Abstract
The ability to regulate intracellular gene expression with exogenous nucleic acids such as small interfering RNAs (siRNAs) has substantial potential to improve the study and treatment of disease. However, most transfection agents and nanoparticle-based carriers that are used for the intracellular delivery of nucleic acids cannot distinguish between diseased and healthy cells, which may cause them to yield unintended widespread gene regulation. An ideal delivery system would only silence targeted proteins in diseased tissue in response to an external stimulus. To enable spatiotemporal control over gene silencing, researchers have begun to develop nucleic acid-nanoparticle conjugates that keep their nucleic acid cargo inactive until it is released from the nanoparticle on-demand by externally applied near-infrared laser light. This strategy can overcome several limitations of other nucleic acid delivery systems, but the mechanisms by which these platforms operate remain ill understood. Here, we perform a detailed investigation of the mechanisms by which silica core/gold shell nanoshells (NSs) release conjugated siRNA upon excitation with either pulsed or continuous wave (CW) near-infrared (NIR) light, with the goal of providing insight into how these nanoconjugates can enable on-demand gene regulation. We demonstrate that siRNA release from NSs upon pulsed laser irradiation is a temperature-independent process that is substantially more efficient than siRNA release triggered by CW irradiation. Contrary to literature, which suggests that only pulsed irradiation releases siRNA duplexes, we found that both modes of irradiation release a mixture of siRNA duplexes and single-stranded oligonucleotides, but that pulsed irradiation results in a higher percentage of released duplexes. To demonstrate that the siRNA released from NSs upon pulsed irradiation remains functional, we evaluated the use of NSs coated with green fluorescent protein (GFP)-targeted siRNA (siGFP-NS) for on-demand knockdown of GFP in cells. We found that GFP-expressing cells treated with siGFP-NS and irradiated with a pulsed laser experienced a 33% decrease in GFP expression compared to cells treated with no laser. Further, we observed that light-triggered gene silencing mediated by siGFP-NS is more potent than using commercial transfection agents to deliver siRNA into cells. This work provides unprecedented insight into the mechanisms by which plasmonic NSs release siRNA upon light irradiation and demonstrates the importance of thoroughly characterizing photoresponsive nanosystems for applications in triggered gene regulation.
Keywords: Nanoshells, small interfering RNA, light-triggered release, photothermal, gene regulation, laser
Graphical Abstract
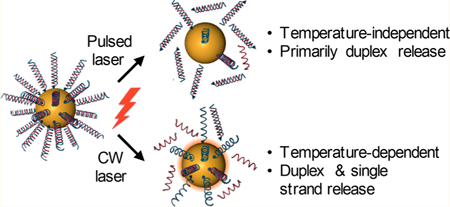
Regulating gene expression via the delivery of small interfering RNA (siRNA) is a promising means to study and treat disease, but the preclinical and clinical use of siRNA is limited by molecular instability, short circulation times, and low cellular uptake.1 Nanoparticle (NP) delivery platforms that either encapsulate or carry gene regulatory molecules on their surfaces can overcome many of these limitations by increasing their stability, improving their biodistribution, and enhancing their delivery into cells.2,3 Accordingly, siRNA nanocarriers are recognized as powerful gene regulatory agents. However, these carriers typically cannot distinguish diseased cells from normal cells and, once inside cells, they often fail to reach the cytosol due to entrapment within intracellular compartments, rendering the attached oligonucleotides ineffective.1 To promote successful cytosolic delivery, siRNA nanocarriers are often coated with additional moieties such as cell penetrating peptides or polymers that improve endosome escape.4,5 Unfortunately, these modified NPs may be more toxic than their unmodified counterparts, and they still cannot distinguish healthy cells from diseased cells, which may cause them to elicit unintended widespread gene regulation and substantial off-target effects. An ideal NP platform for gene regulation would enter cells without additional penetration reagents and keep its siRNA cargo inactive until stimulated by an external trigger. Such a system could facilitate on-demand, localized gene regulation while avoiding impacts to healthy tissue. To meet this need, researchers have recently begun to develop light-responsive nanocarriers for siRNA delivery, and in this paper we use one example platform (siRNA-nanoshell (NS) conjugates) to define, with an unprecedented level of detail, the mechanisms by which these platforms operate to enable temporal control over gene silencing.
