Abstract
Purpose:
Lipid mediators of inflammation are a group of signaling molecules produced by various cells under physiological conditions and modulate the inflammatory process during various pathologic conditions. Although eicosanoids and F2-isoprostanes are recognized lipid mediators of inflammation, there is no consensus yet on the extraction and mass spectrometry method for their analysis in individual human tear samples. Thus, the aim of this study was to develop an optimal method for extraction of lipid mediators of inflammation in the tear film and evaluate mass spectrometry (MS) techniques for their analysis.
Methods:
Basal tears were collected from each eye of 19 subjects using glass microcapillaries. Lipid extraction was done using either varying concentrations of acidified methanol, a modified Folch method or solid phase extraction. Initially an untargeted analysis of the extracts was done using SCIEX TripleTOF 5600 mass spectrometer to identify any lipid mediators of inflammation (eicosanoids) and later a targeted analysis was done using the SCIEX 6500 Qtrap to identify and quantify prostaglandins and isoprostanes. Mass spectra and chromatograms were analyzed using Peakview, XCMS, and Multiquant software.
Results:
Prostaglandins and isoprostanes were observed and quantified using the Qtrap mass spectrometer under MRM mode after solid phase extraction. Extraction with acidified methanol along with the Folch method produced cleaner spectra during mass spectrometry with the Triple TOF mass spectrometer. Lipid mediators of inflammation were not observed in any of the tear samples using the Triple TOF mass spectrometer.
Conclusions:
Solid phase extracti on may be the method of choice for extraction of prostaglandins and isoprostanes in low volumes of tears. The SCIEX Qtrap 6500 in MRM mode may be suitable to identify and quantify similar lipid mediators of inflammation.
Keywords: lipids, inflammatory mediators, tears, mass spectrometry
Lipid mediators of inflammation are a group of signaling molecules that are produced and secreted from many cell types under physiological and pathological conditions. They belong to different groups of molecules including eicosanoids and isoprostanes which are known mediators and regulators of inflammation.1–3 Eicosanoids are generated from 20 carbon polyunsaturated fatty acids (PUFAs). Arachidonic acid (AA) is usually the major substrate that is esterified to phospholipids of cell membranes.4 Phospholipase A2 is an enzyme that metabolizes these cell membrane phospholipids to produce free AA.5 AA is oxidized by three groups of enzymes, cycloxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP 450) to produce various eicosanoids i.e. prostaglandins, leukotrienes, thromboxanes, lipoxins, hydroxyeicosatetraenoic acids (HETEs) and epoxyeicosatrienoic acids.6 Arachidonic acid is also oxidized non-enzymatically in-vivo by free radicals and forms peroxidation products like F2-isoprostanes.7 Although F2-isoprostanes are widely recognized as reliable markers of oxidative stress, they have also been found to be involved in several acute and chronic inflammatory conditions like rheumatic disease, asthma, diabetes etc.3, 8They have also been described as inflammatory mediators that augment nociception.9
The lipid mediators of inflammation are known for their role in vascular homeostasis, protection of the gastric mucosa and platelet aggregation, regulation of immunopathological processes ranging from inflammatory responses to chronic tissue remodeling, asthma, rheumatoid arthritis, cancer and various autoimmune disorders. They regulate many important aspects of immunity, including pathogen recognition through the extracellular and the intracellular receptors, antibody formation, cytokine production, cell proliferation, differentiation, migration and antigen presentation. They have been shown to play roles in either the pathogenesis or resolution of various ocular diseases such as glaucoma, age related macular degeneration, diabetic retinopathy, uveitis and dry eye disease.10–22 On the ocular surface, one of the prostaglandins, prostaglandin E2 (PGE2) has been shown to be upregulated in dry eye disease and leukotriene B4 (LTB4) has been found to increase in tears of contact lens wearers with discomfort (CLD).22, 23 Oxidative stress has also been proposed to be involved in the pathogenesis of meibomian gland dysfunction and dry eye disease, and markers of oxidative stress (lipid peroxides/malondialdehyde) have been found in higher amounts in the tears of intolerant contact lens wearers.24
Lipid mediators of inflammation have been analyzed by various methods such as immunoassays, liquid chromatography, gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS/MS).25–31 Among mass spectrometry methods, LC-MS/MS is the most common method of analysis. Prior to mass spectrometry, sample preparation is done using different extraction methods and the analytes are usually separated chromatographically. Major methods that have been used for extraction include liquid-liquid and solid-phase extraction (SPE). Some studies have only done protein precipitation without extraction, which is not very common as it is prone to higher amount of contamination and matrix effects.32, 33 SPE is the most frequently used technique for extraction of these lipids. SPE protocols have used SPE cartridges with either C1834, 35 or polymeric reversed-phase materials.36–38 Samples are typically acidified using mild acids like formic acid or acetic acid prior to reverse-phase SPE. The organic solvents used for elution of the analytes are then evaporated by a nitrogen stream or a vacuum concentrator. Liquid chromatography (LC) is the most widely used separation technique for eicosanoids typically using reverse phase columns. The mass spectromety method that has most frequently been used in the past few years is tandem mass spectrometry (MS/MS) on triple quadrupole using the multiple reaction monitoring (MRM) technique. Other analyzers like ion traps, hybrid qudrupole-linear ion traps, time of flight (TOF) or Orbitrap instruments also have been used.38–43 Isotope labelled internal standards are generally added to the samples for quantification.
To our knowledge, few studies have evaluated ecosanoids and F2-isoprostanes in the human tear film using mass spectrometry.22, 44, 45 Prior to mass spectrometric analysis, the tear samples have been extracted using solid phase extraction via C18 columns in such tear film related studies.45, 46 Chromatographic separation has been done with LC and the mass analysis with triple quadrouple linear ion trap or Qtrap quadrouple mass spectrometers in MRM mode. 22, 45, 46 The volume of tears collected in these studies is typically quite large—on the order ot 50 −100 µl of basal or reflex tears.45, 46 As basal tears theoretically reflect the normal concentraton of molecular species in the tear film, basal tear analysis are preferred to reflex or flush tear samples. However, basal tears are usually collected at a volume of 5 – 10 µl in one sitting and collection of large volume of tears is tedious and takes multiple times for sample collection requiring pooling. There is no consensus yet on the extraction and mass spectrometry method for analysis of ecosanoids and F2-isoprostanes in individual human tear samples. Thus, the purpose of this study was to develop an extraction method for lipid inflammatory mediators (ecosanoids and F2-isoprostanes) in samples of human tears (5 – 40 µl) and evaluate mass spectrometry methods (direct infusion and LC-MS) for their analysis. Such a method would help in identification of biomarkers for ocular surface disorders like dry eyes and contact lens discomfort. A preliminary comparison of these mediator concentrations in tears has thus been done in contact lens wearers with and without discomfort in this study.
