Abstract
Background:
Bacterial contamination of platelets remains the leading infectious risk from blood transfusion. Pathogen reduction(PR), point-of-release testing(PORt), and secondary bacterial culture(SBC) have been proposed as alternative risk control strategies, but a comprehensive financial comparison has not been conducted.
Methods:
A Markov-based decision-tree was constructed to model the financial and clinical impact of PR, PORt, and SBC, as well as a baseline strategy involving routine testing only. Hospitals were assumed to acquire leukoreduced apheresis platelets on day 3 post-collection, and, in the base case analysis, expiration would occur at the end of day 5(PR and SBC) or 7(PORt). Monte Carlo simulations assessed the direct medical costs for platelet acquisition, testing, transfusion, and possible complications. Input parameters, including test sensitivity and specificity, were drawn from existing literature and costs(2018 US$) were based on a hospital perspective.
Results:
The total costs per unit acquired by the hospital under the baseline strategy, PR, PORt, and SBC were $651.45, $827.82, $686.33 and $668.50, respectively. All risk-reduction strategies decreased septic transfusion reactions and associated expenses, with the greatest reductions from PR. PR would add $191.09 in per-unit acquisition costs, whereas PORt and SBC would increase per-unit testing costs by $31.79 and $17.26, respectively. Financial outcomes were sensitive to platelet dating; allowing 7-day storage with SBC would lead to a cost-savings of $12.41 per transfused unit. Results remained robust in probabilistic sensitivity analyses.
Conclusions:
All three strategies are viable approaches to reducing bacterially contaminated platelet transfusions, although SBC is likely to be the cheapest overall.
Keywords: cost-effectiveness, bacterial contamination, secondary bacterial culture, pathogen reduction, point-of-release testing, platelets, transfusion, Markov model, decision-tree
Introduction
While numerous risk-reduction strategies have dramatically improved the safety of the US blood supply, bacterial contamination of platelets (PLTs) continues to be a substantial cause of transfusion-associated morbidity and mortality1,2,3,4. As a leading risk of infection from blood transfusion, contaminated PLTs are estimated to cause septic transfusion reactions in approximately 1 in 100,000 transfused platelet products, with nearly 20% of these expected to be fatal5. Because the symptoms are often non-specific, the true incidence of serious or fatal septic transfusion reactions may be substantially underestimated6. Even with conservative estimates, this risk far exceeds the combined risk from HIV, HCV, and other blood-borne viral infections7. Transfusion-transmitted infections also can be associated with significant financial consequences8.
Bacterial contamination of transfused PLTs is often attributable to entry of small quantities of skin flora at the time of phlebotomy or collection from asymptomatic donors with subclinical bacteremia1,3. Furthermore, room-temperature storage of PLTs provides a favorable environment for most organisms to grow. Although routine culture is undertaken at blood centers at a minimum of 24 hours after collection and prior to release to hospital transfusion services, bacteria - if present - may still be in a lag phase or may have a long generation time, with levels not sufficient to be detectable at this stage9,10. It is also possible that bacteria in contaminated units may grow poorly in the aerobic conditions of PLT storage10 or may be present as biofilms, making them unavailable for sampling1. It is estimated that only 20-40% of contaminated units are detected through routine primary culture11,12,13, allowing for the possibility of contaminated units being transfused. Primary culture may have particularly low efficacy in detecting slow-growing Gram-positive bacteria1,14,15.
Although implementation of additional safeguards such as diversion pouches and standardized phlebotomy site cleaning have reduced contamination rates by 50–75%, residual risk remains unacceptable16,17,18,19. Draft guidance from the Food and Drug Administration (FDA) was issued in 2016 to promote the use of additional measures to address bacterial contamination1, including pathogen reduction (PR) and secondary bacterial testing. In the US, the only currently available FDA-approved PR technology is a psoralen/UV irradiation system (INTERCEPT), which can treat PLTs at blood collection establishments within 24 hours after collection, and has been shown to effectively inactivate a broad range of viruses, bacteria, and parasites20. Secondary testing is an alternative to PR, and can be conducted by a hospital transfusion service using either a culture-based system or a point-of-release test (e.g., Verax) less than 24 hours prior to transfusion.
Both of these approaches may have disadvantages; PR may be costly since each unit would incur a large fixed fee, and point-of-release testing (PORt) may be difficult to implement given the unpredictable blood supply needs of a transfusion service. Since the FDA guidance was issued, secondary bacterial culture (SBC) has been proposed as a third risk-reduction approach. SBC would be conducted after PLTs had been received by a hospital, in an effort to identify contaminated units that had escaped detection during primary culture. One large academic medical center implemented SBC for all apheresis PLTs and found the approach to be effective at reducing the risk of transfusion-transmitted sepsis22.
There is currently no standardized practice to address the residual risk of bacterially contaminated PLT transfusions. To further guide decision-making in this area, this study provides a comprehensive financial analysis of three alternative risk-reduction strategies.
Materials and Methods
A Markov-based decision tree (TreeAge Pro Suite 2018, Williamstown, MA) modeled the direct medical costs and transfusion outcomes associated with three alternative strategies to reduce bacterial contamination of apheresis PLTs: PR, PORt, and SBC. Outcomes for each of these strategies were compared to those using a “baseline” strategy, characterized by routine testing only. Microsimulations were run to track the path of individual apheresis PLT units under each of these approaches, from the point of acquisition by a hospital transfusion service, through testing and possible disposal due to positive test results, transfusion, or expiration. Accumulated costs and outcomes associated with each unit were tracked. A base-case scenario was modeled and sensitivity analyses using varied input parameters were conducted.
