Abstract
Epileptic spasms during infancy (infantile spasms) represent a serious treatment and social problem despite their rare occurrence. Current treatments include hormonal therapy (adrenocorticotropin-ACTH or corticosteroids) or vigabatrin (per se or in the combination). These treatments are partially effective and with potentially significant adverse effects. Thus, the search for new effective drugs is warranted. We tested efficacy of a novel fusion peptide AQB-565 developed by Aequus Biopharma in a model of infantile spasms consisting of prenatal exposure to betamethasone and repeated postnatal trigger of spasms with N-methyl-D-aspartic acid (NMDA). AQB-565 molecule includes the first 24 amino acids of ACTH, a ten amino acid linker and a modified melanocyte-stimulating hormone molecule. In contrast to ACTH with almost uniform activity over all peripheral and central melanocortin receptor isoforms, AQB is preferentially active on central melanocortin receptors MC3 and MC4. Here, we used equivalent doses of rat ACTH (full molecule) and AQB-565 and compared their efficacy in a prospective randomized test against of repeated bouts of spasms on postnatal days (P)12, P13 and P15 in the rat model. All doses of ACTH (range 0.02-1.0 mg/kg sc) and all doses but one of AQB-565 in the same range suppressed spasms in P15 rats (treatment stopped on P14). There was no dose-dependent effect and both compounds had all-or-none effect that is similar to clinical outcome of hormonal treatment of infantile spasms in children. Thus, AQB-565 may represent a novel treatment of infantile spasms similarly effective as ACTH but with potentially limited side effects.
Keywords: ACTH, infantile spasms model, prenatal betamethasone, postnatal NMDA, AQB-565
1. Introduction
West syndrome (WS; often called infantile spasms) is characterized by a triad consisting of epileptic spasms during infancy (=infantile spasms; with usual onset between 3-12 months of age), interictal irregular asynchronous high-voltage EEG waves (i.e., hypsarrhythmia), and progressive mental deterioration (Dulac et al., 2002). WS is a devastating epilepsy syndrome with occurrence approximately 1:3,200-3,400 live births. The spasms can rarely be controlled with classic anti-seizure medications, but more often respond to the treatment with adrenocorticotropic hormone - ACTH* (Dulac et al., 2002; Go et al., 2012), a Food and Drug Administration (FDA; US)-approved treatment for this syndrome and a drug of choice recommended by the American Academy of Neurology and the Practice Committee of the Child Neurology Society (Go et al., 2012; Pellock et al., 2010). Current therapy of WS also includes vigabatrin and corticosteroids (prednisone, prednisolone) (Go et al., 2012; Lux et al., 2004, 2005; Pellock et al., 2010) as well as combination of hormonal treatment with vigabatrin (O'Callaghan et al., 2017). New treatments for this condition are still critically needed because of incomplete efficacy and significant side effects of these commonly used drugs.
We have developed a model of epileptic spasms in infant rats (Velíšek et al., 2007) consisting of prenatal priming with betamethasone and postnatal trigger of developmentally-specific spasms with N-methyl-D-aspartic acid (NMDA) (Mareš and Velíšek, 1992). Prenatal priming with corticosteroids is essential for the spasms to become sensitive to ACTH treatment (Chachua et al., 2011; Velíšek et al., 2010; Velíšek et al., 2007). EEG in the model shows interictal irregular large amplitude wave activity (rat hypsarrhythmia variant) as well as EEG suppression (electrodecrement) during spasms (Chachua et al., 2011). Vigabatrin and prednisolone are also effective in this model (Chachua et al., 2011) indicating that the model may be valuable for testing new putative treatment for infantile spasms.
