Abstract
Objective
To examine prostate cancer incidence and mortality by arm in the randomized Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial.
Subjects and Methods
Subjects aged 55–74 at 10 screening centers were randomized between 1993 and 2001 to an intervention or usual care arm. Intervention arm men received 6 annual prostate-specific antigen (PSA) tests and 4 annual digital rectal exams. Subjects were followed for prostate cancer (PCa) incidence and for mortality by active follow-up processes and by linkage to state cancer registries and the National Death Index. For cancers identified by active follow-up, trial abstractors recorded the mode of diagnosis (screen detected, symptomatic, other).
Results
38340 men were randomized to the intervention arm and 38343 to usual care. Median follow-up for mortality was 16.9 (intervention) and 16.7 (usual care) years. There were 333 (intervention) and 352 (usual care) PCa cancer deaths, giving rates (per 10,000 PY) of 5.5 and 5.9, respectively, and an RR of 0.93 (95% CI: 0.81–1.08; p=0.38). The rate ratio (RR) for overall PCa incidence was 1.05 (95% CI: 1.01–1.09); by Gleason category, it was 1.17 (95% CI: 1.11–1.23), 1.00 (95% CI: 0.93–1.07) and 0.89 (95% CI; 0.80–0.99) for Gleason 2–6, 7 and 8–10, respectively. By mode of detection, during the trial’s screening phase, 12% of intervention arm versus 27% of usual care arm cases were symptomatic; post-screening these percentages were 18% in each arm.
Conclusion
After almost 17 years median follow-up, there was no significant reduction in prostate cancer mortality in the intervention compared to usual care arm. There was a significant increase in Gleason 2–6 disease and a significant reduction in Gleason 8–10 disease in the intervention compared to usual care arm.
Keywords: prostate cancer, prostate-specific antigen, screening
Introduction
Screening for prostate cancer with prostate-specific antigen (PSA) has been the subject of intense scientific debate for the past two decades. Over the last decade, two large randomized trials have reported initial and extended follow-up results, with disparate findings. The European Randomized Study of Screening for Prostate Cancer (ERSPC) has reported a significant reduction in prostate cancer mortality; in contrast, the U.S.-based Prostate, Lung, Colorectal and Ovarian (PLCO) trial has not found any reduction in prostate cancer mortality 1–5. Recently, a third major trial has reported its findings, and these do not lessen the level of uncertainty over the effectiveness of PSA screening. The Cluster Randomised Trial of PSA Testing for Prostate Cancer (CAP) trial from the UK, which randomized over 400,000 men to either one-time PSA testing or usual care, like PLCO did not show a significant reduction in prostate cancer mortality, with a reported risk ratio of 0.96 (95% CI: 0.85–1.08) through a median of 10 years of follow-up 6. However, the low compliance rate with screening (around 35%) and the fewer than expected number of control arm prostate cancer deaths raised questions about whether the trial was under-powered 7.
Due to the generally slow progression of the disease, long time intervals are needed to assess the effect of screening interventions on prostate cancer mortality. Prior papers examined mortality in PLCO through a median of 15 years and incidence through a median of 11 years3,5. Here we extend follow-up of PLCO for both incidence and mortality by several years, allowing for the assessment of longer term effects of screening. With respect to incidence, in addition to assessing overall rates by arm, we also examine incidence by mode of diagnosis, Gleason score category and metastatic status. Furthermore, we examine prostate cancer deaths in each arm by cancer characteristics, including Gleason score.
Subjects and Methods
The design of the PLCO Trial has been described 1,3. Briefly, randomization at ten U.S. screening centers of subjects aged 55–74 to either an intervention or usual care arm occurred from 1993–2001. Primary exclusion criteria were a history of a PLCO cancer, current cancer treatment, and beginning in 1995, having had more than one PSA blood test in the prior three years. At study entry, participants completed a self-administered baseline questionnaire that included demographics, general risk factors, and screening and medical histories.
The primary endpoint for the prostate component was prostate cancer-specific mortality. Secondary endpoints included overall mortality, prostate cancer incidence and prostate cancer characteristics. The trial was approved by the Institutional Review Board at each screening center and all subjects provided written informed consent.
