Abstract
The strong inverse relationship between low levels of high density lipoproteins (HDLs) and atherosclerotic cardiovascular disease (CVD) led to the designation of HDL as the “good” cholesterol. The atheroprotection is thought to reflect HDL’s capacity to efflux cholesterol from macrophages, followed by interaction with other lipoproteins in the plasma, processing by the liver and excretion into bile. However, pharmacologic increases in HDL-C levels have not led to expected clinical benefits, giving rise to the concept of dysfunctional HDL, in which increases in serum HDL-C are not beneficial due to lost or altered HDL functions and transition to “bad” HDL. It is now understood that the cholesterol in HDL, measured by HDL-C, is neither a marker nor the mediator of HDL function, including cholesterol efflux capacity. It is also understood that besides cholesterol efflux, HDL functionality encompasses many other potentially beneficial functions, including antioxidant, anti-inflammatory, antithrombotic, anti-apoptotic, vascular protective effects that may be critical protective pathways for various cells, including those in the kidney parenchyma. This review highlights advances in our understanding of the role kidneys play in HDL metabolism, including the effects on levels, composition, and functionality of HDL particles, particularly the main HDL protein, apolipoprotein AI (apoAI). We suggest that normal apoAI/HDL in the glomerular filtrate provides beneficial effects, including lymphangiogenesis, that promote resorption of renal interstitial fluid and biological particles. In contrast, dysfunctional apoAI/HDL activates detrimental pathways in tubular epithelial cells and lymphatics that lead to interstitial accumulation of fluid and harmful particles that promote progressive kidney damage.
Keywords: HDL, ApoA-I, kidney, chronic kidney disease, cardiovascular disease
Introduction
Decades of epidemiological and observational studies have documented an inverse relationship between low levels of HDL-cholesterol (HDL-C) and increased risk of cardiovascular disease (CVD) [1]. The strong association provided the framework for labeling HDL as the “good cholesterol” and generated substantial interest in developing treatment strategies for CVD. Recently, however, randomized controlled trials of HDL-C raising therapies, including nicotinic acid, fenofibrate and inhibitors of cholesterol ester transfer protein (CETP), have not shown reduction in cardiovascular event rates [2–5]. Moreover, in Mendelian randomization studies, several polymorphisms and rare mutations were associated with levels of HDL-C, but did not track with CVD risk [6]. Although disappointing, these results have given way to the concept that cholesterol in HDL, which is measured by HDL-C, is neither a marker nor mediator of HDL function. Instead, particle number, composition, and HDL functionality are considered better predictors of CVD risk [7]. These HDL characteristics reflect hepatic metabolism and remodeling occurring in the circulation. The kidneys have not been regarded as important regulators of lipid and lipoprotein metabolism because the glomerular filtration barrier prevents passage of all but the smallest size molecules. However, HDL is not synthesized as an intact particle, but is assembled and remodeled from its constituent lipids, apolipoproteins and enzymes. Metabolism of HDL also does not proceed by removal of the holoparticle, and instead represents metabolism of its individual components. There is increasing evidence that chronic kidney disease (CKD) affects HDL level, particle number, composition and functionality, and participates in the conversion of normal “good” HDL with beneficial functions into dysfunctional “bad HDL” that not only promotes CVD, but also initiates/propagates CKD. How kidneys regulate HDL and how HDL affects kidney function in children has not been studied although there is little evidence of major differences in the biological consequences of “good” and “bad” HDL between adults versus children.
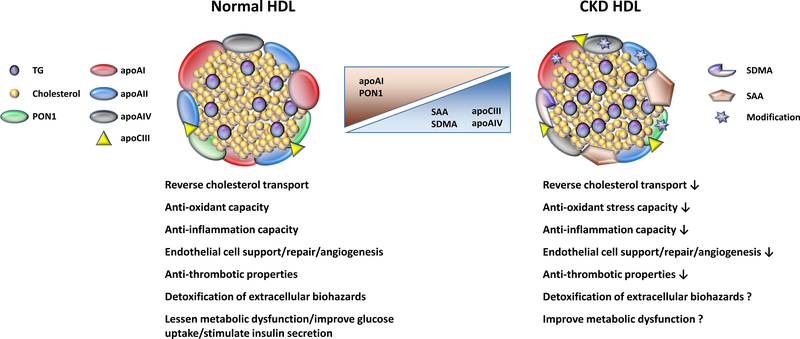
The most recognized function of HDL is atheroprotection, although recent discoveries have revealed a wide range of biological actions that reflect the complexity of its structure and composition. HDL is a heterogeneous and multimolecular complex of particles that includes proteins, lipids, phospholipids and small amphiphilic and lipophilic molecules, which interact with many different cells including hepatocytes, vascular endothelial cells, adipocytes, pancreatic cells, skeletal muscles and macrophages. HDL is well documented to have a number of beneficial biological actions that can influence many tissues and organs (Figure 1). HDL is critical in: 1) reverse cholesterol transport (RCT), a multi-step, multi-organ process to remove excess cholesterol from peripheral cells, transport it in plasma for delivery to the liver where the cholesterol is processed for biliary excretion into the intestines. Modulation of the first step in RCT, cellular cholesterol efflux, has been shown to predict subclinical atherosclerosis, likelihood of coronary artery disease, and acute cardiovascular events in the general population [8–10]. 2) HDL lessens oxidant stress which is linked to the level and activity of constituent enzymes, including paraoxonase, glutathione peroxidase, lipoprotein-associated phospholipase A2 (Lp-PLA2) as well as other HDL components that bind and dispose of endotoxins and oxidized phospholipids, including apoAI and LCAT. 3) HDL lessens inflammation by reducing formation of oxidized lipids and lipoproteins, and by removing oxidized phospholipids and fatty acids from lipoproteins through hepatic uptake. HDL blunts adhesion between circulating monocytes and endothelial cells by suppressing activation of both cells. 4) HDL protects the endothelium through inhibition of monocyte chemotaxis, adhesion molecule expression, enhanced nitric oxide and prostacyclin production. HDL supports repair, migration and proliferation of endothelial cells that promotes angiogenesis while decreasing apoptosis. HDL also increases the number of circulating endothelial progenitor cells. 5) HDL has antithrombotic activities by reducing platelet aggregation, augmenting urokinase-dependent fibrinolysis as well as suppressing adhesion molecules on endothelial cells. 6) HDL detoxifies extracellular biohazards by inactivating hazardous molecules directly on its surface including bacterial lipopolysaccharides and xenobiotics. 7) HDL improves metabolic dysfunctions by stimulating synthesis and secretion of insulin by pancreatic β cells, as well as stimulation of glucose uptake and turnover in skeletal muscle, liver and adipose tissue.
Figure 1. HDL changes in chronic kidney disease (CKD).
HDL in CKD becomes enriched in SAA, SDMA, apoCIII and apoAIV, while decreasing the content of apoAI and PON1. CKD degrades several potentially beneficial functionalities of HDL.
HDL, high density lipoprotein; TG, triglyceride; PON1, paraoxonase 1; apoAI, apolipoprotein AI; apoAII, apolipoprotein apoAII; apoAIV, apolipoprotein IV; apoCIII, apolipoprotein apoCIII; SAA, serum amyloid A; SDMA, symmetric dimethylarginine.
This review will highlight the concept of a significant kidney role in the homeostasis of the HDL particle, specifically focusing on apolipoprotein AI (apoAI), the main protein in HDL, and its effects on podocytes, tubules and renal lymphatics. The discussion is divided into three sections: HDL Changes in CKD, Kidney Regulation of HDL Metabolism, and Kidneys as Targets of CKD-modified HDL.
HDL changes in CKD
CKD affects HDL levels
CKD causes abnormalities in lipids and lipoproteins, the extent and character of which depend on the degree of kidney impairment, underlying etiology, and whether proteinuria, especially nephrotic syndrome, is present. These dyslipidemias have recently been summarized in several excellent reviews [11, 12]. Classically, CKDrelated dyslipidemia is characterized by hypertriglyceridemia and depressed levels of circulating HDL-C. HDL-C reflects reduced synthesis, increased degradation, and abnormal clearance of HDL. Notably, unlike the general population, the link between low HDL-C and acute CVD events and mortality in CKD is unsettled [13–20]. This discrepancy is clearly illustrated by a large study of >33,000 patients on maintenance hemodialysis: patients with HDL-C <30 mg/dl, as well as those with HDL-C >60 mg/dl had a significantly increased risk of total and cardiovascular mortality [14]. The implication of these findings is that conditions that affect HDL composition/functionality, or therapeutic interventions to increase HDL-C levels, may be detrimental.
CKD affects HDL functionality
Cholesterol efflux capacity.