Aequus Biopharma, Inc., synthesized a novel melanocortin fusion peptide AQB-565 optimized for treatment of inflammatory diseases. This 47 amino acid molecule consists of ACTH1-24, a 10 amino acid linker, and the NDP-MSH molecule (an MSH analogue with asparagine, aspartate and proline as the initial three of the 13 amino acids). In comparison to ACTH1-24 using EC50 , AQB-565 is almost 10 times more potent at centrally located melanocortin isoform 3 (MC3) receptors, almost 20 times more potent at MC4 receptors and 20 times more potent at MC5 receptors. Activity at peripheral MC2 receptors preferentially present in adrenals is similar to ACTH (Berenson et al., 2016).
Central effects of ACTH were suggested based on its efficacy against infantile spasms and seizures even in children who have suppressed peripheral production of corticosteroids (Farwell et al., 1984; Willig and Lagenstein, 1982). Consequently, Dr. Baram and colleagues (Brunson et al., 2001) proposed that central MC4 receptors may mediate the effects of ACTH in infantile spasms therapy.
The purpose of this study was to evaluate the effects of AQB-565 preferentially targeting MC3 and MC4 receptors in the infant rat model of epileptic spasms. As standard treatment, head to head comparison with full rat ACTH1-39 molecule in a prospective randomized trial was tested.
2. Methods
2.1. Animals
Experiments described here are consistent with ARRIVE guidelines, were approved by the IACUC of the New York Medical College and carried out consistent with the NIH Guide for the Care and Use of Laboratory Animals, Eighth Edition (2011). Timed pregnant female Sprague-Dawley rats were obtained from Taconic Farms (Germantown, NY, U.S.A). Animals were kept in the AAALAC-accredited animal facility on a regular light:dark cycle (lights on at 07:00, off at 19:00) with free access to food and water. For priming, pregnant Sprague-Dawley rats were injected with two doses of betamethasone (0.4 mg/kg ip each) on gestational day (G) 15 [at 08:00 and 18:00; (Velíšek et al., 2007)]. Delivery of pups regularly occurred on G23, which was also considered as postnatal day (P) 0 for the offspring. On P1, the pups were identified, sexed and weighed and if necessary, the litter was culled to 10; 5 males and 5 females whenever possible. Each experimental subgroup consisted of pups from 3 to 4 different litters, with no more than 2 pups (a male and a female) of a given litter selected for any given experimental subgroup. Body weight was again determined on P7 and on all days of the experiment.
2.2. Induction of spasms
On P12 morning, NMDA spasms were triggered with 7.5 mg/kg of NMDA injected intraperitonealy (i.p.) in the rat pups prenatally exposed to betamethasone. Rats were observed for 90 minutes and latency to onset of the first spasm (threshold) as well as number of spasms per observation period (severity) were recorded. Once the spasms diminished, rats were randomized into treatment groups and the treatment was initiated. Another bout of NMDA-induced spasms was triggered on P13 morning (12 mg/kg of NMDA i.p.) and finally, the spasms were induced on P15 morning (15 mg/kg of NMDA i.p.). The injection volume was always 10 ml/kg of body weight.
