Abstract
A combination of genetic manipulations of donor organs and target specific immunosuppression are instrumental in achieving long-term cardiac xenograft survival. Recently, results from our preclinical pig-to-baboon heterotopic cardiac xenotransplantation model suggests that a three-pronged approach is successful in extending xenograft survival: (1) α−1,3-galactosyl transferase (Gal) gene knockout in donor pigs (GTKO) to prevent Gal-specific antibody mediated rejection; (2) transgenic expression of human complement regulatory proteins (hCRP; hCD46) and human thromboregulatory protein thrombomodulin (hTBM) to avoid complement activation and coagulation dysregulation; and (3) effective induction and maintenance of immunomodulation, particularly through co-stimulation blockade of CD40-CD40L pathways with anti-CD40 (2C10R4) monoclonal antibody (mAb). Using this combination of manipulations, we reported significant improvement in cardiac xenograft survival.
In this study, we are reporting the survival of cardiac xenotransplantation recipients (n=3) receiving xenografts from pigs without the expression of hTBM (GTKO.CD46). We observed that all grafts underwent rejection at an early time point (median 70 days) despite utilization of our previously reported successful immunosuppression regimen and effective control of non-Gal antibody response. These results support our hypothesis that transgenic expression of human thrombomodulin in donor pigs confers an independent protective effect for xenograft survival in the setting of a co-stimulation blockade based immunomodulatory regimen.
Introduction
Recent technological advancements in the field of genetic engineering may facilitate the development of genetically modified pigs with either deletion of certain molecules immunogenic to humans or with expression of human transgenes to overcome various incompatibilities between humans /non-human primates and pigs. We believe that organs from these genetically engineered (GE) animals may overcome the unmet demand of solid organs for patients with end-stage organ failure (1). However, identifying the ideal genetic construct of a pig donor for clinical xenotransplantation remains a challenge. Recently, transplantation of cells and solid organs from GE pig donors has resulted in significant improvement in xenograft survival (2–10).
Thrombomodulin (TBM) is a coagulation regulatory protein that binds thrombin and enhances its activity by converting protein C to activated protein C (aPC). The aPC then inhibits activated factors V and VIII, leading to decreased thrombin formation and prevention of a hypercoagulable state (11). Pig TBM does not demonstrate analogous efficacy within human or non-human primate systems. The lack of a functional TBM is hypothesized to lead to microthrombosis and premature graft loss through a mechanism independent of immunologic graft rejection and is unmitigated by immunosuppressive regimens. The ability to express hTBM in donor pigs has provided a mechanism to overcome this graft loss secondary to microthrombosis. Recently, we have demonstrated long-term cardiac xenotransplantation survival from a GE donor pig, expressing human thrombomodulin (GTKO.CD46. hTBM) using immunosuppression including co-stimulation blockade by anti-CD40 (2C10R4) mAb in pig-to-baboon cardiac xenotransplantation model (4). One question raised from this work is the necessity of human hTBM expression in the presence of anti-CD40 mAb based co-stimulation blockade; it is unclear whether it confers additional graft protection from rejection over co-stimulation blockade alone. In this study, using a similar cardiac xenotransplantation model described by us earlier (3, 4, 12) we substituted GTKO.CD46.hTBM pig hearts with hearts from donor pigs which do not express hTBM (i.e. GTKO.CD46 only) along with our already tested modified immunosuppression regimen which include costimulation blockade by anti-CD40 mAb.
Materials and Methods
Animals
Specific pathogen free (SPF) baboons of either sex weighing 7–15 kg (2–3 years of age) from University of Oklahoma (Norman, OK) were housed in a clean pathogen free facility and were used as recipients. Six to eight week old genetically modified alpha 1,−3-Galactosyltransferase gene knockout (GTKO) pigs of either sex with an overexpression of human CD46 without hTBM (GTKO.hCD46) (n=3) were used as donors (Revivicor Inc., Blacksburg, VA). These GTKO.CD46 pigs were produced by breeding over 6 generations and were homozygous for human CD46 and homozygous Gal knockout. The method of generation of these pigs has been described previously (13). The transgenes of hCD46 were stable at the genomic and protein level (3, 4). The weights of donor pigs were matched with the baboon recipient to accommodate the heart in the abdomen. All animals were used in compliance with guidelines provided by the National Heart, Lung and Blood Institute (NHLBI) Animal Care and Use Committee (IACUC).