CKD degrades many beneficial functions of HDL (Figure 1). HDL cellular cholesterol efflux capacity (CEC), a central process in reverse cholesterol transport is impaired across the spectrum of CKD [21–23]. Monocytes of CKD patients have altered expression of lipoprotein receptors and transporters, including increased CD36, CD68 and reduced ABCA1, together with preferential accumulation of lipids compared to monocytes of control subjects [24, 25]. Only a few studies have described HDL functionality in children with CKD. Shroff et al. first showed that children with CKD have depressed CEC that becomes worse with progressive reduction in renal function [26]. The study also revealed that HDL-mediated endothelial dysfunction correlated with degree of renal impairment and with levels of circulating markers of vascular dysfunction (urate, angiopoietin-2, IL-6), endothelial dysfunction (nitric oxide production, superoxide production, vascular cell adhesion molecule-1 expression), and with clinical measures of arterial disease (aortic pulse wave velocity, carotid intima-media thickness).
Although many studies report reduced CEC in CKD, the precise relationship between CEC and kidney function is complicated. Thus, renal transplantation and recovery of renal function (eGFR ~50 ml/min) may improve endothelial and vascular function, however, CEC remains depressed [27]. Even after stratification of recipients into those with good versus poor graft function, CEC remained profoundly depressed in both transplant groups and was not different compared to patients on hemodialysis. These data suggest that CEC may represent a more severe or advanced disruption of normal HDL functionality, or that reduction in CEC requires long-standing disease and/or comorbidities, and may be especially recalcitrant to therapeutic interventions. It is therefore notable that children with CKD or end-stage renal disease (ESRD) requiring dialysis who did not have long-standing comorbidities or risk factors characteristic of adults with CKD (diabetes, obesity, pre-existing CVD), have HDL that is consistently shown to have profound impairment in anti-inflammatory, anti-oxidative and endothelial protection functions, but not consistent impairment of CEC [26, 28–30]. In a separate cohort of children with CKD, those with depressed CEC were older and already had demonstrable vasculopathy (abnormal aortic pulse wave velocity and increased carotid intima-media thickness), compared to those with normal CEC [26, 28]. This idea that depressed CEC reflects established atherosclerotic vasculopathy, is supported by observations that while reduced CEC is associated with prevalent coronary artery disease in adults with CKD, increased, rather than decreased, CEC was associated with risk of future myocardial infarction, stroke or death [31].
Anti-inflammation, antioxidation and endothelial protection.
HDL from CKD patients has defective anti-inflammatory function, antioxidant capacity, and is less effective in supporting the endothelium, including endothelial cell survival and repair [26, 28, 29]. HDL of CKD patients is also less effective in restoring endothelial cell proliferation following TNF-α stimulation, results that complement observations that uremic serum impairs endothelial cell proliferation [32]. Anti-inflammatory and antiapoptotic actions of HDL are more defective in patients on hemodialysis than those on peritoneal dialysis [33]. Interestingly, although direct activation of ABCA1 by liver X receptor agonist improved CEC to HDL from subjects with moderate-severe CKD, the intervention actually increased the pro-inflammatory effects of the HDL through activation of TLRs and ERK1/2 pathways [34]. Another study showed that while both angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor antagonists (ARB) stabilized HDL cholesterol acceptor function and sustained cellular anti-oxidative effects, they did not improve anti-inflammatory effects [34]. Indeed, ACEI-treatment instead amplified the HDL inflammatory response. It is therefore of particular interest that IL-1 blockade improved HDL functionality in patients with pre-dialysis CKD as well as individuals on maintenance hemodialysis [35]. Specifically, the therapeutic intervention improved HDL anti-inflammatory, anti-oxidative functions and reduced cellular expression of the Nod-like receptor protein (NLRP3) component of the inflammasome, a cytosolic multiprotein complex controlled by interleukin 1β. The therapy did not affect CEC. Several studies in children with CKD have reported abnormal anti-oxidative, anti-inflammatory and endothelial protective effects of HDL. HDL from children with CKD and ESRD caused a significantly amplified inflammatory cytokine response and greater chemotactic response in cultured macrophages compared to HDL from children with normal kidney function [28]. HDL from CKD and ESRD children was less effective than HDL from normal children in the ability to suppress endothelial activation or endothelial adhesion of monocytes. Another study showed that compared to HDL from healthy children, HDL from children with CKD strongly inhibited endothelial cell production of nitric oxide, promoted superoxide production, and increased vascular cell adhesion molecule-1 expression, effects that correlated with CKD grade, with the most profound changes induced by HDL from patients on dialysis [26]. A separate study of HDL from adults and children with stage 24 CKD, indicated that the underlying mechanisms for endothelial cell stimulation of reactive oxygen species and inhibition of NO bioavailability involves symmetric dimethylarginine (SDMA), see below [26, 29]. It is interesting that, unlike the effects on CEC [26], renal transplantation led to a partial recovery in these HDL dysfunctions. Together these studies underscore that CKD-induced abnormalities in HDL functionalities are not synchronized and that a particular therapeutic modality may have variable effects on specific HDL actions.
CKD-related HDL dysfunction in CVD and CKD
Unlike findings in the general population and high-risk individuals, where cellular cholesterol efflux is a strong predictor of acute cardiovascular events [8–10], efflux has not been found to predict cardiovascular outcome in the CKD population [36–38]. Interestingly, a study of 495 kidney transplant recipients with a median follow-up of 7 years found that although efflux capacity did not predict cardiovascular and total mortality, high efflux capacity at baseline was linked to a lower risk for graft failure, independent of apoA-I, HDL-C and creatinine clearance [7]. Both the negative association between CEC and cardiovascular consequences as well as the positive association between CEC and graft survival are surprising, and raise several issues regarding the utility of CEC in CVD and CKD. First, CVD, especially in advanced stages of renal failure, may be different from the atherosclerotic coronary artery disease in the general population. There is ample evidence that cardiac fibrosis, hypertrophy, heart failure and arrhythmia are important causes of CVD in the CKD setting. Therefore, CEC may not be a suitable parameter to predict acute cardiovascular events in this population, and anti-inflammatory, anti-oxidant and antithrombotic activities may be useful alternative or additive parameters. In particular, systemic inflammation and oxidant stress prevail at all stages of CKD and are key mechanisms underlying many adverse consequences of CKD, including CVD. A prospective study observed that high levels of oxidized HDL are associated with increased CIMT, while the combination of high ox-HDL and high interleukin-6 predicts not only a greater increase in carotid intima-media thickness, but also an increased risk for CVD events and CVD-related mortality in maintenance hemodialysis patients [39]. Secondly, considering that HDL and its major apoproteins are metabolized in the kidney, HDL may directly modulate different types of renal resident cells, which express its transporters and receptors and thus directly contribute to parenchymal damage through mechanisms distinct from those in extra-renal cells and tissues (see below).
CKD effects on HDL composition
Biochemical and mass spectrometry analyses have documented a number of changes in the protein and lipid moieties of HDL particles isolated from CKD patients, including reduced levels of apoAI, apoAII, apoM, paraoxonase and higher levels of serum amyloid A (SAA), apoCII, apoCIII, apoAIV, albumin, lipoprotein-associated phospholipase A2 (Lp-PLA2), surfactant protein B (SP-B), and α−1-microglobulin/bikunin precursor (Figure 1) [21, 40–42]. A recent study reporting results of targeted mass spectrometry of HDL particles in over 500 patients with CKD stages I-V, revealed that eGFR >60 ml/min/1.73m2 showed differences to those individuals with eGFR <15 in terms of four HDL proteins: higher retinol binding protein 4, higher apoC-III, lower apolipoprotein L1, and lower vitronectin [43]. One of the most consistent alterations observed in the HDL proteome of CKD patients is increased SAA. Among the 49 proteins altered in HDL of ESRD patients, only SAA levels inversely correlated with its anti-inflammatory potency [40]. Similarly, dramatic enrichment with SAA in HDL of dialysis patients was associated with lower anti-inflammatory capacity, and linked this effect to activation of formylpeptide receptor 2 [42]. In normal subjects, SAA can displace both apoAI and PON1, thus explaining reduced anti-oxidative and anti-inflammatory activity of SAA-enriched HDL [40]. These potentially harmful effects are especially prominent when SAA constitutes >50% of total HDL protein. Interestingly, a post hoc analysis of the 4D study found that SAA enrichment of HDL was associated with risk of cardiovascular events, while HDL enrichment in SP-B associated with all-cause mortality [27]. In contrast, in pre-dialysis patients, SAA, Lp-PLA2 and paraoxonase activity of HDL did not predict cardiovascular outcome [38].
