Abstract
Objectives:
Treatment-resistant depression is a common clinical occurrence among patients with major depressive disorder (MDD), but its neurobiology is poorly understood. We used data collected as part of routine clinical care to study white matter integrity of the brain’s limbic system and its association to treatment response.
Methods:
Electronic medical records of multiple large New England hospitals were screened for patients with an MDD billing diagnosis, and natural language processing was subsequently applied to find those with concurrent diffusion-weighted images, but without any diagnosed brain pathology. Treatment outcome was determined by review of clinical charts. MDD patients (n=29 non-remitters, n=26 partial-remitters, and n=37 full-remitters), and healthy control subjects (n=58) were analyzed for fractional anisotropy (FA) of the fornix and cingulum bundle.
Results:
Failure to achieve remission was associated with lower FA among MDD patients, statistically significant for the medial body of the fornix. Moreover, global and regional-selective age-related FA decline was most pronounced in patients with treatment-refractory, non-remitted depression.
Conclusions:
These findings suggest that specific brain microstructural white matter abnormalities underlie persistent, treatment-resistant depression. They also demonstrate the feasibility of investigating white matter integrity in psychiatric populations using legacy data.
Keywords: Major depressive disorder (MDD), diffusion tensor imaging (DTI), natural language processing (NLP), fornix, limbic system, medical records, treatment-outcome
1. Introduction
Limbic and prefrontal cortical networks crucial for emotional processing are reported to be dysfunctional in some individuals with major depressive disorder (MDD) (Fales et al., 2008); abnormal white matter fibers within these networks may underlie disrupted emotional processing. White matter integrity in major depressive disorder was first evaluated in structural magnetic resonance imaging (MRI) studies, which showed increased rate and severity of white matter hyperintensities (de Groot et al., 2000). Furthermore, associations have been found between white matter hyperintensities and lower rate of treatment response in geriatric (Gunning-Dixon et al., 2010) and in younger depressed subjects (Iosifescu et al., 2006). Of note, such hyperintensities have been associated with white matter tract disruptions as measured in higher apparent diffusion coefficient and lower fractional anisotropy (FA) in diffusion tensor imaging (DTI) studies (Taylor et al., 2001).
The movement of water molecules in the brain is constrained by natural barriers, such as myelin sheaths and nerve fibers. By measuring the direction and magnitude of restricted water molecule movement, DTI can provide information on the organization and orientation of white matter fibers (Basser et al., 1994). Previous DTI studies have reported on temporal and frontal FA abnormalities in young, middle-aged, and older adults with major depressive disorder (Ma et al., 2007; Shimony et al., 2009; Kieseppa et al., 2010), but studies evaluating treatment outcome are inconclusive (Alexopoulos et al., 2008; Taylor et al., 2008).
In this study, we used the DTI-derived FA to quantify white matter integrity primarily in two important limbic tracts: the fornix and cingulum bundle, chosen due to their importance in the interconnectivity and output of the limbic system (and especially the hippocampus), and their previous implication in MDD. The cingulum bundle is a 5-7 mm diameter fiber bundle that connects all parts of the limbic system: From medial regions of the frontal and parietal lobes to parahippocampal and adjacent temporal cortical regions. It further lies within the white matter of the cingulate gyrus that is frequently reported to be structurally and functionally abnormal in major depressive disorder (Fitzgerald et al., 2008; Koolschijn et al., 2009). Structural abnormalities in the cingulum bundle have previously been recognized in unipolar (Schermuly et al., 2010) and bipolar (Benedetti et al., 2011) depression, as well as in adults with a history of childhood abuse (Choi et al., 2009). The fornix is a compact bundle that projects from the hippocampus to the anterior thalamus and mamillary bodies, and serves as the main output for the hippocampus, a structure consistently reported to be implicated in affect regulation (Konarski et al., 2008). Although the fornix is clearly visible on MRI, few studies have investigated its role in pathological processes in psychiatric conditions (Thomas et al., 2011).
Using clinical and diffusion data previously collected as part of routine clinical treatment (legacy data), we hypothesized that alterations in white matter microstructure in the limbic tracts studied, as measured with FA, would be associated with poor long-term treatment outcomes (e.g., failure to achieve remission over an extended period). To our knowledge this is the first study to explore white matter FA in the limbic system of MDD patients using legacy data.
2. Methods
2.1. Subjects
For this retrospective chart review study, ninety-two patients with MDD and fifty-eight healthy control subjects, aged 16 to 75 years, were selected from the electronic medical record system with the aid of computerized tools, billing diagnoses, and diagnostic codes, a method previously described and validated by members of the same research group (Perlis et al., 2011). In short, depressed patients had at least one episode of MDD (ICD-9 296.2x or 296.3x) and were excluded if they had 1) any comorbid Axis I psychiatric disorder, such as schizophrenia or bipolar disorder (but anxiety disorders were allowed, with the exception of OCD), 2) any diagnosis of neurologic disorder, including brain tumors, stroke, dementia, status epilepticus, seizure disorder, CNS vasculitis and multiple sclerosis, 3) substance abuse. Control subjects were free of any mental and neurologic disorder.
2.2. Data acquisition
A detailed description of can be found elsewhere (Perlis et al., 2011). In short, research in major depressive disorder is one of the driving biology projects in which the i2b2 (Informatics for Integrating Biology & the Bedside, http://www.i2b2.org) provides technical expertise (Murphy et al., 2007) to identify, process, and mine the electronic medical record. Data in this study were collected using an i2b2 “datamart” extracted from the Research Patient Data Registry (RPDR; http://rc.partners.org/rpdr) with the i2b2 Workbench software (i2b2 v1.4, Boston, MA, USA) and natural language processing (NLP) technology. i2b2 datamarts are created as project-specific defined extracts from the RPDR.
The RPDR is a centralized data warehouse that stores clinical information, such as billing codes, sociodemographic data, and laboratory results acquired from medical records of inpatient clinical systems, billing systems, laboratory systems, and outpatient electronic medical record systems at Partners HealthCare (Boston, MA, USA). Data in the repository exists for more than 4 million patients (Murphy et al., 2010).
NLP is a technique for extracting specific data embedded in free text based on researcher-defined criteria in the form of keywords and grammatical rules (Zeng et al., 2006). We used NLP to gather qualitative information from clinicians’ electronic notes to verify the billing diagnosis of major depressive disorder (Perlis et al., 2011), and to verify the lack of diagnosed pathology in brain MRIs (i.e., read as “normal” or “unremarkable” in the radiology notes).
MDD patients with no diagnosed pathology on brain MRIs were assigned a clinical outcome status by an experienced research psychiatrist (DVI) by review of clinical charts of the 12 months interval preceding the MRI. The following clinical outcome categories were used: 1) “remitted depression” (full response, patient does not feel depressed and is back to normal), 2) “partial-remitted depression” (fluctuating response or an intermediate state representing partial response not sufficient for remission), and 3) “non-remitted depression” (no response, patient feels persistently depressed after 12 months or more of treatment).
The Institutional Review Boards of Partners Healthcare System and Harvard Medical School approved all aspects of this study.
