Abstract
Bacteria are ubiquitous in the bovine uterus after parturition, but 50 years ago, cows tolerated these bacteria and few animals developed uterine disease. Now, up to 40% of dairy cattle develop postpartum uterine disease. Uterine disease causes infertility by compromising the function of not only the endometrium but also the ovary. Animals defend themselves against pathogens using tolerance and resistance mechanisms. Tolerance is the ability to limit the disease severity induced by a given pathogen burden. Resistance is the ability to limit the pathogen burden and is usually the function of immunity. Endometrial cells contribute to tolerance and have roles in innate immunity and the inflammatory response to pathogens. However, failures in endometrial tolerance and the character of the inflammatory response shape post-partum uterine disease. We propose that uterine health is more dependent on the ability of the endometrium to tolerate pathogens than the ability to resist invading bacteria.
Keywords: bovine, fertility, uterus, ovary, infection, inflammation
INTRODUCTION
Bacteria are ubiquitous in the bovine uterus after parturition, and invasion and growth of bacteria in the endometrium cause postpartum uterine disease (1). Fifty years ago, dairy cows tolerated these bacteria in the uterus, and animals only occasionally developed uterine disease. Now, up to 40% of dairy cattle develop postpartum uterine disease every year, ranging from subclinical endometritis to life-threatening metritis (Figure 1a). These uterine diseases compromise animal health, welfare, and productivity. In particular, endometritis impairs the fertility of dairy cattle by disrupting endometrial function, generating a hostile environment for the embryo in the female genital tract, and by perturbing ovarian follicle function and oocyte health. Emergence of endemic postpartum uterine disease has coincided with intensification of farming and breeding of dairy cattle to increase milk yields by fivefold over the last 50 years. The association between uterine disease and the metabolic stress of dairy production is a concern because intensification of dairy farming will continue, with the milk yield of cows in the United States projected to double in the next 50 years (2). In this review, we outline the fundamental aspects of the postpartum period and uterine disease and then discuss how tolerance and resistance shape the development of postpartum uterine disease, as well as the impact of endometritis on dairy cattle.
Figure 1.
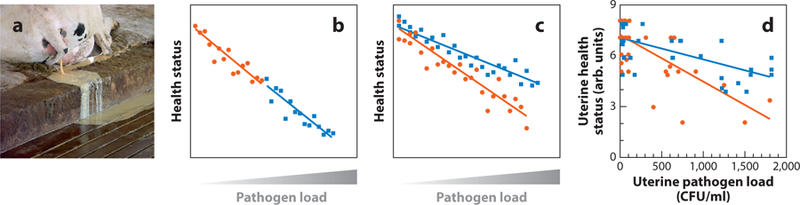
Uterine disease is associated with impaired tolerance. (a) Cows with postpartum uterine disease discharge pus from the uterus. (b,c) Schematic reaction norms of health status against pathogen load for (b) two groups of animals with similar tolerance, where each animal is represented by a symbol (blue squares or orange circles), but the reaction norm for the blue group (blue line) indicates that these animals have impaired immunity, with reduced health status and more pathogens, compared with the orange group (orange line), or (c) two groups with similar immunity, but the reaction norm for the orange group (orange line) indicates that these animals are less tolerant, with reduced health at the same pathogen load as the blue group (blue line) [based on the concepts proposed by Raberg and others (3–6)]. (d) Using data from a previously published study of postpartum uterine clinical health score and uterine bacterial load for dairy cows producing >35 L milk/day (orange circles, n = 56) and <35 L milk/day (blue squares, n = 34), the reaction norm for the cows producing >35 L milk/day (orange line) indicates that these metabolically stressed animals have impaired tolerance, with reduced health at the same pathogen load as the animals producing <35 L milk/day (blue line). Panel d adapted with permission from I.M. Sheldon.
RESILIENCE TO UTERINE INFECTION
Bacteria associated with uterine disease employ virulence factors that cause tissue damage and provoke inflammation in the endometrium. The ability of an organism to counter pathogenic microbes, or the animal’s resilience, depends on resistance and tolerance (see sidebar titled Animal Resilience Depends on Resistance and Tolerance) (3–5). Tolerance is the ability to limit the disease severity induced by a given pathogen burden (3, 5, 6). Resistance is the ability to limit the pathogen burden and is usually the function of immunity. Together tolerance and resistance determine the balance between an animal’s resilience to pathogens and the severity of disease.
We have used reaction norms to examine whether postpartum uterine disease in cows is a failure of tolerance to pathogens and/or the ability of immunity to resist pathogens. Reaction norms are used in populations to compare the health status of organisms with their pathogen load, allowing one to disentangle the relative contributions of resistance (Figure 1b) and tolerance (Figure 1c) (3). Data from a herd of dairy cows with similar genotype, where milk production, uterine health, and bacterial load were recorded (7), were used to determine whether milk yields in early lactation influenced tolerance or resistance to uterine pathogens. Postpartum cows under the metabolic stress of producing >35 L milk/day had worse endometrial health at the same uterine bacterial load than animals producing <35 L milk/day (Figure 1d), implying that metabolic stress perturbs uterine tolerance rather than immunity.
Here, we argue that tolerance to bacteria invading the endometrium is more important than resistance for the development of postpartum uterine disease. If we are correct, this would imply that antibiotics used to treat uterine disease by killing pathogens would have little benefit for health or fertility. Instead, the aim should be to enhance animal tolerance to uterine pathogens and to control risk factors that compromise animal resilience after parturition.
THE POSTPARTUM PERIOD
The events that are required to foster optimal fertility after parturition and expulsion of the placenta are
Prompt involution of the uterus and restoration of a receptive endometrium.
Return of ovarian cyclical activity and ovulation of competent oocytes.
Efficient control of bacteria in the uterus.
Several factors influence uterine involution, including expulsion of the placenta, postpartum uterine muscular contractions, turnover of the extracellular matrix, and regeneration of the uterine caruncles. During the first 30 days postpartum, the weight of the uterus decreases from approximately 9 kg at parturition to approximately 1 kg, and the uterine horns return to their nonpregnant dimensions (8, 9). The epithelium of the endometrium is often damaged during parturition, or during the process of releasing the placenta. Accordingly, inflammation, remodeling, and regeneration of the endometrium are part of postpartum physiology (8, 10). The caruncular tissue undergoes necrosis of the stratum compactum within 7 days postpartum, with the tissues sloughing by 14 days, followed by re-epithelialization of the caruncles by 30 days (10). As epithelialization progresses, the initially flattened epithelial cells develop their columnar appearance, typical of normal endometrium. Neutrophils and lymphoid aggregates are common in the tissue during endometrium repair, and they are likely to be part of the response to tissue damage, as well as to the presence of microbes.
The return of ovarian cyclic activity after parturition requires a coordinated endocrine program by the hypothalamus, pituitary, ovary, and uterus (11–13). Briefly, within days of parturition, the circulating steroid hormone concentrations associated with pregnancy decrease to basal values. Approximately 7 days postpartum there is an increase in plasma follicle stimulating hormone (FSH) concentration, emergence of a cohort of growing follicles, and selection of the first postpartum dominant follicle approximately 10 days after parturition, with subsequent recurrent increases in FSH and emergence of waves of growing follicles every 7 to 10 days. The first postpartum dominant follicle may ovulate to form a corpus luteum, marking the return of ovarian cyclic activity; the dominant follicle may undergo atresia with emergence of subsequent dominant follicles typical of postpartum anestrus; or the dominant follicle may abnormally persist as a follicular cyst. The fate of the first postpartum dominant follicle depends on luteinizing hormone (LH) pulse frequency, and failure to ovulate is usually a consequence of inadequate LH pulse frequency and reduced ovarian follicle estradiol, often associated with the metabolic stress of lactation and uterine bacterial infections (7, 11, 13, 14). Unfortunately, the return of ovarian cyclic activity is abnormal in approximately half of modern dairy cattle.
Uterine Bacteria
Understanding of the microbes found in the postpartum genital tract has changed over the last 20 years. It was thought that the uterus was sterile during pregnancy and became contaminated with nonspecific bacteria from the animal and the environment after parturition. However, there is evidence that the uterus is not sterile and that specific microbes are adapted to the endometrium. Fluorescent probes to image bacteria and 16S ribosomal RNA sequencing of endometrium provide evidence that there is a sparse microbiota in the uterus, even during pregnancy (15, 16). Bacteria identified in these studies include endometrial pathogens, such as Trueperella pyogenes, Fusobacteria species, and Prevotella species. However, the uterine microbiota is substantially less abundant than in the gut or vagina, and the bacterial load is a fraction of that in postpartum uterine disease. Many bacteria in the postpartum uterus likely derive from the vagina, skin, and gut, as well as the environment. However, a bloom in the growth of pathogenic bacteria from the uterine microbiota after parturition may also help establish disease.
Postpartum uterine disease is polymicrobial and the microbial community in the uterus fluctuates during the postpartum period, with cycles of infection, elimination, and reinfection with bacteria. The bacteria most commonly cultured from animals with uterine disease are Escherichia coli, T. pyogenes, Fusobacterium necrophorum, and Prevotella and Bacteroides species (7, 17). Metagenomic techniques have found associations between uterine disease and Bacteroidetes, Fusobacteria, Proteobacteria, and Firmicutes, which are not readily cultured using standard techniques (18–22). Some of the metagenomic studies also find E. coli and T. pyogenes associated with disease, but others do not. Although the bacterial populations vary among animals, between diseases, and with time postpartum, some bacteria are associated with uterine health, such as Peptostreptococcus and Propionibacterium. However, there remains a gap in understanding about which bacteria contribute to the pathogenesis of uterine disease. Taken together, the evidence is that E. coli, T. pyogenes, and anaerobic bacteria are probably the main pathogens causing the clinical signs of uterine disease, but that other pathogens may initiate or contribute to endometrial pathology. There is also evidence that specific coinfections foster disease. For example, T. pyogenes, F. necrophorum, and Prevotella act synergistically to increase the likelihood of disease and the severity of endometritis (23, 24).
Novel strains of E. coli have been isolated from the uterus of animals with uterine disease (25, 26). These endometrial pathogenic E. coli (EnPEC) are more than twice as adherent and invasive for endometrial stromal cells as E. coli isolated from the uterus of clinically unaffected animals (25). In addition, EnPEC stimulate endometrial inflammation and establish disease in animal models. Lipopolysaccharide (LPS, endotoxin) and Type 1 fibrin D-mannose-specific adhesin (commonly known as FimH) are important EnPEC virulence factors. LPS is a major component of the outer membrane of Gram-negative bacteria and provokes a robust inflammatory response when detected in animal tissues (27). Fimbrial adhesins allow bacteria to adhere to host cells, and EnPEC FimH adhesion to endometrial cells is reduced by D-mannose (25).