As noted above, there is increasing evidence that HDL of CKD patients is also enriched in SDMA, the structural isomer of asymmetric dimethylarginine (ADMA), both of which are endogenous products of protein methylation linked to CVD risk and accumulate as kidney function decreases. HDL of patients with CKD show increased content of SDMA, and the SDMA-containing HDL interacts with endothelial TLR2 to enhance NADP– dependent production of ROS while inhibiting endothelial NO bioavailability [29]. In mice, SDMA in HDL caused hypertension and impaired re-endothelialization after carotid injury. A separate study in children with CKD reported that levels of HDL-associated SDMA inhibited NO synthesis, promoted superoxide production, increased expression of vascular cell adhesion molecule 1 in human aortic endothelial cells, and suppressed macrophage cholesterol efflux [26]. To date, most studies of CKD-related changes in HDL composition have focused on the HDL proteome. However, CKD also affects HDL lipidome, including increased triglycerides and lysophospholipids, and decreased phospholipids and cholesterol [21]. The significance of these lipidome changes remains to be determined.
In addition to compositional alteration of HDL, CKD causes post-translational modifications of HDL proteins and lipids by reactive oxygen/nitrogen species or the resulting reactive carbonyls, which is likely a very important mechanism regulating HDL functionality [44]. For example, CKD increases reactive oxygen/nitrogen species and increases myeloperoxidase (MPO) which alters ABCA1-mediated cholesterol efflux, activation of LCAT, and endothelial cell survival [45]. MPO-catalyzed lipoprotein carbamylation involves formation of cyanate (a product of urea) and ɛ-carbamyl-lysine homocitrulline (HCit) [46]. Serum HCit and carbamylated albumin predict mortality in dialysis patients [47]. Oxidative stress also increases reactive lipid aldehydes, including malondialdehyde [48], F2-isoprostane (F2IsoP), and isolevuglandin (IsoLG). IsoLGs are particularly interesting because of their extremely rapid reaction with lysine residues of proteins and proclivity to crosslink and alter protein function. IsoLG is primarily associated with apoAI/HDL. F2-IsoP/IsoLG-protein adducts are found to be significantly increased in plasma of CKD subjects [49–51]. Our new data show that proteinuric patients and animal models have increased urinary F2-IsoP and IsoLG, and IsoL-Gmodified apoAI is more avidly accumulated by proximal tubules.
Overall, there is ample support for the concept that CKD alters the composition of HDL, however, no specific footprint exists for HDL in CKD, and there is no specific constituent that incontrovertibly predicts hard clinical end-points such as CVD and mortality. On the other hand, there is increasing support that HDL parameters may be linked to progressive CKD.
Kidney regulation of HDL metabolism
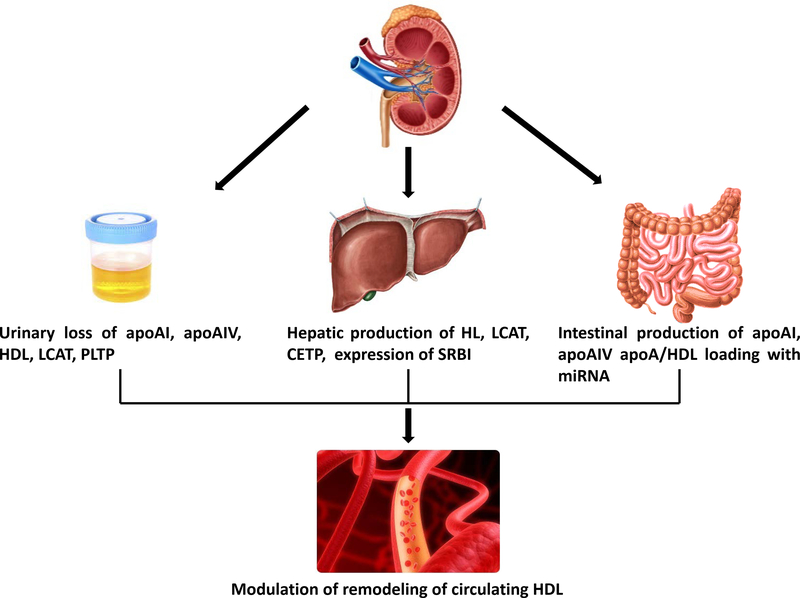
HDL biogenesis begins with hepatic, and to a lesser extent, intestinal synthesis of ApoAI. Once released into the circulation, assembly and maturation of HDL particles proceeds with lipidation of apoAI with cholesterol and phospholipids through interaction with the ABCA1 transporter on various cells, including macrophages, adipocytes, skin fibroblasts and skeletal muscle cells to form nascent preβ-HDL. This is followed by a critical interaction with the enzyme, LCAT, and formation of cholesteryl ester-rich spherical HDL that circulates in the plasma. HDL acquires additional lipids from other circulating lipoproteins, including VLDL and LDL, and from circulating albumin. This remodeling is followed by HDL catabolism by plasma proteins and interaction with hepatic receptors that recycle its components. Scavenger receptor class BI (SRBI) binds cholesterol ester-rich HDL2 particles, which undergo hydrolysis by hepatic lipase that detaches apoAI, returning it back into the circulation. The hepatic catabolism is the primary pathway of clearance of HDL-associated lipids, while endocytic receptors in the liver degrade HDL-associated apolipoproteins. Although kidneys are not featured in the classic overview of HDL homeostasis, kidney participation in HDL homeostasis occurs through: i) filtration and reabsorption of HDL and its components; ii) regulation of extrarenal metabolism of HDL (Figure 2).
Figure 2. Kidney regulation of HDL metabolism.
Kidneys can filter, reabsorb and lose HDL and its components. All these processes are activated when injury involves disruption of the glomerular filtration barrier; renal injury activates hepatic production and metabolism of HDL and its components; renal injury activates intestinal production of apoAI and loads cargo onto HDL modifying the particles.
apoAI, apolipoprotein AI; apoAIV, apolipoprotein IV; HDL, high density lipoprotein; LCAT, lecithin cholesteryl ester acyltransferase; PLTP, phospholipid transfer protein; HL, hepatic lipase; CETP, cholesterol ester transfer protein; SRBI, scavenger receptor BI.
Filtration and reabsorption of HDL and its components
In the normal kidney, the glomerular capillary filtration barrier prevents passage of molecules >60–100kD. All mature spherical HDL subclasses (HDL3, HDL2) exceed this mass, but HDL components such as apoAI (28kD), apoAIV (46kD) and enzymes such as LCAT (67kD), easily cross the filtration barrier. Discoidal pre-β HDL (60–85kD) is similar in size to albumin (66.5kD) and is therefore predicted to cross the normal glomerular filtration barrier. In animals, 30–70% of injected radiolabeled human apoA-I is cleared by the kidneys [52]. In humans, early stages of renal injury and GFR impairment are associated with elevated urinary apoAIV and LCAT, which have been proposed as markers of CKD [53–55]. Similar to apoAI, apoAIV levels are affected by glomerular filtration and tubular handling. As GFR falls, increasing plasma apoAIV likely reflects reduced renal clearance although more avid tubular reabsorption and/or reduced tubular catabolism may be additional contributing factors. It is also possible that decresing kidney function may stimulate compensatory synthesis of apoAIV in the gut. Tubular dysfunction (with or without reduced GFR) further disrupts apoAIV homeostasis because of the additional defect of urinary loss. Indeed, more urinary apoAIV was detected in patients with Dent’s disease, an inherited protein reabsorption defect of the proximal tubular system, supporting the concept that apoAIV is reabsorbed by proximal tubular cells [56]. Importantly, like apoAI, tubular handling of apoAIV does not necessarily match tubular handling of albumin as urinary apoAIV does not parallel albuminuria, at least in the general population [57]. These observations underscore the possibility that filtered apolipoproteins are handled by pathways distinct from those involved in reclaiming filtered albumin. Proteinuria per se can reduce HDL levels. Type 1 diabetics show lower levels of plasma HDL and HDL3 that independently associate with albuminuria, even after adjusting for glycemic control and other risk factors. Proteinuric patients have a shift in HDL particle size distribution toward the larger particles which are less likely to cross the filtration barrier [58].
