Abstract
Adolescent binge drinking renders young drinkers vulnerable to alcohol use disorders in adulthood; therefore, understanding alcohol-induced brain damage and associated cognitive dysfunctions is of paramount importance. Here we investigated the effects of binge-like adolescent intermittent ethanol (AIE) exposure on nonspatial working memory, behavioral flexibility and cholinergic alterations in the nucleus accumbens (NAc) in male and female rats. On postnatal days P25-57 rats were intubated with water or ethanol (at a dose of 5 g/kg) on a 2-day-on/2-day-off cycle and were then tested in adulthood on social recognition and probabilistic reversal learning tasks. During the social recognition task AIE-treated rats spent similar amounts of time interacting with familiar and novel juveniles, indicating an impaired ability to sustain memory of the familiar juvenile. During probabilistic reversal learning, AIE-treated male and female rats showed behavioral inflexibility as indicated by a higher number of trials needed to complete 3 reversals within a session, longer response latencies for lever selection, and for males, a higher number of errors as compared to water-treated rats. AIE exposure also reduced the number of cholinergic interneurons in the NAc in males and females. These findings indicate AIE-related pathologies of accumbal cholinergic interneurons and long lasting cognitive-behavioral deficits, which may be associated with cortico-striatal hypofunction.
Keywords: cholinergic interneurons, ethanol, probabilistic reversal learning, social recognition, nucleus accumbens
Introduction
Adolescence is a developmental period marked by vulnerability to the neurotoxic effects of alcohol (Spear 2018). Alcohol binge drinking during adolescence has been associated with impairment of executive functions (Garcia-Moreno et al. 2008; Sanhueza et al. 2011), such as working memory (Carbia et al. 2017) and brain structural alterations including a reduction of gray matter and volume in the prefrontal cortex (PFC) (Heikkinen et al. 2017; Squeglia et al. 2015). This has been corroborated by animal studies in which rodents subjected to adolescent intermittent ethanol (AIE) treatment have deficits in attention set shift learning (Fernandez and Savage 2017; Gass et al. 2014), reversal learning (Badanich et al. 2016; Coleman et al. 2014; Kuzmin et al. 2012) and extinction of responding (Broadwater and Spear 2013; Gass et al. 2014). These tasks require behavioral flexibility that is predominantly mediated by cortico-striatal circuits (Dalton et al. 2014; Floresco et al. 2008; Ghods-Sharifi et al. 2008; McAlonan and Brown 2003; Ragozzino et al. 1999). AIE-treated rodents show persistent changes in fronto-striatal functional connectivity (Broadwater et al. 2018), neuronal firing in the medial PFC (McMurray et al. 2016; Renteria et al. 2018), neuronal myelination as well as a reduction in frontal cortical volume (Coleman et al. 2014).
Of particular interest are ethanol-induced alterations in the forebrain cholinergic circuits, which is highly involved in learning, memory and attention. AIE treatment reduces gene expression for cholinergic receptors (Coleman et al. 2011), blunts PFC acetylcholine (ACh) efflux during maze tasks (Fernandez and Savage 2017) and leads to the loss of cholinergic neurons in forebrain regions (Boutros et al. 2014; Coleman et al. 2014; Ehlers et al. 2011; Fernandez and Savage 2017; Vetreno et al. 2014). Similar cholinergic neuropathology has been noted in rat forebrain regions after chronic alcohol consumption (Aloe and Tirassa 1992; Cadete-Leite et al. 1995; Savage et al. 2000) or thiamine deficiency (a condition that is common in chronic alcohol consumption) (Hall and Savage 2016; Vedder et al. 2015).
Surprisingly, little is known about alcohol-related alterations of cholinergic interneurons in the nucleus accumbens (NAc). Chronic ethanol consumption during adulthood leads to a 50% reduction in vesicular acetylcholine transporter (VAChT) in the NAc, without change in size or number of NAc cholinergic interneurons (Pereira et al. 2014). To our knowledge, no studies have examined whether or not intermittent ethanol exposure during adolescence produces a loss of ChAT interneurons within the NAc, as it does in the dorsal lateral striatum (Vetreno et al. 2014). In addition, sex differences in the cholinergic cell loss are yet to be explored. Thus, shed light on this issue, we investigated whether AIE exposure leads to morphological changes of cholinergic interneurons in the NAc of male and female rats.
In addition, two tasks that have some dependency on the NAc were used to probe dysfunctional memory and behavioral flexibility following AIE. Social recognition is a nonspatial assessment of short term memory in rodents (Kelly and Tran 1997; Oliveira et al. 2015) that is mediated in part by a circuit linking the ventral hippocampus to the NAc shell. Optogenetic stimulation of neurons in the ventral hippocampus, as well as hippocampal to NAc shell projections modulate social memory storage whereas their inhibition impairs social recognition (Okuyama et al. 2016). Given that the cholinergic system plays a critical role in learning, and other cholinergic nuclei are particularly vulnerable to ethanol toxicity, rats exposed to AIE might display deficits in probabilistic reversal learning, a type of learning that requires behavioral flexibility. Behavioral inflexibility is often observed in individuals with alcohol dependence (Beylergil et al. 2017; Park et al. 2010). There is evidence that the NAc (Cools et al. 2002; Dalton et al. 2014; Mell et al. 2009), in addition to the orbital regions of the PFC (Dalton et al. 2016), plays an important role in probabilistic reversal learning. Therefore, AIE-induced cortico-striato-limbic dysfunction (Crews et al. 2016; Fernandez and Savage 2017; Pascual et al. 2009; Trantham-Davidson et al. 2017) might contribute to impairments in behavioral flexibility following adolescent alcohol exposure.
Our goal was to determine the effects of AIE exposure on nonspatial short-term memory, as measured in the social recognition task, and behavioral flexibility, as measured in the probabilistic reversal-learning task. We also examined whether or not there were any sex differences in how AIE may alter learning and cognition. Lastly, we determined whether AIE leads to alterations in cholinergic interneurons within the NAc.
Experimental Procedures
Subjects
Animal housing and care conditions were consistent with those specified by the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 2010). The protocols used in the present experiments were approved by the Binghamton University Institutional Animal Care and Use Committee.
Subjects consisted of 34 male and 35 female Sprague Dawley rats obtained either from litters bred at the Binghamton University animal facility or purchased from Envigo (NJ, US). Each treatment condition (Control gavage or AIE, see below) included only one male and one female pup from a litter. Each treatment condition had 8-9 male and 8-9 female rats randomly assigned (based on by-chance procedure). All animals were housed in pairs in a temperature and humidity controlled colony under a 12-h light/dark cycle (lights on at 7:00 am). Rats had free access to water and standard rat chow (Purina Lab Diet 5012), except in Experiment 2 where one week prior to the start of experimental sessions they received daily rations of food in order to maintain weights at 85% of their free feeding values.
Adolescent intermittent ethanol treatment
Rats were randomly assigned to either adolescent intermittent ethanol (AIE) or water treatment (Control) groups. During postnatal days P25 – P57 (between 9 am- 12 pm) all rats received 16 intragastric gavages of either ethanol, administered at a dose of 5 g/kg, or distilled water, on a 2-day on/2-day-off cycle. Blood samples were collected via a small incision made along the lateral tail vein 30-60 min following the first and last gavages in order to measure blood ethanol content (BEC) using an AM1 Alcohol Anylazer (Analox Instruments, MA, US). The AM1 assay does have window of error in the range of 18.5 mm/L (see manual), we therefore report BEC in control rats to get a precise estimate of error.
Similar to other studies (Fernandez and Savage 2017; Vetreno et al. 2014), the weights of the AIE and the water-treated rats did not differ during the gavage treatment (sex × treatment × day: F [1, 66]= 2.02; p=0.159, treatment: F[1, 66]=1.55; p=.216). As expected, all subjects gained weight during treatment (day: F[1, 66]=1812.08; p<0.0001). Prior to behavioral testing there were no treatment differences in weight (AIE female=206.75±7.70 g vs. Control female: 201.75±6.71 g; AIE male= 294.00±3.34 g vs. Control male: 295.75±6.06 g; treatment effect: F[1, 28]=0.07, p=0.79; or sex X treatment interaction F[1, 28]=0.29, p=0.59); but of course male rats were heavier than female rats (F[1, 28]=215.91, p<0.0001).