Model Structure:
For each of the modeled strategies, leukoreduced apheresis PLTs were assumed to be received at the transfusion service on day 3 post-collection, with some units defined as “contaminated,” based on published probabilities of contamination. Beginning on day 3, units could be transfused or retained for later use (Figure 1). This process continued up until the expiration date for each unit (day 5 or 7), at which point, unused units would be disposed. A transfusion was designated as either “uncontaminated” or “contaminated”, based on whether or not the transfused unit contained bacteria. Transfusion with contaminated units could result in no clinical complications or either minor or serious clinical sequelae. Clinical sequelae were associated with additional costs. The relative probabilities of these consequences were drawn from active surveillance data of bacterially contaminated PLTs over a seven-year period and a retrospective analysis characterizing culture-positive and septic transfusion reactions at a tertiary academic medical center23,24. Since it has been suggested that the overwhelming majority of septic reactions occur from contaminated units transfused on day 4 or later, the analysis assumed contaminated units transfused on day 3 were less likely to cause serious complications than contaminated units transfused after day 31,13,25,26. It was assumed that the processes and costs associated with transfusion would not differ across strategies.
Figure 1.
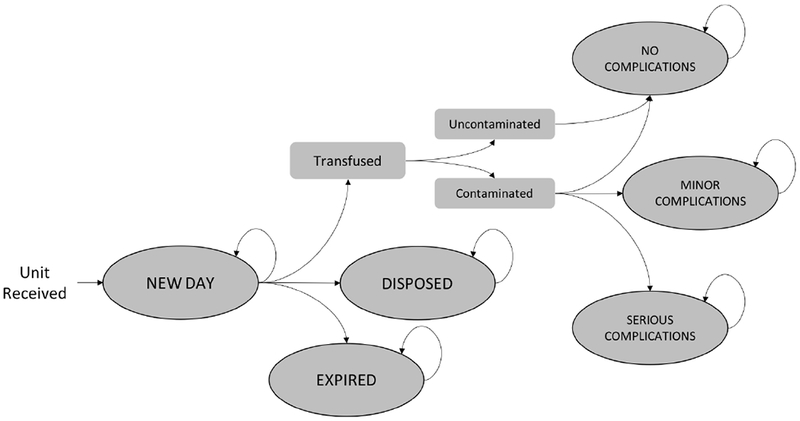
Markov Model Schematic. Plaletets were modeled from the point of receipt at a hospital through possible transfusion, disposal, or expiration. Each day until the end of the simulated period, the unit could transition between Markov states or remain in the same state, as shown by the directed arrows. Beginning on day 3 post collection, a unit could transition from a “New Day” state to a state characterizing transfusion, a disposal state or an expiration state. If the unit was transfused on a particular day, it could result in no complications, minor complications, or serious complications, depending on the contamination status of the unit. The unit could transition to the “Disposed” state due to positive or indeterminate test results under the PORt or SBC strategies. If the unit had not been transfused or disposed prior to reaching its expiration date, it would transition to the “Expired” state.
The baseline strategy was defined by primary culture, irradiation and Zika testing, without additional testing or manipulation to reduce bacterial contamination.
The PR strategy was modeled on the INTERCEPT Blood System for Platelets (Cerus Corporation, CA), which has been FDA-approved to maintain platelet quality through at least day 5 of storage1,20. Sensitivity of PR was based upon reported clinical efficacy of INTERCEPT using samples of bacterially spiked apheresis PLTs27. In this study, PR was 100% effective for all apheresis PLTs spiked with concentrations of either 100 or 1000 colony-forming units/bag. We assumed a base-case sensitivity of 100% under this strategy. It was assumed that PR would eliminate the need for (and costs of) primary culture at the blood collection facility, irradiation, and Zika testing. However, since patients treated with PR PLTs require an increased number of transfusions to achieve a fixed post-transfusion corrected count increment, an inflation factor of 1.14 was incorporated28,29.
The PORt strategy was modeled on the Platelet Pan Genera Detection (PGD) Test (Verax Biomedical, Marlborough, MA), which is a rapid test cleared by the FDA as a safety measure29,30. The “safety measure” label allows for extension of platelet dating through day 71. As under the baseline strategy, primary culture, irradiation and Zika testing would be conducted for all units. PORt was assumed to begin on day 4, as earlier testing is not currently a regulatory requirement1. As per the manufacturer’s instructions, units would be tested within 24 hours of transfusion, and would be retested if transfusion did not occur within this period. Initial test results could be: (1) initially reactive, (2) non-reactive, or (3) indeterminate30, and initially reactive units would be retested in duplicate. If both repeat tests were non-reactive, the sample would be deemed “non-reactive”. Otherwise, the sample would be “repeat-reactive,” and associated units would be discarded. Indeterminate results would be retested up to 2 times to obtain a valid result. After two repeat tests without a valid result, the unit would be discarded.
The SBC strategy model was based upon the BacT/ALERT (bioMerieux, Inc.) system and has been previously described22. Units would be received on day 3 and sampled, with 5mL of each platelet product inoculated into a single aerobic culture bottle. The inoculated bottles would be incubated at 35°C for 3 days or until positive, allowing for platelet storage through day 5 post-collection. The BacT/ALERT system may be used as a safety measure to extend dating through day 7 under certain sampling and storage conditions, but it was conservatively assumed that only storage through day 5 was allowable under the SBC strategy. Any positive culture would result in a follow-up Gram stain, and repeat confirmatory testing. It was assumed that any unit associated with a positive culture would be discarded if it had not already been transfused. Primary culture, irradiation and a Zika test would still be performed.
