Abstract
Background
Hepatic steatosis is a common manifestation of CF-related liver disease(CFLD). Controlled attenuation parameter(CAP) measurement during transient elastography(TE) semiquantifies liver steatosis. We examined the relationship between CAP and CFLD severity, clinical factors and liver stiffness measurements(LSM).
Methods
This is a cross-sectional study of CF patients seen for outpatient care between January 2013-March 2014. CFLD severity was categorized as no CFLD, CFLD without portal hypertension(PHTN) and CFLD with PHTN, based on published criteria.
Results
129 patients (median 18.4y; 57% male) had valid CAP. 70(54%) had no CFLD, 44(34%) CFLD without PHTN, and 15(12%) CFLD with PHTN. The median CAP was 210 dB/m (IQR 181– 239). Steatosis(CAP ≥230 dB/m) was seen in 27% of subjects without CFLD, 48% in CFLD but no PHTN, and 20% in with CFLD and PHTN(P=0.04). CAP was higher for subjects with CFLD without PHTN (P<0.05). There was no CAP difference between subjects with no CFLD and those with CFLD and PHTN (P≥0.65). LSM was not different between no CFLD and CFLD without PHTN (P=0.07), but each of these groups had lower LSM compared to subjects with CFLD and PHTN(P<0.001 for each). Except for direct bilirubin, CAP was not associated with clinical markers of liver disease.
Conclusion
CAP was normal in 86(67%) of patients with CF and was not associated with standard clinical markers of liver disease. CAP was higher in patients with liver disease, which could possibly reflect the loss of steatosis observed with progression to cirrhosis and portal hypertension.
Keywords: Cystic Fibrosis, Children, Adolescents, Transient elastography, Controlled attenuation parameter, Hepatic steatosis
Introduction
The health outcomes of patients with Cystic Fibrosis (CF) have greatly improved over the last 20 years due to advances in diagnosis and therapy of chronic lung disease and malnutrition. However, liver disease associated with CF has emerged over this time as a significant contributor to long-term morbidity and mortality. Liver disease is most often diagnosed late in its course, when cirrhosis and portal hypertension are well established.
The prevalence of hepatic steatosis in patients with CF has been described as 20–60% and can present at any age1. The degree of steatosis can range from mild changes in echogenicity on ultrasound to extensive fatty infiltration2. Focal fat deposition in centrilobular and periportal regions can also occur3. The pathogenesis of steatosis remains obscure; it does not seem to be related to a CF Transmembrane Conductance Regulator secretory defect, but rather to selective nutritional deficiencies and altered phospholipid metabolism4–5. Whether simple steatosis is a risk factor for the development of steatohepatitis and progression to cirrhosis in CF patients remains unclear1.
Ultrasound (US) is a low-cost, safe and widely accessible imaging technique. US is accurate in detecting steatosis if it involves at least 20–30% of hepatocytes6. However, it lacks sensitivity and the ability to consistently detect and quantify hepatic steatosis, and it is operator dependent. Other modalities like magnetic resonance imaging and magnetic resonance spectroscopy are more accurate for the detection of hepatic steatosis but are not widely used due to expense, practicality and limited clinical availability7.
A noninvasive method called the controlled attenuation parameter (CAP) has been developed to assess hepatic steatosis. CAP is based on the radiofrequency ultrasound signal acquired by the transient elastography device (Fibroscan®, Echosens, Paris, France). CAP is an estimate of the ultrasonic attenuation coefficient at 3.5 MHz. These measures are reproducible, as well as operator and machine independent. CAP has been shown to correlate with hepatic steatosis in adults8 independent of the degree of fibrosis and etiology of liver disease. Depending on the applied cut-off value and the estimated prevalence in the population of interest, CAP can provide semi-quantitative assessment of liver steatosis in adults8–11 and children12. To our knowledge, there are very few data on the use of CAP in children and adults with cystic fibrosis.
