Abstract
The ability to constantly anticipate events in the world is critical to human survival. It has been suggested that predictive processing originates from the motor system and that incoming sensory inputs can be altered to facilitate sensorimotor integration. In the current study, we investigated the role of the readiness potentials, i.e. the premotor brain activity registered within the fronto-parietal areas, in sensorimotor integration. We recorded EEG data during three conditions: a motor condition in which a simple action was required, a visual condition in which a visual stimulus was presented on the screen, and a visuomotor condition wherein the visual stimulus appeared in response to a button press. We measured evoked potentials before the motor action and/or after the appearance of the visual stimulus. Anticipating a visual feedback in response to a voluntary action modulated the amplitude of the readiness potentials. We also found an enhancement in the amplitude of the visual N1 and a reduction in the amplitude of the visual P2 when the visual stimulus was induced by the action rather than externally generated. Our results suggest that premotor brain activity might reflect predictive processes in sensory-motor binding and that the readiness potentials may possibly represent a neural marker of these predictive mechanisms.
Keywords: Sensorimotor, Predictive processes, Readiness potentials, Sensory suppression
1. Introduction
The term “agency” refers to the human sense of control over voluntary actions and over the sensory consequences of these actions. Agency implies an endogenous causation, as voluntary actions are performed to produce desired effects in the outside world (Haggard, 2017; Haggard and Chambon, 2012; Moore, 2016). For this reason, intentional actions should be cognitively represented in terms of their anticipated sensory consequences (Hommel, 1996; Koch et al., 2004; Prinz, 1997), facilitating the discrimination between self-produced and externally generated sensations (Clark, 2013; Friston, 2010; Haggard and Chambon, 2012).
Despite the acknowledged significance of predictive processes, the link between intentional actions and their sensory consequences remains unknown. An “internal model” has been proposed; the idea of a system that estimates the outcomes of an action before sensory feedback is available. This system makes use of the efferent motor signal, generated in the motor areas during a voluntary movement, to anticipate a sensory outcome and modulate its processing (Wolpert, 1997; Wolpert et al., 1995). This way, it would be easier to discern the effect of an action from externally generated sensory stimuli (Blakemore et al., 1999, 2001; Blakemore et al., 1998; Weiss et al., 2011). For example, people cannot tickle themselves, as tactile stimuli are “suppressed” when they are self-produced rather than externally generated (Blakemore et al., 1998).
Recent studies supported the notion of an internal model showing that the Supplementary Motor Area (SMA), a brain region located in the dorsomedial frontal cortex, plays an important role during motor preparation and in controlling perceptual processing during voluntary actions (Nachev et al., 2008). Activity within the SMA increases when individuals focus attention on their intent to move, rather than to the movement itself (Lau et al., 2004), and during sensorimotor learning (Chen and Wise, 1996, 2018; Nakamura et al., 1998), reinforcing the idea that premotor activity might be related to motor control. Indeed, transient disruption of the SMA with a Transcranial Magnetic Stimulation (TMS) prepulse can reduce and almost abolish sensory suppression during voluntary action, supporting the idea that the motor command itself contributes to predictive processes (Haggard and Whitford, 2004; Voss et al., 2006).
In primates, SMA neurons fire before limb movements (Brinkman and Porter, 1979; Tanji and Kurata, 1982) and similarly in humans, the onset of intentional actions is always preceded by an increased activity in the motor areas (Kornhuber and Deecke, 1965) as revealed by studies using electroencephalography (EEG). The Readiness Potential – RP (Kornhuber and Deecke, 1965) is a negative deflection maximal at the midline centro-parietal area (Deecke et al., 1969; Kornhuber and Deecke, 1965; Shibasaki and Hallett, 2006) and it represents an electrophysiological measurement of the premotor activity in humans. The RP is usually associated with an increase in neural activity that spreads from the SMA (Lang et al., 1991; Shibasaki and Hallett, 2006; Yazawa et al., 2000) to the primary motor cortex - M1 (Fried et al., 2011; Pedersen et al., 1998). Specifically, the RP comprises an early component that starts around 1 s before movement’s onset reflecting a bilateral activation of the SMA, and a late component reflecting the lateralized activation of M1 (Oken and Phillips, 2009). The RP is followed by a bilateral positive potential distributed over the parietal cortex that starts around the onset of the action (Deecke et al., 1982). Such premotor activity has been related to planning and preparation of voluntary movements (Keller and Heckhausen, 1990; Libet et al. 1982; Libet et al. 1983; Shibasaki and Hallett, 2006).
In the current study, we investigated whether RPs and visual potentials can be modulated by action-effect contingency. Following the notion of an internal model and the idea that the SMA might play a role in predictive processes, we would expect a modulation of the RPs when individuals perform voluntary actions with the intention of causing a sensory event as compared to the production of actions without effects. Specifically, we would expect an increased pre-motor activity possibly driven by sensorimotor learning. Moreover, predictive processes should suppress visual responses when visual stimuli are induced by an intentional movement rather than externally generated. To rule out any effect of action execution (such as speed and/or amplitude of the movement) on the RPs, in a control experiment we measured electromyography (EMG) while participants performed an action with and without receiving a visual feedback.
2. Methods
2.1. Participants
The study was approved by the local ethics committee at the University of Nevada, Reno and followed the principles of the Declaration of Helsinki. Participants read and signed a written informed consent about the experimental protocol and received monetary compensation for taking part in the study. A total of fifteen right-handed healthy volunteers (mean age= 28±2 years) were recruited at the University of Nevada, Reno. All participants had normal or corrected to normal vision and none of them had a history of motor or neurological disease.
Eight of these participants were also tested in the control EMG experiment.
2.2. Stimuli and procedures
During the experiment, participants sat in a quiet and darkened room at 57 cm from the computer screen. The visual stimulus was a 6° diameter white circle flashed on a grey background for 30 ms on a CRT monitor. Motor actions were button presses performed with the right index finger and recorded through the computer’s keyboard. The sound produced by the button press was removed by exposing participants to white noise delivered through earphones. To reduce variability across experimental conditions, white noise was presented in all conditions, even those that didn’t involve voluntary movements.