Input Parameters:
Point estimates and ranges were defined for all input parameters, as shown in Table 1. Parameter estimates were drawn from existing literature, wherever possible. Associated costs from the platelet acquisition, testing/manipulation, transfusion, and transfusion-associated complications are expressed in 2018 US$. These costs include direct medical costs only, and reflect the perspective of a hospital transfusion service.
Table 1.
Input Parameters. Base-case values and ranges for model parameters under each strategy.
| Parameter | Value | Range | Source |
|---|---|---|---|
| Baseline Product Costs (US$) | |||
| Apheresis Platelets (Leukoreduced) | 555.75 | (524.99, 593.93) | [31] |
| Irradiation | 9.01 | (6.76, 11.27) | [32] |
| Zika test | 4.56a | (3.42, 5.70) | [29] |
| Baseline Non-Product Costs (US$) | |||
| Blood Bank | 23.98 | (17.99, 29.98) | [33] |
| Transfusion | 63.11 | (47.33, 78.89) | [33] |
| Pathogen Reduction Costs (US$) | |||
| Pathogen Reduction | 100 | (75, 125) | Assumed |
| Point of Release Testing Costs (US$) | |||
| Materials | 27.05 | (20.29, 33.81) | [29] |
| Labor (Sampling and Test Performance) | 4.39 | (3.29, 5.49) | [29] |
| Labor (Management of Test Results) | 0.35 | (.26, .44) | [29, 32] |
| Labor (Quality Control and Proficiency) | 2.21 | (1.65, 2.76) | [29, 32] |
| Secondary Bacterial Culture Costs (US$) | |||
| Materials | 13.74 | (10.30, 17.17) | [22] |
| Labor | 3.52 | (2.64, 4.40) | [22] |
| Gram Stain (Materials and Labor) | 5.27 | (3.95, 6.59) | [38] |
| Complication Costs (US$) | |||
| Serious Complications (Sepsis) | 34354.53 | (15692.18, 79488.75) | [8] |
| Minor Complications | 2395.87 | (1796.90, 2994.84) | [34] |
| Pathogen Reduction | |||
| Inflation factor for increased units | 1.14 | (1.1, 1.2) | [28] |
| Sensitivity | 100% | (95, 100) | [27] |
| Point-of-Release Testing | |||
| Probability of retesting once | 15.45% | (10, 20) | [35] |
| Probability of retesting twice | 1.40% | (0.8, 2.0) | [35] |
| Transfused before Day 4 | 31.10% | (20, 42) | [29] |
| Initial Indeterminate | 0.46% | (0.2, 0.7) | [30] |
| Repeat Indeterminate | 6.25% | (2.25, 10.5) | [30] |
| Sensitivity - First Result | 60% | (40, 80) | [30] |
| Specificity - First Result | 98.61% | (95, 100) | [30] |
| Sensitivity - Retest | 100% | (95, 100) | [30] |
| Specificity - Retest | 46.90% | (32, 62) | [30] |
| Secondary Bacterial Culture | |||
| Sensitivity | 62.50% | (50, 75) | [22] |
| Specificity | 100% | (95, 100) | [22] |
| Usage | |||
| Outdate Probability (5-day expiration) | 9.5% | (7, 15) | [31] |
| Outdate Probability (7-day expiration) | 5% | (2, 8) | Assumed |
| Probability of Use on Day 3 | 20% | (10, 40) | Assumed |
| Contamination Probability | 0.04% | (0.02, 0.06) | [23] |
| Probability of Serious Complications | 11.11% | (8, 14) | [23] |
| Probability of Minor Complications | 13.89% | (10, 17) | [24] |
| Probability of Serious Complications (Day 3 Use) | 5% | (0, 11) | Assumed |
This value assumes a collection split rate of 1.9 platelet units per donation. The test would be performed on the donation, but this parameter reflects a per-unit cost
Reported estimates of the risk of bacterial contamination of apheresis PLTs that have tested negative in primary culture vary widely, and depend on the precise setting and method2,23,24. In the base-case analysis, it was assumed that the contamination probability in the absence of PR or secondary testing followed a beta distribution with mean 1/250023. Transfusion of contaminated PLTs would result in no clinical consequences in the majority (75%) of cases23. Of those clinical consequences, 44.4% would be minor and 55.6% would be serious24. In this model, “serious” complications included severe, life-threatening, or fatal reactions, as defined using the CDC National Healthcare Safety Network hemovigilance criteria24.
Standard product and non-product costs were obtained from published literature and were based on a hospital perspective. In the absence of PR, it was assumed that the hospital service received leukoreduced apheresis PLTs ($555.75)31, which then underwent irradiation32 and Zika testing29. Non-product costs included expenses associated with the blood bank as well as transfusion itself, and were based on a lean process analysis of platelet transfusion at a large academic medical center in the US33. Complication costs were drawn from a recent systematic review of hospital costs of sepsis in the US8 and from published estimates of the cost of a visit to a hospital-based emergency department34 for a serious and mild complications from contamination, respectively.
Strategy-specific parameters were also drawn from existing literature where available. Under the base-case scenario, the PR strategy was assumed to eliminate bacterial contamination completely: hospitals would receive only uncontaminated units, resulting in no transfusion reactions. A fixed per-unit cost for PR of $100 was assumed.
