Abstract
Background:
Several retrospective studies have suggested that transfusion with red blood cells (RBCs) stored for longer periods is associated with increased mortality. The Age of Blood Evaluation (ABLE) study randomized subjects to receive fresh vs. standard issue RBC units and showed no difference in the primary or secondary endpoints of mortality or change in multi-organ dysfunction syndrome (MODS) score.
Methods:
In this study a subset of 100 ABLE subjects were enrolled to measure coagulation and immune parameters. Samples were collected pre-transfusion and on days 2, 6, 28, and 180 post-transfusion. Levels of 16 coagulation parameters, regulatory and functional T cells, 25 cytokines, and 16 markers of extracellular vesicles (EVs) were determined.
Results:
Changes from baseline in levels of protein C, factor V, and EVs expressing phosphatidyl serine and CTLA-4 (CD152) differed between recipients of fresh and standard storage age RBC units, with the vast majority of coagulation and EV markers and all cytokines tested showing no difference between study arms. Although most analytes showed no difference between subjects in the fresh and standard arms of the study, 6 coagulation parameters, 15 cytokines, and 7 EV parameters changed significantly in the period post-transfusion.
Discussion:
Transfusion of fresh vs. standard issue RBC units does not result in substantial changes in coagulation or immune parameters, up to day 35 of RBC storage. Furthermore, significant changes in multiple coagulation and immune parameters are detectable post-transfusion, though causality cannot be determined based on the current study.
Introduction
Red blood cell (RBC) transfusion is a common therapeutic intervention. In 2013 there were 13.2 million units of RBCs and whole blood transfused in the US, with 12.5% of transfusion prescribed by intensive care medicine services.1 Transfusion related consequences may be due to transfusion transmitted diseases, immune mediated transfusion reactions, and circulatory overload. A potentially additional adverse consequence of transfusion is an RBC storage lesion effect, which is a controversial topic that has inspired multiple randomized, controlled trials (RCTs) over the last several years.
The RBC storage lesion has been thoroughly described and includes both RBC cellular effects and extracellular effects.2 The alterations in RBC energetics that influence the many biochemical changes that occur during storage have been well documented.2 Some of these RBC storage lesion extracellular effects include the development of RBC membrane extracellular vesicles (EVs) that have been shown to mediate immune and coagulation effects in vitro.2 The clinical relevance of RBC storage lesion effects has been debated,3,4 but recent trials in neonates and adult populations have not revealed any effect of RBC storage duration on clinical outcomes.5–9
The ABLE trial was one of the recently conducted trials that examined the effect of RBC storage age on mortality in critically ill adults7. In this RCT patients were randomized to either RBCs stored for ≤7 days (mean of 6.1 days) or to standard issue RBCs (mean of 22 days). To examine if RBC storage age affects immune and coagulation function and EV concentration in transfused critically ill adults, we performed an ancillary study of 100 subjects recruited within the ABLE trial. Our hypotheses were that patients transfused with RBCs in the standard issue arm would have increased pro-inflammatory cytokines, depressed T cell function, and a hypercoagulable profile, as well as increased EV’s of white blood cell, RBC, or platelet origin compared to patients transfused RBCs 7 days or less.
Methods
Study subjects
Participants in the ABLE trial were recruited to participate in longitudinal sampling of blood for this ancillary study from three ABLE clinical sites, the Institut de Cardiologie et de Pneumologie de Québec, Université Laval in Quebec City, the Ottawa General Hospital, and the Ottawa Civic Hospital. The first 100 eligible subjects across the sites were enrolled in the current study. Blood was collected pre-transfusion (day 0) and on days 2, 6, 28, and 180 post-transfusion. The day 0 sample was collected within a 12 hour time period prior to transfusion, and subsequent samples were collected on or within 1, 1, 3, and 21 days before or after the scheduled collection date, respectively. Informed consent was obtained for all participants included in the current study under an IRB approved protocol.
Sample acquisition
On day 0 (pre-transfusion), and days 6, 28, and 180 (post-transfusion), 7 ml blood was collected in EDTA and shipped at ambient temperature overnight to Vitalant Research Institute for Ficoll separation and processing into peripheral blood mononuclear cells (PBMCs) and platelet-rich plasma (PRP), which were then cryopreserved in the vapor phase of liquid nitrogen or at −80°C, respectively. The PBMC samples were used for cellular function assays, and the processing protocol is consistent with prior published work demonstrating retained function of cells cryopreserved after delayed processing.10 On days 0, 2, 6, and 28 additional samples were collected in citrate tubes (7.2 ml) and processed at the clinical site to create platelet-poor plasma (PPP). These samples were centrifuged at 1,500 g for 10 min at 20°C followed by centrifuging at 13,000 g for 10 min at 4°C. Aliquots of 0.5 ml PPP were frozen at −80°C and batch-shipped to Vitalant Research Institute for distribution and analysis of coagulation, cytokine, and EV parameters.
