Abstract
The menstrual cycle is known to impact mood and cognitive function and has been shown to lead to variability in symptoms of obsessive-compulsive disorders and anxiety. Using a within-subject design, the present study examined ovarian hormones, the error-related negativity (ERN), and self-reported checking symptoms in both the mid-follicular and mid-luteal phases of the menstrual cycle. ERN amplitude and checking symptom severity did not vary between the follicular and luteal phases. However, a more negative ERN was associated with greater checking symptoms in the luteal phase of the menstrual cycle, even when controlling for ERN amplitude in the follicular phase. Moreover, changes in checking symptoms between phases were associated with phase-related changes in the ERN. Finally, a significant mediation model was found such that the ERN measured in the luteal phase mediated the association between progesterone in the luteal phase and checking symptoms in the luteal phase. Collectively, the present findings suggest that levels of progesterone in the luteal phase could impact checking symptoms by modulating response monitoring and sensitivity to errors, and that fluctuation in the ERN between menstrual cycle phases may play an important role in the expression of anxious and obsessive-compulsive symptoms.
Keywords: error-related negativity, EEG/ERP, anxiety, checking, OCD, progesterone, menstrual cycle
Introduction
The pubertal period is a pivotal time of risk for internalizing disorders, including both depression and anxiety. While both sexes have equal incidence of mood and anxiety disorders throughout childhood, an imbalance in the incidence of these disorders emerges in the pubertal period, such that women experience depression and anxiety at twice the rate of men by midadolescence (Cohen et al., 1993). For girls, the onset of menstruation is a major facet of pubertal development, and thus, hormonal changes associated with the onset of menses may play a role in increasing internalizing psychopathology and related symptoms.
The menstrual cycle has increasingly been investigated in relation to psychological changes. While studies examining whether transition between menstrual cycle phases is associated with changes in mood and cognition have produced mixed findings, hormones of the menstrual cycle—estradiol and progesterone in particular—have been associated with changes in emotional reactivity (Farage et al., 2008). The menstrual cycle can be divided into three phases that are characterized by distinct fluctuations in endogenous hormones (Farage et al., 2008). The follicular phase begins with menstrual bleeding and typically lasts 13–14 days. During the early part of the follicular phase, women experience low levels of estrogen and progesterone, while the latter part of the follicular phase is characterized by a sharp increase in both estrogen and luteinizing hormone levels. The increase in luteinizing hormone leads into the 16- to 32-hour ovulatory phase, when estrogen levels decrease rapidly and an egg is released. The luteal phase begins after ovulation, lasts for approximately 14 days, and is characterized by a peak of progesterone and estrogen in the mid-luteal phase that is flanked by relatively decreased levels of both hormones in the early- and late-luteal phases. The late-luteal phase is also commonly referred to as the pre-menstrual phase. Natural variability in levels of estradiol and progesterone across the menstrual cycle are depicted in Figure 1.
Figure 1.
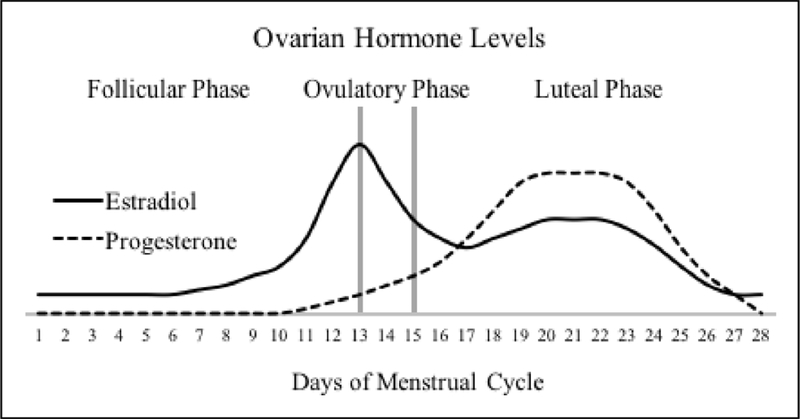
Schematic depicting the natural variability in the menstrual hormones estradiol and progesterone across the menstrual cycle. Reprinted with permission from Mulligan et al., 2018, Psychophysiology, e13268.
In women, greater estradiol has been linked to decreased reactions to negative emotional stimuli, and estrogen therapy has been shown to decrease depressive symptoms in some perimenopausal women (Sakaki & Mather, 2012; Cohen et al., 2003). Similarly, in animal models, estradiol has been shown to reduce anxious behavior (Walf & Frye, 2007; Walf & Frye, 2010). On the other hand, progesterone has been shown to increase reactions to negative stimuli (Sakaki & Mather, 2012). Naturally high levels of progesterone in the mid-luteal compared to the early follicular phase have been related to heightened amygdala activity to negative stimuli (Andreano & Cahill, 2010), and exogenous progesterone administration increased negative mood in women that were in the early follicular phase (Klatzkin et al., 2006). Furthermore, progesterone administration has been shown to increase amygdala reactivity to angry and fearful faces (Van Wingen et al., 2008), and greater levels of progesterone have been associated with increased self-reported proneness to anxiety in the form of excessive doubts, compulsions, obsessions, and unreasonable fears (Avgoustinaki et al., 2012).
In line with these findings, reproductive cycle phases have been linked to risk for onset and exacerbation of obsessive-compulsive disorder (OCD) in women (Gugliemi et al., 2014). Previous studies suggest that pre-existing OCD is often exacerbated in pregnancy and the postpartum period, which are periods characterized by high fluctuation in ovarian hormones (Ross & McLean, 2006; Forray et al., 2010). Further, the premenstrual phase, which occurs during the luteal phase of the menstrual cycle, has also been linked to the exacerbation of OCD symptoms. In a study by Vulink and colleagues (2006), 49 percent of outpatients with OCD reported exacerbated symptoms during the pre-menstrual (or late-luteal) phase. Several other studies find similar results indicating worsening of OCD symptoms in the late-luteal phase of the menstrual cycle (Labad et al., 2005; Williams & Koran, 1997). Thus, hormonal changes that occur over the course of the menstrual cycle may play a role in the severity of OCD symptoms in women.
Given evidence from previous studies that the menstrual cycle may impact severity of OCD symptoms, it is of interest to examine whether biomarkers of OCD and anxiety also vary across the menstrual cycle. For instance, one previous study by Lithgow and Moussavi (2017) examined whether electrovestibulography (EVestG) features, which have been previously proposed to be biomarkers of depression (Lithgow et al., 2015) and have been linked to anxiety (Balaban et al., 2011), vary across the menstrual cycle. They found that EVestG features linked to anxiety differed across early follicular, late follicular and luteal menstrual phases (Lithgow & Moussavi, 2017).