Participants performed three separate blocks of 100 trials. Each block represented a separate condition: visual, motor, or visuomotor. The order of the blocks was counterbalanced across participants. Trials always started with a fixation cross appearing at the center of the screen and disappearing after 2 s (avoiding any contamination of the data induced by the visual potentials evoked by the fixation cross). In the visual condition, the disappearance of the fixation cross signaled the occurrence of the visual stimulus. Participants were asked to maintain their fixation at the center of the screen. The visual stimulus came into sight after a delay ranging between 700 and 900 ms from the disappearance of the fixation cross. This delay range was selected to make the timing of the visual stimulus comparable across the visual and visuomotor conditions. We selected 100 different delays within this range using 2 ms steps, and then randomized these values within the block. The following trial started 1.5 s after the visual stimulus vanished. In the motor condition, participants were instructed to press a button at their own will, but only after the disappearance of the fixation cross. 1.5 s after the button press, the next trial started. The visuomotor condition was a combination of the visual and the motor conditions. When the fixation cross disappeared, participants pressed a button at their own will and received a visual feedback 300 ms later. Like the other two conditions, the inter-trial interval was set to 1.5 s (calculated from the disappearance of the visual stimulus). Fig. 1A shows example procedures for the viusomotor condition.
Fig. 1.
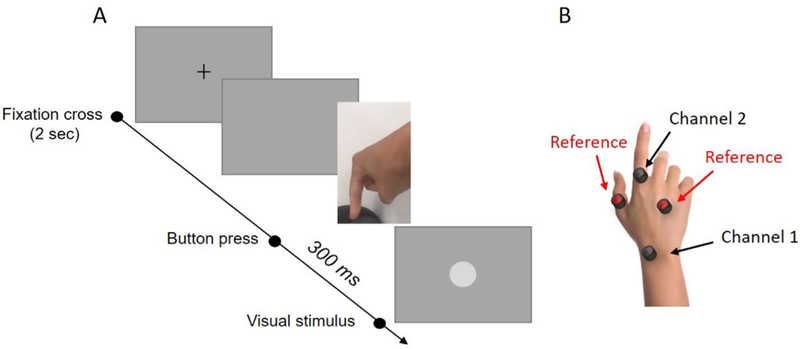
A) Procedures for the visuomotor condition. At the beginning of each trial a fixation cross appeared on the screen for 2 s. After the disappearance of the cross, participants pressed a button and received a visual feedback (a 6° diameter white circle) 300 ms after the button press. In the motor condition, when the cross disappeared, participants had to press a button but received no visual feedback. In the visual condition, no action was required but rather a visual stimulus appeared in the center of the screen. B) Electrodes location in the EMG control experiment.
In a control experiment we measured differences in the electrical activity of finger muscles between the motor and the visuomotor condition. Participants performed a block of 100 trials for each of the two conditions while we recorded muscle activity using EMG. Two electrodes from the Biosemi system (see Fig. 1B, channel 1 and channel 2) were positioned on participants’ posterior right hand to record the activity of the index finger’s muscles. Other two electrodes, used as references during data analysis, were positioned on the same hand: one on the thumb and the other one in the proximity of the digiti minimi. Only for recording purposes, reference electrodes were positioned on the scalp (Cz, Pz, Fz and two other electrodes located between Fz and Cz).
2.3. EEG/ERP analysis
EEG data were continuously recorded with a Biosemi 128 Channel electroencephalography system. We also used 4 extra channels for electrooculography (EOG), two channels on the external side of the eye to detect horizontal movements and two channels above and below the right eye to detect vertical eye movements. Data were analyzed using EEGLAB 12_0_2_6b and ERPLAB 5.0.0.0 running under MATLAB 2014a (The Mathworks, Inc.). EEG recording was sampled for analysis at 256 Hz and filtered with a 0.1-to-30 Hz bandpass filter. Raw data were referenced to the average of the 128 scalp channels. For the motor and the visuomotor conditions, the continuous EEG was segmented into epochs from 2000 ms before the onset of the button press until 100 ms after the motor event. For the visual and the visuomotor conditions, the continuous EEG was segmented into epochs from 300 ms before the onset of the visual stimulus until 500 ms after its presentation. From the original signal we subtracted a baseline calculated as the average voltage of the entire epoch. Epochs containing considerable motor artifacts were detected through visual inspection/EOG and rejected. Afterwards, blink artifacts were identified using EOG data and corrected using an Independent Component Analysis (ICA). Individual Event Related Potentials (ERPs) were calculated as the average of all the epochs for each experimental condition. ERPs were then averaged across participants.
Temporal windows were selected to identify specific ERP components and measure the amplitude and latency of these components. For the readiness potentials (RPs) we measured the voltage at the central channel Cz. Based on data observation, we decided to consider an early RP component extracted from −1000 to −500 ms, and a late RP component isolated from −500 ms until the onset of the action. Lateralized readiness potentials (LRPs) were calculated as the difference between the voltage in the left (contralateral to the moving finger) C3 channel and the right (ipsilateral to the moving finger) C4 channel in a temporal window of 500 ms before the onset of the action. For both RP and LRP, the amplitude was calculated as the mean voltage within a specified time range, marked by a starting and an ending latency. The onset of the RP and LRP was calculated as the fractional area latency (Lopez-Calderon and Luck, 2014). This method defines the latency of the component as the first time point at which a certain percentage (20% in our analysis) of the total area of the component has been reached. The analysis is conducted on rectified data (i.e. absolute values - negative values become positive), within the defined temporal window (from −1000 to 0 ms).