2.3. Treatments
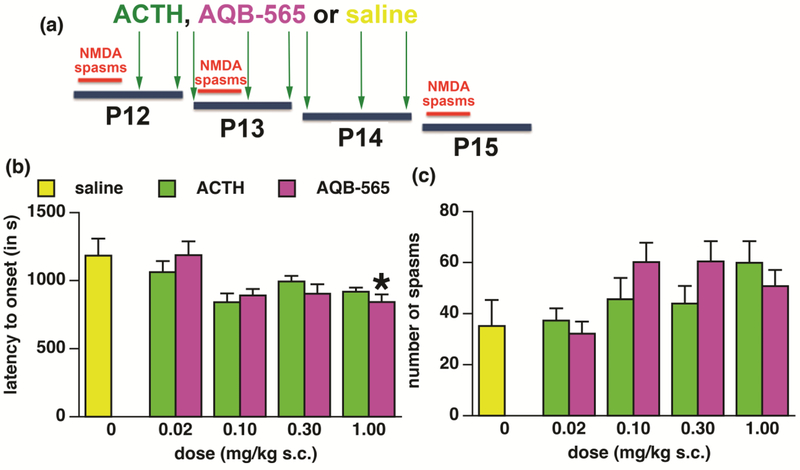
After the spasms on P12, the pups were randomized into the following groups: AQB-565 (provided by Aequus Biopharma, Inc.), ACTH (rat full molecule, 39 amino acids, GenScript RP11305) and the vehicle (normal saline). Rats received AQB-565 or ACTH subcutaneously (sc) in the doses 0.02, 0.10, 0.30, or 1.00 mg/kg of body weight administered always in 1 ml/kg of body weight of vehicle or the same volume of vehicle. The drugs or vehicle were injected according to the following schedule: P12 at 14:00 and 21:00, P13 at 07:00, 14:00 and 21:00 and P14 at 07:00, 14:00 and 21:00. Fig. 1A shows interleaving schedule of induction of spasms and treatments. The purpose of this schedule was to initiate treatment after the spasms similar to human condition, follow randomized rats through treatments prospectively and evaluate long-term effects of treatment on P15 (>12 h after the last treatment administration).
Figure 1-. Experimental design and randomization efficacy on P12.
(a) Design of the experiment. ACTH or AQB-565 were injected sc, twice on P12, thrice on P13 and P14. Doses were 0.02, 0.1, 0.3, and 1.0 mg/kg in saline (volume always 1 ml/kg). Vehicle controls received normal saline (saline) in the volume of 1 ml/kg sc.
(b)Result of the randomization into saline (controls) and ACTH or AQB-565 (treatment) groups prior to the drug administration. Latency to onset of spasms (measured from the NMDA trigger) on P12 in the groups assigned to receive saline, ACTH or AQB-565 later on (mean ± S.E.M.).Numbers of animals: saline=11; ACTH 0.02=12; ACTH 0.1=12; ACTH 0.3 =20; ACTH 1.0=10; AQB-565 0.02=12; AQB-565 0.1=11; AQB-565 0.3=10; AQB-565 1.0=13.
(c) Number of spasms (per observation period) on P12 in the groups designated to receive saline, ACTH or AQB-565 later on (mean ± S.E.M.). There was no significant effect of group designation.
2.4. Statistics
We used parametric one-way ANOVA with the factor of treatment (nine levels: four doses of AQB-565, four doses of ACTH, and vehicle). Post-hoc pairwise comparison was performed using Bonferroni-Dunn test. Level of significance was preset at p<0.05 and was always adjusted for multiple comparisons. Before each analysis, we tested for the effect of sex (using two-way ANOVA with factors of treatment and sex). Males and females were combined for further statistical evaluation if no effect of sex and no interaction between sex and treatment were found.
3. Results
3.1. NMDA syndrome and quality control
At these developmental ages, NMDA injected i.p. elicits a sequence of signs: Initially, twisting tail movements occur beginning at the tip and slowly involving entire tail. Second, there is a non-constant sign of arching during which the animal flexes body and sometimes also the head but still preserves upright position. Finally, animal loses upright position, lies on the side and curls the body, head, and hindlimbs into hyperflexion (emprosthotonus, flexion spasms) lasting several seconds. This is usually followed by a release of the spasms, brief run or crawl and another spasm, etc. At the end of the observation period with diminishing effect of the NMDA trigger, the spasms disappear. There may still be infrequent arching, and the animal often goes through tail twisting again. For quality control we used two approaches. First, we attempted to distribute the rats randomly into control and treatment groups after the first bout of spasms on P12. If complete randomization was not possible (because of relatively small groups and balancing the sex), we randomized against the anticipated effect of treatments. Second, only the rats showing a sign of the NMDA trigger (at least twisting tail movements early after the i.p. NMDA injection) were included into further statistical evaluation to eliminate false positive effects of treatment.