Apparatus
Social recognition test cages
Clean standard rat plastic housing cages (47 × 26 × 20 cm) with fresh bedding were used for the Social Recognition Experiment (Experiment 1).
Operant conditioning chambers
In Experiment 2 twelve operant conditioning chambers (Med Associates Inc, VT, US) were used, each measuring 59 × 55 × 36 cm and placed in a ventilated, sound-attenuating cubicle with an operating fan to mask outside noise. The conditioning chambers were equipped with, a house light, two retractable levers, stimulus light positioned above each lever and a food trough into which food pellets (45 mg each; Bio-Serv, NJ, US) could be delivered from a dispenser.
Procedures
Behavioral experiments were conducted during animals’ light cycle between 9 am −12 pm.
Experiment 1: Social recognition
Following treatment, subjects (AIE Ns: male=8; female=8; Control Ns: male=8; female=8) were aged until early adulthood (P90-95) and then transferred to the testing room and placed into clean individual standard cages 30-min prior to the start if the experiment. The social recognition experiment consisted of two trials separated by a 30-min delay. During Trial 1 experimental rats (AIE and Control, both males and females) were exposed individually to a same-sex juvenile (P25-P30) in a social recognition test cage for 5 min before being removed and placed in a separate cage with fresh bedding (to avoid further exposure to the juvenile rat’s scent). The total time spent observing the juvenile was recorded using an ANY-maze video tracking system (Stoelting, Wood Dale, IL, USA).
After a 30-min delay interval, Trial 2 began with the adult experimental rat being paired with the familiar juvenile from Trial 1 and a second novel same-sex juvenile for 5 min in a clean cage. Social interaction was observed and defined as the direct investigation of the juvenile rats’ anogenital or oral areas. Trial 2 was recorded (ANY-maze) and scored off line by a blind reviewer. The amount of time the experimental rat spent interacted with the two juveniles (familiar and novel) was determined.
Experiment 2: Probabilistic learning and rule reversal.
A different group of rats (AIE Ns: male=8; female=8; Control Ns: male=8; female=8), aged until P90-95, was introduced to food pellets in their home cages one day prior to the beginning of the experimental procedure. The protocol for Experiment 2 was adapted from (Dalton et al. 2014) and consisted of 3 phases: fixed ratio one (FR1) lever press training, retractable lever press training, followed by probabilistic learning and reversal training. All rats were trained in the operant conditioning chambers to press a lever for food during a 30 min session under a FR1 schedule of reinforcement (phase 1). Each response on the active lever was reinforced by a food pellet delivered into the food trough. Responding on the inactive lever was counted but produced no consequences. The program ended either after 30 min or when 60 active lever presses were made, whichever came first. Once the animals met the criterion of at least 50 active lever presses they were trained to press the opposite lever for food until the same criterion was met. The position of the active lever (left or right) was counterbalanced across subjects.
During retractable lever press training (phase 2) rats were familiarized with intertrial intervals and retraction of levers. The session started with the house light turned off. Every 15 sec, one of the levers extended and the house light turned on for the duration of the trial (maximum 10 sec). The rats were trained to press the lever for food within 10 sec of the lever insertion. No response within a 10 sec trial was marked as omission and led to lever retraction, the house light turning off and the termination of the trial. During each of 90 trials a lever press was reinforced with a food pellet with 50% probability. The criterion of 10 or less omissions had to be met before progressing to phase 3, the probabilistic reversal learning.
The last phase of training was conducted over 12 sessions, each consisting of 200 trials. Each trial began with the house light turning on followed by the insertion of two levers. Only one of the levers, randomly determined, served as the active “correct” lever. The rat had 10 sec to press the active lever, otherwise the trial was recorded as an omission or, in the case of a response on the inactive “incorrect” lever, as an error.
Presses on the active “correct” lever were reinforced with food pellets 80% of the time whereas presses on the inactive “incorrect” lever were reinforced 20% of the time. When a rat pressed the active “correct” lever 8 times consecutively (regardless of whether or not presses on this lever were reinforced) the opposite lever was designated the active “correct lever”. This rule continued until the end of the session such that a rat could in theory make a maximum of 25 reversals per session.
Experiment 3: Cholinergic interneurons in the NAc.
Immunohistochemistry
Rats (AIE Ns: male=8; female=9; control Ns: male=8; female=9; approximately half came from social recognition experiment and the other half from probabilistic reversal learning experiment) were deeply anesthetized with sodium pentobarbital (Fatal Plus, Vortech Pharmaceuticals, MI, US) and perfused through the heart first with phosphate buffered saline (PBS) followed by a fixative solution consisting of 4% paraformaldehyde in PBS at 4° C. Brains were removed from the skull and post-fixed for 24 hours in the same fixative and later stored in a 30% sucrose solution made in 0.1 M PBS at 4° C. The brains were then embedded in tissue-freezing medium and coronally sectioned at 40 μm using a microtome (Sm200r; Leica Instruments, Germany). Tissue sections were sequentially collected into 96 well plates containing a cryoprotectant solution (62.8 mg NaH2PO4, 2.18 g Na2HPO4, 160 ml dH2O, 120 ml ethylene glycol and 120 ml glycerol; pH:7.4) and stored at −20° C until immunohistochemistry processing.
For choline acetyltransferease (ChAT) immunohistochemistry, a total of 8 sections per subject were selected across the NAc at equal intervals (every 6th). Sections were first washed 3 times in 0.1 M PBS for 10 min and later incubated in 0.3% hydrogen peroxide (H2O2) for 30 min to diminish endogenous peroxidase activity and triple-washed again in PBS. To facilitate permeabilization and block non-specific binding, sections were placed into a blocking solution consisting of 4% normal rabbit serum, 0.1% Triton-X100 in 0.1 M PBS for 60 min of incubation. Sections were then incubated with the ChAT primary antibody (1:200 dilution of goat monoclonal anti-ChAT; AB144P; Millipore EMD, MA, US) and a blocking solution overnight at 4° C. The following day the sections were triple-rinsed in 0.1 M PBS solution and subsequently placed in a secondary antibody (1:200 anti-goat lgG, BA5000, Vector Laboratories, CA, US) plus the blocking buffer solution for 1 h. To allow for greater binding accessibility to a biotinylated secondary antibody, the sections were next incubated for 2 h in an avidin–horseradish peroxidase mixture in PBS (Elite Vectastain ABC kit; Vector Laboratories, US). Sections were rinsed in PBS and then visualized with a chromogenic agent containing 3,3’diaminobenzidine (0.7 mg/ml DAB) and 0.67 mg/ml Urea H2O2 (SIGMAFAST DAB solution, Sigma Aldrich, MA, US). Tissues were subsequently mounted, dried and coverslipped with the mounting agent Permount (Fisher Scientific, MA, US).
Stereology
We used an unbiased stereological counting method (as described in (Hall and Savage 2016; Olesen et al. 2017) to estimate a population of ChAT interneuron in the NAc. A Zeiss Microscope (Axioscope 2-Plus, NY, US) with an attached digital camera (DVC-1310; DVC Company, TX, US) containing a motorized stage was used in conjunction with Stereoinvestigator software (MBF Bioscience, VT, US). Contours were drawn around the NAc under the 2.5 X magnification and counting was performed at the 40 X magnification. The estimated population of ChAT intemeurons was calculated using the optical fractionator function based on the total number of counted markers, our section-sampling fraction of 1/8, optical dissector height of 20 μm, top guard zone of 2 μm, counting frame of 100 × 100 μm, sampling frame of 150 × 150 μm and calculated average section thickness of 37.2 μm. We ensured that the Gundersen-Jensen estimator of the error coefficient was lower than 0.10, to ensure the precision of the stereological estimates.
Data Analysis
To validate the use of parametric statistics we ensured that (1) our data were normally distributed (Shapiro Wilk Test for normality; values >.05), and (2) variances of the differences across all groups were equal (Levene’s test for homogeneity for between subject ANOVAS, values >0.05 and Greenhouse Geisser test for sphericity for repeated measures/ mixed design ANOVAs; values >0.075.). For all rats, BEC was measured and analyzed using a two-way analysis of variance (ANOVA) with gavage day as a repeated-measures factor and treatment as between-group factor. In Experiment 1, total time of social interaction with the familiar and novel juvenile rats during Trial 2 was recorded and analyzed using a three-way ANOVA with juvenile type (familiar or novel) as a repeated-measures factor and treatment and sex as between-groups factors. A significant interaction was followed by tests of simple effects of juvenile type at each level of treatment.