Visual evoked potentials (VEPs) were also analyzed. One participant was excluded from the analysis because the rate, amplitude, and pattern of spontaneous eye-blinks and eye-movements that occurred after stimulus presentation significantly compromised the quality of the EEG signal (data were not excluded for the RP and LRP analysis). The first visual component, the P1, which is a positive electric potential with a peak normally observed around 100 ms after stimulus onset, was measured in the occipital Oz, O1 and O2 channels. The temporal window for the visual P1 was set between 50 and 150 ms from the onset of the visual stimulus. The concurrent negative potential, the N1, was measured in the same temporal window in the parietal regions, specifically in the Cz, C3 and C4 channels. We also analyzed the succeeding parietal positive potential, the P2, recorded from the Cz and Fz channel in the temporal window between 180 and 220 ms form stimulus onset. The amplitude of the VEPs was calculated with the same procedures used for the premotor potentials.
2.4. EMG analysis
Utilized for the EEG and analyzed using EEGLAB 12_0_2_6b and ERPLAB 5.0.0.0 running under MATLAB 2014a (The Mathworks, Inc.). EMG recording was sampled at 2048 Hz and filtered with a high pass Butterworth filter (cut off at 10 Hz) and with a 60 Hz notch filter. Raw data were referenced to the two external reference channels (see Fig. 1B). The continuous EMG signal was segmented into epochs from 2000 ms before the onset of the button press until 100 ms after the motor event (as for the RPs). From the original signal we subtracted a baseline (the average voltage of the entire epoch). Epochs containing large artifacts were detected through visual inspection and rejected. Data were then rectified and integrated. Specifically, we calculated the area under the curve of the rectified EMG signal, that is the mathematical integral of the absolute value of the EMG signal, within the temporal window between −1000 and 0 ms from the onset of the button press. MEPs were then averaged across participants for each experimental condition.
3. Results
Fig. 2 shows the scalp map (panel A) and the average voltage (panel B) of the RPs and the LRPs in the motor and visuomotor conditions. The two graphs in Fig. 2B show RP and LRP amplitudes in both motor (black) and visuomotor (green) conditions as a function of time, with the 0 and the black dashed line denoting the onset of the action. Expecting a visual feedback in response to a voluntary action significantly modulated premotor cortical activity in a non-monotonic way. The voltage measured in the Cz channel is similar between the motor and the visuomotor from 2000 ms to 1000 ms before movement’s onset. Around 1000 ms before the button press, the electric voltage moved toward negative values and changes in the electric potential were modulated by the presence of the visual feedback. Specifically, the descent was delayed in the visuomotor condition as compared to the motor condition. As a result, in the temporal window ranging from 1000 to 500 ms prior to the button press, the electric potential was significantly more negative in the motor as compared to the visuomotor condition (paired two-tailed t-test, Bonferroni correction for two comparisons represented by the two temporal windows; t14 = −2.44; p = 0.02), as shown in the left panel of Fig. 2A and in the yellow window of the left panel of Fig. 2B. The voltage of the RP became more negative in the succeeding 500 ms, reaching the most negative peak around 200 ms before the movement onset in both the conditions. Also in this temporal window we found a significant difference between experimental conditions, with the RP being more negative in the visuomotor as compared to the motor condition (paired two-tailed t-test, Bonferroni correction for two temporal windows; t14 =2.75; p =0.015), as shown in the right panel of Fig. 2A and in the blue window of the left panel of Fig. 2B. Overall, the RP seemed to be more precipitous, shrunk and delayed in time in the visuomotor as compared to the motor condition. In agreement with this idea we found a significant difference in the latency (calculated from 1000 ms before the onset of the movement) of the RP between the two experimental conditions (paired two-tailed t-test; t14 = 3.17; p = 0.006).
Fig. 2.
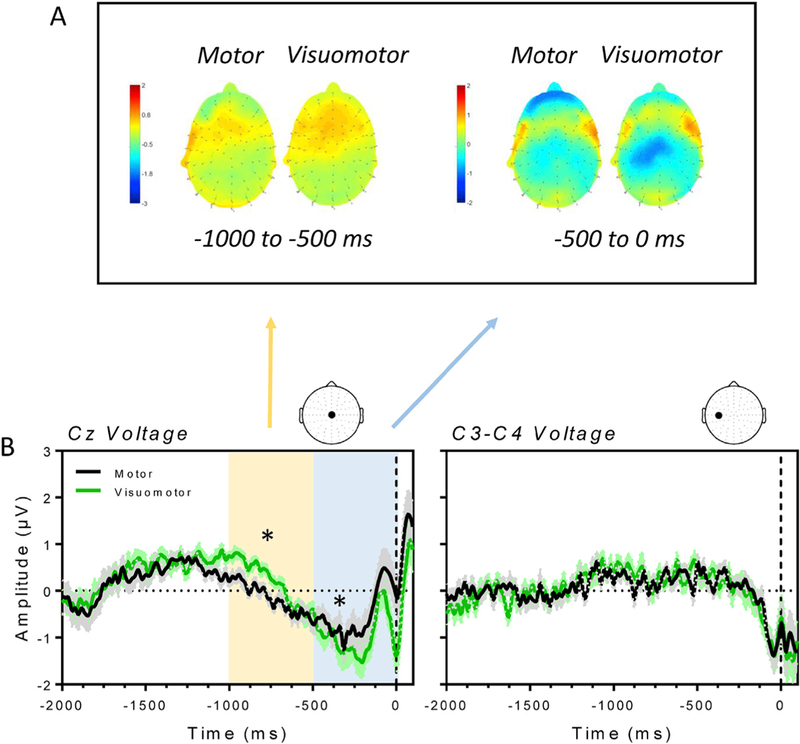
A) Scalp map for the motor and visuomotor condition averaged across two temporal windows. From 1000 to 500 ms before the button press (left panel) and from 500 ms to the onset of the motor action (right panel). The RP is initially more positive in the visuomotor as compared to the motor condition. The negative potential dramatically increases in the 500 ms before the button press with a significant difference between the two conditions. B) Average RPs (±SE; left panel) recorded from the Cz electrode and LRPs (±SE; right panel), measured as the difference between the activity at the C3 and the C4 channel, for the motor (black line) and visuomotor (green line) condition. Time 0 on the x-axis denotes the onset of the motor action. The yellow and the blue window in the left panel denote a significant difference between electric potentials measured in the motor and the visuomotor condition, the same differences reported in the scalp maps.