In these photoresponsive siRNA nanocarriers, the core NP is carefully chosen for its inherent photophysical properties that enable activation by externally applied light. Upon laser excitation, any surface-conjugated siRNA is released to silence the targeted proteins. siRNA is desirable as a gene regulatory agent in these systems because it is more potent and longer-lasting than antisense DNA oligonucleotides, which have been used in the majority of light-triggered nucleic acid-nano-conjugate systems, and it requires no additional chemical modifications to be active inside cells.6,7 siRNA is composed of complementary strands of sense and antisense oligonucleotides, and it acts through RNA interference (RNAi). In RNAi, siRNA duplexes inside cells are recognized by the RNA-induced silencing complex (RISC), which separates the duplex and escorts the antisense strand to its target messenger RNA (mRNA) to inhibit the transcription of the encoded protein.8 Importantly, RISC only recognizes double stranded siRNA,9 so it is critical to ensure that the majority of the siRNA released from photoresponsive NPs retains a duplexed structure. Accordingly, thoroughly characterizing the structure and functionality of the siRNA released from siRNA-NS conjugates upon light exposure was a primary goal of our work.
We used silica core/gold shell NSs as the core material of our platform to study light-triggered siRNA release from nano-carriers because they are specifically engineered to maximally absorb ∼800 nm light, and they have proven safety in human clinical trials.10−13 Gold-based NPs such as NSs that maximally absorb near-infrared (NIR) light (∼700−1200 nm) are ideal for phototherapies because these wavelengths can safely penetrate several centimeters of healthy tissue.10,14 Several gold NP designs have been developed to study the intracellular delivery and light-triggered release of nucleic acids, including nanorods,15,16 nanoshells,17−19 and hollow gold NPs,20,21 among others.22 As mentioned before, however, many of these platforms have utilized additional agents to promote cell uptake that may result in nonspecific off-target gene silencing.5,20 Our overarching goal in this study was to develop siRNA-coated NSs as a platform for light-triggered gene silencing that does not require additional uptake agents. Moreover, we wanted to thoroughly characterize siRNA release from these nanoparticles to understand the parameters that are important for duplex release to induce gene regulation on-demand.
We hypothesized that siRNA-NS conjugates could enter cells without the need for auxiliary transfection agents and that the siRNA could be released from the NS carriers on-demand using NIR laser light to facilitate gene silencing. Importantly, the mode of laser irradiation is a critical experimental design feature, as it is believed to dictate the structure of the released siRNA (Table of contents graphic, Scheme 1). Prior work using DNA-NS conjugates has shown that continuous wave (CW) irradiation causes large temperature increases of bulk NS solutions over time, which may yield duplex denaturation such that primarily single-stranded oligonucleotides are released.19 Alternatively, delivering very short, high energy pulses of laser light to NSs yields a hot-electron transfer effect that breaks the gold−thiol bond attaching the nucleic acids to the NSs without increasing bulk solution temperature.20,23 Jain et al. speculated that this bond dissociation is due to coupling of the NPs’ photoexcited electrons and the gold−sulfur bond vibrations, rather than thermal heating as experienced through CW irradiation.23 Due to these differences, it is commonly believed that CW irradiation leads to release of single-stranded oligonucleotides, while pulsed laser irradiation leads to release of entire duplexes. We aimed to test this belief using a powerful combination of NP characterization and molecular biology techniques. Further, we wanted to characterize the laser parameters required to trigger release under CW or pulsed irradiation, with the expectation that pulsed irradiation would require significantly lower laser powers than CW irradiation due to the high amount of energy delivered per pulse. Additionally, we aimed to accurately characterize the structural differences of the siRNA released by each mode of irradiation and to show that the released siRNA remains functional following irradiation. Finally, we wanted to demonstrate that siRNA-NS conjugates could enable on-demand gene silencing upon pulsed laser irradiation using green fluorescent protein (GFP) as a model target.
Scheme 1.
On-Demand Gene Regulation with siRNA-NS Conjugatesa

aWhen cells treated with siRNA-NS are exposed to a femtosecond pulsed laser, there is no change in temperature and the released siRNA consists primarily of duplexes that can silence the expression of the target gene (e.g., GFP) after being recognized by the RNA induced silencing complex (RISC). Alternatively, CW irradiation causes NSs to generate a substantial amount of heat that releases both single stranded siRNA and duplexes and that can cause nonspecific cell death without achieving the desired gene silencing. In this work, we evaluate light-triggered release of GFP siRNA to induce GFP downregulation in cells.