T. pyogenes is the pathogen most associated with the severity of endometrial pathology, clinical disease, and reduced fertility (28–30). The link between T. pyogenes and disease probably depends on the virulence factor pyolysin. Pyolysin is a cholesterol-dependent cytolysin secreted by the bacterium, and it binds cholesterol-rich domains in the plasma membrane of host cells to form pores, causing cell death by osmotic shock. Endometrial stromal cells are particularly sensitive to pyolysin, compared with endometrial epithelial cells or immune cells (31, 32). The stromal cytolysis caused by pyolysin may explain how T. pyogenes switches from a commensal in the uterus when the epithelium is intact to causing uterine pathology once the epithelium is breached after parturition and bacteria reach the stroma. The importance of E. coli and T. pyogenes for endometritis is supported by the ability to create models of endometritis by infusing E. coli and T. pyogenes into the uteri of naïve cattle (31, 33). However, it is notable that establishment of an animal model of endometritis is also fostered by supplying exogenous progesterone, which may suppress immune defenses, and by debriding the endometrium immediately prior to infusion of bacteria, which disrupts the protective epithelium, allowing the pathogens to adhere to the tissues and invade the underlying stroma.
POSTPARTUM UTERINE DISEASE
The development of disease depends on pathogen virulence and on the ability of the pathogens to overcome an animal’s tolerance and resistance. Many factors influence this balance, including genetics and the environment. The development of postpartum uterine disease in cows is particularly facilitated by several environmental risk factors.
Risk Factors for Uterine Disease
Uterine disease is most common in Bos taurus dairy breeds that have high milk yields, and genetic selection for milk yield is often associated with the increased incidence of uterine disease after parturition. There is an opportunity to select animals for uterine health, and the heritability of metritis ranges from 0.08 to 0.26 (34, 35). However, environmental risk factors may be more important than genetic factors. For example, although some polymorphisms in genes associated with immunity have small effects on uterine health, environmental factors, such as dystocia, parity, and ketosis, are more predictive for uterine disease than the genetic markers (36).
The risk factors for postpartum uterine disease can be classified into three groups: trauma to the female genital tract, disorders of metabolism, and problems with hygiene. Trauma to the female genital tract is associated with retained placenta, dystocia, a large male calf, stillbirths, twins, first parity, and induction of parturition (37–40). Among these risk factors, retained placenta has the strongest association with disease; for example, the odds ratio is >40 for retained placenta causing clinical endometritis (40). Trauma is important in the development of endometritis, as trauma delays uterine involution to limit the physical expulsion of bacteria and damages the endometrial epithelium, allowing bacteria to reach the stroma.
Dairy cows are under metabolic stress because they cannot consume enough food to provide the metabolizable energy required for lactation. Typical cows producing 40 L milk/day require 200 MJ/day, which is three times the energy requirement for resting metabolism. Consequently, postpartum dairy cows catabolize tissues, develop insulin resistance, and have reduced blood concentrations of insulin-like growth factor, glucose, and glutamine and increased concentrations of ketones (41–43). One mechanism underlying the increased risk of disease is that metabolic stress compromises immune cell function (44, 45). Neutrophils have reduced superoxide production, impaired chemotaxis, and lower intracellular glycogen in cows that develop metritis than healthy cows (44, 46). Neutrophils from cows with metritis also had approximately a third of the myeloperoxidase activity and cytochrome c reduction compared with neutrophils from cows with normal uterine health around the time of parturition (45). Impaired neutrophil function is also evident before parturition, which may be important because cows that have stronger initial recruitment of inflammatory cells into the uterus have a more rapid resolution of uterine inflammation postpartum (30).
Deficits in glucose or glutamine, which are the fundamental nutrients used by cells for energy, also reduce endometrial inflammatory responses (47, 48). Limiting the availability of glucose to ex vivo organ cultures of endometrium challenged with LPS or bacterial lipopeptides reduces the secretion of inflammatory mediators, such as the cytokines interleukin (IL)-1β and IL-6 and the chemokine IL-8 [also known as chemokine (C-X-C motif ) ligand 8, CXCL8] (47). Similarly, depletion of glutamine, in the presence of abundant glucose, reduced the IL-1β, IL-6, and IL-8 response to LPS by at least 50% (48). The principal regulator of cellular energy is AMP-activated protein kinase (AMPK), which senses the ratio of AMP to ATP in the cytosol, and a homeostatic level of AMPK activation fosters optimal inflammatory responses (47). Cows also mobilize adipose tissue to satisfy the negative energy balance of lactation, increasing the peripheral plasma concentrations of nonesterified fatty acids (49, 50). These fatty acids are metabolized in tissues to acetyl coenzyme A to provide additional cellular energy, although excess fatty acid oxidation leads to increased production of ketones. During an immune response, tissue cells tend to further exploit fatty acid oxidation to supply nutrients, whereas immune cells exposed to pathogens often increase fatty acid synthesis as part of their inflammatory response (51).
Although it is obvious that bacteria may reach the uterus from the environment, surprisingly, the cleanliness of the environment of the animals is not a dominant risk factor for postpartum uterine disease (40, 52). Rather than the presence of pathogens, development and progression of disease may depend more on risk factors that impair tolerance and immunity to the pathogens. Furthermore, which risk factors are important among trauma, metabolic stress, and hygiene depends on the type of uterine disease.
DEFINITIONS OF UTERINE DISEASE
Uterine disease is diagnosed by veterinarians on the basis of clinical signs. Definitions for the diagnosis of disease are established, and although clinical definitions are limited, it is important that veterinarians and researchers use a consistent approach to diagnose disease (1, 53, 54).
Metritis
Metritis is most common within 10 days of parturition and is characterized by an enlarged uterus, with a purulent uterine discharge, ranging from watery red-brown fluid to viscous off-white pus (Figure 1a), which often has a fetid odor (53). The incidence of metritis varies between breed, country, and herd, but in a study of records from 97,318 cows in the United States, the lactation incidence of metritis, including retained placenta, was 21% (35). The severity of disease can be categorized by the signs of health:
Grade 1 metritis. Animals with an abnormally enlarged uterus and a purulent uterine discharge, but without any systemic signs of ill health.
Grade 2 metritis. Animals with an abnormally enlarged uterus and a purulent uterine discharge, with additional signs of systemic illness, such as decreased milk yield, dullness, and fever.
Grade 3 metritis, also called puerperal metritis or toxic metritis. Animals with an abnormally enlarged uterus and a purulent uterine discharge, with signs of toxemia, such as reduced appetite, cold extremities, and depression.
Metritis warrants treatment because it is painful and causes infertility (55, 56). In a meta-analysis of records from >10,000 animals, metritis increased the time to first insemination by 7.2 days, reduced conception rates to first insemination by 20%, and increased the calving-to-conception interval by 18.6 days (56). Animals with grade 2 or 3 metritis are treated with parenteral broad-spectrum antibiotics for 3 to 5 days. However, a review of 17 studies that employed the third-generation cephalosporin, ceftiofur, to treat metritis found that, whereas 7 studies reported clinical improvement, there was no significant improvement in reproductive performance of the dairy herds (57). In our experience, it is equally important to provide adequate nutrition, nonsteroidal anti-inflammatory drugs, and fluid therapy, if necessary. Of course antibiotics are directed at killing pathogens (resistance), whereas supportive therapy aims to limit tissue damage and return the animal to homoeostasis (tolerance).
Clinical Endometritis
Clinical endometritis is defined as the presence of a purulent or mucopurulent uterine discharge detectable in the vagina of cattle 21 days or more postpartum. Examination of the vagina for purulent discharge with a clean gloved hand is simple, cheap, and effective, with little risk of microbial contamination of the uterus in dairy cattle (7). The presence of pus in the vagina can also be detected using a vaginal speculum or the Metricheck device composed of a rubber diaphragm on the end of a stainless steel rod. A four-point grading system, based on the amount of pus in the vaginal mucus, is readily used to evaluate the severity of clinical endometritis and is prognostic for the outcome of treatment (1, 58). The endometritis grade correlates with the presence of pathogenic organisms associated with uterine disease but not with the presence of nonpathogenic bacteria. However, evaluation of uterine disease is subjective, and there is interoperator and intraoperator variation (54, 59). There is also evidence that in some animals a purulent vaginal discharge associated with cervicitis or vaginitis impairs fertility, independent of endometritis (60). Irrespective of the exact pathology, these purulent conditions reflect an imbalance among pathogen virulence, host tolerance, and resistance.
The incidence of clinical endometritis is approximately 10–20%, with variation between breed, country, and herd. In Canada, 16.9% of 1,865 dairy cows were affected (61), and a survey of 19,870 Holstein cows in Germany found a lactation incidence of 19.2% for endometritis (62). Clinical endometritis is important because even after resolution of the disease, a history of endometritis increases the interval to first insemination by 11 days and delays conception by 32 days, compared with animals that did not have endometritis (63). Cows with clinical endometritis between 20 and 33 days postpartum are also 1.7 times more likely to be culled for reproductive failure than cows without endometritis (61). The main treatments for endometritis are induction of estrus with prostaglandin F2α in animals that have a corpus luteum and intrauterine infusion of antimicrobial agents (58, 64). However, the treatment of endometritis attracts much discussion because there is 33–46% spontaneous self-cure and because animals have reduced fertility even after successful treatment (63, 65, 66).
Subclinical Endometritis
Subclinical endometritis is characterized by inflammation of the endometrium in the absence of clinical signs of endometritis. The importance of subclinical endometritis emerged when cytological evidence of endometritis was associated with reduced fertility (67, 68). The cause of subclinical endometritis is not yet clear and may include resolving bacterial infections, immunopathology, or tissue repair. Approximately twice as many animals with metritis subsequently develop sub-clinical endometritis as animals without metritis (69). In the tissues of animals with subclinical endometritis, there is increased expression of genes encoding inflammatory mediators, such as the chemokines CXCL5 and CXCL8; the cytokines IL1A, IL1B, and TNF; and the acute phase protein HP (haptoglobin) (70–74). For example, 28–41-day postpartum endometrial cytobrush samples from animals with subclinical endometritis, compared with normal animals, had 30-fold higher IL6 and >50-fold higher CXCL8 mRNA expression (71).
Subclinical endometritis is diagnosed when the proportion of neutrophils exceeds operator-defined thresholds, usually approximately 5% of cells, in samples collected by flushing the uterine lumen or by endometrial cytobrush 3 to 9 weeks postpartum (53, 54, 75). Many open questions remain about subclinical endometritis because the etiology is not well established, and cytology does not always correlate with endometrial histopathology (29, 76). The proportion of animals affected also varies widely among studies and with different cellular cutoffs, with the incidence ranging from approximately 11% to >40% of animals. A further issue is that there is no consensus about treatment for subclinical endometritis (75). In most diseases, the acute response by neutrophils to infection is usually replaced by infiltration with macrophages, followed by resolution of inflammation (77, 78). So the persistence of neutrophils in the endometrium 3 to 9 weeks postpartum is unexpected, and the mechanism is currently unexplained.
THE PATHOGENESIS OF UTERINE DISEASE
The increased incidence of uterine disease over the last 50 years coincides with intensification of dairy farming and breeding for increased milk yields to the extent that cows cannot consume enough food to meet the metabolic demand of producing >35 L milk/day (42, 79). It is often argued that metabolic stress suppresses immunity, which leads to the development of postpartum uterine disease (42, 46, 49, 80). Our alternative view is that tolerance is more important than immunity, and that failure of the endometrium to tolerate pathogens causes disease and leads to persistent inflammation until tolerance and homeostasis are reestablished. Examination of reaction norms provides evidence that metabolic stress perturbs uterine tolerance rather than immunity (Figure 1d). Furthermore, the risk factors associated with uterine disease, and the varied polymicrobial infections causing uterine disease, also support our concept that uterine disease is less about which pathogens are present and more about a failure in tolerance to pathogens. However, it is important to realize that once pathogens overcome endometrial tolerance, immunity and inflammation shape the progression and resolution of uterine disease.