The aims of this study were: 1) to determine the prevalence of hepatic steatosis, as determined by CAP, in a cross-sectional sample of pediatric patients with CF, 2) to examine the association of CAP measurements with the presence and severity of CF-related liver disease (CFLD) and 3) to examine the association of CAP measurements with biochemical and clinical parameters including serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), platelet count, body mass index (BMI), pancreatic insufficiency, use of feeding tube, presence of CF-related diabetes and liver stiffness measurements (LSM).
Material and methods
Study design
This was a cross-sectional observation study of children and young adults with cystic fibrosis (age 6–25 years) who underwent transient elastography measurements during routine non-emergent care at Boston Children’s Hospital (BCH) between January 2013 and March 2014. CAP measurements were obtained during transient elastography done for another study of CFLD13. Exclusion criteria were known liver disease other than CFLD, liver transplant and pregnancy. Reported here are CAP/LSM studies performed at each patient’s first visit.
Clinical and biochemical data were obtained from the medical records, including age, sex, weight, height, presence or absence of splenomegaly as determined by physical examination or ultrasound, pulmonary function test results, and prescription for ursodeoxycholic acid at the time of CAP measurement. Biochemical parameters closest to the CAP measurement date were recorded. Findings from the most recent abdominal ultrasound and/or GI endoscopy, if performed, were recorded. Body mass index Z-scores (BMIZ) were calculated for subjects age 6–20 years according to CDC reference charts.
This study was approved by the BCH Institutional Review Board. Written informed consent was obtained from parents, guardians, and subjects ≥18y. Assent was obtained from children 7–17 years.
Transient Elastography
Steatosis as determined by CAP and fibrosis as determined by LSM were measured by transient elastography (TE) by trained research investigators during annual routine care visits using the FibroScan ® device (Echosens, Paris, France). CAP and LSM were obtained by placing an ultrasonic transducer in a right intercostal space to transmit a vibration which induces an elastic shear wave through the right lobe of the liver.
Elastography measures the liver stiffness in kiloPascals (kPa). The velocity of propagation is directly related to tissue stiffness: the harder the tissue (as in hepatic fibrosis), the faster the shear wave propagates. The TE probe uses ultrasound to track the wave and subsequently CAP is calculated as a measure of the ultrasound attenuation, which corresponds to the decrease in amplitude of ultrasound waves as they propagate through the liver, expressed as decibel per meter (dB/m). CAP is calculated automatically by the device, and only if the liver stiffness measurement is valid.
CAP was measured with the M probe at 3.5 MHz, at depths between 25 and 65 mm, since all subjects had a thoracic perimeter 75 – 110 cm. In each subject, 10 valid measurements (as recommended by the manufacturer) were obtained in rapid succession. The adequacy of the measurement was assessed by the FibroScan© device. If the measurement was invalid, owing to either patient characteristics or operator error, then neither LSM nor CAP measurement was given. The test typically was completed in 5–10 minutes. CAP measurements were obtained by technicians who were blinded to clinical data.
CFLD Categories
Patients were categorized into three groups according to clinical, biochemical, sonographic, and endoscopic parameters. The groupings from published guidelines on CFLD were reviewed and modified based on accepted clinical parameters at our institution14–15. Subjects were classified as having no CFLD when all the following criteria were met:
Most recent ALT <1.3 × ULN (upper limit of normal; 30 IU/mL at BCH)
Not prescribed ursodeoxycholic acid
Normal ultrasound (if done).
Subjects were classified as CFLD without portal hypertension (PHTN) when at least one of the following criteria were met:
Most recent ALT >1.3 × ULN
Prescribed ursodeoxycholic acid
Abnormal liver echogenicity without evidence of PHTN.
Subjects were classified as having CFLD with PHTN when at least one of the following were met:
Splenomegaly on physical exam defined as spleen below costal margin
Presence of esophageal varices by traditional or video capsule endoscopy
Platelet count <100,000/mm3
Evidence of PHTN on ultrasound (ascites, reversed portal flow, varices, and/or splenomegaly). Splenomegaly by ultrasound was defined as spleen length-for-age above 90% of upper confidence limit16.