To quantify and characterize siRNA release from NSs upon CW or pulsed laser irradiation, we coated NS with a scrambled form of GFP-targeted siRNA (all sequences used in this work are listed in Table S1). The sense strand was directly attached to the gold surface of NSs via a thiol on its 3′ end, and the antisense strand was attached to the sense strand by complementary base pairs. The 3′ end of the antisense strand was labeled with a Cy5 fluorophore to enable direct detection of both duplexed and single-stranded siRNA (Cy5-siSCR-NS, Figure S1). NSs were also passivated with 5 kDa methoxypoly(ethylene) glycol-thiol (mPEG-SH) for increased stability. Solutions of Cy5-siSCR-NS at a concentration of 5.37E9 NS/mL were irradiated from the side with either an 808 nm CW laser or a femtosecond pulsed 800 nm laser while stirring (Figure S2). Samples undergoing CW irradiation were exposed to 0, 157, 314, 628, 785, 942 mW (corresponding to 0, 5, 10, 20, 25, or 30 W/cm2) laser powers for 30 min to investigate the temperature dependence on siRNA release. Thermal images were taken with an FLIR camera every 5 min, and the highest temperature of the NS solution was recorded. In agreement with the literature,19 we found that the bulk solution temperature dramatically increased upon CW irradiation in accordance with exposure time and laser power, and samples reached temperatures up to 52 °F with the powers used in this study (Figure 1). Importantly, the high temperatures reached during CW irradiation are sufficient to cause nonspecific cell death,24,25 making the laser powers studied here irrelevant for light-triggered gene regulation. Bulk solution temperatures were also investigated under pulsed laser irradiation using 3 mW output power, which is the highest power we investigated for siRNA release, for up to 30 min, and we found that the bulk solution temperature did not change (Figure 1, black dotted line). These results demonstrate that the use of CW irradiation for triggered gene regulation is limited by changes in bulk solution temperature, and any resultant siRNA release is likely a temperature-dependent process.19 Alternatively, pulsed laser irradiation is a temperature-independent process, suggesting that siRNA release is caused by a different mechanism such as breakage of the gold−thiol bond. Next, we tested the ability of CW and pulsed laser irradiation to induce siRNA release, and we thoroughly characterized both the NSs and the released siRNA following laser treatment.
Figure 1.
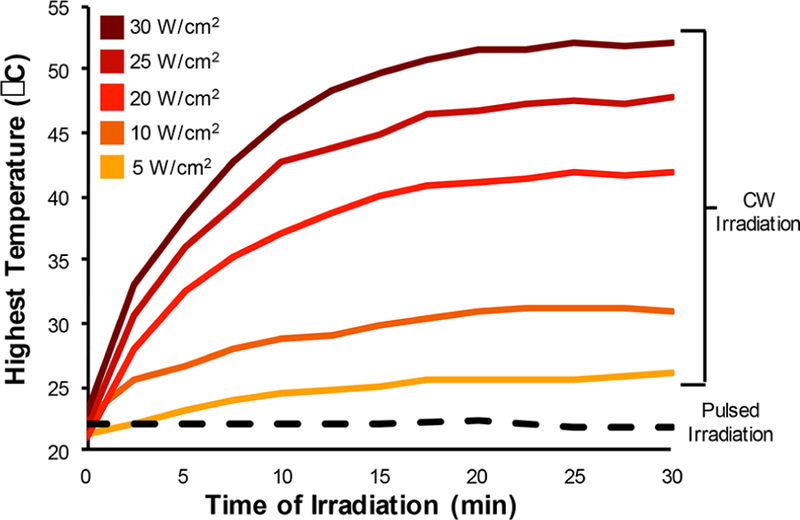
Bulk solution temperature measurements of Cy5-siSCR-NS exposed to 808 nm continuous wave light (solid lines) or 800 nm pulsed light at 3 mW for 30 min (dotted black line).
First, we investigated how CW or pulsed laser irradiation impact NS structure and size by UV−visible spectrophotometry (UV−vis) and dynamic light scattering (DLS) (Figure 2 and Figure S3A,B). CW irradiation with 0, 5, 10, 20, 25, or 30 W/cm2 for 30 min caused no critical changes in the extinction spectrum or hydrodynamic diameter of Cy5-siSCR-NS, indicating that NS are still intact and functionalized with a substantial amount of siRNA and mPEG-SH following CW irradiation (Figure S3A, B). Alternatively, irradiation with 1, 2, or 3 mW pulsed light for 10, 20, or 30 min resulted in substantial structural changes to Cy5-siSCR-NS (Figure 2). Spectrophotometry results demonstrate that the structural integrity of NSs is preserved up to 3 mW output power for 20 min. At 3 mW, the peak extinction at 800 nm decreases, indicating a change in NS structure. Further, this change depends on irradiation time, as samples irradiated with the pulsed laser at 3 mW for 10 min do not experience a change in their extinction spectrum (Figure 2A). DLS measurements show that the hydrodynamic diameter of Cy5-siSCR-NS decreases from 181.3 ± 7.1 nm before laser treatment to 173.7 ± 23 nm and 155.1 ± 7.3 nm after irradiation with 3 mW for 20 or 30 min, respectively. With these higher irradiation times, the hydrodynamic diameter of the laser-treated NS is reduced to the original size of bare NS, whereas lower laser powers and irradiation times do not result in notable changes in hydrodynamic diameter (Figure 2B). This confirms our spectrophotometry results that indicate that the structure of Cy5-siSCR-NS changes with pulsed laser irradiation at 3 mW, and suggests that the majority of both siRNA duplexes and mPEG-SH are removed from NS surfaces upon pulsed laser excitation.