Immunosuppression
Immunosuppressive regimen for all recipient baboons included induction therapy comprised of anti-thymocyte globulin (ATG), anti-CD20 antibody (Rituximab) and co-stimulation blockade with anti-CD40 (Clone 2C10R4) mAb, Cobra venom factor (CVF; Quidel, San Diego, CA) which was used to inhibit complement activation. High dose anti-CD40 mAb, Mycophenolate Mofetil (MMF), and tapered dose of steroids were also administered throughout the course as maintenance therapy. Details of the immunosuppressive regimen are shown in Table 1.
Table.1.
Table of Immunosuppression
| Drugs | Dose | Timing |
|---|---|---|
| Induction: | ||
| ATG | 4–5 mg/Kg | Pre op days −2 & −1 |
| CVF | 50–100 U/Kg | Pre op days −1, 0 & 1 |
| Anti-CD20 ( Rituximab) | 19 mg/Kg | Pre op days −7, 0, 7 & 14 |
| Anti-CD40 (2C10R4) antibody |
50 mg/Kg | Pre op days −1 & 0 |
| Maintenance: | ||
| Anti-CD40 (2C10R4) antibody |
50 mg/Kg | Post op days 3, 7, 10, 14, 19, q weekly |
| MMF | 20 mg/Kg/2hr IV infusion |
BID daily |
| Steroids | 2 mg/Kg | BID, tapered off in 7 weeks |
| Aspirin | 81 mg | Daily |
| Heparin | Maintain ACT 2x baseline |
Continuous infusion |
| Supportive: | ||
| Ganciclovir | 5 mg/Kg/day | Daily |
| Cefazolin | 250 mg | BID for 7 days |
| Epogen | 200 U/ Kg | Daily from −7 to 7 then weekly |
Heterotopic cardiac xenotransplantation and xenograft evaluation
All heterotopic cardiac xenotransplant procedures were performed at a NHLBI core surgical facility, as previously described (4, 14). Cardiac xenograft function was evaluated by continuous telemetry monitoring (15). The health of the recipient baboon was evaluated utilizing , complete blood work (Complete blood count (CBC), chemistry, troponin release assay, serum and plasma collection for non-gal IgG and IgM antibody measurement) at regular intervals (weekly for the first 2 months of xenotransplantation and monthly after this period). A telemetry device was implanted in the donor heart and measured the recipient’s temperature, the xenograft’s left ventricular pressure (LVP) and the electrocardiogram (EKG). A drop in LVP of the xenograft below 60 mmHg correlated with the initiation of the rejection process, which also indicated a decrease in graft contractility, and a pressure below 10 mmHg was an indicator of complete cessation of graft contractility (15). Palpation and ultrasonography of the cardiac xenograft were performed along with blood work of the recipient at a weekly or bi-weekly frequency. Based on xenograft palpation, contractility of the heart was scored as ++++ (fully functional) to zero (non-functional) and blood flow and wall, motion was analyzed by ultrasonography (3).
Coagulation studies
Blood samples from xenotransplantation recipient baboon were collected in sodium citrate solution before transplantation and two times a week after xenotransplantation for 3 months. Plasma obtained after centrifugation was stored in aliquots at –80°C until use. Activated partial thromboplastin time (aPTT), Prothrombin Time (PT) and Fibrinogen were determined by the Coagulation lab of Animal Health Diagnostic Center, Cornel University, (Ithaca, NY).
Measurement of Non Gal IgG and IgM Antibodies
Anti-pig non-gal (IgG and IgM) antibodies were measured in the serum of recipient baboons; they were collected at different times after cardiac xenotransplantation by flow cytometry using GTKO porcine endothelial cells as described previously (4).
Histological evaluation of explanted xenograft
Paraffin sections of explanted cardiac xenografts from multiple sites were stained with hematoxylin and eosin for light microscopy (3, 4). Sections were analyzed semi quantitatively for the presence of myocyte damage, hemorrhage, necrosis, thrombosis, and cellular infiltrates. Percentage of myocyte necrosis was determined in all sections of the heart including apex, interventricular septum (IVS), atria (left and right) and ventricles (right and left). Presence of fibrin microthrombi was also scored or assessed by relative number of thrombi from section to section and the score was defined by the presence of microthrombi as follows: 1+ = >0; 2+ = >1–5; 3+ = >5–10; and 4+ = >10.
Statistical Analysis
GraphPad Prism 7.0 or Microsoft Excel was used to generate all the graphs. Two tailed student t- test were used to determine the level of statistical significance among the groups. Log-rank (Mantel-Cox) test was used for survival analysis.