2.3. Reasons for MRI exam
Subjects enrolled in this study were scanned for several reasons. The majority had MRIs to rule out brain pathology after experiencing transient and remitted clinical symptoms (e.g., transient aphasia, ataxia, vertigo, altered mental state, one-sided transient sensation loss or numbness, syncope, fainting or dizziness) (n=58, 35%). Other patients (n=44, 29%) had non-specific clinical symptoms and received “routine imaging” to rule out any brain pathology. A significant number of patients had primary symptoms of recurrent headaches and/or migraines (n=39, 26%), whereas some patients had a history of non-CNS neoplasms (e.g. breast or lung cancer) and were examined to rule out brain tumors (n=14, 9%). Each subject’s exam was reviewed by a clinical radiologist at the time of scanning and explicitly diagnosed as “normal” or “unremarkable MRI of the brain” in the radiology report. Exam reasons did not differ by group (X2=15.43, df=12, p=0.22; See Table 1 for more details).
Table 1:
Demographic and clinical characteristics of MDD patients classified by long-term treatment outcome, and healthy control subjects
| Major depressive disorder patients |
||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Non-remitters (N=29) | Partial-remitters (N=26) | Full-remitters (N=37) | Healthy control subjects (N=58) | ||||
| Age (years) | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 51.0 | 16.0 | 46.1 | 15.5 | 42.6 | 12.4 | 43.8 | 16.4 | |
| Female | N | % | N | % | N | % | N | % |
| 19 | 66 | 16 | 62 | 23 | 62 | 24 | 41 | |
| Racea | N | % | N | % | N | % | N | % |
| Caucasian | 25 | 86 | 19 | 76 | 30 | 86 | 50 | 86 |
| African American | 1 | 3 | 1 | 4 | 0 | 0 | 2 | 3 |
| Hispanic | 3 | 10 | 3 | 12 | 5 | 14 | 2 | 3 |
| Other | 0 | 0 | 2 | 8 | 0 | 0 | 4 | 7 |
| MRI exam indicationb | N | % | N | % | N | % | N | % |
| 1. Assess for transient aphasia, ataxia, vertigo, altered mental state, one-sided transient sensation loss or numbness | 10 | 35 | 7 | 27 | 9 | 24 | 18 | 31 |
| 2. Routine imaging | 8 | 28 | 6 | 23 | 12 | 32 | 18 | 31 |
| 3. Chronic headaches and/or migraines | 7 | 24 | 8 | 31 | 13 | 35 | 11 | 19 |
| 4. Rule out brain metastasis | 1 | 3 | 3 | 12 | 0 | 0 | 10 | 17 |
| 5. Assess for transient syncope, dizziness, fainting | 3 | 10 | 2 | 8 | 3 | 8 | 1 | 2 |
| Medicationsc | Median | IQR | Median | IQR | Median | IQR | Median | IQR |
| Unique antidepressants | 2 | 2-3 | 3 | 1-4 | 2 | 1-2 | n/a | n/a |
| Total prescriptions | 3 | 2-4 | 4 | 1-6 | 2 | 1-3 | n/a | n/a |
| SSRI prescriptions | 1 | 1-1 | 1 | 1-2 | 1 | 1-1 | n/a | n/a |
| Non-SSRI prescriptions | 2 | 1-3 | 2 | 1-2 | 1 | 1-1 | n/a | n/a |
Abbreviations: MDD, major depressive disorder; N, number of subjects; SD, standard deviation; IQR, interquartile range; n/a, not applicable.
Caucasians were overrepresented in each group (p<0.001).
Exam reasons did not differ by group (X2=15.43, df=12, p=0.22), and all exams were concluded unremarkable.
Antidepressant medications prescribed within 12 months prior to MRI. See supplemental Table S1 for more details on antidepressant prescriptions among MDD patients. Refractory patients were free of any ECT and TMS therapy in the 12 months prior to MRI exam.
2.4. Image acquisition
MRI scans used in this analysis were acquired at Massachusetts General Hospital as part of routine clinical treatment between 1999 and 2009. Diffusion-weighted images (DWIs) with no diagnosed pathology were included only if the following minimum acceptable acquisition criteria were met: 1) 1.5 Tesla, 2) at least 6 diffusion directions, 3) b=1000 s/mm2, and 4) axial plane acquisition.
The majority of subjects had clinical DWIs acquired that comprised 24 axial slices (median; IQR=2 slices) parallel to the AC-PC line covering whole brain, in 6 diffusion directions with b=1000 s/mm2 and with 1 baseline scan with b=0 s/mm2 (7 gradients=53% of study sample; 21% had 35 gradients, 19% had 28 gradients, 5% had 22 gradients, and 16, 23, 39, and 70 gradients comprised less than 1% of the sample). The scan parameters were as follows: FOV 22 cm (median; IQR=0 cm), 128×128 matrix (median; IQR=0 rows x columns), 6 mm slice thickness, producing 1.7×1.7×6mm voxels (median; IQR=0 mm). Scan acquisition did not differ by group for any of the parameters (X2<29.28, p’s>0.11).
2.5. Image analyses
Image data were transferred to a workstation at the Psychiatry Neuroimaging Laboratory (http://pnl.bwh.harvard.edu) and explored for the presence of diffusion-weighted images and verified for acquisition requirements. To quantify FA, we used the software package 3DSlicer (http://www.slicer.org), which provides tools such as diffusion tensor estimation, diffusion tensor scalar measurements, and it also allows for the viewing of ROIs in all three planes simultaneously. Once FA maps of the diffusion tensor were calculated for each subject, atlas-based white matter segmentation was applied, a method described elsewhere (Mori et al., 2008; Oishi et al., 2008). In short, a stereotaxic probabilistic white matter atlas (ICBM DTI-81, http://www.loni.ucla.edu/Atlases/) that fuses DTI-based white matter information with an anatomical template was used to create a hand-segmented white matter parcellation map (WMPM) for which partition criteria were derived from histology-based atlases. Because anatomical images were not available for each subject, as opposed to diffusion-weighted images, we used DWI data to transfer the WMPM fiber tracts into subject DTI space. More specifically, we used a multi-stage registration procedure to optimize the normalization results. First, the atlas template and corresponding parcellation map were sequentially coregistered to each subject’s FA map using a 6-parameter affine registration, a 12-parameter affine registration, followed by non-linear registration utilizing the FLIRT and FNIRT registration tools from the FMRIB Software Library (http://www.fmrib.ox.ac.uk/). Finally, the atlas fiber tracts were resampled into subject DTI space using a nearest neighbor interpolation algorithm and FA was calculated (see Figure 1 A-D for a brief overview of normalization steps). We carefully inspected each registration result and we excluded poor registrations (N=18) from further analyses.
Fig. 1.