3.2. Randomization efficacy on P12
Statistical evaluation determined that in the latency to onset of spasms (measured from the NMDA trigger point), there was main effect of group assignment on P12 (prior to any treatment was given; ANOVA F(8,102)=3.422; p=0.0016; (1-β)=0.975). Fig. 1B shows that this effect, if any, was against the anticipated drug efficacy. The only group significantly different from controls was the group designated to receive AQB-565 1.0 mg/kg dose, yet again significantly shorter latency to onset equals pro-convulsant effect working against future treatment. When we considered number of spasms in designated groups as a more important measure for the outcome, there was no effect of group assignment (Fig. 1C; ANOVA F(8,103)=2.016; p=0.0517; (1-β)=0.800).
3.3. Effects of treatments on P13
On P13, all rats have already received three injections of treatment (two on the afternoon and evening of P12 plus one on the morning of P13). While ANOVA (F(8,82)=2.581; p=0.0145; (1-β)=0.900) showed significant differences in the latency to onset of spasms, Bonferroni-Dunn post-hoc test did not indicate any differences between any of the treatments and the saline (control) group (p value would have to be smaller than 0.0014). There was a similar outcome in the number of spasms on P13. ANOVA (F(8,81)=2.298; p=0.0284; (1-β)=0.853) showed overall significant differences, but Bonferroni-Dunn test did not contrast any treatment against the controls. If we looked on the changes in the number of spasms in each individual rat using a ratio between the number of spasms on P13 and P12 (P13/P12), ANOVA (F(8,81)=0.706; p=0.6854; (1-β)=0.302) did not even show an overall effect of treatment (not illustrated).
3.4. Effects of treatments on P15
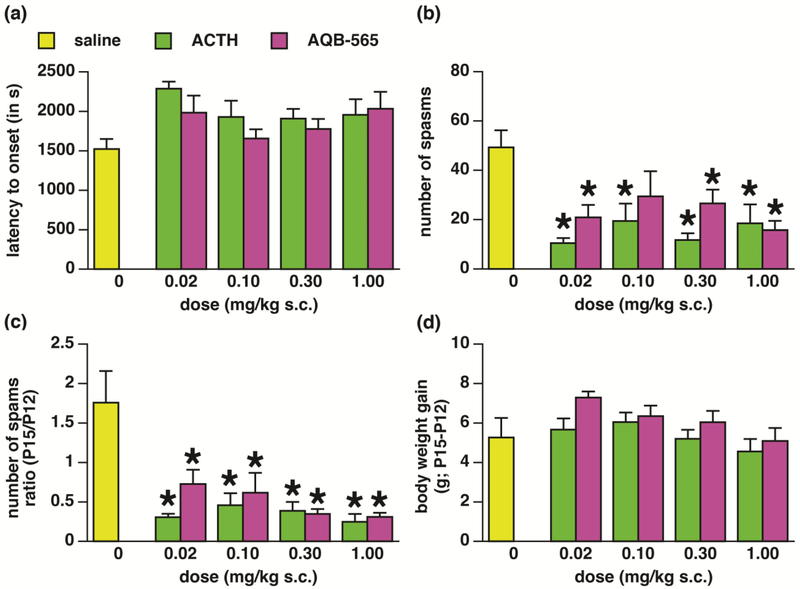
We never found any effects of sex, so male and female data were combined for further analysis. On P15 once the treatment has ceased (on P14), there was no effect of treatments on the latency to onset of spasms (ANOVA F(8,87)=1.998; p=0.0560; (1-β)=0.789; Fig. 2A). However we found significant effect of treatments on the average number of spasms (Fig. 2B; ANOVA F(8,88)=4.918; (1-β)=0.998; p<0.0001). Bonferroni-Dunn post-hoc pairwise analysis with p corrected for number of comparisons (only *p value less than 0.0014 was considered significant) showed that all treatments except for AQB-565 in the dose of 0.1 mg/kg were effective in suppressing number of spasms. However, this analysis was performed on P15 data points and did not take into account changes in numbers of spasms in individual animals between P12 (prior to onset of treatment) and P15. Thus, we also evaluated P15/P12 ratio in the number of spasms. There was a significant main effect of treatment (ANOVA F(8,85)=6.917; p<0.0001; (1-β)=1.000; Fig. 2C). Post-hoc pairwise comparison revealed that all treatments effectively suppressed number of the spasms if the pre-randomization number of spasms on P12 was taken into consideration (again p had to be below 0.0014). The graph indicates that in controls, number of spasms rose about 1.7x between P12 and P15. However, after each treatment and dose fewer spasms were found on P15 compared to P12 (ratio <1.0).