Statistical analyses
Categorical data are described with frequency counts and percentages and compared across outcome categories by Fisher’s exact test. Continuous data are described with median (interquartile range; IQR) and compared by Wilcoxon rank-sum test or Kruskal-Wallis test according to whether two or three categories are compared, respectively. The Wilcoxon rank-sum test compares sums of ranks between two groups and not the medians, such that the medians can be equal even when the test returns a significant P value. Since this occurred for direct bilirubin (DB), an additional comparison for DB=0.1 mg/dL and DB>0.1 mg/dL is presented in Table 3 for added clarity, which was also significant at P=0.01 by Fisher’s exact test. All tests of significance were two-sided, with P<0.05 deemed statistically significant. Analysis was performed with SAS® v9.4 (Cary, NC).
Table 3.
Unadjusted association of baseline CAP measurements with patient and clinical factors. Shown are median (IQR) or n (%).
| Characteristic | CAP < 230 |
CAP ≥ 230 |
|
|---|---|---|---|
| (N = 86) | (N = 43) | P | |
| Growth and nutrition | |||
| BMIZ (aged ≤20y)(n= 76) | −22 (−0.86, 0.54) | 0.33 (−0.57, 0.88) | 0.10 |
| BMI (aged >20y) (n = 53) | 22.1 (20.4, 23.7) | 22.1 (21.1, 24.4) | 0.47 |
| BMI (all subjects) | 20.9(19.2, 22.9) | 21.5(19.9, 24.3) | 0.19 |
| Gastrostomy tube | 10(11.6%) | 7 (16.3%) | 0.58 |
| Labs | |||
| GGT(u/L) (n = 76) | 16(11.25) | 14 (12, 30) | 0.99 |
| ALT (u/L) | 21 (15, 28) | 24 (19, 33) | 0.08 |
| AST (u/L) | 24 (19, 31) | 26 (20, 30) | 0.93 |
| INR (n = 104) | 1.02 (0.98, 1.07) | 1.01 (0.98, 1.04) | 0.23 |
| Albumin (g/dL) (n = 121) | 4.3 (4.0, 4.6) | 4.3 (4.1, 4.6) | 0.99 |
| Direct bilirubin (mg/dL) (n = 96) | 0.1 (0.1, 0.1) | 0.1 (0.1, 0.1) | 0.01 |
| Direct bilirubin = 0.1 mg/dL | 49(75.4%) | 30 (96.8%) | |
| Direct bil irubi n >0.1 mg/d L | 16(24.6%) | 1 (3.2%) | |
| PLT (K cells/uL) (n = 127) | 263(227, 312) | 280 (254, 347) | 0.10 |
| Creatinine (mg/dL) (n = 127) | 0.6 (0.5, 0.8) | 0.6 (0.5, 0.7) | 0.54 |
| Glucose (mg/dL) (n = 123) | 93 (87, 130) | 96 (88, 109) | 0.92 |
| HgbA1C (n = 87) | 5.5 (5.3, 6.1) | 5.4 (5.2, 5.8) | 0.46 |
| Clinical | |||
| UDCA | 30 (349%) | 12 (27.9%) | 0.55 |
| Pancreatic insufficiency | 80 (93.0%) | 43 (100%) | 0.18 |
| Diabetes | 17 (19.8%) | 7 (16.3%) | 0.81 |
| Noninvasive scores | |||
| LSM | 5.3 (4.3, 6.4) | 5.3 (4.3, 6.6) | 0.87 |
| AST:ALT (APRI) (n = 127) | 0.24 (0.16, 0.32) | 0.22(0.15, 0.29) | 0.33 |