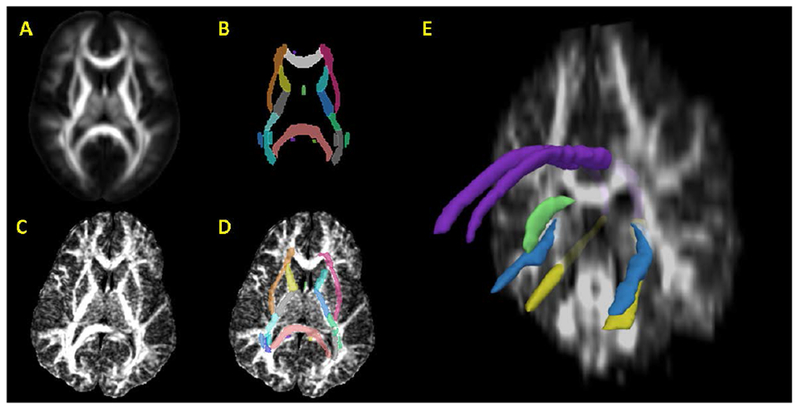
Volumes and regions-of-interest used for FA measurement. From the stereotaxic probabilistic white matter atlas (ICBM-DTI-81; figure A), a hand-segmented white matter parcellation map (WMPM; figure B) was created. The probabilistic white matter atlas was coregistered to each subject’s FA map (figure C) and the WMPM was subsequently resampled into each subject’s image space (figure D). In the WMPM, the cingulum is separated at the axial level of the splenium of the corpus callosum, resulting in cingulum at the level of the cingulate gyrus (dorsal cingulum) and cingulum at the level of the hippocampus (ventral cingulum). The fornix is isolated at the central level of the septum (medial fornix) and in the hippocampal area (lateral fornix). Figure E represents a coronal-plane subject FA map with a region-of-interest model reconstruction, including dorsal cingulum (purple), ventral cingulum (yellow), lateral fornix (light blue), and medial body of the fornix (green).
Normalized white matter labelmaps were used to quantify FA in subregions of the fornix and cingulum bundle (see Figure 1E). The sum of FA in these regions comprised a cumulative (or global) measure of the limbic system.
2.6. Antidepressant medications
Using the i2b2 datamart, we collected information on antidepressants prescribed in the 12 month interval preceding the MRI (see Table 1 in the text for a brief summary and Table S1 in the data supplement for a detailed summary of antidepressant prescriptions). There was no additional temporal relationship between the MRI acquisition (ROI measurement) and the onset of antidepressant treatment in the 12 months interval preceding the MRI, because the collection of legacy data was part of ongoing and routine clinical care. The large number of different antidepressant medications precluded use of antidepressant treatment as a covariate in primary analyses, but we did exploratory analyses comparing the FA results among subjects taking different classes of antidepressants. Refractory patients were free of any electroconvulsive therapy (ECT) or transcranial magnetic stimulation (TMS) in the 12 months preceding the MRI exam.
2.7. Statistical analyses
To test for effects of hemisphere on bilateral white matter tracts in the present study, a repeated measures analysis of covariance (ANCOVA) was performed with study cohort as between-subject factor, hemisphere and tract as within group factors, and age as a covariate. Regions-of-interest (ROI) that showed no hemispheric differences in FA were merged into a single ROI and recalculated for FA.
In primary analyses, we compared FA results among subject groups (i.e., MDD full-remitters, MDD partial-remitters, MDD non-remitters, and control subjects) and between genders (male, female) using multivariate analysis of covariance (MANCOVA), age as covariate, and with factor by covariate interactions. Age-related effects on tract FA were further evaluated with Pearson’s correlations and the equality of the correlation coefficients was compared using Fisher’s Z transformation.
In secondary analyses aiming to detect the impact of chronic SSRI antidepressant treatment on brain morphology, we compared FA results between MDD patients on long-term SSRI treatment and MDD patients with no SSRI exposure in binary logistic regression, adjusted for age, gender, and clinical outcome status.
Multivariate analyses were controlled for multiple comparisons with Bonferroni corrections. The level of significance was set to p<0.05 for all aforementioned statistical analyses.
All analyses were conducted with SPSS 17.0 (Chicago, IL).
3. Results
3.1. Subject enrolment
The RPDR query to create the i2b2 “major depressive disorder datamart” resulted in 108275 patients with a billing diagnosis for major depressive disorder (MDD) of which 5224 had concurrent MRI data and no history of comorbid mental or neurologic disorder. Natural language processing subsequently selected 880 patients with brain MRI scans and with no diagnosed brain pathology, including 320 patients with multiple clinical notes available describing treatment outcome. Finally, a total of 150 subjects (MDD patients, N=92; control subjects, N=58), with a mean age of 45.3±15.4 years (55% female), met all clinical and imaging criteria for further analysis. See Figure S1 in the data supplement for a detailed description of subject enrolment.
3.2. Clinical and demographic characteristics
The 92 study eligible MDD patients were classified as follows: 37 (40%) were full-remitters, 26 (28%) were partial-remitters, and 29 (32%) were non-remitters (Table 1). All study cohorts were matched for age (p=0.12) and race (p=0.66), although Caucasians were overrepresented in each cohort (p<0.001). There was a non-significant (p=0.08) increase in number of females with MDD (62-66%) compared to controls (41%), in agreement with female overrepresentation in most MDD studies (Wisniewski et al., 2009). Further, compared to full-remitters, non-remitters had non-significant increases in the number of antidepressants (p=0.08) and antidepressant classes (p=0.14) prescribed (Table 1), in agreement with clinical guidelines for treating persistent depression using switching and augmentation strategies (Nierenberg et al., 2008).
3.3. Inter-hemispheric and intra-tract differences
Following repeated measures ANCOVA for bilateral tracts, no group by hemisphere (F=0.13, p=0.94), hemisphere by tract (F=1.31, p=0.27), or group by hemisphere by tract interactions were found (F=0.72, p=0.63). Therefore, a mean tract FA was calculated for bilateral tracts.
We further tested for intra-tract differences, resulting in a subregion by tract interaction (F=10.81, df=1, 147, p=0.001). Paired-samples t-tests indicated that lateral fornix and dorsal cingulum measured significantly higher FA than medial fornix and ventral cingulum, respectively (t>8.17, df=149, p<0.001). Therefore, we treated limbic subregions separately in primary analysis, resulting in four regional ROIs (medial fornix, lateral fornix, dorsal cingulum, ventral cingulum), and one global ROI (cumulative FA of regional regions-of-interest).
3.4. Treatment outcome and global FA
Numerically lower cumulative FA was noticed for MDD non-remitters, MDD partial-remitters, and MDD full-remitters compared to control subjects (i.e., −9.9%, −5.3% and −2.4% respectively; Figure 2), although this did not reach statistical significance among groups (F=1.67, df=3, 141, p=0.18) or between genders (F=0.36, df=1, 141, p=0.55). Age was a significant covariate in these analyses (F=19.86, df=1, 141, p=1.68×10−5). There were no significant gender by age, or treatment outcome by age or gender interactions (F<2.38, p>0.05).
Fig. 2.
