Abstract
Objective
Postural instability and gait difficulties (PIGDs) represent debilitating disturbances in Parkinson's disease (PD). Past acetylcholinesterase positron emission tomography (PET) imaging studies implicate cholinergic changes as significant contributors to PIGD features. These studies were limited in quantification of striatal cholinergic synapse integrity. Vesicular acetylcholine transporter (VAChT) PET ligands are better suited for evaluation of high binding areas. We examined associations between regional VAChT expression and freezing of gait (FoG) and falls.
Methods
Ninety‐four PD subjects underwent clinical assessment and VAChT ([18F]FEOBV) PET.
Results
Thirty‐five subjects (37.2%) reported a history of falls, and 15 (16%) had observed FoG. Univariate volume‐of‐interest analyses demonstrated significantly reduced thalamic (p = 0.0016) VAChT expression in fallers compared to nonfallers. VAChT expression was significantly reduced in the striatum (p = 0.0012) and limbic archicortex (p = 0.004) in freezers compared to nonfreezers. Whole‐brain voxel‐based analyses of FEOBV PET complemented these findings and showed more granular changes associated with falling history, including the right visual thalamus (especially the right lateral geniculate nucleus [LGN]), right caudate nucleus, and bilateral prefrontal regions. Freezers had prominent VAChT expression reductions in the bilateral striatum, temporal, and mesiofrontal limbic regions.
Interpretation
Our findings confirm and extend on previous PET findings of thalamic cholinergic deficits associated with falling history and now emphasize right visual thalamus complex changes, including the right LGN. FoG status is associated with reduced VAChT expression in striatal cholinergic interneurons and the limbic archicortex. These observations suggest different cholinergic systems changes underlying falls and FoG in PD. Ann Neurol 2019;85:538–549
Advancing Parkinson's disease (PD) is associated with debilitating postural instability and gait difficulties (PIGDs), such as falls and freezing of gait (FoG).1 The Sydney Multicenter Study of PD found that dopamine nonresponsive PIGDs dominate motor function 15 years after initial assessments and includes frequent falls, occurring in 81% of subjects.2 Another incident cohort reported that 68% of PD subjects exhibited postural instability at 10‐year follow‐up.3 Dopaminergic medication “on” freezing has been reported in 38% of a large series of subjects with PD.4
Absent dopaminergic therapy responses implicates nondopaminergic mechanisms in worsening PIGD motor features. Major populations of central nervous system cholinergic neurons include the basal forebrain (BF) complex, the brainstem pedunculopontine nucleus/lateral dorsal tegmental complex (PPN/LTDC), and striatal cholinergic interneurons. We previously associated PPN/LTDC‐thalamic and BF corticopetal cholinergic projection system degeneration with falls and slow gait speed in PD, respectively.5, 6 Using dopaminergic, acetylcholinesterase (AChE) and β‐amyloid positron emission tomography (PET) imaging, we also reported reduced striatal dopaminergic terminals, reduced diffuse cortical cholinergic terminals, and more severe cortical amyloidopathy in PD freezers compared to nonfreezers.7
Our previous AChE PET imaging studies were limited because of the ligand's inability to reliably estimate tracer hydrolysis rates in high binding areas, such as the striatum or cerebellum.8 This limits identification of potentially relevant fall‐ or FoG‐associated markers.9 [18F]‐FEOBV is a PET radioligand that selectively binds to the vesicular acetylcholine transporter (VAChT).10 An advantage of [18F]‐FEOBV PET is that ligand binding in regions with high cholinergic terminal density can be more accurately estimated.11
The objective of this study is a detailed in vivo examination of regional cerebral, including cortical and subcortical, VAChT expression in PD subjects with PIGD motor features. We hypothesized that distinct distributed patterns of subcortical and cortical cholinergic projection system changes are associated with FoG and falls in PD. Based on our previous AChE studies, we hypothesized a central role for thalamic involvement for falls and cortical changes underlying FoG.
Patients and Methods
Subjects and Clinical Test Battery
This study involved 94 subjects with PD (72 males; 22 females), mean age 67.9 ± 7.6 (standard deviation [SD]; range, 51–93) years. PD subjects met the UK Parkinson's Disease Society Brain Bank clinical diagnostic criteria.12 Subjects with evidence of large vessel stroke or other intracranial lesions on anatomic imaging were excluded. The International Parkinson and Movement Disorder Society revised UPDRS (MDS‐UPDRS) motor examination was performed in the morning in the dopaminergic medication ‘off’ state.
Subjects completed the Montreal Cognitive Assessment (MOCA) with mean score of 26.2 ± 3.3 (range, 10–30).13 Mean duration of disease was 6.0 ± 4.5 (range, 1–30) years. The mean motor examination score on the MDS‐UPDRS was 33.9 ± 13.8 (range, 2–74).14
Thirty‐one PD subjects were taking a combination of dopamine agonist and carbidopa‐levodopa medications, 47 were using carbidopa‐levodopa alone, 10 were taking dopamine agonists alone, and 6 were not receiving dopaminergic drugs. Mean levodopa equivalent dose (LED) was 655.5 ± 397.8 mg (range, 0.0–1,902.5).15 No subjects were treated with anticholinergic or cholinesterase inhibitor drugs. Most subjects had moderate severity of disease: 6 subjects in stage 1, 3 in stage 1.5, 20 in stage 2, 40 in stage 2.5, 20 in stage 3, and 5 in stage 4 of the modified Hoehn and Yahr classification with mean stage of 2.45 ± 0.60.
Fall and FoG Assessment
Participants were asked about a history of falling. A fall was defined as an unexpected event during which a person falls to the ground. The presence or absence of FoG was based on clinical examination and directly observed by the clinician examiner based on a nonzero score on item 3.11 “Freezing of Gait” of the MDS‐UPDRS motor examination.14 For most reliable assessment, FoG classification should be based upon objective confirmation by an experienced observer during clinical assessment rather than on patient recollection.16 This study was approved by the Institutional Review Boards of the University of Michigan School of Medicine and Veterans Affairs Ann Arbor Healthcare System. Written informed consent was obtained from all subjects.
Imaging Techniques
Magnetic resonance imaging (MRI) was performed on a 3 Tesla Philips Achieva system (Philips, Best, The Netherlands). A three‐dimensional (3D) inversion recovery‐prepared turbo field echo was performed in the sagittal plane using repetition time/echo time/inversion time = 9.8/4.6/1041 ms; turbo factor = 200; single average; field of view (FOV) = 240 × 200 × 160 mm; acquired matrix = 240 × 200 × 160 slices; and reconstructed to 1‐mm isotropic resolution.