Figure 2.
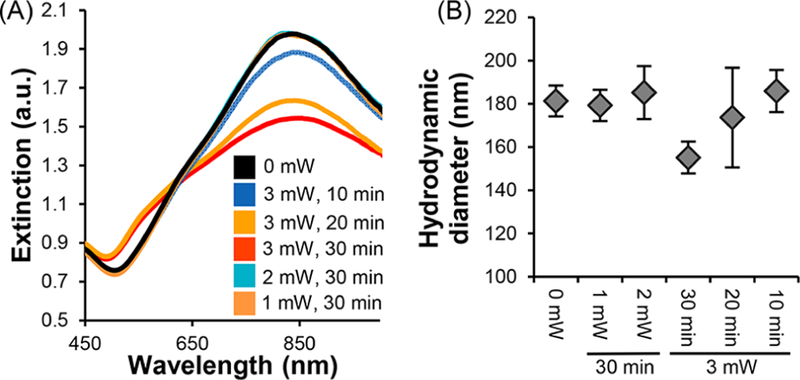
Characterization of Cy5-siSCR-NS irradiated with a femotosecond pulsed laser at 0, 1, 2, or 3 mW for 0, 10, 20, or 30 min. (A) UV−visible extinction spectrum and (B) hydrodynamic diameter measurements after irradiation.
An added benefit of utilizing NS as the core material for investigating light-triggered siRNA release from nanocarriers is that NSs can be detected by forward- and side- scatter profiles in flow cytometry, a technique that has not yet been explored to directly detect fluorescently tagged NSs. We expected that the Cy5 signal from NSs would decrease proportionally to the amount of siRNA released during irradiation. In agreement with our spectrophotometry and DLS data, we found that Cy5- siSCR-NS solutions treated with CW light do not experience a shift in Cy5 fluorescence intensity, indicating that the majority of the Cy5 fluorophores remain attached to NSs following irradiation (Figure S3C). Alternatively, the fluorescence of Cy5- siSCR-NS decreases with increased pulsed laser powers and irradiation times, indicating that the pulsed laser sufficiently triggers release of surface-conjugated molecules (Figure 3A,B).
Figure 3.

Characterization and quantification of Cy5 siRNA release from NSs after pulsed laser irradiation with 0, 1, 2, or 3 mW for 10, 20, or 30 min. (A) Flow cytometer measurements and (B) median fluorescence intensity to assess Cy5 fluorescence signal from NS samples after exposure to the pulsed laser. (C,D) Percent siRNA release following irradiation with 0, 1, 2, or 3 mW pulsed light for 30 min or with 3 mW pulsed light for 0, 10, 20, or 30 min, respectively, to show the power and time dependence of the siRNA release from NSs. (E) Gel electrophoresis of stock siRNA sense strands, antisense strands, or duplexes compared to siRNA that was released from NSs upon pulsed laser irradiation.
We hypothesized that siRNA release from NSs upon pulsed or CW excitation would depend highly on laser power and irradiation time. To investigate this, we quantitatively examined the efficacy of CW and pulsed laser irradiation for triggered siRNA release by detecting the Cy5 signal of released siRNA following laser irradiation. The original loading of siRNA on NSs was determined by first separating the duplexes using urea and comparing the Cy5 signal in the supernatant to a standard curve of known antisense concentration. Following irradiation with the CW laser (0, 5, 10, 20, 25, or 30 W/cm2 for 30 min) or the pulsed laser (0, 1, 2, or 3 mW for 0, 10, 20, or 30 min), samples were centrifuged and the siRNA-containing supernatant was collected. siRNA content in the supernatant was quantified by comparing the Cy5 fluorescence from each sample to a standard curve of known siRNA content. Then, we divided the amount of released siRNA by the original loading to determine the percent siRNA released at each laser power and irradiation time.
We found that the amount of siRNA released under both modes of irradiation was highly dependent on both laser power and irradiation time. For example, CW irradiation resulted in a linear increase in released siRNA at the laser powers studied here, and the maximum siRNA release was 36% with 30 W/cm2 (Figure S4A). Notably, the temperatures reached during CW irradiation at 30 W/cm2 (Figure 1) would cause nonspecific cell death, and therefore this strategy would not be effective for light-triggered gene regulation. Alternatively, pulsed laser irradiation caused an exponential increase in released siRNA (Figure 3C,D). Pulsed irradiation at 1 mW only minimally released siRNA above background levels, but samples treated with 2 mW or 3 mW of pulsed light experienced 33% and 72% siRNA release, respectively (Figure 3C). A similar trend was observed for increasing irradiation times when samples were exposed to 3 mW output power (Figure 3D). These results agree with our DLS and flow cytometry analyses and demonstrate that the pulsed laser is substantially more effective than the CW laser for triggering siRNA release at the laser parameters studied here.