Figure 2-. Effects of treatment on spasms on P15.
(a) Latency to onset of spasms (measured from the NMDA trigger) on P15 in the groups receiving saline, ACTH or AQB-565 on P12, P13, and P15 (mean ± S.E.M.). There was no effect of the group assignment on this parameter. Numbers of animals: saline=13; ACTH 0.02=11; ACTH 0.1=9; ACTH 0.3 =18; ACTH 1.0=6; AQB-565 0.02=9; AQB-565 0.1=8; AQB-565 0.3=10; AQB-565 1.0=12.
(b) Number of spasms (per observation period) on P15 in the groups receiving saline, ACTH or AQB-565 on P12, P13, and P15 (mean ± S.E.M.). There was a significant effect of group assignment. All groups except for AQB-565 0.10 mg/kg sc significantly suppressed number of spasms in comparison to controls (saline-injected).
(c) Ratio of the number of spasms on P15 over P12 calculated for each individual rat (mean ± S.E.M.). There was a significant effect of all treatments used (suppression of the ratio compared to saline controls). Numbers of animals: saline=10; ACTH 0.02=11; ACTH 0.1=9; ACTH 0.3 =18; ACTH 1.0=7; AQB-565 0.02=10; AQB-565 0.1=8; AQB-565 0.3=9; AQB-565 1.0=12.
(d) Body weight gain between P12 (before treatment) and P15 (completion of treatments; mean ± S.E.M.). Neither drug in any dose used affected body weight gain. Numbers of animals: saline=12; ACTH 0.02=12; ACTH 0.1=12; ACTH 0.3 =20; ACTH 1.0=11; AQB-565 0.02=10; AQB-565 0.1=10; AQB-565 0.3=9; AQB-565 1.0=13.
3.5. Body weight gain between P12 and P15
Finally, we followed gain in body weight as a basic indicator of adverse effects. Statistical analysis did not show any main effect of treatments (ANOVA F(8,100)=1.627; p=0.1265; (1-β)=0.686; Fig. 2D) on body weight gain.
4. Discussion
Our current study shows that the new fusion peptide AQB-565 consisting of a combination of ACTH(1-24) and NDP-MSH effectively suppresses spasms in the rat model of epileptic spasms in infancy. The efficacy approximates that of rat ACTH (full molecule of 39 amino acids). None of the treatments (ACTH or AQB-565) in the dose range used here 0.02-1.0 mg/kg affected the food intake over four days of experiment, though a longer study would be required to determine this effect.
While new therapies and treatment approaches for epileptic spasms during infancy (WS) are needed, there is a very limited choice of effective drugs. Preclinical studies investigated variety of potential novel compounds such as GABAB receptor antagonist CGP35348, caspase 1 inhibitor VX-765, or neonatal estradiol. All these compounds were ineffective in a model of multiple-hit symptomatic infantile spasms (Galanopoulou et al., 2017). Estradiol was also ineffective in our model (Chachua et al., 2016) despite its prominent efficacy in a genetic model of infantile spasms based on Arx gene triplet repeat expansion (Olivetti et al., 2014). This indicates that individual subtypes of WS may have very specific sensitivity to treatments. Clinical studies also show that not all etiological varieties of WS would respond to identical or similar treatments [reviewed in (Hussain, 2018)]. There are three notions associated with this conclusion: First, current crude subdivision of WS to cryptogenic and symptomatic conditions is also suggesting differential treatment approach, hormones (ACTH) for the former and vigabatrin for the latter. Second, it can be speculated that genetic varieties of infantile spasms will require variety-specific treatment. Finally, better understanding of the underlying mechanisms for the distinct subtype of epileptic spasms etiologies would allow for more personalized treatments.
