Abstract
BACKGROUND:
Serum cortisol levels have been associated with type 2 diabetes (T2D). However, the role of cortisol in T2D and glycemia is not fully elucidated among African Americans (AAs). We hypothesized that among AAs morning serum cortisol would be positively associated with glycemic measures and prevalent T2D.
METHODS:
We examined the cross-sectional association of baseline morning serum cortisol with fasting plasma glucose (FPG), Hemoglobin A1c (HbA1c), homeostasis model assessment of insulin resistance (HOMA-IR), β-cell function (HOMA-β), and prevalent T2D in the Jackson Heart Study. Linear regression models were used to examine the association of log-transformed cortisol with glycemic traits, stratified by T2D status. Logistic regression was used to examine the association of log-transformed cortisol with prevalent T2D. Models were adjusted for age, sex, education, occupation, systolic blood pressure, waist circumference, physical activity, smoking, beta-blocker/hormone replacement medications and cortisol collection time.
RESULTS:
Among 4,206 AAs (mean age 55 ± 13 years, 64% female), 19% had prevalent T2D. A 100% increase in cortisol among participants without diabetes was associated with 2.7 mg/dL (95% CI: 2.0, 3.3) higher FPG and a 10.0% (95% CI: −14.0, 6.0) lower HOMA-β with no significant association with HbA1c or HOMA-IR. In participants with diabetes, a 100% increase in cortisol was associated with a 23.6 mg/dl (95% CI:13.6, 33.7) higher FPG and a 0.6% (95% CI: 0.3, 0.9) higher HbA1c. Among all participants, quartile 4 vs. 1 of cortisol was associated with a 1.26-fold (95% CI: 1.75, 2.91) higher odds of prevalent T2D.
CONCLUSION:
Higher morning serum cortisol was associated with higher FPG and lower β-cell function among participants without T2D and higher FPG and HbA1c in participants with diabetes. Among all participants, higher cortisol was associated with higher odds of T2D. These findings support a role for morning serum cortisol in glucose metabolism among AAs.
Keywords: cortisol, diabetes, glycemia, adiposity, obesity, insulin resistance
1.0. Introduction
Cortisol has been associated with components of the molecular pathogenesis of type 2 diabetes (T2D) including insulin resistance and impaired β-cell function. Insulin resistance and T2D are comorbities commonly associated with Cushing’s disease. Improper glucose homeostasis is promoted by hypercortisolism via the accumulation of visceral adipose tissue, impairment of insulin signaling in skeletal muscle, and the activation of lipolysis resulting in the release of free fatty acids (Anagnostis et al., 2009). In addition, states of subclinical hypercortisolism are often characterized by a redistribution of subcutaneous fat to visceral fat storage through differentiation and proliferation of adipocytes. This effect is likely mediated via glucocorticoid receptors as they are overexpressed in visceral relative to subcutaneous adipose tissue (Anagnostis et al., 2009). Therefore, due to the promotion of visceral adiposity and the direct as well as indirect interference with insulin signaling, cortisol likely plays a causal role in the pathogenesis of T2D.
Longitudinal studies analyzing the effect of baseline cortisol status and future development of impaired fasting plasma glucose (FPG) as well as incident diabetes suggest that alterations in cortisol precede deleterious changes in glucose metabolism. In the Whitehall II Study, HPA axis dysfunction characterized by elevations in evening cortisol at baseline was associated with increased incident diabetes nine years later (Hackett et al., 2016). Additionally, evidence from the Multiethnic Study of Atherosclerosis suggests that diabetes status at baseline is not associated with longterm changes in diurnal cortisol curve features (Spanakis et al., 2016). Therefore, the existing literature favors the hypothesis that changes in cortisol status occur prior to the development of insulin resistance and T2D.
In cross-sectional studies, elevated morning plasma cortisol has been correlated with greater insulin resistance, decreased β-cell function (insulin secretion) (Kamba et al., 2016), and higher odds of prevalent diabetes (Radin et al., 2016; Schoorlemmer et al., 2009) in majority white and Japanese studies. However, given the higher combined diagnosed and undiagnosed diabetes prevalence is among non-Hispanic black Americans (17.9%) compared to non-Hispanic white Americans (12.4%) (Mendola et al., 2018) and the disparities in microvascular complications, macrovascular complications and mortality(Fuchs, 2016), it is critical to examine cortisol and glycemia in AAs(Joseph and Golden, 2017b).
Despite the evidence of a link between hypercortisolism and dysglycemia, and the prevalence of T2D in AAs, data on the relationship of insulin resistance, β-cell function and glycemia with any measure of cortisol is limited among African Americans (AAs). One study in 61 AAs (20 with T2D) showed higher hair cortisol was associated with elevated glycated hemoglobin (HbA1c), suggesting that chronic exposure to elevated cortisol is associated with dysglycemia (Lehrer et al., 2016). Polymorphisms of the glucocorticoid gene that have been associated with glucocorticoid sensitivity, body fat, and insulin secretion have a higher homozygous prevalence in black vs. white Mississippians, indicating that AAs are potentially more susceptible to the effects of cortisol (Melcescu et al., 2012). Another study revealed a positive association of total salivary cortisol with higher HbA1c among individuals with T2D, but not in those without T2D and lower insulin resistance with higher wake-up and total diurnal cortisol prior to adjustment for adiposity in a combined cohort of whites, AAs, and Hispanic Americans without T2D (Joseph et al., 2015). Despite mechanistic evidence revealing detrimental effects of chronically elevated cortisol on glucose homeostasis supported by analyses of diverse populations, to our knowledge, no studies have examined the association of morning serum cortisol with glycemia and T2D specifically among AAs.
HPA axis measures differ significantly based on race/ethnicity, steeper awakening salivary cortisol response are observed in AAs compared to whites (Bennett et al., 2004). Furthermore, lower morning salivary cortisol, higher bedtime cortisol levels, and a “flatter” diurnal cortisol profile throughout the day are seen in blacks, independent of socioeconomic status (Cohen et al., 2006; DeSantis et al., 2007; Hajat et al., 2010; Karlamangla et al., 2013; Skinner et al., 2011; Zeiders et al., 2014). Though, importantly, “flatter” diurnal cortisol profiles are also seen in white individuals, they are seen in the presence of diseases like diabetes, as compared to healthy individuals(Hackett et al., 2016; Hackett et al., 2014). Though most of these studies have examined salivary cortisol, studying serum cortisol offers assessment of total cortisol (free and proteinbound), rather than free cortisol alone (Kosak et al., 2014), and serum sampling could allow for future development of an easily measurable clinically applicable biomarker and target for disease prevention and treatment.
