Abstract
Objective
To examine the association between muscular strength and incident type 2 diabetes, independent of cardiorespiratory fitness (CRF).
Patients and Methods
A total of 4,681 adults aged 20-100 years who had no type 2 diabetes at baseline were included in the current prospective cohort study. Participants received muscular strength tests and maximal treadmill exercise tests between January 1, 1981 and December 31, 2006. Muscular strength was measured by leg and bench press and categorized as age group- and sex-specific thirds (lower, middle, and upper) of the combined strength score. Type 2 diabetes was defined based on fasting plasma glucose levels, insulin therapy, or physician-diagnoses.
Results
During an average follow-up of 8.3 years, 229 (4.9%) developed type 2 diabetes. Participants with the middle level muscular strength had 32% lower risk of developing type 2 diabetes (HR 0.68 [95% CI 0.49-0.94]) compared with the lower level of muscular strength after adjusting for potential confounders, including estimated CRF. However, no significant association between the upper level of muscular strength and incident type 2 diabetes was observed.
Conclusion
A moderate level of muscular strength is associated with a lower risk of developing type 2 diabetes, independent of estimated CRF. More studies on the dose-response relationship between muscular strength and type 2 diabetes are needed.
Type 2 diabetes (T2D) is projected by the World Health Organization to be the seventh leading cause of death by 2030.1 There are observational and experimental findings indicating that regular physical activity (PA) is associated with reduced risk of T2D.2,3,4 However, the majority of PA literature focuses primarily on aerobic exercise. Likewise, cardiorespiratory fitness (CRF), which is an objective marker for recent PA,5 is inversely associated with the incidence of T2D.6,7,8 Much less is known about other types of PA and fitness on the risk of T2D.
The role of muscular strength in preventing and managing the development of chronic diseases has been increasingly recognized.9 Muscular strength has been associated with lower risks of cardiovascular disease risk factors and cardiovascular disease morbidity and mortality.10 Although genetic factors influence muscular strength, the primary determinant of muscular strength is resistance exercise (RE), which is recommended in addition to aerobic exercise in order to reduce disease incidence and attenuate disease progression.11 Several prospective studies have shown that RE is associated with a lower risk of T2D in both men and women.12,13,14 However, these previous studies were based on self-reported evaluation of RE, where recall bias and social desirability often exist, potentially leading to an underestimation of the true effect of RE on health outcomes.14,15 Therefore, objectively measuring muscular strength may provide better data to assess the effect of RE on T2D prevention.
Several studies have demonstrated that muscular strength is inversely associated with incidence of metabolic syndrome16 and obesity17, which are diagnoses that often precede T2D. However, the association between muscular strength and incident T2D has yet to be examined, particularly after controlling for CRF. Therefore, the objective of this study was to examine the independent association of muscular strength and T2D incidence. We hypothesized that after controlling for potential confounders, muscular strength would have a protective effect on developing T2D.