One of the most replicated findings in the neurobiology of OCD is an enhanced error-related negativity (ERN) event-related potential (ERP). However, no studies have examined whether ovarian hormones impact the ERN. The ERN is a negative deflection in the ERP that peaks approximately 50 milliseconds (ms) after a participant makes an incorrect response, or error (Hajcak & Foti, 2008). Previously source-localized to the anterior cingulate cortex (ACC; Holroyd et al., 1998; Herrmann et al., 2004), the ERN is thought to reflect early error processing activity of the ACC (Olvet & Hajcak, 2008), and to reflect the integration of information regarding pain, threat, and punishment for optimizing goal-directed behavior (Meyer, 2016; Shackman et al., 2011). Importantly, the ERN has been found to be accentuated (i.e., is more negative) in individuals with OCD (Ruchsow et al., 2005; Gehring et al., 2000; Johannes et al., 2001; Hajcak et al., 2008; Endrass et al., 2010; Endrass et al., 2008; Xiao et al., 2011; Klawohn et al., 2016), as well as those with heightened symptoms of OCD (Hajcak & Simons, 2002; Santesso et al., 2006), suggesting that these populations are more vigilant to making errors. In line with these findings, the ACC, the cortical region to which the ERN has been localized, has been shown to be hyperactive in OCD patients as compared to healthy controls during a cognitive task designed to elicit errors (Fitzgerald et al., 2005). Moreover, the ERN has even been shown to be enhanced in unaffected first-degree relatives of OCD patients (Riesel et al., 2011; Carrasco et al., 2013). For these reasons, an overactive ERN has been suggested to be a candidate endophenotype or biomarker of OCD (Riesel et al., 2011).
The ERN has also been found to be increased in individuals with generalized anxiety disorder (GAD; Weinberg et al., 2015; Weinberg et al., 2012; Weinberg et al., 2010; Ladouceur et al., 2006) and social anxiety disorder (SAD; Endrass et al., 2014), as well as non-clinical individuals reporting symptoms of worry and general anxiety (Hajcak et al., 2003; Meyer, 2016). Given these reported associations between an enhanced ERN and multiple clinical disorders, recent studies have investigated the association between the ERN and cross-diagnostic symptoms. In a study by Weinberg and colleagues (2015), the ERN was measured in a sample of healthy controls as well as individuals with generalized anxiety disorder, OCD, major depressive disorder, or a combination of the three disorders. Across all groups, checking symptoms (i.e., inspection of one’s own behaviors to reduce anxiety about potential adverse outcomes) were associated with a larger ERN (Weinberg et al., 2015). Furthermore, in a sample of 515 never-depressed adolescents, Weinberg and colleagues (2016) similarly found that a larger ERN was related to self-reported checking symptoms. In both of these studies, when scores from all anxiety symptom subscales (i.e., panic, social anxiety, claustrophobia, traumatic intrusions, traumatic avoidance, checking, ordering, and cleaning) were entered into a multiple regression predicting ERN amplitude, checking symptoms displayed the most robust relationship with the ERN, over and above other symptom dimensions (Weinberg et al., 2015; Weinberg et al., 2016) As a result of these studies, it has been posited that checking may be the best cross-diagnostic phenotype to characterize individual differences in the ERN (Weinberg et al., 2016).
Checking behaviors, which consist of repetitive checking (e.g., repetitively checking on the safety of loved ones or checking that you locked the door) despite knowing that checking is unnecessary, are the most common compulsion in individuals with OCD (Rasmussen et al., 1992) and have been found to be significant predictors of OCD (Watson et al., 2012). Additionally, previous research has indicated that non-clinical compulsive checkers demonstrate higher levels of perfectionism, worry, and cognitive impairment than anxious controls (Gershuny & Sher 1995), that individuals with high checking symptoms are more likely to have depression and anxiety in the postpartum period (Abramowitz et al., 2010), and that dysfunctional beliefs underlying checking behavior are a risk factor for OCD (Abramowitz et al., 2006). Therefore, checking behaviors are both related to OCD, risk for OCD, and are problematic in their own right. However, no study has yet examined variability in the ERN and checking symptoms across the menstrual cycle. If ovarian hormones impact the ERN, checking symptoms, or their association, then menstrual phases with high balances of those hormones may represent periods of vulnerability or risk for developing OCD or anxiety (Andreano et al., 2018). Moreover, these data could shed important light on the timing of when it is most useful to assess neural mechanisms of risk, as well as when such neural mechanisms of risk might best be targeted via intervention or prevention efforts.
In the current study, we sought to examine for the first time the relationship between ERN and checking symptoms across phases of the menstrual cycle-our primary goal was to determine whether the ERN and checking symptoms might vary across menstrual phases, and if changes in checking symptoms across menstrual phase relate to changes in the ERN. To this end, the present study used a within-subject design to examine whether checking symptoms and the ERN varied in the mid-follicular and mid-luteal phases of the menstrual cycle. Forty undergraduate females completed a hormone assay for estradiol and progesterone, a checking symptom inventory, and a task to elicit the ERN twice-once during the mid-follicular phase and once during the mid-luteal phase. Given previous findings, we hypothesized that the ERN and checking symptoms would both be elevated in the mid-luteal phase. We also hypothesized that greater checking symptoms would be associated with a heightened ERN, greater levels of progesterone, and reduced levels of estradiol. Finally, as an exploratory aim, we aimed to examine whether the ERN and checking symptoms were associated in the mid-follicular and mid-luteal phases and whether the ERN mediates associations between hormones and checking symptoms.
Methods
Participants
Fifty-three female undergraduates from Stony Brook University participated for course credit. Of these, 13 participants were excluded from analyses for not returning for their second assessment. Thus, the final sample consisted of 40 participants. The sample was college-aged (M = 20.78 years, SD = 3.37), and ethnically diverse, including 55% Asian, 25% Caucasian, 12.5% Latino, and 7.5% Black. Demographic information can be found in Table 1. Participants were recruited from the introduction to psychology subject pool. Demographic information was obtained through an initial screening e-mail, and eligibility for participation was determined through an online pre-screen survey that assessed the use of hormonal/oral contraceptives, average menstrual cycle duration, date of onset of previous menses, and regularity of the menstrual cycle. The menstrual cycle length was defined as the number of days from the start of menses in one cycle to the start of menses in the next cycle. Inclusion criteria were age 18–35 years and regular menstrual cycle (average cycle length 28.65 days [SD = 2.97]; average length of menstruation 5.29 days [SD = 0.95]). Exclusion criteria were: taking hormonal/oral birth control within the past 4 months, irregular menstruation, pregnancy or lactation within the past 12 months.