Tolerance
Tolerance is about damage limitation in the face of pathogens, and we propose several mechanisms for tolerance to uterine pathogens. The vulva, vagina, cervix, and cervical mucus plug provide physical barriers to ascending infections during gestation, until they are breached during and after parturition. In humans, commensal Lactobacillus species in the vaginal microbiota provide additional protection against pathogens, probably by competing for nutrients and generating an acidic environment (81). However, cattle have <1% relative abundance of lactobacilli in the vagina compared with 70% in humans and a less acidic vaginal environment of pH 7 in cows compared with pH 4.5 in humans (82). However, infusion of lactobacilli into the prepartum bovine vagina reduced the incidence of postpartum metritis to 15% compared with 38% in control animals (83).
The mucus layer on the apical surface of epithelia provides a substantial obstacle to microbes (Figure 2). The secretion and character of mucus in the genital tract are under the control of ovarian steroids, and during estrus mucus flow increases considerably. Interestingly, the expression of MUC1 mRNA was increased twofold when endometrial epithelial cells were treated with LPS (84) and by a similar amount in endometrial cytobrush samples from cows with clinical endometritis (74). Lysozyme expression is also increased in the endometrium of cows with uterine inflammation, and lysozyme digests the peptidoglycans found in the cell walls of bacteria (85). Antimicrobial peptides and mucosal glycoproteins cover the mucosa of the vagina, cervix, and endometrium, where they also neutralize bacteria and prevent them from reaching the plasma membrane of the epithelia. The principal cysteine-rich, cationic, antimicrobial peptides expressed by bovine endometrial epithelium include β-defensins, lingual antimicrobial peptide (LAP), and tracheal antimicrobial peptide (TAP); several gene transcripts for antimicrobial peptides are more abundant in the face of microbial challenge, which links immunity and tolerance (84, 86).
Figure 2.
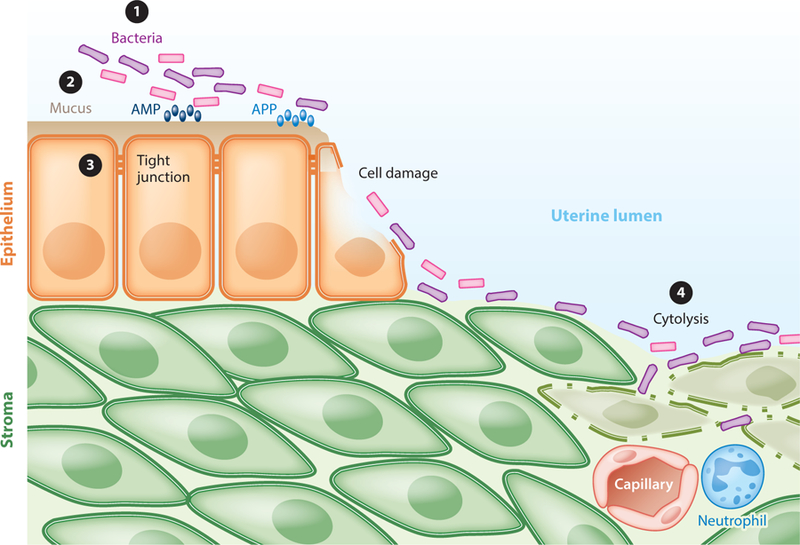
Development of postpartum endometritis. ① After parturition, there is a bloom of bacterial growth in the uterus, and multiple pathogens compete with commensal bacteria in the endometrium. ② The mucus layer in the endometrium helps prevent bacteria from reaching the epithelium, and antimicrobial peptides (AMP) and acute phase proteins (APP) neutralize bacteria and their virulence factors. ③ Tight junctions between epithelial cells prevent bacteria penetrating to the underlying stroma, although, after parturition, epithelial cell damage exposes the stroma. ④ If bacteria reach the stroma, they often cause cell damage and cytolysis and provoke inflammatory responses, including the influx of neutrophils from the peripheral circulation.
The next line of endometrial defense is the columnar epithelium, which has tight junctions between cells to separate the apical and basolateral compartments of the endometrium (Figure 2). Epithelial cells are at least twice as resistant as stromal cells to cytolysis caused by T. pyogenes or pyolysin (31). The greater tolerance of epithelial cells to pyolysin may be because they contain less cholesterol in their plasma membrane than stromal cells. Approximately 90% of the cholesterol in fibroblasts is contained within the plasma membrane, and cholesterol concentrations are tightly regulated by cholesterol uptake, cholesterol efflux transporters, and cholesterol synthesis (87, 88). The first steps in cholesterol synthesis are encompassed by the mevalonate pathway, converting acetyl-CoA to isoprenoids, which are then converted to squalene, and ultimately to cholesterol. The ability of endometrial stromal cells to tolerate pyolysin can be increased using methyl-β-cyclodextrin to reduce cellular cholesterol, statins to inhibit the mevalonate pathway, or squalene synthase inhibitors, or by supplying exogenous isoprenoids (31, 32, 89, 90). For example, endometrial stromal cell viability increased from 14% in controls challenged with pyolysin to 34% in cells treated prior to pyolysin challenge with 10 μM zaragozic acid to inhibit squalene synthase (89). Infusing statins or squalene synthase inhibitors into the postpartum uterus might increase endometrial tolerance to pathogens and limit the severity of uterine disease. Interestingly, statins or inhibition of squalene synthase also modulates innate immunity in the endometrium. In particular, inhibiting squalene synthase reduces the IL-6 and IL-8 inflammatory response to LPS by >50% in endometrial epithelial and stromal cells (91).
The endometrial epithelium is often denuded during the postpartum period, which allows pathogens to reach the extracellular matrix. Damage control is an important aspect of tolerance, and there is evidence that endometritis affects extracellular matrix turnover. Interestingly, genes involved in extracellular matrix homeostasis, such as the matrix metallopeptidase genes MMP1, MMP3, MMP9, and MMP13, are differentially regulated in cows that develop uterine disease when they have severe negative energy balance compared with animals that are in energy balance and do not develop disease (92). Furthermore, the cytokine TNFα increases the production of metallopeptidases MMP-2 and MMP-9 (93).
Although physical barriers and secreted proteins are important for tolerance to microbes in the female genital tract, there are some caveats. First, microbes have evolved countermeasures and strategies to avoid many host defenses. For example, some bacteria produce enzymes that lyse mucus to penetrate the protective layer, and many bacteria secrete proteases that can disrupt defensive peptides and proteins produced by the host (94). Second, immunity is principally responsible for initiating rapid inflammatory responses to resist microbial infections if the tolerance mechanisms are overcome (5, 95).
Resistance
Resistance provides pathogen control and principally depends on innate and adaptive immunity. Innate immunity provides immediate, nonspecific defense against pathogens and does not depend on prior exposure to microbes (27, 95). Adaptive immunity employs antigen-specific receptors, and the response may take several days, depending on prior exposure to the antigen. Innate immunity appears to dominate resistance to pathogens in the endometrium. Cows with uterine disease have increased expression of genes in the endometrium that are typical of innate immunity, including cytokines IL1A, IL1B, IL6, TNF, and IL12A; cytokine receptors IL1R1 and IL1R2; chemokines CXCL5 and CXCL8; PGES; antimicrobial peptides LAP, TAP, DEFB5, and DEFB1; and acute phase proteins HP and SAA3 (70–74, 86, 96, 97). Cows with clinical endometritis also have higher concentrations of IL-1α, IL-1β, and IL-6 in uterine fluid than healthy cows (98, 99). The influx of neutrophils into the uterus is also typical of innate immunity, and this rapid and robust response is important for postpartum uterine health (30).
Innate Immunity
Innate immunity encompasses multiple host resistance mechanisms ranging from acute phase proteins to cytokines (27). Acute phase proteins are synthesized in the liver in response to increased peripheral plasma concentrations of cytokines such as IL-6 (100). Acute phase proteins help restore homeostasis after infection or tissue damage, with roles in hemostasis typified by the action of fibrinogen, antimicrobial effects, and attracting and activating phagocytes. The peripheral plasma concentrations of acute phase proteins, such as α1-acid glycoprotein, haptoglobin, and ceruloplasmin, are associated with the severity of bacterial contamination in the postpartum uterus, particularly the presence of E. coli and T. pyogenes (101, 102). However, the acute phase response is also initiated by trauma (100), and concentrations of α1-acid glycoprotein, haptoglobin, and ceruloplasmin increase with parturition and then decline as uterine involution progresses (102). There is also local expression of genes encoding acute phase proteins in the uterus and ovary, which may provide further localized protection from pathogens (70, 86, 103). Antimicrobial molecules, such as S100 calgranulins and cathelicidin, are also more abundant in the endometrium of animals with uterine disease (92, 104, 105). These proteins are often abundant in neutrophils and are associated with antimicrobial activity and attracting and activating immune cells.
Complement is also an important component of innate defense against microbial infections. The complement system includes approximately 20 proteins, which generate lytic complexes at the surface of pathogens and provide opsonins such as C1b and C3b, which interact with cell surface receptors to promote phagocytosis by neutrophils and macrophages. Components of the complement system, such as the genes C1QA, C1QB, C1QC, C3, and C8, are differentially expressed in the endometrium of diseased postpartum cows with more severe negative energy balance (92).
Much of the innate immune response depends on the detection of microbes by host cells. Innate immunity is founded on the discovery that eukaryotic cells possess pattern recognition receptors that bind pathogen-associated molecular patterns (PAMPs) (106, 107). These PAMPs are usually highly conserved molecules found in prokaryotes but not eukaryotes and include LPS, lipopeptides, flagellin, and microbial RNA and DNA. Receptors such as Toll-like receptors (TLRs) and NOD-like receptors (NLRs) bind PAMPs (27, 95). For example, TLR4 binds to LPS, TLR2/TLR1 and TLR2/TLR6 dimers bind bacterial lipopeptides, and TLR5 binds flagellin. Binding of PAMPs to TLRs activates NF-κB and MAPK intracellular signaling pathways, which result in the production of antimicrobial peptides, such as β-defensins; LAP and TAP; and inflammatory mediators, such as IL-1β, IL-6, IL-8, and prostaglandin E2 (Figure 3). Intracellular pattern recognition receptors, such as NLRP3 (nucleotide-binding domain and leucine rich repeat pyrin 3 domain), principally detect microbes that invade cells, activating the multiprotein inflammasome complex and leading to caspase-1 cleavage of pro-IL-1β to mature IL-1β protein (108). Inflammatory mediators attract and/or activate immune cells to clear bacteria (27, 95). Cytokines bind their cognate receptors, leading to inflammation and further production of antimicrobial peptides, eicosanoids, and reactive oxygen species. Cytokines in the peripheral plasma also drive systemic inflammatory responses, including pyrexia, generalized vasodilation, and release of acute phase proteins from hepatocytes. Chemokines, such as IL-8, attract neutrophils and monocytes to the site of infection to engulf and kill bacteria.
Figure 3.