Tubular epithelial cells express receptors (cubilin/megalin) and transporters (ABCA1 and SRBI) for apolipoproteins and HDL. Cubilin deficiency and proximal tubular reabsorption failure due to Fanconi syndrome increase urinary excretion of apoAI [18]. Cubilin-deficient mice have reduced proximal tubule uptake and increased urinary loss of apoAI as well as albumin, attended by significant decrease in plasma levels of apoAI and HDL3 along with hypoalbuminemia [59]. The larger, more mature HDL2 subclass was not detected in the urine, and plasma level of HDL2 was not reduced compared to mice with intact renal cubilin. These results add to the concept that uptake, endocytosis and lysosomal degradation of apoA-metabolic pathways in the proximal tubule are linked mechanisms that can salvage and return at least some of the filtered apoAI/HDL back into the circulation. Our recent studies add additional insights into tubular handling of apoAI. We generated two tubular injury models: diphtheria toxin (DT) transgenic mouse, which expresses the human DT receptor in proximal tubular epithelial cells and in which DT injection causes acute tubular injury, and the folic acid injury model, where injected folic acid forms crystals in the distal nephron with distal tubular necrosis. Compared to baseline, mice with DT-induced proximal tubular injury had increased urinary KIM-1, a proximal tubular marker of acute kidney injury, that was accompanied by doubling in urinary excretion of apoAI. By contrast, folic acid injury caused only subtle changes in KIM-1 and urinary apoAI. In DT mice, proximal tubule expression of cubilin was reduced and apoAI localized to the apical side.
Several issues concerning renal handling of apoAI/HDL remain unresolved. One issue concerns the pathway (endocytosis, transcytosis and degradation) by which apoAI/HDL is handled by tubular epithelial cells and whether a specific pathway is linked to a particular receptor or transporter. Another issue concerns whether renal salvage or metabolism of normal “good” and modified “bad” apoAI proceeds through the same or different pathways. In preliminary studies, we found that modified apoAI increases uptake by tubular epithelial cells compared to normal apoAI, a result that raises the possibility that potentially harmful lipoproteins/HDL are more avidly taken up and deposited into the renal interstitium, possibly potentiating renal injury.
Regulation of extra-renal metabolism of HDL
Kidneys can also affect HDL metabolism by modulating extrarenal production/metabolism of HDL components, including in liver, plasma and gut. This effect is especially conspicuous in the presence of nephrotic-range proteinuria, which is characterized by greater urinary losses as well as greater renal catabolism of HDL particles, apolipoproteins and enzymes. Even in the absence of reduced GFR, proteinuria decreases HDL levels and alters its metabolism - a subject which has been expertly reviewed elsewhere [11, 12]. Briefly, nephrotic syndrome: i) reduces levels of hepatic lipase (HL) that decreases extraction and hepatic uptake of HDL triglyceride and phospholipid cargo; ii) reduces the adapter molecule PDZ-containing kidney protein 1 (PDZK1), which destabilizes hepatocyte plasma membrane HDL docking receptor, SRBI, which in turn reduces hepatic uptake of HDL-cholesterol; iii) increases serum cholesterol ester transfer protein (CETP), which depletes cholesterol esters while increasing the triglyceride cargo of HDL, limiting the normal HDL maturation; iv) impairs HDL maturation because of increased renal tissue acyl-CoA cholesterol acyltransferase-1 (ACAT-1). Increased cellular ACAT-1 limits cholesterol efflux by catalyzing esterification of intracellular free cholesterol involved in the transfer across the cell membrane. ACAT-1 facilitates intracellular cholesterol retention by competing with intracellular cholesterol ester hydrolase. Thus, cellular ACAT-1, which is increased in CKD, is a major factor in limiting cholesterol efflux in macrophages, vascular and renal cells [12]; v) HDL maturation is further compromised by reduced level and activity of lecithin cholesteryl ester acyltransferase (LCAT) that reflects urinary losses. LCAT deficiency has been suggested to decrease plasma HDL in CKD stage 3 and 4 patients, although the study did not specifically address to what extent urinary loss contributed to the reduced plasma LCAT levels [55]. Overall, these proteinuria-driven abnormalities in plasma and liver enzymes, receptors and transporters impair normal HDL maturation and create particles susceptible to urinary loss as well as enhanced catabolism by the liver and kidneys. In addition to the aberrant hepatic/kidney response, in preliminary studies we recently observed that nephrotic syndrome also compromises intestinal synthesis of apoAI. Since some 30% of apoAI is normally produced by the gut, nephrotic syndrome-induced decline in intestinal synthesis may contribute to the abnormal homeostasis of apoAI in this setting.
Kidney injury also alters the composition of HDL that can impact kidney cells. For example, mass spectrometry of HDL particles across CKD revealed that lower apolipoprotein L1 (apoL1) may alter susceptibility to progressive CKD in AfricanAmericans [43]. Another component of HDL that may directly affect the kidneys is apolipoprotein IV, the third most abundant protein on the HDL particle; apoAIV is increased in CKD HDL [13, 53, 60]. Recent observational and GWAS studies support an association between levels of apoAIV and reduced eGFR [61, 62]. Importantly, CKD causes accumulation of toxins that can directly modify the structure and composition of apoAI/HDL. Indeed, modification of lipids and lipoproteins have recently been proposed as a new class of uremic toxins [63]. Thus, similar to the observations with the whole HDL particle, its component apolipoproteins, apoAI and apoAII, CKD may also degrade apoAIV that further impairs cellular binding, cholesterol efflux, anti-inflammatory, antioxidative, anti-apoptotic and cellular proliferative effects of the normal ApoAIV. This interesting possibility requires further investigation.
Kidneys as targets of CKD-modified HDL
Levels of apoAI/HDL and CKD
Although most studies have focused on the relationship between HDL and CVD, increasing evidence supports the concept that level, composition and function of HDL predict acute and progressive CKD. Epidemiologic studies have reported that low HDLC level is a significant risk factor for developing renal dysfunction in apparently healthy individuals [64]. Conversely, higher HDL cholesterol concentration has been associated with lower incidence of AKI after cardiac surgery [65]. In people with existing CKD (stage 2–3), those with low HDL-C had worsening of kidney function and earlier entry into dialysis, independent of other risk factors, such as diabetes and hypertension, and portend poor prognosis [18, 66–68]. In diabetics, low HDL-C and high triglyceride levels independently predicted onset of diabetic nephropathy [69], while in type 2 diabetes, LDL/HDL ratio and low levels of apoAI/HDL particles were linked to the occurrence of diabetic nephropathy [70]. A recent retrospective longitudinal analysis of >10,000 subjects, found HDL-C/apoAI ratios as well as triglyceride and HDL-C levels independently predicted increased risk of CKD, suggesting that particle size of HDL and LDL contributes to the development of CKD [17, 18]. An observational study of the association between HDL-C and several CKD endpoints in almost 2 million male veterans followed for a median of 9 years, found HDL-C <30 mg/dl had 10–20% higher risk for CKD and/or progressive CKD compared to those with HDL-C >40 mg/dl [18]. In contrast, the recent Randomized Evaluation of the Effects of Anacetrapib through Lipid Modification (REVEAL) trial, specifically aiming to increase HDL-C, reported a higher frequency of low eGFR <60 ml/min/1.73m2 in the treatment group with higher levels of HDL-C [68]. A recent genetic study did not confirm the causal effect of HDL-C on kidney function, despite an association of some HDL-associated single-nucleotide polymorphisms with eGFR [71]. Finally, the Study of Heart and Renal Protection (SHARP) trial not only found no benefit of lipid lowering treatment on progression of kidney disease, but the baseline HDL-C levels did not affect the negative observation [72].
In aggregate, and similar to the discussion of the relationship between HDL-C and CVD, it is clear that renal disease reduces levels of apoAI/HDL. However, since levels of apoAI/HDL-C may not reflect the critical properties of the lipoproteins (particle number, composition, function), it is currently unclear if low apoAI/HDL constitutes an actual risk for CKD progression. On the other hand, since kidneys filter, reabsorb, catabolize and excrete components of HDL, it is possible that lipoprotein interaction with renal cells may provide beneficial effects or convey an injurious stimulus (abnormal structure, composition, cargo) to the renal parenchyma.
ApoAI/HDL interaction with kidney cells
ApoAI/HDL can interact with several renal cells, including glomerular endothelial cells, podocytes, mesangial, and proximal tubule epithelial cells. All these cells express lipoprotein transporters/receptors, but their response to injury is variable (Figure 3A). In vivo, mice with diabetic nephropathy showed intra-renal accumulation of lipids associated with a significant reduction in expression of transporters/receptors. In vitro, hyperglycemia reduced the expression of all cholesterol transporters in mesangial and proximal tubule epithelial cells, together with reduced ability to mediate cholesterol efflux [73]. A different study showed podocytes exposed to sera from albuminuric diabetic patients have greater cholesterol loading than cells exposed to sera of diabetics without albuminuria, despite similar lipid profiles and duration of diabetes [74]. The increased lipid loading was not due to greater cholesterol uptake or synthesis, but impairment in cholesterol efflux linked to downregulation in ABCA1. Induction of cholesterol efflux with cyclodextrin in cultured podocytes and in diabetic mice preserved podocyte functions and lessened albuminuria. A different kidney response was seen in CKD induced by 5/6 nephrectomy, a non-diabetic CKD model. Lipid accumulation in the remnant kidney model upregulated both ABCA1 and SRBI linked to upregulation of tubular reabsorption of filtered protein-bound lipids in the remnant kidney [73].