Next, we evaluated whether the siRNA released from NSs retains the proper structure to mediate gene regulation inside cells. As previously mentioned, the released siRNA must be duplexed in order to be recognized by RISC to guide degradation of its target mRNA. Therefore, it is critical to investigate the structure of the released siRNA under each mode of irradiation. It is generally believed that the increased temperatures generated during CW irradiation primarily cause only antisense strands to be released due to duplex denaturation,26 whereas pulsed laser irradiation releases entire duplexes by breakage of the gold−thiol bond.23,27 Here, we employed gel electrophoresis to directly analyze the siRNA structure based on its size, which is directly related to the number of oligonucleotides in the sequence. After irradiating solutions of Cy5-siSCR-NS, we pelleted the NPs by centrifugation, lyophilized the supernatant, and ran gel electrophoresis on 100 ng of the released siRNA. Contrary to literature,16,18,19 we found that CW irradiation leads to a mixture of both duplexes and antisense strands being released from NSs (Figure S4B). This underscores the importance of the experimental method we utilized for this work. Alter-natively, and as expected, the siRNA released with the pulsed laser was primarily double-stranded, as the brightest band from these samples appears at ∼40 base pairs (Figure 3E). To quantitatively compare the amount of duplexes and antisense strands released from NSs, we evaluated the band intensities from the gel electrophoresis experiments and calculated the ratio of duplex to antisense released for both modes of irradiation at the highest laser powers used. Pulsed laser irradiation with 3 mW exposure and CW irradiation with 30 W/cm2 exposure resulted in duplex:antisense band intensity ratios of 3.2 and 1.23, respectively. Overall, this shows that pulsed laser irradiation releases a higher percentage of duplexes than CW irradiation with substantially lower incident laser powers. Our findings demonstrate that researchers should carefully characterize their photoresponsive nucleic acid carriers using a variety of techniques and also suggest that more studies are needed to identify laser parameters for CW irradiation that may enable duplex release without causing substantial temperature increases.
We also performed experiments to ensure that the siRNA released from NSs upon laser treatment retains its functionality and is not damaged during laser irradiation. For this study, we designed siRNA-NS conjugates to silence green fluorescent protein (GFP, siGFP-NS) in U373.eGFP cells. We used this cell line because it stably expresses GFP and therefore serves as an ideal platform to investigate light-triggered gene silencing by fluorescence analysis. First, we collected released siRNA from the supernatant of siGFP-NS conjugates following pulsed laser irradiation, and transfected cells with 100 nM of this released siRNA. Cells transfected with GFP siRNA that had been released from NSs experienced substantial downregulation of GFP expression compared to cells transfected with scrambled (SCR) siRNA that had been released from NSs at both 48 and 96 h, indicating that the siRNA is still functional following laser treatment (Figure S5). Further, this data shows that siRNA-mediated GFP silencing is prominent for at least 4 days following transfection, which is an important feature for gene regulation.
Next, we directly evaluated the ability of siRNA-NS conjugates to enter U373.eGFP cells and mediate on-demand gene silencing. First, we treated U373.eGFP cells with Cy5-siSCR-NS for 3 h to evaluate cell uptake by fluorescence imaging, which showed a substantial amount of uptake at this time point (Figure 4A). These results were confirmed by flow cytometry, which revealed a 22-fold enhancement in Cy5 signal from cells treated with Cy5-siSCR-NS compared to untreated cells (Figure S6). To examine light-triggered gene knockdown in U373.eGFP cells, the cells were treated with siGFP-NS or siSCR-NS at 1.4E10 NS/mL in complete cell culture media for 3 h, then placed into sterile cuvettes for laser treatment with 3 mW pulsed laser for 20 min while stirring. After laser treatment, the cells were plated in 24-well plates and incubated for 4 days prior to GFP analysis. Flow cytometry revealed that cells treated with siGFP-NS and the laser experienced a 33% decrease in normalized median fluorescence compared to cells treated with siSCR-NS (Figure 4B) or siGFP-NS and no laser. To ensure that this GFP downregulation is a result of GFP knockdown and not cell confluency in the well plate, we plated U373.eGFP cells at 5000−45 000 cells/well, incubated them overnight, and analyzed their GFP expression. This showed that cell confluency does not impact GFP expression in a population of cells (Figure S7), which confirms that the GFP knockdown we observed is due to the combined application of siGFP-NS and the pulsed laser irradiation. Interestingly, the average original siRNA loading on siGFP-NS was 1095 siRNA/NS, which correlates to 25.4 nM siRNA added to cells, and ∼18 nM siRNA released (given that up to 72% of the siRNA releases upon 30 min irradiation at 3 mW). Therefore, the amount of siRNA that is delivered with siGFP-NS is at least 5X less than the 100 nM siRNA used for the transfections previously discussed. These results indicate that NS-mediated delivery of siRNA is more effective for gene silencing than using commercially available transfection reagents. Further, utilizing NSs as the core nanomaterial enables on-demand control over gene silencing, which will ultimately avoid undesired side effects from nonspecific and widespread gene regulation.
