles between divergent QTL genotypes. Physiological Genomics. 2014;46(4):123–37. 10.1152/physiolgenomics.00105.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen KA, Rondeau EB, Minkley DR, Leong JS, Nugent CM, Danzmann RG, et al. The Arctic charr (Salvelinus alpinus) genome and transcriptome assembly. PLOS ONE. 2018. September 13;13(9):e0204076 10.1371/journal.pone.0204076 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Lien S, Gidskehaug L, Moen T, Hayes BJ, Berg PR, Davidson WS, et al. A dense SNP-based linkage map for Atlantic salmon (Salmo salar) reveals extended chromosome homeologies and striking differences in sex-specific recombination patterns. BMC Genomics. 2011;12(1):615–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houston RD, Taggart JB, Cézard T, Bekaert M, Lowe NR, Downing A, et al. Development and validation of a high density SNP genotyping array for Atlantic salmon (Salmo salar). BMC Genomics. 2014;15:90–90. 10.1186/1471-2164-15-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yáñez JM, Naswa S, López ME, Bassini L, Correa K, Gilbey J, et al. Genomewide single nucleotide polymorphism discovery in Atlantic salmon (Salmo salar): validation in wild and farmed American and European populations. Molecular Ecology Resources. 2016;16(4):1002–11. 10.1111/1755-0998.12503 [DOI] [PubMed] [Google Scholar]
- 21.Correa K, Lhorente JP, López ME, Bassini L, Naswa S, Deeb N, et al. Genome-wide association analysis reveals loci associated with resistance against Piscirickettsia salmonis in two Atlantic salmon (Salmo salar L.) chromosomes. BMC Genomics. 2015;16(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palti Y, Gao G, Liu S, Kent MP, Lien S, Miller MR, et al. The development and characterization of a 57K single nucleotide polymorphism array for rainbow trout. Molecular Ecology Resources. 2015;15(3):662–72. 10.1111/1755-0998.12337 [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez AP, Lubieniecki KP, Fukui S, Withler RE, Swift B, Davidson WS. Detection of quantitative trait loci (QTL) related to grilsing and late sexual maturation in Atlantic salmon (Salmo salar). Marine Biotechnology. 2014;16(1):103–10. 10.1007/s10126-013-9530-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutierrez AP, Yáñez JM, Fukui S, Swift B, Davidson WS. Genome-wide association study (GWAS) for growth rate and age at sexual maturation in Atlantic salmon (Salmo salar). Plos One. 2015;10(3):e0119730–e0119730. 10.1371/journal.pone.0119730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai HY, Hamilton A, Guy DR, Tinch AE, Bishop SC, Houston RD. Verification of SNPs associated with growth traits in two populations of farmed atlantic salmon. International Journal of Molecular Sciences. 2015;17(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez-Pena D, Gao G, Baranski M, Moen T, Cleveland BM, Brett Kenney P, et al. Genome-wide association study for identifying loci that affect fillet yield, carcass, and body weight traits in rainbow trout (Oncorhynchus mykiss). Frontiers in Genetics. 2016;7(NOV). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Correa K, Bangera R, Figueroa R, Lhorente JP, Yanez JM. The use of genomic information increases the accuracy of breeding value predictions for sea louse (Caligus rogercresseyi) resistance in Atlantic salmon (Salmo salar). Genetics, Selection, Evolution : GSE. 2017;49(1):15–15. 10.1186/s12711-017-0291-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vallejo RL, Liu S, Gao G, Fragomeni BO, Hernandez AG, Leeds TD, et al. Similar genetic architecture with shared and unique quantitative trait loci for bacterial cold water disease resistance in two rainbow trout breeding populations. Frontiers in Genetics. 2017;8(OCT):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Vallejo RL, Palti Y, Gao G, Marancik DP, Hernandez AG, et al. Identification of single nucleotide polymorphism markers associated with bacterial cold water disease resistance and spleen size in rainbow trout. Frontiers in Genetics. 2015;6(SEP):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbey J, Cauwelier E, Coulson MW, Stradmeyer L, Sampayo JN, Armstrong A, et al. Accuracy of assignment of Atlantic salmon (Salmo salar L.) to rivers and regions in Scotland and northeast England based on single nucleotide polymorphism (SNP) markers. PLoS ONE. 2016;11(10):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Zhao Z, Zhang X, Zheng X, Li J, Jiang Y, et al. Development and evaluation of the first high-throughput SNP array for common carp (Cyprinus carpio). BMC Genomics. 2014. April 24;15:307 10.1186/1471-2164-15-307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutierrez AP, Turner F, Gharbi K, Talbot R, Lowe NR, Peñaloza C, et al. Development of a medium density combined-species SNP array for Pacific and European oysters (Crassostrea gigas and Ostrea edulis). G3: Genes, Genomes, Genetics. 2017;7(7):2209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu S, Sun L, Li Y, Sun F, Jiang Y, Zhang Y, et al. Development of the catfish 250K SNP array for genome-wide association studies. BMC research notes. 2014;7:135–135. 