For Experiment 2, we calculated the following factors for the probabilistic learning/reversal phases: (1) the total number of errors made before the first discrimination in each session was completed using a three-way ANOVA (session × treatment × sex); (2) the number of trials needed to complete the first 3 reversals in one session by summing the number of all trials (from the previous sessions in which 3 reversals were not reached and the number of trials from the session in which 3 reversals were completed); (3) the number of reversals completed per session (aka a number of blocks composed of 8 consecutive correct responses that occurred after the first discrimination of 8 correct consecutive responses was completed); (4) the average latency to make a response from the onset of each trial across the 12 sessions, (5) the total numbers of errors (sum of omissions and incorrect responses) and omissions made per session.
Furthermore, in order to assess whether AIE treatment altered reward or response-related negative feedback we analyzed each animal's choices according to the response-outcome from a preceding trial. Specifically, the likelihood that a rat followed a correct, rewarded lever choice with the same lever choice was assessed as a percentage of win-stay. A percentage of win-stay calculated from the number of trials on which a rat chose the correct lever that was rewarded in a previous trial (“stay”) divided by the total number of trials with rewarded (correct or incorrect) responses (“win”) *100.
Conversely, lose-shift percentage indicated how likely rats were to switch lever choices after receiving negative feedback (i.e., no reward) for a non-rewarded correct response on the preceding trial. These values were calculated from the number of trials on which a rat switched responding onto the other lever after not being rewarded for correct response in the previous trial (“shift”), divided by the total number of non-rewarded correct responses (“lose”)* 100. The percent of win-stay and lose-shift was analyzed using separate three-way (treatment × sex × session) ANOVAs.
The estimated population of ChAT interneurons was calculated using the optical fractionator function and group differences were analyzed using a two-way ANOVA with treatment and sex as between-group factors. We calculated the overall density of ChAT-labeled cells per mm3 in the NAc by dividing the sum of ChAT counts by the sum of the volumes sampled across 8 sections and group differences were analyzed using a two-way ANOVA with treatment and sex as between-groups factors.
Significant interactions were followed by tests of simple effects of treatment at each level of the sex factor. A main effect of session was further analyzed by conducting multiple comparisons with Bonferroni corrections.
Results
Blood Ethanol Content (BEC)
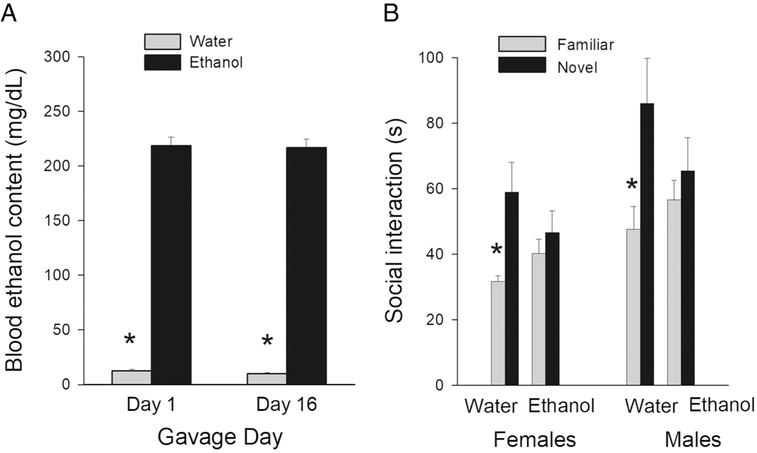
Control-gavages rats had BEC values that averaged 9-12 mg/dL, and those levels have been stated to be within the range of noise for this assay (see Vore et al, 2017). Thus, for AIE rats (both experiments), when correct for this assay error, reached approximately 190 mg/dL, rather than 200 mg/dL. The BEC of AIE rats higher than those of the water-treated rats after the first and last gavage treatments (Days 1 and 16) (see Figure 1A; F[1, 68]=1360.78; p< 0.001), but no treatment × day interaction nor main effect of gavage day. When the AIE rats were examined there was not an effect of session, F(1, 34)=0.02; p> 0.15. Thus, there was no ethanol tolerance in BEC across the duration of treatment.
Figure 1.
A: Mean (± SEM) blood ethanol content after the first and last ethanol exposure in AIE- or water-treated rats. * represents a significant treatment effect, p < 0.05.
B. Social recognition test: Mean (± SEM) time engaged in social interaction with familiar and novel juveniles for AIE- or water-treated male and female rats. * represents a significant difference in the amount of time spent interacting with familiar and novel juveniles, p< 0.05.
Experiment 1: Social Recognition
During the social recognition test, Control male and female rats, which were subjected to water-gavage treatment, spent more time interacting with a novel juvenile rat than with a familiar juvenile (see Figure 1B). In contrast, AIE male and female rats spent similar amounts of time interacting with novel or familiar juvenile rats (see Figure 1B), demonstrating a lack of social recognition. A three-way ANOVA with juvenile type (familiar or novel) as a repeated-measures factor and treatment and sex as between-groups factors revealed a significant novel/familiar × treatment interaction, F(1, 28)=6.796; p< 0.05, but no novel/familiar × treatment × sex interaction, F(1, 28)=0.211; p=0.65. Tests of simple effects of novel/familiar at each level of treatment revealed a significant difference in novel/ familiar type activity for Control rats, F(1, 28)=22.95; p< 0.01, but not for AIE-treated rats, F(1, 28)=1.22; p> 0.10.
Experiment 2: Probabilistic Learning and Rule Reversal.
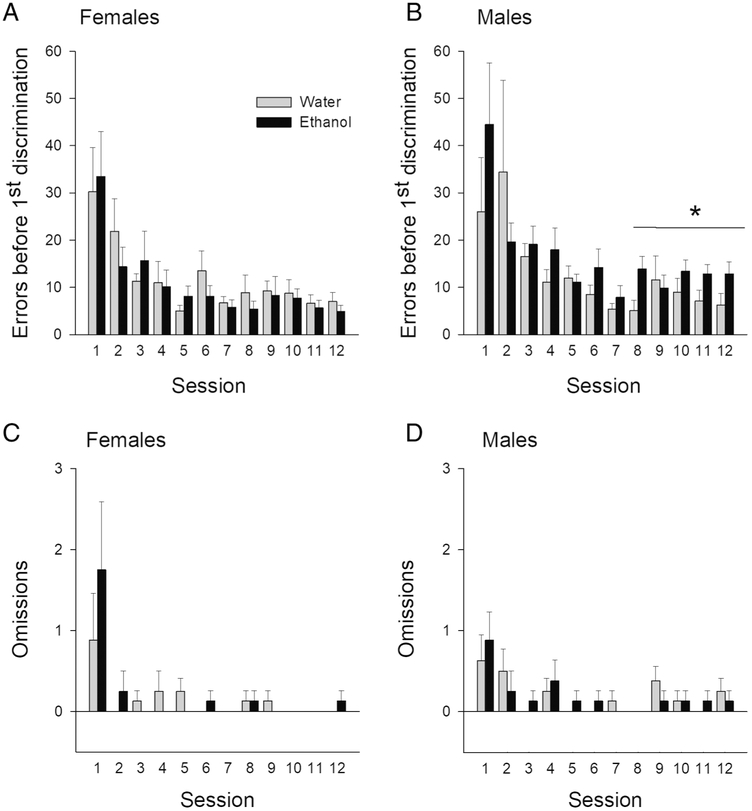
We first examined whether the groups differed in the number of errors made before completing the first successful discrimination in each session as this measure would reveal deficits in probabilistic learning. In general, rats made fewer learning errors in the later sessions than in early sessions. However, AIE-treated male rats, relative to Control male rats, made more errors when learning the first discrimination rule of each session, particularly in the later sessions (see Figure 2A). Separate three-way ANOVAs (session × treatment × sex) for the first 6 and the last 6 sessions revealed a significant main effect of session, F(5, 140)=7.23; p< 0.001 in the first 6 sessions, and a significant treatment × sex interaction in the last 6 sessions, F(1, 28)=6.07; p< 0.05. Tests of simple effects of treatment as a function of sex revealed that AIE-treated males made significantly more errors when completing the first discrimination in the last 6 sessions of training, F(1, 14)=6.28; p<0.05, whereas female AIE-treated rats did not different from controls (see Figure 2B) on learning and sustaining the probabilistic rule, F(1, 14)=0.90; p=0.39. AIE and control groups did not differ in the number of omissions they made (see Figure 2C and D). A three-way ANOVA (session × treatment × sex) showed a significant session effect (F[11, 308]=8.33; p< 0.05), but no session × treatment × sex interaction, (F[11, 308]=0.66; p= 0.72). Post hoc tests with Bonferroni correction for multiple comparisons revealed that session 1 was significantly different from all other sessions and session 2 was different from sessions 7 and 11, p ‘s<.05, suggesting that AIE-treated rats were capable of responding and similarly to the control, they made fewer omissions with additional training.