Methods
The research was approved by an institutional review board (IRB) and followed the tenets of the Declaration of Helsinki. Informed consent was obtained from all the subjects after explanation of the nature and possible consequences of the study prior to sample collection and analysis. There were two arms in the study: first was the untargeted analysis of the tear samples using SCIEX TripleTOF 5600 (SCIEX, Toronto, Canada) mass spectrometer to look for lipid mediators of inflammation (eicosanoids) and the second was the targeted analysis using the SCIEX 6500 Qtrap (SCIEX, Toronto, Canada) to look for prostaglandins and isoprostanes. For the unatargeted analysis, ten µl tear samples were collected from each eye of 15 subjects using glass microcapillary tubes (Drummond Microcaps, Drummond Scientific Company, PA). The edges of the tubes were placed near the lateral canthus area over the tear prism for capillary collection, which were expelled into amber glass vials using a bulb dispenser. The microcapillary tubes were of known length (32mm) which when filled in full accommodate 5 µl of tears per tube. Multiple tubes were used to collect the tears till the required amount (10 µl from each eye for the untargeted analysis and 20 µl from each eye for the targeted analysis) was obtained. If the tubes did not fill completely with tears samples, the length of tears collected was measured using a slit-lamp graticule and the volume was calculated using the formula {(length collected (in mm)/32)*5} µl. The experimental error was around 0.1µl (1%). Tears from the two eyes of each subject were pooled together and stored at - 80°C until extraction. Standards for prostaglandin E2 (PGE2), thromboxane B2 (TXB2) and leukotriene B4 (LTB4) from the three major eicosanoid groups (prostaglandins, thromboxanes and leukotrienes) were purchased from Cayman Chemical Company (Ann Arbor, MI) and prepared to final concentrations of 500, 100, 10, 2, 1, 0.1, 0.01 and 0.001 ng/ml in methanol. The lowest concentration of the standard that had a signal-to-noise ratio of at least 3:1 was estimated as the limit of detection. The peak intensity of the spectra for various concentrations were fit as a function of corresponding concentrations to examine the linearity of the assays. A regression line was calculated by methods of least squares and fit. The coefficient of determination (r2) provided the degree of linearity.
For the targeted analysis 20 µl tear samples were collected from each eye of four subjects as none of the lipid mediators of inflammation were observed using ten microliter tear samples (unpublished data). Tear collection and storage was done in a similar way as described above. Prostaglandin F2α (PGF2α) along with its oxidized metabolites (isoprostanes), 8-iso-15(R)-PGF2α, 8-iso-PGF2α, 15(R)-PGF2α, 2,3-dinor-8-iso-PGF2α were purchased from Cayman Chemical Company (Ann Arbor, MI) and prepared to final concentrations of 500, 100, 10, 2, 1, 0.1, 0.01 and 0.001 ng/ml using methanol. Limits of detection were calculated, and the linearity of the assays was examined using the methods described above.
Extraction
Methods 1 through 4 were used to extract tear samples for untargeted analysis and method 5 was used to extract tear samples for targeted analysis.
Method 1
A method used by de Grauw et al., was adapted for use with human tears.47 Three tear samples (10 µl each) in duplicate were extracted in amber glass vials using 500 µl of 15% methanol and 0.1% formic acid. Protein precipitation was done using accuSpin Micro 17 centrifuge (Fisher Scientific, Hampton, NH) at 1400 revolution per minute (rpm) for 10 minutes. Four hundred µl of the supernatant was collected and dried under vacuum (Eppendorf Vacufuge Plus, Eppendorf North America, Inc., Hauppauge, NY). It was then reconstituted with 15% methanol to 50 µl and used for mass spectrometric analysis.
Method 2
Three tear samples (10 µl each) were extracted using 500 µl of 80% methanol and 0.1% formic acid. Protein precipitation was done using accuSpin Micro 17 centrifuge at 10,000 rpm for 10 minutes. Four hundred µl supernatant was collected and dried under vacuum (Eppendorf Vacufuge Plus, Eppendorf North America Inc., Hauppauge, NY) It was then reconstituted with 80% methanol to 50 µl and used for mass spectrometric analysis.
Method 3
Three tear samples (10 µl each) were extracted using chloroform:methanol:1M ammonium acetate aqueous solution (8:4:3) modified from the method by Folch et al.,48 to bring the solution to 450 µl. The lower phase containing lipids was collected in a glass vial and immediately used for mass spectrometric analysis.
Method 4
A total of six tear samples, two each collected from non-contact lens wearers, contact lens wearers without discomfort and contact lens wearers with discomfort were used in this extraction method. Each of the tear samples was 10 µl in volume. The tears were extracted using 500 µl of 80% methanol and 0.1% formic acid as this gave cleaner spectrum in initial mass spectrometric analyses. Protein precipitation was done using AccuSpin Micro 17 centrifuge at 17,000 rpm for 10 minutes. Four hundred µl supernatant was collected and dried under vacuum (Eppendorf Vacufuge Plus, Eppendorf North America, Inc., Hauppauge, NY). It was then reconstituted with 0.1% formic acid to 50 µl, which was then used for LC-MS.
Method 5
An extraction method used by Prasain et al., was adapted for use with tears.49 Four tear samples (40 µl each collected from the two eyes of each subject); two each from contact lens wearers with discomfort and without discomfort were extracted using a Strata X-AW 33µ polymeric weak anion solid phase extraction cartridge (Phenomenex, Torrance, CA). Methanol-water (80/20, v/v containing 1% acetic acid) was used as the extraction solvent to elute the prostaglandins and F2-isoprostanes. The extracted samples were evaporated to dryness under a stream of nitrogen gas and reconstituted in 50 µl of methanol:water (1:1 v/v containing 1% acetic acid) for LC-MS/MS analysis.