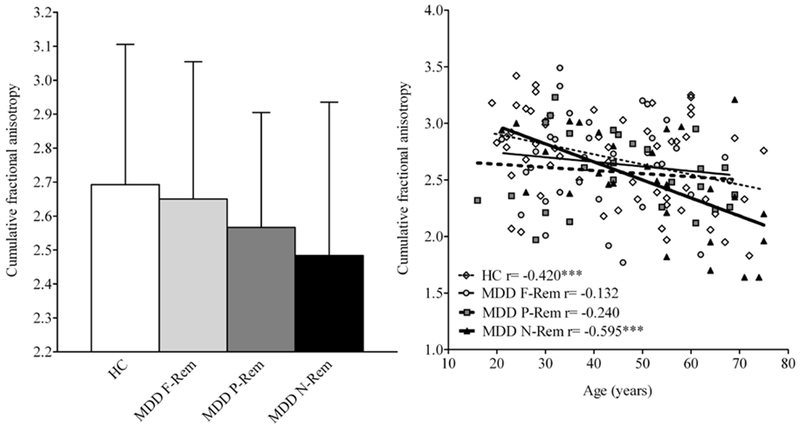
Bar diagram with cumulative FA (left figure) and scatter plot correlating cumulative FA across the age range (16-75 years; right figure) among MDD patients classified by long-term treatment outcome and healthy control subjects. Left: Numerically lower cumulative FA was noticed for non-remitters, partial-remitters, and full-remitters compared to control subjects (i.e.,−9.9%, −5.3%, and −2.4%, respectively), although this did not reach statistical significance among groups (p=0.18). Right: Age was a significant covariate (F=19.86, df=1, 141, p=1.68×10−5) in the MANCOVA model, and follow up with Pearson’s correlations showed statistically significant reduction in global FA with increasing age for MDD non-remitters and healthy control subjects, but not for MDD full-remitters or MDD partial-remitters. Fisher’s Z transformation showed stronger, linear decrease in global FA with age for non-remitters compared to full-remitters (p<0.05), partial-remitters (p<0.10, trend), but not control subjects (p=0.16). Abbreviations: HC, Healthy Control; MDD, Major depressive disorder; F-Rem, Full-remittters; P-Rem, Partial-remitters, N-Rem, Non-remitters.
Pearson correlations showed statistically significant reduction in global FA with increasing age for non-remitters (r=−0.59, p<0.01) and control subjects (r=−0.42, p<0.01) (Figure 2). Fisher’s Z transformation showed larger decrease in global FA with age for non-remitters compared to full-remitters (Z=2.12, p<0.05) and a trend for larger decrease compared to partial-remitters (Z=1.54, p<0.10), but not control subjects (Z=1.00, p=0.16).
3.5. Treatment outcome and regional FA
The medial fornix revealed a significant main effect for group (F=4.28, df=3, 141, p=0.006) and gender (F=10.94, df=1, 141, p=0.001), as well as a significant covariate effect for age (F=27.00, df=1, 141, p=7.00×10−7), but there were no significant gender by age, or treatment outcome by age or gender interactions (F<2.81, p>0.01) (Figure 3 and supplementary Table S2). Bonferroni-adjusted post-hoc analyses revealed that, compared to control subjects, non-remitters had 19.2% lower FA (p=0.009), whereas partial-remitters and full-remitters had non-significant lower FA (12.5%, p=0.24 and 12.3%, p=0.13, respectively). Females had higher FA than males (p=0.001).
Fig. 3.
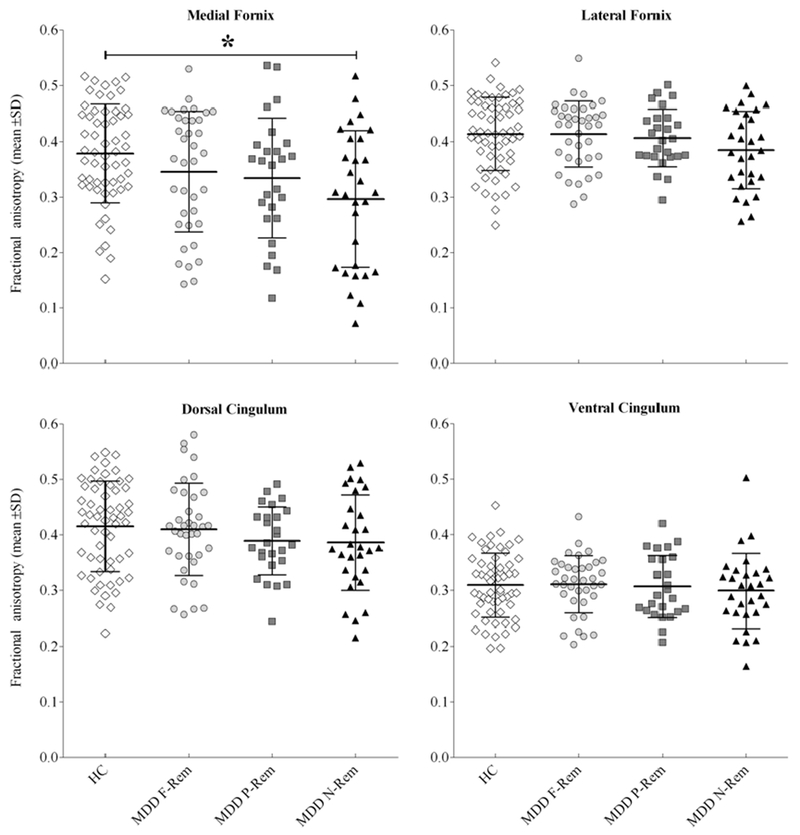
Scatter plot of fractional anisotropy by region-of-interest among MDD patients classified by long-term treatment outcome and healthy control subjects. Each data point represents one individual, and bars represent group mean±SD. Failure to achieve remission was associated with lower FA among MDD patients, statistically significant for the medial fornix. Bonferroni-adjusted post-hoc analyses revealed that, compared to control subjects, MDD non-remitters had 19.2% lower FA (p=0.009), whereas MDD partial-remitters and MDD full-remitters had numerically lower FA (12.5%, p=0.24 and 12.3%, p=0.13, respectively). See supplemental table 2 for more details on fractional anisotropy by region-of-interest and patient cohort. Abbreviations: HC, Healthy Control; MDD, Major depressive disorder; F-Rem, Full-remittters; P-Rem, Partial-remitters, N-Rem, Non-remitters. *F=4.28, df=3, 141, p=0.006.
The other regions of the fornix and cingulum bundle did not reveal significant main or interaction effects, but age as a covariate in the model was associated with the dorsal cingulum (F=5.16, df=1, 141, p=0.025), ventral cingulum (F=6.18, df=1, 141, p=0.014), and lateral fornix (F=12.53, df=1, 141, p=5.42×10−4).
Reduction in FA with increasing age was found for all tracts in each study cohort, except for the dorsal cingulum in partial-remitters (Figure 4). Among depressed patients, only non-remitters showed strong and significant age-related FA decline (r between −0.51 and −0.54, p<0.01), except for the ventral cingulum (r=−0.28, p>0.05). Fisher’s Z transformation revealed stronger, linear decrease in dorsal cingulum FA with age for non-remitters compared to partial-remitters (Z=2.82, p<0.01), full-remitters (Z=2.18, p<0.05), and control subjects (Z=1.50, p<0.10, trend). See Table S3 in the data supplement for a detailed summary of correlation coefficients by ROI among major depressive disorder patients and control subjects.