PATIENTS AND METHODS
Study Population
Participants for this study were recruited via the Aerobics Center Longitudinal Study (ACLS), which is a prospective study on the association of clinical and lifestyle factors with health outcomes in men and women. A total of 4,832 participants aged 20-100 years received muscular strength tests and maximal treadmill exercise tests as part of medical examinations at the Cooper Clinic in Dallas, Texas, between 1981-2006. Participants who reported a history of myocardial infarction, stroke, or cancer (n=77) were excluded. Participants who reported physician-diagnosed diabetes, were diagnosed with diabetes according to a fasting plasma glucose level ≥7.0 mmol/l (126 mg/dl), or had current insulin therapy (n=74) at baseline were excluded, resulting in 4,681 men and women in the current study. Participants were predominantly non-Hispanic whites, well-educated, and belonged to middle and upper socioeconomic strata.18 The Cooper Institute Institutional Review Board approved the study protocol annually, and all participants gave their written informed consent before data collection at baseline and during follow-up examinations. Further details of the ACLS design, sampling procedures, and data collection have been previously described.18,19
Clinical Examination
Participants underwent comprehensive medical examinations at baseline, which included body weight and height assessments, blood chemistry analyses, electrocardiography, physical examination, and a detailed medical history questionnaire. In addition, participants completed a clinical evaluation that included a muscular strength test and a maximal treadmill exercise test. Body mass index (BMI) was computed as weight (kilograms) divided by height (meters) squared. Participants were asked about their smoking status (current smoker, or non-smoker); alcohol consumption (heavy alcohol consumption, defined as average intake of >7 drinks per week for women, and >14 drinks per week for men, or non-heavy alcohol consumption); personal history of hypertension (defined by blood pressure ≥140/90 mmHg or physician diagnosed hypertension), and hypercholesterolemia (defined by total cholesterol ≥240 mg/dl or physician diagnosed hypercholesterolemia); parental history of diabetes (yes or no); meeting the aerobic PA guidelines (active, defined as reporting ≥500 MET-min/week aerobic PA during leisure time in the three months before the examination, or inactive) by a medical history questionnaire.
Muscular strength was assessed in both the upper and lower body by resistance weight machines (Universal Equipment, Cedar Rapids, IA) using a standardized strength testing protocol. Upper body strength was assessed with a 1-repetition maximum (1-RM) supine bench press, and lower body strength was assessed with a 1-RM seated leg press. Initial loads were set as 70% of body weight for bench press and 100% for leg press. Participants completed a series of lifts with incremental increases in weight lifted (2.27-4.45 kg) with short rest periods between each lift until the maximum effort was achieved. The maximal effort was usually achieved after five or fewer lifts. Participants were able to lift the initial load at least 1 time. The intraclass correlations in 1-RM bench press and leg press were tested as R=0.90, and R=0.83, respectively, in a subgroup of 246 men who underwent two muscular strength assessments within one year.16 Therefore, the muscular strength variable used in the current study was regarded as reliable.
A muscular strength score, which was expressed as weight lifted per kilogram body weight, was computed by combining the 1-RM values for the bench press and leg press, and dividing the scores into five different age groups: 20-29, 30-39, 40-49, 50-59, and ≥60, based on sex-specific distribution. In each age group, the scores were standardized using the formula: standardized score=(value - mean)/standard deviation (SD). Since there is no consensus for the categorization of muscular strength, we followed our previous study methods.20,21 Therefore, thirds of the age- and sex-specific muscular strength scores were used in the analysis.
Estimated CRF was assessed by using a modified Balke protocol22 and was quantified as the total duration of a treadmill test, which is highly correlated with directly measured maximal oxygen uptake (r=0.92).23 The maximum metabolic equivalents (1 metabolic equivalent (MET)=3.5 ml oxygen uptake/kg/min) was estimated from the final treadmill speed and grade following ACSM equation,24 consistent with our early studies.5,7 Participants were encouraged to reach their maximum effort, and the test was terminated when the participant reached volitional exhaustion or the doctor stopped the test for medical reasons. Estimated CRF was dichotomized as low fitness or high fitness corresponding to the lower and the upper 50%, respectively, of the age- and sex-specific distribution of treadmill exercise duration from this particular population, following our previous study methods.21 Details of the clinical examination and measures of muscular strength and estimated CRF have been previously described.16,19,25
Ascertainment of T2D
T2D was diagnosed at a follow-up examination according to the American Diabetes Association criteria, which defines T2D as a fasting plasma glucose concentration ≥7.0 mmol/l (126 mg/dl),26 insulin therapy, or physician-diagnosed T2D. Follow-up time was calculated from the baseline examination to the first event of T2D or the last follow-up observation through 2006 for participants who did not develop T2D.