Measurement of cytokines and coagulation factors
To determine the inflammatory and coagulation profile of PPP, 16 coagulation factors and 27 cytokines were measured. Coagulation factors Va, VIIIa, VII, as well as antithrombin III, prothrombin time (PT), partial thromboplastin time (PTT), tissue plasminogen activator (TPA), D-dimer and protein C were measured on a Diagnostica Stago coagulation analyzer, while factor II, factor IX, and factor X were measured on a Dade Behring-Siemens device. Concentrations of prothrombin fragments 1+2, thrombomodulin, PAI-1, and soluble endothelial protein C receptor (EPCR) were measured using commercially available ELISAs kits. In addition, 25 cytokines were measured using Multiplex MAG kits (Millipore): granulocyte macrophage colony stimulating factor (GM-CSF), interferon (IFN)-γ, interleukin (IL)-12(p70), IL-17A, IL-1β, IL-2, IL-21, IL-23, IL-6, IL-7, IL-8, CXCL11/ITAC, CCL3/MIP-1α, CCL4/MIP-1β, tumor necrosis factor (TNF)-α, CXCL10/IP-10, epidermal growth factor (EGF), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), β2 microglobulin, cystatin C, myeloperoxidase (MPO), platelet derived growth factor (PDGF)-AB/BB, CCL5/RANTES, soluble intercellular adhesion molecule (sICAM)-1, and soluble vascular cell adhesion molecule (sVCAM)-1. A Bio-Plex 200 instrument (Bio-Rad) was used for cytokine data acquisition.
Characterization of Extracellular Vesicles
To characterize EVs, PFP samples were stained and acquired as previously described11 using 14 different fluorochrome-conjugated antibodies in three separate panels, including CD235a-FITC, CD62P-APC, CD3-PerCP/Cy5.5, CD19-Alexa/700, CD28-FITC, CD16-V450, CD62L-APC, CD11b-PE/Cy7, CD66-PE (Biolegend), CD15-FITC (ExAlpha), CD152-APC, CD14-APC/Cy7, CD108a-PE, and CD41a-PerCP/Cy5.5 (BD Biosciences). Samples were washed with a 0.22 μm centrifugal filter (Millipore) at 500g for 5 minutes. EVs were then harvested from the top of the filter after washing, and data were acquired by an LSR II flow cytometer (BD Biosciences). Analysis of data was performed using FlowJo 7.6.5 software (Tree Star).
Treg and effector T cell quantification
For Tregs, PBMCS were stained with LIVE/DEAD Fixable Aqua Dead Cell Stain (ThermoFisher) washed, and then stained with anti-CD4 AF700 and anti-CD25 PE-Cy7 (BD Biosciences). Cells were washed and fixed and permeabilized using a FoxP3 Fix/Perm Kit (eBioscience) and intracellularly stained with anti-CD3-Pacific Blue (BD Biosciences) and anti- FoxP3 FITC (eBioscience). For Th-17 cells, 106 PBMCs were rested overnight and then stimulated with PMA (75 ng/ml) and ionomycin (1 μg/ml, Sigma-Aldrich) and incubated for 1 hour before addition of GolgiStop (BD Biosciences) and brefeldin A (1 μg/ml, Sigma-Aldrich) for an additional 5-hour incubation. Cells were washed and stained with LIVE/DEAD Fixable Aqua Dead Cell Stain. Cells were then washed and stained with anti-CD4 AF700 and anti-CD8 APC/H7 (BD Biosciences). Cells were washed and then fixed and permeabilized using BD Cytofix/Cytoperm Kits then stained with anti-IFN-γ PE-Cy7 and anti-CD3 Pacific Blue (BD Biosciences), and IL-17 AF647 (eBioscience). Samples were acquired on a BD LSR II. A minimum of 200,000 events were recorded for each sample. Data were analyzed using FlowJo software (Treestar).
Statistical analyses
Subject demographic and clinical case characteristics between the fresh and standard arms were compared by t-test and chi-square tests. Analysis for differences between treatment arms was performed testing for group by time interaction in longitudinal analyses using mixed model repeated measures analysis of variance (ANOVA). All ANOVA analyses used an autoregressive covariance structure. Cytokine and EV data were log transformed prior to analysis. To determine which analytes changed significantly over time, the fresh and standard groups were analyzed separately using within-group repeated measures mixed model ANOVA, comparing the baseline value with each subsequent time point. Analytes were considered to have changed significantly if any of the follow-up time points differed from the baseline value by a two-sided p value of <0.05. False discovery rate (FDR) correction for multiple comparisons was performed using the method of Benjamini-Hochberg.12 Statistical analyses were performed using SAS software, version 9.4, with the exception of correction for multiple comparisons, which was performed using R software.