Mass spectrometry
Untargeted analysis
A SCIEX TripleTOF 5600 (SCIEX, Toronto, Canada) mass spectrometer was used to analyze the initial 15 tear samples to look at possible lipid mediators of inflammation globally. Mass spectrometry was performed using either direct infusion (DI) or in conjunction with LC. In the DI method, mass spectrometry of the six samples extracted using methods 1 and 2 was done in negative ion mode. Ammonium hydroxide was used as an additive to promote formation of anions of lipids for detection. The resultant solution was directly injected to Triple-TOF 5600 mass spectrometer and detected in the range of m/z 200 −1200 with a flow rate of 7 µl /min, declustering potential of −40 volts (V), collision energy of −10 V, and ionspray voltage of - 4500 V. The three samples extracted using method 3 were also analyzed by the DI method. Ammonium acetate was used as an additive and MS and SWATH-MS were performed in these samples in negative ion mode. Since LTB4 has previously been identified in positive ion mode, 39, 50 tear samples were also analyzed in positive ion mode. SWATH-MS parameters in negative ion mode included a voltage of −4500V, collision energy of −34eV with collision energy spread of 40eV. The parameters in positive ion mode included a voltage of 5500V, collision energy of 40 eV with collision energy spread of 40 eV. The acquisition time was 6 minutes in each ion mode. MS/MS were performed on all precursor ions in the range of m/z 200 to 1200 at every Dalton step in each mode.
In the second mass spectrometry procedure for the untargeted analysis, the six samples extracted using method 4 were analyzed using a NanoLC combined with a Triple Time-Of-Flight (TOF) mass spectrometer. An aliquot (5 µL) of each sample was loaded onto a Nano cHiPLC 200 μm ID x 6 mm ChromXP C18-CL 3 μm, 120 Å reverse-phase trap cartridge (Eksigent, Dublin, CA) at 2 μl/min flow rate using an Eksigent autosampler. After washing the cartridge for 5 min with 0.1% formic acid in LC-MS grade water, the bound metabolites were flushed onto a Nano cHiPLC column (200 μm ID x 15 cm ChromXP C18-CL) with a 20 min, linear gradient of 5–95% acetonitrile in 0.1% formic acid at 1000 nl/min using an Eksigent 415 NanoLC system. The column was washed with 95% acetonitrile and 0.1% formic acid for 5 min and then re-equilibrated with 5% acetonitrile and 0.1% formic acid for 5 min. The SCIEX TripleTOF 5600 mass spectrometer was used to analyze the metabolite profile. The IonSpray voltages for positive and negative modes were +/−2300 V and the declustering potential was +/− 80 V. Ionspray and curtain gases were set at 10 psi and 25 psi, respectively. The interface heater temperature was 120 °C. Eluted compounds were subjected to a time-of-flight survey scan from m/z 50–1000 to determine the top twenty most intense ions for MSMS analysis. Product ion time-of-flight scans to obtain the tandem mass spectra of the selected parent ions over the range from m/z 50–1000 were collected over 50 msec intervals using a collision energy spread of 15 eV with a set collision point of 35 eV. Spectra were centroided and de-isotoped by Analyst software, version 1.6 TF (Sciex, Toronto, Canada). Standard solutions were analyzed by the direct infusion method as described above to determine the limits of detection.
Targeted analysis
A SCIEX 6500 Qtrap (SCIEX, Toronto, Canada) mass spectrometer was used to analyze the last four samples extracted as described in Method 5 above for targeted analysis of prostaglandins and isoprostanes. Liquid chromatography (LC)-electrospray ionization - mass spectrometry (ESI-MS/MS) was performed. Gradient separation of prostaglandins and isoprostanes occurred using an Eksigent MicroLC (SCIEX, Concord, ON) 200 system over an Eksigent 3C18-AQ-120A column (P/N 805 – 10022) 50o Celsius. HPLC was operated using a binary gradient consisting of 0.1% formic acid in LC-MS grade water (A) with 0.1% formic acid in 50% methanol-acetonitrile (B) at a flow rate of 45 µl/min. The gradient schedule was 48% B from 0 to 1 minute, 63% B from 1 to 2.5 minutes, 98% B from 2.5 to 3 minutes, 48% B from 3 to 4 minutes at which the analysis ended. Ten µl of each sample was injected for analysis.
Data acquisition occurred on the Sciex 6500 Qtrap mass spectrometer operating in negatively polarity multiple reacting monitoring (MRM) mode. Ionization occurred via electrospray ionization. The IonDrive Turbo V source was equipped with a 50 μm Eksigent micro ESI probe to facilitate reduced flow rates associated with micro flow HPLC. Mass spectrometry instrument parameters were as follows: Turbo V source temperature (TEM) was set at 375 ° C; collision gas (CAD) was nitrogen set at 9 psi; curtain gas (CUR) was nitrogen set at 20 psi; the nebulizing gas (GS1) was nitrogen set at 50 psi; the drying gas (GS2) was nitrogen set at 30 psi and the ion spray voltage (IS) was set at 4500 V. MRM compound mass transitions and parameters are listed in Table 1.
Table 1.
Mass spectrometry instrument parameters for multiple reaction monitoring (MRM) analysis of prostaglandins and isoprostanes.
| Analyte | Q1 | Q3 | CE (volts) | CXP (volts) | DP (volts) | EP (volts) |
|---|---|---|---|---|---|---|
| PGE2 | 351 | 271 | −20 | −14 | −35 | −12 |
| 351 | 189 | −25 | −12 | −35 | −12 | |
| PGF2α | 353 | 193 | −30 | −10 | −55 | −12 |
| 353 | 309 | −25 | −4 | −65 | −3 | |
| 8-iso-PGF2α | 353 | 193 | −30 | −10 | −55 | −12 |
| 353 | 309 | −25 | −4 | −65 | −3 | |
| 15(R)-PGF2α | 353 | 193 | −30 | −10 | −55 | −12 |
| 353 | 309 | −25 | −4 | −65 | −3 | |
| 8-iso-15(R)-PGF2α | 353 | 193 | −30 | −10 | −55 | −12 |
| 353 | 309 | −25 | −4 | −65 | −3 | |
| 2,3-dinor-8-iso-PGF2α | 325 | 237 | −15 | −14 | −55 | −5 |
| 325 | 137 | −25 | −12 | −45 | −12 | |
| PGF2α-d4 | 357 | 197 | −35 | −10 | −55 | −8 |
| 357 | 313 | −25 | −16 | −55 | −8 | |
Q1 and Q3 are parent and diagnostic daughter ion m/z respectively. CE= collision energy; CXP=collision cell exit potential; DP=declustering potential; EP=entrance potential.