Statistical Analysis
Data were summarized using descriptive statistics. Continuous variables were presented as mean and SD, while categorical variables were summarized by numbers and percentages. Differences in baseline characteristics for participants across three levels of muscular strength were assessed using general linear model for continuous variables and chi-square test for categorical variables. Cox proportional hazard regression was used to compute hazard ratios (HRs) and 95% CIs of T2D, according to exposure categories. Multivariable analyses were adjusted for age (years) and sex in Model 1; for age (years), sex, BMI (kg/m2), current smoking, heavy alcohol drinking, parental history of diabetes, personal history of hypertension, personal history of hypercholesterolemia, abnormal electrocardiogram, impaired fasting glucose, and meeting the aerobic PA guidelines in Model 2. Model 3 additionally adjusted for estimated CRF (METs) as a continuous variable.
A joint association of muscular strength (lower, middle, and upper strength) and estimated CRF (low and high fitness) on T2D incidence was conducted. The effects of each combination of muscular strength and estimated CRF status were compared with the reference group (lower strength combined with low estimated CRF). The proportional hazards assumption was tested by examining the log-log survival plots.
To determine whether the association between muscular strength and incident T2D varied by age (age<45 or age≥45)27, BMI (BMI<25 or BMI≥25), current smoking, heavy alcohol drinking, personal history of hypertension, hypercholesterolemia, abnormal electrocardiogram, impaired fasting glucose, and meeting the aerobic PA guidelines, the interactions of these dichotomized baseline characteristics were tested in the Cox regression.
We examined potential effect modification by sex on the association between muscular strength and T2D incidence using interaction terms in the Cox regression and by comparing risk estimates in sex-stratified analyses. No significant interaction was found between muscular strength and T2D incidence by sex (P=.41), and trends of developing T2D in men and women were similar. Therefore, the results were presented in pooled analyses. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Two-sided tests were used, and a P value<.05 was considered statistically significant.
RESULTS
Among the 4,681 participants, 229 (4.9%) developed T2D during an average follow-up of 8.3±6.3 years (minimum, 1 year; maximum, 25 years). Participants’ baseline characteristics by thirds of muscular strength are summarized in Table 1. The number of participants who met the aerobic PA guidelines and estimated CRF values were higher in participants with higher muscular strength (both P<.001), whereas BMI, the number of participants who had history of hypertension, reported impaired fasting glucose, and had heavy alcohol drinking were lower in participants with higher muscular strength (all P<.05).
TABLE 1.
Baseline Characteristics of Participants in Aerobics Centre Longitudinal Study, 1981-2006, by Thirds of Muscular Strength
| Characteristics | Muscular strength (thirds) |
P value for linear trend |
|||
|---|---|---|---|---|---|
| All (n=4681) |
Lower (n=1560) |
Middle (n=1559) |
Upper (n=1562) |
||
| Age (years) | 43.3 (9.5) | 43.7 (9.5) | 43.4 (9.4) | 42.9 (9.7) | .07 |
| Sex (male) | 4139 (88.4) | 1378 (88.3) | 1380 (88.5) | 1381 (88.4) | .99 |
| BMI (kg/m2) | 25.1 (3.3) | 25.8 (3.8) | 25.0 (3.2) | 24.5 (2.8) | <.001 |
| Current smokers status | 554 (11.8) | 204 (13.1) | 189 (12.1) | 161 (10.3) | .05 |
| Heavy alcohol drinking | 1131 (24.2) | 395 (25.3) | 399 (25.6) | 337 (21.6) | .01 |
| History of hypertension | 1024 (21.9) | 396 (25.4) | 318 (20.4) | 310 (19.9) | <.001 |
| History of hypercholesterolemia | 1071 (22.9) | 384 (24.6) | 359 (23.0) | 328 (21.0) | .05 |
| Abnormal ECG | 217 (4.6) | 70 (4.5) | 81 (5.2) | 66 (4.2) | .41 |
| Parental history of diabetes | 70 (1.5) | 23 (1.5) | 20 (1.3) | 27 (1.7) | .59 |
| Impaired fasting glucose | 1927 (41.2) | 681 (43.7) | 639 (41.0) | 607 (38.9) | .02 |
| Meeting the aerobic PA guidelines | 2112 (45.1) | 632 (40.5) | 680 (43.6) | 800 (51.2) | <.001 |
| Cardiorespiratory fitness (METs) | 12.5 (2.5) | 11.8 (2.4) | 12.4 (2.4) | 13.4 (2.5) | <.001 |
BMI = body mass index; ECG = electrocardiogram; PA = physical activity; METs = metabolic equivalent tasks
Data are presented in mean (SD) unless indicated as number (%).