Table 1.
Demographic information, hormone levels, and checking symptoms
| M | SD | |||
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 20.78 | 3.37 | ||
| Education (years) | 13.94 | 1.19 | ||
| Race | ||||
| Caucasian | 25% | |||
| Black | 7.5% | |||
| Latino | 12.5% | |||
| Asian | 55% | |||
| Follicular Phase | Lutea Phase | |||
| M | SD | M | SD | |
| Hormones (pg/mL) | ||||
| Estradiol | 2.48 | 0.69 | 2.78 | 0.71 |
| Progesterone | 152.69 | 81.68 | 356.19 | 194.41 |
| IDAS-II Checking | 6.12 | 2.90 | 6.20 | 2.49 |
Information on the average menstrual cycle length and the date of onset of previous menses was used to schedule eligible participants for the initial assessment. Order of menstrual phase tested was counterbalanced across participants. Of the 40 participants, 23 (57.5%) were initially tested during the mid-follicular phase (6 to 8 days following the start of menstruation) of their menstrual cycle, and 17 (42.5%) were initially tested during the mid-luteal phase (6 to 8 days before the projected start of menstruation) of their menstrual cycle. For the second assessment, each participant was scheduled during the alternate phase of her cycle. Informed consent was obtained prior to participation and the research protocol was approved by the Institutional Review Board at Stony Brook University.
Measures
Inventory of Depression and Anxiety Symptoms (IDAS-II):
The IDAS-II is a 99-item selfreport questionnaire that measures factor-analytically derived symptom dimensions of depression and anxiety (Watson et al. 2007; Watson et al., 2012). Each item measures symptoms over the past two weeks on a 5-point Likert scale ranging from 1 (Not at all) to 5 (Extremely). The IDAS-II has good internal consistency, test-retest reliability, and convergent and discriminant validity with diagnoses and self-report measures (Watson et al., 2012). National norms have recently been reported for the IDAS-II (Nelson et al., 2017). It has also been found to have good clinical utility, as the IDAS-II scales are reported to be good to excellent predictors of their associated DSM-5 diagnoses (Stasik-O’Brien et al., 2018). For the purposes of this study, checking symptom scores were derived from the checking subscale within the IDAS-II.
Procedure
Participants attended two laboratory visits: one during the mid-follicular phase and the other during the mid-luteal phase. There was an average of two weeks between visits (M = 15.24 days, SD = 3.85). All participants first provided written informed consent, and then completed self-report questionnaires. Participants then provided a salivary sample for hormone assay. All samples were assayed for salivary estradiol and progesterone using an enzyme immunoassay kit (Salimetrics, State College, PA). For estradiol assay, the test uses 100 ul of saliva, has a minimum detection limit of 0.1 pg/mL (range from 1 – 32 pg/mL), and average intra- and inter-assay variation coefficients were 7% and 6% respectively. There is minimal cross-reactivity to estriol and estrone, and no detected cross-reactivity with progesterone. For progesterone assay, 50 ul of saliva were collected. There is a minimum detection limit of 5 pg/mL (range from 10 – 2430) and average intra- and inter-assay coefficients of variation were 4% and 5.5% respectively. There is minimal cross-reactivity to corticosterone and no detected cross-reactivity to estradiol. After collection of the salivary sample and EEG setup, participants completed the flanker task (described below) while EEG was recorded. Participants completed additional EEG tasks in a random order and results from other tasks are presented elsewhere (Mulligan et al., 2018).
The arrowhead version of the flankers task was administered using Presentation software (Neurobehavioral Systems, Inc., Albany, CA, USA) and was similar to the version used in previous studies (Hajcak & Foti, 2008; Jackson et al., 2015). The task consisted of 330 trials presented over 11 blocks of 30 trials. On each trial, five horizontally aligned white arrowheads were presented for 200 ms. Participants were instructed to quickly indicate the direction of the central arrowhead using the left or right mouse button. Half the trials were compatible (e.g., < < < < < or > > > > >) and half were incompatible (e.g., < < > < < or > > < > >); trial type was randomly determined. Incompatible trials are often perceived as more difficult than compatible trials due to their incompatible flanking arrows. Thus, participants are more likely to make errors on incompatible trials. A variable inter-trial interval of 600 to 1000 ms followed the response. At the end of every block, participants received feedback based on their performance on the screen; if accuracy was at 75% or lower, the message “Please try to be more accurate” was displayed to increase attention to the task; when more than 90% of responses were correct, the message “please try to respond faster” was shown to increase the likelihood of the participant committing more errors; otherwise the message “You are doing a great job” was presented. On error trials, it is typically immediately apparent to the participant that they have just made a mistake. Thus, this task is designed to elicit error-related neural activity on trials where the participant chooses the direction of the central arrow erroneously.
EEG Recording and Processing
Continuous EEG was recorded using an elastic cap with 34 electrode sites placed according to the 10/20 system. Electrooculogram (EOG) was recorded using four additional facial electrodes: two placed approximately 1 cm outside of the right and left eyes, and two placed approximately 1 cm above and below the right eye. All electrodes were sintered Ag/AgCl electrodes. Data were recorded using the Active Two BioSemi system (BioSemi, Amsterdam, Netherlands). The EEG was digitized with a sampling rate of 1024 Hz using a low-pass fifth order sinc filter with a half-power cutoff of 204.8 Hz. A common mode sense active electrode producing a monopolar (i.e., nondifferential) channel was used as recording reference. EEG data were analyzed using Brain Vision Analyzer (Brain Products, Gilching, Germany). Data were referenced offline to the average of left and right mastoids and band-pass filtered (0.1 to 30 Hz, with a 24 dB/oct roll-off).
Response-locked epochs were extracted with a duration of 1,500 ms, including a 500 ms pre-response and 1000 ms post-response interval; these segments were then corrected for eye movement artifacts using a regression-based approach (Gratton, Coles, & Donchin, 1983). Epochs containing a voltage greater than 50 μV between sample points, a voltage difference of 300 μV within a segment, or a maximum voltage difference of less than 0.50 μV within 100 ms intervals were automatically rejected. Additional artifacts were identified and removed based on visual inspection. The −500 to −300 ms pre-response interval was used as the baseline. Response-locked ERPs were averaged separately for error and correct trials in the mid-follicular and midluteal phases. The number of trials per condition that remained after artifact rejection at the FCz electrode site were as follows: error follicular (M = 34.20, SD = 21.23), error luteal (M = 31.62, SD = 16.82), correct follicular (M = 286.42, SD = 34.86), and correct luteal (M = 282.80, SD = 34.56). No subjects were excluded from the sample for committing too few errors (i.e., all subjects committed six or more errors throughout the course of the task).