PET imaging was performed in 3D imaging mode with a Siemens ECAT Exact HR+ tomograph or Biograph 6 TruPoint PET/computed tomography scanner (Siemens Molecular Imaging, Inc., Knoxville, TN), which acquire 63 transaxial slices (slice thickness: 2.4 mm) over a 15.2‐cm axial FOV. Images were corrected for scatter and motion. Subjects were scanned in the dopaminergic medication “on” state.
[18F]FEOBV was prepared as described previously.17, 18 [18F]‐FEOBV delayed dynamic imaging was performed over 30 minutes (in six 5‐minute frames) starting 3 hours after an intravenous bolus dose injection of 8 mCi [18F]‐FEOBV.11 PET imaging frames were spatially coregistered within subjects with a rigid‐body transformation to reduce the effects of subject motion during the imaging session.19 Statistical parametric mapping (SPM) software (SPM12; Wellcome Trust Centre for Neuroimaging, University College, London, England [https://www.fil.ion.ucl.ac.uk/spm/software/spm12/]) was used for PET‐MRI registration using the cropped T1‐weighted MR volumetric scan. Freesurfer software (http://surfer.nmr.mgh.harvard.edu) was used to define cortical and subcortical MR gray volumes of interest (VOIs).
A white matter reference tissue approach was used to determine VAChT binding as previously reported.20, 21 This approach departs from previously used cerebellar gray matter reference regions.11 This is appropriate as cholinergic terminal changes may occur in cerebellar cortices in parkinsonian disorders,22 which may potentially bias findings using this reference region in these particular patient populations. Distribution volume ratios (DVRs) were calculated from ratio of summed six delayed imaging frames (3 hours after injection) for gray matter target and white matter reference tissues.21
VOI and Voxel‐Based Brain PET Analysis
VOI and voxel‐based methods provide complementary information. The following bilaterally averaged VOIs were defined for the striatum (putamen and caudate nucleus), thalamus, cerebellar gray matter, brainstem, limbic archicortex (hippocampus and amygdala), and neocortex based on combination of individual labels from the Mindboggle‐101 data set segmented in FreeSurfer.
Complementary whole‐brain voxel‐based [18F]FEOBV PET analyses were performed to explore more granular regional brain VAChT binding changes that may not be captured or missed by predefined VOIs. Voxel‐based statistical analysis was performed using SPM12 software on the parametric [18F]FEOBV DVR images of all subjects. For SPM analysis, all brain images were spatially normalized to Montreal Neurological Institute template space using DARTEL normalization protocol and smoothed with a Gaussian kernel of 4‐mm full width half maximum to adjust the anatomical variability between the individual brains and to enhance the signal‐to‐noise ratio.
Statistical Analysis
Standard pooled‐variance t test or approximate t tests based on rank normalization were used for statistical group comparisons (SAS version 9.3; SAS institute Inc., Cary, NC). Step‐wise logistic regression was performed using fall or FoG status as the outcome parameter and VOI‐based regional VAChT binding as PET regressors. Analyses were performed using SAS software (version 9.3; SAS institute). Statistical inferences were made on meeting two‐tailed testing requirement for α < 0.05 and Holm‐Bonferroni correction for multiple testing for all clinical group comparisons and brain PET VOI analyses.
To complement the VOI‐based analyses, we performed two main exploratory whole‐brain voxel‐wise analyses to compare the total group of fallers versus nonfallers and total group of freezers versus nonfreezers, respectively. For this purpose, we designed a two‐sample voxel‐based t test to compare different groups. We thresholded statistical parametric maps at p = 0.0125 with a minimum cluster size of 50 voxels. We then identified clusters of significant voxels in anatomic subregions that were consistent with our hypotheses and/or concordant with regional cerebral results demonstrated by these VOI analyses. Statistically significant clusters, corrected for multiple comparisons using family‐wise error (FWE), were identified using the small volume (radius of VOI at 5 mm) voxel‐based method as previously reported.23
Finally, we performed post‐hoc exploratory analyses to further evaluate any regional brain findings of these main voxel‐based analyses by comparing subsets of patients of various combinations of fall and FoG status for more pure mechanistic analyses underlying fall and FoG status, respectively. Because some of the regional brain locomotor or motor control region may represent anatomically small structures (ie, clusters smaller than 50 voxels) we did not filter out the parametric images for a minimal cluster size. Significant clusters were also identified by the small volume FWE correction method.23
Results
Clinical Findings: Falls and Freezing Status
Thirty‐five subjects (37.2%) reported a history of falls. Fallers had longer duration of disease, greater motor disease severity, higher Hoehn & Yahr scores, higher LED, higher frequency of reporting acting out of dreams, and worse cognitive performance than nonfallers; however, there were no significant differences in sex distribution or age (Table 1).
Table 1.
Mean (±SD) Values of Demographic, Clinical, Cognitive Data in the Patients With PD Without Versus With Falls (Total n = 94)
| PD Nonfallers (n = 59) | PD Fallers (n = 35) | Statistical Significance | |
|---|---|---|---|
| Age, yr | 67.2 ± 7.3 | 68.9 ± 8.2 | t = 1.1; p = 0.29 |
| Sex (females/males) | 16/43 | 6/29 | χ2 = 1.2; p = 0.26 |
| Duration of motor disease (yr) | 4.9 ± 3.5 | 8.0 ± 5.4 | t = 3.5; p = 0.0008 |
| Hoehn and Yahr stage | 2.2 ± 0.6 | 2.8 ± 0.6 | t = 4.7; p < 0.0001 |
| Motor MDS‐UPDRS | 29.9 ± 10.5 | 40.7 ± 15.5 | t = 4.0; p = 0.0001 |
| Montreal Cognitive Assessment | 27.0 ± 2.3 | 25.0 ± 4.2 | t = 2.4; p = 0.02 |
| History of acting out dreams (yes/no) | 25/34 | 25/9 (n = 34) | χ2 = 0.4; p = 0.004 |
| LED (mg/day) | 571.2 ± 333.9 | 797.6 ± 457.7 | t = 2.5; p = 0.014 |
Sex distribution is presented as proportions. Levels of statistical difference between groups are also presented.
LED = levodopa equivalent dose; PD = Parkinson's disease; SD = standard deviation.