Heavy alcohol drinking (defined as average intake of >7 drinks per week for women, and >14 drinks per week for men); personal history of hypertension (defined by blood pressure≥140/90 mmHg, or physician diagnosed hypertension), and hypercholesterolemia (defined by total cholesterol ≥240 mg/dl, or physician diagnosed hypercholesterolemia); parental history of diabetes (yes or no); meeting the aerobic PA guidelines (≥500 MET-min/week aerobic PA during leisure time in the three months before the examination).
Table 2 shows adjusted hazard ratios of incident T2D by the thirds of muscular strength. Participants with the middle level muscular strength had 39% (HR 0.61 [95% CI 0.44-0.85]) reduced risk of T2D in Model 1 after adjusting for age and sex. However, there was no significant association between incident T2D and the upper level of muscular strength compared with the lower third of muscular strength (HR 0.77 [95% CI 0.56-1.04]). Similar results were observed in both Models 2 and 3 after further adjustment for potential confounders and estimated CRF.
TABLE 2.
Hazard Ratio of Incident Type 2 Diabetes by Thirds of Muscular Strength
| Muscular strength (thirds) |
N | No. of cases |
Adjusted hazard ratio (95% CI) |
||
|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | |||
| Lower | 1560 | 88 | 1.00 [reference] |
1.00 [reference] |
1.00 [reference] |
| Middle | 1559 | 63 | 0.61 (0.44-0.85) |
0.67 (0.48-0.92) |
0.68 (0.49-0.94) |
| Upper | 1562 | 78 | 0.77 (0.56-1.04) |
0.96 (0.70-1.32) |
1.07 (0.78-1.48) |
Adjusted for age (years) and sex.
Adjusted for model 1 plus BMI (kg/m2), current smoking status, heavy alcohol drinking (average intake of >7 drinks per week for women, and >14 drinks per week for men), parental history of diabetes, personal history of hypertension (blood pressure≥140/90 mmHg, or physician diagnosed hypertension), personal history of hypercholesterolemia (total cholesterol ≥240 mg/dl, or physician diagnosed hypercholesterolemia), abnormal electrocardiogram, impaired fasting glucose, and meeting the aerobic PA guidelines (≥500 MET-min/week aerobic PA during leisure time in the three months before the examination).
Adjusted for model 2 plus cardiorespiratory fitness (METs).
The joint association of muscular strength and estimated CRF with incident T2D was assessed. Participants with the middle third of muscular strength had 37% (HR 0.63 [95% CI 0.42-0.95]) and 46% (HR 0.54 [95% CI 0.33-0.89]) reduced risk of T2D in both the low and high estimated CRF groups, respectively, compared with low estimated CRF and lower strength group. However, no significant results were found in participants with the upper third of muscular strength in both low (HR 1.20 [95% CI 0.79-1.82]) and high (HR 0.67 [95% CI 0.43-1.03]) estimated CRF.