10.1186/1756-0500-7-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matukumalli LK, Lawley CT, Schnabel RD, Taylor JF, Allan MF, Heaton MP, et al. Development and characterization of a high density SNP genotyping assay for cattle. PLoS ONE. 2009. April;4(4):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kranis A, Gheyas AA, Boschiero C, Turner F, Yu L, Smith S, et al. Development of a high density 600K SNP genotyping array for chicken. BMC Genomics. 2013;14:59–59. 10.1186/1471-2164-14-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vallejo RL, Leeds TD, Gao G, Parsons JE, Martin KE, Evenhuis JP, et al. Genomic selection models double the accuracy of predicted breeding values for bacterial cold water disease resistance compared to a traditional pedigree-based model in rainbow trout aquaculture. Genetics Selection Evolution. 2017;49(1):17–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida GM, Bangera R, Carvalheiro R, Correa K, Figueroa R, Lhorente JP, et al. Genomic Prediction Accuracy for Resistance Against Piscirickettsia salmonis in Farmed Rainbow Trout. G3: Genes, Genomes, Genetics. 2017. December 18;8(2):719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bangera R, Correa K, Lhorente JP, Figueroa R, Yáñez JM. Genomic predictions can accelerate selection for resistance against Piscirickettsia salmonis in Atlantic salmon (Salmo salar). BMC genomics. 2017. December;18(1):121 10.1186/s12864-017-3487-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robledo D, Matika O, Hamilton A, Houston RD. Genome-Wide Association and Genomic Selection for Resistance to Amoebic Gill Disease in Atlantic Salmon. G3: Genes, Genomes, Genetics. 2018. April 1;8(4):1195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai HY, Hamilton A, Tinch AE, Guy DR, Bron JE, Taggart JB, et al. Genomic prediction of host resistance to sea lice in farmed Atlantic salmon populations. Genetics Selection Evolution. 2016. June 29;48(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayllon F, Kjærner-Semb E, Furmanek T, Wennevik V, Solberg MF, Dahle G, et al. The vgll3 locus controls age at maturity in wild and domesticated Atlantic salmon (Salmo salar L.) males. PLoS Genetics. 2015;11(11):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barson NJ, Aykanat T, Hindar K, Baranski M, Bolstad GH, Fiske P, et al. Sex-dependent dominance at a single locus maintains variation in age at maturity in salmon. Nature. 2015;000(7485):1–4. [DOI] [PubMed] [Google Scholar]
- 43.Christensen KA, Gutierrez AP, Lubieniecki KP, Davidson WS. TEAD3, implicated by association to grilsing in Atlantic salmon. Aquaculture. 2017. October;479:571–8. [Google Scholar]
- 44.Lundrigan T a., Reist JD, Ferguson MM. Microsatellite genetic variation within and among Arctic charr (Salvelinus alpinus) from aquaculture and natural populations in North America. Aquaculture. 2005;244(1–4):63–75. [Google Scholar]
- 45.Blackie CT, Morrissey MB, Danzmann RG, Ferguson MM. Genetic divergence among broodstocks of Arctic charr Salvelinus alpinus in eastern Canada derived from the same founding populations. Aquaculture Research. 2011;42(10):1440–52. [Google Scholar]
- 46.Parsons KJ, Sheets HD, Skúlason S, Ferguson MM. Phenotypic plasticity, heterochrony and ontogenetic repatterning during juvenile development of divergent arctic charr (Salvelinus alpinus). Journal of Evolutionary Biology. 2011;24:1640–52. 10.1111/j.1420-9101.2011.02301.x [DOI] [PubMed] [Google Scholar]
- 47.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Catchen J, Hohenlohe PA, Bassham S, Amores A, Cresko WA. Stacks: An analysis tool set for population genomics. Molecular Ecology. 2013;22(11):3124–40. 10.1111/mec.12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Danzmann RG. LINKMFEX: linkage analysis package for out-crossed families with male and female exchange of the mapping parrent (Internet). 2018. https://uoguelphca-my.sharepoint.com/:f:/g/personal/rdanzman_uoguelph_ca/EjW14Zxt43RAqoHe6_t5QvMBqwkT3dfYzmfzmMdnU7hJXA
- 50.Zerbino DR, Birney E. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Research. 2008;18(5):821–9. 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schulz MH, Zerbino DR, Vingron M, Birney E. Oases: Robust de novo RNA-seq assembly across the dynamic range of expression levels. Bioinformatics. 2012;28(8):1086–92. 10.1093/bioinformatics/bts094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006. July 1;22(13):1658–9. 10.1093/bioinformatics/btl158 [DOI] [PubMed] [Google Scholar]
- 53.Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009. July 15;25(14):1754–60. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25(16):2078–9. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yano A, Guyomard R, Nicol B, Jouanno E, Quillet E, Klopp C, et al. An immune-related gene evolved into the master sex-determining gene in rainbow trout, Oncorhynchus mykiss. Current Biology. 2012;22(15):1423–8. 10.1016/j.cub.2012.05.045 [DOI] [PubMed] [Google Scholar]
- 56.Küttner E, Moghadam HK, Skúlason S, Danzmann RG, Ferguson MM. Genetic architecture of body weight, condition factor and age of sexual maturation in Icelandic Arctic charr (Salvelinus alpinus). Molecular Genetics and Genomics. 2011;286(1):67–79. 10.1007/s00438-011-0628-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TSV)
Base pairs given indicate the location of the SNP, and the INFO column contains the 71mer sequence (SNP at base pair position 36) utilized in the array design.
(VCF)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.