Results
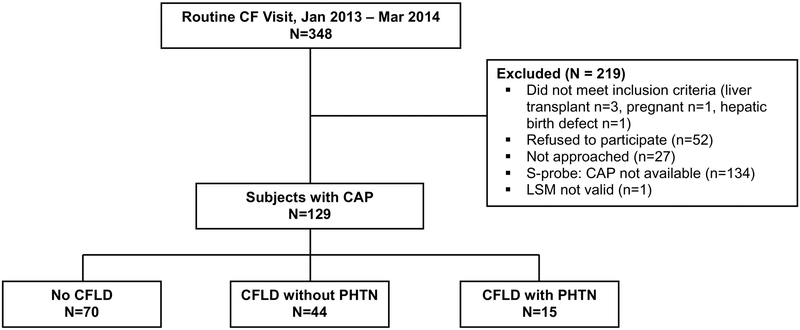
A total of 348 patients with CF were seen for routine care during the study period, representing 76% of all CF patients under 26 years in the BCH CF Patient Registry. Of these, 219 were excluded due to failure to meet inclusion criteria, refusal to participate, patient not approached due to being unavailable for enrollment, small (S) probe used (which is unable to calculate CAP), and invalid TE measurement. A total of 129 subjects with thoracic parameters > 75 cm and valid medium (M)-probe CAP measurements and determinable liver disease status comprised the sample for this study. Derivation of the study population is shown in Figure 1.
Figure 1.
Patient flowchart.
Subjects were 18.4 (15.8, 23.1) years of age, with 2% <12y, 42% 12–17y, and 56% ≥18y. CF was diagnosed by either sweat test or genetic test, with 57 (46%) homozygous and 55 (45%) heterozygous for the Δ F508 CFTR mutation. Eleven (9%) subjects had no Δ F508 mutations; genotype results were unknown for 6 subjects. Among 75 subjects who underwent abdominal ultrasound, abnormalities were found in 32 (43%), in the form of abnormal liver echogenicity in 19 (25%) and signs of PHTN in 13 (17%). ALT and AST were >1.3 times the ULN in 19 (15%) and 8 (6%) of all subjects, respectively. Using the criteria described above, 70 (54%) subjects were classified as no CFLD, 44 (34%) as CFLD without PHTN, and 15 (12%) as CFLD with PHTN (Table 1).
Table 1.
Subject characteristics (n = 129). Shown are median (IQR) or n (%).
| Characteristic | By disease severity |
||||
|---|---|---|---|---|---|
| Overall |
No CFLD |
CFLD without PHTN |
CFLD with PHTN |
||
| (n = 129) | (n = 70) | (n = 44) | (n = 15) | P* | |
| Demographics | |||||
| Male sex | 73(57%) | 39(56%) | 24 (55%) | 10(67%) | 0.77 |
| Age at LSM (y) | 18.4(15.8. 23.1) | 18.2(14.9. 22.2) | 18.5(16.7, 23.3) | 22.2(17.1.23.8) | 0.09 |
| 3–6 | 1(1%) | 1(1%) | 0(0%) | 0(0%) | |
| 7–11 | 2(1%) | 1(1%) | 1(2%) | 0(0%) | |
| 12–17 | 54 (42%) | 32 (46%) | 18 (41%) | 4(27%) | |
| 18–25 | 72(56%) | 36(51%) | 25(57%) | 11 (73%) | |
| Homozygous delta F508 | 57 (46%) | 28 (42%) | 25(58%) | 4(29%) | 0.10 |
| Heterozygous delta F508 | 55(45%) | 29 (44%) | 18 (42%) | 8(57%) | 0.59 |
| Growth and nutrition | |||||
| BMIZ (n = 76 age ≤ 20y) | −0.07 (−0.70.0.60) | 0.13 (−0.57. 0.75) | −0.36 (−0.88. 0.46) | −0.64 (−1.15, −0.43) | 0.16 |