Figure 2.
Errors and omissions during probabilistic learning and reversal: Mean (± SEM) number of errors made by AIE- or water-treated female (A) and male (B) rats during learning the probabilistic reward rule. Mean (± SEM) number of omissions in each session made by AIE- or water-treated female (C) and male (D) across 12 sessions. * represents a significant treatment effect in the last 6 sessions, p< 0.05.
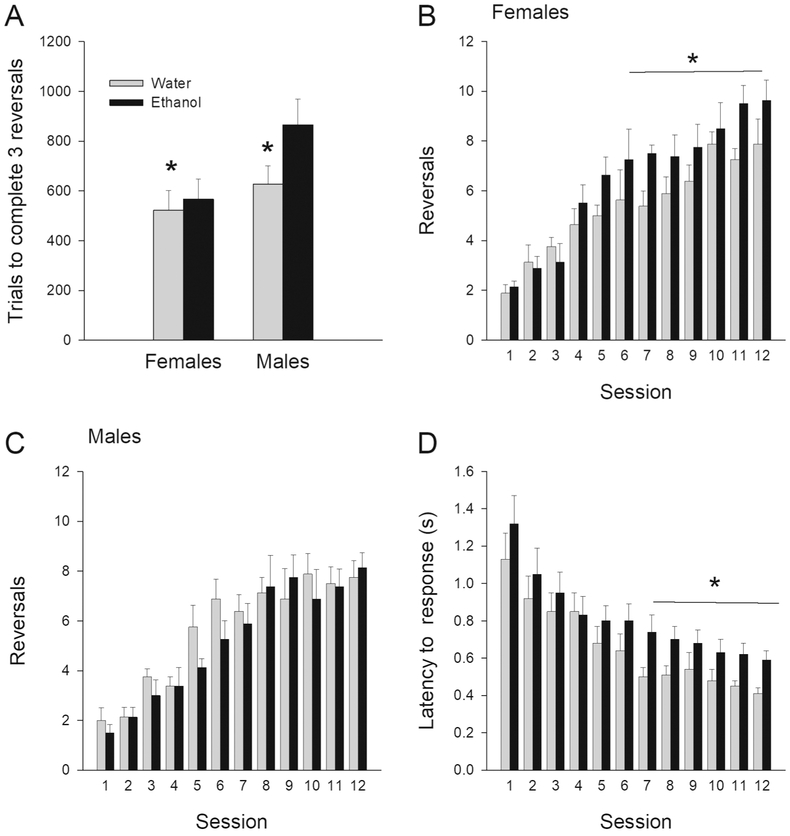
When the probabilistic rule was switched or reversed, we asked the question whether or not the treatment conditions differed in the number of trials needed to complete the first 3 reversals within a session. This measure provides insight into the initial ability of rats to adjust behavioral strategies. Figure 3A shows that AIE-treated rats required more trials to complete first 3 reversals than Control rats, regardless of sex. A two-way ANOVA (treatment × sex) confirmed this observation revealing a significant treatment effect, F(1, 28)=5.594; p< 0.05, but not treatment × sex interaction F(1, 28)=1.297; p=0.26, nor main effect of sex, F(1, 28)=2.76; P=0.108. Thus, AIE-treated rats tended to be initially impaired at implementing shifting strategies.
Figure 3.
Metrics during probabilistic rule reversal: Mean (± SEM) number of trials needed to complete the first 3 reversals in a session by AIE- or water-treated male and female rats (A). Mean (± SEM) number of reversals completed across 12 sessions by AIE- or water-treated (B) females and (C) males. Mean (± SEM) response latencies across 12 sessions for AIE- or water-treated rats (D; no sex differences so males and females combined). * represents a significant treatment effect, p< 0.05.
Additional analyses compared the total number of reversals completed per session over the 12 days of training, using a three-way ANOVA, with treatment and sex as between-groups factor and sessions as repeated-measures factor. This analysis revealed a significant treatment × sex interaction, F(1, 28)=4.591; p< 0.05, and main effect of session, F(11, 308)=41.361; p< 0.001. Tests of simple effects of treatment as a function of sex revealed a significant treatment effect for female rats (F[1, 14]=5.65; p< 0.05), but not for male rats. Surprisingly, AIE female rats completed a higher number of reversals per session than water-treated female rats (see Figure 3B), suggesting that with extended experience with rule shifting, they eventually could adapt behavior more effectively. AIE males reached a similar number of reversals per session as compared to the Control males (see Figures 3C).
With respect to response latencies, on average, AIE-treated rats took longer to make a response than control rats, and this was particularly pronounced in the last 6 sessions (see Figure 3D). A three-way ANOVA (session × treatment × sex) revealed a significant main effect of sessions, F(11, 308)=36.24; p< 0.001. Given the session effect, we conducted separate three-way ANOVAs analyze latencies to make a response in the first 6 sessions and also contrasted with the last 6 sessions. A three-way ANOVA for the first 6 sessions revealed a significant main effect of session, F(5, 140)=29.667; p< 0.001. A three-way ANOVA for the last 6 sessions revealed a significant main effects of session, F(5, 140)=4.677; p< 0.001, and treatment, F(1, 28)=5.447; p< 0.05. There was no significant session × treatment × sex interactions.
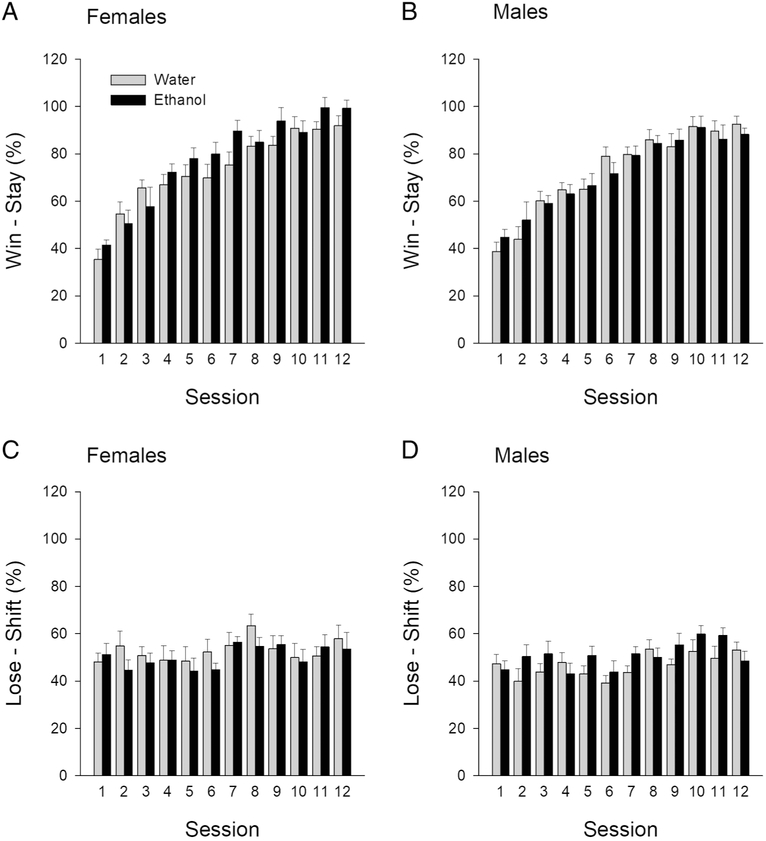
To determine whether AIE treatment altered reward sensitivity we analyzed a percent win-stay across 12 sessions. A three-way ANOVA (treatment × sex × session) revealed a significant session effect, F(11, 308)= 68.74; p< 0.05, but no treatment × sex × session interaction, F(11, 308)=1.137; p=0.33. This finding suggests that all rats, regardless of the treatment, showed response-outcome learning and that AIE treatment produced no changes in reward sensitivity (see Figure 4A and B). To determine whether AIE treatment produced changes in response-related negative feedback we analyzed a percent lose-shift across 12 sessions. A three-way ANOVA (treatment × sex × session) revealed a significant session effect (F[11, 308]=2.82; p< 0.05), but no treatment × sex × session interaction (F[11, 308]=0.84; p=0.59), suggesting that animals, regardless of the treatment adjusted behavior based on negative feedback (see Figure 4C and D).