The ERN and correct-related negativity (CRN) were scored as the average voltage in the window from 0 to 100 ms after the response at electrode FCz. The ΔERN was calculated by subtracting the CRN from the ERN. The ΔERN represents the difference in neural activity between error and correct trials. We compute this subtraction-based difference score to isolate the error-related activity. Behavioral measures included the number of errors, as well as average reaction times (RTs) on error and correct trials.
Results
Phase-related differences in hormones, checking, and error-related brain activity.
Consistent with previous findings, levels of progesterone were higher during the midluteal phase, M = 356.19, SD = 194.41, compared to the mid-follicular phase, M = 150.96, SD = 83.33, t(37) = −7.58,p < .001. Additionally, levels of estradiol were also higher during the mid-luteal phase, M = 2.81, SD = .72, compared to the mid-follicular phase, M = 2.48, SD = .70, t(35) = −2.57,p < .05. Self-reported checking did not vary by phase, mid-luteal: M = 6.20, SD = 2.49, mid-follicular: M = 6.12, SD = 2.90, t(40) = −.19, p = .85.
To examine ERP activity, a repeated-measures ANOVA was conducted with response (error vs. correct) and phase (mid-luteal vs. mid-follicular) entered as within-subject variables. While the main effect of response (error vs. correct) was significant, F(1, 39) = 130.12,p < .001, neither phase (F(1, 39) = 2.24, p = .14) nor the interaction between phase and response (F(1, 39) = .006, p = .94) was significant. These results suggest that ERN was more negative than the CRN, and this effect did not vary by phase.
Associations between checking, error-related brain activity, and hormone levels
Checking symptoms did not correlate with progesterone during the follicular (r(38) = .06, p = .72) or the luteal phase (r(38) = .14, p = .39). Additionally, checking symptoms did not correlate with estradiol during the follicular (r(38) = .03, p = .87) or the luteal phase (r(38) = − .10, p = .53). We then examined whether error-related brain activity related to hormone levels during both the mid-luteal and mid-follicular phase. Results suggested that while the ΔERN measured during the mid-follicular phase was not related to estradiol (r(38) = −.12, p = .49) or progesterone (r(38) = −.07, p = .68) in the mid-follicular phase and the ΔERN measured during the mid-luteal phase was not related to estradiol in mid-luteal phase (r(38) = −.17, p = .32), the ΔERN measured during the mid-luteal phase was significantly related to levels of progesterone measured during the mid-luteal phase, r(38) = −35,p < .05. However, when we controlled for accuracy and reaction time (i.e., RT) during the flankers task, this relationship was no longer significant, r(34) = −.20, p = .26.
Phase-related differences in the association between checking and error-related brain activity.
We also examined the relationship between checking symptoms and the ΔERN during the mid-luteal and the mid-follicular phase. Results suggested that while the ΔERN measured during the mid-follicular phase was not related to checking symptoms measured in the mid-follicular (r(40) = −.23, p = .15) or the mid-luteal phase (r(40) = −.21, p = .20), the ΔERN measured during the mid-luteal phase was related to checking symptoms reported during the mid-luteal phase, r(40) = −.47,p < .01 and during the mid-follicular phase, r(40) = −.34,p < .05. These results suggest that the ERN and checking symptoms may be associated specifically during the midluteal phase, and not in the mid-follicular phase of the menstrual cycle.1
When we controlled for accuracy and RT during the flankers task, the relationship between the ΔERN measured during the mid-luteal phase and checking symptoms during the mid-luteal phase remained significant, r(36) = −.43,p < .01. However, the relationship between the ΔERN measured during the mid-luteal phase and checking symptoms during the mid-follicular phase was no longer significant, r(36) = −.25, p = .13. We depict the relationship between the ΔERN measured during the mid-luteal phase and checking during the mid-luteal phase in Figure 2: we conducted a median-split based on levels of checking during the mid-luteal phase (left; top = low checking; bottom = high checking). Waveforms for error, correct and the difference (error minus correct), as well as topographical head maps are also depicted (error minus correct, 0 – 100 ms). As can be seen in Figure 2, the ΔERN is larger (i.e., more negative) in individuals characterized by increased levels of checking during the mid-luteal phase. A scatter plot (Figure 2, right) depicts the relationship between the ΔERN during the mid-luteal phase and checking symptoms.
Figure 2.
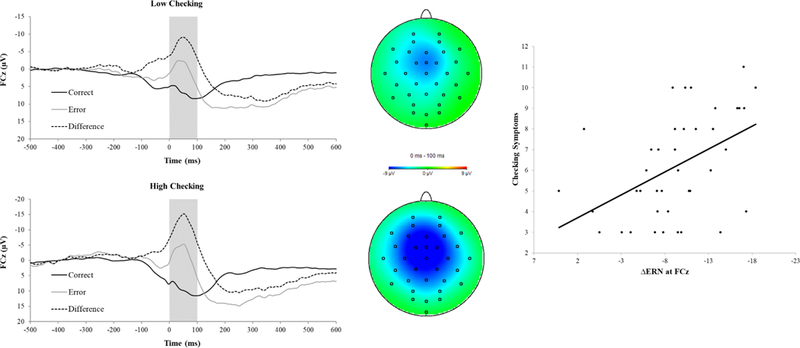
Response-locked ERPs (left) for error and correct trials measured during the mid-luteal phase, and corresponding topographic maps for the correct-error difference (middle) in individuals low (top) and high (bottom) in checking symptoms. In the mid-luteal phase, individuals with high checking symptoms showed a more negative ΔERN (i.e., the difference in amplitude between correct and error conditions) as compared to individuals with low checking symptoms. The scatter plot (right) depicts the association between ΔERN and checking symptoms in the mid-luteal phase and the line represents the line of best fit.
To further examine the specificity of the relationship between the ΔERN measured during the mid-luteal phase and checking, we z-scored and combined checking symptoms reported across both phases. We then conducted a simultaneous multiple regression wherein the both the ΔERN measured during the mid-luteal phase and the ΔERN measured during the mid-follicular phase were entered as predictors, and checking symptom scores were entered as the dependent variable. The regression model was significant (F(2, 39) = 4.90, p < .05) with an R2 of .21. Results suggested that while the ΔERN measured during the mid-follicular phase did not significantly predict checking symptoms, B = .08, t = .45, p = .66, the AERN measured during the mid-luteal phase significantly predicted checking symptoms, B = −.51, t = −2.63,p < .05. This suggests that the relationship between the ΔERN measured during the mid-luteal phase and checking symptoms was significant even when controlling for the ΔERN measured during the mid-follicular phase—that is, checking symptoms were predicted by variance in the ΔERN that is specific to the mid-luteal phase.