Thus, using data from the Jackson Heart Study (JHS), a large AA cohort study, we examined the cross-sectional association of morning serum cortisol with fasting plasma glucose (FPG), Hemoglobin A1c (HbA1c), homeostasis model assessment of insulin resistance (HOMA-IR), and β-cell function (HOMA-β) and prevalent T2D. We hypothesized that, among AAs, morning serum cortisol would be positively associated with measures of glycemia, including FPG and HbA1c in those with prevalent T2D, and positively associated with insulin resistance, but negatively associated with β-cell function, in those without T2D.
2.0. Methods
2.1. Study Population
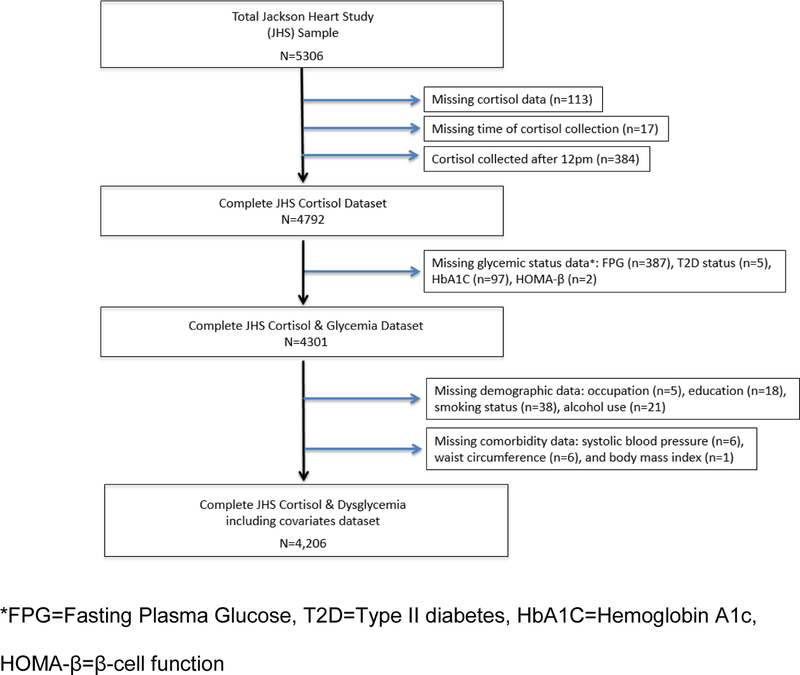
The JHS is a prospective cohort study of 5,306 AA adults, aged 21–94 years from the tricounty area of metropolitan Jackson, Mississippi. The baseline examination was performed between 2000–2004, with two subsequent follow-up examinations between 2005–2008 and 2009–2013. The design of the study has been described elsewhere (Taylor et al., 2005). The JHS was approved by the institutional review boards of the participating institutions and informed consent was obtained from all participants. For this analysis, analysis was performed using data from the baseline exam. Participants were excluded if they had missing data on exposures, outcomes or important covariates including cortisol (n=113), time of cortisol collection (n=17) or cortisol collected after 12pm (n=384), T2D status (n=5), HbA1c (n=97), FPG (n=387), HOMA-β cell function (n=2), occupation (n=5), education (n=18), smoking status (n=38), alcohol use (n=21), systolic blood pressure (n=6), waist circumference (n=6), and body mass index (n=1), as shown in Figure 1. The final analytic cohort included 4,206 participants.
Figure 1.
The Jackson Heart Study Cortisol and Diabetes Cohort The total Jackson Heart Study includes 5,306 participants, however, participants were excluded if they had missing data on exposures, outcomes or important covariates yielding a sample in this study of 4,206 subjects. Excluded subjects were from missing data for the following variables: cortisol (n=113), time of cortisol collection (n=17) or cortisol collected after 12pm (n=384), Type II diabetes (T2D) status (n=5), Hemoglobin A1c (HbA1c) (n=97), Fasting Plasma Glucose (FPG) (n=387), HOMA-β cell function (n=2), occupation (n=5), education (n=18), smoking status (n=38), alcohol use (n=21), systolic blood pressure (n=6), waist circumference (n=6), and body mass index (n=1).
2.2. Assessment of Morning Serum Cortisol
Normal diurnal cortisol regulation follows a circadian pattern, in which levels are typically high upon waking, rise during the first 30–40 min post-awakening and decline across the day, reaching a nadir in the late evening around 11pm-midnight (Joseph and Golden, 2017a). In the JHS, serum cortisol was collected fasting in the morning between 8am and 12pm. Serum cortisol levels were measured by chemiluminescent immunoassay performed on an immunoassay system (ADVIA Centaur; Siemens). Intra-assay coefficients of variation, were 9.1% and 7.7% for high and low cortisol concentrations, respectively.
2.3. Outcomes assessment
Fasting plasma glucose and insulin were measured on a Vitros 950 or 250, OrthoClinical Diagnostics analyzer (Raritan, NJ) using standard procedures that met the College of American Pathologists accreditation requirement (Carpenter et al., 2004). A high-performance liquid chromatography system (Tosoh Corporation, Tokyo, Japan) was used to measure HbA1c concentrations. Insulin resistance and β-cell function were estimated using HOMA-IR = (fasting plasma glucose [mmol/L] × fasting plasma insulin [mU/mL]) ÷ 22.5 and HOMA-β = (20 × fasting plasma insulin) ÷ (fasting plasma glucose – 3.5)% (Matthews et al., 1985). Prediabetes was defined as HbA1c 5.7–6.4% and/or fasting blood glucose 100–125 mg/dL (American Diabetes Association, 2010). T2D was defined as HbA1c ≥ 6.5% (48 mmol/mol), fasting blood glucose ≥ 126 mg/dL, taking T2D medications and/or with a self-reported physician diagnosis (American Diabetes Association, 2010).
2.4. Covariates
Baseline information was obtained during clinic visits or at home using standardized questionnaires including: demographics, occupation (management/professional versus not), level of education (1) Less than high school, 2) High school graduate, or completion of a General Educational Development (GED) degree as equivalent to graduating from high school, 3) attended vocational school, trade school or college), tobacco use (current smoking versus not), alcohol use (any alcohol intake in the past 12 months versus not), medical conditions and current prescription medication usage (hormone replacement therapy/β-blocker medications). Calibrated devices were used by certified technicians and nurses to measure participants’ weight and height. Body mass index (BMI) was calculated as weight (kilograms)/ height2 (meters). Waist circumference in centimeters was calculated as the average of two measurements around the umbilicus. Resting seated blood pressure was measured twice at 5-minute intervals using an appropriately sized cuff with standard Hawksley random-zero instruments and measurements were averaged for analysis. Physical activity was categorized according to the American Heart Association 2020 Cardiovascular health guidelines as poor, intermediate or ideal health, as described previously (Joseph et al., 2016b; Lloyd-Jones et al., 2010).