FoG was present in 15 subjects (16.0%). Freezers were older, had longer duration of disease, higher mean Hoehn and Yahr score, more severe motor disease, higher LED, and worse cognitive performance than nonfreezers (Table 2). There were no significant differences in sex distribution or frequency of reporting acting out of dreams between groups.
Table 2.
Mean (±SD) Values of Demographic, Clinical, and Cognitive Data
| PD without FoG (n = 79) | PD With FoG (n = 15) | Statistical Significance | |
|---|---|---|---|
| Age, yr | 66.9 ± 6.8 | 73.1 ± 9.4 | t = 3.1; p = 0.003 |
| Sex (females/males) | 20/59 | 2/13 | χ2 = 1.0; p = 0.32 |
| Duration of motor disease (yr) | 5.4 ± 4.5 | 9.1 ± 3.7 | t = 3.8; p = 0.0003 |
| Hoehn and Yahr stage | 2.3 ± 0.5 | 3.2 ± 0.6 | t = 6.1; p < 0.0001 |
| Motor MDS‐UPDRS | 31.2 ± 12.6 | 48.5 ± 10.7 | t = 5.0; p < 0.0001 |
| Montreal Cognitive Assessment | 26.8 ± 2.7 | 23.5 ± 4.7 | t = 3.2; p = 0.0019 |
| Acting out dreams (yes/no) | 43/36 | 7/7 (n = 14) | χ2 = 0.4; p = 0.004 |
| LED (mg/day) | 579.1 ± 366.3 | 1,057.9 ± 312.0 | t = 4.7; p < 0.0001 |
Sex distribution is presented as proportions. Levels of statistical differences between groups are also presented.
FoG = freezing of gait; LED = levodopa equivalent dose; PD = Parkinson's disease; SD = standard deviation.
Regional Cerebral FEOBV VOI PET Findings in Fallers
Univariate VOI analyses (corrected for multiple testing) demonstrated significantly reduced thalamic (p = 0.0016) VAChT expression in fallers compared to nonfallers (Table 3). None of the other regions retained significance after correction for multiple testing. In a post‐hoc analysis, we explored whether this finding was lateralized and entered the left and right thalamic VOIs in a backward step‐wise logistic regression analysis yielding right thalamic VAChT binding as the single regressor meeting the model's entry criteria (Wald χ2 = 9.1; p = 0.0025).
Table 3.
Mean (±SD) Values of Bilaterally Averaged [11C]FEOBV VAChT Distribution Volume Ratios in the Patients With PD Without Versus With Falls
| PD Nonfallers (n = 59) | PD Fallers (n = 35) | Statistical Significance | |
|---|---|---|---|
| Brainstem | 1.31 ± 0.09 | 1.26 ± 0.13 | t = 2.1; p = 0.042 |
| Cerebellum | 1.34 ± 0.22 | 1.26 ± 0.19 | t = 1.5; p = 0.15 |
| Thalamus | 1.91 ± 0.18 | 1.80 ± 0.24 | t = 3.3; p = 0.0016* |
| Striatum | 4.50 ± 0.54 | 4.33 ± 0.69 | t = 1.5; p = 0.14 |
| Limbic archicortex | 1.89 ± 0.15 | 1.84 ± 0.18 | t = 1.5; p = 0.15 |
| Neocortex | 1.05 ± 0.08 | 1.04 ± 0.08 | t = 0.9; p = 0.38 |
Levels of statistical differences between groups are also presented (values with an asterisk remain significant after correction for Holm‐Bonferroni multiple testing).
FEOBV = fluoroethoxybenzovesamicol; PD = Parkinson's disease; SD = standard deviation; VAChT = vesicular acetylcholine transporter.
Regional Cerebral FEOBV VOI PET Findings in Freezers
Univariate VOI analysis showed reduced striatal and limbic archicortical VAChT binding in freezers compared to nonfreezers (Table 4). These two regions were entered in backward step‐wise logistic regression analysis yielding striatal VAChT binding as the single regressor meeting the model's entry criteria (Wald χ2 = 8.6; p = 0.0034). In a post‐hoc analysis, we explored whether this involved the lateralized caudate nucleus versus the lateralized putamen. The left and right putamina and caudate nuclei VAChT VOIs were entered in backward step‐wise logistic regression analysis yielding the right caudate nucleus VAChT binding as the single regressor meeting the model's entry criteria (Wald χ2 = 9.3; p = 0.0023).
Table 4.
Mean (±SD) Values of Bilaterally Averaged [11C]FEOBV VAChT Distribution Volume Ratios in the Patients With PD Without Versus With FoG
| PD Without FoG (n = 79) | PD With FoG (n = 15) | Statistical Significance | |
|---|---|---|---|
| Brainstem | 1.30 ± 0.10 | 1.24 ± 0.15 | t = 1.6; p = 0.12 |
| Cerebellum | 1.32 ± 0.22 | 1.25 ± 0.18 | t = 1.0; p = 0.33 |
| Thalamus | 1.89 ± 0.20 | 1.76 ± 0.21 | t = 2.1; p = 0.04 |
| Striatum | 4.53 ± 0.56 | 3.98 ± 0.64 | t = 3.3; p = 0.0012* |
| Limbic archicortex | 1.90 ± 0.15 | 1.76 ± 0.17 | t = 3.0; P = 0.004* |
| Neocortex | 1.05 ± 0.08 | 1.04 ± 0.09 | t = 0.5; p = 0.6 |
Levels of statistical differences between groups are also presented (values with an asterisk remain significant after correction for Holm‐Bonferroni multiple testing).
FEOBV = fluoroethoxybenzovesamicol; FoG = freezing of gait; PD = Parkinson's disease; SD = standard deviation; VAChT = vesicular acetylcholine transporter.
Post‐Hoc Confounder Variable Analysis
A post‐hoc analysis of covariance (ANCOVA) was performed to determine possible confounder effects of significant clinical variables (LED, MOCA score, and duration of disease) where LED and duration of disease are proxy variables for overall disease severity. The total model for fall status was significant (F (4,89) = 3.53; p = 0.01) with significant effects for thalamic VACHT binding (F = 6.17; p = 0.015), borderline for MOCA scores (F = 3.30; p = 0.072), but not significant for duration of disease or LED. Similar ANCOVA for FoG status demonstrated a significant overall model (F (4,89) = 3.26; p = 0.015) with significant effect for striatal VAChT binding (F = 8.14; p = 0.0054), but no significant covariate effects for duration of disease, MOCA, or LED.