Intervention arm men received PSA tests at baseline and annually for 5 more years, and digital rectal exams (DRE) at baseline and annually for 3 more years. Participants also received chest radiographs annually for four years and flexible sigmoidoscopy at baseline and year three or five. PSA results were classified as abnormal if levels were greater than 4 ng/m. DRE results were considered abnormal if there was nodularity or induration of the prostate, or if the examiner judged other criteria to be suspicious for cancer, including asymmetry. Participants and their physicians were notified in writing of a suspicious abnormality on screening. The diagnostic process subsequent to a positive screen was managed by participants’ health care providers and was not dictated by the trial.
From 1993 to 2010, incident cancers and deaths were ascertained primarily by a mailed Annual Study Update questionnaire. Medical records relating to cancer were obtained and abstracted by certified tumor registrars. Tumor-related characteristics abstracted included Gleason score and TNM stage (clinical and pathologic, if available). Beginning in 2011, PLCO switched to a centralized follow-up process that utilized primarily passive linkages to state cancer registries to assess cancer incidence and linkages to the National Death Index (NDI) to assess mortality. A portion of subjects declined to be re-consented (“refusers”) and thus opted out of extended follow-up. Non-refusing subjects (about 85% of those alive in 2011) were followed for prostate cancer incidence through the end of 2014 and for mortality through the end of 2015; refusers were followed for incidence until the end of 2009 and for mortality until their date of refusal (generally in 2011).
For the state cancer registry linkages, all states where the PLCO screening centers were located were included in the linkage effort, as well as some adjacent states with large enough expected number of cases based on last known addresses of PLCO subjects. For all non-refusing subjects, their personally identifying information (PII) was sent to their “home registry” (i.e., the registry of the state where their screening center was located) and generally to the registries of adjacent states.
For the original analysis period (through 13 years of follow-up or December 31, 2009), the underlying cause of death was determined by a blinded endpoint verification process that utilized relevant medical records. For the extended follow-up period, as deaths were ascertained primarily by NDI, medical records were not available and therefore the underlying cause of death from the NDI was used to determine the endpoint. The endpoint verified classifications used in the original report were also used here.
Mode of diagnosis
PLCO abstractors attempted to record the reason for the initial medical visit that led to the eventual diagnosis of cancer. Reasons were categorized by the abstractors as symptomatic, follow-up of a positive PLCO trial screen, and other; for other, abstractors recorded a verbatim text description of what they felt was the reason. Study authors reviewed the verbatim text to further classify the original “other” category into “non-PLCO trial screen” and “incidental/other/unclear”. In addition, a few cases originally classified as “other” were re-classified as symptomatic based on terms such as “pain” or “hematuria”. For the non-PLCO trial screen category, any mention of “DRE”, “PSA” or “screening” qualified, as long as there was no mention of symptoms or incidental findings. For cancers only identified from the cancer registry linkages, mode of diagnosis could not be ascertained.
Statistical Methods
Chi-squared tests were used to compare differences in proportions. Incidence and mortality rates were computed by dividing number of events by person-years (PYs) at risk; corresponding risk-ratios (RRs) were computed from these rates. Incidence rates were computed for overall prostate cancer, as well as for Gleason categories and for metastatic disease.
Results
A total of 38340 and 38343 men were randomized to the intervention and usual care arms, respectively. Table 1 shows the demographics and medical history of trial participants by arm. Median (25th/75th) follow-up for incidence was 15.3 (10.6/17.8) and 15.1 (10.5/17.7) years in the intervention and usual care arms, respectively; median follow-up for mortality was 16.9 (14.0/19.2) years in the intervention arm and 16.7 (13.4/19.1) years in the usual care arm. Of those alive at the time of the transition to centralized follow-up (end of 2011), 11.2% of intervention arm versus 15.2% of usual care arm men refused further follow-up; refusers in each arm were slightly older at the time of transition than non-refusers (median age 77 and 76 for intervention and usual care arm refusers, respectively, versus median age 75 in each arm for non-refusers).
Table 1.