Results
Clinical cohort characteristics
The ancillary study of coagulation and immune parameters enrolled 100 subjects from the parent ABLE trial of 1430 subjects.7 Clinical outcomes were recorded in the parent ABLE trial, and the ancillary study investigators were blinded to subject randomization arm and clinical outcomes until the laboratory testing and reporting were complete. In the ancillary study 51 subjects were randomized to standard issue RBC units and 49 were randomized to receive blood stored less than 8 days (“fresh” blood) (Table 1A). The groups were balanced in terms of age, gender, Apache II score, and other potential confounders measured. The mix of subjects in the ancillary study was more heavily weighted to medical as opposed to surgical patients compared to the parent ABLE trial (96% vs. 71% medical, p=0.0001). As in the parent ABLE trial, there was no significant difference in clinical outcomes between the fresh and standard blood arms in the ancillary study (Table 1B). There was no significant difference in 28-day mortality between the subjects in the ancillary study and the parent ABLE trial, respectively (22% vs. 30%, p=0.12).
Table1A.
Cohort characteristics
| Variable | Group | ||
|---|---|---|---|
| Standard blood (N=49) |
Fresh blood (N=51) |
p value | |
| Median Age (years) | 66 | 68 | 0.80 |
| Female gender | 51.0% | 49.0% | 0.841 |
| Apache score (admission) | 21.8 ± 6.5 | 22.9 ± 6.5 | 0.373 |
| Glasgow coma score (admission) | 14.4 ± 1.3 | 13.8 ± 3.2 | 0.196 |
| Severe lung disease | 5.9% | 4.1% | 0.680 |
| Previous MI | 5.9% | 10.2% | 0.426 |
| Disabling stroke history | 3.9% | 4.1% | 0.967 |
| Chronic renal failure | 2.0% | 10.2% | 0.108 |
| Diabetes | 11.8% | 8.2% | 0.548 |
| Cancer history | 5.9% | 10.2% | 0.426 |
| Immuno-suppressive therapy | 9.8% | 14.3% | 0.490 |
| Deep vein thrombosis | 0.0% | 2.0% | 0.490 |
| Blood type: A | 37.2% | 38.8% |
0.959 |
| B | 9.8% | 10.2% | |
| AB | 5.9% | 8.2% | |
| O | 47.1% | 42.9% | |
| Hemoglobin | 74.8 ± 72.5 | 76.0 ± 73.7 | 0.437 |
| # of RBC transfusions (median) | 2 | 2 | 0.66 |
| FFP product | 17.6% | 18.4% | 0.925 |
| Platelet product | 17.6% | 14.3% | 0.647 |
| Cryoprecipitate product | 3.9% | 4.1% | 0.967 |
MI = myocardial infarction, FFP = fresh frozen plasma
Table 1B.
Clinical events between study arms
| Event rate (%) | p value | ||
|---|---|---|---|
| Fresh | Standard | ||
| Acute lung injury | 8.3 | 9.8 | 0.81 |
| ARDS | 8.3 | 5.9 | 0.64 |
| Pulmonary edema | 18.7 | 25.5 | 0.48 |
| Cardiovascular failure | 4.2 | 13.7 | 0.11 |
| Cardiac ischemia | 12.5 | 13.7 | 0.83 |
| Deep intravascular thrombosis | 6.2 | 5.8 | 0.92 |
| Severe sepsis | 4.2 | 5.8 | 0.71 |
| Septic shock | 6.2 | 3.9 | 0.56 |
| MODS | 14.6 | 7.8 | 0.27 |
| Pneumonia | 18.2 | 13.7 | 0.39 |
| Surgical site infection | 6.2 | 3.9 | 0.58 |