Repeatability of the methods
Repeatability of the methods described above were checked by repeating all the experiments described above except for the Folch extraction method, SWATH-MS and solid phase extraction method. Folch extraction was not done in the repeat experiments as this extraction method has not been used to identify lipid mediators of inflammation in other biological specimens and lipid mediators of inflammation were not found in the initial experiment using this extraction method. Similarly, SWATH-MS was not done in the repeat experiment as the lipid mediators of inflammation were not found using this technique in the initial experiment. Solid phase extraction method was not repeated as it was an adaptation of an already successful method.
Data analysis
The m/z peaks that were obtained from tear samples extracted using methods 1,2 and 3 and that underwent DI-MS assay, were manually analyzed using Peakview software 1.3 (SCIEX, Toronto, Canada). Fragmentation patterns of the parent ions were compared with those of standards and published lipid-maps standards for identification of the molecules. The retention times in LC, the m/z peaks and the peak areas obtained from the three groups of tear samples that were extracted using method 4, and underwent LC-MS and LC-MS/MS assay were analyzed using XCMSonline software (Scripps, La Jolla, CA). The differences in intensities of mass spectra of lipid molecule ions obtained from these three groups of tear samples were compared using ANOVA with post hoc analysis. The threshold for highly significant features was p< 0.01 and for significant features was p< 0.05. The fold change threshold for highly significant features was 1.5. For the tear samples that were extracted using method 5, data collection and instrument operation was done using Analyst software 1.6.2 (SCIEX, Toronto, Canada). Data was analyzed using Multiquant software 3.0.1 (SCIEX, Toronto, Canada). The concentration of prostaglandins and isoprostanes in tears were quantified using standard curves ranging from 0.05 to 2.5 ng/ml for PGE2 series ions and from 0.5 to 250 ng/ml for the 2,3-dinor-PGF2α series. PGF2α-d4 was used as an internal standard.
Results
Subject Demographics and Contact Lens Wear
Nineteen subjects were enrolled in the study. Thirteen (68.42%) of them were females. The most predominant race was Caucasian (47.36%) followed by Asians (36.84%) and African-Americans (15.80%). Eight of the total 19 subjects wore contact lenses and four of those that wore the lenses had contact lens discomfort. The subjects were chosen as per the method development requirements and do not follow any other criteria. A subject with contact lens discomfort was one who was classified as having contact lens related dry eyes when evaluated with a contact lens dry eye questionnaire (CLDEQ-long form) and had two or more hours of uncomfortable contact lens wear out of the total duration of wear per day. All contact lens wearing subjects wore soft contact lenses for at least four hours a day and at least four days a week.
Limits of Detection Calculation for Standards
The identity of the three representative eicosanoid standards (PGE2,TXB2 and LTB4) were confirmed using extracted ion chromatograms (XICs) and mass spectra of precursor and product ions using the direct infusion-mass spectrometry method (Figure 1). Similarly, the identity of PGF2α and the four isoprostanes (8-iso-PGF2α, 8-iso-15(R)-PGF2α, 15(R)-PGF2α and 2,3-dinor-8-iso-PGF2α) was confirmed using the characteristic retention times during HPLC. (Figure 2). The limit of detection for PGE2, TXB2, LTB4, PGF2α, 8-iso-15(R)-PGF2α, 8-iso-PGF2α, 15(R)-PGF2α and 2,3-dinor-8-iso-PGF2α were found to be 10 ng/ml, 1 ng/ml, 10 ng/ml 5 pg/ml, 5 pg/ml, 5 pg/ml, 5 pg/ml and 100 pg/ml respectively. All of the mass spectrometry assays for the standards were found to be linear with r2 values between 0.90 and 0.99 (Figure 3). Figure 4 shows the XICs for the three representative lipid standards.
Figure 1.
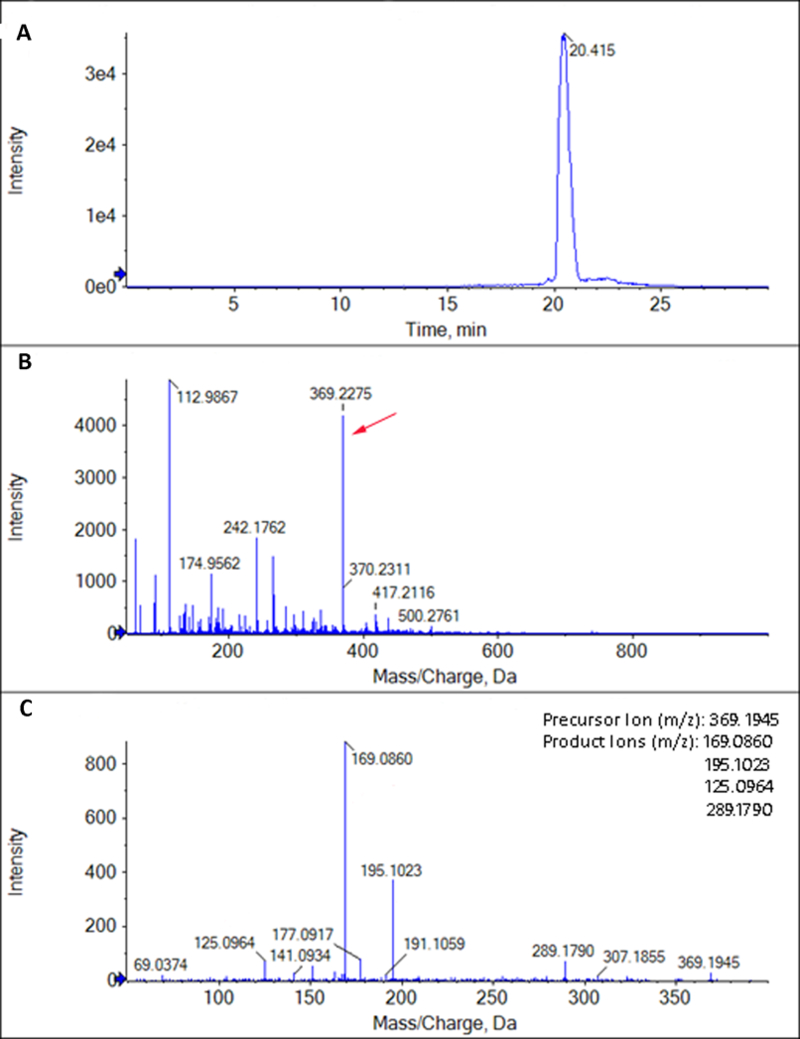
Representative mass spectrometry extracted ion chromatogram and mass spectra (A. XIC: Extracted Ion Chromatogram; B.MS: Mass Spectrum; C.MS/MS: Tandem mass spectrum) of one of the representative standards (TXB2) that underwent direct infusion – mass spectrometry (DI-MS). Limit of detection for TXB2 was found to be 1 ng/ml and the identity was confirmed by comparing the characteristic fragmentation ions found by MS/MS with published LIPID MAPS standards. The identity of the other standards was confirmed by similar methods.