Changes in checking, hormones, and error-related brain activity across phases.
Although neither ΔERN nor checking scores varied overall between mid-follicular and mid-luteal phases, it is possible that intra-individual between-phase increases in ΔERN were related to phase-related changes in checking scores (e.g., if some participants increased in their checking, whereas others decreased in checking - and these were associated with a corresponding increase and decrease in ΔERN, respectively). Thus, as an exploratory analysis, we also examined the relationship between changes in checking symptoms and the ΔERN across phases by creating regression-based change scores. This method has been shown to be useful in calculating difference scores between conditions (Meyer, Lerner, De Los Reyes, Laird, & Hajcak, 2017). Specifically, checking symptoms during the mid-follicular phase were entered predicting checking symptoms during the mid-luteal phase, and the unstandardized residual scores were saved as a measure of change in checking symptoms across the two phases. A similar change score was computed for the ΔERN. As can be seen in Figure 3, which depicts a scatter plot of the relationship between changes in the ΔERN and changes in checking symptoms, individuals who experienced an increase in checking symptoms from the mid-follicular to the mid-luteal phase also were characterized by an increase in the ΔERN from the mid-follicular to the mid-luteal phase, r(40) = −.38, p < .05.
Figure 3.
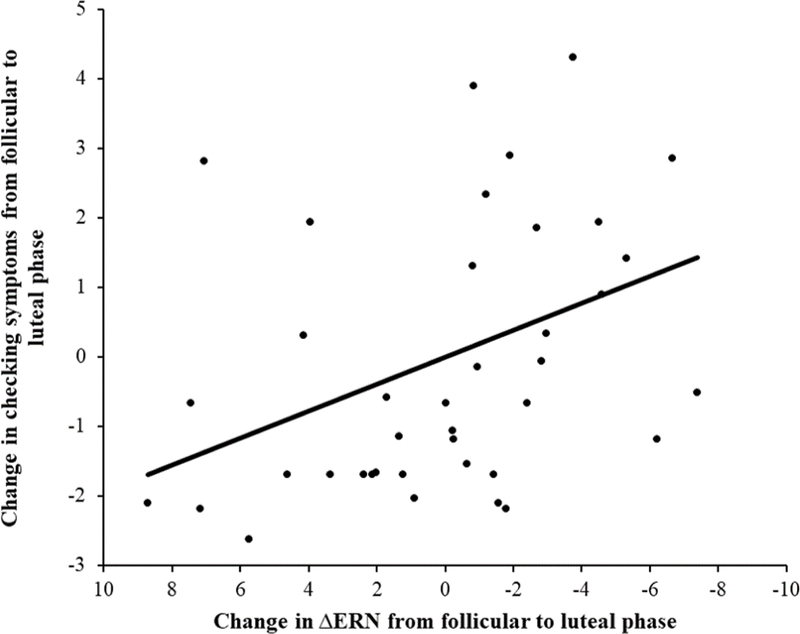
Scatter plot depicting the association between change in ΔERN from the mid-follicular to the mid-luteal phase, and change in checking symptoms from the mid-follicular to the mid-luteal phase. The line represents the line of best fit.
Mediation Models
Next, we examined an exploratory mediation model wherein the pathway between progesterone and checking symptoms was mediated by the ΔERN. We examined this model in both phases. For the mediation analyses, variables were entered into model 4 of the PROCESS Macro for SPSS (Preacher & Hayes, 2004). In the first mediation model (Figure 4), the direct path between progesterone during the mid-luteal phase and checking during the mid-luteal phase was not significant, effect = .00, SE = .01, t = .27, p = .79, 95% CI [−.0100 to .0130]. However, the path from progesterone to the ΔERN, as well as the path from the ΔERN to checking symptoms were both significant, effect = −.01, SE = .00, t = −2.33,p < .05, and effect = −.22, SE = .08, t = −2.88, p < .01, respectively. Additionally, results suggested significant mediation: the indirect path from progesterone to checking via the ΔERN measured during the mid-luteal phase reached significance, effect = .002, SE = .001, 95% CI [.0002 to .0046]. We examined this same model in the mid-follicular phase. In this model, none of the direct paths, nor the indirect path reached significance, all ps > .10.
Figure 4.
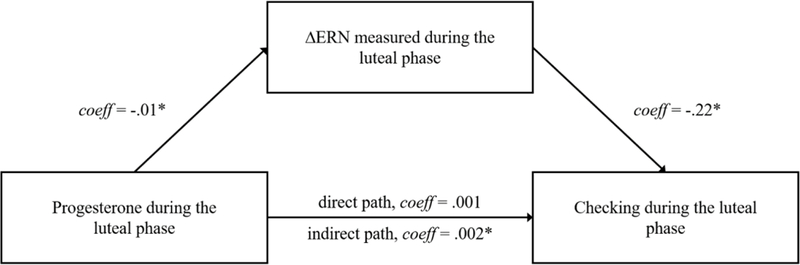
Schematic depicting the a significant mediation model in which the ΔERN measured in the mid-luteal phase mediates an indirect association between progesterone in the mid-luteal phase and checking symptoms in the mid-luteal phase.
Finally, we examined an exploratory mediation model wherein the pathway from estradiol and checking symptoms was mediated by the ERN, and we examined this model in both menstrual phases. In the first mediation model, the path between ΔERN in the mid-luteal phase and checking symptoms during the mid-luteal phase was significant, effect = −.23, SE = .07, t = −3.26, p < .01. However, no other direct paths, nor the indirect path reached significance, all ps > .10. Furthermore, in the mid-follicular phase, none of the direct paths, nor the indirect path reached significance, all ps > .10.
Discussion
The present study examined the impact of cyclic changes in ovarian hormones assessed during the mid-follicular and mid-luteal menstrual phases on associations between the ERN and checking symptoms. Results indicated that participants had higher levels of both estradiol and progesterone in the mid-luteal phase as compared to the mid-follicular phase, consistent with previous work demonstrating that levels of progesterone are low in the mid-follicular phase and high in the mid-luteal phase (Farage et al., 2008). This is also consistent with previous findings that levels of estradiol begin rising in the mid-follicular phase and are moderate in the mid-luteal phase (Farage et al., 2008).