Figure 4.
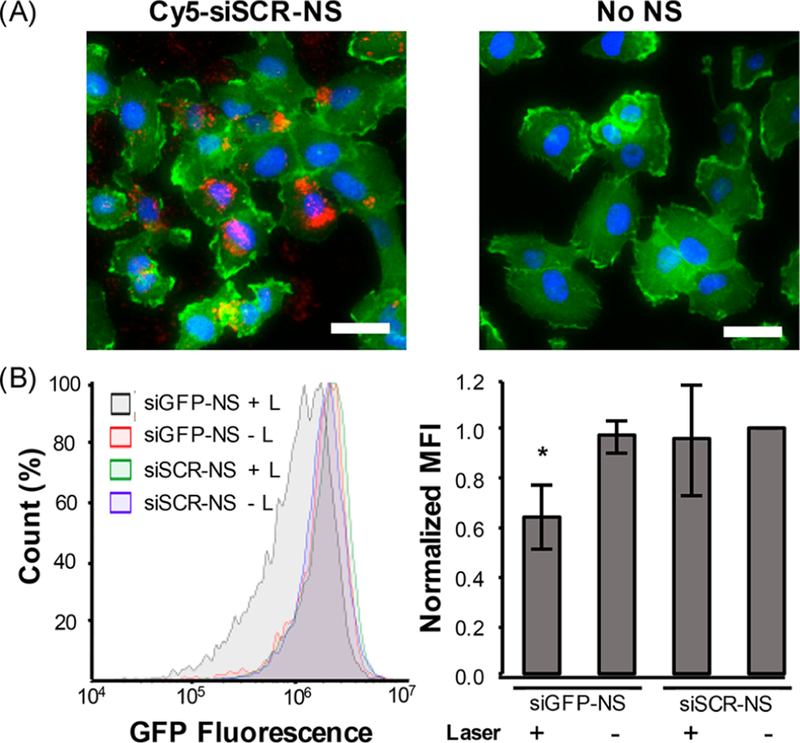
Analysis of siRNA-NS uptake by U373.eGFP cells and GFP silencing upon pulsed laser irradiation. (A) Fluorescence imaging of Cy5-siSCR-NS (red) uptake by U373.eGFP cells 3 h postaddition to the culture medium (scale = 50 μm; GFP = green, nuclei = blue). Control cells were treated with no NS. (B) Flow cytometry of GFP expression in U373.EGFP cells treated with siGFP-NS or siSCR-NS for 3 h and then irradiated for 30 min with 0 or 3 mW pulsed light. The data shown represents GFP expression 4 days after irradiation compiled from four independent experiments. *p < 0.05 by one-way ANOVA with posthoc Tukey−Kramer.
In total, the results of this work provide several new insights into the use of photoresponsive gold-based nanoparticles for on-demand gene regulation. Our overarching goal was to evaluate light-triggered siRNA release from NSs upon CW or pulsed laser irradiation and to demonstrate the importance of carefully choosing laser parameters for maximal release and gene knockdown. We developed siRNA-coated NSs and thoroughly characterized their use for on-demand gene regulation using pulsed and CW NIR light. While most light-triggered release studies have evaluated the release of DNA from NPs,16,19,27,28 here we evaluated the release of siRNA, which provides many benefits for gene regulation over DNA.6,7 Since siRNA has a unique secondary structure, it cannot be assumed that the release conditions will match those of DNA, making it imperative that primarily duplexes be released from NSs to enable RNAi. To evaluate this, we used gel electrophoresis to carefully characterize the siRNA that is released from NSs upon light activation. Using this technique, we demonstrated that pulsed laser irradiation primarily releases siRNA duplexes, making it usable for RNAi. We also demonstrated for the first time that CW irradiation releases not just single-stranded oligonucleotides, as previously expected, but also duplexes of siRNA, although duplex release upon CW irradiation is not as efficient as pulsed laser-induced release. As a result, the temperatures reached during CW irradiation would cause nonspecific cell death, making CW irradiation at the conditions tested in this study impractical for gene regulation purposes. Additional studies evaluating shorter CW irradiation times with higher laser powers may enable siRNA-NS conjugates to be utilized for on-demand gene regulation using either pulsed or CW NIR light.