Despite our best efforts to create dose responses with both investigated drugs, the response was all-or none. This is the type of response typical for physiological activity of hormones and similar to the effects of ACTH seen in children with infantile spasms (Hrachovy et al., 1980; Snead et al., 1989).
The current study combined with previous preclinical data indicate that hormonal treatments, besides the vigabatrin, still prevail in efficacy in preclinical models of spasms during infancy (Briggs et al., 2014). Carisbamate (developed by Johnson & Johnson Pharmaceutical Research) looked promising (Ono et al., 2011) but it was never marketed. Multiple etiologies, genetic variety, and our insufficient understanding of the underlying mechanisms are likely the culprit in lack of development of novel non-hormonal treatments for infantile spasms.
Highlights:
Efficacy of a novel peptide AQB-565 was compared to ACTH in a rodent model of infantile spasms
Both AQB-565 and ACTH suppressed spasms in an all-or-none fashion with similar efficacy
Hormonal or peptide treatments still have superior efficacy in infantile spasms
Acknowledgments
Funding: This work was supported by Aequus Biopharma, Inc. JV was partially supported by the NIH award NS- 092786. The sponsors had no influence on the design of the experiments, collection, analysis or interpretation of the data.
Footnotes
Declaration of Interest: None, except for the funding source.
ACTH: adrenocorticotropic hormone, adrenocorticotropin, corticotropin; ANOVA: analysis of variance; EEG: electroencephalogram; G: gestational day; i.p.: intraperitoneal(ly); MC: melanocortin; MSH-melanocyte stimulating hormone; NMDA: N-methyl-D-aspartic acid; P: postnatal day; s.c.: subcutaneous(ly)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Berenson R, Matthews MA, Wallis W, Dua R, Moore M, Terkeltaub R, Clegg CA, 2016. Melanocortin Fusion Peptide (AQB-565) Optimized for Melanocortin Receptor Engagement Significantly Reduces Inflammation in an In Vivo model of Acute Gout [abstract]. Arthritis Rheumatol. 68 (Suppl 10), 2266. [Google Scholar]
- Briggs SW, Mowrey W, Hall CB, Galanopoulou AS, 2014. CPP-115, a vigabatrin analogue, decreases spasms in the multiple-hit rat model of infantile spasms. Epilepsia 55, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Khan N, Eghbal-Ahmadi M, Baram TZ, 2001. Corticotropin (ACTH) acts directly on amygdala neurons to down-regulate corticotropin-releasing hormone gene expression. Ann Neurol 49, 304–312. [PMC free article] [PubMed] [Google Scholar]
- Chachua T, Di Grazia P, Chern CR, Johnkutty M, Hellman B, Lau HA, Shakil F, Daniel M, Goletiani C, Velíšková J, Velíšek L, 2016. Estradiol does not affect spasms in the betamethasone-NMDA rat model of infantile spasms. Epilepsia 57, 1326–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachua T, Yum M-S, Velíšková J, Velíšek L, 2011. Validation of the rat model of cryptogenic infantile spasms. Epilepsia 52, 1666–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac O, Soufflet C, Chiron C, Kaminska A, 2002. What is West syndrome? Int Rev Neurobiol 49, 1–22. [DOI] [PubMed] [Google Scholar]