2.5. Statistical Analysis
The 1,100 participants excluded (Figure 1) were older, less educated, less likely to hold professional jobs, were less physically active, and had higher cortisol, waist circumference, HbA1c and diabetes prevalence (all p<0.05; Supplemental Table 1). Baseline characteristics of participants were presented and compared across quartiles of morning serum cortisol using chi-square for categorical variables, analysis of variance (ANOVA) for parametric continuous variables and Kruskal-Wallis test for non-parametric continuous variables. Due to positively skewed distributions, cortisol, HOMA-IR and HOMA-β were log-transformed prior to analysis. We used linear regression models to examine the association of log-transformed morning serum cortisol with FPG, HbA1c among all participants and HOMA-IR and HOMA-β, among participants without T2D. Demographic, socioeconomic and biological factors previously shown to be associated with serum cortisol and glycemia were selected a priori and included in the models (Clow et al., 2004; Cohen et al., 2006; Hajat et al., 2010; Joseph et al., 2016a; Joseph et al., 2017a; Joseph et al., 2015). The models were adjusted for age, sex, education, occupation, systolic blood pressure, waist circumference, physical activity, smoking, β-blocker, and estrogen replacement medications (Model 1) and, additionally, time of cortisol collection (Model 2). Both models were stratified by glycemic status. We used logistic regression to estimate the odds ratios (OR, 95% confidence interval-CI) of prevalent T2D by log-morning serum cortisol. Based on prior studies demonstrating differences in cortisol profile in those with a diagnosis of diabetes compared to individuals with normoglycemia, we stratified by glycemic status (Hackett et al., 2016; Hackett et al., 2014). We tested for effect modification by age, sex, waist circumference, time of cortisol collection and T2D status by inserting multiplicative interaction terms into the fully adjusted model and using the likelihood ratio test (Supplemental Table 3). We found significant effect modification for waist circumference and body mass index. Thus, we presented stratified models for waist circumference in tertiles (Supplemental Table 4) and body mass index in categories < 25, 25–29.99, ≥ 30 kg/m2 (Supplemental Table 5). Statistical significance was defined as two-sided alpha <0.05 in the main analysis and <0.10 for interactions (Joseph et al., 2017a). Analyses were performed using Stata 13.1 (Statacorp, College Station, TX).
3.0. Results
In a sample of 4,206 participants, the majority were female (63.7%), high school graduates or more (81.1%) and non-smokers (87.3%), with nearly half reporting poor physical activity and an average BMI in the obese range (31.7 ± 7.2 kg/m2) (Table 1). Male sex, lower education, smoking and alcohol consumption all significantly increased in higher morning serum cortisol quartiles (p<0.01, Table 1). Participants with higher morning cortisol had lower waist circumference (WC) and BMI (p<0.001). Table 1 demonstrates higher FPG and HbA1c per higher quartile of morning serum cortisol (p<0.0001). Demographics of participants by category of dysglycemia (all, normoglycemia, prediabetes, and T2D) are provided in Supplemental Table 2.
Table 1.
Characteristics of Participants in the Jackson Heart Study by Quartiles of Morning Serum Cortisol
| All | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
|---|---|---|---|---|---|---|
| Baseline Characteristics ♦ | n=4206 | n=1079 | n=1046 | n=1043 | N=1038 | p-value |
| Age, years | 54.97 (12.76) | 52.02 (11.85) | 54.37 (12.94) | 57.21 (12.16) | 56.38 (13.45) | <0.0001 |
| Female, sex (%) | 63.67 | 81.46 | 67.11 | 57.81 | 47.59 | 0.006 |
| Education | ||||||
| < High School (%) | 18.90 | 13.72 | 17.21 | 19.94 | 24.95 | |
| high school graduate/GED | 18.12 | 17.33 | 17.30 | 18.60 | 19.27 | |
| attended vocational school, trade school or college | 62.98 | 68.95 | 65.49 | 61.46 | 55.78 | 0.000 |
| Occupation, Management/Professional | 36.47 | 40.59 | 38.05 | 35.95 | 31.12 | 0.000 |
| Poor AHA Physical Activity† | 48.48 | 47.36 | 46.94 | 49.28 | 50.39 | 0.365 |
| Current Smoking (%) | 12.72 | 10.94 | 10.90 | 12.27 | 16.86 | 0.000 |
| Alcohol Intake (%) | 46.77 | 44.58 | 46.27 | 44.01 | 52.31 | 0.000 |
| Body-mass Index (kilograms/meter2) | 31.67 (7.20) | 32.93 (7.24) | 32.34 (7.45) | 31.43 (7.21) | 29.93 (6.53) | <0.0001 |
| Waist circumference (cm) | 100.42 (16.10) | 101.45 (16.48) | 101.35 (16.34) | 100.18 (15.40) | 98.64 (16.00) | 0.0001 |
| Systolic blood pressure (mmHg) | 127.16 (16.44) | 124.53 (15.20) | 125.72 (15.53) | 129.10 (17.34) | 129.41 (17.09) | <0.0001 |
| Glucose (mg/dL) | 100.30 (33.21) | 93.75 (23.21) | 98.64 (28.77) | 102.42 (33.54) | 106.64 (43.07) | <0.0001 |
| Hemoglobin A1c (%) | 5.91 (1.21) | 5.74 (0.93) | 5.88 (1.09) | 5.97 (1.27) | 6.05 (1.48) | <0.0001 |
| Cortisol (µg/dL) | 9.2 (6.9, 12) | 5.7 (4.8, 6.4) | 8.1 (7.5, 8.6) | 10.5 (9.9, 11.2) | 14.6 (13.2, 16.6) | 0.0001 |
| Log-Transformed Cortisol | 2.20 (0.43) | 1.66 (0.30) | 2.09 (0.08) | 2.35 (0.75) | 2.72 (0.18) | <0.0001 |
| Cortisol Collection Time (24-hour time) | 10.75 (0.62) | 10.84 (0.58) | 10.77 (0.61) | 10.71 (0.63) | 10.66 (0.64) | <0.0001 |
| Homeostatic model assessment of insulin resistance ‡ | 3.03 (2.19, 4.37) | 3.16 (2.33, 4.38) | 3.13 (2.24, 4.38) | 2.96 (2.09, 4.39) | 2.89 (2.11, 4.29) | 0.0385 |
| Homeostatic model assessment of βcell function (%) ‡ | 191.79 (141.13, 266.63) | 214.80 (161.54, 300.97) | 195.35 (146.51, 276.34) | 177.60 (134.61, 244.18) | 174.05 (124.99, 243.36) | 0.0001 |
| Hormone Replacement Therapy (%) | 15.00 | 13.53 | 14.24 | 15.34 | 16.96 | 0.139 |
| Beta-Blocker Medications (%) | 9.70 | 7.04 | 10.13 | 10.64 | 11.08 | 0.007 |
| Prevalent Diabetes (%) | 18.95 | 13.53 | 18.64 | 19.56 | 24.28 | <0.001 |
GED=General Educational Development (degree equivalent to graduation from high school)
Mean (SD), median (interquartile range) or percentages are listed, p-values calculated using chi-square (categorical variables), ANOVA (parametric continuous variables) and Kruskal-Wallis test (non-parametric continuous variables)
AHA = American Heart Association, Ideal physical activity recommendations were defined by AHA “2020” guidelines. Physical Activity was considered poor if participant performed no physical activity.