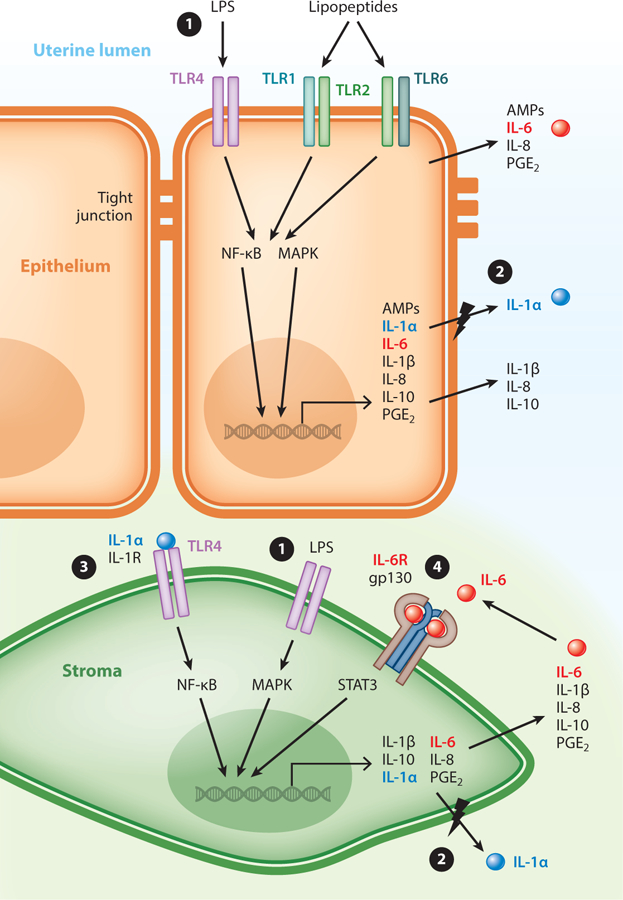
The innate immune response in the endometrium. ① Endometrial epithelial (orange) and stromal (green) cells express functional Toll-like receptor 4 (TLR4) and TLR2/TLR1 and TLR2/TLR6 heterodimers. Binding of pathogen-associated molecular patterns to TLRs activates the NF-κB and mitogen-activated protein kinase (MAPK) signaling pathways, which lead to the transcription of genes that encode several inflammatory mediators, including antimicrobial peptides (AMPs), cytokines, chemokines, and prostaglandins. Whereas most inflammatory mediators are released from cells into the extracellular fluid, in the polarized epithelial cells specific inflammatory mediators are secreted apically. ② Cells release the intracellular cytokine interleukin (IL)-1α (blue spheres) if cells are also damaged (lightning bolt). ③ In a paracrine manner, IL-1α can bind to the IL-1R of nearby cells, further activating the NF-κB and MAPK signaling pathways. ④ Epithelial and stromal cells respond differently to IL-6 (red spheres), and in stromal cells IL-6 has a positive feedback through the IL-6R/gp130 receptor heterodimer and signal transducer and activator of transcription-3 (STAT3) signaling to enhance the secretion of IL-6 and IL-8. Abbreviations: LPS, lipopolysaccharide; PGE2, prostaglandin E2.
Innate Immunity in the Endometrium
Pattern recognition receptors are principally expressed by hematopoietic cells such as neutrophils, macrophages, and dendritic cells (27, 95). However, bovine endometrial epithelial and stromal cells also express most TLR genes (84). These epithelial and stromal cells respond to bacteria, LPS, and lipopeptides via TLR1, TLR2, TLR4, and TLR6, activating the NF-κB and MAPK pathways, which stimulate the production of inflammatory mediators, including IL-1β, IL-6, IL-8, and prostaglandin E2 (109–111). In addition, antimicrobial peptide LAP and TAP gene transcription is increased by a hundredfold when epithelial cells are stimulated with LPS (84). The polarized epithelial cells also spatially direct some of their inflammatory responses (Figure 3), with apical challenge using LPS stimulating predominantly apical secretion of IL-8 and basolateral secretion of prostaglandins (112). More striking is that apical or basolateral challenge of epithelial cells with LPS always directs IL-6 secretion apically (113). These spatial responses may help attract immune cells to the site of infection, regulate the immune response, or be associated with physiological roles for some of these inflammatory mediators. For example, LPS switches epithelial cell secretion from prostaglandin F2α to prostaglandin E2, and this is not reversed by administering oxytocin to mimic the luteolytic signal (114). This prostaglandin switch has important implications for fertility, as LPS may counter the physiological production of prostaglandin F2α from epithelial cells for luteolysis.
Excessive inflammation leads to immunopathology or septic shock, and so a series of checks and balances are in place to scale inflammation to meet the level of microbial threat during the progression of an infection and to resolve inflammation after infections are cleared (115). One example in the bovine endometrium is a positive feedback role for the IL-6 receptor (IL6R) and signal transducer and activator of transcription-3 (STAT3) (116). Stromal cell IL6R binds the IL-6 released during the initial stage of infection, leading to activation of STAT3, which in turn stimulates further increases in the secretion of IL-6 and IL-8 (Figure 3).
Damage-associated molecular patterns (DAMPs) are also used to modulate the intensity of the inflammatory response initiated by PAMPs (115). Pattern recognition receptors bind DAMPs to help cells sense danger (117, 118). Dying or damaged mammalian cells release DAMPs such as HMGB1, IL-1α, mitochondrial DNA, and ATP (117). Endometrial epithelial and stromal cells employ IL-1α as the principal DAMP (98). Cells accumulate IL-1α intracellularly in response to LPS, and IL-1α is released only when cells are damaged (Figure 3). This may be important for pyogenes infections where pyolysin forms pores in the plasma membrane of endometrial cells (31, 32, 89). The IL-1α then binds to the cognate receptor, IL-1R, to stimulate other cells to release more inflammatory mediators, such as IL-6. Interestingly, innate immune responses are also activated by pore-forming toxins that induce ion fluxes across the plasma membrane (119, 120). However, whereas T. pyogenes stimulates inflammatory responses, pyolysin did not stimulate inflammation in bovine endometrial or immune cells (31).
Innate immunity is an evolutionarily ancient system, so it is not surprising that it is integrated with other cellular homeostatic and metabolic pathways (78). Cows with a severe negative energy balance have persistent uterine inflammation, whereas animals with mild negative energy balance recover their energy balance and repair their endometrium by two weeks after parturition (92). There is even evidence that reduced appetite prior to parturition increases the risk of postpartum uterine disease (38). The response to pathogen molecules is also energetically expensive (121). A striking example is that animals use >1 kg of glucose in the first 12 h after challenge with LPS (121). Endometrial tissue exposed to LPS in vitro also has an increased demand for glucose (47). Metabolic stress compromising innate immunity may result in inefficient clearance of bacteria from the endometrium.
Adaptive Immunity
Adaptive immunity in the endometrium also contributes to the development of endometritis, but there remain gaps in knowledge. Early work identified areas rich in T cells and B cells in the postpartum endometrium, often comprising lymphocytic foci within the stroma (10, 28). More generalized adaptive immune responses are also evident in postpartum animals, with increased abundance of antibodies (122). Furthermore, these increased levels of circulating antibodies are associated with less postpartum uterine disease (123). However, the relative importance of adaptive immunity in countering uterine infections is unclear, as preliminary data on vaccines for metritis containing components of E. coli, F. necrophorum, and/or T. pyogenes suggest that vaccines can provide some protection against disease (124, 125), but other studies do not support this concept (126). Furthermore, postpartum endometritis often occurs after successive calvings, and so adaptive immunity does not appear to provide long-term protection. Loss of function is a cardinal sign of inflammation, whether associated with innate or adaptive immunity. Because the principal function of the endometrium is reproduction, it is not surprising that endometritis reduces fertility (61, 63, 67, 68). The mechanisms linking endometritis with fertility are not fully defined but may include damaging effects of bacterial toxins on sperm, inappropriate endocrine signaling by inflammatory mediators such as cytokines and prostaglandins, perturbation of sperm transport and competence, a reduced ability to nurture the early embryo, and impaired implantation and placentation. However, beyond the direct effects of endometritis on reproductive physiology in the uterus, there is also evidence that uterine disease affects ovarian function.
IMPACT OF UTERINE DISEASE ON OVARIAN FUNCTION
Although failures in damage limitation and pathogen control shape uterine disease, the ovary was previously considered to be tolerant to infections. However, links between the postpartum uterus and ovary were proposed because the first dominant follicle after parturition is twice as likely to be in the ovary contralateral to the previously gravid uterine horn (127, 128). The role of uterine disease in this link between uterus and ovary was highlighted by observations that dairy cows with uterine infections have slower growth of the first postpartum dominant follicle from 7 to 16 days after parturition, have lower peripheral plasma estradiol concentrations, and are less likely to ovulate (7). A subsequent study showed not only reduced follicle growth and estradiol secretion in cows with uterine infections but also lower circulating concentrations of progesterone after formation of the first postpartum corpus luteum (129). Subclinical endometritis 21 days postpartum did not affect follicle growth, but even 9 weeks after parturition intrafollicular estradiol concentrations were lower in cows with uterine infection compared with normal cows (130).
There is an intimate vascular connection between the uterus and ovary, with the venous drainage from the uterus serving an important route for prostaglandin F2α to reach the ovarian artery to initiate luteolysis (131). This vasculature is markedly enlarged in the postpartum period, and so PAMPs and inflammatory mediators may also use this local route to reach the ovary (Figure 4). The concentration of LPS in follicular fluid aspirated from dominant follicles in vivo is correlated with the severity of uterine disease (132). In follicles collected after slaughter, follicles that contained higher concentrations of LPS had lower concentrations of estradiol and lower expression of mRNA for the steroidogenic enzymes CYP17A1 and CYP19A1 (133). Follicular fluid LPS concentrations are also higher in follicles of cows that did not ovulate the first postpartum follicle compared with cows that ovulated (134).
Figure 4.
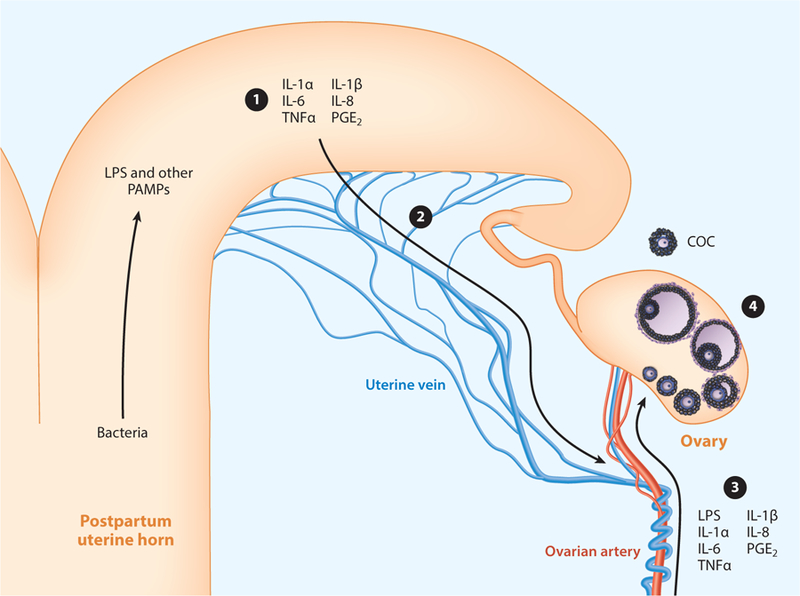
Uterine disease affects ovarian function. ① Pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), and inflammatory mediators, such as cytokines and prostaglandin E2 (PGE2), are abundant in the uterus of animals with postpartum endometritis. ② The PAMPs and inflammatory mediators reach the vasculature via the uterine vein and ③ then may reach the ovary by countercurrent mechanisms with the ovarian arterial circulation, local lymphatics, or the peripheral circulation. ④ PAMPs and inflammatory meditators perturb most of the stages of ovarian follicle and oocyte development, depicted in the ovary, ranging from primordial follicles to ovulation of the cumulus-oocyte complex (COC). Abbreviations: IL, interleukin; TNFα, tumor necrosis factor alpha.