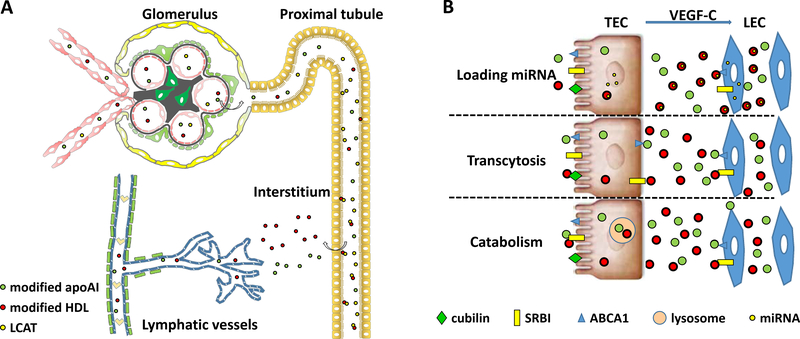
Figure 3. Kidneys as targets of HDL.
A. HDL and its components are filtered by glomeruli and can be lost in urine or reabsorbed by tubular epithelial cells, reclaimed by renal lymphatic vessels, transported in lymph for return to the circulation. B. TECs catabolism, transcytosis and modification of apoAI/HDL through their receptors and transporters. LECs can be directly influenced by apoAI or indirectly by TECs-secreted VEGF-C or modified HDL.
HDL, high density lipoprotein; LCAT, lecithin cholesteryl ester acyltransferase; apoAI, apolipoprotein AI; TEC, tubular epithelial cells; VEGF-C, vascular endothelial growth factor-C; LEC, lymphatic endothelial cells; SRBI, scavenger receptor BI.
In our preliminary studies in proteinuric mice, we found increased expression of ABCA1, SRBI and cubilin in tubular epithelial cells accompanied by greater kidney uptake of filtered apoAI. Cultured tubular epithelial cells (TEC) exposed to apoAI modified by IsoLG further increased expression of ABCA1 and SRBI, and caused more avid tubular cell uptake of the modified apoAI compared to normal unmodified apoAI. These results are notable because albuminuric humans and mice have increased urinary levels of both apoAI and the reactive aldehyde which readily modifies apoAI, namely, IsoLG. Our in vitro data indicates that both normal and modified apoAI are taken up by TEC, although intriguingly, the modified apoAI is more avidly taken up and increases expression of lysosomal marker LAMP-1. Whether modified-apoAI activates lysosomal pathways for degradation of normal or modified apoAI is currently unknown. These findings fit well with data that oxidized HDL enhances cellular oxidative stress and transforms tubule epithelial cells to a more dysfunctional pro-inflammatory phenotype with reduced migration but enhanced apoptosis [75]. SRBI and SRBII were shown to mediate interaction with SAA that increased pro-inflammatory signaling pathways in the kidney. Transgenic mice with pLiv-11-directed liver/kidney overexpression of hSR-BI or hSR-BII were used to show enhanced inflammation and tissue injury, suggesting an important role of the SRBI receptor family in SAA-induced inflammation and immune response [76]. Together, these results suggest the possibility that modified apoAI/HDL in the glomerular filtrate can regulate the phenotype and functionality of kidney cells.
In addition to regulating glomerular podocytes and tubular epithelial cells, our most recent studies reveal that apoAI/HDL can affect lymphatic endothelial cells (LECs) and lymphatic vessels, which are potent regulators of many biologic processes in health and disease. In many organs, immune cells, especially macrophages, stimulate lymphangiogenesis by producing VEGF-C and –D that activate VEGF receptor-3 via nuclear factor kappa-B and prospero-related homeobox 1 (Prox-1). In the kidney, tubular epithelial cells secrete VEGF-C and load microRNA onto HDL, which can affect LECs. The crosstalk between TECs and LECs could be affected by “bad” apoAI/HDL prevailing in CKD (unpublished data, Figure 3B). ApoAI has a direct effect on LECs. In preliminary in vivo studies, albuminuric kidney injury increases urinary apoAI and leads to a dramatically more dense and complex renal lymphatic network. Our data also reveal that LEC exposure to apoAI increases cell viability, lowers migration, and lessens production of reactive oxygen species. We show that the reabsorbed apoAI co-localizes with renal lymphatic vessels expressing SRBI. These results make the novel observations that apoAI can modulate lymphangiogenesis either as a direct effect on LECs, or indirectly by affecting TEC/LEC crosstalk, and that in the kidney the source for apoAI is the glomerular ultrafiltrate. Thus, urinary apoAI taken up into the renal interstitial space may be in a unique position to regulate the renal lymphatic network, which in turn can have profound physiologic and pathophysiologic consequences (Figure 3).
Normal apoAI/HDL protects against kidney injury
As with CVD, supplementation of normal apoAI or HDL may ameliorate kidney injuries. Normal HDL can significantly reduce renal ischemia/reperfusion injury and severity of ischemic acute renal failure [77]. The mechanism for these protective effects involves reduction of the expression of adhesion molecules, resulting in reduction of polymorphonuclear leukocyte infiltration and oxidative stress [77, 78]. In a rat model of cecal ligation and puncture (CLP), apoAI mimetic, L-4F, improved glomerular filtration rate, decreased tubular injury and protected kidney function in an HDL-dependent manner [79]. Proteinuria, glomerular and tubulointerstitial injury in apoE−/− mice were ameliorated by the administration of apoA-1 mimetic peptide [80]. We also found that apoAI mimetic peptide protects podocyte differentiation, density, and improves proteinuric renal injury and proteinuria-driven atherosclerosis in the podocyte injury proteinuric model. These findings support the idea that normal apoAI and apoAI mimetics can be renoprotective. Recently, we also targeted modification of apoAI/HDL with pentylpyridoxamine (PPM), a reactive aldehyde scavenger which interacts with IsoLG nearly 2000-times faster than IsoLG reacts with lysine on apoAI. In vitro, IsoLGapoAI increased lymphatic endothelial cell viability and migration vs unmodified apoAI. This response was significantly abrogated by exposure to PPM. In vivo, proteinuric mice treated with PPM showed significantly reduced urinary excretion of IsoLG, reduction in albuminuria and decrease in urinary KIM-1, together with significant reduction in lymphangiogenesis.
Conclusion
While liver, skeletal muscle, adipose tissue and macrophages are considered to be the primary sites of lipoprotein metabolism, the kidney is increasingly recognized as having a significant role in the homeostasis of individual components of HDL, which in turn, affect level, composition and functionality of the HDL particles. The primary target of lipoproteins has also expanded beyond the cardiovascular system and now includes many different organs, including the kidneys. Kidneys as targets for lipoproteins may be especially prominent in renal injuries that are accompanied by proteinuria (taken as an indicator of disruption in the glomerular filtration barrier) that may permit escape of more and greater variety of lipoproteins into the urinary space. As in other tissues, interactions between the normal apoAI/HDL from the filtrate and kidney cells provides beneficial or protective effects. In contrast, appearance of modified apoAI/HDL in the filtrate (predicted by the CKD setting) enhances the tubular uptake of potentially harmful lipoproteins which activates tubular and interstitial cells, particularly lymphatic endothelial cells that may initiate and perpetuate damaging response in the kidneys.
Footnotes
Disclosures: None.