n = 3,409 participants with HOMA-IR and HOMA-β without diabetes at baseline, (Q1 933, Q2 851, Q3 839, Q4 786)
Upon examining the cross-sectional association of log-morning serum cortisol with FPG and HbA1c) (Table 2), we found a positive association of with FPG (β=11.22, p<0.001), with the strongest association in those with T2D (β=23.62, p<0.001). A similar relationship was seen with log-morning serum cortisol and HbA1c among all participants (β=0.28, p<0.001) and those with T2D (β=0.59, p<0.001), demonstrating that a 100% increase in log-morning serum cortisol was associated with a 0.59% increase in HbA1c in those with T2D. These findings remained significant in the fully adjusted models.
Table 2.
The Association of log-serum cortisol with fasting plasma glucose and hemoglobin A1c in the Jackson Heart Study
| Fasting Plasma Glucose, Multivariable Linear Regression Model Beta-Coefficient (95% CI) | |||
| Unadjusted | Model 1 | Model 2 | |
| All Participants | 11.29 (8.99, 13.60), p<0.001 | 11.98 (9.61, 14.34), p<0.001 | 11.22 (8.85, 13.59), p<0.001 |
| Participants without Diabetes (n=3,409) | 3.57 (2.90, 4.25), p<0.001 | 3.06 (2.39, 3.73), p<0.001 | 2.65 (1.99, 3.32), p<0.001 |
| Participants with Diabetes (n=797) | 22.60 (12.75, 32.45), p<0.001 | 25.05 (15.01, 35.09), p<0.001 | 23.62 (13.57, 33.67), p<0.001 |
| Hemoglobin A1c, Multivariable Linear Regression Model Beta-Coefficient (95% CI) | |||
| All Participants | 0.26 (0.18, 0.35), p<0.001 | 0.29 (0.21, 0.38), p<0.001 | 0.28 (0.20, 0.37), p<0.001 |
| Participants without Diabetes (n=3,409) | −0.02 (−0.05, 0.02), p=0.344 | −0.03 (−0.07, 0.01), p=0.119 | −0.03 (−0.07, 0.01), p=0.117 |
| Participants with Diabetes (n=797) | 0.50 (0.21, 0.80), p<0.001 | 0.62 (0.31, 0.92), p<0.001 | 0.59 (0.29, 0.90), p<0.001 |
Model 1 – Adjusted for age, sex, education, occupation, systolic blood pressure, waist circumference, current smoking, physical activity, hormone replacement therapy, beta-blocker medications Model 2 – Model 1 + time of cortisol collection
Fasting Plasma Glucose Interpretation: A 100% increase in cortisol is associated with an average beta-coefficient unit change in fasting plasma glucose (mg/dL). In the continuous unadjusted model, a 100% increase in serum cortisol is associated with a 11 mg/dl increase in fasting plasma glucose.
Hemoglobin A1c (HbA1c) Interpretation: A 100% increase in cortisol is associated with an average beta-coefficient unit change in HbA1c (%). In the continuous unadjusted model, a 100% increase in serum cortisol is associated with a 0.26 increase in HbA1c.
In regard to the relation of morning serum cortisol with HOMA-IR and HOMA-β in participants without T2D, no association was observed between log-morning serum cortisol and HOMA-IR in the fully-adjusted analyses (p>0.05). However, there was a significant negative association of log-morning serum cortisol with HOMA-β (β=−0.10, p<0.001) in the fully-adjusted analysis (Table 3).
Table 3.
The Association of log-morning serum cortisol with log-Homeostatic model assessment of Insulin Resistance and β-cell Function among participants without diabetes in the Jackson Heart Study
| Log-Homeostatic model assessment of insulin resistance, Multivariable Linear Regression Model Beta-Coefficient (95% CI), n=3,409 | |||
| Unadjusted | Model 1 | Model 2 | |
| Participants without diabetes♦ (n=3409) | −0.07 (−0.12, −0.031), p=0.001 | 0.03 (−0.01, 0.07), p=0.108 | 0.02 (−0.02, 0.06), p=0.370 |
| Participants with normoglycemia (n=1883) | −0.11 (−0.16, −0.06), p<0.001 | 0.00 (−0.05, 0.06), p=0.880 | −0.01 (−0.06, 0.04), p=0.705 |
| Participants with prediabetes (n=1526) | −0.07 (−0.13, −0.01), p=0.027 | 0.06 (−0.00, 0.11), p=0.06 | 0.04 (−0.01, 0.10), p=0.138 |
| Log-Homeostatic model assessment of β-cell Function, Multivariable Linear Regression Model Beta-Coefficient (95% CI), n=3,409 | |||
| Unadjusted | Model 1 | Model 2 | |
| Participants without diabetes (n=3409) | −0.24 (−0.28, −0,20), p<0.001 | −0.11 (−0.14, −0.07), p<0.001 | −0.10 (−0.14, −0.06), p<0.001 |
| Participants with normoglycemia (n=1883) | −0.21 (−0.26, −0.15), p<0.001 | −0.09 (−0.14, −0.04), p=0.001 | −0.08 (−0.14, −0.03), p<0.001 |
| Participants with prediabetes (n=1526) | −0.27 (−0.33, −0.21), p<0.001 | −0.12 (−0.18, −0.07), p<0.001 | −0.12 (−0.17, −0.06), p<0.001 |
Includes subjects with prediabetes and normoglycemia
Model 1 – Adjusted for age, sex, education, occupation, systolic blood pressure, waist circumference, current smoking, physical activity, hormone replacement therapy, beta-blocker medications Model 2 – Model 1 + time of cortisol collection
Log-Homeostatic model assessment of insulin resistance (HOMA-IR) Interpretation: A 100% increase in cortisol is associated with a beta-coefficient 100% change in HOMA-IR (%). In the continuous unadjusted model, a 1% increase in serum cortisol is associated with a 7% lower HOMA-IR.
Log-Homeostatic model assessment of β-cell Function (HOMA-β) Interpretation: A 100% increase in cortisol is associated with a beta-coefficient 100% increase in HOMA-β. In the continuous unadjusted model, a 100% increase in serum cortisol is associated with a 24% lower HOMA-β.
Using logistic regression, a 1-unit increase in log-cortisol was associated with higher odds of prevalent T2D in fully-adjusted analyses (OR: 2.07, 95% CI: 1.66, 2.59). The fourth quartile compared to the first of morning cortisol was also associated with higher odds of prevalent T2D (OR: 2.26, 95% CI: 1.75, 2.91, p<0.001) (Table 4).