Voxel‐Based Whole‐Brain FEOBV PET Analyses
Whole‐brain voxel‐based analyses were performed to compared the total of 35 fallers to 59 nonfallers (analysis 1) and a total of 15 subjects with FoG compared to 79 nonfreezers (analysis 2).
Main Whole‐Brain Voxel‐Based Analysis 1: PD Fallers Versus Nonfallers (All Fallers)
Significant FEOBV binding reductions (FWE‐corrected p values ranging from 0.032 to 0.040) were found in the total group of PD fallers (n = 35) versus nonfallers (n = 59) in the dorsomedial thalamus (right greater than left), right lateral geniculate nucleus (LGN), right pulvinar, right head of the caudate nucleus, prefrontal, right temporopolar, right mesiotemporal, right more than left insula, superior vermis, and bilateral superior cerebellar peduncle regions (Fig 1). Additional FEOBV binding reductions were present in the anterior cingulate cortex. There were no areas of significantly increased FEOBV binding in fallers compared to nonfallers.
Figure 1.
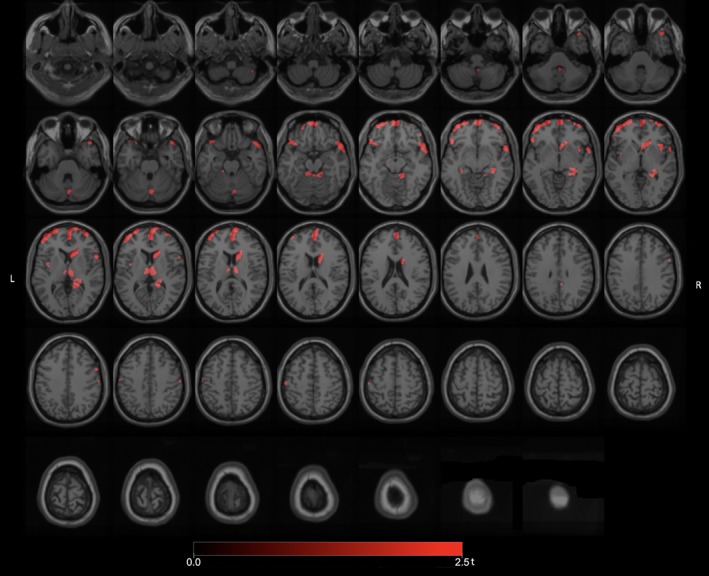
Main voxel‐based SPM analysis comparing the total group of PD fallers versus nonfallers. Significant FEOBV binding reductions were found in the total group of PD fallers versus nonfallers in the dorsomedial thalamus (right greater than left), right LGN, right pulvinar, right head of the caudate nucleus, prefrontal, right temporopolar, right more than left insula, superior vermis, and bilateral superior cerebellar peduncle regions. Additional FEOBV binding reductions were present in the anterior cingulate cortex. FEOBV = fluoroethoxybenzovesamicol; LGN = lateral geniculate nucleus; PD = Parkinson's disease.
Main Whole‐Brain Voxel‐Based Analysis 2: PD Freezers Versus Nonfreezers (All Freezers)
Whole‐brain voxel‐based analysis comparing freezers (n = 15) to nonfreezers (n = 79) demonstrated significant FEOBV binding reductions (FWE‐corrected p values ranging from 0.034 to 0.041) in the left hippocampal region and bilateral prefrontal and bilateral anterior cinguli (Fig 2). Additional reductions were observed in the striatum, including right more than left caudate and accumbens nuclei and putamina, bilateral limbic archicortex, bilateral gyri recti, right LGN, and right midcingulate cortex regions (Fig 2).
Figure 2.
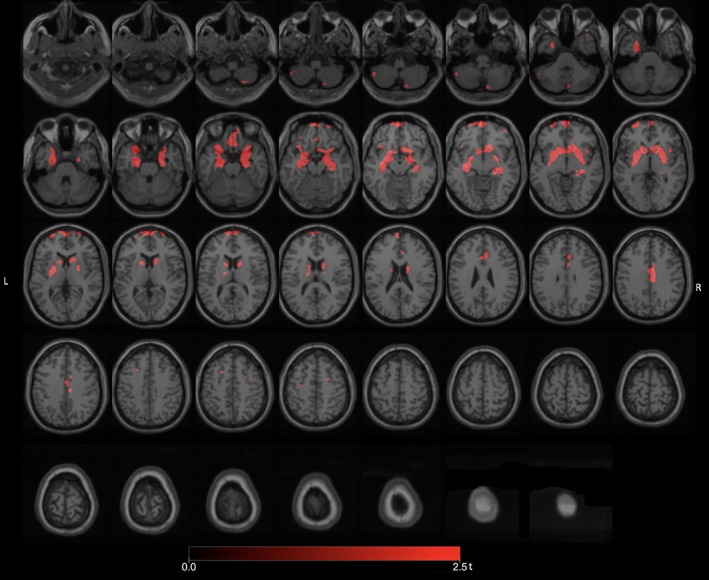
Main voxel‐based SPM analysis comparing the PD freezers to the nonfreezers demonstrated significant VAChT binding reductions in the left hippocampal region and bilateral prefrontal and bilateral anterior cinguli. Additional reductions were seen in the striatum, including right more than left caudate and accumbens nuclei and putamina, bilateral limbic archicortex, bilateral gyri recti, right LGN, and right mid‐cingulate cortex regions. LGN = lateral geniculate nucleus; PD = Parkinson's disease; VAChT = vesicular acetylcholine transporter.
Post‐Hoc Exploratory Voxel‐Based Analysis of Specific Subsets of Variable Combinations of Fall and FoG Status
Finally, we compared subsets of subjects with variable combination of falls and/or FoG status to allow a more “pure” mechanistic assessment of intrinsic fall and FoG phenomena. The first post‐hoc analysis compared PD fallers without FoG (n = 24) versus PD nonfallers without FoG (n = 55) for more pure assessment of fall patterns. The second post‐hoc analysis compared fallers with FoG (n = 11) to fallers without FoG (n = 24) to better capture the intrinsic pattern changes associated with pure freezing motor behaviors.
Post‐Hoc Exploratory Voxel‐Based Analysis 1: PD Fallers Without FoG Versus PD Nonfallers Without FoG (Pure Falls)
We compared the number of PD fallers without FoG to PD nonfallers also without FoG to explore a more neurobiological pattern of intrinsic fall mechanisms. Significant (FWE‐corrected p values ranging from 0.033 to 0.046) clusters included the right LGN, right caudate nucleus, right premotor cortex, right lateral temporal, right frontal eye field, right temporopolar cortex, right posterior cingulum, right proximal lingual gyrus, and bilateral prefrontal regions (Fig 3). Additional reductions were observed in the right more than left sensorimotor cortices.