References
- 1.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J (2009) Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302:1993–2000. doi: 10.1001/jama.2009.1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B (2007) Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 357:2109–2122. doi: 10.1056/NEJMoa0706628 [DOI] [PubMed] [Google Scholar]
- 3.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W (2011) Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 365:2255–2267. doi: 10.1056/NEJMoa1107579 [DOI] [PubMed] [Google Scholar]
- 4.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS (2012) Effects of Dalcetrapib in Patients with a Recent Acute Coronary Syndrome. N Engl J Med. doi: 10.1056/NEJMoa1206797 [DOI] [PubMed] [Google Scholar]
- 5.Keene D, Price C, Shun-Shin MJ, Francis DP (2014) Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ 349:g4379. doi: 10.1136/bmj.g4379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansen H, Samani NJ, Schunkert H (2014) Mendelian randomization studies in coronary artery disease. Eur Heart J 35:1917–1924. doi: 10.1093/eurheartj/ehu208 [DOI] [PubMed] [Google Scholar]
- 7.Annema W, von Eckardstein A (2013) High-density lipoproteins. Multifunctional but vulnerable protections from atherosclerosis. Circ J 77:2432–2448 [DOI] [PubMed] [Google Scholar]
- 8.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ (2011) Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 364:127–135. doi: 10.1056/NEJMoa1001689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW (2014) HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 371:2383–2393. doi: 10.1056/NEJMoa1409065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, Kastelein JJ, Boekholdt SM, Khaw KT, Wareham N, Rader DJ (2015) Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 3:507–513. doi: 10.1016/S2213-8587(15)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal S, Zaritsky JJ, Fornoni A, Smoyer WE (2017) Dyslipidaemia in nephrotic syndrome: mechanisms and treatment. Nat Rev Nephrol 14:70. doi: 10.1038/nrneph.2017.175 [DOI] [PubMed] [Google Scholar]
- 12.Vaziri ND (2016) HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat Rev Nephrol 12:37–47. doi: 10.1038/nrneph.2015.180 [DOI] [PubMed] [Google Scholar]
- 13.Kronenberg F (2018) HDL in CKD-The Devil Is in the Detail. J Am Soc Nephrol 29:1356–1371. doi: 10.1681/asn.2017070798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moradi H, Streja E, Kashyap ML, Vaziri ND, Fonarow GC, Kalantar-Zadeh K (2014) Elevated high-density lipoprotein cholesterol and cardiovascular mortality in maintenance hemodialysis patients. Nephrol Dial Transplant 29:1554–1562. doi: 10.1093/ndt/gfu022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamprea-Montealegre JA, Sharrett AR, Matsushita K, Selvin E, Szklo M, Astor BC (2014) Chronic kidney disease, lipids and apolipoproteins, and coronary heart disease: the ARIC study. Atherosclerosis 234:42–46. doi: 10.1016/j.atherosclerosis.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 16.Rahman M, Yang W, Akkina S, Alper A, Anderson AH, Appel LJ, He J, Raj DS, Schelling J, Strauss L, Teal V, Rader DJ; CRIC Study Investigators (2014) Relation of serum lipids and lipoproteins with progression of CKD: The CRIC study. Clin J Am Soc Nephrol 9:1190–1198. doi: 10.2215/CJN.09320913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae JC, Han JM, Kwon S, Jee JH, Yu TY, Lee MK, Kim JH (2016) LDL-C/apoB and HDL-C/apoA-1 ratios predict incident chronic kidney disease in a large apparently healthy cohort. Atherosclerosis 251:170–176. doi: 10.1016/j.atherosclerosis.2016.06.029 [DOI] [PubMed] [Google Scholar]
- 18.Bowe B, Xie Y, Xian H, Balasubramanian S, Al-Aly Z (2016) Low levels of high-density lipoprotein cholesterol increase the risk of incident kidney disease and its progression. Kidney Int 89:886–896. doi: 10.1016/j.kint.2015.12.034 [DOI] [PubMed] [Google Scholar]
- 19.Zewinger S, Speer T, Kleber ME, Scharnagl H, Woitas R, Lepper PM, Pfahler K, Seiler S, Heine GH, Marz W, Silbernagel G, Fliser D (2014) HDL Cholesterol Is Not Associated with Lower Mortality in Patients with Kidney Dysfunction. J Am Soc Nephrol 25:1073–1082. doi: 10.1681/ASN.2013050482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silbernagel G, Genser B, Drechsler C, Scharnagl H, Grammer TB, Stojakovic T, Krane V, Ritz E, Wanner C, Marz W (2014) HDL Cholesterol, Apolipoproteins, and Cardiovascular Risk in Hemodialysis Patients. J Am Soc Nephrol 26:484–492. doi: 10.1681/ASN.2013080816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holzer M, Birner-Gruenberger R, Stojakovic T, El-Gamal D, Binder V, Wadsack C, Heinemann A, Marsche G (2011) Uremia alters HDL composition and function. J Am Soc Nephrol 22:1631–1641. doi: 10.1681/ASN.2010111144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto S, Yancey PG, Ikizler TA, Jerome WG, Kaseda R, Cox B, Bian A, Shintani A, Fogo AB, Linton MF, Fazio S, Kon V (2012) Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol 60:2372–2379. doi: 10.1016/j.jacc.2012.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaseda R, Tsuchida Y, Yang HC, Yancey PG, Zhong J, Tao H, Bian A, Fogo AB, Linton MRF, Fazio S, Ikizler TA, Kon V (2018) Chronic kidney disease alters lipid trafficking and inflammatory responses in macrophages: effects of liver X receptor agonism. BMC Nephrol 19:17. doi: 10.1186/s12882-018-0814-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogacev KS, Zawada AM, Emrich I, Seiler S, Bohm M, Fliser D, Woollard KJ, Heine GH (2014) Lower Apo A-I and lower HDL-C levels are associated with higher intermediate CD14++CD16+ monocyte counts that predict cardiovascular events in chronic kidney disease. Arterioscler Thromb Vasc Biol 34:2120–2127. doi: 10.1161/ATVBAHA.114.304172 [DOI] [PubMed] [Google Scholar]
- 25.Ganda A, Yvan-Charvet L, Zhang Y, Lai EJ, Regunathan-Shenk R, Hussain FN, Avasare R, Chakraborty B, Febus AJ, Vernocchi L, Lantigua R, Wang Y, Shi X, Hsieh J, Murphy AJ, Wang N, Bijl N, Gordon KM, de Miguel MH, Singer JR, Hogan J, Cremers S, Magnusson M, Melander O, Gerszten RE, Tall AR (2017) Plasma metabolite profiles, cellular cholesterol efflux, and non-traditional cardiovascular risk in patients with CKD. J Mol Cell Cardiol 112:114–122. doi: 10.1016/j.yjmcc.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shroff R, Speer T, Colin S, Charakida M, Zewinger S, Staels B, Chinetti-Gbaguidi G, Hettrich I, Rohrer L, O’Neill F, McLoughlin E, Long D, Shanahan CM, Landmesser U, Fliser D, Deanfield JE (2014) HDL in children with CKD promotes endothelial dysfunction and an abnormal vascular phenotype. J Am Soc Nephrol 25:2658–2668. doi: 10.1681/asn.2013111212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopecky C, Haidinger M, Birner-Grunberger R, Darnhofer B, Kaltenecker CC, Marsche G, Holzer M, Weichhart T, Antlanger M, Kovarik JJ, Werzowa J, Hecking M, Saemann MD (2015) Restoration of Renal Function Does Not Correct Impairment of Uremic HDL Properties. J Am Soc Nephrol 26:565–575. doi: 10.1681/ASN.2013111219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaseda R, Jabs K, Hunley TE, Jones D, Bian A, Allen RM, Vickers KC, Yancey PG, Linton MF, Fazio S, Kon V (2015) Dysfunctional high-density lipoproteins in children with chronic kidney disease. Metabolism 64:263–273. doi: 10.1016/j.metabol.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Krankel N, Kania G, Zewinger S, Akhmedov A, Shi Y, Martin T, Perisa D, Winnik S, Muller MF, Sester U, Wernicke G, Jung A, Gutteck U, Eriksson U, Geisel J, Deanfield J, von Eckardstein A, Luscher TF, Fliser D, Bahlmann FH, Landmesser U (2013) Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 38:754–768. doi: 10.1016/j.immuni.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 30.Meier SM, Wultsch A, Hollaus M, Ammann M, Pemberger E, Liebscher F, Lambers B, Fruhwurth S, Stojakovic T, Scharnagl H, Schmidt A, Springer A, Becker J, Aufricht C, Handisurya A, Kapeller S, Rohrl C, Stangl H, Strobl W (2015) Effect of chronic kidney disease on macrophage cholesterol efflux. Life Sci 136:1–6. doi: 10.1016/j.lfs.