Results
Survival of Non-hTBM expressing donor pig heart in Baboon
All heterotopic cardiac xenotransplantation’s from GTKO.hCD46 donor pigs were performed without any technical difficulty or perioperative complications. Recipient baboons were successfully extubated immediately following surgery and were active, eating, and generally well soon after the operation. Use of left ventricular telemetry implant and serial echocardiography confirmed vigorous xenograft contractility following transplantation. All the recipient baboons maintained their hematological parameters. WBC hematocrit and platelet counts are shown in Fig 1. The platelet numbers, after an initial drop immediately after surgery, were maintained at a healthy level. Heparin infusion was given to maintain the intravenous lines and to keep the activated clotting time (ACT) to 2X the normal level. However, all three xenografts were eventually rejected after 9, 70 and 98 days respectively (median 70 days) and these outcomes were significantly lower than previously reported (median 298 days p<0.0042) in the GTKO.CD46.hTBM cohort, as shown in Fig 2 (A).
Figure 1. Hematological Parameters;
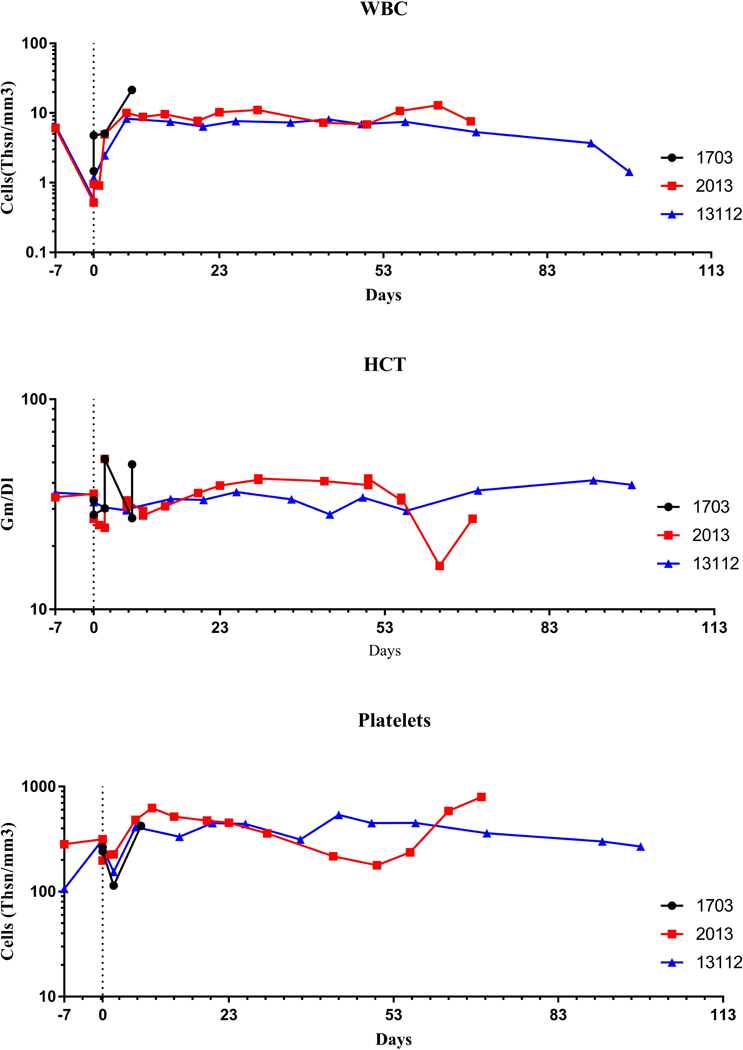
(A). White blood Counts (WBC), (B). Hematocrit (HCT) and (C). Platelets of cardiac xenograft recipient receiving from non-hTBM donor pig (i.e. GTKO.CD46)
Figure 2. Cardiac xenograft survivals and histopathological examination;
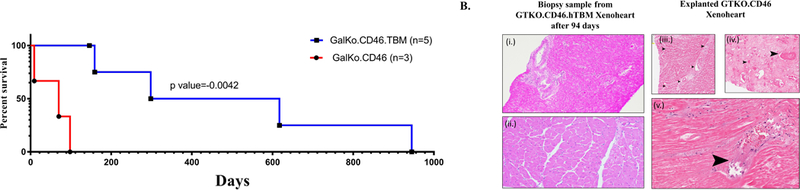
(A) Cardiac xenograft survivals of both GTKO.CD46 and GTKO.CD46.hTBM xenograft in recipient baboons. (B) Hematoxylin and eosin staining of biopsy of xenograft expressing GTKO.CD46.hTBM gene (i (20X) & ii (400X)) without any sign of rejection or thrombotic microangiopathy; and, explanted GTKO.CD46 xenograft without hTBM expression (iii, iv & v (20X)) indicating myocardial destruction and widespread fibrin micro-thrombi. (Arrows).