Moreover, we fitted data using a linear regression model (in the temporal window between −1000 ms and −200 ms) to test for differences in the pattern of descend of the electric potential across conditions. Results, reported in Fig. 3, showed a significant difference in the slope between the two linear fit representing the motor and the visuomotor condition (ANCOVA, F(1,406) =915.3, P < 0.0001). In the motor condition, the slope of the best linear fit was equal to −0.0017 ± 3.292e-005 while in the visuomotor condition was equal to −0.0032 ± 3.48 e–005. On the other side, no significant difference was found in the LRPs between experimental conditions (Fig. 2B, right panel).
Fig. 3.
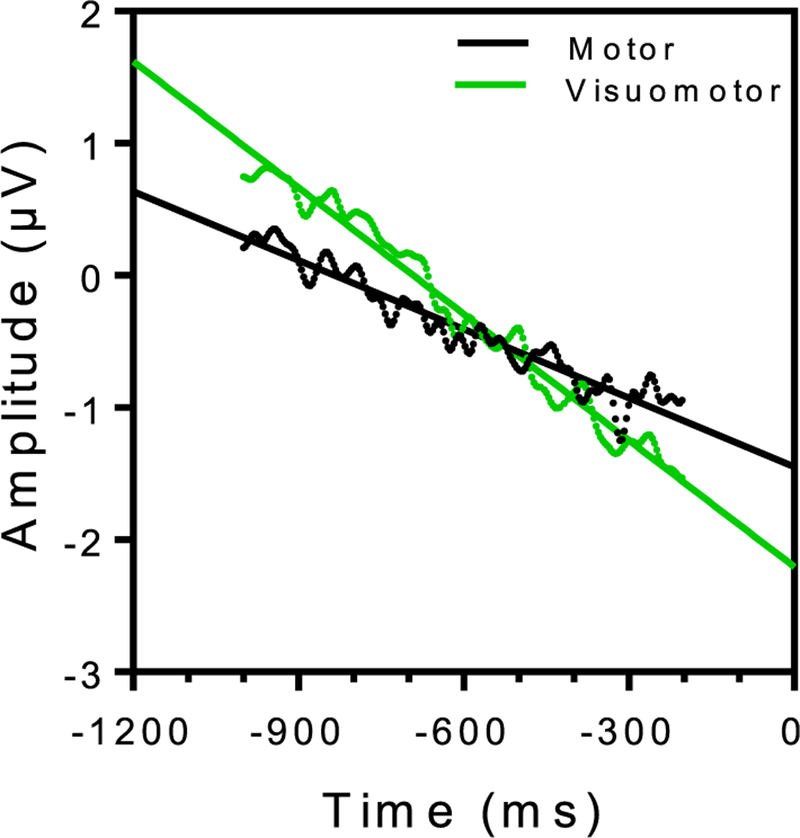
Linear fits of the negative descend characterizing the RPs in the motor (black dots and line) and the visuomotor (green dots and line) condition. Data were fitted only for latencies from 1000 to 200 ms prior to the onset of the motor action.
Fig. 4 shows the scalp map (panel A) and the average voltage (panel B) of the P1 component of the VEPs. The graph in Fig. 4B plots the amplitude of the VEPs in both visual (black) and visuomotor (green) conditions as a function of time, with time 0 and the black dashed line denoting the onset of the visual stimulus. The positive deflection measured in the occipital area between 50 and 150 ms after stimulus onset, slightly increased in amplitude and extended more to the right hemisphere in the visuomotor condition as compared to the visual condition. However, differences were not statistically significant.
Fig. 4.
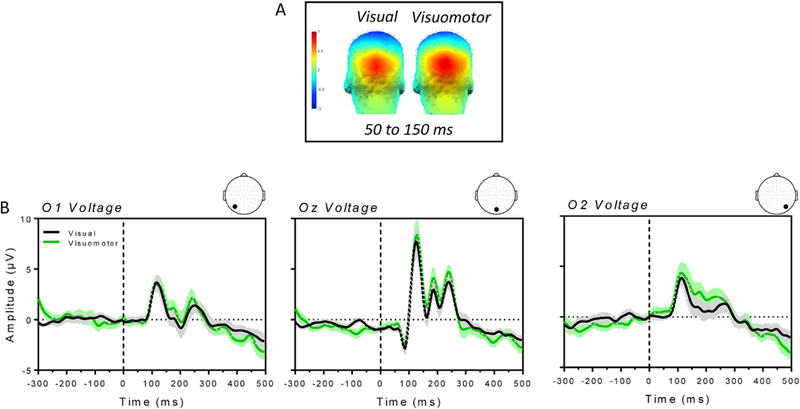
A) Scalp map for the visual and visuomotor condition averaged between 50 and 150 ms (time window of the P1) after stimulus onset. B) Average VEPs ( ± SE) recorded from the occipital Oz, O1 and O2 channels for the visual (black line) and visuomotor (green line) condition. Time 0 on the x-axis denotes the onset of the visual stimulus.