Table 4.
The Association of log-morning serum cortisol with Prevalent Diabetes in the Jackson Heart Study
| Logistic Regression Model – Odds Ratio (95% CI) for Prevalent Diabetes | |||
| Unadjusted | Model 1 | Model 2 | |
| Log-Cortisol | 1.86 (1.53 – 2.24), p<0.001 | 2.13 (1.71 – 2.65), p<0.001 | 2.07 (1.66 – 2.59), p<0.001 |
| Morning Serum Cortisol in Quartiles | |||
| Quartile 1 | Referent | Referent | Referent |
| Quartile 2 | 1.46 (1.16 – 1.85), p=0.001 | 1.45 (1.13 – 1.86), P=0.004 | 1.44 (1.12 – 1.85), p=0.005 |
| Quartile 3 | 1.55 (1.23 – 1.96), p<0.001 | 1.50 (1.17 – 1.94), p=0.002 | 1.47 (1.14 – 1.90), p=0.003 |
| Quartile 4 | 2.05 (1.64 – 2.57), p<0.001 | 2.32 (1.80 – 2.99), P<0.001 | 2.26 (1.75 – 2.91), p<0.001 |
Model 1 – Adjusted for age, sex, education, occupation, systolic blood pressure, waist circumference, current smoking, physical activity, hormone replacement therapy, beta-blocker medications Model 2 – Model 1 + time of cortisol collection
For the logistic regression, the odds ratios are expressed as a percentage of higher prevalence per log unit increase (continuous) and a percentage of higher prevalence of diabetes per quartile compared to Quartile 1.
Interpretation: In the continuous analysis (unadjusted) a 1-unit increase in log-cortisol is associated with an 86% higher odds of prevalent diabetes and Quartile 4 compared with Quartile 1 was associated with a 105% higher odds of prevalent diabetes
Given the significant effect modification of waist circumference on the association of log-morning serum cortisol with FPG (p<0.0001), HbA1c (p=0.0001), HOMA-IR (p=0.0003) and HOMA-β (p=0.0650), we stratified our results by tertiles of waist circumference (Supplemental Tables 3 and 4). Stronger associations (larger β-coefficients) were seen in the relationship of cortisol with FPG and HbA1c in the third tertile of waist circumference tertile (β=17.04, p<0.0001; β=0.49, p<0.0001, respectively) compared to the first (β=6.51, p<0.0001; β=0.12, p=0.035, respectively). Similar results were observed when stratified by BMI categories (Supplemental Table 5). In the highest tertile of WC, there was a positive association between log-morning serum cortisol and insulin resistance (HOMA-IR) (β=0.08, p=0.038), but no associations in the first and second tertiles. The inverse association of log-morning serum cortisol with HOMA-β was strongest in the lowest tertile of WC (β=−0.13, p<0.0001), present in the second tertile (β=−0.08, p=0.023), and not significant in the first tertile. Results did not differ in significance between models 1 and 2.
4.0. Discussion
In the present study, we demonstrated that higher morning serum cortisol was: 1) positively associated with FPG among individuals with and without T2D and with HbA1c among individuals with T2D; 2) associated with FPG more strongly among individuals with vs. without T2D; 3) inversely associated with β-cell function in those without T2D and not associated with HOMA-IR among individuals without T2D; and 4) associated with higher odds of prevalent diabetes. These findings demonstrate robust associations of morning serum cortisol with glucose metabolism among AAs and are in line with our hypothesis of a relationship between cortisol and glycemia in T2D (Champaneri et al., 2013; Joseph and Golden, 2017a).
In studies that have not specifically looked at diverse populations or racial differences in AAs, evidence suggests that hypercortisolism is associated with glycemia and insulin resistance, commonly studied in the context of metabolic syndrome, as demonstrated in a critical review of the literature by Anagnostis, et al. (Anagnostis et al., 2009). One study cited in this review, for example, demonstrated that higher fasting serum cortisol levels were associated with insulin resistance (HOMA-IR) in a population of 370 men, 66 with impaired glucose tolerance and 27 with diabetes, in the United Kingdom (Phillips et al., 2000). Similar relationships have been observed between morning serum cortisol and insulin resistance in Japanese and Latino populations without a diagnosis of diabetes (Adam et al., 2010; Kamba et al., 2016). A population of 1,181 individuals in Amsterdam, and 1,614 older predominantly Caucasian individuals in Southern California, showed higher morning serum cortisol was associated with a risk of prevalent diabetes (Radin et al., 2016; Schoorlemmer et al., 2009). Our findings in a population of AA individuals are consistent with these and other prior morning serum cortisol studies in whites, Hispanic Americans and Japanese populations demonstrating an association of higher morning serum cortisol with greater insulin resistance in individuals with and without diabetes (Kamba et al., 2016), insulin resistance in overweight individuals (Adam et al., 2010), and higher odds of prevalent diabetes (Radin et al., 2016; Schoorlemmer et al., 2009).
We found that morning serum cortisol measures are positively associated with both higher HbA1c and FPG in AAs with T2D. The association of morning serum cortisol with higher glycemia in AAs is in line with previous findings revealing a flatter salivary cortisol diurnal profile was associated with higher glycemia (HbA1c and FPG) among participants with T2D, but not participants without T2D, including 31% black, 43% Hispanic, and 25% white individuals (Joseph and Golden, 2017a; Joseph et al., 2015). Hackett, et al, also observed a flatter diurnal cortisol curve associated with fasting glucose and T2D (Hackett et al., 2016; Hackett et al., 2014). These studies are evidence for a differential relationship between cortisol and dysglycemia in those with and without T2D. However, our current study adds two major novel components: 1) revealing a similar differential relationship between HPA axis dysfunction and glycemia based on diabetes status, utilizing morning serum cortisol, which would require far less burden to collect for patients, as compared to multiple measures of salivary cortisol across the day; and 2) it is unique in that it was completed in a large AA population.