Figure 2.
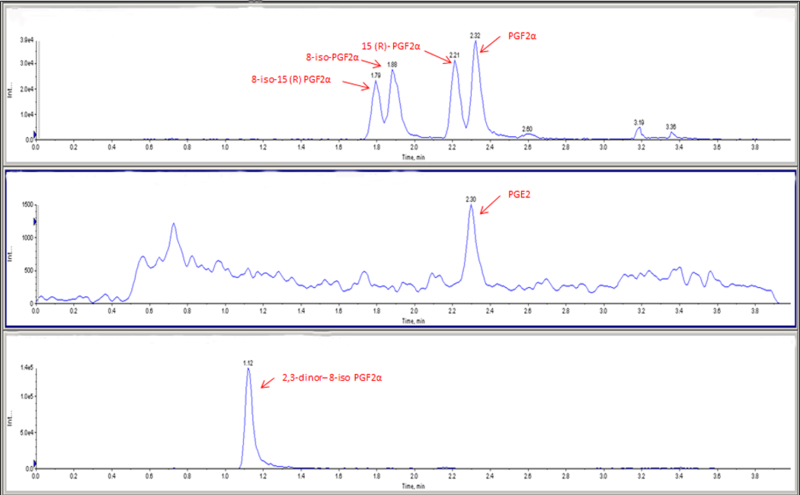
Extracted Ion Chromatograms for standards (A. (8-iso-15(R)-PGF2α, 8-iso-PGF2α, 15(R)-PGF2α, PGF2α); B. PGE2 and C. 2,3-dinor-8-iso-PGF2α). The standards underwent liquid chromatography mass spectrometry (LC - MS) in multiple reaction monitoring (MRM) mode.
Figure 3.
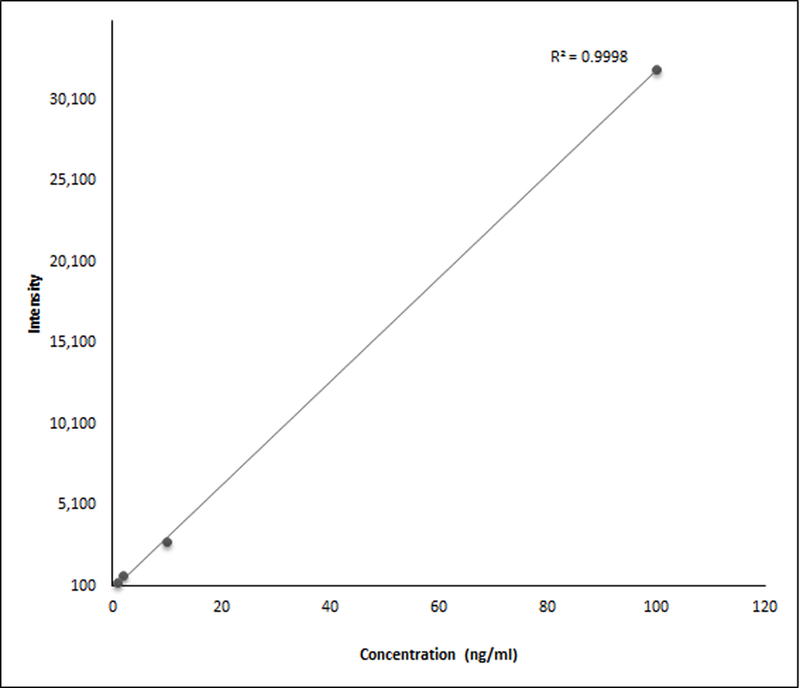
A representative standard curve (fitted with regression line) for TXB2. It shows a linear relationship between the concentration and signal intensity (R2 =0.99). Similar standard curves were used to assess the linearity of the assays for all the standards that were used.
Figure 4.
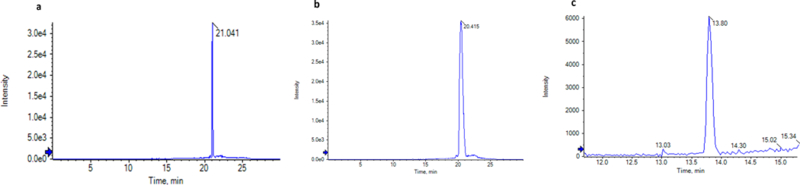
Extracted Ion Chromatogram (XIC) for standards (A. PGE2, B. TXB2, and C. LTB4) that went under direct infusion – mass spectrometry (DI – MS).
Identification of Lipid Species in Tears
Analysis of the mass spectrum peaks did not show any eicosanoids but revealed a good amount of sodium and potassium salts and other contaminants in the tear samples that were extracted using method one (Figure 5A). The contaminants were reduced and more lipid species were observed in the mass spectrum of the tear samples extracted using method two (Figure 5B). Repeat experiments also showed similar mass spectra in both cases.
Figure 5.
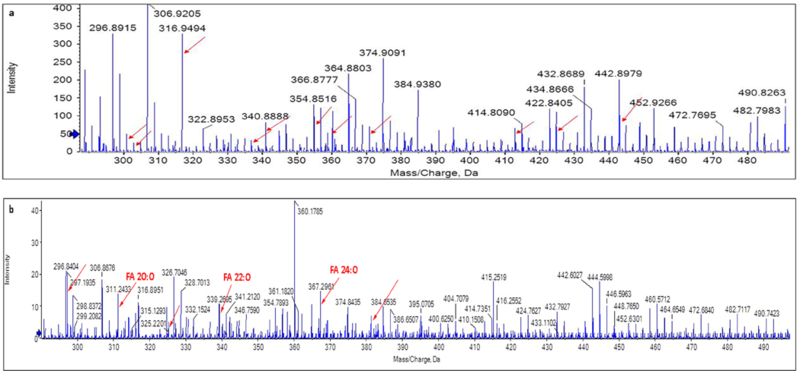
A. Mass spectrum image using extraction method one and direct infusion – mass spectrometry (DI-MS). Arrows in the plot point to m/z of salts and other contaminants. B. Mass spectrum image using extraction method two and DI-MS. Arrows in the plot point to m/z of lipid species extracted using method one and not detectable previously.