Fig. 5 shows the scalp map (panel A) and the average voltage (panel B) of the more parietal N1 and P2 components of the VEPs. The graph in Fig. 5B plots the amplitude of the electric potentials recorded in the left C3, central Cz and right C4 channel for the visual (black) and visuomotor (green) condition. We first tested for differences between the two conditions in the amplitude of the N1, the negative deflection occurring ~100 ms post-stimulus presentation, across the three channel locations. The N1 showed a greater amplitude at the left channel location in the visuomotor condition compared to the visual condition, as shown in the left scalp map of Fig. 5A and in the blue window in the graph of Fig. 5B reporting C3 voltage. There was a marginally significant difference between conditions in the amplitude of the N1 recorded at the C3 channel (paired two-tailed t-test, Bonferroni correction for the three channels; t13 = 2.65; p = 0.018). We also analyzed the parietal P2 component (in a temporal window between 100 and 200 ms post-stimulus onset) at the Cz and Fz channel. We found a significant reduction of the electric potential for the visuomotor condition with respect to the visual condition at the Cz channel location (paired one-tailed t-test, Bonferroni correction for the two channels; t13 = 2.39; p = 0.01). Such reduction is shown in the scalp map in Fig. 4A (right panel) and in the yellow window in the graph of Fig. 4B reporting the Cz voltage. No significant difference between the two conditions was found in the Fz channel.
Fig. 5.
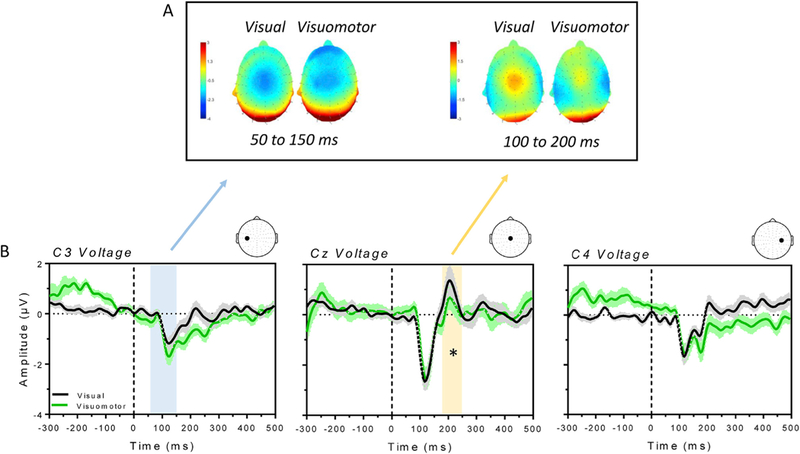
A) Scalp map for the visual and visuomotor condition averaged between 50 and 150 ms (left panel, latency of the N1) and 100–200 ms (right panel, latency of the P2) after stimulus onset. B) Average VEPs ( ±SE) recorded from the parietal Cz, C3 and C4 channels for the visual (black line) and visuomotor (green line) condition. Time 0 on the x-axis denotes the onset of the visual stimulus. The blue and the yellow window highlight a marginally significant and a significant difference between the two experimental conditions, respectively.
Fig. 6 shows the results for the control EMG experiment. Average voltage recorded and averaged across the two channels is plotted for the motor (black) and visuomotor (green) condition as a function of time, with time 0 and the black dashed line reflecting the onset of the motion action. There was no difference in the muscular activity between the two experimental conditions.
Fig. 6.
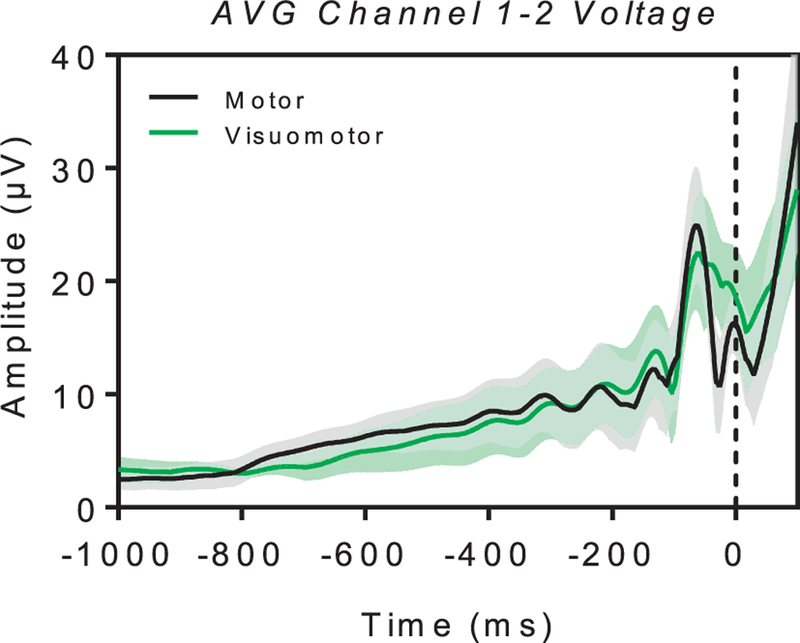
EMG data (AVG voltage ± SE) for the average of the two external channels in the motor (black line) and the visuomotor (green line) condition. Time 0 on the x-axis and the black dashed line denote the onset of the motor action as recorded from the computer keyboard.
4. Discussion
In the current study, we investigated a neural marker related to predictive processing and more in general the sense of agency. First, we showed that premotor brain activity, i.e. the RP, is modulated by stimulus expectancy. Second, we showed a motor-induced modulation of visual cortical responses that occurred at different stages of visual processing. Early parietal responses occurring ~100 ms after stimulus presentation were enhanced, while late parietal responses (~200 ms after) were suppressed when the visual stimulus was the outcome of a motor action rather than externally generated.
Freely voluntary movements are preceded by preparatory processes that have been associated with motor planning and intentions (DEECKE et al., 1984; Keller and Heckhausen, 1990; Benjamin Libet et al., 1983; H Shibasaki et al., 1980; Vaughan et al., 1968). Premotor activity may be related to predictive processes as intentional actions are performed to produce an effect in the environment. Previous neurophysiological studies already supported the importance of the motor system for predictive processes, showing that TMS over pre-supplementary motor area (pre-SMA) produces a decrease in intentional binding (Moore, 2016). Moreover, the simultaneous acquisition of EEG and fMRI confirmed that SMA is a crucial region contributing to the sustained activity of the RP before movement (Nguyen et al., 2014). Our data are consistent with the involvement of SMA in predictive processes. Indeed, attending to a sensory feedback in response to a voluntary movement modulated the amplitude of the RP and delayed the latency of its onset, suggesting that premotor activity within the fronto-parietal regions might play a crucial role in predictive processes and more in general in the sense of agency.