| Bacteremia | 10.3 | 2.0 | 0.07 |
| Cardiac arrest | 2.1 | 2.0 | 0.94 |
| Death | 22.9 | 21.6 | 0.83 |
ARDS = acute respiratory distress syndrome
Age of blood effect on coagulation factors
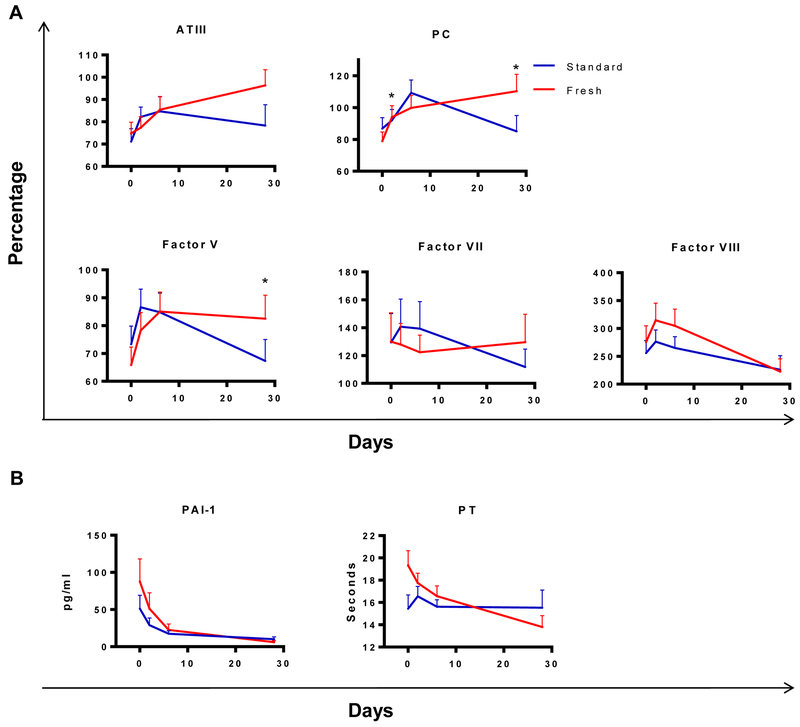
There were minimal differences between treatment arms in coagulation results measured longitudinally from day 0 to day 28. Of 16 coagulation parameters measured, factor V and protein C showed a significant difference between treatment arms, with factor V showing a more rapid decrease from day 6 to 28 in the standard arm and protein C showing a more rapid increase between days 0 and 2 in the fresh blood arm (Table 2, Figure 1A). If p values were FDR corrected none of the differences remained significant. Among the analytes that changed over time compared to day 0, the most common longitudinal pattern observed for the clotting factors was an increase from day 0 to day 2 (Figure 1A, supplemental Figure 1A), and these changes were seen in recipients of fresh and standard aged blood. Analytes that showed significant changes over time are shown in figures, and those that did not show significant changes over time are shown in supplemental figures. Finally, multiple analytes showed an initial decrease or a mixed pattern between groups with no consistent increase or decrease between days 0 and 2 (Figure 1B and supplemental Figure 1B).
Table 2.
Effects of RBC storage age on coagulation factors
| Group by day interaction p-value | Between group comparison of change from baseline to | |||
|---|---|---|---|---|
| 2 days | 6 days | 28 days | ||
| PT | 0.10 | |||
| PTT | 0.26 | |||
| D-dimer | 0.72 | |||
| Factor II | 0.19 | |||
| Factor V | 0.02 | 0.46 | 0.071 | 0.004 |
| FactorVII | 0.82 | |||
| Factor VIII | 0.4 | |||
| Factor IX | 0.68 | |||
| Factor X | 0.91 | |||
| AT-III | 0.44 | |||
| Protein C | 0.03 | 0.045 | 0.52 | 0.018 |
| Fibrinogen | 0.95 | |||
| Thrombomodulin | 0.99 | |||
| EPCR | 0.07 | |||
| TPA | 0.38 | |||
| PAI-1 | 0.98 | |||
Figure 1: Longitudinal analysis of coagulation parameters after transfusion.
Mean levels of coagulation parameters that changed significantly over time are shown on days 0 (pre-transfusion) and 2, 6, and 28 post-transfusion in recipients of fresh (red) and standard (blue) aged blood. (A) Values as a percentage of normal control plasma levels are shown for analytes that significantly increased after day 0. The analytes protein C and factor V showed a significant difference in the change from day 0 to one or more time points between recipients of fresh vs. standard blood. (B) Analytes that significantly decreased in at least one treatment arm after day 0 are shown. Error bars represent SEM. *p<0.05 comparing the difference in change from day 0 to day 2 or day 0 to day 28 between fresh and standard transfusion arms.