Demographics of PLCO population
| Intervention (N=38340 ) | Usual Care (N=38343) | ||
|---|---|---|---|
| Age (at baseline) | 55–59 | 12387 (32.3) | 12372 (32.3) |
| 60–64 | 12012 (31.3) | 12015 (31.3) | |
| 65–69 | 8877 (23.2) | 8885 (23.2) | |
| 70–74 | 5064 (13.2) | 5071 (13.2) | |
| Race/Ethnicity | Non-Hispanic White | 33043 (88.3) | 32136 (88.3) |
| Non-Hispanic Black | 1713 (4.6) | 1657 (4.6) | |
| Hispanic | 816 (2.2) | 787 (2.2) | |
| Asian | 1532 (4.1) | 1476 (4.1) | |
| Other | 322 (0.9) | 329 (0.9) | |
| Education | College Graduate | 15294 (40.9) | 14656 (40.5) |
| Family history of prostate cancer | 2737 (7.5) | 2589 (7.3) | |
| PSA test within the past 3 years | Once | 13252 (38.8) | 13135 (39.5) |
| More than once | 3588 (10.5) | 3760 (11.3) |
Note: Percentages exclude unknowns (except for age).
Prostate Cancer Mortality and Incidence
A total of 333 deaths from prostate cancer were observed in the intervention arm versus 352 in the usual care arm, giving rates of 5.5 and 5.9 (per 10,000 PY), respectively, and a RR of 0.93 (95% CI: 0.81–1.08; p=0.38) (Table 2). Figure 1 shows prostate cancer deaths over study time in each arm.
Table 2.
Prostate cancer incidence and mortality rates by arm
| Intervention |
Usual Care | RR (95% CI); p-value 1 | |
|---|---|---|---|
| N (rate per 10,000 PY) | N (rate per 10,000 PY) | ||
| PCa Mortality | 333 (5.5) | 352 (5.9) | 0.93 (0.81–1.08); 0.38 |
| PCa Incidence | |||
| All Prostate Cancer | 5574 (106.4) | 5287 (101.2) | 1.05 (1.01–1.09); <0.001 |
| Gleason 2–6 (Biopsy) | 3095 (59.0) | 2648 (50.6) | 1.17 (1.11–1.23); <0.001 |
| Gleason 7 (Biopsy) | 1510 (28.8) | 1511 (28.9) | 1.00 (0.93–1.07); 0.92 |
| Gleason 8–10 (Biopsy) | 630 (12.0) | 708 (13.6) | 0.89 (0.80– 0.99); 0.03 |
| Gleason 8–10 (Best) | 654 (12.5) | 749 (14.3) | 0.87 (0.78–0.97); 0.01 |
| Metastatic (at diagnosis) | 134 (2.6) | 158 (3.0) | 0.85 0.67–1.06); 0.15 |
RR for intervention versus usual care arm.
Figure 1.
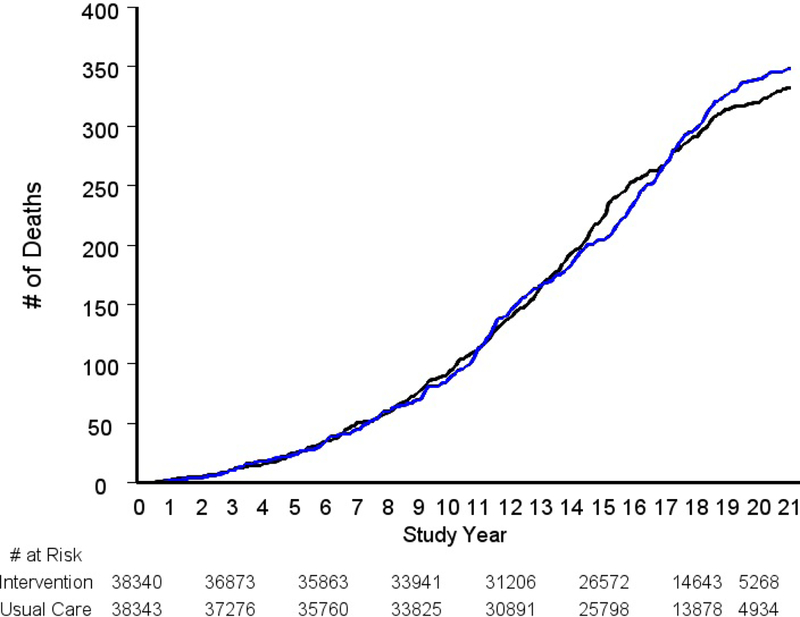
Cumulative prostate cancer deaths by trial arm. Black is intervention arm, blue is usual care arm.