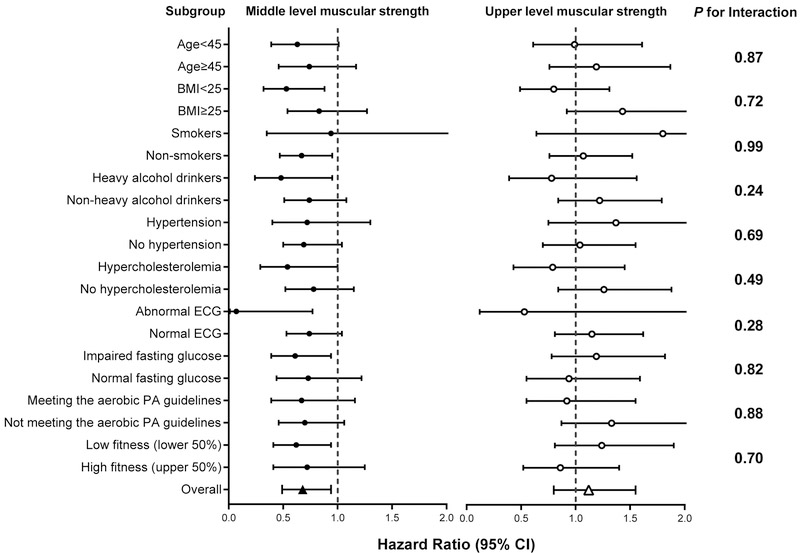
Joint analyses examining the interactions between muscular strength and other baseline characteristics with incident T2D were conducted (Figure 1). None of these interactions were significant (P-values were from.24 to .99), suggesting that the association between muscular strength and incident T2D was not different by lifestyle factors (smoking, drinking, meeting the aerobic PA guidelines), or health conditions (overweight, hypertension, hypercholesterolemia, abnormal electrocardiogram (ECG), and fasting glucose conditions).
FIGURE 1.
Hazard ratio of incident type 2 diabetes by muscular strength in subgroups. The reference group for all subgroup analyses was the lower third muscular strength group. All hazard ratios were adjusted for age (not in age-stratified analyses), sex, BMI (not in BMI-stratified analyses), current smoking status (not in smoking-stratified analyses), heavy alcohol drinking (not in drinking-stratified analyses), parental history of diabetes, personal history of hypertension (not in hypertension-stratified analyses), personal history of hypercholesterolemia (not in hypercholesterolemia-stratified analyses), abnormal ECG (not in ECG-stratified analyses), impaired fasting glucose (not in fasting glucose-stratified analyses), meeting the aerobic PA guidelines (not in meeting the aerobic PA guidelines-stratified analyses), and estimated CRF (not in fitness-stratified analyses). The P-values for the interactions between the levels of muscular strength (lower, middle, and upper) and dichotomized baseline characteristics are depicted on the right. No subgroup analyses were conducted for parental history of diabetes due to the small sample size (n=70; 1.5% of sample) and limited number of type 2 diabetes cases in this group (n=6). BMI = body mass index; ECG = electrocardiogram; PA = physical activity.
DISCUSSION
Moderate level of muscular strength was associated with a significantly lower risk of developing T2D compared with lower muscular strength; however, upper muscular strength was not significantly associated with the risk of T2D after controlling for potential confounders, including estimated CRF (Table 2).
In our sample, muscular strength and estimated CRF were moderately correlated (age and sex adjusted: R=0.27, P<.001), which suggests that the protective mechanism between moderate muscular strength and T2D is somewhat independent of the protective mechanism between CRF and T2D. As CRF is inversely associated with the incidence of T2D,6,7,8 these results highlight the importance of achieving moderate muscular strength and higher CRF to reduce the risk of developing T2D, although the additive benefits of having both moderate or higher muscular strength and higher CRF should be further investigated.
Previous studies with the ACLS dataset have shown that muscular strength is inversely associated with the development of metabolic syndrome,16 obesity,17 and all-cause and cancer mortality20 independent of CRF. Our results showing the benefits of moderate muscular strength on T2D prevention align with the findings from the other ACLS studies; however, the protective effect on T2D in the upper strength group was not statistically significant.