Histopathology of explanted xenografts
Hematoxylin and eosin stain analyses of paraffin sections were performed on all explanted xenografts and representative images are shown in Fig 2B. Histopathological findings from explanted xenografts (after 70 and 98 days for baboon #2013 and #13112 respectively) demonstrate acute interstitial hemorrhage, diffuse coagulative myocytes necrosis and numerous micro-thrombi throughout the graft surrounded by dilated capillaries and vascular congestion (Fig 2B. iii, iv & v). Percentage of necrosis and presence of microthrombi in two of three GTKO.CD46 without hTBM expression of explanted xenograft were semi-quantitatively evaluated and shown in table 2. Biopsy specimen collected at the same time (94 days after cardiac xenotransplantation) from grafts expressing TBM (from our earlier experiments (4)) did not show any signs of rejection or thrombosis. (Fig 2B. i & ii). Immunohistochemistry to detect anti pig antibodies was inconclusive due to non-specific antibody binding.
Table 2.
Histopathology of rejected xenoheart and biopsy sample
| GTKO. CD46 Xenograft | GTKO. CD46. TBM Xenograft |
|||||
|---|---|---|---|---|---|---|
| B#2013 (Explant after 70 days) |
B#13112 (Explant after 98 days) |
Biopsy (after 94 days) |
||||
| Heart site | Percent Necrosis* |
Microthrombi | Percent Necrosis* |
Microthrombi | Percent Necrosis |
Microthrombi |
|
Left ventricle |
100% | 4+ | 100% | 4+ | 0 | 0 |
|
Left atrium |
100% | 3+ | 100% | 0 | 0 | 0 |
|
Right atrium |
100% | 4+ | 100% | 0 | 0 | 0 |
| Septum | 100% | 3+ | 100% | 4+ | 0 | 0 |
| Apex | 100% | 3+ | 100% | 4+ | 0 | 0 |
|
Right ventricle |
100% | 4+ | 100% | 4+ | 0 | 0 |
100 % necrosis means that in every section examined all the myocytes were necrotic.
Coagulation profile, troponin release and ultrasonography examination
Prothrombin Time (PT) and activated partial thromboplastin time (aPTT) were found to be increased, whereas fibrinogen levels were decreased in all the recipients receiving GTKO.CD46 grafts without hTBM expression as compared with recipients receiving GTKO.CD46.hTBM xenografts (Fig 3A).
Figure 3. Coagulation, Troponin release and Ultrasonography:
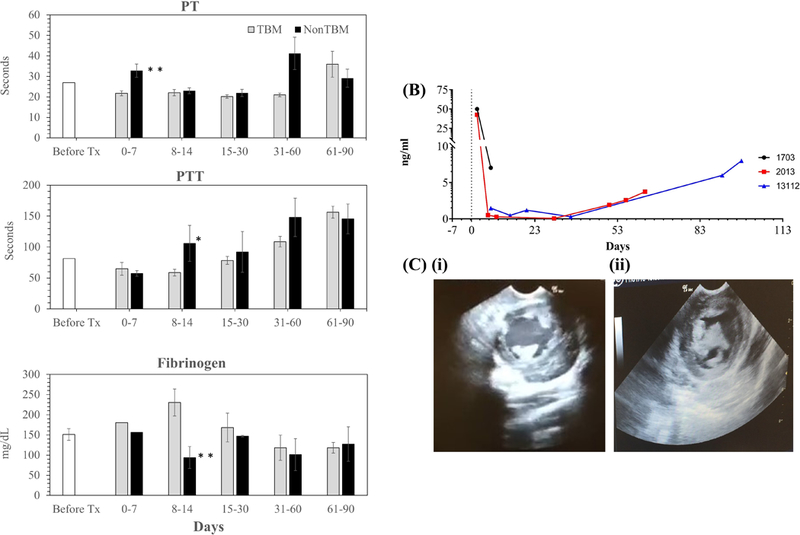
(A).Coagulation profile (e.g. (i) PT, (ii) PTT & (iii) Fibrinogen levels) of recipient baboon receiving non TBM and TBM expressing donor xenoheart;(pvalue=*<0.5; **<0.05) (B) Troponin Release, and, (C) Ultrasonography of GTKO.CD46 xenograft (i.e. (i) a representative ultrasound screenshot showing an empty cavity of transplanted xenoheart just after the xenotransplantation and (ii) with clot filled in transplanted heart during the rejection) from recipient baboon receiving non TBM donor pig.