Sensitivity and specific parameters for the PORt strategy were drawn from post-marketing surveillance data comparing Verax PGD results to concurrent culture30. It was assumed that 0.46% of samples were initially indeterminate, and among those, 6.25% were repeatedly indeterminate30. The initial read of the PORt was assumed to have a sensitivity of 60% and specificity of 98.61%. Among those samples with an initially positive read, it was assumed the sensitivity of the repeat test was 100%, and the specificity of the repeat test was 46.90%. The model also incorporated the possibility of retesting units if they were not used within 24 hours of a test; it was assumed that 15.45% of units were tested twice, and 1.4% of units were tested 3 times35. PORt would be associated with a per-unit test cost, as well as labor costs from sampling and test performance, management of test results, and quality control. This approach has been used in previous cost analyses of PORt29.
Parameters associated with SBC were obtained from a feasibility study conducted at a large academic medical center22. While 7 of 8 contaminated units were detected by culture within 24 hours, only 5 of these 8 units were successfully interdicted prior to transfusion. Therefore, it was conservatively assumed that the base-case sensitivity of SBC was 62.5%, to reflect the probability that SBC would detect contaminated units prior to transfusion. A base-case specificity of 100% was assumed. Incorporated costs included expenses for the sampling kit and other materials, as well as for labor.
Analysis:
In the base-case scenario, a cost associated with each strategy was estimated for each “effective” unit received by the hospital transfusion service, which accounted for increased unit requirements under PR. A cost per transfused unit was also estimated, as some of the units received by the hospital would be disposed due to test results or expiration. Results were projected over an annual period to reflect estimated outcomes for a large hospital transfusion service, assuming 20,000 transfused units each year. Each simulation was run using 1,000,000 individual trials. For each unit, we tracked if the unit was associated with a contaminated transfusion or an uncontaminated transfusion, or if the unit was disposed due to indeterminate results, positive test results, or expiration. Under the base-case scenario, it was assumed that units expired after day 5 in the baseline strategy and the PR and SBC strategies, but that PORt allowed for extended use through day 7. However, efforts are underway to identify approaches to extend the limited supply of PLTs in order to meet demand36. Therefore, as PR and SBC may be approved for extended platelet dating, additional analyses were conducted varying these expiration dates. To account for uncertainty in input parameter estimates, probabilistic sensitivity analyses varying parameters simultaneously were conducted using 10,000 samples of 10,000 trials each. Costs were varied by 25% in either direction using an adjustment factor sampled from a triangular distribution (mode=1; min=0.75, max=1.25) and probabilities were drawn from beta distributions, using the 95% CI reported by the original data source wherever possible.
Results
In the base-case scenario, all three risk reduction strategies would be expected to increase total costs per unit acquired by a hospital transfusion service, as compared to the baseline strategy. Among the risk reduction strategies compared, total costs would be lower under the SBC strategy than the PR or PORt strategies (Table 2). The average total cost in the baseline strategy, incorporating expenses from product acquisition, testing, transfusion, and complications, was $651.45 per unit, whereas the average total costs under PR, PORt, and SBC were $827.82, $686.33, and $668.50, respectively. Differences in expected costs between strategies was primarily driven by product acquisition (PR added up-front costs and was associated with an inflation factor of 1.14 due to low platelet increments28) and testing costs. Relative to the baseline approach, PR added $191.09 in per-unit acquisition costs ($749.00 vs. $557.91). PORt and SBC increased per-unit testing costs by $31.79 ($45.37 vs. $13.58) and $17.26 ($30.84 vs. $13.58), respectively. Complication-related costs also differed across strategies, with the baseline strategy, PORt, and SBC associated with additional costs from transfusion of contaminated PLTs.
Table 2.
Results from Base-Case Scenario and Varied Platelet Dating. Financial and clinical impact of baseline strategy and three alternative risk reduction approaches. Unit costs are expressed per “effective” unit received by a hospital transfusion service from a blood collection agency. Annual costs assume 20,000 transfused units per year.