| BMI (n = 53 age> 20y) | 22.1 (20.6. 24.3) | 22.1 (20.4. 24.1) | 22.1 (21.1.24.4) | 21.4(203. 26.3) | 0.66 |
| Gastrostomy tube | 17(13%) | 7(10%) | 7(16%) | 3(20%) | 0.39 |
| Labs | |||||
| GGT (u/L), n = 76 | 16(11,27) | 13(11,17) | 18 (13,31) | 26(18.47) | |
| ALT (u/L) | 22(16. 30) | 20(16.25) | 25 (17.47) | 26(14, 30) | |
| AST (u/L) | 25(20. 30) | 23(19,29) | 27 (20. 37) | 26(23. 33) | |
| ALT >1.3 × ULN | 19(15%) | 0(0%) | 16 (36%) | 3(20%) | |
| AST> 13 × ULN | 8(6%) | 0(0%) | 6(14%) | 2(13%) | |
| INR, n = 104 | 1.01 (0.98.1.06) | 1.01 (0.98.1.06) | 1.01 (0.99,1.05) | 1.05(055.1.19) | |
| Albumin (g/dL), n = 121 | 4.3 (4.0.4.6) | 4.3 (4.1.4.6) | 4.3(35,4.6) | 43(3.6.4.5) | |
| Direct bilirubin (mg/dL), n = 96 | 0.1 (0.1. 0.1) | 0.1 (0.1. 0.1) | 0.1 (0.1, 0.1) | 0.1 (0.1. 0.2) | |
| PLT (K cells/uL), n = 127 | 269 ( 239. 335) | 280(254.330) | 262(241.342) | 147(73, 309) | |
| HgbA1C, n = 87 | 5.4 (5.2. 5.8) | 5.4 (5.2. 5.7) | 5.5(53. 6L1) | 5.5(52. 7.6) | |
| Clinical | |||||
| Asdtes | 1(1%) | 0(0%) | 0(0%) | 1(7%) | |
| Meconium ileus, n = 74 | 20(27%) | 11 (28%) | 9(31%) | 0(0%) | |
| Ursodeoxycholic acid | 42(33%) | 0(0%) | 29 (66%) | 13(87%) | |
| Pancreatic insuffidency | 123(95%) | 64(91%) | 44 (100%) | 15(100%) | |
| Diabetes | 24(19%) | 8(11%) | 11 (25%) | 5(33%) | |
P value from Kruskal-Wallis test or Fisher exact test. P value not shown for laboratory and clinical variables since they were used to either define or are known to be related to the CF liver disease severity categories.
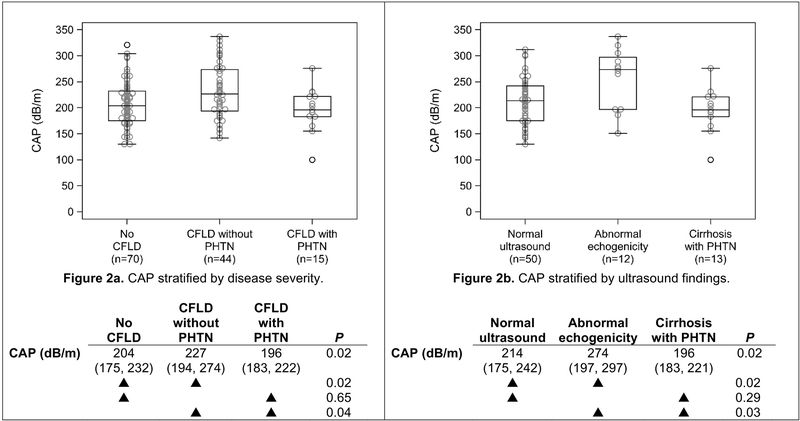
TE measurements by liver disease severity are shown in Table 2. Steatosis was defined as CAP ≥230 dB/m. The median CAP measurement for the entire sample was 210 dB/m (IQR 181– 239) and 43 (33%) had steatosis. Steatosis was seen in 27% of subjects without CFLD, 48% of those with CFLD but no PHTN, and 20% of those with CFLD and PHTN (P=0.04). CAP measurement (and steatosis prevalence) was statistically higher for subjects categorized as CFLD without PHTN than either of the other two categories (P<0.05), while there was no difference between subjects categorized as no CFLD and those with CFLD and PHTN (P≥0.65). Liver stiffness measurements (LSM) were not statistically different between subjects with no CFLD and those categorized as CFLD without PHTN (P=0.07), but each of these groups had statistically lower LSM compared to subjects with CFLD and PHTN (P<0.001 for each), as previously reported13.