Prostate cancer incidence rates by arm, overall and by cancer characteristics, are given in Table 2. There were a total of 5574 and 5287 incident cases in the intervention and usual care arms, respectively, giving rates of 106.3 and 101.1 (per 10,000 PY) and a RR for the intervention versus usual care arm of 1.05 (95% CI: 1.01–1.09). Figure 2A shows cumulative prostate cancer cases by arm over time. The absolute difference in number of cases in the intervention versus usual care arm appears relatively constant after about study year 7.
Figure 2A.
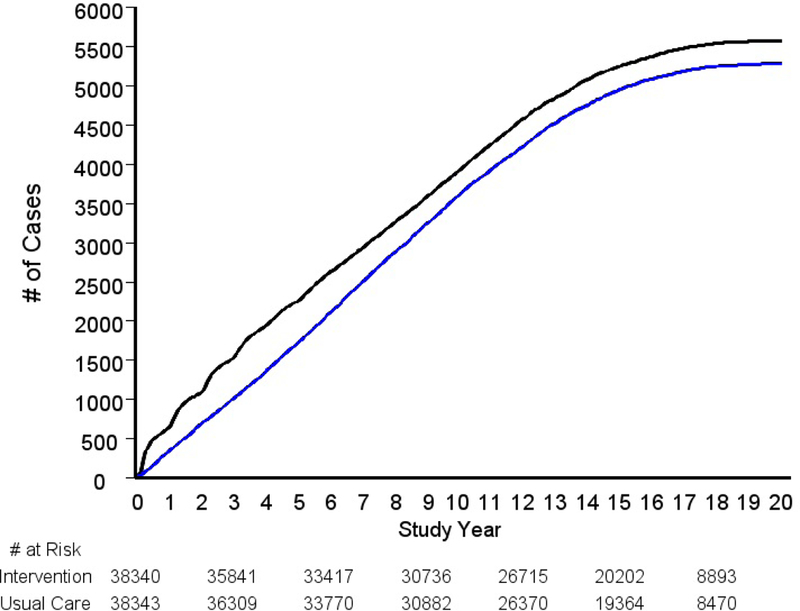
Cumulative prostate cancer cases by trial arm. Black is intervention arm, blue is usual care arm.
By (biopsy) Gleason category, the RR was significantly elevated for Gleason 2–6 disease (RR=1.17, 95% CI: 1.11–1.23; p <0.001), near unity for Gleason 7 disease (RR=1.00), and significantly below one for Gleason 8–10 disease (RR=0.89; 95% CI:0.80–0.99; p=0.03). Figure 2B shows cumulative cases over time by arm for each Gleason category.
Figure 2B.
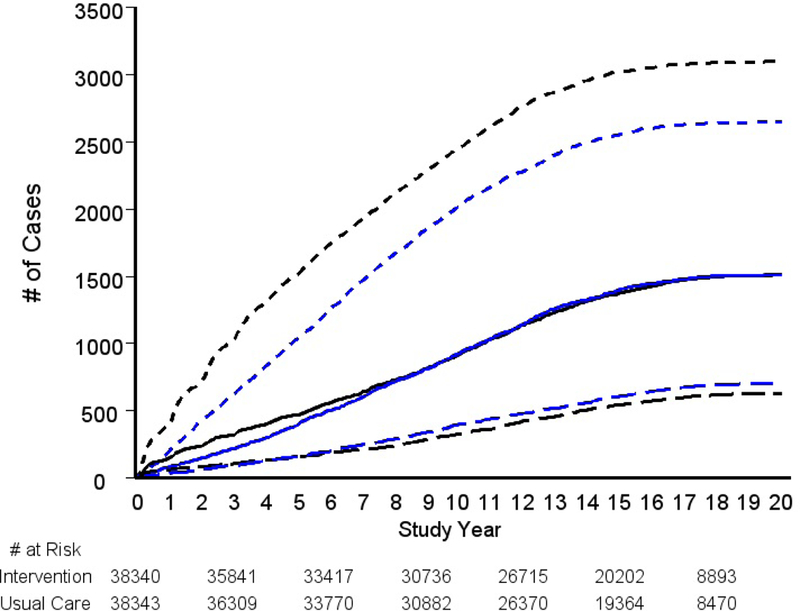
Cumulative prostate cancer cases by arm by Gleason category. Black lines are intervention arm, blue lines are usual care arm. Dotted, solid, and dashed lines are (biopsy) Gleason 2–6, Gleason 7 and Gleason 8–10 cases, respectively.