| Base-Case Scenario | Varied Platelet Dating | |||||
|---|---|---|---|---|---|---|
| Baseline | Pathogen Reduction: 5-day expiration | Point-of-Release Testing: 7-day expiration | Secondary Culture: 5-day expiration | Pathogen Reduction: 7-day expiration | Secondary Culture: 7-day expiration | |
| Unit Costs (US$): Mean (SD) | ||||||
| Acquisition | 557.91 (14.32) | 749.00 (16.30) | 557.91 (14.32) | 557.91 (14.32) | 749.02 (16.29) | 557.91 (14.31) |
| Testing/Manipulation | 13.58 (1.38) | 0.00 (0.00) | 45.37 (22.91) | 30.84 (1.45) | 0.00 (0.00) | 30.84 (1.44) |
| Transfusion | 78.82 (26.91) | 78.82 (26.91) | 82.06 (22.08) | 78.78 (26.95) | 82.73 (20.85) | 82.74 (20.89) |
| Complications | 1.15 (232.26) | 0.00 (0.00) | 0.86 (183.79) | 0.97 (215.26) | 0.00 (0.00) | 0.55 (159.79) |
| Total | 651.45 (234.3) | 827.82 (31.48) | 686.33 (187.46) | 668.50 (217.44) | 831.75 (26.47) | 672.05 (161.79) |
| Unit Disposition (%) | ||||||
| Uncontaminated Transfusion | 90.45% | 90.49% | 94.20% | 90.44% | 95.01% | 94.97% |
| Contaminated Transfusion | 0.04% | 0.00% | 0.02% | 0.01% | 0.00% | 0.02% |
| Disposed | 0.00% | 0.00% | 0.77% | 0.04% | 0.00% | 0.03% |
| Expired | 9.51% | 9.51% | 5.01% | 9.51% | 4.99% | 4.98% |
| Total Cost per Transfused Unit (US$): Mean (SD) | 719.92 (174.54) | 914.82 (149.31) | 728.44 (134.44) | 739.05 (169.96) | 875.46 (101.31) | 707.51 (117.82) |
| Annual Costs (Million US$): Mean (SD) | 14.40 (3.49) | 18.30 (2.99) | 14.57 (2.69) | 14.78 (3.40) | 17.51 (2.03) | 14.15 (2.36) |
Across strategies, greater than 90% of units acquired by a hospital transfusion service resulted in uncontaminated transfusions (Table 2). All three risk reduction strategies were associated with a decrease in contaminated transfusions relative to the baseline strategy, with the greatest decrease from PR. In the base-case scenario, for a fixed number of units received by a hospital, a PR strategy would be expected to result in 90.49% of these units being used in uncontaminated transfusions, 0.0% used in contaminated transfusions, and 9.51% of units expiring before use. The PORt strategy, which incorporated 7-day extended platelet dating, was associated with a greater proportion of uncontaminated units being transfused (94.20%) and a correspondingly lower proportion of units expiring than any other strategy (5.01%). However, PORt was associated with a slightly greater proportion of contaminated transfusions (0.02%) than PR or SBC since PORt was assumed not to be performed on day 3. SBC was associated with 90.44% of units being used in uncontaminated transfusions and 0.01% in contaminated transfusions. In the base-case, the costs per transfused unit under PR, PORt, and SBC were $914.82, $728.44, and $739.05, respectively. This would translate to annual costs for transfusing 20,000 units of $18.30 million, $14.57 million, and $14.78 million. Among these 20,000 transfused units, approximately 8 units would be expected to be contaminated under the baseline strategy. PR would be expected to eliminate transfusion of contaminated units, approximately 4 transfused units would be contaminated using PORt, and approximately 3 transfused units would be contaminated with SBC.
Financial outcomes under alternative platelet dating are shown in the last two columns of Table 2. Allowing either of the PR or SBC strategies to extend platelet life through day 7 would increase the proportion of units being used in uncontaminated transfusions and decrease the total costs per transfused unit. SBC with 7-day approval would be the least costly strategy per transfused unit, and would lead to a cost-savings of $12.41 ($707.51 vs. $719.92) when compared to the baseline approach. Even with 7-day approval, PR would remain the most expensive strategy, with a total cost of $875.46 per transfused unit.
Expected financial and health outcomes under PORt were sensitive to the proportion of units being transfused on day 3 (and thus untested) and the likelihood of serious complications occurring due to these PLTs. Implementing PORt on day 3 PLTs would reduce the risk of complications by approximately 12%, but would incur additional per-unit testing costs of $7.59, although these estimates would vary based on assumptions of day 3 usage. PORt costs were also sensitive to the probability of a unit not being transfused within 24 hours of testing and thus requiring additional testing. Across strategies, a decrease in sensitivity increased the potential for contaminated transfusions and associated costs, and a decrease in specificity increased costs from confirmatory testing and product wastage.
Results from the probabilistic sensitivity analysis, varying input parameters simultaneously, are shown in Table 3, and are consistent with results from the base-case scenario. Adopting SBC or PORt as strategies would add approximately $16.93 ($668.16 vs $651.23) and $35.00 ($686.23 vs $651.23) per unit to a baseline approach, respectively, but would nearly eliminate contaminated transfusions. PR would eliminate contaminated transfusions, but would add $176.55 ($827.78 vs $651.23) per unit.
Table 3.
Probabilistic Sensitivity Analysis Results. Financial and clinical impact of baseline strategy and three alternative risk reduction approaches, varying input parameters simultaneously. Unit costs are expressed per “effective” unit received by a hospital transfusion service from a blood collection agency. Annual costs assume 20,000 transfused units per year.