Fig. 4.
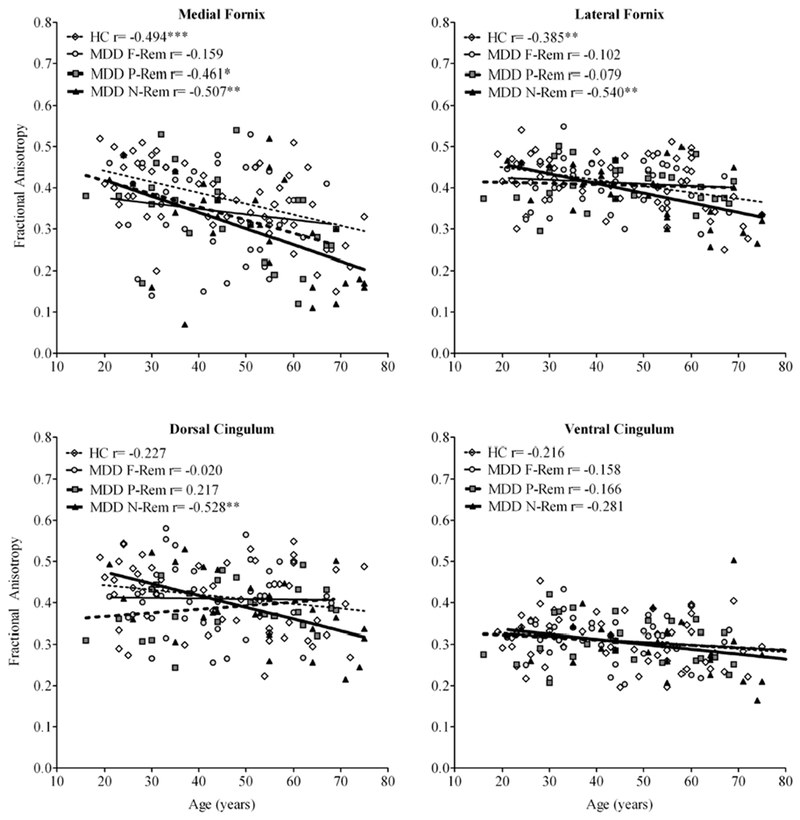
Scatter plots of fractional anisotropy across the age range (16-75 years) by region-of-interest among MDD patients classified by long-term treatment outcome and healthy control subjects. Age revealed a significant covariate-effect for the medial and lateral fornix (p<0.001), and dorsal and ventral cingulum bundle (p<0.05) in MANCOVA. Follow-up correlational analyses indicated lower FA with increasing age for all regions-of-interest in each patient cohort (except for the dorsal cingulum in partial-remitters), but in particular for non-remitters. See supplemental table 3 for full details on correlations between fractional anisotropy and age, by region-of-interest and patient cohort.
Fisher’s Z transformation revealed stronger, linear decrease in medial fornix FA with age for MDD non-remitters compared to MDD full-remitters (p<0.10, trend), but not MDD partial remitters or control subjects (p>0.10). Fisher’s Z transformation revealed stronger, linear decrease in lateral fornix FA with age for MDD non-remitters compared to MDD partial-remitters (p<0.05), MDD full-remitters (p<0.05), but not control subjects (p>0.10). Fisher’s Z transformation revealed larger linear decrease in dorsal cingulum FA with age for MDD non-remitters compared to MDD partial-remitters (p<0.01), MDD full-remitters (p<0.05), and control subjects (p<0.10, trend). Fisher’s Z transformation revealed no significant differences in ventral cingulum between correlations among groups. *p<0.05, **p<0.01, ***p<0.001. Abbreviations: HC, Healthy Control; MDD, Major depressive disorder; F-Rem, Full-remittters; P-Rem, Partial-remitters, N-Rem, Non-remitters.
3.6. Antidepressant medication effects on FA
We explored FA results between MDD patients that were solely prescribed SSRIs (N=19) versus non-SSRIs (N=13). In logistic regression, SSRI treatment was not associated with significant changes in global or regional FA measures compared to treatment with non-SSRI antidepressants (p>0.05), and this result remained non-significant after step-wise adjusting for age, gender and treatment outcome.
4. Discussion
In this study we investigated fractional anisotropy of limbic white matter fibers in a large sample of MDD patients classified by long-term treatment outcome, and control subjects. Our results suggest lower global and regional FA are associated with failure to achieve remission, statistically significant for the medial body of the fornix. Furthermore, global and regional selective age-related FA decline was most prominent for patients with persistent depression. It is estimated that 1-out-of-2 individuals treated for MDD will fail to achieve full remission after adequate dose and duration of at least two antidepressant treatments (Fava, 2003), making persistent depression a common clinical occurrence among MDD patients. Our findings contribute to the growing body of literature suggesting that specific brain microstructural white matter abnormalities underlie poor response to antidepressant treatments (Iosifescu et al., 2006; Sheline et al., 2010; Zhou et al., 2010).
A novel aspect of our study is the use of legacy data. This approach has advantages in efficiency and feasibility because the data has already been collected and the sheer size of electronic medical record systems may potentially result in large study samples. Moreover, study results may have increased ecological validity (i.e., representing “real patients”, not just volunteers in a research study) and reduced sampling bias. Given however the limitations of this approach discussed later in this paper, we believe that the use of legacy data will not replace studies with prospectively acquired data, but rather complement the methods for data acquisition to study questions of scientific interest related to psychiatric populations, especially in situations where prospective studies may be impractical, time-consuming, or prohibitively expensive.
To our knowledge, this is the first large scale DTI study to report reduced FA in the medial fornix of patients who are persistently depressed. The significant main effect of treatment outcome accompanied with a non-significant age-interaction suggests that alterations in medial fornix FA affect both younger and older patients. Therefore, reduced medial fornix FA may be an early indicator for persistent depression. Despite the central location of the fornix in the limbic system and its involvement in several cognitive functions, no previous studies have evaluated this structure in major depressive disorder, and only few reports exist for other psychiatric disorders such as schizophrenia (Fitzsimmons et al., 2009) and bipolar disorder (Barnea-Goraly et al., 2009). Recently, lower fornix FA has been associated with greater severity of psychotic symptoms in schizophrenia (Abdul-Rahman et al., 2011), and Huang and coworkers (Huang et al., 2011) demonstrated disrupted FA in the medial fornix of people with Alzheimer’s disease by using the same white matter atlas as reported in this work. Also, our finding of the potential value of the fornix to predict clinical status is consistent with recent studies in neurodegenerative disease in which fornix FA reliably discriminates between normal controls, mild cognitive impairment, and Alzheimer’s disease (Pievani et al., 2010; Bozoki et al., 2011; Oishi et al., 2011). Replication studies in mood disorders are needed. Further, we did not find statistically significant group differences for the other regions-of-interest, but MDD non-remitters generally had lower FA than MDD full-remitters and control subjects, consistent with previous results (Alexopoulos et al., 2008). Since there are reports suggesting the presence (Kubicki et al., 2002) and absence (Nestor et al., 2008) of hemispheric asymmetry with respect to bilateral tract FA, we included hemisphere in the statistical model, but we found no asymmetry effects for either the cingulum or fornix.