Though our study can make no conclusions on causation, prior studies may suggest two plausible mechanisms for the observed association between higher levels of morning serum cortisol and dysglycemia including central and peripheral mechanistic hypotheses (Joseph et al., 2015). First, in regards to the central hypothesis, both animal and human studies of central regulation of the HPA axis have associated hyperglycemia with hippocampal atrophy and a hypothesized reduction in hypothalamic inhibition by the hippocampus (Bruehl et al., 2009; Stranahan et al., 2008). Additionally, individuals with diabetes may have impaired pituitary feedback mechanisms (Bruehl et al., 2009; Hudson et al., 1984). Second, peripherally, it has been shown that cortisol yields hyperglycemia and insulin resistance through increasing lipolysis and gluconeogenesis in the liver (Goodpaster et al., 1997; Kelley et al., 2001; Santomauro et al., 1999). We observed no association between morning serum cortisol and insulin resistance (HOMA-IR) in the total analytic sample of individuals without T2D. This is supported by the observation that lower wake-up and morning cortisol are associated with greater adiposity (Champaneri et al., 2012; Joseph et al., 2017b; Kumari et al., 2010; Kumari et al., 2011). Importantly, longitudinal trajectory may factor into the directionality of the between adiposity and cortisol. In subclinical hypercortisolism or Cushing’s disease one experiences hypercortisolism throughout the day associated with adiposity at diagnosis(Di Dalmazi et al., 2015), however, in a longitudinal study of adiposity, gain in BMI was associated with lower morning cortisol(Joseph et al., 2017b). Though there was no significant adjusted finding linking cortisol and insulin resistance in our study, when stratifying by tertiles of WC, those with the highest WC (third tertile) demonstrated a positive association of morning serum cortisol with HOMA-IR. This is consistent with the prior study by Joseph et al, which demonstrated that waist circumference attenuated the negative relationship between wake-up salivary cortisol and HOMA-IR (Joseph et al., 2015). These findings may be explained by the hypothesis that in individuals with greater adiposity, insulin resistance may be amplified by increased levels of 11β-HSD1 (Incollingo Rodriguez et al., 2015). The enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) converts cortisol to its active form. Animal studies have shown that overexpression of 11β-HSD1 yields lower serum, but higher adipocyte corticosterone (Incollingo Rodriguez et al., 2015; Masuzaki et al., 2001), which could then yield higher levels of active cortisol and impact adipocyte insulin resistance. Further, the complex relationship between hypercortisolism and adiposity and insulin resistance may be due to associations between subclinical hypercortisolism with visceral rather than central adiposity in metabolic syndrome (Min, 2016).
The observation that the negative relationship between cortisol and HOMA-β was seen most strongly in the lowest WC tertile, suggests that the relationship between cortisol and β-cells may be moderated by lack of adiposity. This finding should be taken with caution, however, given the average BMI of our sample was in the obese category (32 kg/m2), although 619 individuals had a normal BMI (<25 kg/m2). These results are consistent with studies in normoglycemia, where cortisol inhibits insulin release from pancreatic β-cells (Delaunay et al., 1997; Ling et al., 1998). It is possible that among individuals with lower levels of adiposity, the first defect is a decrease in β-cell function and changes in insulin resistance and then hyperglycemia may come later in the pathophysiology. A model of overexpression of the glucocorticoid receptor (GR) in mouse islet cells demonstrated that progressing from hyperglycemia and impaired glucose utilization to T2D may be related to GR sensitivity exerting functional changes on β-cell activity (Davani et al., 2004). Persistent hypercortisolism changes GR sensitivity in that it causes GRs to saturate and cortisol-receptor interaction to plateau (Granner et al., 2015). Therefore, perhaps modest hypercortisolism is associated with impaired β-cell function, but the effect plateaus with limited availability of GRs as they become saturated with even further increased cortisol levels as seen in hyperglycemia and adiposity. We plan to evaluate the nuances of the relationship between cortisol and adiposity in future studies.
Prior research demonstrates the importance of conducting studies dedicated to HPA axis measures in AA populations due to differential cortisol dynamics among various race/ethnicities, which could lead to differential outcomes. HPA axis measures vary in that AAs show steeper awakening salivary cortisol response, lower morning salivary cortisol, higher bedtime cortisol levels, and a “flatter” diurnal cortisol profile throughout the day in blacks, independent of socioeconomic status, compared to Caucasians (Bennett et al., 2004; Cohen et al., 2006; DeSantis et al., 2007; Hajat et al., 2010; Karlamangla et al., 2013; Skinner et al., 2011; Zeiders et al., 2014). These racial differences in HPA axis measures may affect studying clinical outcomes in AAs. For example, one study in a sample of 3,730 subjects (42% AA) demonstrated higher fasting serum cortisol levels are associated with a glucocorticoid receptor polymorphism that differs significantly in prevalence between non-Hispanic white and AAs (Whirledge et al., 2017), with another study looking at the clinical outcomes of body fat and insulin resistance associated with glucocorticoid receptor genotype that showed homozygosity of the polymorphism more prevalent in AAs (Melcescu et al., 2012). In two studies that accounted for AA race in clinical outcomes, one demonstrated blunted morning and nocturnal salivary, but not morning serum cortisol was found to be correlated with greater coronary-artery intimal medial thickness in obese individuals of both races (n=55 AAs) (Toledo-Corral et al., 2013), and another that higher hair cortisol was associated with elevated glycated hemoglobin (HbA1c) in 61 AAs (20 with T2D) (Lehrer et al., 2016). With more understanding of these mechanisms, it may become clinically important to identify genetic risk for diabetes and cardiovascular disease within HPA axis genes, or in using related biomarkers, as possible early risk stratification, or for individual treatment targets in future research. Ours is the first, however, to assess the association between cortisol and clinical outcomes of glycemia (fasting glucose, prevalent diabetes, HOMA-B, HOMA-IR) in a large AA population study. Yet, as demonstrated by the literature, more research is needed to consider cardiovascular outcomes, and associated underlying genetic and pathophysiologic underpinnings of these relationships in AA populations.
Our study has several strengths. First, the Jackson Heart Study include a large sample of AAs, thus, allowing the study of a subpopulation for which there has been limited data on HPA axis dysfunction and dysglycemia, as well as the comparison of individuals with T2D to those without. Our study suggests that quantifying cortisol may be a reliable and clinically meaningful biomarker in glycemia as demonstrated that our findings with serum cortisol corroborate prior findings completed with salivary cortisol. We also conducted a comprehensive assessment of the cortisol and glycemic relationship across various levels of glycemia, accounting for various levels of WC and including multiple measures of glucose metabolism and insulin resistance (FPG, A1C and HOMA-IR), thus allowing an assessment of different facets of the pathophysiology of obesity and T2D.
Our study does have some limitations. First, due to the nature of cross-sectional studies, the temporality between the exposure and the outcome cannot be determined. Second, given longitudinal measures of cortisol such as 24-hour urinary or complete diurnal cortisol curve are challenging to collect in a large sample size of 4,206 individuals, serum cortisol was only collected at one point in time during the day. However, serum cortisol (which measures free and protein bound cortisol) correlates well with salivary cortisol (measuring free cortisol only) in diagnosing Addison’s disease and Cushing’s syndrome (Restituto et al., 2008). The advantage of morning serum cortisol is that it can be collected along with routine fasting labs, thus increasing clinical utility compared to multiple measurements of salivary cortisol throughout the day. However, though we did demonstrate that regardless of variation of morning collection time of cortisol, results did not vary, our data is limited in that we do not have wake up time for participants to include as a covariate. Importantly, the participants in the JHS are from one geographic area in the southeastern United States and may not be representative of all AAs, thus limiting generalizability. Though we explored WC, our sample average BMI was 32 kg/m2, thus did not allow the exploration of the whole spectrum of BMI, especially individuals with normal BMI.