Figure 3.
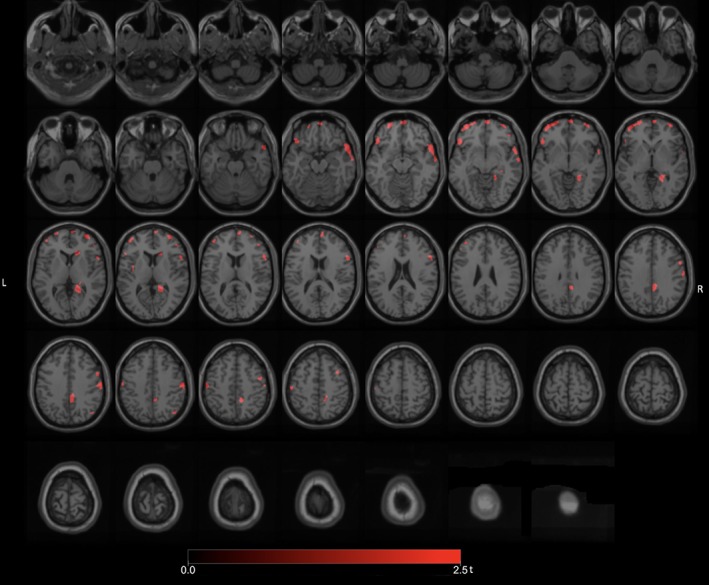
Post‐hoc exploratory voxel‐based SPM analysis comparing the subset of PD fallers without FoG to PD nonfallers also without FoG. There were more isolated reductions in the right LGN, right caudate nucleus, right premotor cortex, right frontal eye field, right temporopolar cortex, right lateral temporal, right posterior cingulum, right proximal lingual gyrus, and bilateral prefrontal regions. Additional reductions were seen in the right more than left sensorimotor cortices. FoG = freezing of gait; LGN = lateral geniculate nucleus; PD = Parkinson's disease.
Post‐Hoc Exploratory Voxel‐Based Analysis 2: PD Fallers With FoG Versus PD Fallers Without FoG (Pure FoG)
We also compared fallers with FoG to fallers without FoG to better capture the intrinsic changes underlying freezing motor behaviors and found significant clusters of reduced VAChT binding (FWE‐corrected p levels ranging from 0.034 to 0.046) in the left hippocampus, right temporal lobe, anterior cingulum, and cerebellum (Fig 4). Additional reductions were observed in the bilateral basal ganglia, limbic archicortex, right LGN, and right insula.
Figure 4.
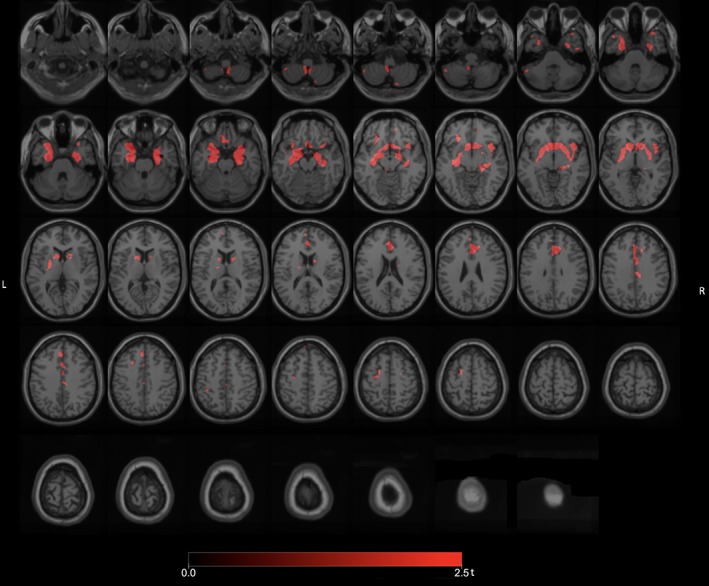
Post‐hoc exploratory voxel‐based SPM analysis comparing fallers with FoG to fallers without FoG showing more specific cholinergic transporter reductions in the left hippocampus, right temporal lobe, anterior cingulum, and cerebellum. Additional reductions were seen in the bilateral basal ganglia, limbic archicortex, right LGN, and right insula. FoG = freezing of gait; LGN = lateral geniculate nucleus.
Finally, a post‐hoc confounder analysis to determine any possible effect of LED medication effects on the regional cholinergic binding did not significantly change the main findings.
Discussion
Although falls and FoG are interconnected episodic phenomena,1 our findings show that these represent partially distinct entities with probable differing pathophysiology. VOI‐based analysis identified the thalamus and striatum as critical regions contributing to falls and FoG in PD, respectively. Thalamic cholinergic deficits, for example, are associated with impaired postural reflexes, whose underlying pathophysiology may differ from that leading to FoG.24, 25 We found that history of falls is associated with cholinergic projection system changes in which the thalamus is likely a key node whereas FoG is associated with changes for which the caudate nucleus is likely a key node.
[18F]FEOBV is a specific VAChT ligand and marker of cholinergic terminals.10 The regional distribution of [18F]FEOBV binding in human brain is identical to the distribution of cholinergic terminals described in postmortem human brain studies.26 The diminished [18F]FEOBV binding we describe in brain regions of PD falling and/or freezing subjects compared to those without these PIGD features is most compatible with degeneration (or greater degeneration) of cholinergic terminals. This inference is consistent with preclinical studies demonstrating that lesions of the BF complex or PPN result in significantly diminished [18F]FEOBV binding in target regions of these projections.27, 28 Similarly, Schmitz et al demonstrated a good correlation between cortical [18F]FEOBV binding deficits and basal forebrain nuclei atrophy in subjects with probable early Alzheimer's disease.29 Another important point for interpreting our results is that whereas basal forebrain projections to neocortex were conceived historically as a diffuse projection system, recent data indicate that subpopulations of BF cholinergic neurons project to relatively restricted cortical regions.30 The VOI and VB cortical [18F]FEOBV binding reductions we describe likely reflect degeneration or dysfunction of subpopulations of BF cholinergic neurons. Diminished [18F]FEOBV binding in the amygdala and hippocampal formation of PD subjects with FoG likely reflects disproportionate degeneration of more rostral BF cholinergic projection neurons. Similarly, diminished frontal cortical [18F]FEOBV binding in PD subjects with history of falls likely reflects preferential loss of subpopulations of more caudal BF cholinergic projection neurons.