For Gleason 8–10 disease defined by “best” Gleason score (prostatectomy Gleason score if available, otherwise biopsy Gleason), the RR was 0.87 (95% CI: 0.78–0.97; p=0.01). The proportions of cases with prostatectomy Gleason score available were 33.1% and 29.2% in the intervention and usual care arms, respectively. The RR for metastatic disease was 0.85 (95% CI: 0.67–1.06).
Mode of Diagnosis
Figure 3 shows the number and proportion of cases with each mode of diagnosis by time period and trial arm. During the screening period (T0-T5), intervention arm cases were significantly less likely to be diagnosed symptomatically (12%) than usual care arm cases (27%), p < 0.001; conversely, they were significantly more likely to be diagnosed through screening (80% vs. 54%) (p <0.001). In contrast, in the post-screening period, the distribution of mode of diagnosis was similar across arms. In the post-screening compared to screening period, the proportion of cases diagnosed through symptoms increased in the intervention arm (12% to 18%) but decreased in the usual care arm (27% to 18%) (p < 0.001).
Figure 3.
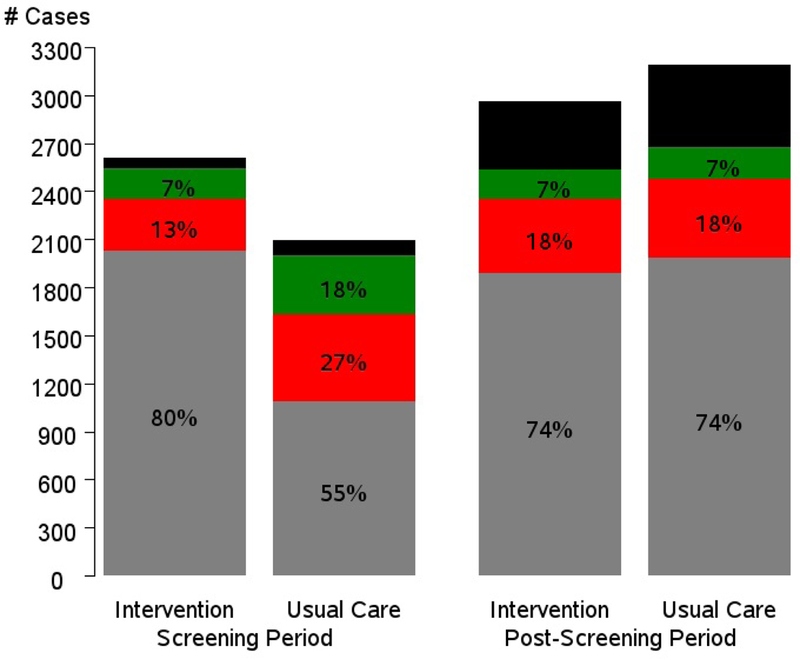
Number and percent of prostate cancer cases by trial arm, trial period and mode of detection. Gray is screen-detected cases, red is symptomatically detected, green is other-detected and black is not ascertained. Percentages exclude not ascertained.
Table 3 examines symptomatic diagnosis by trial arm and cancer characteristics. In each trial arm, symptomatic diagnosis was significantly greater in Gleason 8–10 cases (21.6% and 27.5% in the intervention and usual care arms, respectively) than in Gleason 2–7 cases (14.6% and 21.0%), p < 0.001. Additionally, in each arm, symptomatic diagnosis was significantly greater in metastatic cases (34.0% and 43.1% in the intervention and usual care arms, respectively) than in non-metastatic cases (15.0% and 21.4%), p < 0.001.
Table 3.