Our study also showed that pulsed laser irradiation with 3 mW for 30 min can induce up to 72% release of the conjugated siRNA, and the released siRNA is still functional to enable on-demand GFP knockdown in GFP-expressing U373 cells. Excitingly, we found that siGFP-NS are substantially more effective for GFP knockdown than unconjugated siRNA that is complexed with a commercial transfection reagent, as 100 nM siRNA was required for gene silencing with Dharmafect, but only 25.4 nM siRNA was delivered with siGFP-NS. By taking the 35% siRNA release after 20 min irradiation into account, we effectively administered only ∼9 nM siRNA to cells for GFP knockdown, which is a remarkable improvement in gene silencing efficiency. Furthermore, unlike previously reported systems,4,5 our platform does not require any additional materials to enhance cellular uptake. This is a key feature of the siRNA-NS conjugates reported here, as cell penetrating peptides and polymers may yield unintended gene regulation in healthy tissues in the absence of light activation. The ability to enable on-demand gene silencing without the use of additional chemical modifications makes these siRNA-NS conjugates highly desirable for practical applications. Overall, this platform offers temporal control over gene silencing with low concentrations of siRNA to overcome the common limitations with other nanoparticle systems, and our results demonstrate that light-triggered siRNA release facilitated through siRNA-NS conjugates is a promising means of enabling gene regulation on-demand.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under Award Number R35GM119659 (PI: Day). R.S.R. received support from the American Association of University Women through a Dissertation Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the views of the funding agencies.
ABBREVIATIONS
- NP
nanoparticle
- NS
nanoshell
- RNAi
RNA interference
- RISC
RNA-induced silencing complex
- GFP
green fluorescent protein
- SCR
scramble
- CW
continuous wave
- siRNA
small interfering RNA
- RNA
ribonucleic acid
- DLS
dynamic light scattering
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.nanolett.8b00681.
Experimental procedures, irradiation setup, additional characterization data, and supporting GFP expression analysis (PDF)
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Wang T; Shigdar S; Shamaileh HA; Gantier MP; Yin W; Xiang D; Wang L; Zhou SF; Hou Y; Wang P; et al. Challenges and Opportunities for siRNA-Based Cancer Treatment. Cancer Lett 2017, 387, 77–83. [DOI] [PubMed] [Google Scholar]
- (2).Weissleder R; Kelly K; Sun EY; Shtatland T; Josephson L Cell-Specific Targeting of Nanoparticles by Multivalent Attachment of Small Molecules. Nat. Biotechnol 2005, 23, 1418–1423. [DOI] [PubMed] [Google Scholar]
- (3).Riley RS; Day ES Frizzled7 Antibody-Functionalized Nanoshells Enable Multivalent Binding for Wnt Signaling Inhibition in Triple Negative Breast Cancer Cells. Small 2017, 13, 1700544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Yuan H; Fales AM; Vo-Dinh T TAT Peptide-Functionalized Gold Nanostars: Enhanced Intracellular Delivery and Efficient NIR Photothermal Therapy Using Ultralow Irradiance. J. Am. Chem. Soc 2012, 134, 11358–11361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Huang X; Lai Y; Braun GB; Reich NO Modularized Gold Nanocarriers for TAT-Mediated Delivery of siRNA. Small 2017, 13, 1602473. [Google Scholar]
- (6).Bertrand JR; Pottier M; Vekris A; Opolon P; Maksimenko A; Malvy C Comparison of Antisense Oligonucleotides and siRNAs in Cell Culture and in Vivo. Biochem. Biophys. Res. Commun 2002, 296, 1000–1004. [DOI] [PubMed] [Google Scholar]
- (7).Watts J; Corey D Gene Silencing by siRNAs and Antisense Oligonucleotides in the Laboratory and the Clinic. J. Pathol 2012, 226, 365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Hannon GJ RNA Interference. Nature 2002, 418, 244–251. [DOI] [PubMed] [Google Scholar]
- (9).Meister G; Tuschl T Mechanisms of Gene Silencing by Double-Stranded RNA. Nature 2004, 431, 343–349. [DOI] [PubMed] [Google Scholar]
- (10).Riley RS; Day ES Gold Nanoparticle-Mediated Photo-thermal Therapy: Applications and Opportunities for Multimodal Cancer Treatment. Wiley Interdiscip. Rev. Nanomedicine Nano-biotechnology 2017, 9, e1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Day ES; Zhang L; Thompson PA; Zawaski JA; Kaffes CC; Gaber MW; Blaney SM; West JL Vascular-Targeted Photothermal Therapy of an Orthotopic Murine Glioma Model. Nanomedicine (London, U. K.) 2012, 7, 1133–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Nanospectra Biosciences, Inc. Pilot Study of AuroLase(tm) Therapy in Refractory and/or Recurrent Tumors of the Head and Neck https://clinicaltrials.gov/ct2/show/NCT00848042?term=auroshell&rank=2 (accessed May 23, 2016).