Falling is a serious axial motor impairment in PD. There is converging evidence that cholinergic input from the PPN/LDTC to the thalamus may play an important role in the pathophysiology of falls in PD.5, 7, 24, 25 Our present study, using a more specific PET cholinergic terminal ligand and a different population of subjects, confirms our previous AChE PET imaging findings that PD fallers have lower density of thalamic cholinergic nerve terminals compared to nonfallers.5 This inference is consistent with a postmortem study that found evidence of lower PPN cholinergic neuron counts in PD subjects with falls during life compared to nonfallers.24 Although the human thalamus also receives cholinergic inputs from the BF complex, these are sparser than PPN/LDTC afferents and it is likely that the considerable majority of thalamic terminals arise in the PPN/LDTC complex.31 This is particularly true for visual thalamic nuclei such as the pulvinar and LGN, which contain only sparse BF terminals.31 Although cholinergic thalamic afferents likely play a critical role in the pathophysiology of falls in PD, PPN/LTDC‐thalamic cholinergic degeneration typically occurs in the setting of BF losses,32 suggesting that extensive brain cholinergic projection system deficits are also implicated in falls in PD.
Our post‐hoc VOI analysis identified the right thalamus as the most significant hypocholinergic brain region associated with falls. A significant limitation of the VOI‐based approach, however, is that anatomic resolution is limited to predefined VOIs and/or Freesurfer parcellation. This particularly limits the analysis of the thalamus, where no validated nuclear parcellation is available at the present time. Complex phenomena like falls and FoG likely reflect circuit‐level alterations whereas the VOI‐based analyses identify critical nodes; it may underestimate the extent of circuit level changes. To explore circuit level alterations, we supplemented the VOI analysis with a voxel‐based analysis. The voxel‐based analysis suggests a more specific role of the right visual thalamus, including the pulvinar and LGN, in the pathophysiology of falls in PD. Most models of vision treat the LGN as a passive relay station to the primary visual cortex. Accumulating data, however, indicate that the pulvinar and LGN are important nodes in corticothalamocortical circuits modulating attentional function and the coordination of cortical regions for attending to stimuli and tasks.33
We can only speculate as to why we find asymmetric abnormalities in the visual thalamus (fallers) and caudate (freezers). The ventral attention network, which responds to unexpected stimuli and is highly integrated with the visual system, lateralizes to the nondominant hemisphere.34 Hypothetically, lateralization of unique brain function(s) may require higher ipsilateral neural network or neuronal metabolic demands, which may, in turn, increase vulnerability to early degeneration.
These observations suggest that impaired processing of visual information relevant for safe ambulation may be compromised in PD fallers. It is conceivable that vulnerability of the right visual thalamus complex may be related to previous observations of a subtle left hemineglect in PD consisting of a directional (right hemifield) bias of initial visual exploration.35 Relative deficiency of detecting salient environmental visual cues from the left hemifield may be a potential mechanism to explain increased fall risk with right LGN dysfunction. There is also evidence of perceptual asymmetry in perceptuomotor tasks without visual input. An upper body truncal rotation experiment showed evidence of contraction of left external hemispace relative to the right hemispace, possibly affecting generation and execution of motor commands through disease progression in PD.36
The voxel‐based analysis implicates other brain regions as important contributors to the etiology of falls, notably the frontal cortex. Reduced cortical cholinergic signaling likely results in decreased transfer of cortical sensory and movement cues to subcortical structures, such as the striatum, degrading sensorimotor integration.37 Similarly, altered neocortical regulation of movement may underlie some forms of FoG.38 FoG is a debilitating feature of PD that becomes more frequent with advancing disease. The magnitude of nigrostriatal dopaminergic denervation is an important pathophysiological element of FoG.39 Our study confirms that the presence of FoG is related to longer duration of disease, more severe parkinsonian motor ratings, and higher LED levels. Striatal dopamine deficits, however, are likely not the only factor in FoG. First, our confounder covariate analysis did not show a significant covariate effect for LED. Second, the presence of FoG and its degree of responsiveness to dopaminergic medications correlates with exposure to anticholinergic drugs.4 Freezing in PD may result from striatal dopamine loss and cortical cholinergic deafferentation, yielding striatal circuitry that lacks information about the efficacy of gait, posture, and movement and that is impaired in selecting and sequencing motor actions, resulting in slow and reluctant movements or fails to initiate movement altogether.37
Past imaging studies found evidence of disruption of cortical function in FoG, including regions or networks involved in executive functions and sensorimotor perception in PD.38, 40, 41, 42 For example, a resting‐state fMRI brain connectivity study identified reduced connectivity in right cortical frontoparietal “executive‐attention” and right occipitotemporal “visual” networks in PD with FOG suggesting a role of network connectivity disruption.41 A diffusion tensor MRI study showed evidence of reduced connectivity between connectivity of the PPN with the cerebellum, thalamus, and multiple regions of the frontal cortex.43 Moreover, these structural differences were observed solely in the right hemisphere of patients with FoG.
Our voxel‐based analysis suggests specific roles of striatal cholinergic interneurons, especially of the right caudate nucleus, and limbic archicortical structures in FoG. Unlike the predominant motor connections of the putamen, the caudate nucleus is a node in more cognitive and affective circuits.44 FoG is notably exacerbated by anxiety and often arises in situations where there are competing cognitive demands. These observations suggest that nonmotor (cognitive, affective, and emotional) functions of the caudate nucleus are relevant for FoG. Degeneration or dysfunction of caudate cholinergic interneurons may disrupt corticostriatal information flow underlying the integration of goal directed behavior and sensorimotor integration and is a plausible substrate for altered network behavior underlying FoG.45
Dysfunctional limbic circuitry may underlie freezing of gait in PD. A recent resting‐state brain MRI found abnormal connectivity between the right amygdala and striatum in freezers compared to nonfreezers.46 Hippocampal abnormalities may point to altered spatial sensorimotor integration functions. FoG is notoriously associated with anxiety, and the amygdalar cholinergic abnormalities we detected suggest a concrete substrate for anxiety as a determinant of FoG in PD.47
REM sleep without atonia may be a comorbid feature of patients with PD exhibiting FoG.48 We previously reported reduced limbic, cortical, and brainstem‐thalamic acetylcholinesterase hydrolysis rates in PD patients with REM sleep behavior, suggesting partially shared cholinergic pathophysiology of these two phenomena.49 We did not find a significant difference in the frequency of dream enactment behaviors in the freezers versus nonfreezers. Our sample size, however, was small. We found an unexpected significant difference in reporting of dream enactment behavior in the fallers compared to the nonfallers. Given that isolated falls typically precede freezing motor behaviors during the natural course of the disease in PD,50 it is possible that brainstem‐thalamic cholinergic changes may be a greater determinant of this parasomnia than more anterior striatal or limbic changes. Further research is needed to explore this hypothesis.