Occasional loss of white matter fiber integrity in individual tracts may not be catastrophic, but the cumulative effect of small deteriorations across the limbic system may have some impact at the functional level. However, our findings also suggest that white matter alterations in the limbic system are more likely to be regional than global, which is also true for morphology studies where regional effects (e.g., hippocampus) are more often observed than global effects (e.g., whole brain volume) (Konarski et al., 2008).
We studied the effect of gender because there are reports suggesting gender effects on antidepressant outcome (Steffens et al., 2005), whereas others have not found such an association (Taylor et al., 2008). The significant higher FA in the medial fornix of females observed in our study is additional evidence for gender differences in regional white matter microstructure of adults (Szeszko et al., 2003), however, the non-significant patient cohort by gender interaction suggests that gender-related changes are present in all depressed groups, regardless of treatment outcome. Since white matter abnormalities in MDD may be related to vascular risk factors (Iosifescu et al., 2005), our finding is consistent with the increased cardiovascular risk in males before age 55.
We found no associations between any FA measure and treatment with SSRI versus non-SSRI antidepressants. Similarly, in a recent study (van Tol et al., 2010) omitting SSRI users from analyses did not affect the results with respect to anterior cingulate gray and white matter volume reduction. Longitudinal research studies with larger samples and medication-controlled trials are needed to more definitively determine the long-term effects of SSRIs on brain morphology, white matter microstructure, vascular change and its relationship to depression.
Our findings of age-related decline in global and regional limbic FA for both control and depressed subjects are consistent with DTI studies of healthy aging reporting regionally diverse decline in white matter microstructure across the lifespan (Kochunov et al., 2010). Interestingly, we found that this effect was most notable for MDD patients who were persistently depressed, which suggests accelerated limbic white matter degeneration in the process of aging. However, the association between white matter and age may not necessarily be linear (Hsu et al., 2010), which may also account for observed group differences. Notably, compared to healthy controls and non-remitters, the partial and full-remitters showed milder age-related white matter decline in all regions-of-interest reported in this study. This finding may be partially explained by the potential neuroprotective effects of antidepressants. A large body of the literature supports the association between antidepressant effects and neuroplastic and neurotrophic effects (mediated by BDNF, Bcl-2, etc) (e.g., see reviews in Duman et al., 2006; Castrén et al., 2010). Although we do not fully understand why antidepressants are effective in certain individuals but not others, it is likely that neurotrophic effects will only be present in the individuals showing treatment response (as a necessary step in the process of clinical improvement). Following this line of reasoning, one could hypothesize that in treatment responders antidepressants also could show neuroprotective effects versus age-related effects. If the deceleration of age-related white matter decline is mediated by neuroprotective effects of antidepressant treatment, and if such effects are only present in treatment responders, then this may explain some aging patterns observed in our study. Further work is needed to better understand these relationships.
Cingulate cortex and hippocampus have been associated with selective attention to emotional stimuli in non-depressed individuals (Fossati et al., 2003) and these regions have been reported functionally abnormal in MDD patients in response to positive and negative emotional stimuli (Anand et al., 2005). In particular, medial prefrontal and hippocampal hypo-activity and temporal hyper-activity have been associated with positive and negative affect disturbances in treatment-resistant depression (Kumari et al., 2003). Interestingly, MDD treatment nonresponders have demonstrated alterations compared to MDD responders in the effective connectivity of limbic–(sub)cortical pathways, including involvement of the cingulum, hippocampus and anterior thalamus (Seminowicz et al., 2004). Building upon these prior findings, altered white matter connectivity in fronto-temporal regions, including the cingulum bundle and fornix (as shown in this report), may contribute to mood-congruent information processing biases. MDD patients with severe white matter alterations in these pathways may be at increased risk for relapse and refractory depression.
4.1. Limitations
We acknowledge several limitations to our work. The ROI-based approach in this study enabled a priori testing of the brain regions hypothesized and minimizing the risk of multiple comparisons, but further studies are needed to explore other brain regions potentially affected in mood disorders. Also, because there are concerns in drawing longitudinal inferences about developmental or disease processes from cross-sectional data (Kraemer et al., 2000), further research of longitudinal design is needed to confirm cross-sectional trends such as reported in this work.
We also acknowledge specific limitations related to the use of legacy data. First, even though we used a set of minimally acceptable criteria for MRI acquisition to restrict the variability in MRI technology and to maintain acceptable standards of research-quality data, inter-subject differences in image parameters across groups remained, such as in the number of diffusion directions. The number of diffusion directions may represent a confounding variable for anisotropy measurements (Giannelli et al., 2010), although it has been demonstrated with in-vivo data that with different diffusion directions, values of FA may vary at the voxel level, but not at the level of ROI analyses (including limbic system fibers) (Ni et al., 2006). It has also been suggested that the effects of different diffusion directions on differences in DTI contrasts are small relative to low signal-to-noise (SNR) ratio (Landman et al., 2007). Despite the fact that SNR has less effect on the reproducibility of FA in ROI-based methods than voxel-wise methods (Farrell et al., 2007), it remains to be seen how SNR affects legacy data. Further investigation on the effect of SNR on studies with legacy data is deferred to future work. Also, the majority of DWIs in our study had the minimum number of 6 diffusion-weighted directions. While the choice for a small number of gradients is practical in the clinical setting as it enables a short scanning time, it has been suggested that 20 or 30 unique directions may be better for robust estimation of anisotropy (Jones, 2004). Of note, the variability in MRI technology in our study was primarily within, and not between groups. Therefore, it was expected and found that this variation equally affected diagnostic groups. One concern, however, with such increased within-group variation is that it increases variation in the outcome measures, and thus it lowers the statistical power to detect real differences. Also, the alignment of fiber bundles was an important step in the application of white matter parcellation. In the absence of anatomical volumes, our normalization process was limited to the use of DWI data. We used FA maps for the coregistration process, because the ICBM DTI-81 is partly created from DTI data and visually similar to the FA map. Replication studies with standardized MRI acquisition and image normalizations are needed to confirm our study findings.
Second, we relied on clinical characterization based on review of medical records, where absent or insufficient data is prone to some level of misclassification of clinical outcomes. Also, variables that may influence the course of depression such as illness duration and onset of depression were not consistently recorded in the clinical notes we analyzed and therefore not included in our analyses. Further, the absence of a specific temporal relationship between the MRI and the onset of antidepressant treatment in the 12 months interval preceding the MRI was unavoidable given the fact that the collection of legacy data was part of ongoing and routine clinical care (and unrelated to psychiatric diagnosis or treatment). Future studies with legacy data may be able to focus on MRI explorations at early phases of treatment and to collect a broader range of clinical features so that the effects of medications and disease characteristics can be further evaluated.