Little is known about tolerance and resistance in the ovary. The physical protection provided to the oocyte by the ovarian follicle and the zona pellucida, and the suspended development of primordial follicles and meiotic arrest, are concordant with the concept of tolerance. However, healthy ovarian follicles do not contain hematopoietic immune cells, so ovarian follicle resistance must rely on the granulosa cells or oocyte (132, 135). Granulosa cells isolated from growing or dominant ovarian follicles express TLR1–10 mRNA, and LPS or bacterial lipopeptides stimulate the secretion of IL-1β, IL-6, CXCL1, CXCL2, CXCL3, and IL-8 protein from granulosa cells (135–137). The functional role for TLR4 and TLR2 in granulosa cells was confirmed using small interfering RNA to deplete gene expression and by inhibiting the MAPK signaling pathway (135, 136). The abundance of IL-6 secreted by granulosa cells from beef or dairy animals is similar to the macrophage response to LPS (135). LPS also limits granulosa cell estradiol production by reducing the expression of CYP17A1 and CYP19A1 and reducing aromatase protein levels (132, 133, 136). Although there are direct effects of LPS on granulosa cell function, there may also be indirect effects because IL-6, IL-8, and TNFα perturb granulosa cell steroidogenesis in vitro (138–140). In addition, 2-day treatment with 100 ng/ml IL-6 perturbed the proliferation of granulosa cells from emerging and dominant follicles, with 32% and 55% fewer cells than controls, respectively (138). The impact of uterine infection may also affect even the earliest stages of follicle development. In ex vivo experiments, LPS reduced the primordial ovarian follicle pool, with an associated increase in primordial follicle activation and loss of primordial follicle regulatory proteins (141).
Resistance mechanisms are evident in cumulus-oocyte complexes, as LPS stimulates the secretion of IL-6 (135). The LPS also activates cumulus expansion in vitro, and inappropriate timing of cumulus expansion may contribute to infertility, because expansion is closely coordinated with ovulation. Furthermore, LPS or IL-6 might reach the oocyte via the cytoplasmic trans-zonal projections from granulosa cells that synapse on the oolemma. Indeed, LPS increases the incidence of meiotic arrest and germinal vesicle breakdown failure in bovine oocytes (135). Treatment of cumulus-oocyte complexes with LPS or a bacterial lipoprotein also perturbs the expression of GDF9 and NLRP5, which are involved in oocyte maturation (142). The effects of infection on fertility are evident not only during disease but also for some time after because oocyte development takes approximately 120 days from the primordial follicle stage to ovulation of a cumulus-oocyte complex. Thus, in cows inseminated 60 to 120 days after parturition, the ovulated oocytes will have started their development during the early postpartum period when they may have been exposed to uterine disease. This effect is similar to how greater body condition loss from 3 to 5 weeks postpartum reduces conception rate several weeks later (2, 143).
Another mechanism linking uterine disease to ovarian dysfunction is that PAMPs and cytokines perturb the function of the hypothalamus and the pituitary, reducing the release of gonadotrophin-releasing hormone and LH, which regulate ovarian function (14, 144). In postpartum cows, LPS in the uterus suppresses the LH surge and prevents ovulation (14). In addition, during the follicular phase, LPS decreases LH pulse frequency, decreases estradiol, and interrupts the LH surge and ovulation in cattle (145, 146). However, the peripheral plasma concentrations of FSH are unaffected by uterine disease in cattle, so that recurrent waves of follicles develop in the ovary, even during uterine disease (7).
One consideration about the links between the uterus and ovary is that ovarian steroid hormones can also influence the development of postpartum uterine disease. Progesterone is immunosuppressive, whereas estradiol may enhance immunity and was used to treat uterine disease (147). However, there are conflicting data about how estradiol could increase uterine resistance. Exogenous estradiol infused into the bovine uterus during the postpartum period increased the abundance of bacteria in the uterus (148). Furthermore, the stage of the estrous cycle, or exogenous progesterone or estradiol, did not modulate innate immunity in ex vivo organ cultures of bovine endometrium (149). Similarly, treatment with estradiol or progesterone, or inhibitors of estradiol or progesterone nuclear receptors, did not affect IL-1β, IL-6, or IL-8 protein or IL1B, IL6, CXCL8, or CCL5 gene expression by endometrial cells or macrophages (149). More work is needed on how ovarian steroids affect the resilience of the uterus to pathogens.
CONCLUSIONS
Although there is a clear understanding of the clinical aspects and implications of postpartum uterine disease, and some of the mechanisms of pathology, there are important outstanding questions. The most obvious question is why modern, high-milk-yield cows are so susceptible to metritis and endometritis. Allied to this is what can be done to prevent uterine disease. Answering these questions is vital for sustainable intensification of the dairy industry over the next 50 years (2). Genetic selection for more resilient animals that are less susceptible to uterine disease is a priority for the dairy industry. However, here we argue that the increasing incidence of postpartum uterine disease could be caused by failures in tolerance to bacteria in the endometrium. Once the pathogens overcome endometrial tolerance, a prompt and robust innate immune response is essential to counter them. However, there is evidence that the metabolic stress associated with lactation compromises both tolerance and immunity. We propose that failures in endometrial tolerance to pathogenic bacteria and the subsequent innate immune response shape postpartum uterine disease.
Failures in tolerance would better explain why antibiotics used in animals with uterine disease to kill the pathogens are of little benefit to fertility. We suggest that prevention is better than a cure for uterine disease, not only because the disease causes pain and suffering but also because uterine infections perturb ovarian function and oocyte health. Development of new ways to counter postpartum uterine disease will come from improved understanding of tolerance and resistance in the postpartum genital tract. The best current advice is to optimize animal nutrition and management to increase the tolerance of parturient and postpartum animals so that they are better able to limit the impact of uterine pathogens without developing uterine disease.
Tolerance:
the ability to limit the disease severity induced by a given pathogen burden
Resistance:
the ability to limit pathogen burden, usually the function of immunity
Pathogen:
an organism that causes disease in a host
ANIMAL RESILIENCE DEPENDS ON RESISTANCE AND TOLERANCE.
The nomenclature for tolerance and resistance in animals is somewhat confused by semantics and scientific traditions because it originates from the field of plant ecology. The simplest approach is to consider an animal’s resilience to infection as the combination of the animal’s tolerance and resistance to pathogens (3). Tolerance is the ability to limit the disease severity induced by a given pathogen burden (4–6). Resistance is the ability to limit the pathogen burden and is usually the function of immunity. However, tolerance has also been described using words such as resilience and endurance. Tolerance is often equated with damage control, whereas resistance largely depends on immunity for pathogen control. Although resistance mechanisms are widely studied, there is a scarcity of knowledge about the mechanisms of tolerance in animals. However, tolerance has the advantage over immunity in that tolerance helps prevent disease from developing, whereas immunity is a response to the progression of infection. Unfortunately, resistance is often negatively correlated with tolerance (6). Thus, selecting for milk yield in the face of uterine disease may inadvertently reduce tolerance. In our view, prevention is better than cure, and tolerance in cows warrants further investigation to facilitate sustainable agriculture.
Reaction norm:
a curve that relates, for a given genotype, the contribution of environmental variation to observed phenotypic variation
Cytokines:
signaling molecules that mediate and regulate immunity, inflammation, and hematopoiesis
Chemokines:
proteins that stimulate chemotaxis—movement of leukocytes toward sites of infection
Acute phase protein:
protein that changes serum concentration by >25% in response to inflammatory cytokines and helps counter infections
Pathogen-associated molecular patterns (PAMPs):
molecules found in prokaryotes that are recognized by the innate immune system in eukaryotes
Sepsis:
life-threatening organ dysfunction caused by a dysregulated host response to infection
ACKNOWLEDGMENTS
We thank Sian Owens, John Harwood, Stephen LeBlanc, Robert Gilbert, Geoffrey Dahl, Jose Santos, and many collaborators, whose discussions helped to frame our thoughts. We apologize to investigators whose research could not be cited due to space limitations. Work in the authors’ laboratories is supported by the United Kingdom Biotechnology and Biological Sciences Research Council (BB/K006592/1) and the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (R01HD084316).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Sheldon IM, Cronin J, Goetze L, Donofrio G, Schuberth HJ. 2009. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod 81:1025–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Britt JH, Cushman RA, Dechow CD, Dobson H, Humblot P, et al. 2018. Invited review: learning from the future: a vision for dairy farms and cows in 2067. J. Dairy Sci 101:3722–41 [DOI] [PubMed] [Google Scholar]
- 3.Raberg L, Sim D, Read AF. 2007. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 318:812–14.Provides a framework for exploring resistance and tolerance in animals.
- 4.Schneider DS, Ayres JS. 2008. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat. Rev. Immunol 8:889–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medzhitov R, Schneider DS, Soares MP. 2012. Disease tolerance as a defense strategy. Science 335:936–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Read AF, Graham AL, Raberg L. 2008. Animal defenses against infectious agents: Is damage control more important than pathogen control? PLOS Biol 6:e1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheldon IM, Noakes DE, Rycroft AN, Pfeiffer DU, Dobson H. 2002. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction 123:837–45.Evidence for how postpartum uterine disease affects uterine health and ovarian follicle growth and development in vivo.
- 8.Gier HT, Marion GB. 1968. Uterus of the cow after parturition: involutional changes. Am. J. Vet. Res 29:83–96 [PubMed] [Google Scholar]
- 9.Sheldon IM, Noakes DE, Rycroft AN, Dobson H. 2003. The effect of intrauterine administration of estradiol on postpartum uterine involution in cattle. Theriogenology 59:1357–71 [DOI] [PubMed] [Google Scholar]
- 10.Wagner WC, Hansel W. 1969. Reproductive physiology of the post partum cow. l. Clinical histological findings. J. Reprod. Fertil 18:493–500 [DOI] [PubMed] [Google Scholar]
- 11.Beam SW, Butler WR. 1997. Energy balance and ovarian follicle development prior to the first ovulation postpartum in dairy cows receiving three levels of dietary fat. Biol. Reprod 56:133–42 [DOI] [PubMed] [Google Scholar]
- 12.Crowe MA. 2008. Resumption of ovarian cyclicity in post-partum beef and dairy cows. Reprod. Domest. Anim 43(Suppl. 5):20–28 [DOI] [PubMed] [Google Scholar]
- 13.Cheong SH, Sa Filho OG, Absalon-Medina VA, Pelton SH, Butler WR, Gilbert RO. 2016. Metabolic and endocrine differences between dairy cows that do or do not ovulate first postpartum dominant follicles. Biol. Reprod 94:18. [DOI] [PubMed] [Google Scholar]
- 14.Peter AT, Bosu WT, DeDecker RJ. 1989. Suppression of preovulatory luteinizing hormone surges in heifers after intrauterine infusions of Escherichia coli endotoxin. Am. J. Vet. Res 50:368–73 [PubMed] [Google Scholar]
- 15.Karstrup CC, Klitgaard K, Jensen TK, Agerholm JS, Pedersen HG. 2017. Presence of bacteria in the endometrium and placentomes of pregnant cows. Theriogenology 99:43–47.Visualization of bacteria in the endometrium of animals during pregnancy showing that the uterus is not sterile.