5.0. Conclusion
In conclusion, our study demonstrates an association of morning serum cortisol with multiple measures of glucose metabolism (increasing FPG and A1C, and decreasing beta-cell function) and prevalent T2D among AAs. Further, we demonstrate that the relationship between cortisol and dysglycemia may be modified by adiposity. Given the increasing magnitude of association of cortisol with FPG and HbA1c in those with versus without T2D and the association with lower β-cell function, future studies should assess the association of longitudinal changes in glucose and diabetes control based on serum cortisol among AAs. This would set the stage for targeted pharmacologic intervention to potentially treat T2D using cortisol modulating pharmacotherapies. Ultimately, this work will inform the design and assessment of potential interventions targeting the HPA axis and cortisol pathway in the prevention and treatment of dysglycemia and T2D.
Supplementary Material
Highlights.
Examined the role of serum cortisol in glycemia and T2D in African Americans (AAs)
Cortisol was associated with dysglycemia and insulin resistance, modified by adiposity
Quartile 4 vs. 1 of serum cortisol was associated with a 1.26-fold higher T2D odds
These findings support a role for morning serum cortisol in glycemia among AAs
6.0. Acknowledgements
The authors thank the other investigators, the staff, and the participants of the JHS for their valuable contributions. The JHS is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Conflict of interest
The authors have no conflicts of interest to disclose.
References
- Adam TC, Hasson RE, Ventura EE, Toledo-Corral CM, Le K, Mahurka S, Lane CJ, Weigensberg MJ, Goran MI, 2010. Cortisol is Negatively Associated with Insulin Sensitivty in Overweight Latino Youth. J Clin Endocrinol Metab 95, 4729–4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association, A., 2010. Diagnosis and classification of diabetes mellitus. Diabetes Care 33 Suppl 1, S62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP, 2009. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab 94, 2692–2701. [DOI] [PubMed] [Google Scholar]
- Bennett GG, Merritt MM, Wolin KY, 2004. Ethnicity, education, and the cortisol response to awakening: a preliminary investigation. Ethn Health 9, 337–347. [DOI] [PubMed] [Google Scholar]
- Bruehl H, Wolf OT, Convit A, 2009. A blunted cortisol awakening response and hippocampal atrophy in type 2 diabetes mellitus. Psychoneuroendocrinology 34, 815–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, Skelton T, Jensen R, Sarpong D, 2004. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci 328, 131–144. [DOI] [PubMed] [Google Scholar]
- Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, DeSantis AS, Diez Roux A., Shrager S, Golden SH, 2013. Diurnal salivary cortisol is associated with body mass index and waist circumference: the Multiethnic Study of Atherosclerosis. Obesity (Silver Spring) 21, E56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, Diez Roux A., Golden SH, 2012. Diurnal salivary cortisol and urinary catecholamines are associated with diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis. Metabolism 61, 986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F, 2004. The awakening cortisol response: methodological issues and significance. Stress 7, 29–37. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T, 2006. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med 68, 41–50. [DOI] [PubMed] [Google Scholar]
- Davani B, Portwood N, Bryzgalova G, Reimer MK, Heiden T, Ostenson CG, Okret S, Ahren B, Efendic S, Khan A, 2004. Aged transgenic mice with increased glucocorticoid sensitivity in pancreatic beta-cells develop diabetes. Diabetes 53 Suppl 1, S51–59. [DOI] [PubMed] [Google Scholar]
- Delaunay F, Khan A, Cintra A, Davani B, Ling ZC, Andersson A, Ostenson CG, Gustafsson J, Efendic S, Okret S, 1997. Pancreatic beta cells are important targets for the diabetogenic effects of glucocorticoids. J Clin Invest 100, 2094–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG, 2007. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J Adolesc Health 41, 3–13. [DOI] [PubMed] [Google Scholar]
- Di Dalmazi G, Pasquali R, Beuschlein F, Reincke M, 2015. Subclinical hypercortisolism: a state, a syndrome, or a disease? Eur J Endocrinol 173, M61–71. [DOI] [PubMed] [Google Scholar]
- Fuchs VR, 2016. Black Gains in Life Expectancy. JAMA 316, 1869–1870. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE, 1997. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 46, 1579–1585. [DOI] [PubMed] [Google Scholar]
- Granner DK, Wang JC, Yamamoto KR, 2015. Regulatory Actions of Glucocorticoid Hormones: From Organisms to Mechanisms. Adv Exp Med Biol 872, 3–31. [DOI] [PubMed] [Google Scholar]
- Hackett RA, Kivimaki M, Kumari M, Steptoe A, 2016. Diurnal Cortisol Patterns, Future Diabetes, and Impaired Glucose Metabolism in the Whitehall II Cohort Study. J Clin Endocrinol Metab 101, 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett RA, Steptoe A, Kumari M, 2014. Association of diurnal patterns in salivary cortisol with type 2 diabetes in the Whitehall II study. J Clin Endocrinol Metab 99, 4625–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat A, Diez-Roux A, Franklin TG, Seeman T, Shrager S, Ranjit N, Castro C, Watson K, Sanchez B, Kirschbaum C, 2010. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology 35, 932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hudson MS, Rothschild AJ, Vignati L, Schatzberg AF, Melby JC, 1984. Abnormal results of dexamethasone suppression tests in nondepressed patients with diabetes mellitus. Arch Gen Psychiatry 41, 1086–1089. [DOI] [PubMed] [Google Scholar]
- Incollingo Rodriguez AC, Epel ES, White ML, Standen EC, Seckl JR, Tomiyama AJ, 2015. Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: A systematic review. Psychoneuroendocrinology 62, 301–318. [DOI] [PubMed] [Google Scholar]
- Joseph JJ, Echouffo-Tcheugui JB, Carnethon MR, Bertoni AG, Shay CM, Ahmed HM, Blumenthal RS, Cushman M, Golden SH, 2016a. The association of ideal cardiovascular health with incident type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis. Diabetologia 59, 1893–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JJ, Echouffo-Tcheugui JB, Kalyani RR, Yeh HC, Bertoni AG, Effoe VS, Casanova R, Sims M, Correa A, Wu WC, Wand GS, Golden SH, 2016b. Aldosterone, Renin, and Diabetes Mellitus in African Americans: The Jackson Heart Study. J Clin Endocrinol Metab 101, 1770–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JJ, Echouffo-Tcheugui JB, Talegawkar SA, Effoe VS, Okhomina V, Carnethon MR, Hsueh WA, Golden SH, 2017a. Modifiable Lifestyle Risk Factors and Incident Diabetes in African Americans. Am J Prev Med 53, e165–e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JJ, Golden SH, 2017a. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann N Y Acad Sci 1391, 20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JJ, Golden SH, 2017b. Diabetes in Native Populations and Underserved Communities in the USA, in: Dagogo-Jack S. (Ed.), Diabetes Mellitus in Developing Countries and Underserved Communities Springer, Cham. [Google Scholar]