The potential benefit in the upper strength group was noticeably diminished in the full model (Model 3) after adjusting for all potential confounders including estimated CRF (Table 2). In fact, a secondary analysis indicated that the upper strength group had 49% higher risk of T2D compared with the moderate strength group (HR 1.49 [95% CI 1.05-2.10]) in the full model after excluding the lower strength group. While these findings are not clear at this time, one possible explanation is that the health benefits in the upper strength group are considerably confounded by other baseline factors, such as estimated CRF, BMI, and meeting the aerobic PA guidelines (Table 1). Furthermore, approximately two-thirds of individuals in the upper strength group had high estimated CRF; however, one-third of individuals in the lower strength group had high estimated CRF. Therefore, randomized controlled trials (RCTs) designed to mitigate the effects of these confounding factors are needed to clarify the relationships and underlying mechanisms between muscular strength and T2D.
Since muscular strength is partially determined by RE,28 the protective effect of moderate muscular strength against T2D may be due to the benefits of RE. In fact, RE helps maintain or increase lean body mass,29 which improves glycemic control by augmenting skeletal muscle storage of glucose.30 Other effects reported for RE may include reducing visceral adiposity,28 which has been associated with insulin resistance.31
Several studies indicated that RE was associated with a reduced risk of T2D.12,13,14 However, in some of these results, no additional benefits on reducing the risk of T2D were found with higher levels of RE,12,14 which aligns with our findings of no reduced risk of T2D in the upper strength group. Information on RE is not available for most participants in the current study; however, in a subgroup of 656 (14%) participants, muscular strength and RE frequency (days per week) were moderately correlated (age and sex adjusted: r=0.25, P<.001). Thus, while greater frequency of RE training likely contributed to greater muscular strength in this sample, this moderate correlation also indicates that there are other factors, such as a genetic predisposition, beyond RE that determine muscular strength and could influence T2D risk.
Emerging data suggest that greater amounts of aerobic exercise may not yield additional health benefits.32,33 Similar to aerobic exercise, long-term excessive RE may cause pathological structural changes. One meta-analysis found that high-intensity RE training was associated with an increase of ~11% in arterial stiffness, but this association only existed in young participants (<40 years).34 However, our participants (average age: 43.4 years, range: 20-100 years old) were not as young as the study sample in the meta-analysis.
There are limitations of the current study. Participants in our study were predominantly non-Hispanic whites, well-educated, and belonged to middle and upper socioeconomic strata, which may limit the generalizability of the results. Conversely, homogeneity in ethnicity and socioeconomic strata reduces potential confounding by different races/ethnicities, education, and income levels. Differentiation between type 2 and type 1 (insulin-dependent) diabetes was not available, because there was no information about the type of diabetes in the current study. However, a previous ACLS study showed only 2.7% of men diagnosed with diabetes also reported insulin use.5 All 2.7% men were diagnosed with diabetes after age 30, indicating that there are likely few men with type 1 diabetes (known as juvenile diabetes) in the ACLS study.5 Due to the small sample size and limited number of T2D cases in women, a separate analysis in women was not conducted. Additionally, no diet information is a limitation of the current study, since diet is another important factor related to T2D. Since most participants in this study did not have multiple clinic visits, data analyses considering weight gain and physical activity change, which are related to incident diabetes, were not available. Future studies with more completed follow-up sample investigating the association between muscular strength and incident T2D are needed.
Strengths of the current study include the availability of an objective and standardized assessment of total muscular strength from both upper and lower body and estimated CRF within a large cohort of men and women with comprehensive clinic follow-up. These objective assessments decrease the measurement errors in most self-reported exercise studies. Due to the extensive and comprehensive baseline physical examination in the clinic, undetected T2D cases are less likely to exist in the current study.