- 16.Moore SG, Ericsson AC, Poock SE, Melendez P, Lucy MC. 2017. Hot topic: 16S rRNA gene sequencing reveals the microbiome of the virgin and pregnant bovine uterus. J. Dairy Sci 100:4953–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huszenicza G, Fodor M, Gacs M, Kulcsar M, Dohmen MJW, et al. 1991. Uterine bacteriology, resumption of cyclic ovarian activity and fertility in postpartum cows kept in large-scale dairy herds. Reprod. Domest. Anim 34:237–45 [Google Scholar]
- 18.Machado VS, Oikonomou G, Bicalho ML, Knauer WA, Gilbert R, Bicalho RC. 2012. Investigation of postpartum dairy cows’ uterine microbial diversity using metagenomic pyrosequencing of the 16S rRNA gene. Vet. Microbiol 159:460–69 [DOI] [PubMed] [Google Scholar]
- 19.Santos TM, Bicalho RC. 2012. Diversity and succession of bacterial communities in the uterine fluid of postpartum metritic, endometritic and healthy dairy cows. PLOS ONE 7:e53048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng Y, Wang Y, Hang S, Zhu W. 2013. Microbial diversity in uterus of healthy and metritic postpartum Holstein dairy cows. Folia Microbiol 58:593–600 [DOI] [PubMed] [Google Scholar]
- 21.Wagener K, Prunner I, Pothmann H, Drillich M, Ehling-Schulz M. 2015. Diversity and health status specific fluctuations of intrauterine microbial communities in postpartum dairy cows. Vet. Microbiol 175:286–93 [DOI] [PubMed] [Google Scholar]
- 22.Knudsen LR, Karstrup CC, Pedersen HG, Agerholm JS, Jensen TK, Klitgaard K. 2015. Revisiting bovine pyometra: new insights into the disease using a culture-independent deep sequencing approach. Vet. Microbiol 175:319–24 [DOI] [PubMed] [Google Scholar]
- 23.Olson JD, Ball L, Mortimer RG, Farin PW, Adney WS, Huffman EM. 1984. Aspects of bacteriology and endocrinology of cows with pyometra and retained fetal membranes. Am. J. Vet. Res 45:2251–55 [PubMed] [Google Scholar]
- 24.Ruder CA, Sasser RG, Williams RJ, Ely JK, Bull RC, Butler JE. 1981. Uterine infections in the post-partum cow: II. Possible synergistic effect of Fusobacterium necrophorum and Corynebacterium pyogenes. Theriogenology 15:573–80 [Google Scholar]
- 25.Sheldon IM, Rycroft AN, Dogan B, Craven M, Bromfield JJ, et al. 2010. Specific strains of Escherichia coli are pathogenic for the endometrium of cattle and cause pelvic inflammatory disease in cattle and mice. PLOS ONE 5:e9192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bicalho RC, Machado VS, Bicalho ML, Gilbert RO, Teixeira AG, et al. 2010. Molecular and epidemiological characterization of bovine intrauterine Escherichia coli. J. Dairy Sci 93:5818–30 [DOI] [PubMed] [Google Scholar]
- 27.Moresco EM, LaVine D, Beutler B. 2011. Toll-like receptors. Curr. Biol 21:R488–93 [DOI] [PubMed] [Google Scholar]
- 28.Bonnett BN, Martin SW, Gannon VP, Miller RB, Etherington WG. 1991. Endometrial biopsy in Holstein-Friesian dairy cows. III. Bacteriological analysis and correlations with histological findings. Can. J. Vet. Res 55:168–73 [PMC free article] [PubMed] [Google Scholar]
- 29.Westermann S, Drillich M, Kaufmann TB, Madoz LV, Heuwieser W. 2010. A clinical approach to determine false positive findings of clinical endometritis by vaginoscopy by the use of uterine bacteriology and cytology in dairy cows. Theriogenology 74:1248–55 [DOI] [PubMed] [Google Scholar]
- 30.Gilbert RO, Santos NR. 2016. Dynamics of postpartum endometrial cytology and bacteriology and their relationship to fertility in dairy cows. Theriogenology 85:1367–74 [DOI] [PubMed] [Google Scholar]
- 31.Amos MR, Healey GD, Goldstone RJ, Mahan S, Duvel A, et al. 2014. Differential endometrial cell sensitivity to a cholesterol-dependent cytolysin links Trueperella pyogenes to uterine disease in cattle. Biol. Reprod 90:54.Dissection of the role of pyolysin in the bovine endometrium and identifying the sensitivity of stromal cells to pyolysin.
- 32.Preta G, Lotti V, Cronin JG, Sheldon IM. 2015. Protective role of the dynamin inhibitor Dynasore against the cholesterol-dependent cytolysin of Trueperella pyogenes. FASEB J 29:1516–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayliffe TR, Noakes DE. 1982. Effects of exogenous oestrogen and experimentally induced endometritis on absorption of sodium benzylpenicillin from the cow’s uterus. Vet. Rec 110:96–98 [DOI] [PubMed] [Google Scholar]
- 34.Lin HK, Oltenacu PA, Van Vleck LD, Erb HN, Smith RD. 1989. Heritabilities of and genetic correlations among six health problems in Holstein cows. J. Dairy Sci 72:180–86 [DOI] [PubMed] [Google Scholar]
- 35.Zwald NR, Weigel KA, Chang YM, Welper RD, Clay JS. 2004. Genetic selection for health traits using producer-recorded data. I. Incidence rates, heritability estimates, and sire breeding values. J. Dairy Sci 87:4287–94 [DOI] [PubMed] [Google Scholar]
- 36.Pinedo PJ, Galvao KN, Seabury CM. 2013. Innate immune gene variation and differential susceptibility to uterine diseases in Holstein cows. Theriogenology 80:384–90 [DOI] [PubMed] [Google Scholar]
- 37.Markusfeld O. 1984. Factors responsible for post parturient metritis in dairy cattle. Vet. Rec 114:539–42 [DOI] [PubMed] [Google Scholar]
- 38.Huzzey JM, Veira DM, Weary DM, von Keyserlingk MA. 2007. Prepartum behavior and dry matter intake identify dairy cows at risk for metritis. J. Dairy Sci 90:3220–33 [DOI] [PubMed] [Google Scholar]
- 39.Dubuc J, Duffield TF, Leslie KE, Walton JS, LeBlanc SJ. 2010. Risk factors for postpartum uterine diseases in dairy cows. J. Dairy Sci 93:5764–71 [DOI] [PubMed] [Google Scholar]
- 40.Potter T, Guitian J, Fishwick J, Gordon PJ, Sheldon IM. 2010. Risk factors for clinical endometritis in postpartum dairy cattle. Theriogenology 74:127–34 [DOI] [PubMed] [Google Scholar]
- 41.Wathes DC, Cheng Z, Fenwick MA, Fitzpatrick R, Patton J. 2011. Influence of energy balance on the somatotrophic axis and matrix metalloproteinase expression in the endometrium of the postpartum dairy cow. Reproduction 141:269–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chagas LM, Bass JJ, Blache D, Burke CR, Kay JK, et al. 2007. Invited review: new perspectives on the roles of nutrition and metabolic priorities in the subfertility of high-producing dairy cows. J. Dairy Sci 90:4022–32 [DOI] [PubMed] [Google Scholar]
- 43.Doepel L, Lessard M, Gagnon N, Lobley GE, Bernier JF, et al. 2006. Effect of postruminal glutamine supplementation on immune response and milk production in dairy cows. J. Dairy Sci 89:3107–21 [DOI] [PubMed] [Google Scholar]
- 44.Cai TQ, Weston PG, Lund LA, Brodie B, McKenna DJ, Wagner WC. 1994. Association between neutrophil functions and periparturient disorders in cows. Am. J. Vet. Res 55:934–43 [PubMed] [Google Scholar]
- 45.Hammon DS, Evjen IM, Dhiman TR, Goff JP, Walters JL. 2006. Neutrophil function and energy status in Holstein cows with uterine health disorders. Vet. Immunol. Immunopathol 113:21–29 [DOI] [PubMed] [Google Scholar]
- 46.Galvao KN, Flaminio MJ, Brittin SB, Sper R, Fraga M, et al. 2010. Association between uterine disease and indicators of neutrophil and systemic energy status in lactating Holstein cows. J. Dairy Sci 93:2926–37 [DOI] [PubMed] [Google Scholar]
- 47.Turner ML, Cronin JG, Noleto PG, Sheldon IM. 2016. Glucose availability and AMP-activated protein kinase link energy metabolism and innate immunity in the bovine endometrium. PLOS ONE 11:e0151416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Noleto PG, Saut JP, Sheldon IM. 2017. Short communication: Glutamine modulates inflammatory responses to lipopolysaccharide in ex vivo bovine endometrium. J. Dairy Sci 100:2207–12 [DOI] [PubMed] [Google Scholar]
- 49.Wathes DC. 2012. Mechanisms linking metabolic status and disease with reproductive outcome in the dairy cow. Reprod. Domest. Anim 47(Suppl. 4):304–12 [DOI] [PubMed] [Google Scholar]
- 50.Sordillo LM, Contreras GA, Aitken SL. 2009. Metabolic factors affecting the inflammatory response of periparturient dairy cows. Anim. Health Res. Rev 10:53–63 [DOI] [PubMed] [Google Scholar]
- 51.O’Neill LA, Kishton RJ, Rathmell J. 2016. A guide to immunometabolism for immunologists. Nat. Rev. Immunol 16:553–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noakes DE, Wallace L, Smith GR. 1991. Bacterial flora of the uterus of cows after calving on two hygienically contrasting farms. Vet. Rec 128:440–42 [DOI] [PubMed] [Google Scholar]
- 53.Sheldon IM, Lewis GS, LeBlanc S, Gilbert RO. 2006. Defining postpartum uterine disease in cattle. Theriogenology 65:1516–30.Sets out the definitions and methods for scoring postpartum uterine disease.