Third, given the large database available to our study for collecting clinical and MRI data, larger final sample sizes may have been expected. Our application of strict data inclusion criteria was necessary to ensure an acceptable level of research-quality data, but it inevitably resulted in significantly reduced group sample sizes. For example, subjects in this study were only included if their brain MRIs were explicitly described as “normal” or “unremarkable” in the radiology notes; however, a less conservative approach would have been to exclude subjects only if their brain MRIs were described as “abnormal” or “remarkable”. Furthermore, we expect future studies with legacy data to identify larger samples sizes, because a greater number of institutions have adopted electronic medical record systems in recent years (Jha et al., 2009) and a greater number of institutions are making electronic medical record data available for research (Murphy et al., 2010).
4.2. Conclusions
Our data suggest that specific brain microstructural white matter abnormalities underlie persistent, treatment-refractory depression, which broadens our understanding of the pathophysiology of depression and of specific brain abnormalities associated with treatment response. We also demonstrated the feasibility of using clinical and diffusion MRI data that had previously been collected as part of routine clinical treatment to study white matter fiber integrity in the limbic system of MDD patients. This approach is time and cost-effective and has the potential to generate large study samples reflective of the real population with the illness studied.
Supplementary Material
Acknowledgements
The authors thank Victor Castro, B.S., for his assistance in collecting data from the research patient data registry; Sergey Goryachev, M.S., for his assistance with natural language processing; Kenneth Nesbitt, for providing access to MRI data from Massachusetts General Hospital’s radiology department; and Dr. Bouix and Dr. Kubicki, for their advice in the development of the imaging study.
Grant support
This research is supported as a Driving Biological Project (PI: Dr. Iosifescu) with the i2b2 grant (National Institutes of Health, NIH U54-LM008748; PI: Dr. Kohane). Dr. Iosifescu is also supported by the National Institutes of Health Career Development Award K23 MH067111. Dr. Shenton is supported by grants from the National Institutes of Health (R01 MH 50740), and the Department of Veterans Affairs (VA) (VA Merit Award and VA Schizophrenia Research Center Grant). Dr. Perlis is supported by a grant from the National Institute of Mental Health (MH086026).
Footnotes
Conflicts of interest
Dr. Iosifescu has received research support from Aspect Medical Systems, Forest Laboratories and Janssen Pharmaceutica; he has been a speaker for Eli Lilly & Co., Forest Laboratories, Pfizer, Inc. and Reed Medical Education. Dr. Perlis has received consulting fees from Concordant Rater Systems, Proteus Biomedical, and RIDventures. All other contributors have none to declare.
References
- Abdul-Rahman MF, Qiu A, Sim K, 2011. Regionally specific white matter disruptions of fornix and cingulum in schizophrenia. PLoS One 6(4): e18652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Klimstra S, et al. , 2008. Microstructural white matter abnormalities and remission of geriatric depression. American Journal of Psychiatry 165(2): 238–244. [DOI] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, et al. , 2005. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biological Psychiatry 57(10): 1079–1088. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Chang KD, Karchemskiy A, Howe ME ,Reiss AL, 2009. Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biological Psychiatry 66(3): 238–244. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J ,LeBihan D, 1994. MR diffusion tensor spectroscopy and imaging. Biophysical Journal 66(1): 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Yeh PH, Bellani M, Radaelli D, Nicoletti MA, Poletti S, et al. , 2011. Disruption of white matter integrity in bipolar depression as a possible structural marker of illness. Biological Psychiatry 69(4): 309–317. [DOI] [PubMed] [Google Scholar]
- Castrén E, Rantamäki T, 2010. The role of BDNF and its receptors in depression and antidepressant drug acction: Reactivation of developmental plasticity. Developmental Neurobiology 70(5): 289–297. [DOI] [PubMed] [Google Scholar]
- Bozoki AC, Korolev IO, Davis NC, Hoisington LA ,Berger KL, 2011. Disruption of limbic white matter pathways in mild cognitive impairment and Alzheimer’s disease: A DTI/FDG-PET Study. Human Brain Mapping. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Jeong B, Rohan ML, Polcari AM ,Teicher MH, 2009. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological Psychiatry 65(3): 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J ,Breteler MM, 2000. Cerebral white matter lesions and depressive symptoms in elderly adults. Archives of General Psychiatry 57(11): 1071–1076. [DOI] [PubMed] [Google Scholar]
- Duman RS, Montegia LM, 2006. A neurotrophic model for stress-related mood disorders. Biological Psychiatry 59: 1116–1127. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, et al. , 2008. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biological Psychiatry 63(4): 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell JA, Landman BA, Jones CK, Smith SA, Prince JL, van Zijl PC, et al. , 2007. Effects of signal-to-noise ratio on the accuracy and reproducibility of diffusion tensor imaging-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5 T. Journal of Magnetic Resonance Imaging 26(3): 756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, 2003. Diagnosis and definition of treatment-resistant depression. Biological Psychiatry 53(8): 649–659. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J ,Daskalakis ZJ, 2008. A meta-analytic study of changes in brain activation in depression. Human Brain Mapping 29(6): 683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons J, Kubicki M, Smith K, Bushell G, Estepar RS, Westin CF, et al. , 2009. Diffusion tractography of the fornix in schizophrenia. Schizophrenia Research 107(1): 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, et al. , 2003. In search of the emotional self: an fMRI study using positive and negative emotional words. American Journal of Psychiatry 160(11): 1938–1945. [DOI] [PubMed] [Google Scholar]
- Giannelli M, Cosottini M, Michelassi MC, Lazzarotti G, Belmonte G, Bartolozzi C, et al. , 2010. Dependence of brain DTI maps of fractional anisotropy and mean diffusivity on the number of diffusion weighting directions. Journal of Applied Clinical Medical Physics 11(1): 2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Walton M, Cheng J, Acuna J, Klimstra S, Zimmerman ME, et al. , 2010. MRI signal hyperintensities and treatment remission of geriatric depression. Journal of Affective Disorders 126(3): 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JL, Van Hecke W, Bai CH, Lee CH, Tsai YF, Chiu HC, et al. , 2010. Microstructural white matter changes in normal aging: a diffusion tensor imaging study with higher-order polynomial regression models. Neuroimage 49(1): 32–43. [DOI] [PubMed] [Google Scholar]
- Huang H, Fan X, Weiner M, Martin-Cook K, Xiao G, Davis J, et al. , 2011. Distinctive disruption patterns of white matter tracts in Alzheimer’s disease with full diffusion tensor characterization. Neurobiology of Aging. DOI: 10.1016/j.neurobiolaging.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosifescu DV, Papakostas GI, Lyoo IK, Lee HK, Renshaw PF, Alpert JE, et al. , 2005. Brain MRI white matter hyperintensities and one-carbon cycle metabolism in non-geriatric outpatients with major depressive disorder (Part I). Psychiatry research 140(3): 291–299. [DOI] [PubMed] [Google Scholar]