The present study did not find overall differences in ERN or checking symptoms between menstrual phases. Results indicated that although the ΔERN measured during the mid-follicular phase was not related to checking symptoms measured in either phase, the ΔERN measured during the mid-luteal phase was related to checking symptoms reported during the mid-luteal phase, even when controlling for the ΔERN measured during the mid-follicular phase. In previous studies, estradiol and progesterone have been suggested to have opposing effects on emotional reactivity. Specifically, greater estradiol decreases responsiveness to negative stimuli while greater progesterone increases responsiveness to negative stimuli (Sakaki & Mather, 2012; Andreano et al., 2018). Thus, our results suggest that ERN and checking symptoms may be associated specifically during the mid-luteal phase of the menstrual cycle, and that hormonal profiles that naturally occur during the mid-luteal phase (i.e., greater progesterone relative to estradiol) may impact the severity of experienced checking symptoms by way of impacting neural systems linked to performance monitoring and error sensitivity. Consistent with this possibility, greater changes in checking symptoms between phases were associated with greater changes in the ΔERN between phases, suggesting that variability in the ERN between menstrual phases is associated with variability in checking symptoms between menstrual phases. Furthermore, a mediation analysis revealed that the ΔERN in the mid-luteal phase mediated the association between progesterone levels and checking symptoms in the mid-luteal phase. Thus, results from our mediation models indicate that progesterone may impact the intensity of checking symptoms by modulating neural sensitivity to errors.
Taken together, the present findings present novel evidence that associations between the ERN and checking symptoms may be impacted by menstrual cycle phase. The findings presented here bolster previous research suggesting the ERN is potentiated in individuals with elevated obsessive-compulsive symptoms (Hajcak & Simons, 2002; Santesso et al., 2006), and checking symptoms, specifically (Weinberg et al., 2015; Weinberg et al., 2016). The current findings build on previous research by suggesting that, in women, the ERN may only be associated with checking symptoms in the luteal phase of the menstrual cycle. Future studies in women of reproductive age may be able to account for more variance in associations between the ERN and checking symptoms by examining menstrual phase, or by assessing the ERN in the luteal phase of the menstrual cycle.
The present findings bear similarity to recent research from our group that revealed that greater variability in the neural response to monetary gains between the mid-follicular and midluteal phases was associated with greater depressive symptoms (Mulligan et al., 2018). Taken together, these studies suggest that hormonal influences inherent in the menstrual cycle may impact neurobiological processes underlying reward and error processing, which, in turn, may impact the expression of internalizing symptoms. Additionally, our present finding that change in ERN across phases predicted change in checking symptoms across phases also aligns with findings from the study by Mulligan and colleagues (2018) in that both studies observed a role for variability in neural signals across menstrual phases in impacting symptoms. This may imply that the change or fluctuation of hormone levels across phases, rather than absolute hormone levels, impact depressive and obsessive-compulsive symptoms. This may also explain why the late luteal or premenstrual phase, a phase characterized by falling levels of estradiol and progesterone, is a particularly vulnerable for increases in OCD and related behaviors. The premenstrual phase, which is characterized by steady decline of both hormones, may be a time of exacerbation of symptoms due to the marked withdrawal from hormones occurring in that time period. Future studies seeking to replicate and extend on these findings in clinical samples have potential to illuminate when it is most useful to assess neural mechanisms of risk. Additionally, the present study sets the stage for future work examining the impact of ovarian hormones and menstrual cycle phases on potential interventions and experimental manipulations
Contrary to previous studies which find exacerbation of OCD in the late-luteal phase (Vulink et al., 2006; Labad et al., 2005; Williams & Koran, 1997), the present study did not find significant differences in checking symptoms between mid-follicular and mid-luteal menstrual phases. This could be because the present study examined differences in checking symptoms between the mid-follicular and mid-luteal phases, as opposed to the late-luteal phase. It could also be because the present study utilized a non-clinical sample. Thus, future studies could extend the current work by examining the ERN in OCD patients and examining whether checking symptoms and amplitude of the AERN differ between the follicular and late-luteal phases. Moreover, the IDAS-II checking subscale assesses mean levels of checking symptoms over the past two weeks, which was the approximate duration between the two assessments. Thus, the IDAS may not have been sensitive to changes in checking symptoms that fluctuate more rapidly over the course of a menstrual cycle. Future studies could utilize self-report measures of checking symptoms with greater temporal precision via ecological momentary assessment to detect shorter-term variation in symptoms.
Additionally, the present study did not find significant differences in ERN amplitude between menstrual phases. This could also be due to the timing of our data collection within the menstrual cycle. Estradiol is low in the early-follicular phase and high in the late-follicular phase. Given that our follicular assessment took place in the mid-follicular phase, we may not have been able to observe the full impact of peaking estradiol in the late-follicular phase, which may have had greater effects on ERN amplitude. Finally, hormone levels did not directly relate to checking symptoms in the present study, nor did they relate to ΔERN amplitude after controlling for accuracy and reaction time. This could be due to our limited sample size, limited variability in change in estradiol levels between phase (M = −0.30, SD = .77), or that the present study utilized a non-clinical sample.
The present study had several limitations that warrant consideration. First, due to the undergraduate sample and exclusion criterion of hormonal contraceptive use, results may not generalize to older or younger female populations, or women who are on hormonal contraceptives. Future studies should examine whether the present pattern of findings are also evident in women on hormonal contraceptives and whether these effects appear in adolescence and persist through adulthood. Second, although hormone measures were used to verify that assessments of mid-follicular and mid-luteal phases were correctly timed, the current study did not include other biological indicators of menstrual cycle phase and relied on day count to time menstrual phases. Therefore, it’s possible that women were in other distinct hormonal periods of their follicular and luteal phases, which may have diluted the findings and limited our ability to see menstrual cycle effects on the ERN and checking symptoms. Accurate tracking of menstrual cycle phases can be done utilizing ovulation kits, which often involve urine sampling (Poromaa et al., 2014). Future studies may wish to employ multiple biological measures of menstrual phase to confirm accurate timing of assessments. Third, a limitation of the IDAS-II checking subscale is that the scale consists of only three items. Therefore, in addition to utilizing this or other scales of checking symptoms, future studies might consider incorporating a behavioral measure of checking propensity to allow for a multi-method assessment approach. Finally, the present study examined continuous checking symptoms in a non-clinical sample, and thus it will be important for future studies to examine the relevance of these findings in clinical samples.
In conclusion, the present study examined the impact of menstrual cycle phase and ovarian hormones on the ERN and checking symptoms and found that a more negative ERN was associated with greater checking symptoms in the mid-luteal phase of the menstrual cycle, even when controlling for ERN amplitude in the mid-follicular phase. Also, greater changes in checking symptoms between phases were associated with greater changes in the ΔERN between phases. Finally, the ΔERN in the mid-luteal phase mediated the association between progesterone levels and checking symptoms in the mid-luteal phase. Collectively, our findings suggest that the ERN and checking symptoms may be impacted by naturally-occurring hormonal variation related to the menstrual cycle, and that these hormonal changes may impact the severity of checking symptoms by modulating neural mechanisms associated with response monitoring and sensitivity to errors.