- 54.de Boer MW, LeBlanc SJ, Dubuc J, Meier S, Heuwieser W, et al. 2014. Invited review: systematic review of diagnostic tests for reproductive-tract infection and inflammation in dairy cows. J. Dairy Sci 97:3983–99 [DOI] [PubMed] [Google Scholar]
- 55.Stojkov J, von Keyserlingk MA, Marchant-Forde JN, Weary DM. 2015. Assessment of visceral pain associated with metritis in dairy cows. J. Dairy Sci 98:5352–61 [DOI] [PubMed] [Google Scholar]
- 56.Fourichon C, Seegers H, Malher X. 2000. Effect of disease on reproduction in the dairy cow: a meta-analysis. Theriogenology 53:1729–59 [DOI] [PubMed] [Google Scholar]
- 57.Haimerl P, Heuwieser W. 2014. Invited review: antibiotic treatment of metritis in dairy cows: a systematic approach. J. Dairy Sci 97:6649–61 [DOI] [PubMed] [Google Scholar]
- 58.Sheldon IM, Noakes DE. 1998. Comparison of three treatments for bovine endometritis. Vet. Rec 142:575–79 [DOI] [PubMed] [Google Scholar]
- 59.Sannmann I, Heuwieser W. 2015. Technical note: intraobserver, interobserver, and test-retest reliabilities of an assessment of vaginal discharge from cows with and without acute puerperal metritis. J. Dairy Sci 98:5460–66 [DOI] [PubMed] [Google Scholar]
- 60.Denis-Robichaud J, Dubuc J. 2015. Determination of optimal diagnostic criteria for purulent vaginal discharge and cytological endometritis in dairy cows. J. Dairy Sci 98:6848–55 [DOI] [PubMed] [Google Scholar]
- 61.LeBlanc SJ, Duffield TF, Leslie KE, Bateman KG, Keefe GP, et al. 2002. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J. Dairy Sci 85:2223–36 [DOI] [PubMed] [Google Scholar]
- 62.Gernand E, Rehbein P, von Borstel UU, Ko¨ nig S. 2012. Incidences of and genetic parameters for mastitis, claw disorders, and common health traits recorded in dairy cattle contract herds. J. Dairy Sci 95:2144–56 [DOI] [PubMed] [Google Scholar]
- 63.Borsberry S, Dobson H. 1989. Periparturient diseases and their effect on reproductive performance in five dairy herds. Vet. Rec 124:217–19 [DOI] [PubMed] [Google Scholar]
- 64.LeBlanc SJ, Duffield TF, Leslie KE, Bateman KG, Keefe GP, et al. 2002. The effect of treatment of clinical endometritis on reproductive performance in dairy cows. J. Dairy Sci 85:2237–49 [DOI] [PubMed] [Google Scholar]
- 65.Steffan J, Adriamanga S, Thibier M. 1984. Treatment of metritis with antibiotics or prostaglandin F2a and influence of ovarian cyclicity in dairy cows. Am. J. Vet. Res 45:1090–94 [PubMed] [Google Scholar]
- 66.Griffin JFT, Hartigan PJ, Nunn WR. 1974. Non-specific uterine infection and bovine fertility. I. Infection patterns and endometritis during the first seven weeks post-partum. Theriogenology 1:91–106 [DOI] [PubMed] [Google Scholar]
- 67.Kasimanickam R, Duffield TF, Foster RA, Gartley CJ, Leslie KE, et al. 2004. Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows. Theriogenology 62:9–23.Recognition of the importance of subclinical endometritis in cattle.
- 68.Gilbert RO, Shin ST, Guard CL, Erb HN, Frajblat M. 2005. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology 64:1879–88 [DOI] [PubMed] [Google Scholar]
- 69.Lima FS, Vieira-Neto A, Vasconcellos GS, Mingoti RD, Karakaya E, et al. 2014. Efficacy of ampicillin trihydrate or ceftiofur hydrochloride for treatment of metritis and subsequent fertility in dairy cows. J. Dairy Sci 97:5401–14 [DOI] [PubMed] [Google Scholar]
- 70.Fischer C, Drillich M, Odau S, Heuwieser W, Einspanier R, Gabler C. 2010. Selected pro-inflammatory factor transcripts in bovine endometrial epithelial cells are regulated during the oestrous cycle and elevated in case of subclinical or clinical endometritis. Reprod. Fertil. Dev 22:818–29 [DOI] [PubMed] [Google Scholar]
- 71.Ghasemi F, Gonzalez-Cano P, Griebel PJ, Palmer C. 2012. Proinflammatory cytokine gene expression in endometrial cytobrush samples harvested from cows with and without subclinical endometritis. Theriogenology 78:1538–47 [DOI] [PubMed] [Google Scholar]
- 72.Gabler C, Drillich M, Fischer C, Holder C, Heuwieser W, Einspanier R. 2009. Endometrial expression of selected transcripts involved in prostaglandin synthesis in cows with endometritis. Theriogenology 71:993–1004 [DOI] [PubMed] [Google Scholar]
- 73.Gabler C, Fischer C, Drillich M, Einspanier R, Heuwieser W. 2010. Time-dependent mRNA expression of selected pro-inflammatory factors in the endometrium of primiparous cows postpartum. Reprod. Biol. Endocrinol 8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kasimanickam R, Kasimanickam V, Kastelic JP. 2014. Mucin 1 and cytokines mRNA in endometrium of dairy cows with postpartum uterine disease or repeat breeding. Theriogenology 81:952–58 [DOI] [PubMed] [Google Scholar]
- 75.Wagener K, Gabler C, Drillich M. 2017. A review of the ongoing discussion about definition, diagnosis and pathomechanism of subclinical endometritis in dairy cows. Theriogenology 94:21–30 [DOI] [PubMed] [Google Scholar]
- 76.Bogado Pascottini O, Hostens M, Dini P, Vandepitte J, Ducatelle R, Opsomer G. 2016. Comparison between cytology and histopathology to evaluate subclinical endometritis in dairy cows. Theriogenology 86:1550–56 [DOI] [PubMed] [Google Scholar]
- 77.Serhan CN, Chiang N, Van Dyke TE. 2008. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol 8:349–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kotas ME, Medzhitov R. 2015. Homeostasis, inflammation, and disease susceptibility. Cell 160:816–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kerestes M, Faigl V, Kulcsar M, Balogh O, Foldi J, et al. 2009. Periparturient insulin secretion and whole-body insulin responsiveness in dairy cows showing various forms of ketone pattern with or without puerperal metritis. Domest. Anim. Endocrinol 37:250–61 [DOI] [PubMed] [Google Scholar]
- 80.LeBlanc SJ. 2012. Interactions of metabolism, inflammation, and reproductive tract health in the post-partum period in dairy cattle. Reprod. Domest. Anim 47(Suppl. 5):18–30 [DOI] [PubMed] [Google Scholar]
- 81.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, et al. 2011. Vaginal microbiome of reproductive-age women. PNAS 108(Suppl. 1):4680–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller E, Beasley D, Dunn R, Archie E. 2016. Lactobacilli dominance and vaginal pH: Why is the human vaginal microbiome unique? Front. Microbiol 7:1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deng Q, Odhiambo JF, Farooq U, Lam T, Dunn SM, Ametaj BN. 2014. Intravaginal lactic acid bacteria modulated local and systemic immune responses and lowered the incidence of uterine infections in periparturient dairy cows. PLOS ONE 10:e0124167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davies D, Meade KG, Herath S, Eckersall PD, Gonzalez D, et al. 2008. Toll-like receptor and antimicrobial peptide expression in the bovine endometrium. Reprod. Biol. Endocrinol 6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoelker M, Salilew-Wondim D, Drillich M, Christine GB, Ghanem N, et al. 2012. Transcriptional response of the bovine endometrium and embryo to endometrial polymorphonuclear neutrophil infiltration as an indicator of subclinical inflammation of the uterine environment. Reprod. Fertil. Dev 24:778–93 [DOI] [PubMed] [Google Scholar]
- 86.Chapwanya A, Meade KG, Doherty ML, Callanan JJ, Mee JF, O’Farrelly C. 2009. Histopathological and molecular evaluation of Holstein-Friesian cows postpartum: toward an improved understanding of uterine innate immunity. Theriogenology 71:1396–407 [DOI] [PubMed] [Google Scholar]
- 87.Goldstein JL, Brown MS. 1990. Regulation of the mevalonate pathway. Nature 343:425–30 [DOI] [PubMed] [Google Scholar]
- 88.Lange Y, Swaisgood MH, Ramos BV, Steck TL. 1989. Plasma membranes contain half the phospholipid and 90% of the cholesterol and sphingomyelin in cultured human fibroblasts. J. Biol. Chem 264:3786–93 [PubMed] [Google Scholar]
- 89.Griffin S, Preta G, Sheldon IM. 2017. Inhibiting mevalonate pathway enzymes increases stromal cell resilience to a cholesterol-dependent cytolysin. Sci. Rep 7:17050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Griffin S, Healey GD, Sheldon IM. 2018. Isoprenoids increase bovine endometrial stromal cell tolerance to the cholesterol-dependent cytolysin from Trueperella pyogenes. Biol. Reprod 99:749–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Healey GD, Collier C, Griffin S, Schuberth HJ, Sandra O, et al. 2016. Mevalonate biosynthesis intermediates are key regulators of innate immunity in bovine endometritis. J. Immunol 196:823–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wathes DC, Cheng Z, Chowdhury W, Fenwick MA, Fitzpatrick R, et al. 2009. Negative energy balance alters global gene expression and immune responses in the uterus of postpartum dairy cows. Physiol. Genom 39:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hashizume K, Takahashi T, Shimizu M, Todoroki J, Shimada A, et al. 2003. Matrix-metalloproteinases-2 and −9 production in bovine endometrial cell culture. J. Reprod. Dev 49:45–53 [DOI] [PubMed] [Google Scholar]
- 94.Baxt LA, Garza-Mayers AC, Goldberg MB. 2013. Bacterial subversion of host innate immune pathways. Science 340:697–701 [DOI] [PubMed] [Google Scholar]
- 95.Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140:805–20 [DOI] [PubMed] [Google Scholar]
- 96.Galvao KN, Santos NR, Galvao JS, Gilbert RO. 2011. Association between endometritis and endometrial cytokine expression in postpartum Holstein cows. Theriogenology 76:290–99 [DOI] [PubMed] [Google Scholar]
- 97.Herath S, Lilly ST, Santos NR, Gilbert RO, Goetze L, et al. 2009. Expression of genes associated with immunity in the endometrium of cattle with disparate postpartum uterine disease and fertility. Reprod. Biol. Endocrinol 7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Healy LL, Cronin JG, Sheldon IM. 2014. Endometrial cells sense and react to tissue damage during infection of the bovine endometrium via interleukin 1. Sci. Rep 4:7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim IH, Kang HG, Jeong JK, Hur TY, Jung YH. 2014. Inflammatory cytokine concentrations in uterine flush and serum samples from dairy cows with clinical or subclinical endometritis. Theriogenology 82:427–32 [DOI] [PubMed] [Google Scholar]
- 100.Baumann H, Gauldie J. 1994. The acute phase response. Immunol. Today 15:74–8 [DOI] [PubMed] [Google Scholar]
- 101.Smith BI, Donovan GA, Risco CA, Young CR, Stanker LH. 1998. Serum haptoglobin concentrations in Holstein dairy cattle with toxic puerperal metritis. Vet. Rec 142:83–85 [DOI] [PubMed] [Google Scholar]
- 102.Sheldon IM, Noakes DE, Rycroft A, Dobson H. 2001. Acute phase protein response to postpartum uterine bacterial contamination in cattle. Vet. Rec 148:172–75 [DOI] [PubMed] [Google Scholar]
- 103.Lecchi C, Dilda F, Sartorelli P, Ceciliani F. 2012. Widespread expression of SAA and Hp RNA in bovine tissues after evaluation of suitable reference genes. Vet. Immunol. Immunopathol 145:556–62 [DOI] [PubMed] [Google Scholar]
- 104.Swangchan-Uthai T, Chen Q, Kirton SE, Fenwick MA, Cheng Z, et al. 2013. Influence of energy balance on the antimicrobial peptides S100A8 and S100A9 in the endometrium of the post-partum dairy cow. Reproduction 145:527–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ledgard AM, Smolenski GA, Henderson H, Lee RS. 2015. Influence of pathogenic bacteria species present in the postpartum bovine uterus on proteome profiles. Reprod. Fertil. Dev 27:395–406 [DOI] [PubMed] [Google Scholar]
- 106.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. 1996. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973–83.Discovery of a role for Toll in invertebrates, founding the field of innate immunity.