Mode of diagnosis by prostate cancer characteristics and trial arm.
| Intervention | Usual Care | ||
|---|---|---|---|
| % Symptomatic (total N) 1 |
% Symptomatic (total N) 1 |
p-value (I vs. C) | |
| Gleason 8–10 | 21.6 (561) | 27.5 (619) | 0.02 |
| Gleason 2–7 | 14.6 (4404) | 21.0 (3949) | <0.0001 |
| p-value (Gleason 8–10 vs 2–7) |
<0.0001 | 0.0003 | |
| Metastatic Disease | 37.8 (111) | 49.6 (133) | 0.06 |
| No metastatic disease | 15.0 (4971) | 21.4 (4537) | <0.0001 |
| p-value (Metastatic vs. non metastatic) |
<0.0001 | <0.0001 |
Total with known mode of diagnosis
Cancer characteristics of PCa Deaths
Table 4 shows the cancer characteristics by arm of the prostate cancer deaths. In each arm, Gleason 8–10 cases comprised slightly less than half of the deaths (44% and 48% in the intervention and usual care arms, respectively). By risk category, 28% of intervention and 33% of usual care arm prostate cancer deaths were metastatic cases (at diagnosis), 35% (intervention) and 33% (usual care) were high D’Amico risk (including T3, T4 or N1 cases), and 37% (intervention) and 34% (usual care) were low or intermediate D’Amico risk. There were no statistically significant differences by arm. Among those dying of prostate cancer, the median time from diagnosis to death significantly decreased with increasing Gleason score (8.8, 7.0 and 3.5 years for Gleason 2–6, 7 and 8–10, respectively; p <0.0001) and with increasing D’Amico Risk category (9.9, 7.9, 6.7, 7.0 and 1.8 years for low, intermediate, high, T3/4 or N1, and metastatic disease, respectively; p < 0.0001). Time to death did not significantly differ by trial arm (median 6.0 versus 5.1 years for intervention and usual care arms, respectively; p=0.16).
Table 4.
Deaths from prostate cancer – characteristics of cancers
| Intervention | Usual Care | P-value for comparison |
All | |
|---|---|---|---|---|
| N(%) | N(%) | |||
| Total PCa deaths | 333 | 352 | 685 | |
| Gleason Category | ||||
| 2–6 | 73 (26) | 57 (20) | 0.20 | 130 (23) |
| 7 | 82 (30) | 92 (32) | 174 (31) | |
| 8–10 | 123 (44) | 137 (48) | 260 (46) | |
| Unknown | 55 | 66 | 121 | |
| D’Amico Risk Category | ||||
| Low | 42 (14) | 32 (10) | 0.47 | 74 (12) |
| Intermediate | 67 (23) | 75 (24) | 142 (24) | |
| High | 82 (28) | 79 (26) | 161 (27) | |
| T3/T4 or N1 | 22 (7) | 22 (7) | 44 (7) | |
| Metastatic Disease | 82 (28) | 101 (33) | 183 (30) | |
| Unknown | 38 | 43 | 81 |
Note: Unknowns were excluded from the denominator for percentages and from calculations for p-value.
Discussion
Over a median follow-up of almost 17 years, there was no significant reduction in prostate cancer mortality in the PLCO intervention as compared to usual care arm, with an RR of 0.93 (9% CI: 0.81–1.08). This is the fourth report of endpoint data from the prostate component of the PLCO trial. With increasing median length of follow-up, the mortality RR has steadily decreased, from 1.11 initially to the current 0.93 (see Supplemental Appendix). Although the current follow-up was, on average, about 12 years beyond the final scheduled screening round in the trial, further follow-up for mortality of trial participants is planned to be able to assess longer term effects of screening. With respect to prostate cancer incidence, the overall rate ratio was significantly elevated in the intervention versus usual care arm, although the magnitude of the increase was small (RR=1.05, 95% CI: 1.01–1.09). The rate was elevated from the initiation of screening and has persisted through the latest long-term follow-up, indicating overdiagnosis, although the RR has decreased steadily with increasing length of median follow-up (see Supplemental Appendix). The RR varied substantially by Gleason category, being increased for Gleason 2–6 disease, null for Gleason 7 disease, and significantly decreased for Gleason 8–10 disease. This demonstrates that overdiagnosis in the trial was restricted to low-grade disease.