Highlights.
Larger error response (ERN) related to greater checking symptoms in the luteal phase.
Change in ERN and checking symptoms between menstrual phases were correlated.
Luteal ERN mediated links between luteal progesterone level and checking symptoms.
Hormonal fluctuations may impact checking symptoms via neural sensitivity to errors.
Acknowledgments
This work was supported by the following grant: NIMH T32 MH 093311.
Footnotes
Declarations of interest: none.
To correct for our number of t-tests and correlations, which consisted of 16 tests, we have employed the Benjamini-Hochberg step-up procedure (Benjamini & Hochberg, 1995) with a false discovery rate of .15. All comparisons and associations reported as significant survived this correction.
As with the original submission, there are no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramowitz JS, Khandker M, Nelson CA, Deacon BJ, & Rygwall R (2006). The role of cognitive factors in the pathogenesis of obsessive-compulsive symptoms: A prospective study. Behaviour Research and Therapy, 44(9), 1361–1374. [DOI] [PubMed] [Google Scholar]
- Abramowitz JS, Meltzer-Brody S, Leserman J, Killenberg S, Rinaldi K, Mahaffey BL, & Pedersen C (2010). Obsessional thoughts and compulsive behaviors in a sample of women with postpartum mood symptoms. Archives of women’s mental health, 13(6), 523–530. [DOI] [PubMed] [Google Scholar]
- Andreano JM, & Cahill L (2010). Menstrual cycle modulation of medial temporal activity evoked by negative emotion. NeuroImage, 53(4), 1286–1293. 10.1016/j.neuroimage.2010.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Touroutoglou A, Dickerson B, & Barrett LF (2018). Hormonal cycles, brain network connectivity, and windows of vulnerability to affective disorder. Trends in neurosciences, 41(10), 660–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avgoustinaki PD, Mitsopoulou E, Chlouverakis G, Triantafillou T, Venihaki M, Koukouli S, & Margioris AN (2012). Sex steroids and personality traits in the middle luteal phase of healthy normally menstruating young professional women. Hormones (Athens), 11(3), 333–343. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society. Series B (Methodological), 289–300. [Google Scholar]
- Carrasco M, Harbin SM, Nienhuis JK, Fitzgerald KD, Gehring WJ, & Hanna GL (2013). Increased error-related brain activity in youth with obsessive-compulsive disorder and unaffected siblings. Depression and Anxiety, 30(1), 39–46. [DOI] [PubMed] [Google Scholar]
- Cohen P, Cohen J, Kasen S, Velez CN, Hartmark C, Johnson J, … & Streuning EL (1993). An epidemiological study of disorders in late childhood and adolescence—I. Age- and gender-specific prevalence. Journal of child psychology and psychiatry, 34(6), 851–867. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Soares CN, Poitras JR, Prouty J, Alexander AB, & Shifren JL (2003). Short-Term Use of Estradiol for Depression in Perimenopausal and Postmenopausal Women: A Preliminary Report. American Journal of Psychiatry, 160(8), 1519–1522. 10.1176/appi.ajp.160.8.1519 [DOI] [PubMed] [Google Scholar]
- Endrass T, Klawohn J, Schuster F, & Kathmann N (2008). Overactive performance monitoring in obsessive-compulsive disorder: ERP evidence from correct and erroneous reactions. Neuropsychologia, 46(7), 1877–1887. [DOI] [PubMed] [Google Scholar]
- Endrass T, Riesel A, Kathmann N, & Buhlmann U (2014). Performance monitoring in obsessive-compulsive disorder and social anxiety disorder. Journal of Abnormal Psychology, 123(4), 705. [DOI] [PubMed] [Google Scholar]
- Endrass T, Schuermann B, Kaufmann C, Spielberg R, Kniesche R, & Kathmann N (2010). Performance monitoring and error significance in patients with obsessive-compulsive disorder. Biological psychology, 84(2), 257–263. [DOI] [PubMed] [Google Scholar]
- Farage MA, Osborn TW, & MacLean AB (2008). Cognitive, sensory, and emotional changes associated with the menstrual cycle: a review. Archives of gynecology and obstetrics, 278(4), 299. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, & Taylor SF (2005). Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biological psychiatry, 57(3), 287–294. [DOI] [PubMed] [Google Scholar]
- Forray A, Focseneanu M, Pittman B, McDougle CJ, & Epperson CN (2010). Onset and exacerbation of obsessive-compulsive disorder in pregnancy and the postpartum period. The Journal of clinical psychiatry, 71(8), 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Himle J, & Nisenson LG (2000). Action-monitoring dysfunction in obsessive-compulsive disorder. Psychological science, 11(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Gershuny BS, & Sher KJ (1995). Compulsive checking and anxiety in a nonclinical sample: Differences in cognition, behavior, personality, and affect. Journal of Psychopathology and Behavioral Assessment, 17(1), 19–38. [Google Scholar]
- Gratton G, Coles MG, & Donchin E (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55(4), 468–484. 10.1016/0013-4694(83)90135-9 [DOI] [PubMed] [Google Scholar]
- Guglielmi V, Vulink NC, Denys D, Wang Y, Samuels JF, & Nestadt G (2014). Obsessive-compulsive disorder and female reproductive cycle events: Results from the OCD and reproduction collaborative study. Depression and anxiety, 31(12), 979–987. [DOI] [PubMed] [Google Scholar]
- Hajcak G, & Foti D (2008). Errors are aversive: Defensive motivation and the error-related negativity. Psychological science, 19(2), 103–108. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Franklin ME, Foa EB, & Simons RF (2008). Increased error-related brainactivity in pediatric obsessive-compulsive disorder before and after treatment. American Journal of Psychiatry, 165(1), 116–123. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, & Simons RF (2003). Anxiety and error-related brain activity. Biological psychology, 64(1–2), 77–90. [DOI] [PubMed] [Google Scholar]
- Hajcak G, & Simons RF (2002). Error-related brain activity in obsessive-compulsive undergraduates. Psychiatry research, 110(1), 63–72. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Römmler J, Ehlis AC, Heidrich A, & Fallgatter AJ (2004). Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe). Cognitive Brain Research, 20(2), 294–299. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Dien J, & Coles MG (1998). Error-related scalp potentials elicited by hand and foot movements: evidence for an output-independent error-processing system in humans. Neuroscience letters, 242(2), 65–68. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Nager W, Rada D, Dengler R, Emrich HM, … & Dietrich DE (2001). Discrepant target detection and action monitoring in obsessive-compulsive disorder. Psychiatry Research: Neuroimaging, 108(2), 101–110. [DOI] [PubMed] [Google Scholar]