CONCLUSION
We found that moderate muscular strength, but not upper muscular strength, was associated with a reduced risk of developing T2D independent of estimated CRF. These results indicate that very high levels RE training may not be necessary to obtain the significant health benefits on T2D prevention. More observational studies with larger sample size and randomized controlled trials on the dose-response relationship of RE and muscular strength with T2D and related chronic diseases (e.g., metabolic syndrome, cardiovascular diseases) among diverse populations are clearly warranted.
Acknowledgments.
The authors thank the Cooper Clinic physicians and technicians for collecting the baseline data and staff at the Cooper Institute for data entry and data management.
Grant Support: This study was supported by the National Institutes of Health grants (AG06945, HL62508, DK088195, and HL133069). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Steven Blair has received unrestricted research grants from The Coca-Cola Company, but these grants were not used to support this manuscript.
Abbreviations and Acronyms:
- T2D
type 2 diabetes
- PA
physical activity
- CRF
cardiorespiratory fitness
- RE
resistance exercise
- ACLS
Aerobics Center Longitudinal Study
- BMI
body mass index
- 1-RM
1-repetition maximum
- MET
metabolic equivalent task
- HR
hazard ratio
Footnotes
Potential Competing Interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yuehan Wang, Department of Kinesiology, Iowa State University, Ames, IA; Department of Physiology, Radboud University Medical Center, Nijmegen, The Netherlands.
Duck-chul Lee, Department of Kinesiology, Iowa State University, Ames, IA.
Angelique G. Brellenthin, Department of Kinesiology, Iowa State University, Ames, IA.
Xuemei Sui, Department of Exercise Science, University of South Carolina, Columbia, SC.
Timothy S. Church, Department of Preventive Medicine, Pennington Biomedical Research Center, Baton Rouge, LA.
Carl J. Lavie, Department of Cardiovascular Diseases, John Ochsner Heart and Vascular Institute, Ochsner Clinical School, University of Queensland School of Medicine, New Orleans, LA.
Steven N. Blair, Department of Exercise Science, University of South Carolina, Columbia, SC.
References
- 1.World Health Organization. Diabetes [fact sheet]. http://www.who.int/mediacentre/factsheets/fs312/en/. Accessed March 6, 2018
- 2.Myers J, Atwood JE, Froelicher V. Active lifestyle and diabetes. Circulation. 2003;107(19):2392–2394. [DOI] [PubMed] [Google Scholar]
- 3.Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30(3):744–752. [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee DC, Sui X, Church TS, Lee IM, Blair SN. Associations of cardiorespiratory fitness and obesity with risks of impaired fasting glucose and type 2 diabetes in men. Diabetes Care. 2009;32(2):257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN. The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med. 1999;130(2):89–96. [DOI] [PubMed] [Google Scholar]
- 7.Sui X, Hooker SP, Lee IM, et al. A prospective study of cardiorespiratory fitness and risk of type 2 diabetes in women. Diabetes Care. 2008;31(3):550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawakami R, Sawada SS, Lee IM, et al. Long-term Impact of Cardiorespiratory Fitness on Type 2 Diabetes Incidence: A Cohort Study of Japanese Men. J Epidemiol. 2018;28(5):266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84(3):475–482. [DOI] [PubMed] [Google Scholar]
- 10.Artero EG, Lee DC, Lavie CJ, et al. Effects of muscular strength on cardiovascular risk factors and prognosis. J Cardiopulm Rehabil Prev. 2012;32(6):351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollock ML, Franklin BA, Balady GJ, et al. AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription: An advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association; Position paper endorsed by the American College of Sports Medicine. Circulation. 2000;101(7):828–833. [DOI] [PubMed] [Google Scholar]