Troponin release is an established indicator of cardiac xenograft injury. Troponin release was found to be increased (Fig 3b) in all cardiac xenograft recipients receiving a pig heart from non-hTBM expressing GTKO.CD46 donors. Rejection was correlated with a drop in left ventricular pressure demonstrated by telemetry. A significant drop in LVP was correlated with progressive graft thickening noted on echocardiography (Fig 3C). A marked reduction in contractility and wall motion was also observed via echocardiography at this time point. Besides shorter survival of these xenografts, we also observed significant adhesions around the xenograft at the time of graft explantation consistent with inflammatory adhesions previously observed by our group and others (16–20).
Measurement of Non-Gal antibody titers
In order to assess the humoral immunity of recipient baboons, non-Gal IgG and IgM were measured and found to be at their baseline level except for one recipient where IgM antibody level was increased (Fig 4), at the time of rejection of non-hTBM expressing GTKO.CD46 pig donor heart. In contrast, both IgM and IgG non-Gal antibody levels were increased when xenograft rejection was triggered by withdrawal of anti-CD40 mAb in a previous experiment (4).
Figure 4. Non-Gal IgG and IgM levels;
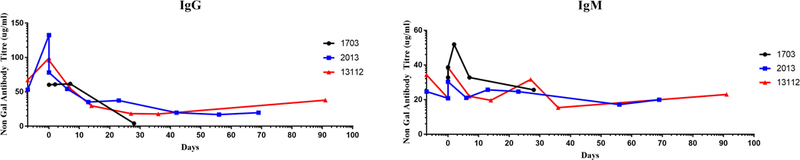
Anti pig non-Gal (A) IgG and (B) IgM antibodies levels in mean fluorescence intensity (MFI) was measured in cardiac xenograft grafts baboon recipient’s serum by flow cytometry using GTKO porcine endothelial cells.
Discussion
Hyperacute xenograft rejection mediated by preformed antibodies (21) and host complement pathways (22) attacking donor vascular endothelium has been effectively prevented by knocking out α-galactosyltransferase (GTKO) (13) and by the transgenic expression of complement regulatory proteins in the donor (5, 23). Xenograft survival for heterotopic solid organs from GE donor pigs along with immunosuppression targeting both B-cell and T-cell mediated responses has been prolonged to nearly three years (4, 5, 24–26).
Coagulation dysregulation is also a major limitation for the success of xenotransplantation. Its occurrence is partly due to activation of the complement cascade by preexisting antibodies and it is partly due to some molecular incompatibilities between pig and NHP/human of major components of coagulation pathways which ultimately develops into thrombotic microangiopathy and concomitant disseminated intravascular coagulation (DIC)(27). In this study we have observed an increase inboth PT, as wll as aPTT and a decrease in fibrinogen levels immedialtely after cardiac xenotransplantation in the recipients receiving GTKO.CD46 xenograft as compared to the GTKO.CD46.TBM xenografts. We believe that this coagulation dysfunction might have triggered the late or delayed xenograft rejection in these recipients. In comparison, recipients receiving cardiac xenografts with hTBM expression maintained coagulation, profiles and ultimately demonstrated prolonged the cardiac xenograft survival.
A number of investigators have tested various forms of clinical and experimental systemic anticoagulation agents to prevent the development and mitigate the effects of DIC (28–30). For example, Cowan et al. have shown that daily treatment of recombinant human anti thrombin (rhAT) can considerably extend the survival of nephrectomized baboons receiving life-supporting renal xenografts (29) However, Cozzi et al. were unable to demonstrate prolonged survival in a renal pig-to-cynomolgus monkey xenotransplantation model after the rhAT treatment (30). Likewise, Byrne et al (28) have also reported that the addition of systemic antiplatelet or anticoagulation therapy does not increase xenograft survival in pig-to baboon heterotopic cardiac xenotransplantation. However in our experience, in the absence of hTBM expression on the donor hearts, all grafts developed microthrombi despite aggressive and continuous anticoagulation therapy with heparin. This anticoagulation therapy was similar in all the recipients receiving hTBM and non-hTBM expressing xenografts.