Table 2.
Fibroscan measurements (n = 129). Shown are median (IQR) or n (%).
| Characteristic | By disease severity |
||||
|---|---|---|---|---|---|
| Overall |
No CFLD |
CFLD without PHTN |
CFLD with PHTN |
||
| (n = 129) | (n = 70) | (n =44) | (n = 15) | P* | |
| CAP (dB/m), median (IQR) | 210 (181,239) | 204 (175, 232) | 227 (194, 274) | 196 (183, 222) | 0.02 |
| Steatosis (CAP≥230) | 43 (33%) | 19 (27%) | 21 (48%) | 3 (20%) | 0.04 |
| LSM (kPa), median (IQR) | 53 (4.3, 6.4) | 43 (4.2, 5.7) | 5.4 (4.5, 65) | 113 (6.4, 24.8) | <0.0001 |
P value from Kruskal-Wallis test or Fisher exact test.
Association of steatosis, as defined by CAP score ≥230 dB/m, with patient and clinical factors is shown in Table 3. With the exception of direct bilirubin (DB), CAP measurements were not associated with clinical and biochemical markers of liver disease. Median DB was 0.1 (range 0.1 – 1.4) mg/dL in subjects without steatosis compared to 0.1 (range 0.1 – 0.2) mg/dL in subjects with steatosis (P=0.01). Notably, neither group had elevated direct bilirubin levels.
The association of CAP with liver disease severity and the association of CAP with ultrasound measurement exhibited similar trends to one another (Figure 2). In both cases, CAP for the least-extreme (no CFLD; normal ultrasound) and most-extreme (CFLD with PHTN) groups were not statistically different from one another (P=0.65 and P=0.29, respectively), while the least- and most-extreme groups were each statistically different from the group between these extremes (CFLD without PHTN; abnormal echogenicity; P<0.05 for each).
Figure 2.
CAP, stratified by CF liver disease severity (Figure 2a) and ultrasound findings (Figure 2b). Below each figure is a summary table showing the median (IQR) CAP for each category. The symbol indicates pairwise comparisons, which are not adjusted for multiple comparisons. CFLD = cystic fibrosis liver disease; PHTN = portal hypertension.
Discussion
To our knowledge, this is the first study evaluating CAP for the assessment of steatosis in CF patients. CAP appears to be a promising tool for the non-invasive detection and quantification of steatosis in patients with CFLD. CAP measurements can be evaluated simultaneously with LSM to assess hepatic fibrosis. Desai et al. demonstrated a difference in CAP between no steatosis and steatosis, and between grades of steatosis in children12. Aqul et al. showed a correlation between liver stiffness measurement (LSM) with presence and severity of liver disease in children and young adults with CF13. An optimal CAP threshold for steatosis of 232 dB/m was demonstrated in a meta-analysis in chronic liver disease and 225 dB/m in pediatric patients17,12. In our study, we used 230 dB/m as the threshold value. More data and studies are needed to predict accurate thresholds that are disease specific, according to age and BMI.
Identifying patients who are at risk for CFLD and potential hepatic complications is an ongoing challenge. Long-term follow-up of different cohorts of CF patients carefully monitored for hepatic involvement indicates a cumulative incidence of liver disease ranging between 27% and 35%, without incident cases after the age of 18 years. The prevalence of hepatic steatosis has been described to be as high as 20%–60% in patients with CF and can present at any age. Approximately 5 to 10% of all CF patients will develop multi-lobular cirrhosis during the first decade of life. Most of these will present with related complications during the second decade, rarely in the pediatric age group.