The tears that were extracted using method three (Folch method) and MS/ SWATH-MS showed a variety of lipids found in tears in both positive and negative ion modes but not the eicosanoids. Many free fatty acids (Figure 6) along with (O-acyl)-ω-hydroxy fatty acids (OAHFAs) (Figure 7) were found in negative ion mode. Table 2 provides the theoretical m/z, experimental m/z and the mass error of the FFAs that were observed.
Figure 6.
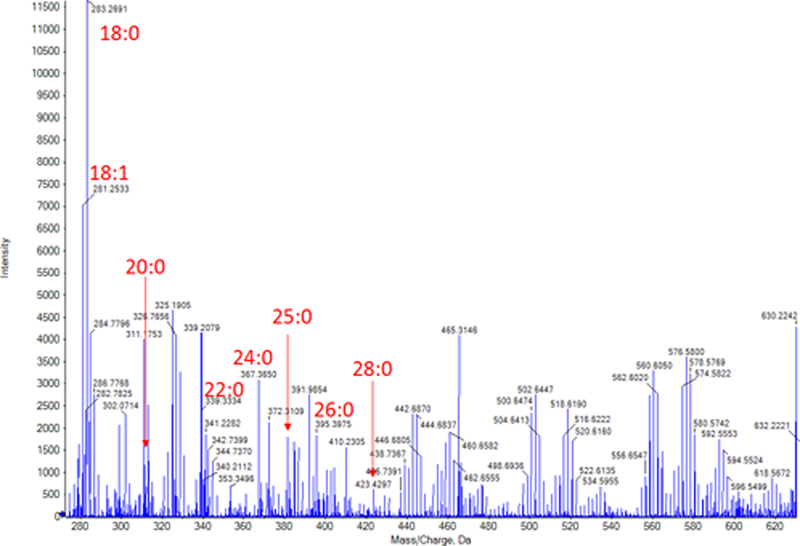
Mass spectrum of tears extracted using Folch method. Many free fatty acid (FFA) peaks were observed. Bold numbers and arrows indicate FFAs and their peaks.
Figure 7.
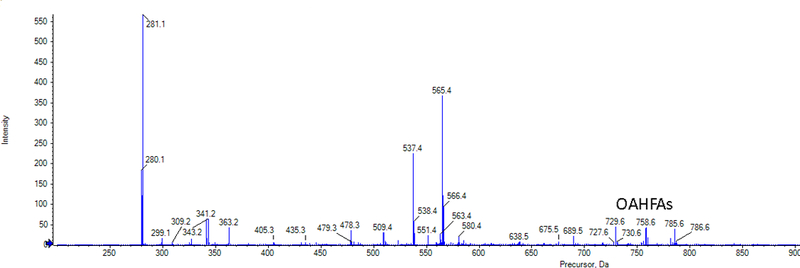
Extracted Ion Chromatogram of lipids seen in negative ion mode for samples extracted with Folch method and direct infusion (time-of-flight) – mass spectrometry (TOF-MS). (O-acyl)-ω-hydroxy fatty acids were observed during MS/MSall.
Table 2.
Major free fatty acids observed in negative mode during MS/SWATH-MS of tears that were extracted using the Folch Method.
| Composition | Theoretical m/z | Experimental m/z | Mass Error (ppm) |
|---|---|---|---|
| C18H35O2 (18:0) | 283.2643 | 283.2691 | 16.94 |
| C18H33O2 (18:1) | 281.2486 | 281.2533 | 16.71 |
| C20H39O2 (20:0) | 311.2956 | 311.2923 | 10.60 |
| C21H41O2 (21:0) | 325.3112 | 325.3163 | 15.67 |
| C22H43O2 (22:0) | 339.3269 | 339.3334 | 19.15 |
| C23H45O2 (23:0) | 353.3425 | 353.3501 | 21.50 |
| C24H47O2 (24:0) | 367.3582 | 367.3650 | 18.51 |
| C25H49O2 (25:0) | 381.3738 | 381.3825 | 22.81 |
| C26H51O2 (26:0) | 395.3895 | 395.3975 | 20.23 |
| C28H55O2 (28:0) | 423.4202 | 423.4297 | 22.43 |
In the positive ion mode, LTB4 was not observed but other predominant lipid molecules found in tears like cholesteryl esters, wax esters and phospholipids were observed (Supplementary Figure S1).
Tear samples that were extracted using solid phase extraction and analyzed using LC-MS/MS in MRM mode showed PGE2, PGF2α, 8-iso-15(R)-PGF2α, 8-iso-PGF2α, 15(R)-PGF2α, and 2,3-dinor-8-iso-PGF2α (Figure 8).
Figure 8.
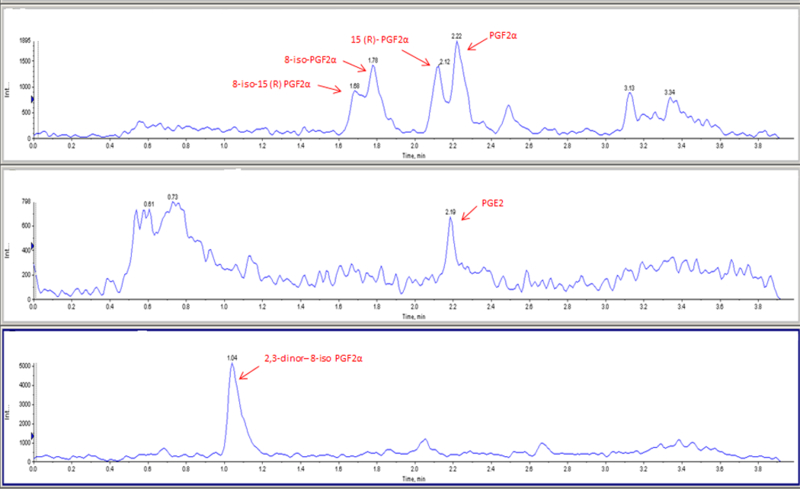
Extracted Ion Chromatograms of a tear sample showing various lipid molecules (A. (8-iso-15(R)-PGF2α, 8-iso-PGF2α, 15(R)-PGF2α, PGF2α); B. PGE2 and C. 2,3-dinor-8-iso-PGF2α).