- Joseph JJ, Wang X, Diez Roux AV, Sanchez BN, Seeman TE, Needham BL, Golden SH, 2017b. Antecedent longitudinal changes in body mass index are associated with diurnal cortisol curve features: The multi-ethnic study of atherosclerosis. Metabolism 68, 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JJ, Wang X, Spanakis E, Seeman T, Wand G, Needham B, Golden SH, 2015. Diurnal salivary cortisol, glycemia and insulin resistance: The multi-ethnic study of atherosclerosis. Psychoneuroendocrinology 62, 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamba A, Daimon M, Murakami H, Otaka H, Matsuki K, Sato E, Tanabe J, Takayasu S, Matsuhashi Y, Yanagimachi M, Terui K, Kageyama K, Tokuda I, Takahashi I, Nakaji S, 2016. Association between Higher Serum Cortisol Levels and Decreased Insulin Secretion in a General Population. PLoS One 11, e0166077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Friedman EM, Seeman TE, Stawksi RS, Almeida DM, 2013. Daytime trajectories of cortisol: demographic and socioeconomic differences--findings from the National Study of Daily Experiences. Psychoneuroendocrinology 38, 2585–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, Williams KV, Price JC, McKolanis TM, Goodpaster BH, Thaete FL, 2001. Plasma fatty acids, adiposity, and variance of skeletal muscle insulin resistance in type 2 diabetes mellitus. J Clin Endocrinol Metab 86, 5412–5419. [DOI] [PubMed] [Google Scholar]
- Kosak M, Hana V, Hill M, Simunkova K, Lacinova Z, Krsek M, Marek J, 2014. Serum cortisol seems to be a more appropriate marker for adrenocortical reserve evaluation in ACTH test in comparison to salivary cortisol. Physiol Res 63, 229–236. [DOI] [PubMed] [Google Scholar]
- Kumari M, Chandola T, Brunner E, Kivimaki M, 2010. A nonlinear relationship of generalized and central obesity with diurnal cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab 95, 4415–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Shipley M, Stafford M, Kivimaki M, 2011. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II study. J Clin Endocrinol Metab 96, 1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer HM, Dubois SK, Maslowsky J, Laudenslager ML, Steinhardt MA, 2016. Hair cortisol concentration and glycated hemoglobin in African American adults. Psychoneuroendocrinology 72, 212–218. [DOI] [PubMed] [Google Scholar]
- Ling ZC, Khan A, Delauny F, Davani B, Ostenson CG, Gustafsson JA, Okret S, Landau BR, Efendic S, 1998. Increased glucocorticoid sensitivity in islet betacells: effects on glucose 6-phosphatase, glucose cycling and insulin release. Diabetologia 41, 634–639. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD, American Heart Association Strategic Planning Task, F., Statistics, C., 2010. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 121, 586–613. [DOI] [PubMed] [Google Scholar]
- Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS, 2001. A transgenic model of visceral obesity and the metabolic syndrome. Science 294, 2166–2170. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC, 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419. [DOI] [PubMed] [Google Scholar]
- Melcescu E, Griswold M, Xiang L, Belk S, Montgomery D, Bray M, Del Ben KS, Uwaifo GI, Marshall GD, Koch CA, 2012. Prevalence and cardiometabolic associations of the glucocorticoid receptor gene polymorphisms N363S and BclI in obese and non-obese black and white Mississippians. Hormones (Athens) 11, 166–177. [DOI] [PubMed] [Google Scholar]
- Mendola ND, Chen TC, Gu Q, Eberhardt MS, Saydah S, 2018. Prevalence of Total, Diagnosed, and Undiagnosed Diabetes Among Adults: United States, 2013–2016. NCHS Data Brief, 1–8. [PubMed]
- Min L, 2016. Functional Hypercortisolism, Visceral Obesity, and Metabolic Syndrome. Endocr Pract 22, 506–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DI, Walker BR, Reynolds RM, Flanagan DE, Wood PJ, Osmond C, Barker DJ, Whorwood CB, 2000. Low birth weight predicts elevated plasma cortisol concentrations in adults from 3 populations. Hypertension 35, 1301–1306. [DOI] [PubMed] [Google Scholar]
- Radin JM, Al-Delaimy WK, Kritz-Silverstein D, Wingard D, Barrett-Connor E, Laughlin GA, 2016. The Association of Cortisol with Prevalent and Incident Type 2 Diabetes in Older Community-Dwelling Adults. Global Journal of Epidemiology and Public Health 3, 1–7. [Google Scholar]
- Restituto P, Galofre JC, Gil MJ, Mugueta C, Santos S, Monreal JI, Varo N, 2008. Advantage of salivary cortisol measurements in the diagnosis of glucocorticoid related disorders. Clin Biochem 41, 688–692. [DOI] [PubMed] [Google Scholar]
- Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, Strassmann PG, Wajchenberg BL, 1999. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 48, 1836–1841. [DOI] [PubMed] [Google Scholar]
- Schoorlemmer RM, Peeters GM, van Schoor NM, Lips P, 2009. Relationships between cortisol level, mortality and chronic diseases in older persons. Clin Endocrinol (Oxf) 71, 779–786. [DOI] [PubMed] [Google Scholar]
- Skinner ML, Shirtcliff EA, Haggerty KP, Coe CL, Catalano RF, 2011. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Dev Psychopathol 23, 1167–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanakis EK, Wang X, Sanchez BN, Diez Roux AV, Needham BL, Wand GS, Seeman T, Golden SH, 2016. Lack of significant association between type 2 diabetes mellitus with longitudinal change in diurnal salivary cortisol: the multiethnic study of atherosclerosis. Endocrine 53, 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP, 2008. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci 11, 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HA Jr., Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB, 2005. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis 15, S6–4-17. [PubMed] [Google Scholar]
- Toledo-Corral CM, Myers SJ, Li Y, Hodis HN, Goran MI, Weigensberg MJ, 2013. Blunted nocturnal cortisol rise is associated with higher carotid artery intima-media thickness (CIMT) in overweight African American and Latino youth. Psychoneuroendocrinology 38, 1658–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whirledge SD, Jewell CM, Barber LM, Xu X, Katen KS, Garantziotis S, Cidlowski JA, 2017. Generating diversity in human glucocorticoid signaling through a racially diverse polymorphism in the beta isoform of the glucocorticoid receptor. Lab Invest 97, 1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiders KH, Hoyt LT, Adam EK, 2014. Associations between self-reported discrimination and diurnal cortisol rhythms among young adults: The moderating role of racial-ethnic minority status. Psychoneuroendocrinology 50, 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.