- (13).Nanospectra Biosciences, Inc. Efficacy Study of AuroLase Therapy in Subjects with Primary and/or Metastatic Lung Tumors https://clinicaltrials.gov/ct2/show/NCT01679470?term=auroshell&rank=3 (accessed May 23, 2016).
- (14).Weissleder R A Clearer Vision for in Vivo Imaging. Nat. Biotechnol 2001, 19, 316–317. [DOI] [PubMed] [Google Scholar]
- (15).Wijaya A; Schaffer SB; Pallares IG; Hamad-Schifferli K Selective Release of Multiple DNA Oligonucleotides from Gold Nanorods. ACS Nano 2009, 3, 80–86. [DOI] [PubMed] [Google Scholar]
- (16).Huschka R; Zuloaga J; Knight MW; Brown LV; Nordlander P; Halas NJ Light-Induced Release of DNA from Gold Nanoparticles: Nanoshells and Nanorods. J. Am. Chem. Soc 2011, 133, 12247–12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Huschka R; Barhoumi A; Liu Q; Roth J. a; Ji L; Halas NJ Gene Silencing by Gold Nanoshell-Mediated Delivery and Laser-Triggered Release of Antisense Oligonucleotide and siRNA. ACS Nano 2012, 6, 7681–7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Barhoumi A; Huschka R; Bardhan R; Knight MW; Halas NJ Light-Induced Release of DNA from Plasmon-Resonant Nanoparticles: Towards Light-Controlled Gene Therapy. Chem. Phys. Lett 2009, 482, 171–179. [Google Scholar]
- (19).Goodman AM; Hogan NJ; Gottheim S; Li C; Clare SE; Halas NJ Understanding Resonant Light-Triggered DNA Release from Plasmonic Nanoparticles. ACS Nano 2017, 11, 171–179. [DOI] [PubMed] [Google Scholar]
- (20).Braun GB; Pallaoro A; Wu G; Missirlis D; Zasadzinski J. a; Tirrell M; Reich NO Laser-Activated Gene Silencing via Gold Nanoshell-siRNA Conjugates. ACS Nano 2009, 3, 2007–2015. [DOI] [PubMed] [Google Scholar]
- (21).Lu W; Zhang G; Zhang R; Flores LG; Huang Q; Gelovani JG; Li C Tumor Site – Specific Silencing of NF-κB p65 by Targeted Hollow Gold Nanosphere − Mediated Photothermal Transfection. Cancer Res 2010, 70, 3177–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Jones MR; Millstone JE; Giljohann D. a; Seferos DS; Young KL; Mirkin C. a. Plasmonically Controlled Nucleic Acid Dehybridization with Gold Nanoprisms. ChemPhysChem 2009, 10, 1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Jain PK; Qian W; El-Sayed MA Ultrafast Cooling of Photoexcited Electrons in Gold Nanoparticle-Thiolated DNA Conjugates Involves the Dissociation of the Gold-Thiol Bond. J. Am. Chem. Soc 2006, 128, 2426–2433. [DOI] [PubMed] [Google Scholar]
- (24).Hildebrandt B; Wust P; Ahlers O; Dieing A; Sreenivasa G; Kerner T; Felix R; Riess H The Cellular and Molecular Basis of Hyperthermia. Crit. Rev. Oncol. Hematol 2002, 43, 33–56. [DOI] [PubMed] [Google Scholar]
- (25).Melamed JR; Edelstein RS; Day ES Elucidating the Fundamental Mechanisms of Cell Death Triggered by Photothermal Therapy. ACS Nano 2015, 9, 6–11. [DOI] [PubMed] [Google Scholar]
- (26).Lee SE; Liu GL; Kim F; Lee LP Remote Optical Switch for Localized and Selective Control of Gene Interference. Nano Lett 2009, 9, 562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Poon L; Zandberg W; Hsiao D; Erno Z; Sen D; Gates BD; Branda NR Photothermal Release of Single-Stranded DNA from the Surface of Gold Nanoparticles Through Controlled Denaturating and Au-S Bond Breaking. ACS Nano 2010, 4, 6395–6403. [DOI] [PubMed] [Google Scholar]
- (28).Takahashi H; Niidome Y; Yamada S Controlled Release of Plasmid DNA from Gold Nanorods Induced by Pulsed near-Infrared Light. Chem. Commun 2005, 2247–2249. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