| Baseline | Pathogen Reduction | Point-of-Release Testing | Secondary Culture | |
|---|---|---|---|---|
| Unit Costs (US$): Mean (SD) | ||||
| Acquisition | 557.91 (0.14) | 748.95 (11.64) | 557.91 (0.14) | 557.91 (0.14) |
| Testing/Manipulation | 13.58 (0.01) | 0.00 (0.00) | 45.4 (3.71) | 30.83 (1.79) |
| Transfusion | 78.83 (4.40) | 78.83 (4.40) | 82.02 (1.79) | 78.80 (4.49) |
| Complications | 0.91 (2.09) | 0.00 (0.00) | 0.75 (1.89) | 0.61 (1.73) |
| Total | 651.23 (4.92) | 827.78 (12.38) | 686.23 (4.76) | 668.16 (5.09) |
| Unit Disposition (%) | ||||
| Uncontaminated Transfusion | 90.48% | 90.51% | 94.16% | 90.46% |
| Contaminated Transfusion | 0.04% | 0.00% | 0.02% | 0.01% |
| Disposed | 0.00% | 0.00% | 0.77% | 0.04% |
| Expired | 9.49% | 9.49% | 5.06% | 9.49% |
| Total Cost per Transfused Unit (US$): Mean (SD) | 719.48 (40.49) | 914.53 (52.79) | 728.65 (16.72) | 738.50 (42.43) |
| Annual Costs (Million US$): Mean (SD) | 14.39 (0.81) | 18.29 (1.06) | 14.57 (0.33) | 14.77 (0.85) |
Discussion
Currently, there is no universally-accepted approach to mitigate the residual risk of bacterially contaminated PLTs. This analysis assessed alternative risk-reduction approaches and a baseline strategy centered on routine testing only. The model demonstrated that all three strategies – PR, PORt, and SBC – would reduce contaminated transfusions, but based on our model input parameters for test sensitivity, reduction in this risk would be somewhat greater with PR than PORt or SBC. The PORt strategy was modeled using a sensitivity estimate of 60%, resulting in a possibility of false-negative test results. In addition, since PORt is not recommended for use on day 3 post-collection, there is potential for contaminated units to be transfused on day 3. Incorporating PORt on day 3 would slightly decrease the risk of contaminated transfusions, but would increase per-unit costs under this approach. The SBC strategy modeled could also result in false-negative results; although it has been reported that SBC can detect contamination in 87.5% of PLTs within 24 hours of sampling, this model conservatively assumed that SBC would be only 62.5% sensitive in detecting contamination prior to transfusion22.
For a fixed number of units obtained by a hospital transfusion service, all three risk-reduction strategies would increase total costs relative to a baseline approach. However, SBC is likely to be less costly per unit than PR or PORt. Adoption of SBC would increase the per-unit costs by approximately $17.05 ($668.50 vs. $651.45), relative to a baseline approach with routine testing only. SBC is expected to result in lower testing costs than PORt, and would avoid the logistical challenge of anticipating daily PLT testing requirements, which may be difficult to predict. Under PORt, units may be tested twice or more if not transfused within 24 hours of initial testing, but testing associated with SBC would be done only once per unit. SBC would, however, incur per-unit testing costs for all units, including those that would ultimately expire. PORt, in theory, would be unnecessary for units that would never be transfused.
PR would also be an effective risk reduction strategy, but would add significant per-unit costs to the baseline approach. While PR would eliminate additional expenses associated with irradiation and other testing, the marginal cost of implementing PR far exceeds these reductions. Extension of platelet usage through day 7 would reduce wastage under this strategy, although possible damage to the PLTs from PR may limit benefits of this extension28.
At the time this manuscript was accepted, PORt was the only FDA-approved strategy that allowed for extended PLT dating through day 7. Thus, under this model’s assumptions, PORt is expected cost less per transfused unit than the baseline strategy, SBC, or PR, due to an increase in the proportion of units actually being transfused. Allowing SBC or PR to extend use through day 7 would decrease the proportion of units expiring associated with these strategies, leading to lower overall costs per transfused unit than the other approaches. Under 7-day usage, SBC would be cost-saving per transfused unit. Given the effectiveness and relative low costs of SBC as a risk-reduction strategy, further research into the safety of extended platelet dating under this approach is warranted.
Recent FDA draft guidance proposes that an alternative SBC-based approach involving secondary culture on Day 4, instead of Day 3, may allow for extended usage through Day 7.39 While this Day 4 SBC variant would reduce wastage due to expired platelets, it may also come with a risk from transfusion of contaminated Day 3 units. It’s plausible that this risk could be mitigated by testing on Day 3, as under the SBC strategy modeled in this analysis. Since this new FDA draft guidance had not been released at the time of manuscript acceptance, Day 4 SBC was not explicitly modeled.
This study has a number of limitations. First, the analysis focuses exclusively on apheresis PLTs, and did not model risks from pooled or whole blood-derived PLTs. However, approximately 92% of the PLTs transfused annually in the US are apheresis PLTs, rendering this a reasonable simplification31. The analysis also focused on bacterial contamination only, and did not account for potential effects of these approaches on non-bacterial infections. It has been shown that PR can effectively inactivate cytomegalovirus and West Nile virus, among certain other viruses and parasites, and would be expected to eliminate additional costs associated with these tests32. Incorporating these additional pathogens would be expected to increase overall costs for the baseline strategy, PORt, and SBC. However, averted costs from not needing to test for these additional pathogens are small, relative to the cost of PR itself32.
The model also did not explicitly incorporate variation in the concentration of bacteria in contaminated products or relate the concentration to the sensitivity of testing approaches or expenses for complications. However, the relationship between contaminated PLTs and clinical consequences is not predictable, as many of the patients who receive PLTs have comorbid medical conditions and/or are on other treatments (e.g. antibiotics) which may affect their risk of infection.
In addition, the specific cost and probability input parameters used in this model may not reflect all hospital settings and may not be appropriate in certain international contexts. In particular, results for PORt are sensitive to assumptions about usage such as the proportion of units transfused on day 3 and the precise schedule for testing units each day. Results are also sensitive to assumptions about usage on day 7; it is conceivable that older units of platelets may not yield the same corrected count increment as units that had not been stored as long. Accounting for increased transfusions when using day 7 platelets would increase costs under PORt. This model was also conservative in the input cost estimates associated with PR; it was assumed that PR would increase per-unit acquisition costs by $100, but it has been reported that costs of PR may be as high as $165 per unit37.