Recently, organs from genetically modified pigs expressing human complement and coagulation regulatory proteins have been capable of controlling activation of primate complement and have thus been protected from hyper acute rejection (30), prolonging xenograft survival (1, 4, 14, 31). We have previously reported the longest pig-to baboon heterotopic cardiac xenograft survival of GTKO.CD46. hTBM xenograft along with immunosuppression and co-stimulation blockade by high dose of anti-CD40 (2C10R4)(4), whereas low doses of anti-CD40 mAb treatment followed by its early withdrawal from the immunosuppressive regimen resulted in poor xenograft outcomes (32). However, in the study presented here, despite high doses of anti-CD40 mAb treatment, cardiac xenograft survival was significantly reduced and explanted xenografts demonstrated microvascular thrombosis, which, in our opinion, is due to the lack of hTBM expression on donor pig hearts. Iwase et al. have also previously reported a poor xenograft outcome from donor hearts that do not express hTBM (7). Recently, Iwase et al. in another study have reported a long term kidney xenograft survival using both anti CD40 mAb and multigene expressing GE donor pigs (six gene; GTKO.CD46.CD55.EPCR.TFPI.CD47) without hTBM, but the recipientwhich had a donor pig kidney expressing GTKO.CD46.hTBM developed consumptive coagulopathy within a short period following transplantation (33). The authors have mentioned that hTBM expression was modest on the graft; it is possible that stronger expression of hTBM on the xenograft would have prolonged graft survival in this case. However, there are few other unpublished studies from donor pig kidney and other organs demonstrating long-term xenograft survival without hTBM expression. Previously, differences between pig hearts and kidneys have been shown on a molecular level by others (34); it is possible that expression of hTBM on donor hearts is essential to prevent thrombotic microangiopathy and very late development of consumptive coagulopathy while this is not essential in donor kidneys. We have also reported shorter cardiac xenograft survival (median 71 days) from GTKO.hCD46 donor pigs while administering anti-CD154 antibody based immunosuppression (3).
Lack of hTBM aggravates thrombosis and a complete deficiency of hTBM in homozygous mice has been demonstrated to causes embryonic lethality (35). However, administration of hTBM attenuates the consequences of thrombin-induced thromboembolism in mice and rats (36). Thrombomodulin has been shown to be involved in the regulation of coagulation, complement, and anti-inflammation and cell proliferation, which have a protective role against microvascular thrombosis (36, 37). Furthermore, our results presented earlier also suggest that hTBM expression in the donor organ along with anti-CD40 mAb treatment may help to prolong the xenograft survival (38).,
Based on the above observations, we conclude that a modified immunosuppression regimen with anti-CD40 (2C10R4) antibody alone is not sufficient to extend cardiac xenograft survival. Lack of hTBM may have induced thrombotic microangiopathy that could have led to graft rejection. It is difficult to dissect the rejection mechanism because xenografts from our earlier studies were also rejected in a similar manner after terminating anti CD40 mAb. There are several other immune and non-immune mechanisms of xenograft rejection besides antibody-mediated rejection, which may require further donor genetic modifications and adjustments in immunosuppressive therapies. The above results strengthen the idea that each of these mechanisms must be addressed separately, as they each function independently to influence xenograft rejection. Modification of antigenicity of the donor, regulation of complement activation and thrombogenicity all through genetic engineering of donor pigs and appropriate choice of immunomodulatory regimens may each contribute towards the advancement of xenotransplantation as a treatment modality of end organ failure and its future clinical application.
Acknowledgements:
We acknowledge the staff of the Laboratory of Animal Medicine and Surgery (LAMS) and the Division of Veterinary Resources (DVR) for their support in surgical procedures and care of animals. The Flow cytometry core of NHLBI for their help in FACS analyses and Patricia Jackson for her administrative help. We also acknowledge Elizabeth Bowery for proofreading the manuscript.
Abbreviations
- ACT
Activated Clotting Time
- ACUC
Animal Care and Use Committee
- CVF
Cobra Venom Factor
- EKG
Electrocardiogram
- GTKO
Alpha 1–3 Galactosyltransferase Gene Knockout
- hTBM
Human Thrombomodulin
- LVP
Left Ventricular Pressure
- MMF
Mycophenolate Mofitel
- NHLBI
National Heart, Lung and Blood Institute
Footnotes
Disclosure:
The following authors of this manuscript have conflicts of interest to disclose as described by the Xenotransplantation journal.
David Ayares is the CEO & president of Revivicor, Inc.
Muhammad Mohiuddin, Avneesh K. Singh, Billeta Lewis and Laura DiChiacchio are part of Cardiac Xenotransplantation Program at the University of Maryland. This program is funded by United Therapeutics Inc.