Figure 4.
Reward strategies adapted during probabilistic rule reversal: Percent win-stay achieved by AIE- or water-treated female (A) and male (B) rats in each of the 12 sessions. Percent lose-shift achieved by AIE- or water-treated female (C) and male (D) rats in each of the 12 sessions.
Experiment 3: Cholinergic interneurons in the NAc.
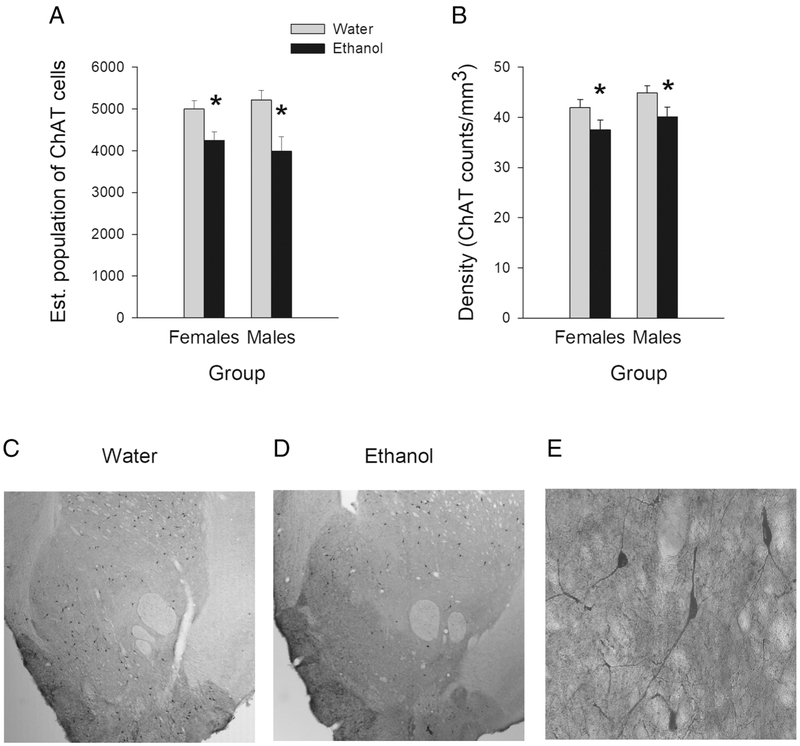
Figure 5 displays cell counts, cell density and representative NAc sections with ChAT positive cells. Adult rats treated with ethanol during adolescence had fewer cholinergic interneurons (ChAT+ cells) within the NAc than rats previously treated with water (see Figure 5A). A two-way ANOVA with treatment and sex as between-groups factors on the estimated population of ChAT+ cells revealed a significant treatment effect, F (1, 30)=15.389; p< 0.01, but no sex effect or treatment × sex interaction. The density of cholinergic interneurons in the NAc was lower in AIE animals than in Control animals (see Figure 5B). A two-way ANOVA (treatment × sex) revealed a significant treatment effect (F[1, 30]=6.927; p< 0.05), but no sex differences or treatment × sex interaction.
Figure 5.
Data and images of NAc cholinergic interneurons following adolescent intermittent ethanol exposure: (A) Mean (± SEM) estimated population of ChAT positive cells in the NAc of AIE- or water-treated male and female rats. (B) Mean (± SEM) density of ChAT positive cells in the NAc in AIE- or water-treated male and female rats. Representative NAc sections showing ChAT+ cells in water-treated (C) or AIE-treated animals (D). These representative images were taken at the 2.5 X magnification that was used to trace the NAc area. (E) Representative image of ChAT positive cells at the 40 X magnification that was used to count cells. * represent a significant treatment effect, p< 0.05.
Discussion
In the present study, we evaluated behavioral dysfunctions and neuropathology of the ventral striatum cholinergic system attributed to AIE exposure. BEC levels during AIE treatment reached about 190 mg/dl, regardless of sex, and showed little change throughout the duration of treatment. Human adolescents reach higher BAC levels than adults (Donovan 2009), with the youngest drinkers often reaching the highest BAC, up to 3-times the standard intoxicated level of 0.08 g/dL. A growing percentage of adolescent binge drinkers fall into a category of high-intensity drinking, consuming two or three times the 4-5 drink/2-hr binge threshold and reaching very high BAC (Patrick and Azar 2017). The AIE model represents binge to high-intensity drinking, and therefore sheds light on the long-term consequences of this type of developmental alcohol exposure.
Social recognition, a form of short-term memory, was dramatically impaired in adulthood following AIE. During a social recognition task, control animals interacted more with the novel juvenile than with the familiar juvenile, indicating their ability to remember and recognize a familiar animal. In contrast, AIE-treated rats spent similar amounts of time interacting with familiar and novel juveniles. This failure to recognize a familiar juvenile (after a short delay) demonstrates impaired non-spatial short-term memory in AIE-treated rats. Our results are congruent with previous studies demonstrating deficits in social working memory after early postnatal or adolescent ethanol exposure (Kelly and Tran 1997; Oliveira et al. 2015). Social recognition is mediated by the neuronal circuit that involves the PFC, anterior cingulate cortex (ACC), NAc, basolateral amygdala and dorsal and ventral hippocampus (Kogan et al. 2000; Maaswinkel et al. 1996; Okuyama et al. 2016; Tanimizu et al. 2017; Terranova et al. 1994; van Wimersma Greidanus and Maigret 1996). A significant expression of immediate-early gene c-Fos (aka a biomarker for neuronal activity) was observed in these brain regions during social recognition tasks in rodents (Tanimizu et al. 2017). However, ibotenic acid lesions of the hippocampus (Kogan et al. 2000) or optogenetic inhibition of ventral hippocampal cell bodies or their axonal terminals in the NAc (Okuyama et al. 2016) resulted in social recognition deficits. AIE treatment has been shown to cause persistent dysfunction or morphological alterations in some of the brain regions that are important for social recognition (Boutros et al. 2014; Broadwater et al. 2018; Coleman et al. 2014; Fernandez and Savage 2017; Jury et al. 2017; Pascual et al. 2009). This is interesting as spatial working memory, which is more dependent on the dorsal hippocampus (Chawla et al. 2018; Fanselow and Dong 2010), is not impaired after AIE (Fernandez and Savage 2017; Vetreno and Crews 2012).
A key novel finding of this study was that AIE-treated rats also showed impairments in probabilistic learning and rule reversal; however, some effects were sex dependent. A sex difference emerged when the number of errors made to complete the first discrimination was examined: AIE-treated male rats, relative to controls, made more errors before completing the first discrimination in each session. This suggests that probabilistic reinforcement learning was impaired in AIE-treated males. Although they were able to reach a similar number of reversals across sessions to the control rats, AIE-treated rats, both male and female, required more trials to complete the first 3 reversals in a session, suggesting an initial impairment in the ability to adjust behavior according to changes in reward outcome. Specifically, when the response-outcome contingency changed after 8 consecutive correct responses, AIE-treated rats showed cognitive-behavioral inflexibility, as manifested in a higher number of trials required to complete 3 reversals in a session. An unexpected finding was that with extended experience on the probabilistic reversal task, female rats exposed to AIE were eventually able to make more reversals per session, relative to female control rats. This is suggestive that female rats exposed to AIE, unlike male rats exposed to AIE, display a capacity for cognitive recovery.
On the other hand, AIE treatment resulted in response latencies that were longer than for control rats in both males and females, indicating that AIE-treated animals required more time to make a decision on regarding the appropriate action. AIE-treated animals appear to have difficulties with complex information processing, as indicated by longer latencies for lever selection, a higher number of trials needed to meet the criterion of 3 reversals per session and, for males, a higher number of errors in acquiring probabilistic reward learning. Notably, AIE treatment did not alter animals’ sensitivity to either negative or in particular, rewarded feedback, as lose-shift and win-stay behavior was comparable across groups. This latter finding suggests that the increase in response latencies are unlikely to be attributable to motivational deficits.