The input parameters used may also not be appropriate in the event of changes to FDA guidance or regulations. For example, if FDA requires usage of both an aerobic and an anaerobic culture bottle for the SBC approach, costs under this strategy would increase slightly. Nevertheless, the probabilistic nature of this analysis incorporated wide ranges in input parameters to reflect possible variation across platelet units, patients, and hospital settings.
Overall, this analysis has shown that PR, PORt, and SBC all represent financially viable approaches for reducing bacterial contamination of PLTs and associated clinical consequences. While all strategies would increase overall costs, SBC is likely to incur the lowest per-unit cost for risk-reduction and could lead to cost-savings per transfused unit, especially if the outdate is extended to Day 7.
Acknowledgments
Funding: This work was supported in part by the National Institutes of Health (5R01AI120938 and 1R01AI128779 to A.A.R.T).
Footnotes
Conflicts of Interest: EB, EG, PN, PL and AT are co-investigators or principal investigators on Mirasol clinical trials assessing the efficacy of pathogen reduction. EG is a co-investigator on a Cerus clinical trial. PN consults for Terumo BCT.
References
- [1].FDA-CBER, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion,” March 2016[Online]. Available: https://www.fda.gov/downloads/Guidances/Blood/UCM425952.pdf [Accessed 1 August 2018].
- [2].Kuehnert MJ, Roth VR, Haley NR, et al. , “Transfusion-transmitted bacterial infection in the United States, 1998 through 2000,” Transfusion, vol. 41, pp. 1493–1499, 2001. [DOI] [PubMed] [Google Scholar]
- [3].Yomtovian R, “Bacterial contamination of blood: lessons from the past and road map for the future,” Transfusion, vol. 44, pp. 450–460, 2004. [DOI] [PubMed] [Google Scholar]
- [4].Kaufman RM, Djulbegovic B, Gernsheimer T, et al. , “Platelet transfusion: a clinical practice guideline from the AABB,” Annals of Internal Medicine, vol. 162, no. 3, pp. 205–213, 2015. [DOI] [PubMed] [Google Scholar]
- [5].Ramirez-Arcos S, DiFranco C, McIntyre T, et al. , “Residual risk of bacterial contamination of platelets: six years of experience with sterility testing,” Transfusion, vol. 57, pp. 2174–2181., 2017. [DOI] [PubMed] [Google Scholar]
- [6].Morrow JF, Braine HG, Kickler TS, et al. , “Septic reactions to platelet transfusions: a persistent problem,” Journal of the American Medical Association, vol. 266, no. 4, pp. 555–558, 1991. [PubMed] [Google Scholar]
- [7].Stramer SL, “Current risks of transfusion-transmitted agents: a review,” Archives of Pathology & Laboratory Medicine, vol. 131, no. 5, pp. 702–707, 2007. [DOI] [PubMed] [Google Scholar]
- [8].Arefian H, Heublein S, Scherag A, et al. , “Hospital-related cost of sepsis: A systematic review,” Journal of Infection, vol. 74, pp. 107–117, 2017. [DOI] [PubMed] [Google Scholar]
- [9].AuBuchon JP, Cooper LK, Leach MF, et al. , “Experience with universal bacterial culturing to detect contamination of apheresis platelet units in a hospital transfusion service,” Transfusion, vol. 42, no. 7, pp. 855–861, 2002. [DOI] [PubMed] [Google Scholar]
- [10].Benjamin RJ and Wagner SJ, “The residual risk of sepsis: modeling the effect of concentration on bacterial detection in two-bottle culture systems and an estimation of false-negative culture rates,” Transfusion, vol. 47, pp. 1381–1389, 2007. [DOI] [PubMed] [Google Scholar]
- [11].Murphy WGW, Foley MM, Doherty CC, et al. , “Screenng platelet concentrates for bacterial contamination: low numbers of bacteria and slow growth in contaminated units mandate an alternative approach to product safety,” Vox Sanguinis, vol. 95, no. 1, pp. 13–19, 2008. [DOI] [PubMed] [Google Scholar]
- [12].Benjamin R, “Transfusion-related sepsis: a silent epidemic,” Blood, vol. 127, pp. 380–381, 2016. [DOI] [PubMed] [Google Scholar]
- [13].Dumont LJ, Kleinman S, Murphy JR, et al. , “Screening of single-donor apheresis platelets for bacterial contamination: the PASSPORT study results,” Transfusion, vol. 50, pp. 589–599, 2010. [DOI] [PubMed] [Google Scholar]
- [14].“Fatalities Reported to FDA Following Blood Collection and Transfusion Annual Summary for Fiscal Year 2016,”[Online]. Available: https://www.fda.gov/downloads/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TransfusionDonationFatalities/UCM598243.pdf.