Prostaglandins and isoprostanes were quantified in the tear samples from the four subjects that were extracted using extraction method 5. The concentration of these lipid molecules were not found to be significantly different between the two groups (Tables 3).
Table 3.
Concentration (in ng/ml of tears) of selected prostaglandins and isoprostanes among contact lens wearers with and without discomfort (No CLD).
| PGE2 | PGF2α | 8-iso-15(R) – PGF2α | 8-iso-PGF2α | 15(R)-PGF2α | 2,3-dinor-8-iso-PGF2α | |
|---|---|---|---|---|---|---|
| Mean (± SD) | Mean (± SD) | Mean (± SD) | Mean (± SD) | Mean (± SD) | Mean (± SD) | |
| No CLD1 subjects (n=2) | 1.28 (0.40) | 0.11 (0.06) | 0.11 (0.06) | 0.12 (0.06) | 0.09 (0.06) | 8.66 (4.29) |
| CLD subjects (n=2) | 1.28 (1.81) | 1.40 (1.83) | 1.28 (1.68) | 1.33 (1.73) | 1.40 (1.83) | 1035.44 (1450.40) |
| p – value | 1.00 | 0.67 | 0.67 | 0.67 | 0.67 | 0.67 |
CLD = CONTACT LENS DISCOMFORT
Discussion
This study aimed to identify the best possible extraction and analytical method using mass spectrometry for lipid mediators of inflammation in low volume (10 – 40 µl) of human tear samples. Four extraction and two mass spectrometry methods were first evaluated in the non-targeted analysis of tears to identify any lipid mediators of inflammation (eicosanoids, isoprostanes and others) that may be found in the tear samples using the SCIEX TripleTOF 5600 mass spectrometer. Since these non-targeted experiments could not identify any of the desired lipid inflammatory mediators, a final targeted analysis of tears was done using solid-phase extraction and LC-ESI-MS/MS with the SCIEX Qtrap 6500 mass spectrometer to identify and quantify prostaglandins (PGE2, PGF2α) and isoprostanes (8-iso-15(R)-PGF2α, 8-iso-PGF2α, 15(R)-PGF2α, and 2,3-dinor-8-iso-PGF2α) that have been shown to play a role in many inflammatory conditions. A comparison of the extraction and mass spectrometry methods used for non-targeted analysis of tears and their limitations in detecting lipid inflammatory mediators are discussed below. Limits of detection for four lipid mediator standards (PGE2, PGF2α, TXB2 and LTB4) were calculated and were found to be higher than the limits of detection in three previous studies that looked at similar molecules in tears, albeit in much larger volumes.22, 45, 46 Limits of detection for isoprostanes in tears have been reported for the first time in this study. The features of targeted analysis of tears and results are also discussed.
Several factors related to tear samples, extraction approach and the mass spectrometry methods might have led to the observed results while using the SCIEX Triple TOF 5600 instrument. The tear-related factors include volume of collected tears, method of tear collection and type of tear collected. A volume of 10 µl tears was collected from each eye of enrolled subjects, that were analyzed using this instrument, which is different from other studies looking at lipid mediators of inflammation where 40 to 100 µl tears were collected.22, 45, 46 Investigators in these aforementioned studies have either collected tears multiple times and pooled them or bought larger volume of tears from a commercial company, although details of this are not clear.22, 45, 46 It is tedious to collect a high volume of tears at one point of time and also uncomfortable to tear donors, with reflex tearing very likely. Pooling of tears collected at different time points and in different environments may lead to an inconsistent molecular profile as the concentration of various molecules in the tears may change throughout the day and at environments with different temperatures and humidities.51 The method of tear collection and the type of tear collected can also play a role in the detection and quantification of lipid species. Capillary collection of basal tears has been recommended for lipid species identification in tears as the reflex tears and tear-saline mixture do not best represent the lipid molecules present in a normal tear film.52 Glass microcapillaries were used in this study to collect basal tears while fiber rods have been used to collect reflex tears in a study by Shim et al.22 A mixture of tears and saline solution has also been used for identifying some lipid mediators of inflammation.45
The extraction approach related factors for not detecting the desired lipid mediators of inflammation using the SCIEX Triple TOF 5600 mass spectrometer include the inclusion/exclusion of extraction in the sample preparation procedure, type of extraction done and the drying method for concentration of sample. The extraction approach used in the present study differed from other studies that looked at lipid mediators of inflammation in tears.22, 45, 46 Liquid Chromatography-Mass Spectrometry without extraction was performed in the study by Shim et al.22 A liquid-liquid extraction with either methanol and formic acid or with chloroform, methanol and water was done in this study. Extraction not only separates the desired molecules from matrix but also helps in removal of unwanted contaminants thus lowering the matrix effect. The second extraction method utilized in this study gave cleaner spectra with detection of many lipids. Thus, it is better to add an extraction method, even when various molecules are separated with LC-MS, prior to mass spectrometry. Methanol and formic acid were used in this study for extraction while solid phase extraction was utilized by English et al., in their study.46 Solid phase extraction with a reverse phase has been used to extract eicosanoids in body fluid and tissues but it requires a large volume of sample to pass through the column to extract the required molecules.31, 53, 54When solid phase extraction was done in this study with 10 µl of PGE2 standard in acidified methanol and weaker signal (peak height of the specific m/z spectrum reduced) were observed when compared to direct infusion of the same standard (unpublished data). This reduction may be due to sticking of some amount of the standard to the walls of the extraction tubes or the column itself. Thus, when a low volume sample like 10 µl is available it may be better to directly infuse the sample into the mass spectrometer rather than pass it through an extraction column. In the study by Walter et al., methanol was used for extraction and the resultant mixture was dried under nitrogen before suspending it into the HPLC mobile phase.45 Acidified methanol and HPLC has been used in one of the methods in the current study but the drying was done using an eppendorf vacufuge. Vacuum drying has been suggested for eicosanoids and the results have been shown to be at par with drying under nitrogen.55
The mass spectrometry related factors include the sensitivity of mass spectrometer and the analytical approach used, which might have played a role in our inability to detect these molecules in low volume samples when analyzing them with the SCIEX Triple TOF 5600 instrument. A Triple TOF instrument was used in this study while studies of tears for similar lipid mediators of inflammation have used Qtrap instruments that have higher sensitivities compared to the Triple TOF instruments. 22, 45, 46 The limits of detection in this study was also found to be higher for the three representative lipid mediators of inflammation (10pg/µl, 1pg/µl and 10 pg/µl for PGE2, TXB2 and LTB4) than in another study (0.100 pg/µl, 0.200 pg/µl and 0.200 pg/µl).46 An untargeted approach was used in this study while other studies used a targeted approach in multiple reaction monitoring (MRM) mode.22, 45, 46
Since any of the desired lipid mediators of inflammation were not observed using the liquid-liquid extraction and SCIEX Triple TOF 5600 mass spectrometer, an approach similar to other studies in the literature using solid-phase extraction and a Qtrap mass spectrometer in the MRM mode was used in this study.22, 45, 46 A published method that was used to identify prostaglandins and isoprostanes in urine samples was adapted for tear samples.49 Since this method required larger sample volume, 20 µl of tears were collected from each eye of the two contact lens wearing subjects without discomfort and pooled together to give 40 µl. Same volume was collected from the two contact lens wearing subjects with discomfort and the tears from two eyes were pooled together for extraction and analysis.