Age of blood effect on cellular immune and cytokine expression
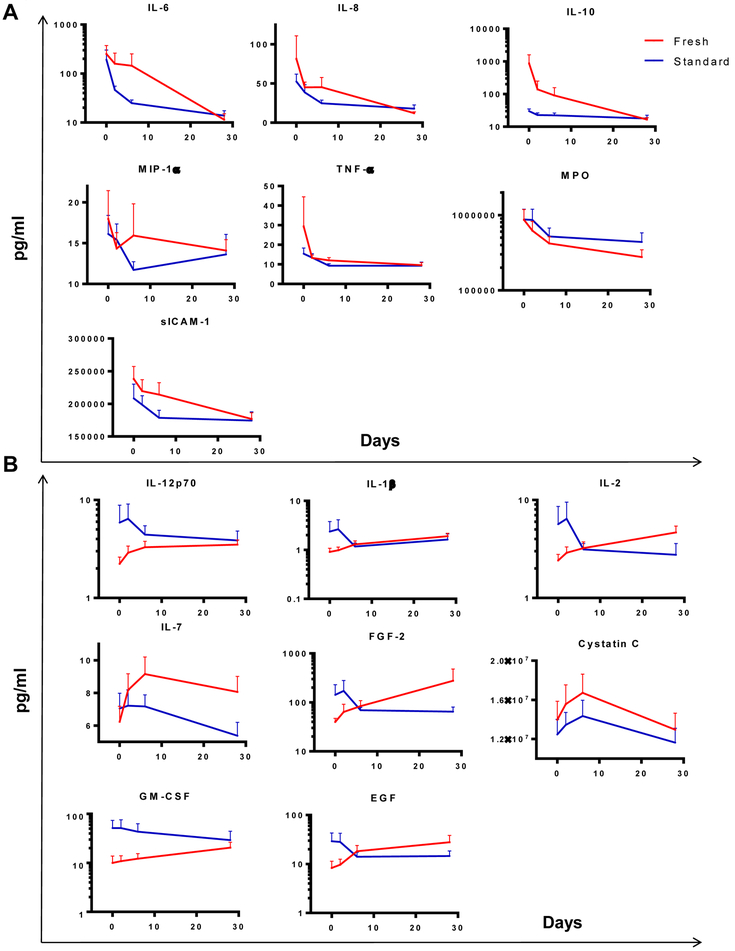
There was no significant difference over time in Treg levels, in the capacity of CD4+ T cells to secrete IL-17, or of CD8+ T cells to secrete IFN-γ in response to mitogen stimulation (supplemental Table 1, supplemental Figure 2A). Of 25 cytokines measured, none significantly differed at any time points between the fresh and standard blood arms (supplemental Table 1). Though not different between groups, levels of multiple cytokines changed significantly over time compared to day 0. The most common pattern of responses showed a decrease in predominantly pro-inflammatory cytokines post-transfusion (Figure 2A, supplemental Figure 2B), with the remainder showing an increase or mixed responses post-transfusion (Figure 2B, supplemental Figure 2C).
Figure 2: Longitudinal analysis of cytokine levels after transfusion.
Mean levels of analytes are shown on days 0 (pre-transfusion) and 2, 6, and 28 post-transfusion in recipients of fresh (red) and standard (blue) aged blood. (A) Cytokines that decreased significantly after day 0 are shown. (B) Cytokines that significantly increased after day 0 are shown. Error bars represent SEM.
Age of blood effect on extracellular vesicles
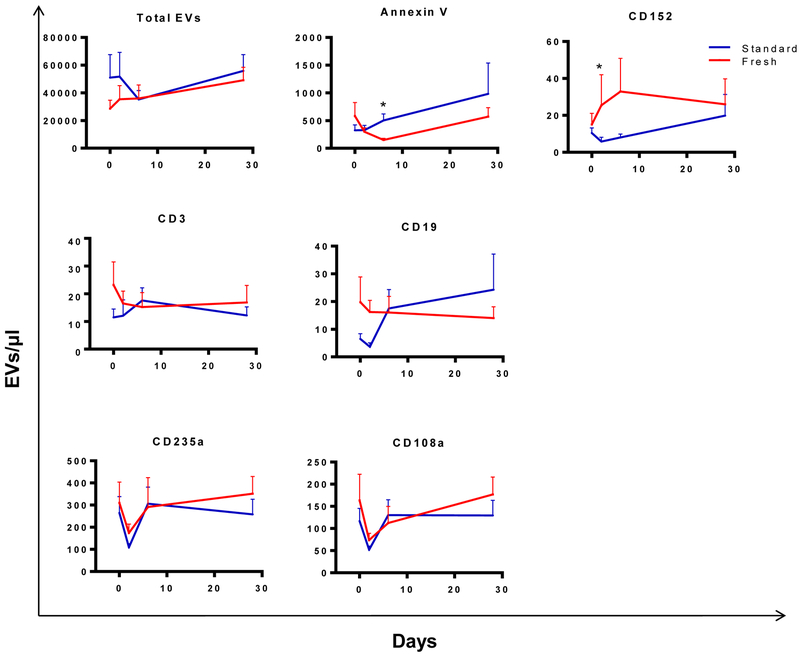
Total EV concentrations did not differ between the two treatment arms (Table 3). EVs expressing phosphatidyl serine bind annexin V, and these decreased more between days 0 and 6 in recipients of standard compared to fresh blood (Figure 3). In addition, EVs expressing the immune checkpoint molecule CD152 (CTLA-4) diverged post-transfusion in the two treatment arms, with CD152+ EVs rising in the fresh arm and falling in the standard blood arm. These differences did not remain significant after correction for multiple comparisons. The remaining EV markers showed no difference over time between treatment arms. EVs derived from B cells (CD19), T cells (CD3), and RBCs (CD235a, CD108a) all changed significantly post-transfusion compared to day 0, with a pattern of an early decrease followed by recovery (Figure 3). EVs bearing markers for granulocytes (CD15, CD66b) and platelets (CD41a, CD62P) did not change significantly after transfusion, though they showed the same pattern of an early decrease post-transfusion (supplemental Figure 3).