The observed reduction in the incidence of Gleason 8–10 disease in the intervention arm is of interest. It is not known whether and to what extent Gleason grade progresses over time. A reduction in incidence with a screening intervention would seem to imply that there is progression of Gleason grade over time. The CAP trial reported a statistically significant between-arm difference (control minus intervention) in the proportion of trial men with Gleason 8–10 disease of 0.58 (95% CI: 0.06 to 1.09) per 1,000 (7.45 per 1,000 of control versus 6.88 per 1,000 of intervention arm men) 6. Based on the reported number of cases and person years, the RR for Gleason 8–10 disease in CAP, intervention compared to control arm, was 0.91. The RR of 0.89 observed in PLCO for Gleason 8–10 disease was of similar magnitude, although for a longer follow-up period (median 10 years in CAP versus 15 years in PLCO). Since the reduction in Gleason 8–10 disease in the PLCO intervention arm was modest, and Gleason 8–10 disease only comprised about half of usual care arm deaths, the reduction in Gleason 8–10 disease alone would not be expected to lead to a significant reduction in mortality.
A modeling paper examining three Swedish epidemiologic studies of men not receiving PSA screening showed a relationship between lead time and Gleason grade; specifically, that the likelihood of high-grade disease increased with longer lead time 8. Such a result would imply that Gleason grade may increase over time in men, and that thus screening could result in lower Gleason scores at diagnosis.
The data here on mode of diagnosis showed similar proportions of screen-detected and symptomatic disease across trial arms in the post-screening phase, whereas during the screening phase there was a higher proportion of screen-detected disease and a lower proportion of symptomatic disease in the intervention as compared to usual care arm. These data are consistent with previously reported data on PSA screening rates in PLCO, where during the screening phase the PSA testing rate was approximately two-fold higher in the intervention than usual care arm, whereas in the post-screening phase PSA testing rates were high but similar across arms 5,9. The high rate of usual care arm PSA testing (contamination) in PLCO explains the only modest increase in prostate cancer incidence in the intervention versus usual care arm over the length of the trial. Within each trial arm, we also found significantly higher rates of symptomatic detection for higher Gleason grade (8–10) versus lower grade disease and for metastatic versus non-metastatic disease. There are little data in the literature on mode of detection by tumor characteristics in an actively screened population.
Although the rate of prostate cancer death is substantially lower for low or intermediate grade (Gleason 2–6 or 7) disease than for high-grade (Gleason 8–10) disease, and for low or intermediate risk D’Amico category disease compared to high D’Amico risk or metastatic disease, the absolute number of deaths from low/intermediate grade and low/intermediate D’Amico risk disease in this heavily screened population comprised a substantial proportion of all observed prostate cancer deaths. Slightly over half of all prostate cancer deaths were from Gleason 2–7 cases, and somewhat over one third were from low and intermediate D’Amico risk disease.
The CAP and ERSPC trials did not report the breakdown of prostate cancer deaths (by arm or overall) by prostate cancer characteristics. A recent study examined the tumor characteristics of all prostate cancer deaths in Denmark from 1995–2013 10. With respect to Gleason score, the results were similar to those seen in PLCO; specifically, 53% and 31% of deaths were Gleason 8–10 and Gleason 7, respectively, as compared to 46% and 31% in PLCO (across both arms). However, for stage, the results were different, with 47% of the Danish deaths being metastatic stage, compared to only 30% in PLCO. Although PSA screening has never been recommended in Denmark, use is believed to be common, based in part on the increasing incidence of prostate cancer over the study period, with a three-fold rise in age-adjusted incidence from 1995 to 2009. However, the overall intensity of PSA use in Denmark was likely less than in the PLCO control arm, let alone the intervention arm. A study of approximately 10,000 deaths from prostate cancer in the UK in men diagnosed with the disease between 1997 and 2006 showed that 58% of deaths were metastatic stage (of those with known stage), a substantially higher proportion than seen in PLCO 11. However, PSA use in the UK during this period was believed to be low, and the proportion of all prostate cancer cases that were metastatic stage was much higher in the UK study, 20% (of those with known stage), than in PLCO, about 2.5%. The UK study did not examine the Gleason score distribution of deaths.