- Farwell J, Milstein J, Opheim K, Smith E, Glass S, 1984. Adrenocorticotropic hormone controls infantile spasms independently of cortisol stimulation. Epilepsia 25, 605–608. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, Mowrey WB, Liu W, Li Q, Shandra O, Moshe SL, 2017. Preclinical Screening for Treatments for Infantile Spasms in the Multiple Hit Rat Model of Infantile Spasms: An Update. Neurochem Res 42, 1949–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go CY, Mackay MT, Weiss SK, Stephens D, Adams-Webber T, Ashwal S, Snead OC 3rd, Child Neurology, S., American Academy of, N., 2012. Evidence-based guideline update: medical treatment of infantile spasms. Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 78, 1974–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrachovy RA, Frost JD Jr., Kellaway P, Zion T, Nau H, 1980. A controlled study of ACTH therapy in infantile spasms. Epilepsia 21, 631–636. [DOI] [PubMed] [Google Scholar]
- Hussain SA, 2018. Treatment of infantile spasms. Epilepsia Open 3, 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, O'Callaghan FJ, Verity CM, Osborne JP, 2004. The United Kingdom Infantile Spasms Study comparing vigabatrin with prednisolone or tetracosactide at 14 days: a multicentre, randomised controlled trial. Lancet 364, 1773–1778. [DOI] [PubMed] [Google Scholar]
- Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, O'Callaghan FJ, Verity CM, Osborne JP, 2005. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet Neurol 4, 712–717. [DOI] [PubMed] [Google Scholar]
- Mareš P, Velíšek L, 1992. N-methyl-D-aspartate (NMDA)-induced seizures in developing rats. Brain Res Dev Brain Res 65, 185–189. [DOI] [PubMed] [Google Scholar]
- O'Callaghan FJ, Edwards SW, Alber FD, Hancock E, Johnson AL, Kennedy CR, Likeman M, Lux AL, Mackay M, Mallick AA, Newton RW, Nolan M, Pressler R, Rating D, Schmitt B, Verity CM, Osborne JP, participating, i., 2017. Safety and effectiveness of hormonal treatment versus hormonal treatment with vigabatrin for infantile spasms (ICISS): a randomised, multicentre, open-label trial. Lancet Neurol 16, 33–42. [DOI] [PubMed] [Google Scholar]
- Olivetti PR, Maheshwari A, Noebels JL, 2014. Neonatal estradiol stimulation prevents epilepsy in Arx model of X-linked infantile spasms syndrome. Sci Transl Med 6, 220ra212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, Moshe SL, Galanopoulou AS, 2011. Carisbamate acutely suppresses spasms in a rat model of symptomatic infantile spasms. Epilepsia 52, 1678–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellock JM, Hrachovy R, Shinnar S, Baram TZ, Bettis D, Dlugos DJ, Gaillard WD, Gibson PA, Holmes GL, Nordl DR, O'Dell C, Shields WD, Trevathan E, Wheless JW, 2010. Infantile spasms: a U.S. consensus report. Epilepsia 51, 2175–2189. [DOI] [PubMed] [Google Scholar]
- Snead OC, Benton JW Jr., Hosey LC, Swann JW, Spink D, Martin D, Rej R, 1989. Treatment of infantile spasms with high-dose ACTH: efficacy and plasma levels of ACTH and cortisol. Neurology 39, 1027–1031. [DOI] [PubMed] [Google Scholar]
- Velíšek L, Chachua T, Yum MS, Poon KL, Velíšková J, 2010. Model of cryptogenic infantile spasms after prenatal corticosteroid priming. Epilepsia 51 Suppl 3, 145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velíšek L, Jehle K, Asche S, Velíšková J, 2007. Model of infantile spasms induced by N-methyl-D-aspartic acid in prenatally impaired brain. Ann Neurol 61, 109–119. [DOI] [PubMed] [Google Scholar]
- Willig RP, Lagenstein I, 1982. Use of ACTH fragments of children with infantile spasms. Neuropediatrics 13, 55–58. [DOI] [PubMed] [Google Scholar]