Table 3.
Effects of RBC storage age on extracellular vesicles
| Group by day interaction p-value |
Between group comparison of change from baseline to | |||
|---|---|---|---|---|
| 2 days | 6 days | 28 days | ||
| EV concentration | 0.84 | |||
| Annexin V | 0.005 | 0.99 | 0.03 | 0.4 |
| CD3 | 0.07 | |||
| CD14 | 0.2 | |||
| CD16 | 0.58 | |||
| CD19 | 0.006 | 0.058 | 0.21 | 0.69 |
| CD28 | 0.06 | |||
| CD152 | 0.02 | 0.004 | 0.08 | 0.7 |
| CD41a | 0.57 | |||
| CD62L | 0.23 | |||
| CD108a | 0.22 | |||
| CD235a | 0.03 | 0.97 | 0.11 | 0.18 |
| CD11b | 0.85 | |||
| CD15 | 0.76 | |||
| CD62P | 0.36 | |||
| CD66b | 0.95 | |||
Figure 3: Longitudinal analysis of extracellular vesicle levels after transfusion.
Mean levels of EV parameters that changed significantly over time are shown. EVs staining with annexin V or anti-CD152 differed between the fresh and standard RBC arms at day 2 post-transfusion. Error bars represent SEM. *p<0.05 comparing the difference in change from day 0 to day 2 between fresh and standard transfusion arms.
Post-transfusion coagulation and immune parameters correlated with mortality and organ dysfunction
A secondary aim of the ABLE ancillary study was to identify changes between baseline and day 2 in coagulation or immune parameters that would predict mortality or organ dysfunction in these critically ill patients. Both treatment arms were combined for Cox regression analysis of whether or not post-transfusion fluctuation in coagulation or immune markers predicted mortality or MODS. Because of the potential impact of group assignment on the two outcome measures of interest (mortality and MODS), all p-values were adjusted for the study group. Increases in IFN-γ+ CD8+ T cells, IL-1β, EGF and decreases in EVs expressing the granulocyte markers CD11b and CD66b were associated with increased mortality. Increases in GM-CSF, IL-2, IL-17A, β2-microglobulin, and cystatin C, and decreases in D-dimer, factor II, IL-12p70, IL-23, and CD11b EVs levels were associated with higher MODS (Table 4).
Table 4.
Changes in factors associate with mortality or MODS
| Predictors of mortality | Predictors of MODS | |||
|---|---|---|---|---|
| p-value | HR (cb) | p-value | HR (cb) | |
| D-dimer | 0.005 | 0.69 (0.54 – 0.90) | ||
| Factor II | 0.007 | 0.99 (.98 – 0.99) | ||
| CD8+IFN-γ+ | 0.03 | 1.07 (1.01 – 1.14) | ||
| GM-CSF | 0.036 | 1.01 (1.00 – 1.03) | ||
| IL-12p70 | 0.0006 | 0.69 (0.55 – 0.85) | ||
| IL-17A | 0.01 | 1.08 (1.02 – 1.15) | ||
| IL-1β | 0.009 | 1.75 (1.15 – 2.66) | ||
| IL-2 | 0.018 | 1.21 (1.03 – 1.42) | ||
| IL-23 | 0.041 | 0.99 (0.98 – 1.00) | ||
| EGF | 0.017 | 1.03 (1.00 – 1.06) | ||
| β2 microglobulin | 0.022 | 1.04 (1.00 – 1.07) | ||
| Cystatin C | 0.001 | 1.16 (1.06 – 1.28) | ||
| EV CD11b | <0.0001 | 0.79 (0.70 – 0.88) | 0.0006 | 0.99 (0.99 – 0.99) |
| EV CD66b | 0.007 | 0.99 (0.99 – 0.99) | ||
HR: hazard ratio, cb: confidence bounds. HRs reflect the change in the hazard that is associated with the following magnitude of change in particular variables:
10 units of change: GM-CSF, IL-23, and EGF
100 units of change: EV CD11b and CD66b
1 million units of change: Cystatin C
10 million units of change: β2 microglobulin
Discussion
In this study a large panel of coagulation and immune parameters were measured in a subset of ABLE trial participants. The goal of the study was to identify laboratory parameters that might differ between randomization arms in the ABLE study participants, and we hypothesized that recipients of older RBCs would show increased levels of soluble markers of inflammation and coagulation compared to recipients of fresh RBCs. The parent ABLE trial found no difference in clinical outcome between recipients of fresh and standard issue RBC units. Similar to the clinical findings, we found no differences in cytokine levels between recipients of fresh vs. standard aged RBC units, and minimal differences in coagulation marker and EV levels that did not remain significant after correction for multiple comparisons. Although there were no appreciable changes between the study arms, significant modulation of coagulation parameters, cytokines, and EV subsets was observed. Coagulation factors showed a mixed picture, with some analytes increasing post-transfusion, but the majority showing no significant change over time. Analysis of regulatory T cells and effector CD4+ and CD8+ T cells showed no significant change over time post-transfusion. The most common pattern of cytokine responses was a decrease in pro-inflammatory cytokines post-transfusion, though multiple cytokines showed no change or a modest increase post-transfusion. Finally EV levels were more consistent, with most showing a decrease two days post-transfusion with subsequent recovery in levels.