References
- 1.MOHIUDDIN MM. Clinical xenotransplantation of organs: why aren’t we there yet? PLoS Med 2007: 4: e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.THOMPSON P, CARDONA K, RUSSELL M, BADELL IR, SHAFFER V, KORBUTT G, et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am J Transplant 2011: 11: 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MOHIUDDIN MM, CORCORAN PC, SINGH AK, AZIMZADEH A, HOYT RF JR., THOMAS ML, et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant 2012: 12: 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MOHIUDDIN MM, SINGH AK, CORCORAN PC, THOMAS ML, 3RD, CLARK T, LEWIS BG, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun 2016: 7: 11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.HIGGINBOTHAM L, MATHEWS D, BREEDEN CA, SONG M, FARRIS AB, 3RD, LARSEN CP, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation 2015: 22: 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.KIM J, KIM DH, CHOI HJ, LEE HJ, KANG HJ, PARK CG, et al. Anti-CD40 antibody-mediated costimulation blockade promotes long-term survival of deep-lamellar porcine corneal grafts in non-human primates. Xenotransplantation 2017: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.IWASE H, EKSER B, SATYANANDA V, BHAMA J, HARA H, EZZELARAB M, et al. Pig-to-baboon heterotopic heart transplantation—exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation 2015: 22: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IWASE H, LIU H, WIJKSTROM M, ZHOU H, SINGH J, HARA H, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation 2015: 22: 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IWASE H, LIU H, SCHMELZER E, EZZELARAB M, WIJKSTROM M, HARA H, et al. Transplantation of hepatocytes from genetically engineered pigs into baboons. Xenotransplantation 2017: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SHAH JA, PATEL MS, ELIAS N, NAVARRO-ALVAREZ N, ROSALES I, WILKINSON RA, et al. Prolonged Survival Following Pig-to-Primate Liver Xenotransplantation Utilizing Exogenous Coagulation Factors and Costimulation Blockade. Am J Transplant 2017: 17: 2178–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.PETERSEN B, RAMACKERS W, TIEDE A, LUCAS-HAHN A, HERRMANN D, BARG-KUES B, et al. Pigs transgenic for human thrombomodulin have elevated production of activated protein C. Xenotransplantation 2009: 16: 486–495. [DOI] [PubMed] [Google Scholar]
- 12.MOHIUDDIN MM, SINGH AK, CORCORAN PC, HOYT RF, THOMAS ML, 3RD, AYARES D, et al. Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation. J Thorac Cardiovasc Surg 2014: 148: 1106–1113; discussion 1113–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.PHELPS CJ, KOIKE C, VAUGHT TD, BOONE J, WELLS KD, CHEN SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 2003: 299: 411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CHAN JL, SINGH AK, CORCORAN PC, THOMAS ML, LEWIS BG, AYARES DL, et al. Encouraging experience using multi-transgenic xenografts in a pig-to-baboon cardiac xenotransplantation model. Xenotransplantation 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.HORVATH KA, CORCORAN PC, SINGH AK, HOYT RF, CARRIER C, THOMAS ML 3RD, et al. Left ventricular pressure measurement by telemetry is an effective means to evaluate transplanted heart function in experimental heterotopic cardiac xenotransplantation. Transplant Proc 2010: 42: 2152–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.BUHLER L, AWWAD M, BASKER M, GOJO S, WATTS A, TRETER S, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation 2000: 69: 2296–2304. [DOI] [PubMed] [Google Scholar]
- 17.HARRIS DG, GAO Z, SIEVERT EP, BENIPAL P, CHENG X, BURDORF L, et al. Transgenic Human Thrombomodulin Expression Reduces Xenogeneic Thrombosis: a Promising Means of Reducing Pig Lung Xenograft Thrombotic Injury. J Heart Lung Transpl 2014: 33: S108–S108. [Google Scholar]
- 18.LIN WL, CHEN CC, SHI GY, MA CY, CHANG CF, WU HL. Monocytic thrombomodulin promotes cell adhesion through interacting with its ligand, Lewisy. Immunol Cell Biol 2017: 95: 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CHUNG DR, CHITNIS T, PANZO RJ, KASPER DL, SAYEGH MH, TZIANABOS AO. CD4+ T cells regulate surgical and postinfectious adhesion formation. J Exp Med 2002: 195: 1471–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MENZIES D Postoperative adhesions: their treatment and relevance in clinical practice. Ann R Coll Surg Engl 1993: 75: 147–153. [PMC free article] [PubMed] [Google Scholar]