In our study, 67% of subjects had CAP measurement in the normal range (<230 dB/m) and curiously CAP was not associated with clinical and biochemical markers of liver disease. There are several possible explanations for this. As the pathogenesis of steatosis remains obscure, we might be missing the clinical and biochemical markers that truly correlate with CAP. On the other hand, we may simply need more patients to illustrate an association, or a longitudinal study to investigate whether serial correlation exists with clinical and biochemical markers of liver disease. In addition, we might be observing a sample bias as most of our study sample is relatively healthy.
In the subset of patients with abnormal ultrasound echogenicity, CAP measurements were higher than the CAP measurement for patients with normal ultrasound. This may suggest an association of CAP with steatosis. CAP was significantly lower in patients with evidence of cirrhosis and portal hypertension on ultrasound compared to patients with only increased liver echogenicity (85% vs. 33% normal CAP; P=0.03). We theorize that this may indicate that as the fibrosis increases and cirrhosis develops, steatosis decreases and fat may be replaced by fibrous tissue. Larger, longitudinal studies are need to investigate the progression of CFLD.
The main limitation of our study is that it is based on a single center with a relatively healthy CF population and a small sample size of patients with CFLD. Furthermore, we lack a gold standard method to evaluate steatosis and compare CAP measurements. Most patients with CFLD do not need clinical liver biopsies and ultrasound is a crude measurement of steatosis. Furthermore, only a subset of our study sample (58%) had ultrasound data available for assessment of association with CAP. This is because routine ultrasound is not the standard of care and are only done if there is a suspicion for liver disease. However, it is possible that with the absence of ultrasound data, this potentially introduces the possibility of misclassification of patients. In addition, ALT > 1.3 × ULN was included as part of the classification criteria. We acknowledge that an elevated ALT may not be an exclusively specific marker for CFLD, and thus may add to the risk for misclassification of patients. Further, the use of ursodeoxycholic acid was part of the classification criteria. Since the practice at our institution is to treat patients with CF with persistent elevation of aminotransferases with ursodeoxycholic acid, subjects prescribed ursodeoxycholic acid were categorized as having CFLD even if the most recent ALT was normal. The CFLD categories are broad to be inclusive and to avoid missing subjects with CFLD.
There is a possibility that there is confounding interplay between CAP measurement and liver fibrosis. Although there are no data in CF patients, it has been reported in adults with biopsy proven non-alcoholic fatty liver disease that high CAP values could be associated with overestimation of liver stiffness measurements, particularly in subjects with lower stages of fibrosis18. Additional studies are needed to see if this relationship exists in other chronic liver diseases.
This preliminary study is the first to examine CAP and steatosis in patients with cystic fibrosis. CAP measurements were normal in two-thirds of CF patients. CAP measurements were associated with ultrasound findings. Larger studies are needed for more experience using CAP in children with cystic fibrosis. Ultimately, CAP/TE is an important tool which could be used for early detection and surveillance of CFLD.
Highlights.
CAP is a non-invasive tool to assess liver steatosis.
CAP was normal in a majority of children/young adults with Cystic Fibrosis.
CAP is higher in patients with CFLD without portal hypertension.
Acknowledgments
Echosens (Paris, France) provided the FibroScan® machine, technical support, and training of investigators for the purpose of this study. Echosens had no role in study design, collection/analysis/interpretation of data, writing the manuscript, or the decision to submit the manuscript for publication.
Intramural funding was provided by the Division of Gastroenterology, Hepatology and Nutrition at Boston Children’s Hospital. Statistical work was partially funded by National Institute of Health/ National Institute of Diabetes and Digestive and Kidney Diseases grant # P30DK34854.
Footnotes
Conflict of Interest Statement
Drs. Lee and Jonas have received research support from Echosens® in the form of the transient elastography hardware and software. No other support was provided.