There is evidence that probabilistic reversal learning is mediated by cortico-striato-limbic circuits (Amodeo et al. 2017; Dalton et al. 2014; Dalton et al. 2016). Inactivation of medial orbitofrontal cortex (OFC) or NAc shell (but not core) leads to a decrease in a number of reversals completed by rats during this task (Dalton et al. 2014; Dalton et al. 2016). Patients with OFC damage have been reported to perform deficiently on the probabilistic reversal-learning task (Tsuchida et al. 2010). Inactivation of (prelimbic) PFC in rodents leads to improved performance by increasing the number of reversals completed (Dalton et al. 2016) and in healthy individuals behavioral reversals are accompanied by significant signal change in the PFC and ventral striatum (Cools et al. 2002; Evers et al. 2005). In addition, reversal deficits have been also documented after damage to OFC (Boulougouris et al. 2007), PFC (Rygula et al. 2010), amygdala (Izquierdo and Murray 2004; Schwartzbaum and Poulos 1965), hippocampus (Shohamy et al. 2009), pedunculopontine tegmental nucleus (Syed et al. 2016) and dorsal striatum (Grospe et al. 2018). It has been well documented that these regions are affected by ethanol neurotoxicity (Broadwater et al. 2018; Coleman et al. 2014; Fernandez and Savage 2017; Gass et al. 2014; Vetreno et al. 2014). This being the case, it is reasonable to propose that the alterations in probabilistic learning induced by AIE may be driven by pathophysiological alterations in mOFC and ventral striatal circuits.
In this regard, it is notable that in addition to cognitive-behavioral dysfunctions, we observed a reduction in ChAT positive cells within the NAc of AIE-treated rats, compared to Control rats. The reduction in the cholinergic population was 15% in females and 25% in males, but this difference was not statistically significant. These findings indicate that intermittent ethanol exposure during adolescence leads to a significant long-term loss of cholinergic interneurons in the NAc. Although it has been reported that the number of cholinergic interneurons within dorsal lateral striatum is reduced after AIE (Vetreno et al, 2014), this is the first study to demonstrate a loss of cholinergic interneurons in the ventral striatum, specifically the NAc. This data supports the notion that cholinergic neurons, regardless of location, are very sensitive to AIE and do not fully recover even with an extended period without ethanol exposure.
Furthermore, this cholinergic pathology suggests a novel mechanism by which AIE may modulate NAc dopamine dysfunction. It is well known that binge-type ethanol exposure during adolescence alters neurotransmitter signaling within the NAc, particularly dopamine function, some of which is sex-specific (Badanich et al. 2007; Cozzoli et al. 2016; Maldonado-Devincci et al. 2010; Pascual et al. 2009; Shniko et al. 2017). The dopamine reward system is regulated by cholinergic interneurons within the NAc (Hanada et al, 2018). Cholinergic tone within the NAc modulates behavioral responses to both drugs and natural reward (Hoebel et al. 2007; Williams and Adinoff, 2008). Evidence suggests that acetylcholine activates the dopamine release through stimulation α4β2 nAChRs and M5 muscarinic receptors within the NAc (Hanada et al. 2018). Thus, the loss of cholinergic interneurons within the NAc by AIE will impact dopamine-dependent behaviors. Further investigation into cholinergic-dopamine interactions within the NAc following AIE will provide novel insights into behavioral change following prolonged adolescent drinking.
These results correspond with previous studies demonstrating ethanol-induced hypofunction of the cholinergic system, as measured by a reduced population of cells expressing ChAT and a reduction of ACh efflux. A loss of cholinergic neurons in forebrain regions, such as medial septal nucleus, diagonal band, and nucleus basalis magnocellularis, has been observed after AIE treatment (Boutros et al. 2014; Ehlers et al. 2011; Fernandez and Savage 2017; Swartzwelder et al. 2015; Vetreno et al. 2014), prolonged ethanol consumption (Arendt et al. 1989; Cadete-Leite et al. 2003; Lukoyanov et al. 2003; Savage et al. 2000) and prenatal exposure (Swanson et al. 1995). Chronic ethanol consumption can also decrease behavior-stimulated ACh efflux in the hippocampus and cortex (Casamenti et al. 1993; Melis et al. 1996). Similar deficits in ACh efflux in the PFC have been observed after AIE treatment (Fernandez and Savage 2017). Interestingly, the degree of ethanol-induced cholinergic damage depends on the timing of ethanol exposure. For example, binge ethanol exposure during adolescence, but not adulthood, can lead to global loss of cholinergic cells (Vetreno et al. 2014). This might explain a discrepancy between our results and a previous study demonstrating no morphological changes in size and density of cholinergic striatal interneurons after adult chronic ethanol consumption (Pereira et al. 2014).
Cholinergic interneurons in the NAc control local circuits (Cachope et al. 2012; Higley et al. 2011; Threlfell et al. 2012), modulate the activity of GABAergic projection neurons (Witten et al. 2010), as well as dopamine release (Cachope et al. 2012). They have been implicated in associative learning (Brown et al. 2012; Joshua et al. 2008) as well as attention set shift task (Aoki et al. 2015). Rats with lesions to cholinergic interneurons in the NAc have been shown to make more errors than non-lesioned rats on tasks that require attention shifts from a stimulus to a novel stimulus (Aoki et al. 2015). In addition, the NAc has been implicated in other forms of cognitive flexibility including the maze-based strategy set shifting, (Floresco et al. 2006), delayed matching-to-sample (Burk and Mair 2001), matching to position (Reading and Dunnett 1991) and probabilistic reversal-learning tasks (Dalton et al. 2014). In the present study, we demonstrate that AIE treatment can impair probabilistic learning/reversal, non-spatial short-term memory and lead to pathology of cholinergic interneurons in the NAc. There is also evidence that the PFC, another brain region involved in behavioral flexibility and working memory, is affected by AIE exposure (Badanich et al. 2016; Broadwater and Spear 2013; Broadwater et al. 2018; Coleman et al. 2014; Fernandez and Savage 2017; Gass et al. 2014; Kuzmin et al. 2012). Thus, it is tempting to suggest here that AIE treatment leads to cognitive-behavioral dysfunction and that ethanol-induced cortico-striato-limbic pathology might play a role in this process.
In conclusion, the present study demonstrates AIE-induced long lasting impairment of working memory as measured by a social recognition task and behavioral inflexibility as measured in a probabilistic reversal-learning task. These data are the first to demonstrate the loss of cholinergic interneurons after AIE-treatment and constitute new evidence for ethanol-induced cortico-striatal hypofunctionality. Our results might contribute to better understanding of behavioral and cholinergic neuropathologies caused by adolescence binge drinking and point toward the cholinergic system as a potential target in the development of new pharmacotherapies for alcohol use disorders.
Highlights:
Adolescent intermittent ethanol exposure reduces the number of cholinergic interneurons in the nucleus accumbens in both sexes.
Short-term social memory is impaired by adolescent intermittent ethanol exposure in both sexes.
Male rats exposed to intermittent ethanol during adolescence display less effective probabilistic reward learning with extended training.
Intermittent ethanol during adolescence reduces the effectiveness of both sexes to adjust initial reversal strategies.
Rats exposed to intermittent ethanol during adolescence, regardless of sex, require more time to make decisions.