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 31.Li XM, Tang WH, Mosior MK, Huang Y, Wu Y, Matter W, Gao V, Schmitt D, Didonato JA, Fisher EA, Smith JD, Hazen SL (2013) Paradoxical Association of Enhanced Cholesterol Efflux With Increased Incident Cardiovascular Risks. Arterioscler Thromb Vasc Biol 33:1696–1705. doi: 10.1161/ATVBAHA.113.301373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chitalia VC, Murikipudi S, Indolfi L, Rabadi L, Valdez R, Franses JW, Edelman ER (2011) Matrix-embedded endothelial cells are protected from the uremic milieu. Nephrol Dial Transplant 26:3858–3865. doi: 10.1093/ndt/gfr337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holzer M, Schilcher G, Curcic S, Trieb M, Ljubojevic S, Stojakovic T, Scharnagl H, Kopecky CM, Rosenkranz AR, Heinemann A, Marsche G (2015) Dialysis Modalities and HDL Composition and Function. J Am Soc Nephrol 26:2267–2276. doi: 10.1681/asn.2014030309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaseda R, Tsuchida Y, Gamboa JL, Zhong J, Zhang L, Yang H, Dikalova A, Bian A, Davies S, Fogo AF, Linton MF, Brown NJ, Ikizler TA, Kon V (2018) Angiotensin receptor blocker vs ACE inhibitor effects on HDL functionality in patients on maintenance hemodialysis. Nutr Metab Cardiovasc Dis 28:582–591. doi: 10.1016/j.numecd.2018.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hung AM TY, Nowak K, Sarkar S, Chonchol M, Salas N, Dikalova A, Huang J, Linton MF, Ikizler TA, Kon V (2017) IL-1 Inhibition Improves and HDL Functionality in Patients with Stages 3 to 5 Chronic Kidney Disease. ASN Kidney Week 2017;11/02/17 (https://www.asn-online.org/education/kidneyweek/2017/program-abstract.aspx?controlId=2783530) [Google Scholar]
- 36.Kopecky C, Ebtehaj S, Genser B, Drechsler C, Krane V, Antlanger M, Kovarik JJ, Kaltenecker CC, Parvizi M, Wanner C, Weichhart T, Saemann MD, Tietge UJ (2017) HDL Cholesterol Efflux Does Not Predict Cardiovascular Risk in Hemodialysis Patients. J Am Soc Nephrol 28:769–775. doi: 10.1681/asn.2016030262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauer L, Kern S, Rogacev KS, Emrich IE, Zawada A, Fliser D, Heinemann A, Heine GH, Marsche G (2017) HDL Cholesterol Efflux Capacity and Cardiovascular Events in Patients With Chronic Kidney Disease. J Am Coll Cardiol 69:246–247. doi: 10.1016/j.jacc.2016.10.054 [DOI] [PubMed] [Google Scholar]
- 38.Untersteller K, Meissl S, Trieb M, Emrich IE, Zawada AM, Holzer M, Knuplez E, Fliser D, Heine GH, Marsche G (2018) HDL functionality and cardiovascular outcome among nondialysis chronic kidney disease patients. J Lipid Res 59:1256–1265. doi: 10.1194/jlr.P085076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Honda H, Ueda M, Kojima S, Mashiba S, Michihata T, Takahashi K, Shishido K, Akizawa T (2012) Oxidized high-density lipoprotein as a risk factor for cardiovascular events in prevalent hemodialysis patients. Atherosclerosis 220:493–501. doi: 10.1016/j.atherosclerosis.2011.10.038 [DOI] [PubMed] [Google Scholar]
- 40.Weichhart T, Kopecky C, Kubicek M, Haidinger M, Doller D, Katholnig K, Suarna C, Eller P, Tolle M, Gerner C, Zlabinger GJ, van der Giet M, Horl WH, Stocker R, Saemann MD (2012) Serum amyloid A in uremic HDL promotes inflammation. J Am Soc Nephrol 23:934–947. doi: 10.1681/ASN.2011070668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mange A, Goux A, Badiou S, Patrier L, Canaud B, Maudelonde T, Cristol JP, Solassol J (2012) HDL proteome in hemodialysis patients: a quantitative nanoflow liquid chromatography-tandem mass spectrometry approach. PLoS One 7:e34107. doi: 10.1371/journal.pone.0034107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tolle M, Huang T, Schuchardt M, Jankowski V, Prufer N, Jankowski J, Tietge UJ, Zidek W, van der Giet M (2012) High-density lipoprotein loses its anti-inflammatory capacity by accumulation of pro-inflammatory-serum amyloid A. Cardiovasc Res 94:154–162. doi: 10.1093/cvr/cvs089 [DOI] [PubMed] [Google Scholar]
- 43.Rubinow KB, Henderson CM, Robinson-Cohen C, Himmelfarb J, de Boer IH, Vaisar T, Kestenbaum B, Hoofnagle AN (2017) Kidney function is associated with an altered protein composition of high-density lipoprotein. Kidney Int 92:1526–1535. doi: 10.1016/j.kint.2017.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao B, Tang C, Heinecke JW, Oram JF (2010) Oxidation of apolipoprotein A-I by myeloperoxidase impairs the initial interactions with ABCA1 required for signaling and cholesterol export. J Lipid Res 51:1849–1858. doi: jlr.M004085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shao B, Heinecke JW (2011) Impact of HDL oxidation by the myeloperoxidase system on sterol efflux by the ABCA1 pathway. J Proteomics 74:2289–2299. doi: S1874-3919(11)00143-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kraus LM, Kraus AP, Jr, (2001) Carbamoylation of amino acids and proteins in uremia. Kidney Int Suppl 78:S102–107. doi: 10.1046/j.1523-1755.2001.59780102.x [DOI] [PubMed] [Google Scholar]
- 47.Koeth RA, Kalantar-Zadeh K, Wang Z, Fu X, Tang WH, Hazen SL (2013) Protein carbamylation predicts mortality in ESRD. J Am Soc Nephrol 24:853–861. doi: 10.1681/ASN.2012030254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montuschi P, Barnes PJ, Roberts LJ 2nd, (2004) Isoprostanes: markers and mediators of oxidative stress. FASEB J 18:1791–1800. doi: 10.1096/fj.04-2330rev [DOI] [PubMed] [Google Scholar]
- 49.Shao B (2012) Site-specific oxidation of apolipoprotein A-I impairs cholesterol export by ABCA1, a key cardioprotective function of HDL. Biochim Biophys Acta 1821:490–501. doi: 10.1016/j.bbalip.2011.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikizler TA, Morrow JD, Roberts LJ, Evanson JA, Becker B, Hakim RM, Shyr Y, Himmelfarb J (2002) Plasma F2-isoprostane levels are elevated in chronic hemodialysis patients. Clin Nephrol 58:190–197 [DOI] [PubMed] [Google Scholar]
- 51.May-Zhang LS, Yermalitsky V, Huang J, Pleasent T, Borja MS, Oda MN, Jerome WG, Yancey PG, Linton MF, Davies SS (2018) Modification by isolevuglandins, highly reactive gamma-ketoaldehydes, deleteriously alters HDL structure and function. J Biol Chem 293:9176–9187. doi: 10.1074/jbc.RA117.001099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woollett LA, Spady DK (1997) Kinetic parameters for high density lipoprotein apoprotein AI and cholesteryl ester transport in the hamster. J Clin Invest 99:1704–1713. doi: 10.1172/JCI119334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kronenberg F, Kuen E, Ritz E, Konig P, Kraatz G, Lhotta K, Mann JF, Muller GA, Neyer U, Riegel W, Riegler P, Schwenger V, von Eckardstein A (2002) Apolipoprotein A-IV serum concentrations are elevated in patients with mild and moderate renal failure. J Am Soc Nephrol 13:461–469 [DOI] [PubMed] [Google Scholar]
- 54.Mack S, Coassin S, Vaucher J, Kronenberg F, Lamina C; ApoA-IV-GWAS Consortium (2017) Evaluating the Causal Relation of ApoA-IV with Disease-Related Traits - A Bidirectional Two-sample Mendelian Randomization Study. Sci Rep 7:8734. doi: 10.1038/s41598-017-07213-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calabresi L, Simonelli S, Conca P, Busnach G, Cabibbe M, Gesualdo L, Gigante M, Penco S, Veglia F, Franceschini G (2015) Acquired lecithin:cholesterol acyltransferase deficiency as a major factor in lowering plasma HDL levels in chronic kidney disease. J Intern Med 277:552–561. doi: 10.1111/joim.12290 [DOI] [PubMed] [Google Scholar]
- 56.Lingenhel A, Lhotta K, Neyer U, Heid IM, Rantner B, Kronenberg MF, Konig P, von Eckardstein A, Schober M, Dieplinger H, Kronenberg F (2006) Role of the kidney in the metabolism of apolipoprotein A-IV: influence of the type of proteinuria. J Lipid Res 47:2071–2079. doi: 10.1194/jlr.M600178-JLR200 [DOI] [PubMed] [Google Scholar]
- 57.Stangl S, Kollerits B, Lamina C, Meisinger C, Huth C, Stockl A, Dahnhardt D, Boger CA, Kramer BK, Peters A, Kronenberg F (2015) Association between apolipoprotein AIV concentrations and chronic kidney disease in two large population-based cohorts: results from the KORA studies. J Intern Med 278:410–423. doi: 10.1111/joim.12380 [DOI] [PubMed] [Google Scholar]