- 21.GALILI U Interaction of the natural anti-Gal antibody with alpha-galactosyl epitopes: a major obstacle for xenotransplantation in humans. Immunol Today 1993: 14: 480–482. [DOI] [PubMed] [Google Scholar]
- 22.DALMASSO AP, VERCELLOTTI GM, FISCHEL RJ, BOLMAN RM, BACH FH, PLATT JL. Mechanism of complement activation in the hyperacute rejection of porcine organs transplanted into primate recipients. Am J Pathol 1992: 140: 1157–1166. [PMC free article] [PubMed] [Google Scholar]
- 23.LOVELAND BE, MILLAND J, KYRIAKOU P, THORLEY BR, CHRISTIANSEN D, LANTERI MB, et al. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in non-immunosuppressed baboons. Xenotransplantation 2004: 11: 171–183. [DOI] [PubMed] [Google Scholar]
- 24.KIM SC, WAKWE W, HIGGINBOTHAM LB, MATHEWS DV, BREEDEN CP, STEPHENSON AC, et al. Fc-Silent Anti-CD154 Domain Antibody Effectively Prevents Nonhuman Primate Renal Allograft Rejection. Am J Transplant 2017: 17: 1182–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DONS EM, MONTOYA C, LONG CE, HARA H, ECHEVERRI GJ, EKSER B, et al. T-cell-based immunosuppressive therapy inhibits the development of natural antibodies in infant baboons. Transplantation 2012: 93: 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.HIGGINBOTHAM L, FORD ML, NEWELL KA, ADAMS AB. Preventing T cell rejection of pig xenografts. Int J Surg 2015: 23: 285–290. [DOI] [PubMed] [Google Scholar]
- 27.ROBSON SC, COOPER DK, D’APICE AJ. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation 2000: 7: 166–176. [DOI] [PubMed] [Google Scholar]
- 28.BYRNE GW, DAVIES WR, OI K, RAO VP, TEOTIA SS, RICCI D et al. Increased immunosuppression, not anticoagulation, extends cardiac xenograft survival. Transplantation 2006: 82: 1787–1791. [DOI] [PubMed] [Google Scholar]
- 29.COWAN PJ, AMINIAN A, BARLOW H, BROWN AA, DWYER K, FILSHIE RJ, et al. Protective effects of recombinant human antithrombin III in pig-to-primate renal xenotransplantation. Am J Transplant 2002: 2: 520–525. [DOI] [PubMed] [Google Scholar]
- 30.COZZI E, SIMIONI P, BOLDRIN M, SEVESO M, CALABRESE F, BALDAN N, et al. Effects of long-term administration of high-dose recombinant human antithrombin in immunosuppressed primate recipients of porcine xenografts. Transplantation 2005: 80: 1501–1510. [DOI] [PubMed] [Google Scholar]
- 31.COZZI E, BHATTI F, SCHMOECKEL M, CHAVEZ G, SMITH KGC, ZAIDI A, et al. Long-term survival of nonhuman primates receiving life-supporting transgenic porcine kidney xenografts. Transplantation 2000: 70: 15–21. [PubMed] [Google Scholar]
- 32.MOHIUDDIN MM, SINGH AK, CORCORAN PC, HOYT RF, THOMAS ML, 3RD, LEWIS BG, et al. Role of anti-CD40 antibody-mediated costimulation blockade on non-Gal antibody production and heterotopic cardiac xenograft survival in a GTKO.hCD46Tg pig-to-baboon model. Xenotransplantation 2014: 21: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.IWASE H, HARA H, EZZELARAB M, LI T, ZHANG Z, GAO B, et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation 2017: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.KNOSALLA C, YAZAWA K, BEHDAD A, BODYAK N, SHANG H, BUHLER L, et al. Renal and cardiac endothelial heterogeneity impact acute vascular rejection in pig-to-baboon xenotransplantation. Am J Transplant 2009: 9: 1006–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.HEALY AM, RAYBURN HB, ROSENBERG RD, WEILER H. Absence of the blood-clotting regulator thrombomodulin causes embryonic lethality in mice before development of a functional cardiovascular system. Proc Natl Acad Sci U S A 1995: 92: 850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.GOMI K, ZUSHI M, HONDA G, KAWAHARA S, MATSUZAKI O, KANABAYASHI T, et al. Antithrombotic effect of recombinant human thrombomodulin on thrombin-induced thromboembolism in mice. Blood 1990: 75: 1396–1399. [PubMed] [Google Scholar]
- 37.KUMADA T, DITTMAN WA, MAJERUS PW. A role for thrombomodulin in the pathogenesis of thrombin-induced thromboembolism in mice. Blood 1988: 71: 728–733. [PubMed] [Google Scholar]
- 38.COOPER DK, EZZELARAB MB, HARA H, IWASE H, LEE W, WIJKSTROM M, et al. The pathobiology of pig-to-primate xenotransplantation: a historical review. Xenotransplantation 2016: 23: 83–105 [DOI] [PubMed] [Google Scholar]