Transfusion has been postulated to have an immune modulatory effect,13 and several pre-clinical and observational clinical studies have suggested that older blood accentuates transfusion-related immune modulation (reviewed in14). In the ABLE trial only transfused subjects were enrolled, so it was not possible to compare transfused vs. non-transfused subjects. In addition, critical illness is known to induce both a pro- and anti-inflammatory immune responses.15–17 The data in the current manuscript provide important information about which parameters might be affected by transfusion, though conclusive evidence of which changes were caused by transfusion would require study of similar subjects randomized to receive transfusion or not. Of note, measures of effector and regulatory T cells did not show significant changes from baseline when measured at 180 days (Supplemental Figure 2A), which argues against a prolonged systemic suppression of adaptive immune responses after transfusion.
Interest in EVs as an immune mechanism has increased with the evolving ability to measure these sub-cellular particles, and it is now possible to determine the cell of origin of EVs by staining for cell surface markers.18,19 It is known that the content of EVs in RBC units changes with blood storage.19–22 We hypothesized that it would be unlikely that RBC unit-derived EVs would be detectable in the circulation of transfusion recipients, both due to dilution in the recipient and data from mouse models showing that these particles are rapidly cleared by the reticuloendothelial system.23,24 However, the study design did allow testing of whether presumably endogenously generated EVs were differentially modulated after receipt of fresh vs. standard aged blood. We predicted that RBC-derived EVs would increase post-transfusion, but on the contrary we found a decrease in the RBC markers CD235a (expressed on all RBCs) and CD108a (expressed on activated RBCs). The EV staining panels included more than one marker specific for a given cell type, such as CD41a and CD62P for platelets and CD15 and CD66b for granulocytes. Each of these marker pairs showed concordant changes in EV concentration, increasing confidence that the flow cytometry assay was accurately detecting EVs derived from a given cell type.
The study includes a number of strengths, including the randomized study design, standardized sample processing and testing in each of the participating laboratories, and robust statistical analysis. However, there are several limitations that should be noted. The main diagnoses for the population studied were primarily medical, so the findings of this study may not apply to surgical patients. This study only assessed transfused patients, so no conclusions can be drawn about the safety of RBC transfusion. A large number of analytes were studied, increasing the chance that changes seen were due to chance. However, given that the primary comparison showed no difference between arms for the vast majority of analytes studied, we feel confident that the storage age of blood in the range studied did not have large effects on coagulation or immune parameters. The fresh vs. standard issue arms of the trial measured the effect of storage age, but only one unit of blood transfused to the standard blood recipients was stored >35 days in the current study. Accordingly, these study results do not shed light on whether or not blood stored for >35 days could modulate coagulation or immune parameters. Recent data demonstrated that blood stored >35 days causes increased non-transferrin bound iron in healthy control subjects, so the effects of blood storage >35 days is still an active research question.25
In summary, measurement of a broad array of coagulation, cytokine, and EV parameters revealed very few differences between recipients of stored vs. fresh blood, concordant with the lack of clinical difference seen in the parent randomized clinical trial. A pattern of immune modulation was seen in these critically ill subjects after ICU admission and transfusion, and future studies will be needed to determine the relative roles of transfusion and critical illness itself in affecting recipient immune responses.