A limitation of our study is that assessment of FoG was not based on special maneuver to identify specific subtypes of freezing motor behaviors, like making turns or passing through narrow doorways. The absence of a specific provocative FoG assessment protocol may also explain the relatively low frequency of freezing behaviors in our study sample. Consequently, this may result in a relative underestimation of effect size estimates of our study. However, our assessment protocol of direct observation of freezing behavior will result in a very high specificity of FoG classification.
In this study, we found that differential degeneration of cholinergic projections play distinct roles in postural and gait disturbances of PD. Our VOI analysis findings independently confirm previous observations that impaired integrity of thalamic cholinergic nerve terminals contributes to the pathophysiology of falls in PD. Our voxel‐based analysis suggests that visual thalamus, in particular the right LGN, is a key node in disturbed circuit function underlying falls. FoG may be the result of striatal cholinergic interneurons and limbic cholinergic nerve terminals, with the right caudate nucleus as a key node in disturbed control of gait.
Author Contributions
N.B. and M.M. designed the study. N.B., M.M., R.L.A., R.K., P.K., Z.Z., and R.A.K. acquired and/or analyzed the data. N.B., R.A., and M.M. drafted the manuscript, which was reviewed and revised by all co‐authors.
Potential Conflicts of Interest
Nothing to report.
Acknowledgment
Study funded by National Institutes of Health (P01 NS015655, RO1 NS070856, and P50 NS091856), Department of Veterans Affairs grant (I01 RX001631), and the Michael J. Fox Foundation.
The authors thank Christine Minderovic, Cyrus Sarosh, Virginia Rogers, the PET technologists, cyclotron operators, and chemists for their assistance. We are indebted to the subjects who participated in this study.
References
- 1. Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson‘s disease: a review of two interconnected, episodic phenomena. Mov Disord 2004;19:871–884. [DOI] [PubMed] [Google Scholar]
- 2. Hely MA, Morris JG, Reid WG, Trafficante R. Sydney Multicenter Study of Parkinson‘s disease: non‐L‐dopa‐responsive problems dominate at 15 years. Mov Disord 2005;20:190–199. [DOI] [PubMed] [Google Scholar]
- 3. Williams‐Gray CH, Mason SL, Evans JR, et al. The CamPaIGN study of Parkinson‘s disease: 10‐year outlook in an incident population‐based cohort. J Neurol Neurosurg Psychiatry 2013;84:1258–1264. [DOI] [PubMed] [Google Scholar]
- 4. Perez‐Lloret S, Negre‐Pages L, Damier P, et al. Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease. JAMA Neurol 2014;71:884–890. [DOI] [PubMed] [Google Scholar]
- 5. Bohnen NI, Muller ML, Koeppe RA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology 2009;73:1670–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bohnen NI, Frey KA, Studenski S, et al. Gait speed in Parkinson disease correlates with cholinergic degeneration. Neurology 2013;81:1611–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bohnen NI, Frey KA, Studenski S, et al. Extra‐nigral pathological conditions are common in Parkinson‘s disease with freezing of gait: an in vivo positron emission tomography study. Mov Disord 2014;29:1118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koeppe RA, Frey KA, Snyder SE, Meyer P, Kilbourn MR, Kuhl DE. Kinetic modeling of N‐[11C]methylpiperidin‐4‐yl propionate: alternatives for analysis of an irreversible positron emission tomography tracer for measurement of acetylcholinesterase activity in human brain. J Cereb Blood Flow Metab 1999;19:1150–1163. [DOI] [PubMed] [Google Scholar]
- 9. Marcone A, Garibotto V, Moresco RM, et al. [11C]‐MP4A PET cholinergic measurements in amnestic mild cognitive impairment, probable Alzheimer‘s disease, and dementia with Lewy bodies: a Bayesian method and voxel‐based analysis. J Alzheimers Dis 2012;31:387–399. [DOI] [PubMed] [Google Scholar]
- 10. Kilbourn MR, Hockley B, Lee L, et al. Positron emission tomography imaging of (2R,3R)‐5‐[(18)F]fluoroethoxybenzovesamicol in rat and monkey brain: a radioligand for the vesicular acetylcholine transporter. Nucl Med Biol 2009;36:489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petrou M, Frey KA, Kilbourn MR, et al. In vivo imaging of human cholinergic nerve terminals with (‐)‐5‐18F‐fluoroethoxybenzovesamicol: biodistribution, dosimetry, and tracer kinetic analyses. J Nucl Med 2014;55:396–404. [DOI] [PubMed] [Google Scholar]
- 12. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson‘s disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 14. Goetz CG, Fahn S, Martinez‐Martin P, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson‘s Disease Rating Scale (MDS‐UPDRS): Process, format, and clinimetric testing plan. Mov Disord 2007;22:41–47. [DOI] [PubMed] [Google Scholar]
- 15. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson‘s disease. Mov Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 16. Snijders AH, Haaxma CA, Hagen YJ, Munneke M, Bloem BR. Freezer or non‐freezer: clinical assessment of freezing of gait. Parkinsonism Relat Disord 2012;18:149–154. [DOI] [PubMed] [Google Scholar]
- 17. Shao X, Hoareau R, Runkle AC, et al. Highlighting the versatility of the Tracerlab synthesis modules. Part 2: fully automated production of [11C]labelled radiopharmaceuticals using a Tracerlab FXC‐Pro . J Labelled Comp Radiopharm 2011;54:819–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shao X, Hoareau R, Hockley BG, et al. Highlighting the versatility of the Tracerlab synthesis modules. Part 1: fully automated production of [18F]labelled radiopharmaceuticals using a Tracerlab FXFN . J Labelled Comp Radiopharm. 2011;54:292–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Minoshima S, Koeppe RA, Fessler JA, et al. Integrated and automated data analysis method for neuronal activation studying using O15 water PET In: Uemura K, Lassen NA, Jones T, Kanno I, eds. Quantification of Brain Function to Tracer Kinetics and Image Analysis in Brain PET. Tokyo: Excerpta Medica; 1993:409–418. [Google Scholar]