Acknowledgments
Funding sources: The research was supported by the Developmental Alcohol Exposure Research Center at Binghamton University (P50AA017823); The Developmental and Neuroadaptations in Alcohol and Addiction Training grant (T32AA025606) and NIAAA R01 grant to LMS (RO1AA021775) and a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada to SBF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aloe L, Tirassa P (1992) The effect of long-term alcohol intake on brain NGF-target cells of aged rats. Alcohol 9: 299–304. [DOI] [PubMed] [Google Scholar]
- Amodeo LR, McMurray MS, Roitman JD (2017) Orbitofrontal cortex reflects changes in response-outcome contingencies during probabilistic reversal learning. Neuroscience 345: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki S, Liu AW, Zucca A, Zucca S, Wickens JR (2015) Role of Striatal Cholinergic Interneurons in Set-Shifting in the Rat. J Neurosci 35: 9424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt T, Allen Y, Marchbanks RM, Schugens MM, Sinden J, Lantos PL, Gray JA (1989) Cholinergic system and memory in the rat: effects of chronic ethanol, embryonic basal forebrain brain transplants and excitotoxic lesions of cholinergic basal forebrain projection system. Neuroscience 33: 435–62. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Maldonado AM, Kirstein CL. (2007) Chronic ethanol exposure during adolescence increases basal dopamine in the nucleus accumbens septi during adulthood. Alcohol Clin Exp Res, 31, 895–900. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Fakih ME, Gurina TS, Roy EK, Hoffman JL, Uruena-Agnes AR, Kirstein CL (2016) Reversal learning and experimenter-administered chronic intermittent ethanol exposure in male rats. Psychopharmacology (Berl) 233: 3615–26. [DOI] [PubMed] [Google Scholar]
- Beylergil SB, Beck A, Deserno L, Lorenz RC, Rapp MA, Schlagenhauf F, Heinz A, Obermayer K (2017) Dorsolateral prefrontal cortex contributes to the impaired behavioral adaptation in alcohol dependence. NeuroImage Clinical 15: 80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeley CP, Cains S, Smith R, Bracci E (2011) Ethanol affects striatal interneurons directly and projection neurons through a reduction in cholinergic tone. Neuropsychopharmacology 36: 1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW (2007) Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res 179: 219–28. [DOI] [PubMed] [Google Scholar]
- Boutros N, Semenova S, Liu W, Crews FT, Markou A (2014) Adolescent intermittent ethanol exposure is associated with increased risky choice and decreased dopaminergic and cholinergic neuron markers in adult rats. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Spear LP (2013) Consequences of ethanol exposure on cued and contextual fear conditioning and extinction differ depending on timing of exposure during adolescence or adulthood. Behav Brain Res 256: 10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater MA, Lee SH, Yu Y, Zhu H, Crews FT, Robinson DL, Shih YI (2018) Adolescent alcohol exposure decreases frontostriatal resting-state functional connectivity in adulthood. Addict Biol 23: 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MT, Tan KR, O'Connor EC, Nikonenko I, Muller D, Luscher C (2012) Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature 492: 452–6. [DOI] [PubMed] [Google Scholar]
- Burk JA, Mair RG (2001) Effects of dorsal and ventral striatal lesions on delayed matching trained with retractable levers. Behav Brain Res 122: 67–78. [DOI] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, Lovinger DM, Cheer JF (2012) Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell reports 2: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadete-Leite A, Andrade JP, Sousa N, Ma W, Ribeiro-da-Silva A (1995) Effects of chronic alcohol consumption on the cholinergic innervation of the rat hippocampal formation as revealed by choline acetyltransferase immunocytochemistry. Neuroscience 64: 357–74. [DOI] [PubMed] [Google Scholar]
- Cadete-Leite A, Pereira PA, Madeira MD, Paula-Barbosa MM (2003) Nerve growth factor prevents cell death and induces hypertrophy of basal forebrain cholinergic neurons in rats withdrawn from prolonged ethanol intake. Neuroscience 119: 1055–69. [DOI] [PubMed] [Google Scholar]
- Carbia C, Cadaveira F, Lopez-Caneda E, Caamano-Isorna F, Rodriguez Holguin S, Corral M (2017) Working memory over a six-year period in young binge drinkers. Alcohol 61: 17–23. [DOI] [PubMed] [Google Scholar]
- Casamenti F, Scali C, Vannucchi MG, Bartolini L, Pepeu G (1993) Long-term ethanol consumption by rats: effect on acetylcholine release in vivo, choline acetyltransferase activity, and behavior. Neuroscience 56: 465–71. [DOI] [PubMed] [Google Scholar]
- Chawla MK, Sutherland VL, Olson K, McNaughton BL, Barnes CA (2018) Behavior-driven arc expression is reduced in all ventral hippocampal subfields compared to CA1, CA3, and dentate gyrus in rat dorsal hippocampus. Hippocampus 28: 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG Jr., He J, Lee J, Styner M, Crews FT (2011) Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol Clin Exp Res 35: 671–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG Jr., Liu W, Oguz I, Styner M, Crews FT (2014) Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol Biochem Behav 116: 142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW (2002) Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci 22: 4563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Kaufman MN, Nipper MA, Hashimoto JG, Wiren KM, Finn DA. (2016). Functional regulation of PI3K-associated signaling in the accumbens by binge alcohol drinking in male but not female mice. Neuropharmacology. 105:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, Broadwater MA, Robinson DL (2016) Adolescent Alcohol Exposure Persistently Impacts Adult Neurobiology and Behavior. Pharmacol Rev 68: 1074–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton GL, Phillips AG, Floresco SB (2014) Preferential involvement by nucleus accumbens shell in mediating probabilistic learning and reversal shifts. J Neurosci 34: 4618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton GL, Wang NY, Phillips AG, Floresco SB (2016) Multifaceted Contributions by Different Regions of the Orbitofrontal and Medial Prefrontal Cortex to Probabilistic Reversal Learning. J Neurosci 36: 1996–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan JE (2009) Estimated blood alcohol concentrations for child and adolescent drinking and their implications for screening instruments. Pediatrics 123: e975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT (2011) Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience 199: 333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers EA, Cools R, Clark L, van der Veen FM, Jolles J, Sahakian BJ, Robbins TW (2005) Serotonergic modulation of prefrontal cortex during negative feedback in probabilistic reversal learning. Neuropsychopharmacology 30: 1138–47. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW (2010) Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65: 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez GM, Savage LM (2017) Adolescent binge ethanol exposure alters specific forebrain cholinergic cell populations and leads to selective functional deficits in the prefrontal cortex. Neuroscience 361: 129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT (2008) Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res 190: 85–96. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S, Vexelman C, Magyar O (2006) Dissociable roles for the nucleus accumbens core and shell in regulating set shifting. J Neurosci 26: 2449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Moreno LM, Exposito J, Sanhueza C, Angulo MT (2008) [Prefrontal activity and weekend alcoholism in the young]. Adicciones 20: 271–9. [PubMed] [Google Scholar]
- Gass JT, Glen WB Jr., McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, Yaxley R, Floresco SB, Chandler LJ (2014) Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology 39: 2570–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghods-Sharifi S, Haluk DM, Floresco SB (2008) Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiol Learn Mem 89: 567–73. [DOI] [PubMed] [Google Scholar]
- Grospe GM, Baker PM, Ragozzino ME (2018) Cognitive Flexibility Deficits Following 6-OHDA Lesions of the Rat Dorsomedial Striatum. Neuroscience 374: 80–90. [DOI] [PubMed] [Google Scholar]
- Hall JM, Savage LM (2016) Exercise leads to the re-emergence of the cholinergic/nestin neuronal phenotype within the medial septum/diagonal band and subsequent rescue of both hippocampal ACh efflux and spatial behavior. Exp Neurol 278: 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada Y, Kawahara Y, Oshishi YN, Shuto T, Kuroiwa M, Greengard P, Nishi A. (2018). p11 in cholinergic interneurons of the nucleus accumbens is essential for dopamine responses to rewarding stimuli. eNeuro. ENEURO.0332-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen N, Niskanen E, Kononen M, Tolmunen T, Kekkonen V, Kivimaki P, Tanila H, Laukkanen E, Vanninen R (2017) Alcohol consumption during adolescence is associated with reduced grey matter volumes. Addiction 112: 604–613. [DOI] [PubMed] [Google Scholar]
- Herring BE, Mayfield RD, Camp MC, Alcantara AA (2004) Ethanol-induced Fos immunoreactivity in the extended amygdala and hypothalamus of the rat brain: focus on cholinergic interneurons of the nucleus accumbens. Alcohol Clin Exp Res 28: 588–97. [DOI] [PubMed] [Google Scholar]
- Higley MJ, Gittis AH, Oldenburg IA, Balthasar N, Seal RP, Edwards RH, Lowell BB, Kreitzer AC, Sabatini BL (2011) Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PLoS One 6: e19155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA (2004) Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. J Neurophysiol 91: 2023–39. [DOI] [PubMed] [Google Scholar]