The features of the RP waveform (i.e. amplitude and latency) can be modulated by multiple factors, such as movement selection (Baker et al., 2011), attention (Spring et al., 2016) and neuromuscular fatigue (Lang et al., 1983). To exclude the possibility that the modulation of the RPs found in our study might be induced by differences in the muscular activity during movements (for example amplitude, speed of movements) between conditions, we ran a control EMG experiment. Results showed no significant differences in motor performance between the visuomotor and the motor condition, confirming that the effect that we found on the RP results from higher cognitive processes, such as sensorimotor integration and/or visuomotor learning (Holst and Mittelstaedt, 1971).
It has been suggested that an efferent copy of the motor command alerts sensory cortices about the upcoming sensory feedback, modulating their response properties (Creutzfeldt et al., 1989; Flinker et al., 2010; Greenlee et al., 2011; Niziolek et al., 2013). For instance, ERP studies reported suppressed auditory responses to self-generated speech (Blakemore et al., 1999, 1998) and reduced tactile sensitivity following self-initiated hand movements, (Dunn et al., 1998; Golob and Starr, 2000; Tremblay et al., 2014). Results from our study showed an attenuation of the P2 that may indicate visual suppression. The P2 component has not been entirely characterized yet, mainly because it can be modulated by a diverse number of factors. This ERP component is generated within parieto-occipital regions and seems to be involved in several cognitive processes, such as memory (Hackley et al., 1990; Hillyard et al., 1973) and selective attention (Sanmiguel et al., 2013). In general, the P2 might be related to learning and matching sensory information with stored memory. More interesting is that the amplitude of the auditory P2 is reduced during self-initiated sounds (Mifsud et al., 2016) and after button-press-initiated auditory stimuli, as compared to externally initiated sounds (Di Russo et al. 2002). Analogously, we showed a reduction of the visual P2 amplitude in response to a visual stimulus that was generated by a voluntary action, as compared to a visual stimulus externally generated.
Results from this study also showed an enhancement of an early visual component, the N1, a negative parietal component showing a peak between 100 and 130 ms after stimulus onset. The information-processing contributions of the N1 are not well understood. Previous studies suggested that the N1 component might reflect the operation of a discriminative process that is applied to a restricted area of visual space (Molholm et al., 2004). Specifically, N1 amplitude is greater for attended-location stimuli compared with stimuli presented under neutral or distributed attention conditions. In our study, the visual stimulus was attended in both the visual and the visuomotor condition and the spatial location of the stimulus did not vary across conditions. However, the N1 was enhanced when the attended visual stimulus was caused by the participants’ intentional action and the enhancement was restricted to the left hemisphere, contralateral to the moving hand. Remarkably, a modulation of the N1 has also been related to the integration of stimuli from different modalities (Murray et al., 2002; Rossion et al., 2000; Vogel and Luck, 2000) and to more general ventral stream functions related to the visual processing of the structural features of objects (Nguyen et al., 2014). Similarly, the modulation that we observed could be related to sensorimotor integration.
On the whole, the current study suggests that the brain constantly anticipates events in the world. Internal sensory predictions may possibly be generated prior to voluntary movements, within supplementary motor areas of the brain, to help discriminating self-produced from externally generated sensory stimuli. Such premotor signal can modulate sensory cortices integrating voluntary actions with a representation of the expected sensory feedbacks. Future studies should further investigate potential correlation between neurophysiological and behavioral effects of sensorimotor binding.
Acknowledgments
This research has been supported by EY023268 to Fang Jiang, EY10834 to Michael Webster, and P20 GM103650. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Baker KS, Mattingley JB, Chambers CD, Cunnington R, 2011. Attention and the readiness for action. Neuropsychologia 49 (12), 3303–3313. 10.1016/j.neuropsychologia.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Frith CD, Wolpert DM, 1999. Spatio-temporal prediction modulates the perception of self-produced stimuli. J. Cognit. Neurosci 11 (5), 551–559. 10.1162/089892999563607. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Frith CD, Wolpert DM, 2001. The cerebellum is involved in predicting the sensory consequences of action. Neuroreport 12 (9), 1879–1884. 10.1097/00001756-200107030-00023. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert DM, Frith CD, 1998. Central cancellation of self-produced tickle sensation. Nat. Neurosci 1 (7), 635–640. 10.1038/2870. [DOI] [PubMed] [Google Scholar]
- Brinkman C, Porter R, 1979. Supplementary motor area in the monkey: activity of neurons during performance of a learned motor task. J. Neurophysiol 42 (3), 681–709. [DOI] [PubMed] [Google Scholar]
- Chen L, Wise S, 1996. Evolution of directional preferences in the supplementary eye field during acquisition of conditional oculomotor associations. Evolut. Direct. Preferences Suppl. Eye Field during Acquisit. Cond. Oculomot. Assoc 16 (9), 3067–3081. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/8622136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wise SP, 2018. Supplementary Eye Field Contrasted with the Frontal Eye Field during Acquisition of Conditional Oculomotor Associations, vol. 73 (3). [DOI] [PubMed] [Google Scholar]