This investigation has several limitations. As noted, when the follow-up process switched from active participant contact to passive linkage, endpoint verification was no longer employed and the study relied on the underlying cause of death from NDI to ascertain the primary endpoint. However, an analysis of death certificate versus endpoint verification classification for deaths occurring during the endpoint verification phase of the trial showed minimal differences in the prostate cancer mortality RR by the two methods. Further, comparisons of mortality over time can be influenced by treatment differences within stage between trial arms. Although data on treatment of prostate cancer cases are not presented in this paper, data in earlier reports indicate very comparable treatment distributions between arms within stage, and there is every reason to believe that this held true through follow-up since referral and treatment practices are generally uniform in the US medical care system 3.
Because the PLCO trial involved screening for four different cancers, and extended follow-up mortality results have not been reported yet for all of the cancer sites, we do not report all-cause mortality results here. Rather, a future paper will report on all-cause mortality after all of the trial’s four individual cancer-specific mortality results have been reported.
Extended follow-up of the PLCO trial over a median of almost 17 years showed no significant reduction in prostate cancer mortality in the intervention versus usual care arms. A substantial percentage of prostate cancer deaths in each arm were due to non-advanced disease at the time of diagnosis. There was significantly increased prostate cancer incidence in the intervention arm, specifically of Gleason 2–6 disease, indicating overdiagnosis. However, a significant reduction in the incidence of Gleason 8–10 disease in the intervention arm was also observed.
Supplementary Material
Acknowledgments:
Cancer incidence data have been provided by the following state cancer registries: Alabama, Arizona, California, Colorado, District of Columbia, Hawaii, Idaho, Maryland, Michigan, Minnesota, Missouri, Nevada, Ohio, Pennsylvania, Texas, Utah, Virginia and Wisconsin. All are supported in part by funds from the Centers for Disease Control and Prevention, National Program for Central Registries, local states, or by the National Cancer Institute, Surveillance, Epidemiology, and End Results Program. The results reported here and the conclusions derived are the sole responsibility of the authors.
Dr. Andriole reports personal fees from 3D Biopsy, personal fees from Augmenix, other from Blue Earth Diagnostics, other from Medivation, other from Progenics, grants from PCF, grants from Peter Michael Foundation, grants from St. Louis Men’s Group Against Cancer, grants from Barnes-Jewish Hospital Foundation. Dr. Crawford reports consulting with Bayer, MDx, Genomic Health, Janssen, Ferring and Dendreon.
Footnotes
Conflicts of Interest: No other potential conflicts.
References
- 1.Andriole GL, Grubb RL, Buys SS, et al. Mortality results from a randomized prostate cancer screening trial. New Engl J Med 2009; 360: 1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality in a randomized European study. New Engl J Med 2010; 360: 1320–1328. [DOI] [PubMed] [Google Scholar]
- 3.Andriole GL, Crawford ED, Grubb RL et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial: Mortality results after 13 years of follow-up. J Natl Cancer Inst 2012; 104: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014; 384: 2027–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinsky PF, Prorok PC, Yu K, et al. Extended Mortality Results for Prostate Cancer Screening in the PLCO Trial with Median 15 Years Follow-up. Cancer 2017; 123: 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin RM, Donovan JL, Turner EL, et al. Effect of low-intensity PSA-based screening intervention on prostate cancer mortality: The CAP randomized clinical trial. JAMA 2018; 319: 883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinsky PF. Power of a trial investigating a low-intensity PSA-based screening intervention. JAMA 2018; 320: 600. [DOI] [PubMed] [Google Scholar]
- 8.Assel M, Dahlin A, Ulmert D, et al. Association between lead time and prostate cancer grade: evidence of grade progression from long-term follow-up of large population-based cohorts not subject to prostate-specific antigen screening. Eur Urol 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinsky PF, Black A, Kramer BS, et al. Assessing contamination and compliance in the prostate component of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Clinical Trials 2010; 7: 303–311. [DOI] [PubMed] [Google Scholar]
- 10.Helgstrand JT, Roder MA, Klemann N, et al. Diagnostic characteristics of lethal prostate cancer. Eur J Cancer 2017; 84: 18–26. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhury S, Robinson D, Cahill D, et al. Causes of death in men with prostate cancer: an analysis of 50,000 men form the Thames Cancer Registry. BJU Int 2013; 112: 182–189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