- Joshua M, Adler A, Mitelman R, Vaadia E, Bergman H (2008) Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditioning trials. J Neurosci 28: 11673–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jury NJ, Pollack GA, Ward MJ, Bezek JL, Ng AJ, Pinard CR, Bergstrom HC, Holmes A (2017) Chronic Ethanol During Adolescence Impacts Corticolimbic Dendritic Spines and Behavior. Alcohol Clin Exp Res 41: 1298–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Tran TD (1997) Alcohol exposure during development alters social recognition and social communication in rats. Neurotoxicol Teratol 19: 383–9. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Silva AJ (2000) Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus 10: 47–56. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Liljequist S, Meis J, Chefer V, Shippenberg T, Bakalkin G (2012) Repeated moderate-dose ethanol bouts impair cognitive function in Wistar rats. Addict Biol 17: 132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukoyanov NV, Pereira PA, Paula-Barbosa MM, Cadete-Leite A (2003) Nerve growth factor improves spatial learning and restores hippocampal cholinergic fibers in rats withdrawn from chronic treatment with ethanol. Exp Brain Res 148: 88–94. [DOI] [PubMed] [Google Scholar]
- Maaswinkel H, Baars AM, Gispen WH, Spruijt BM (1996) Roles of the basolateral amygdala and hippocampus in social recognition in rats. Physiol Behav 60: 55–63. [DOI] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Badanich KA, Kirstein CL (2010). Alcohol during adolescence selectively alters immediate and long-term behavior and neurochemistry. Alcohol, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ (2003) Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res 146: 97–103. [DOI] [PubMed] [Google Scholar]
- McMurray MS, Amodeo LR, Roitman JD (2016) Consequences of Adolescent Ethanol Consumption on Risk Preference and Orbitofrontal Cortex Encoding of Reward. Neuropsychopharmacology 41: 1366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis F, Stancampiano R, Imperato A, Carta G, Fadda F (1996) Chronic ethanol consumption in rats: correlation between memory performance and hippocampal acetylcholine release in vivo. Neuroscience 74: 155–9. [DOI] [PubMed] [Google Scholar]
- Mell T, Wartenburger I, Marschner A, Villringer A, Reischies FM, Heekeren HR (2009) Altered function of ventral striatum during reward-based decision making in old age. Frontiers in human neuroscience 3: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S (2016) Ventral CA1 neurons store social memory. Science 353: 1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen MV, Needham EK, Pakkenberg B (2017) The Optical Fractionator Technique to Estimate Cell Numbers in a Rat Model of Electroconvulsive Therapy. Journal of visualized experiments : JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AC, Pereira MC, Santana LN, Fernandes RM, Teixeira FB, Oliveira GB, Fernandes LM, Fontes-Junior EA, Prediger RD, Crespo-Lopez ME, Gomes-Leal W, Lima RR, Maia Cdo S (2015) Chronic ethanol exposure during adolescence through early adulthood in female rats induces emotional and memory deficits associated with morphological and molecular alterations in hippocampus. J Psychopharmacol 29: 712–24. [DOI] [PubMed] [Google Scholar]
- Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J, Heinz A (2010) Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J Neurosci 30: 7749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C (2009) Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem 108: 920–31. [DOI] [PubMed] [Google Scholar]
- Patrick M, Azar B (2017) High Intensity Drinking. Alcohol Res: Curr Rev 39: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira PA, Neves J, Vilela M, Sousa S, Cruz C, Madeira MD (2014) Chronic alcohol consumption leads to neurochemical changes in the nucleus accumbens that are not fully reversed by withdrawal. Neurotoxicol Teratol 44: 53–61. [DOI] [PubMed] [Google Scholar]
- Prado VF, Martins-Silva C, de Castro BM, Lima RF, Barros DM, Amaral E, Ramsey AJ, Sotnikova TD, Ramirez MR, Kim HG, Rossato JI, Koenen J, Quan H, Cota VR, Moraes MF, Gomez MV, Guatimosim C, Wetsel WC, Kushmerick C, Pereira GS, Gainetdinov RR, Izquierdo I, Caron MG, Prado MA (2006) Mice deficient for the vesicular acetylcholine transporter are myasthenic and have deficits in object and social recognition. Neuron 51: 601–12. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP (1999) Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci 19: 4585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading PJ, Dunnett SB (1991) The effects of excitotoxic lesions of the nucleus accumbens on a matching to position task. Behav Brain Res 46: 17–29. [DOI] [PubMed] [Google Scholar]
- Renteria R, Baltz ET, Gremel CM (2018) Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nature communications 9: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygula R, Walker SC, Clarke HF, Robbins TW, Roberts AC (2010) Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. J Neurosci 30: 14552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza C, Garcia-Moreno LM, Exposito J (2011) Weekend alcoholism in youth and neurocognitive aging. Psicothema 23: 209–14. [PubMed] [Google Scholar]
- Savage LM, Candon PM, Hohmann HL (2000) Alcohol-induced brain pathology and behavioral dysfunction: using an animal model to examine sex differences. Alcohol Clin Exp Res 24: 465–75. [PubMed] [Google Scholar]
- Schwartzbaum JS, Poulos DA (1965) Discrimination behavior after amygdalectomy in monkeys: learning set and discrimination reversals. J Comp Physiol Psychol 60: 320–8. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Hopkins RO, Sage J, Gluck MA (2009) Distinct hippocampal and basal ganglia contributions to probabilistic learning and reversal. Journal of cognitive neuroscience 21: 1821–33. [DOI] [PubMed] [Google Scholar]
- Shnitko TA, Spear LP, Robinson DL. (2016). Adolescent binge-like alcohol alters sensitivity to acute alcohol effects on dopamine release in the nucleus accumbens of adult rats. Psychopharm, 233:361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2018) Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci 19: 197–214. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, Pfefferbaum A (2015) Brain development in heavy-drinking adolescents. Am J Psychiatry 172: 531–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson DJ, King MA, Walker DW, Heaton MB (1995) Chronic prenatal ethanol exposure alters the normal ontogeny of choline acetyltransferase activity in the rat septohippocampal system. Alcohol Clin Exp Res 19: 1252–60. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Acheson SK, Miller KM, Sexton HG, Liu W, Crews FT, Risher ML (2015) Adolescent Intermittent Alcohol Exposure: Deficits in Object Recognition Memory and Forebrain Cholinergic Markers. PLoS One 10: e0140042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed A, Baker PM, Ragozzino ME (2016) Pedunculopontine tegmental nucleus lesions impair probabilistic reversal learning by reducing sensitivity to positive reward feedback. Neurobiol Learn Mem 131: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimizu T, Kenney JW, Okano E, Kadoma K, Frankland PW, Kida S (2017) Functional Connectivity of Multiple Brain Regions Required for the Consolidation of Social Recognition Memory. J Neurosci 37: 4103–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova JP, Perio A, Worms P, Le Fur G, Soubrie P (1994) Social olfactory recognition in rodents: deterioration with age, cerebral ischaemia and septal lesion. Behav Pharmacol 5: 90–98. [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ (2012) Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75: 58–64. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Centanni SW, Garr SC, New NN, Mulholland PJ, Gass JT, Glover EJ, Floresco SB, Crews FT, Krishnan HR, Pandey SC, Chandler LJ (2017) Binge-Like Alcohol Exposure During Adolescence Disrupts Dopaminergic Neurotransmission in the Adult Prelimbic Cortex. Neuropsychopharmacology 42: 1024–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida A, Doll BB, Fellows LK (2010) Beyond reversal: a critical role for human orbitofrontal cortex in flexible learning from probabilistic feedback. J Neurosci 30: 16868–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wimersma Greidanus TB, Maigret C (1996) The role of limbic vasopressin and oxytocin in social recognition. Brain Res 713: 153–9. [DOI] [PubMed] [Google Scholar]
- Vedder LC, Hall JM, Jabrouin KR, Savage LM (2015) Interactions between chronic ethanol consumption and thiamine deficiency on neural plasticity, spatial memory, and cognitive flexibility. Alcohol Clin Exp Res 39: 2143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Broadwater M, Liu W, Spear LP, Crews FT (2014) Adolescent, but not adult, binge ethanol exposure leads to persistent global reductions of choline acetyltransferase expressing neurons in brain. PLoS One 9: e113421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT (2012) Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience 226: 475–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vore AS, Doremus-Fitzwater T, Gano A, Deak T. (2017). Adolescent Ethanol Exposure Leads to Stimulus-Specific Changes in Cytokine Reactivity and Hypothalamic-Pituitary-Adrenal Axis Sensitivity in Adulthood. Front Behav Neurosci. 11:78. doi: 10.3389/fnbeh.2017.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K (2010) Cholinergic interneurons control local circuit activity and cocaine conditioning. Science 330: 1677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]