- 107.Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085–88.Discovery of a role for Tlr4 in recognition of LPS in mice, founding the field of innate immunity in mammals.
- 108.Schroder K, Tschopp J. 2010. The inflammasomes. Cell 140:821–32 [DOI] [PubMed] [Google Scholar]
- 109.Herath S, Fischer DP, Werling D, Williams EJ, Lilly ST, et al. 2006. Expression and function of Toll-like receptor 4 in the endometrial cells of the uterus. Endocrinology 147:562–70.First evidence for the role of innate immunity in bovine endometrial cells.
- 110.Cronin JG, Turner ML, Goetze L, Bryant CE, Sheldon IM. 2012. Toll-Like receptor 4 and MyD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium. Biol. Reprod 86:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Turner ML, Cronin JC, Healey GD, Sheldon IM. 2014. Epithelial and stromal cells of bovine endometrium have roles in innate immunity and initiate inflammatory responses to bacterial lipopeptides in vitro via Toll-like receptors TLR2, TLR1 and TLR6. Endocrinology 155:1453–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.MacKintosh SB, Schuberth HJ, Healy LL, Sheldon IM. 2013. Polarised bovine endometrial epithelial cells vectorially secrete prostaglandins and chemotactic factors under physiological and pathological conditions. Reproduction 145:57–72 [DOI] [PubMed] [Google Scholar]
- 113.Healy LL, Cronin JG, Sheldon IM. 2015. Polarized epithelial cells secrete interleukin 6 apically in the bovine endometrium. Biol. Reprod 92:151. [DOI] [PubMed] [Google Scholar]
- 114.Herath S, Lilly ST, Fischer DP, Williams EJ, Dobson H, et al. 2009. Bacterial lipopolysaccharide induces an endocrine switch from prostaglandin F2a to prostaglandin E2 in bovine endometrium. Endocrinology 150:1912–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Blander JM, Sander LE. 2012. Beyond pattern recognition: five immune checkpoints for scaling the microbial threat. Nat. Rev. Immunol 12:215–25 [DOI] [PubMed] [Google Scholar]
- 116.Cronin JG, Kanamarlapudi V, Thornton CA, Sheldon IM. 2016. Signal transducer and activator of transcription-3 licenses Toll-like receptor 4-dependent interleukin (IL)-6 and IL-8 production via IL-6 receptor-positive feedback in endometrial cells. Mucosal Immunol 9:1125–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen GY, Nuñez G. 2010. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol 10:826–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Matzinger P. 2002. The danger model: a renewed sense of self. Science 296:301–5 [DOI] [PubMed] [Google Scholar]
- 119.McNeela EA, Burke Á, Neill DR, Baxter C, Fernandes VE, et al. 2010. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLOS Pathog 6:e1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. 2006. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126:1135–45 [DOI] [PubMed] [Google Scholar]
- 121.Kvidera SK, Horst EA, Abuajamieh M, Mayorga EJ, Fernandez MV, Baumgard LH. 2017. Glucose requirements of an activated immune system in lactating Holstein cows. J. Dairy Sci 100:2360–74 [DOI] [PubMed] [Google Scholar]
- 122.Dhaliwal GS, Murray RD, Woldehiwet Z. 2001. Some aspects of immunology of the bovine uterus related to treatments for endometritis. Anim. Reprod. Sci 67:135–52 [DOI] [PubMed] [Google Scholar]
- 123.Machado VS, Bicalho MLS, Gilbert RO, Bicalho RC. 2014. Short communication: relationship between natural antibodies and postpartum uterine health in dairy cows. J. Dairy Sci 97:7674–78 [DOI] [PubMed] [Google Scholar]
- 124.Nolte O, Morscher J, Weiss HE, Sonntag H. 2001. Autovaccination of dairy cows to treat post partum metritis caused by Actinomyces pyogenes. Vaccine 19:3146–53 [DOI] [PubMed] [Google Scholar]
- 125.Machado VS, Bicalho ML, Meira Junior EB, Rossi R, Ribeiro BL, et al. 2014. Subcutaneous immunization with inactivated bacterial components and purified protein of Escherichia coli, Fusobacterium necrophorum and Trueperella pyogenes prevents puerperal metritis in Holstein dairy cows. PLOS ONE 9:e91734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Freick M, Kunze A, Passarge O, Weber J, Geidel S. 2017. Metritis vaccination in Holstein dairy heifers using a herd-specific multivalent vaccine: effects on uterine health and fertility in first lactation. Anim. Reprod. Sci 184:160–71 [DOI] [PubMed] [Google Scholar]
- 127.Kamimura S, Ohgi T, Takahashi M, Tsukamoto T. 1993. Postpartum resumption of ovarian activity and uterine involution monitored by ultrasonography in Holstein cows. J. Vet. Med. Sci 55:643–47 [DOI] [PubMed] [Google Scholar]
- 128.Sheldon IM, Dobson H. 2000. Effect of administration of eCG to postpartum cows on folliculogenesis in the ovary ipsilateral to the previously gravid uterine horn and uterine involution. J. Reprod. Fertil 119:157–63 [PubMed] [Google Scholar]
- 129.Williams EJ, Fischer DP, Noakes DE, England GC, Rycroft A, et al. 2007. The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology 68:549–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Green MP, Ledgard AM, Beaumont SE, Berg MC, McNatty KP, et al. 2011. Long-term alteration of follicular steroid concentrations in relation to subclinical endometritis in postpartum dairy cows. J. Anim. Sci 89:3551–60 [DOI] [PubMed] [Google Scholar]
- 131.Hixon JE, Hansel W. 1974. Evidence for preferential transfer of prostaglandin F2alpha to the ovarian artery following intrauterine administration in cattle. Biol. Reprod 11:543–52 [DOI] [PubMed] [Google Scholar]
- 132.Herath S, Williams EJ, Lilly ST, Gilbert RO, Dobson H, et al. 2007. Ovarian follicular cells have innate immune capabilities that modulate their endocrine function. Reproduction 134:683–93.Finding of LPS in ovarian follicular fluid and recognition that bovine granulosa cells have roles in innate immunity.
- 133.Magata F, Horiuchi M, Echizenya R, Miura R, Chiba S, et al. 2014. Lipopolysaccharide in ovarian follicular fluid influences the steroid production in large follicles of dairy cows. Anim. Reprod. Sci 144:6–13 [DOI] [PubMed] [Google Scholar]
- 134.Cheong SH, Sa Filho OG, Absalon-Medina VA, Schneider A, Butler WR, Gilbert RO. 2017. Uterine and systemic inflammation influences ovarian follicular function in postpartum dairy cows. PLOS ONE 12:e0177356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bromfield JJ, Sheldon IM. 2011. Lipopolysaccharide initiates inflammation in bovine granulosa cells via the TLR4 pathway and perturbs oocyte meiotic progression in vitro. Endocrinology 152:5029–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Price JC, Bromfield JJ, Sheldon IM. 2013. Pathogen-associated molecular patterns initiate inflammation and perturb the endocrine function of bovine granulosa cells from ovarian dominant follicles via TLR2 and TLR4 pathways. Endocrinology 154:3377–86 [DOI] [PubMed] [Google Scholar]
- 137.Price JC, Sheldon IM. 2013. Granulosa cells from emerged antral follicles of the bovine ovary initiate inflammation in response to bacterial pathogen-associated molecular patterns via Toll-like receptor pathways. Biol. Reprod 89:119. [DOI] [PubMed] [Google Scholar]
- 138.Alpizar E, Spicer LJ. 1994. Effects of interleukin-6 on proliferation and follicle-stimulating hormone-induced estradiol production by bovine granulosa cells in vitro: dependence on size of follicle. Biol. Reprod 49:38–43 [DOI] [PubMed] [Google Scholar]
- 139.Spicer LJ. 1998. Tumor necrosis factor-α (TNF-α) inhibits steroidogenesis of bovine ovarian granulosa and thecal cells in vitro. Involvement of TNF-α receptors. Endocrine 8:109–15 [DOI] [PubMed] [Google Scholar]
- 140.Shimizu T, Kaji A, Murayama C, Magata F, Shirasuna K, et al. 2012. Effects of interleukin-8 on estradiol and progesterone production by bovine granulosa cells from large follicles and progesterone production by luteinizing granulosa cells in culture. Cytokine 57:175–81 [DOI] [PubMed] [Google Scholar]
- 141.Bromfield JJ, Sheldon IM. 2013. Lipopolysaccharide reduces the primordial follicle pool in the bovine ovarian cortex ex vivo and in the murine ovary in vivo. Biol. Reprod 88:98. [DOI] [PubMed] [Google Scholar]
- 142.Sheldon IM, Price JC, Turner ML, Bromfield JJ, Cronin GJ. 2014. Uterine infection and immunity in cattle. In Reproduction in Domestic Ruminants VIII, ed. JL Juengel A Miyamoto C Price, Reynolds LP, Smith RF, Webb R, pp. 415–30. Ashby-de-la-Zouch, UK: Context Prod. [Google Scholar]
- 143.Britt JH. 1992. Impacts of early postpartum metabolism on follicular development and fertility. Bov. Pract 24:39–43 [Google Scholar]
- 144.Karsch FJ, Battaglia DF, Breen KM, Debus N, Harris TG. 2002. Mechanisms for ovarian cycle disruption by immune/inflammatory stress. Stress 5:101–12 [DOI] [PubMed] [Google Scholar]
- 145.Suzuki C, Yoshioka K, Iwamura S, Hirose H. 2001. Endotoxin induces delayed ovulation following endocrine aberration during the proestrous phase in Holstein heifers. Domest. Anim. Endocrinol 20:267–78 [DOI] [PubMed] [Google Scholar]
- 146.Lavon Y, Leitner G, Goshen T, Braw-Tal R, Jacoby S, Wolfenson D. 2008. Exposure to endotoxin during estrus alters the timing of ovulation and hormonal concentrations in cows. Theriogenology 70:956–67 [DOI] [PubMed] [Google Scholar]
- 147.Lewis GS. 2003. Steroidal regulation of uterine resistance to bacterial infection in livestock. Reprod. Biol. Endocrinol 1:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sheldon IM, Noakes DE, Rycroft AN, Dobson H. 2004. Effect of intrauterine administration of oestradiol on postpartum uterine bacterial infection in cattle. Anim. Reprod. Sci 81:13–23 [DOI] [PubMed] [Google Scholar]
- 149.Saut JP, Healey GD, Borges AM, Sheldon IM. 2014. Ovarian steroids do not impact bovine endometrial cytokine/chemokine responses to E. coli or LPS in vitro. Reproduction 148:593–606 [DOI] [PubMed] [Google Scholar]