- Jackson F, Nelson BD, & Proudfit GH (2015). In an uncertain world, errors are more aversive: Evidence from the error-related negativity. Emotion, 15(1), 12. [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Morrow AL, Light KC, Pedersen CA, & Girdler SS (2006). Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology, 31(10), 1208–1219. 10.1016/j.psyneuen.2006.09.002 [DOI] [PubMed] [Google Scholar]
- Klawohn J, Endrass T, Preuss J, Riesel A, & Kathmann N (2016). Modulation of hyperactive error signals in obsessive-compulsive disorder by dual-task demands. Journal of abnormal psychology, 125(2), 292. [DOI] [PubMed] [Google Scholar]
- Labad J, Menchón JM, Alonso P, Segalàs C, Jiménez S, & Vallejo J (2005). Female reproductive cycle and obsessive-compulsive disorder. The Journal of clinical psychiatry, 66(4), 428–35. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, & Ryan ND (2006). Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry, 47(10), 1073–1082. [DOI] [PubMed] [Google Scholar]
- Lithgow BJ, & Moussavi Z (2017). Physiological Differences in the Follicular, Luteal, and Menstrual Phases in Healthy Women Determined by Electrovestibulography: Depression, Anxiety, or Other Associations?. Neuropsychobiology, 76(2), 72–81. [DOI] [PubMed] [Google Scholar]
- Meyer A (2016). Developing psychiatric biomarkers: A review focusing on the error-related negativity as a biomarker for anxiety. Current Treatment Options in Psychiatry, 3(4), 356–364. [Google Scholar]
- Meyer A, Lerner MD, De Los Reyes A., Laird RD, & Hajcak G (2017). Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology, 54(1), 114–122. [DOI] [PubMed] [Google Scholar]
- Mulligan EM, Nelson BD, Infantolino ZP, Luking KR, Sharma R, & Hajcak G (2018). Effects of menstrual cycle phase on electrocortical response to reward and depressive symptoms in women. Psychophysiology, e13268. [DOI] [PubMed] [Google Scholar]
- Nelson GH, O’Hara MW, & Watson D (2018). National norms for the expanded version of the inventory of depression and anxiety symptoms (IDAS-II). Journal of clinical psychology, 74(6), 953–968. [DOI] [PubMed] [Google Scholar]
- Poromaa IS, & Gingnell M (2014). Menstrual cycle influence on cognitive function and emotion processing from a reproductive perspective. Frontiers in Neuroscience, 8 10.3389/fnins.2014.00380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2004). SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers, 36(4), 717–731. 10.3758/BF03206553 [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, & Eisen JL (1992). The epidemiology and differential diagnosis of obsessive-compulsive disorder In Zwangsstorungen/obsessive-compulsive disorders (pp. 1–14). Springer, Berlin, Heidelberg. [Google Scholar]
- Riesel A, Endrass T, Kaufmann C, & Kathmann N (2011). Overactive error-related brain activity as a candidate endophenotype for obsessive-compulsive disorder: evidence from unaffected first-degree relatives. American Journal of Psychiatry, 168(3), 317–324. [DOI] [PubMed] [Google Scholar]
- Ross LE, & McLean LM (2006). Anxiety disorders during pregnancy and the postpartum period: A systematic review. The Journal of clinical psychiatry. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Grön G, Reuter K, Spitzer M, Hermle L, & Kiefer M (2005). Error-related brain activity in patients with obsessive-compulsive disorder and in healthy controls. Journal of Psychophysiology, 19(4), 298–304. [Google Scholar]
- Sakaki M, & Mather M (2012). How reward and emotional stimuli induce different reactions across the menstrual cycle. Social and personality psychology compass, 6(1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, & Schmidt LA (2006). Error-related electrocortical responses are enhanced in children with obsessive-compulsive behaviors. Developmental neuropsychology, 29(3), 431–445. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, & Davidson RJ (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience, 12(3), 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasik-O’Brien SM, Brock RL, Chmielewski M, Naragon-Gainey K, Koffel E, McDade-Montez E, … & Watson D (2018). Clinical Utility of the Inventory of Depression and Anxiety Symptoms (IDAS). Assessment, 1073191118790036. [DOI] [PubMed] [Google Scholar]
- Van Wingen GA, Van Broekhoven F, Verkes RJ, Petersson KM, Bäckström T, Buitelaar JK, & Fernandez G (2008). Progesterone selectively increases amygdala reactivity in women. Molecular psychiatry, 13(3), 325. [DOI] [PubMed] [Google Scholar]
- Vulink NC, Denys D, Bus L, & Westenberg HG (2006). Female hormones affect symptom severity in obsessive-compulsive disorder. International clinical psychopharmacology, 21(3), 171–175. [DOI] [PubMed] [Google Scholar]
- Walf AA, & Frye CA (2007). Estradiol decreases anxiety behavior and enhances inhibitory avoidance and gestational stress produces opposite effects. Stress, 10(3), 251–260. [DOI] [PubMed] [Google Scholar]
- Walf AA, & Frye CA (2010). Estradiol reduces anxiety-and depression-like behavior of aged female mice. Physiology & behavior, 99(2), 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, … & Ruggero CJ (2012). Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II). Assessment, 19(4), 399–420. [DOI] [PubMed] [Google Scholar]
- Watson D, O’hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, … & Stuart S (2007). Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychological assessment, 19(3), 253. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Klein DN, & Hajcak G (2012). Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. Journal of Abnormal Psychology, 121(4), 885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Kotov R, & Proudfit GH (2015). Neural indicators of error processing in generalized anxiety disorder, obsessive-compulsive disorder, and major depressive disorder. Journal of abnormal psychology, 124(1), 172. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Meyer A, Hale-Rude E, Perlman G, Kotov R, Klein DN, & Hajcak G (2016). Error-related negativity (ERN) and sustained threat: Conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology, 53(3), 372–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Olvet DM, & Hajcak G (2010). Increased error-related brain activity in generalized anxiety disorder. Biological psychology, 85(3), 472–480. [DOI] [PubMed] [Google Scholar]
- Williams KE, & Koran LM (1997). Obsessive-compulsive disorder in pregnancy, the puerperium, and the premenstruum. The Journal of clinical psychiatry, 58(7), 330–4. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Wang J, Zhang M, Li H, Tang Y, Wang Y, … & Fromson, J. A. (2011). Error-related negativity abnormalities in generalized anxiety disorder and obsessive-compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(1), 265–272. [DOI] [PubMed] [Google Scholar]