Supplementary Material
Acknowledgments
We thank the study coordinators at each clinical site, Irene Watpool, Tracy McArdle, and Marie-Claude Ferlan. This study was supported by U.S. Department of Defense contracts W81XWH-10–1-0023, W81XWH-2–0028 and NHLBI R01 HL095470.
Footnotes
Conflict of interest: None
References
- 1.The 2103 AABB Blood Collection, Utilization, and Patient Blood Management Survey Report. AABB; 2015.
- 2.Spinella PC, Doctor A. Role of transfused red blood cells for shock and coagulopathy within remote damage control resuscitation. Shock Augusta Ga. 2014;41 Suppl 1:30–34. [DOI] [PubMed] [Google Scholar]
- 3.Tobian AAR, Ness PM. Red Cells - Aging Gracefully in the Blood Bank. N. Engl. J. Med 2016; [DOI] [PubMed] [Google Scholar]
- 4.Glynn SA, Klein HG, Ness PM. The red blood cell storage lesion: the end of the beginning. Transfusion (Paris). 2016;56(6):1462–1468. [DOI] [PubMed] [Google Scholar]
- 5.Fergusson DA, Hebert P, Hogan DL, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308:1443–51. [DOI] [PubMed] [Google Scholar]
- 6.Steiner ME, Ness PM, Assmann SF, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N. Engl. J. Med 2015;372(15):1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lacroix J, Hébert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N. Engl. J. Med 2015;372(15):1410–1418. [DOI] [PubMed] [Google Scholar]
- 8.Heddle NM, Cook RJ, Arnold DM, et al. Effect of Short-Term vs. Long-Term Blood Storage on Mortality after Transfusion. N. Engl. J. Med 2016;375(20):1937–1945. [DOI] [PubMed] [Google Scholar]
- 9.Cooper DJ, McQuilten ZK, Nichol A, et al. Age of Red Cells for Transfusion and Outcomes in Critically Ill Adults. N. Engl. J. Med 2017; [DOI] [PubMed] [Google Scholar]
- 10.Lanteri MC, Heitman JW, Owen RE, et al. Comprehensive analysis of west nile virus-specific T cell responses in humans. J Infect Dis. 2008;197:1296–306. [DOI] [PubMed] [Google Scholar]
- 11.Inglis HC, Danesh A, Shah A, et al. Techniques to improve detection and analysis of extracellular vesicles using flow cytometry. Cytom. Part J. Int. Soc. Anal. Cytol 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc 1995;B 57:289–300. [Google Scholar]
- 13.Vamvakas EC, Blajchman MA. Deleterious clinical effects of transfusion-associated immunomodulation: fact or fiction? Blood. 2001;97:1180–95. [DOI] [PubMed] [Google Scholar]
- 14.Muszynski JA, Spinella PC, Cholette JM, et al. Transfusion-related immunomodulation: review of the literature and implications for pediatric critical illness. Transfusion (Paris). in press; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. J. Exp. Med 2011;208(13):2581–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackman RP, Utter GH, Muench MO, et al. Distinct roles of trauma and transfusion in induction of immune modulation after injury. Transfusion (Paris). 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muszynski JA, Thakkar R, Hall MW. Inflammation and innate immune function in critical illness. Curr. Opin. Pediatr 2016;28(3):267–273. [DOI] [PubMed] [Google Scholar]
- 18.Vagida M, Arakelyan A, Lebedeva A, et al. Flow analysis of individual blood extracellular vesicles in acute coronary syndrome. Platelets. 2016;1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danesh A, Inglis HC, Jackman RP, et al. Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood. 2014;123(5):687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veale MF, Healey G, Sparrow RL. Effect of additive solutions on red blood cell (RBC) membrane properties of stored RBCs prepared from whole blood held for 24 hours at room temperature. Transfusion (Paris). 2011;51 Suppl 1:25S–33S. [DOI] [PubMed] [Google Scholar]
- 21.Almizraq R, Tchir JD, Holovati JL, Acker JP. Storage of red blood cells affects membrane composition, microvesiculation, and in vitro quality. Transfusion (Paris). 2013; [DOI] [PubMed] [Google Scholar]
- 22.Grisendi G, Finetti E, Manganaro D, et al. Detection of microparticles from human red blood cells by multiparametric flow cytometry. Blood Transfus. Trasfus. Sangue 2015;13(2):274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai CP, Mardini O, Ericsson M, et al. Dynamic Biodistribution of Extracellular Vesicles in Vivo Using a Multimodal Imaging Reporter. ACS Nano. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saunderson SC, Dunn AC, Crocker PR, McLellan AD. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014;123(2):208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rapido F, Brittenham GM, Bandyopadhyay S, et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. J. Clin. Invest 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.