- Iosifescu DV, Renshaw PF, Lyoo IK, Lee HK, Perlis RH, Papakostas GI, et al. , 2006. Brain white-matter hyperintensities and treatment outcome in major depressive disorder. British Journal of Psychiatry 188: 180–185. [DOI] [PubMed] [Google Scholar]
- Jha AK, DesRoches CM, Campbell EG, Donelan K, Rao SR, Ferris TG, et al. , 2009. Use of electronic health records in U.S. hospitals. New England Journal of Medicine 360(16): 1628–1638. [DOI] [PubMed] [Google Scholar]
- Jones DK, 2004. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magnetic Resonance in Medicine 51(4): 807–815. [DOI] [PubMed] [Google Scholar]
- Kieseppa T, Eerola M, Mantyla R, Neuvonen T, Poutanen VP, Luoma K, et al. , 2010. Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. Journal of Affective Disorders 120(1–3): 240–244. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Williamson DE, Lancaster J, Fox P, Cornell J, Blangero J, et al. , 2010. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiology of Aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarski JZ, McIntyre RS, Kennedy SH, Rafi-Tari S, Soczynska JK ,Ketter TA, 2008. Volumetric neuroimaging investigations in mood disorders: bipolar disorder versus major depressive disorder. Bipolar Disorder 10(1): 1–37. [DOI] [PubMed] [Google Scholar]
- Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE ,Kahn RS, 2009. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Human Brain Mapping 30(11): 3719–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL ,Kupfer D, 2000. How can we learn about developmental processes from cross-sectional studies, or can we? American Journal of Psychiatry 157(2): 163–171. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, et al. , 2002. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. American Journal of Psychiatry 159(5): 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Mitterschiffthaler MT, Teasdale JD, Malhi GS, Brown RG, Giampietro V, et al. , 2003. Neural abnormalities during cognitive generation of affect in treatment-resistant depression. Biological Psychiatry 54(8): 777–791. [DOI] [PubMed] [Google Scholar]
- Landman BA, Farrell JA, Jones CK, Smith SA, Prince JL, Mori S, 2007. Effects of diffusion weighting schemes on the reproducibility of DTI-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5T. Neuroimage 36(4): 1123–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Li L, Shu N, Liu J, Gong G, He Z, et al. , 2007. White matter abnormalities in first-episode, treatment-naive young adults with major depressive disorder. American Journal of Psychiatry 164(5): 823–826. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, et al. , 2008. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40(2): 570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SN, Mendis M, Hackett K, Kuttan R, Pan W, Phillips LC, et al. , 2007. Architecture of the open-source clinical research chart from Informatics for Integrating Biology and the Bedside. American Medical Informatics Association Annual Symposium Proceedings: 548–552. [PMC free article] [PubMed] [Google Scholar]
- Murphy SN, Weber G, Mendis M, Gainer V, Chueh HC, Churchill S, et al. , 2010. Serving the enterprise and beyond with informatics for integrating biology and the bedside (i2b2). Journal of the American Medical Informatics Association 17(2): 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Niznikiewicz M, Gurrera RJ, McCarley RW ,Shenton ME, 2008. Neuropsychological disturbance in schizophrenia: a diffusion tensor imaging study. Neuropsychology 22(2): 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H, Kavcic V, Zhu T, Ekholm S, Zhong J, 2006. Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain. American Journal of Neuroradiology 27(8): 1776–1781. [PMC free article] [PubMed] [Google Scholar]
- Nierenberg AA, Ostacher MJ, Huffman JC, Ametrano RM, Fava M ,Perlis RH, 2008. A brief review of antidepressant efficacy, effectiveness, indications, and usage for major depressive disorder. Journal of Occupational and Environmental Medicine 50(4): 428–436. [DOI] [PubMed] [Google Scholar]
- Oishi K, Mielke MM, Albert M, Lyketsos CG, Mori S, 2011. The fornix sign: A potential sign for Alzheimer’s disease based on diffusion tensor imaging. Journal of Neuroimaging. DOI: 10.1111/j.1552-6569.2011.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Li X, et al. , 2008. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage 43(3): 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Iosifescu DV, Castro VM, Murphy SN, Gainer VS, Minnier J, et al. (2011) Using electronic medical records to enable large-scale studies in psychiatry: Treatment resistant depression as a model. Psychological Medicine DOI: 10.1017/S0033291711000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pievani M, Agosta F, Pagani E, Canu E, Sala S, Absinta M, et al. , 2010. Assessment of white matter tract damage in mild cognitive impairment and Alzheimer’s disease. Human Brain Mapping 31(12): 1862–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermuly I, Fellgiebel A, Wagner S, Yakushev I, Stoeter P, Schmitt R, et al. , 2010. Association between cingulum bundle structure and cognitive performance: an observational study in major depression. European Psychiatry 25(6): 355–360. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, et al. , 2004. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage 22(1): 409–418. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Pieper CF, Barch DM, Welsh-Boehmer K, McKinstry RC, MacFall JR, et al. , 2010. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Archives of General Psychiatry 67(3): 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimony JS, Sheline YI, D’Angelo G, Epstein AA, Benzinger TL, Mintun MA, et al. , 2009. Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biological Psychiatry 66(3): 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, Pieper CF, Bosworth HB, MacFall JR, Provenzale JM, Payne ME, et al. , 2005. Biological and social predictors of long-term geriatric depression outcome. International Psychogeriatrics 17(1): 41–56. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Vogel J, Ashtari M, Malhotra AK, Bates J, Kane JM, et al. , 2003. Sex differences in frontal lobe white matter microstructure: a DTI study. Neuroreport 14(18): 2469–2473. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Kuchibhatla M, Payne ME, Macfall JR, Sheline YI, Krishnan KR, et al. , 2008. Frontal white matter anisotropy and antidepressant remission in late-life depression. PLoS One 3(9): e3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Payne ME, Krishnan KR, Wagner HR, Provenzale JM, Steffens DC, et al. , 2001. Evidence of white matter tract disruption in MRI hyperintensities. Biol Psychiatry 50(3): 179–183. [DOI] [PubMed] [Google Scholar]
- Thomas AG, Koumellis P, Dineen RA, 2011. The fornix in health and disease: an imaging review. Radiographics 31(4): 1107–1121. [DOI] [PubMed] [Google Scholar]
- van Tol MJ, van der Wee NJ, van den Heuvel OA, Nielen MM, Demenescu LR, Aleman A, et al. , 2010. Regional brain volume in depression and anxiety disorders. Archives of General Psychiatry 67(10): 1002–1011. [DOI] [PubMed] [Google Scholar]
- Wisniewski SR, Rush AJ, Nierenberg AA, Gaynes BN, Warden D, Luther JF, et al. , 2009. Can phase III trial results of antidepressant medications be generalized to clinical practice? A STAR*D report. American Journal of Psychiatry 166(5): 599–607. [DOI] [PubMed] [Google Scholar]
- Zeng QT, Goryachev S, Weiss S, Sordo M, Murphy SN ,Lazarus R, 2006. Extracting principal diagnosis, co-morbidity and smoking status for asthma research: evaluation of a natural language processing system. Biomedcentral Medical Informatics and Decision Making 6: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Qin LD, Chen J, Qian LJ, Tao J, Fang YR, et al. , 2010. Brain microstructural abnormalities revealed by diffusion tensor images in patients with treatment-resistant depression compared with major depressive disorder before treatment. European Journal of Radiology. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