- [15].Brecher ME, Blajchman MA, Yomtovian R, et al. , “Addressing the risk of bacterial contamination of platelets within the United States: a history to help illuminate the future,” Transfusion, vol. 53, no. 1, pp. 221–231, 2013. [DOI] [PubMed] [Google Scholar]
- [16].McDonald CP, Roy A, Mahajan P, et al. , “Relative values of the interventions of diversion and improved donor-arm disinfection to reduce the bacterial risk from blood transfusion,” Vox Sanguinis, vol. 86, pp. 178–182, 2004. [DOI] [PubMed] [Google Scholar]
- [17].de Korte DM, Marcelis JH, “Platelet concentrates: reducing the risk of transfusion-transmitted bacterial infections,” International Journal of Clinical Transfusion Medicine, vol. 2, p. 9, 2014. [Google Scholar]
- [18].Liumbruno GM, Catalano L, Piccinini V, et al. , “Reduction of the risk of bacterial contamination of blood components through diversion of the first part of the donation of blood and blood components,” Blood Transfusion, vol. 7, no. 2, pp. 86–93, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jenkins C, Ramirez-Arcos S, Goldman M, et al. , “Bacterial contamination in platelets: incremental improvements drive down but do not eliminate risk,” Transfusion, vol. 51, pp. 2555–2565, 2011. [DOI] [PubMed] [Google Scholar]
- [20].“The INTERCEPT Blood System for Platelets Package Insert. Cerus Corporation,” 2018[Online]. Available: https://interceptusa.com/images/resources/Package_Inserts/INTERCEPT_BloodSystem_INT2530B_DS_Package_Insert_May-2018.pdf [Accessed 1 August 2018].
- [21].“Mirasol Pathogen Reduction Technology (PRT) System,” 2018[Online]. Available: https://www.terumobct.com/mirasol [Accessed 1 August 2018].
- [22].Bloch EM, Marshall CE, Boyd JS, et al. , “Implementation of secondary bacterial culture testing of platelets to mitigate residual risk of septic transfusion reactions,” Transfusion, vol. 58, no. 7, pp 1647–1653, 2018. [DOI] [PubMed] [Google Scholar]
- [23].Hong H, Xiao W, Lazarus H, et al. , “Detection of septic transfusion reactions to platelet transfusions by active and passive surveillance,” Blood, vol. 127, pp. 496–502, 2016. [DOI] [PubMed] [Google Scholar]
- [24].Erony SM, Marshall CE, Gehrie EA, et al. , “The epidemiology of bacterial culture–positive and septic transfusion reactions at a large tertiary academic center: 2009 to 2016,” Transfusion, vol. 58, no. 8, pp 1933–1939, 2018. [DOI] [PubMed] [Google Scholar]
- [25].Benjamin RJ, “Blood Products Advisory Committee, September 21, 2012, transcripts accessible at http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/BloodProductsAdvisoryCommittee/UCM325690.pdf,” [Online].
- [26].Braine HG, Kickler TS, Charache P, et al. , “Bacterial sepsis secondary to platelet transfusion: an adverse effect of extended storage at room temperature,” Transfusion, vol. 26, no. 4, pp. 391–393, 1986. [DOI] [PubMed] [Google Scholar]
- [27].Schmidt M, Hourfar MK, Sireis W, et al. , “Evaluation of the effectiveness of a pathogen inactivation technology against clinically relevant transfusion-transmitted bacterial strains,” Transfusion, vol. 55, pp. 2104–2112, 2015. [DOI] [PubMed] [Google Scholar]
- [28].McCullough J, Vesole DH, Benjamin RJ, et al. , “Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation: the SPRINT Trial,” Blood, vol. 104, no. 5, pp. 1534–1540, 2004. [DOI] [PubMed] [Google Scholar]
- [29].Li JW, Brecher ME, Jacobson JL, et al. , “Addressing the risk of bacterial contamination in platelets: a hospital economic perspective,” Transfusion, vol. 57, pp. 2321–2328, 2017. [DOI] [PubMed] [Google Scholar]
- [30].Verax. Platelet PGD Test [package insert]. 2009, [Online]. Available: https://www.veraxbiomedical.com//wp-content/uploads/2018/01/US_Platelet_PGD_Test.pdf [Accessed 1 August 2018]3
- [31].Ellingson KD, Sapiano MRP, Haass KA, et al. , “Continued decline in blood collection and transfusion in the United States–2015,” Transfusion, vol. 57, pp. 1588–1598, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McCullough J, Goldfinger D, Gorlin J, et al. , “Cost implications of implementation of pathogen-inactivated platelets,” Transfusion, vol. 55, pp. 2312–2320, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Riley W, Smalley B, Pulkrabek S, et al. , “Using lean techniques to define the platelet (PLT) transfusion process and cost-effectiveness to evaluate PLT dose transfusion strategies,” Transfusion, vol. 52, pp. 1957–1967, 2012. [DOI] [PubMed] [Google Scholar]
- [34].Ho V, Metcalfe L, Dark C, et al. , “Comparing utilization and costs of care in freestanding emergency departments, hospital emergency departments, and urgent care centers,” Annals of Emergency Medicine, vol. 70, no. 6, pp. 846–857, 2017. [DOI] [PubMed] [Google Scholar]
- [35].Vauthrin M, Greene M, Weinstein R, “Verax platelet PGD test workflow strategy,” Transfusion, vol. 56, p. 198A, 2016. [Google Scholar]
- [36].Kaufman RM, “Platelets: testing, dosing and the storage lesion - recent advances,” Hematology, vol. 2006, no. 1, pp. 492–496, 2006. [DOI] [PubMed] [Google Scholar]
- [37].Webert KE, Cserti CM, Hannon J, et al. Proceedings of a consensus conference: pathogen inactivation – making decisions about new technologies. Transfusion Medicine Reviews, vol. 22, pp. 1–34, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Centers for Medicare & Medicaid Services, “Clinical Laboratory Fee Schedule,” April 2018[Online]. Available: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html [Accessed 1 August 2018].
- [39].FDA-CBER. Bacterial risk control strategies for blood collection establishments and transfusion services to enhance the safety and availability of platelets for transfusion. December 2018. [cited 2019 January 4]. Available from: https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM627407.pdf. [Online].