- 58.Soto-Miranda E, Carreon-Torres E, Lorenzo K, Bazan-Salinas B, Garcia-Sanchez C, Franco M, Posadas-Romero C, Fragoso JM, Lopez-Olmos V, Madero M, RodriguezPerez JM, Vargas-Alarcon G, Perez-Mendez O (2012) Shift of high-density lipoprotein size distribution toward large particles in patients with proteinuria. Clin Chim Acta 414:241–245. doi: 10.1016/j.cca.2012.09.028 [DOI] [PubMed] [Google Scholar]
- 59.Aseem O, Smith BT, Cooley MA, Wilkerson BA, Argraves KM, Remaley AT, Argraves WS (2014) Cubilin maintains blood levels of HDL and albumin. J Am Soc Nephrol 25:1028–1036 doi: 10.1681/asn.2013060671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kollerits B, Krane V, Drechsler C, Lamina C, Marz W, Ritz E, Wanner C, Kronenberg F; German Diabetes and Dialysis Study Investigators (2012) Apolipoprotein A-IV concentrations and clinical outcomes in haemodialysis patients with type 2 diabetes mellitus--a post hoc analysis of the 4D Study. J Intern Med 272:592–600. doi: 10.1111/j.1365-2796.2012.02585.x [DOI] [PubMed] [Google Scholar]
- 61.Lamina C, Friedel S, Coassin S, Rueedi R, Yousri NA, Seppala I, Gieger C, Schonherr S, Forer L, Erhart G, Kollerits B, Marques-Vidal P, Ried J, Waeber G, Bergmann S, Dahnhardt D, Stockl A, Kiechl S, Raitakari OT, Kahonen M, Willeit J, Kedenko L, Paulweber B, Peters A, Meitinger T, Strauch K, Group KS, Lehtimaki T, Hunt SC, Vollenweider P, Kronenberg F (2016) A genome-wide association metaanalysis on apolipoprotein A-IV concentrations. Hum Mol Genet 25:3635–3646. doi: 10.1093/hmg/ddw211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peters KE, Davis WA, Ito J, Winfield K, Stoll T, Bringans SD, Lipscombe RJ, Davis TME (2017) Identification of Novel Circulating Biomarkers Predicting Rapid Decline in Renal Function in Type 2 Diabetes: The Fremantle Diabetes Study Phase II. Diabetes Care 40:1548–1555. doi: 10.2337/dc17-0911 [DOI] [PubMed] [Google Scholar]
- 63.Florens N, Calzada C, Lyasko E, Juillard L, Soulage CO (2016) Modified Lipids and Lipoproteins in Chronic Kidney Disease: A New Class of Uremic Toxins. Toxins (Basel) 8(12). doi: 10.3390/toxins8120376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kones R (2013) Molecular sources of residual cardiovascular risk, clinical signals, and innovative solutions: relationship with subclinical disease, undertreatment, and poor adherence: implications of new evidence upon optimizing cardiovascular patient outcomes. Vasc Health Risk Manag 9:617–670. doi: 10.2147/VHRM.S37119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith LE, Smith DK, Blume JD, Linton MF, Billings FT 4th (2017) High-Density Lipoprotein Cholesterol Concentration and Acute Kidney Injury After Cardiac Surgery. J Am Heart Assoc 6(12). doi: 10.1161/jaha.117.006975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Perkovic V, Hegbrant J, Strippoli GF (2014) HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev 5:CD007784. doi: 10.1002/14651858.CD007784.pub2 [DOI] [PubMed] [Google Scholar]
- 67.Baragetti A, Norata GD, Sarcina C, Rastelli F, Grigore L, Garlaschelli K, Uboldi P, Baragetti I, Pozzi C, Catapano AL (2013) High density lipoprotein cholesterol levels are an independent predictor of the progression of chronic kidney disease. J Intern Med 274:252–262. doi: 10.1111/joim.12081 [DOI] [PubMed] [Google Scholar]
- 68.REVEAL Collaborative Group, Bowman L, Chen F, Sammons E, Hopewell JC, Wallendszus K, Stevens W, Marquez E, Wiviott S, Cannon CP, Braunwald E, Collins R, Landray MJ(2017) Randomized Evaluation of the Effects of Anacetrapib through Lipid-modification (REVEAL)-A large-scale, randomized, placebo-controlled trial of the clinical effects of anacetrapib among people with established vascular disease: Trial design, recruitment, and baseline characteristics. Am Heart J 187:182–190. doi: 10.1016/j.ahj.2017.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Russo GT, De Cosmo S, Viazzi F, Pacilli A, Ceriello A, Genovese S, Guida P, Giorda C, Cucinotta D, Pontremoli R, Fioretto P (2016) Plasma Triglycerides and HDLC Levels Predict the Development of Diabetic Kidney Disease in Subjects With Type 2 Diabetes: The AMD Annals Initiative. Diabetes Care 39:2278–2287. doi: 10.2337/dc161246 [DOI] [PubMed] [Google Scholar]
- 70.Russo GT, Giandalia A, Romeo EL, Muscianisi M, Ruffo MC, Alibrandi A, Bitto A, Forte F, Grillone A, Asztalos B, Cucinotta D (2017) HDL subclasses and the common CETP TaqIB variant predict the incidence of microangiopatic complications in type 2 diabetic women: A 9years follow-up study. Diabetes Res Clin Pract 132:108–117. doi: 10.1016/j.diabres.2017.07.026 [DOI] [PubMed] [Google Scholar]
- 71.Coassin S, Friedel S, Kottgen A, Lamina C, Kronenberg F (2016) Is High-Density Lipoprotein Cholesterol Causally Related to Kidney Function? Evidence From Genetic Epidemiological Studies. Arterioscler Thromb Vasc Biol 36:2252–2258. doi: 10.1161/atvbaha.116.308393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haynes R, Staplin N, Emberson J, Herrington WG, Tomson C, Agodoa L, Tesar V, Levin A, Lewis D, Reith C, Baigent CMJ ; SHARP Collaborative Group (2014) Evaluating the contribution of the cause of kidney disease to prognosis in CKD: results from the Study of Heart and Renal Protection (SHARP). Am J Kidney Dis 64:40–48. doi: 10.1053/j.ajkd.2013.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsun JG, Yung S, Chau MK, Shiu SW, Chan TM, Tan KC (2014) Cellular cholesterol transport proteins in diabetic nephropathy. PLoS One 9:e105787. doi: 10.1371/journal.pone.0105787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merscher-Gomez S, Guzman J, Pedigo CE, Lehto M, Aguillon-Prada R, Mendez A, Lassenius MI, Forsblom C, Yoo T, Villarreal R, Maiguel D, Johnson K, Goldberg R, Nair V, Randolph A, Kretzler M, Nelson RG, Burke GW 3rd, Groop PH, Fornoni A; FinnDiane Study Group (2013) Cyclodextrin protects podocytes in diabetic kidney disease. Diabetes 62:3817–3827. doi: 10.2337/db13-0399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gao X, Wu J, Qian Y, Fu L, Wu G, Xu C, Mei C (2014) Oxidized high-density lipoprotein impairs the function of human renal proximal tubule epithelial cells through CD36. Int J Mol Med 34:564–572. doi: 10.3892/ijmm.2014.1799 [DOI] [PubMed] [Google Scholar]
- 76.Baranova IN, Souza ACP, Bocharov AV, Vishnyakova TG, Hu X, Vaisman BL, Amar MJ, Chen Z, Remaley AT, Patterson AP, Yuen PST, Star RA, Eggerman TL (2017) Human SR-BII mediates SAA uptake and contributes to SAA pro-inflammatory signaling in vitro and in vivo. PLoS One 12:e0175824. doi: 10.1371/journal.pone.0175824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thiemermann C, Patel NS, Kvale EO, Cockerill GW, Brown PA, Stewart KN, Cuzzocrea S, Britti D, Mota-Filipe H, Chatterjee PK (2003) High density lipoprotein (HDL) reduces renal ischemia/reperfusion injury. J Am Soc Nephrol 14:1833–1843 [DOI] [PubMed] [Google Scholar]
- 78.Milasan A, Jean G, Dallaire F, Tardif JC, Merhi Y, Sorci-Thomas M, Martel C (2017) Apolipoprotein A-I Modulates Atherosclerosis Through Lymphatic Vessel-Dependent Mechanisms in Mice. J Am Heart Assoc 6(9). doi: 10.1161/JAHA.117.006892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moreira RS, Irigoyen M, Sanches TR, Volpini RA, Camara NO, Malheiros DM, Shimizu MH, Seguro AC, Andrade L (2014) Apolipoprotein A-I mimetic peptide 4F attenuates kidney injury, heart injury, and endothelial dysfunction in sepsis. Am J Physiol Regul Integr Comp Physiol 307:R514–524. doi: 10.1152/ajpregu.00445.2013 [DOI] [PubMed] [Google Scholar]
- 80.Vaziri ND, Kim HJ, Moradi H, Farmand F, Navab K, Navab M, Hama S, Fogelman AM, Quiroz Y, Rodriguez-Iturbe B (2010) Amelioration of nephropathy with apoA-1 mimetic peptide in apoE-deficient mice. Nephrol Dial Transplant 25:3525–3534. doi: 10.1093/ndt/gfq274 [DOI] [PMC free article] [PubMed] [Google Scholar]