Portions of this study were presented at The Liver Meeting (the annual meeting of the American Association for the Study of Liver Diseases) on November 11, 2016 in Boston, MA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Staufer K, Halilbasic E, Trauner M, Kazemi-Shirazi L. Cystic fibrosis related liver disease--another black box in hepatology. Int J Mol Sci 2014;15:13529–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palermo JJ. 50 Years Ago in The Journal of Pediatrics: Cystic Fibrosis with Extensive Fat Replacement of the Liver. J Pediatr 2016;168:49. [DOI] [PubMed] [Google Scholar]
- 3.Fields TM, Michel SJ, Butler CL, Kriss VM, Albers SL. Abdominal manifestations of cystic fibrosis in older children and adults. AJR Am J Roentgenol 2006;187:1199–203. [DOI] [PubMed] [Google Scholar]
- 4.Treem WR, Stanley CA. Massive hepatomegaly, steatosis and secondary plasma carnitine deficiency in an infant with cystic fibrosis. Pediatrics 1989; 83(6); 993–7. [PubMed] [Google Scholar]
- 5.Innis SM, Davidson AG, Chen A, Dyer R, Melnyk S, James SJ. Increased plamsa homocysteine and S-adenosylhomocysteine and decreased methionine is associated with altered phosphatidylcholine and phosphatidylethanolamine in cystic fibrosis. J Pediatr 2003; 143(3): 351–6. [DOI] [PubMed] [Google Scholar]
- 6.Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol 2009;51:1061–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohte AE, van Werven JR, Bipat S, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol 2011;21:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Ledinghen V, Vergniol J, Capdepont M, Chernak F, Hiriart JB, Cassinotto C, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol 2014;60:1026–31. [DOI] [PubMed] [Google Scholar]
- 9.Kumar M, Rastogi A, Singh T, Behari C, Gupta E, Garg H, et al. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis: does etiology affect performance? J Gastroenterol Hepatol 2013;28:1194–201. [DOI] [PubMed] [Google Scholar]
- 10.Myers RP, Pollett A, Kirsch R, Pomier-Layrargues G, Beaton M, Levstik M, et al. Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int 2012;32:902–10. [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz Y, Yesil A, Gerin F, Ergelen R, Akin H, Celikel CA, et al. Detection of hepatic steatosis using the controlled attenuation parameter: a comparative study with liver biopsy. Scand J Gastroenterol 2014;49:611–6. [DOI] [PubMed] [Google Scholar]
- 12.Desai NK, Harney S, Raza R, Al-Ibraheemi A, Shillingford N, Mitchell PD, et al. Comparison of Controlled Attenuation Parameter and Liver Biopsy to Assess Hepatic Steatosis in Pediatric Patients. J Pediatr 2016; 173:160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aqul A, Jonas MM, Harney S, Raza R, Sawicki GS, Mitchell PD, et al. Correlation of transient elastography with severity of Cystic Fibrosis-related liver disease. JPGN 2017; 64:505–511. [DOI] [PubMed] [Google Scholar]
- 14.Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros 2011;10 Suppl 2:S29–36. [DOI] [PubMed] [Google Scholar]
- 15.Flass T, Narkewicz MR. Cirrhosis and other liver disease in cystic fibrosis. J Cyst Fibros 2013; 12(2); 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Megremis SD, Valchonikolis IG, Tsilimigaki AM. Spleen length in childhood with US: normal values based on age, sex and somatometric parameters. Radiology 2004; 231:129–34. [DOI] [PubMed] [Google Scholar]
- 17.Shi KQ, Tang JZ, Zhu XL, Ying L, Li DW, Gao J, et al. Controlled attenuation parameter for the detection of steatosis severity in chronic liver disease: A meta-analysis of diagnostic accuracy. J Gastroenterol Hepatol 2014; 29(6): 1149–58. [DOI] [PubMed] [Google Scholar]
- 18.Petta S, Wong VWS, Camma C, Hiriart JB, Wong GLH, Marra F, et al. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. [DOI] [PubMed]