- Clark A, 2013. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav. Brain Sci 36 (3), 181–204. 10.1017/S0140525X12000477. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O, Ojemann G, Lettich E, 1989. Neuronal activity in the human lateral temporal lobe II. Responses to the subjects own voice. Exp. Brain Res 77, 476–489. 10.1007/BF00249600. [DOI] [PubMed] [Google Scholar]
- Deecke L, Heise B, Kornhuber HH, Lang M, Lang W, 1984. Brain potentials associated with voluntary manual tracking: bereitschaftspotential, conditioned premotion positivity, directed attention potential, and relaxation potential: anticipatory activity of the limbic and frontal cortex. Ann. N. Y. Acad. Sci 425 (1), 450–464. 10.1111/j.1749-6632.1984.tb23567.x. [DOI] [PubMed] [Google Scholar]
- Deecke L, Scheid P, Kornhuber HH, 1969. Distribution of readiness potential, premotion positivity, and motor potential of the human cerebral cortex preceding voluntary finger movements. Exp. Brain Res 7 (2), 158–168. 10.1007/BF00235441. [DOI] [PubMed] [Google Scholar]
- Deecke L, Weinberg H, Brickett P, 1982. Magnetic fields of the human brain accompanying voluntary movement: Bereitschaftsmagnetfeld. Exp. Brain Res 48 (1), 144–148. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7140885. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martínez A, Sereno MI, Pitzalis S, Hillyard SA, 2002. Cortical sources of the early components of the visual evoked potential. Hum. Brain Mapp 15 (2), 95–111. 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn BR, Dunn D. a, Languis M, Andrews D, 1998. The relation of ERP components to complex memory processing. Brain Cognit 36 (3), 355–376. 10.1006/brcg.1998.0998. [DOI] [PubMed] [Google Scholar]
- Flinker A, Chang EF, Kirsch HE, Barbaro NM, Crone NE, Knight RT, 2010. Single-trial speech suppression of auditory cortex activity in humans. J. Neurosci.: The Official Journal of the Society for Neuroscience 30 (49), 16643–16650. 10.1523/JNEUROSCI.1809-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried I, Mukamel R, Kreiman G, 2011. Internally generated preactivation of single neurons in human medial frontal cortex predicts volition. Neuron 69 (3), 548–562. 10.1016/j.neuron.2010.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, 2010. The free-energy principle: a unified brain theory? Nat. Rev. Neurosci 11 (2), 127–138. 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- Golob EJ, Starr a., 2000. Age-related qualitative differences in auditory cortical responses during short-term memory. Clin. Neurophysiol.: Official Journal of the International Federation of Clinical Neurophysiology 111 (12), 2234–2244. 10.1016/S1388-2457(00)00468-5. [DOI] [PubMed] [Google Scholar]
- Greenlee JDW, Jackson AW, Chen F, Larson CR, Oya H, Kawasaki H, et al. , 2011. Human auditory cortical activation during self-vocalization. PLoS One 6 (3). 10.1371/journal.pone.0014744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackley SA, Woldorff M, Hillyard SA, 1990. Cross-modal selective attention effects on retinal, myogenic, brainstem, and cerebral evoked potentials. Psychophysiology 27 (2), 195–208. 10.1111/j.1469-8986.1990.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Haggard P, 2017. Sense of agency in the human brain. Nat. Rev. Neurosci 18 (4), 196–207. 10.1038/nrn.2017.14. [DOI] [PubMed] [Google Scholar]
- Haggard P, Chambon V, 2012. Sense of agency. Curr. Biol 22 (10), R390–R392. 10.1016/j.cub.2012.02.040. [DOI] [PubMed] [Google Scholar]
- Haggard P, Whitford B, 2004. Supplementary motor area provides an efferent signal for sensory suppression. Cognit. Brain Res 19 (1), 52–58. 10.1016/j.cogbrainres.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW, 1973. Electrical signs of selective attention in the human brain. Science 182 (4108), 177–180. 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Holst E, Mittelstaedt H, 1971. The principle of reafference: interactions between the central nervous system and the peripheral organs. In: Dodwell PC (Ed.), Perceptual Processing: Stimulus Equivalence and Pattern Recognition, (1950), pp. 41–72. Retrieved from. http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:The+Principle+of+Reafference+:+Interactions+Between+the+Central+Nervous+System+and+the+Peripheral+Organs#3.
- Hommel B, 1996. The cognitive representation of action: automatic integration of perceived action effects. Psychol. Res 59 (3), 176–186. 10.1007/BF00425832. [DOI] [PubMed] [Google Scholar]
- Keller I, Heckhausen H, 1990. Readiness potentials preceding spontaneous motor acts: voluntary vs. involuntary control. Electroencephalogr. Clin. Neurophysiol 76 (4), 351–361. 10.1016/0013-4694(90)90036-J. [DOI] [PubMed] [Google Scholar]
- Koch I, Keller P, Prinz W, 2004. The ideomotor approach to action control: implications for skilled performance. Int. J. Sport Exerc. Psychol 2 10.1093/mind/os-IX.33.1. [DOI] [Google Scholar]
- Kornhuber HH, Deecke L, 1965. Hirnpotentialanderungen bei willkurbewegungen und passiven bewegungen des menschen: bereitschftspotential und reafferente potentiale. Pflugers Archiv Fur Die Gesamte Physiologie 284, 1–17. [PubMed] [Google Scholar]
- Lang W, Cheyne D, Kristeva R, Beisteiner R, Lindinger G, Deecke L, 1991. Three-dimensional localization of SMA activity preceding voluntary movement - a study of electric and magnetic fields in a patient with infarction of the right supplementary motor area. Exp. Brain Res 87 (3), 688–695. 10.1007/BF00227095. [DOI] [PubMed] [Google Scholar]
- Lang W, Lang M, Kornhuber A, Deecke L, Kornhuber HH, 1983. Human cerebral potentials and visuomotor learning. Pflueg. Arch. Eur. J. Physiol 399 (4), 342–344. 10.1007/BF00652762. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Haggard P, Passingham RE, 2004. Attention to intention. Science 303 (5661), 1208–1210. 10.1126/science.1090973. [DOI] [PubMed] [Google Scholar]
- Libet B, Gleason CA, Wright EW, Pearl DK, 1983. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential): the unconscious initiation of a freely voluntary act. Brain 106 (3), 623–642. 10.1093/brain/106.3.623. [DOI] [PubMed] [Google Scholar]