- 12.Grontved A, Pan A, Mekary RA, et al. Muscle-strengthening and conditioning activities and risk of type 2 diabetes: a prospective study in two cohorts of US women. PLoS Med. 2014;11(1):e1001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grontved A, Rimm EB, Willett WC, Andersen LB, Hu FB. A prospective study of weight training and risk of type 2 diabetes mellitus in men. Arch Intern Med. 2012;172(17):1306–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiroma EJ, Cook NR, Manson JE, et al. Strength Training and the Risk of Type 2 Diabetes and Cardiovascular Disease. Med Sci Sports Exerc. 2017;49(1):40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(sup2):S1–14. [PubMed] [Google Scholar]
- 16.Jurca R, Lamonte MJ, Barlow CE, Kampert JB, Church TS, Blair SN. Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc. 2005;37(11):1849–1855. [DOI] [PubMed] [Google Scholar]
- 17.Jackson AW, Lee DC, Sui X, et al. Muscular strength is inversely related to prevalence and incidence of obesity in adult men. Obesity (Silver Spring, Md). 2010;18(10):1988–1995. [DOI] [PubMed] [Google Scholar]
- 18.Blair SN, Kannel WB, Kohl HW, Goodyear N, Wilson PW. Surrogate measures of physical activity and physical fitness. Evidence for sedentary traits of resting tachycardia, obesity, and low vital capacity. Am J Epidemiol. 1989;129(6):1145–1156. [DOI] [PubMed] [Google Scholar]
- 19.Blair SN, Kohl HW 3rd, Barlow CE, Paffenbarger RS Jr., Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273(14):1093–1098. [PubMed] [Google Scholar]
- 20.Ruiz JR, Sui X, Lobelo F, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ (Clinical research ed). 2008;337:a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Artero EG, Lee DC, Ruiz JR, et al. A prospective study of muscular strength and all-cause mortality in men with hypertension. J Am Coll Cardiol. 2011;57(18):1831–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959;10(6):675–688. [PubMed] [Google Scholar]
- 23.Pollock ML, Bohannon RL, Cooper KH, et al. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92(1):39–46. [DOI] [PubMed] [Google Scholar]
- 24.American College of Sports Medicine. ACSM's guidelines for exercise testing and prescription. Lippincott Williams & Wilkins, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Kohl HW 3rd, Gordon NF, Scott CB, Vaandrager H, Blair SN. Musculoskeletal strength and serum lipid levels in men and women. Med Sci Sports Exerc. 1992;24(10):1080–1087. [PubMed] [Google Scholar]
- 26.American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38 Suppl:S8–s16. [DOI] [PubMed] [Google Scholar]
- 27.The National Institute of Diabetes and Digestive and Kidney Diseases. Risk Factors for Type 2 Diabetes [article online]. https://www.niddk.nih.gov/health-information/diabetes/overview/risk-factors-type-2-diabetes. Accessed March 30, 2018. [Google Scholar]
- 28.Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116(5):572–584. [DOI] [PubMed] [Google Scholar]
- 29.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–896. [DOI] [PubMed] [Google Scholar]
- 30.Eves ND, Plotnikoff RC. Resistance training and type 2 diabetes: considerations for implementation at the population level. Diabetes care. 2006;29(8):1933–1941. [DOI] [PubMed] [Google Scholar]
- 31.Kohrt WM, Kirwan JP, Staten MA, Bourey RE, King DS, Holloszy JO. Insulin resistance in aging is related to abdominal obesity. Diabetes. 1993;42(2):273–281. [PubMed] [Google Scholar]
- 32.Schnohr P, O'Keefe JH, Marott JL, Lange P, Jensen GB. Dose of jogging and long-term mortality: the Copenhagen City Heart Study. J Am Coll Cardiol. 2015;65(5):411–419. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong ME, Green J, Reeves GK, Beral V, Cairns BJ. Frequent physical activity may not reduce vascular disease risk as much as moderate activity: large prospective study of women in the United Kingdom. Circulation. 2015;131(8):721–729. [DOI] [PubMed] [Google Scholar]
- 34.Miyachi M Effects of resistance training on arterial stiffness: a meta-analysis. Br J Sports Med. 2013;47(6):393–396. [DOI] [PubMed] [Google Scholar]