- 20. Aghourian M, Legault‐Denis C, Soucy JP, et al. Quantification of brain cholinergic denervation in Alzheimer‘s disease using PET imaging with [18F]‐FEOBV. Mol Psychiatry 2017;22:1531–1538. [DOI] [PubMed] [Google Scholar]
- 21. Nejad‐Davarani S, Koeppe RA, Albin RL, et al. Quantification of brain cholinergic denervation in dementia with Lewy bodies using PET imaging with [(18)F]‐FEOBV. Molecular psychiatry. 2018. Aug 6. 10.1038/s41380-018-0130-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gilman S, Koeppe RA, Nan B, et al. Cerebral cortical and subcortical cholinergic deficits in parkinsonian syndromes. Neurology 2010;74:1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Worsley KJ, Marrett S, Neelin P, et al. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 1996;4:58–73. [DOI] [PubMed] [Google Scholar]
- 24. Karachi C, Grabli D, Bernard FA, et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Invest 2010;120:2745–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muller ML, Albin RL, Kotagal V, et al. Thalamic cholinergic innervation and postural sensory integration function in Parkinson‘s disease. Brain 2013;136(pt 11):3282–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Albin RL, Bohnen NI, Muller M, et al. Regional vesicular acetylcholine transporter distribution in human brain: a [(18) F]fluoroethoxybenzovesamicol positron emission tomography study. J Comp Neurol 2018;526:2884–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parent M, Bedard MA, Aliaga A, et al. PET imaging of cholinergic deficits in rats using [18F]fluoroethoxybenzovesamicol ([18F]FEOBV). Neuroimage 2012;62:555–561. [DOI] [PubMed] [Google Scholar]
- 28. Cyr M, Parent MJ, Mechawar N, et al. PET imaging with [(18)F]fluoroethoxybenzovesamicol ([(18)F]FEOBV) following selective lesion of cholinergic pedunculopontine tegmental neurons in rat. Nucl Med Biol 2014;41:96–101. [DOI] [PubMed] [Google Scholar]
- 29. Schmitz TW, Mur M, Aghourian M, Bedard MA, Spreng RN; Alzheimer‘s Disease Neuroimaging Initiative . Longitudinal Alzheimer‘s degeneration reflects the spatial topography of cholinergic basal forebrain projections. Cell Rep 2018;24:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ballinger EC, Ananth M, Talmage DA, Role LW. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron 2016;91:1199–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heckers S, Geula C, Mesulam M. Cholinergic innervation of the human thalamus: dual origin and differential nuclear distribution. J Comp Neurol 1992;325:68–82. [DOI] [PubMed] [Google Scholar]
- 32. Bohnen NI, Muller MLTM, Kotagal V, et al. Heterogeneity of cholinergic denervation in Parkinson‘s disease without dementia. J Cereb Blood Flow Metab 2012;32:1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Halassa MM, Kastner S. Thalamic functions in distributed cognitive control. Nat Neurosci 2017;20:1669–1679. [DOI] [PubMed] [Google Scholar]
- 34. Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist 2014;20:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ebersbach G, Trottenberg T, Hattig H, et al. Directional bias of initial visual exploration. A symptom of neglect in Parkinson‘s disease. Brain 1996;119 (pt 1):79–87. [DOI] [PubMed] [Google Scholar]
- 36. Wright WG, Gurfinkel V, King L, Horak F. Parkinson‘s disease shows perceptuomotor asymmetry unrelated to motor symptoms. Neurosci Lett 2007;417:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sarter M, Albin RL, Kucinski A, Lustig C. Where attention falls: Increased risk of falls from the converging impact of cortical cholinergic and midbrain dopamine loss on striatal function. Exp Neurol 2014;257C:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Snijders AH, Leunissen I, Bakker M, et al. Gait‐related cerebral alterations in patients with Parkinson‘s disease with freezing of gait. Brain 2011;134(pt 1):59–72. [DOI] [PubMed] [Google Scholar]
- 39. Snijders AH, Takakusaki K, Debu B, et al. Physiology of freezing of gait. Ann Neurol 2016;80:644–659. [DOI] [PubMed] [Google Scholar]
- 40. Bartels AL, Leenders KL. Brain imaging in patients with freezing of gait. Mov Disord 2008;23(suppl 2):S461–S467. [DOI] [PubMed] [Google Scholar]
- 41. Tessitore A, Amboni M, Esposito F, et al. Resting‐state brain connectivity in patients with Parkinson‘s disease and freezing of gait. Parkinsonism Relat Disord 2012;18:781–787. [DOI] [PubMed] [Google Scholar]
- 42. Shine JM, Matar E, Ward PB, et al. Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson‘s disease. Brain 2013;136(pt 4):1204–1215. [DOI] [PubMed] [Google Scholar]
- 43. Fling BW, Cohen RG, Mancini M, et al. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain 2013;136(pt 8):2405–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986;9:357–381. [DOI] [PubMed] [Google Scholar]
- 45. Bradfield LA, Bertran‐Gonzalez J, Chieng B, Balleine BW. The thalamostriatal pathway and cholinergic control of goal‐directed action: interlacing new with existing learning in the striatum. Neuron 2013;79:153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gilat M, Ehgoetz Martens KA, Miranda‐Dominguez O, et al. Dysfunctional limbic circuitry underlying freezing of gait in Parkinson‘s disease. Neuroscience 2018;374:119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Martens KA, Hall JM, Gilat M, et al. Anxiety is associated with freezing of gait and attentional set‐shifting in Parkinson‘s disease: a new perspective for early intervention. Gait Posture 2016;49:431–436. [DOI] [PubMed] [Google Scholar]
- 48. Videnovic A, Marlin C, Alibiglou L, et al. Increased REM sleep without atonia in Parkinson disease with freezing of gait. Neurology 2013;81:1030–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kotagal V, Albin RL, Muller ML, et al. Symptoms of rapid eye movement sleep behavior disorder are associated with cholinergic denervation in Parkinson disease. Ann Neurol 2012;71:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lord S, Galna B, Yarnall AJ, et al. Natural history of falls in an incident cohort of Parkinson‘s disease: early evolution, risk and protective features. J Neurol 2017;264:2268–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]