- Libet B, Wright EW, Gleason CA, 1982. Readiness-potentials preceding unrestricted “spontaneous” vs. pre-planned voluntary acts. Electroencephalogr. Clin. Neurophysiol 54 (3), 322–335. 10.1016/0013-4694(82)90181-X. [DOI] [PubMed] [Google Scholar]
- Lopez-Calderon J, Luck SJ, 2014. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front. Hum. Neurosci 8 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifsud NG, Beesley T, Watson TL, Whitford TJ, 2016. Attenuation of auditory evoked potentials for hand and eye-initiated sounds. Biol. Psychol 120, 61–68. 10.1016/j.biopsycho.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Molholm S, Ritter W, Javitt DC, Foxe JJ, 2004. Multisensory visual-auditory object recognition in humans: a high-density electrical mapping study. Cerebr. Cortex 14 (4), 452–465. 10.1093/cercor/bhh007. [DOI] [PubMed] [Google Scholar]
- Moore JW, 2016. What is the sense of agency and why does it matter? Front. Psychol 10.3389/fpsyg.2016.01272. [DOI] [PMC free article] [PubMed]
- Murray MM, Wylie GR, Higgins B. a, Javitt DC, Schroeder CE, Foxe JJ, 2002. The spatiotemporal dynamics of illusory contour processing: combined high-density electrical mapping, source analysis, and functional magnetic resonance imaging. J. Neurosci.: The Official Journal of the Society for Neuroscience 22 (12), 5055–5073. https://doi.org/22/12/5055 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M, 2008. Functional role of the supplementary and presupplementary motor areas. Nat. Rev. Neurosci 9 (11), 856–869. 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sakai K, Hikosaka O, 1998. Neuronal activity in medial frontal cortex during learning of sequential procedures. J. Neurophysiol 80 (5), 2671–2687. Retrieved from. http://www.ncbi.nlm.nih.gov/pubmed/9819272. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Breakspear M, Cunnington R, 2014. Reciprocal interactions of the SMA and cingulate cortex sustain premovement activity for voluntary actions. J. Neurosci 34 (49), 16397–16407. 10.1523/JNEUROSCI.2571-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niziolek CA, Nagarajan SS, Houde JF, 2013. What does motor efference copy represent? Evidence from speech production. J. Neurosci 33 (41), 16110–16116. 10.1523/JNEUROSCI.2137-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken BS, Phillips TS, 2009. Evoked potentials: clinical. Encyclopedia of Neuroscience 19–28.
- Pedersen JR, Johannsen P, Bak CK, Kofoed B, Saermark K, Gjedde A, 1998. Origin of human motor readiness field linked to left middle frontal gyrus by MEG and PET. Neuroimage 8 (2), 214–220. 10.1006/nimg.1998.0362. [DOI] [PubMed] [Google Scholar]
- Prinz W, 1997. Perception and action planning. Eur. J. Cognit. Psychol 9 (2), 129–154. 10.1080/713752551. [DOI] [Google Scholar]
- Rossion B, Gauthier I, Tarr MJ, Despland P, Bruyer R, Linotte S, Crommelinck M, 2000. The N170 occipito-temporal component is delayed and enhanced to inverted faces but not to inverted objects: an electrophysiological account of face-specific processes in the human brain. Neuroreport 11 (1), 69–74. 10.1097/00001756-200001170-00014. [DOI] [PubMed] [Google Scholar]
- Sanmiguel I, Todd J, Schröger E, 2013. Sensory suppression effects to self-initiated sounds reflect the attenuation of the unspecific N1 component of the auditory ERP. Psychophysiology 50 (4), 334–343. 10.1111/psyp.12024. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Barrett G, Halliday E, Halliday AM, 1980. Cortical potentials following voluntary and passive finger movements. Electroencephalogr. Clin. Neurophysiol 50 (3–4), 201–2013. 10.1016/0013-4694(80)90147-9. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Hallett M, 2006. What is the Bereitschaftspotential? Clin. Neurophysiol 117 (11), 2341–2356. 10.1016/j.clinph.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Spring JN, Place N, Borrani F, Kayser B, Barral J, 2016. Movement-related cortical potential amplitude reduction after cycling exercise relates to the extent of neuromuscular fatigue. Front. Hum. Neurosci 10 10.3389/fnhum.2016.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J, Kurata K, 1982. Comparison of movement-related activity in two cortical motor areas of primates. J. Neurophysiol. (Bethesda) 48 (0022–3077), 633–653. Retrieved from file: //t/1982/Tanji_JNueorphsyiology_1982.pdf. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Ross B, Inoue K, McClannahan K, Collet G, 2014. Is the auditory evoked P2 response a biomarker of learning? Front. Syst. Neurosci 8 10.3389/fnsys.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan HG, Costa LD, Ritter W, 1968. Topography of the human motor potential. Electroencephalogr. Clin. Neurophysiol 25 (1), 1–10. 10.1016/0013-4694(68)90080-1. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ, 2000. The visual N1 component as an index of a discrimination process. Psychophysiology 37 (2), 190–203. 10.1111/1469-8986.3720190. [DOI] [PubMed] [Google Scholar]
- Voss M, Ingram JN, Haggard P, Wolpert DM, 2006. Sensorimotor attenuation by central motor command signals in the absence of movement. Nat. Neurosci 9 (1), 26–27. 10.1038/nn1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C, Herwig A, Schütz-Bosbach S, 2011. The self in action effects: selective attenuation of self-generated sounds. Cognition 121 (2), 207–218. 10.1016/j.cognition.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, 1997. Computational approaches to motor control. Trends Cognit. Sci 1 (6), 209–216. 10.1016/S1364-6613(97)01070-X. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI, 1995. An internal model for sensorimotor integration. Science 269 (5232), 1880–1882. 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- Yazawa S, Ikeda A, Kunieda T, Ohara S, Mima T, Nagamine T, et al. , 2000. Human presupplementary motor area is active before voluntary movement: subdural recording of Bereitschaftspotential from medial frontal cortex. Exp. Brain Res 131 (2), 165–177. 10.1007/s002219900311. [DOI] [PubMed] [Google Scholar]