The Sciex 6500 Qtrap method had a comparable sensitivity to that of commercial assays, with limits of detection of 5–10 pg/ml. PGE2 was identified in both contact lens wearers with and without discomfort. It has been found in tears of subjects with dry eyes but had not been examined in tears of CLD subjects.22, 45 PGF2α and its metabolites 8-iso-15(R)-PGF2α, 8-iso-PGF2α, 15(R)-PGF2α, and 2,3-dinor-8-iso-PGF2α were identified in all cases and controls. This has not been observed in tears in any other studies as far as our knowledge goes. F2-isoprostanes have been detected in human retina and aqueous humor of glaucoma patients.56, 57 Their presence in tears indicates oxidative stress and possible sub-clinical inflammation in ocular surface and during contact lens related discomfort. Since this was a pilot study and only two samples were compared, a larger sample size will be necessary to confirm the quantitative results.
Although lipid mediators of inflammation were not observed in this study using the SCIEX Triple TOF 5600 mass spectrometer, various fatty acids were observed. The major free fatty acids (Table 1 and Figure 5) that were found in this study were similar to the ones that have been found in human meibum by other investigatigators.58,59 As tears were evaluated in this study and not meibum, the number of free fatty acids found were less in comparison to a study by Chen et al.59 A class of fatty acids that has gained more attention recently in lipidomic studies of the tear film are the (O-acyl)-ω-hydroxy fatty acids that were also identified in the present research.60, 61 These fatty acids are thought to have a role in allowing the interaction of the non-polar lipid layer with the aqueous layer of the tear film and in reducing evaporation.62
Conclusions
Liquid-liquid extraction using 80% methanol and 0.1% formic acid may be suitable for extraction of lipids in low volume of tears. Solid phase extraction may be the method of choice for extraction of prostaglandins and isoprostanes in tears for volumes as low as 40 µl. The SCIEX Qtrap 6500 in MRM mode may be more suitable to identify and quantify specific lipid mediators of inflammation when used in conjunction with solid phase extraction. The SCIEX Triple TOF 5600 mass spectrometer may not be sensitive enough, under the given experimental conditions, for identification of lipid mediators in tears although it could be used for identification of major lipid classes found in tears. Further analysis with larger sample sizes may be considered to quantify these mediators.
Supplementary Material
Mass spectra for tears extracted using the Folch method and analyzed using (time-of-flight) – mass spectrometry (TOF-MS). A. Extracted Ion Chromatogram (XIC) for all lipid species. B. Spectrum for cholesteryl esters observed during MS/MSall in positive-ion mode. C. Spectrum for wax esters observed during MS/MSall in positive-ion mode. D. Spectrum for phospholipids observed during MS/MSall in positive-ion mode.
Acknowledgements
The results of this study were presented in part at the Association for Research in Vision and Ophthalmology Annual Meeting, Seattle, Washington, May 1 – 5, 2016.
The authors would like to thank Stephen Barnes, PhD (Director, Targeted Metabolomics and Proteomics Laboratory (TMPL), UAB School of Medicine) for his expert opinion and helpful discussions, Alireza Arabshahi and Taylor Berryhill (TMPL) for their help in analyzing samples using the MRM method.
Purchase of the mass spectrometers in the TMPL came from funds provided by the following NCRR grants: AB Sciex 6500 – UAB Health Services Foundation General Endowment Fund, AB Sciex 5600 TripleTOF – S10 RR027822–01. Funds for the operation of the TMPL come in part from the UAB O’Brien Acute Kidney Injury Center (P30 DK079337).
Grant Support: Supported in part by the UAB Vision Science Research Center Core Grant NIH P30 EY003039.
Footnotes
Commercial Relationship Disclosures: The authors have no proprietary or commercial interests in any concept or product discussed in this article.
References
- 1.Tilley SL, Coffman TM, Koller BH. Mixed messages: Modulation of inflammation and immune responses by prostaglandins and thromboxanes. Journal of Clinical Investigation 2001;108:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. New England Journal of Medicine 1990;323:645–655. [DOI] [PubMed] [Google Scholar]
- 3.Basu S Bioactive eicosanoids: role of prostaglandin F(2alpha) and F(2)-isoprostanes in inflammation and oxidative stress related pathology. Molecules and cells 2010;30:383–391. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res 2008;47:147–155. [DOI] [PubMed] [Google Scholar]
- 5.Dennis EA, Cao J, Hsu YH, et al. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev 2011;111:6130–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol 2015;15:511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milne GL, Musiek ES, Morrow JD. F2-Isoprostanes as markers of oxidative stress in vivo: An overview. Biomarkers 2005;10:10–23. [DOI] [PubMed] [Google Scholar]
- 8. Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free radical biology & medicine 2000;28:505–513. [DOI] [PubMed] [Google Scholar]
- 9.Evans AR, Junger H, Southall MD, et al. Isoprostanes, novel eicosanoids that produce nociception and sensitize rat sensory neurons. The Journal of pharmacology and experimental therapeutics 2000;293:912–920. [PubMed] [Google Scholar]
- 10.Bhattacherjee P. Prostaglandins and inflammatory reactions in the eye. Methods Find Exp Clin Pharmacol 1980;2:17–31. [PubMed] [Google Scholar]
- 11.Bhattacherjee P The role of arachidonate metabolites in ocular inflammation. Prog Clin Biol Res 1989;312:211–227. [PubMed] [Google Scholar]
- 12.Bito LZ. Prostaglandins and other eicosanoids: their ocular transport, pharmacokinetics, and therapeutic effects. Trans Ophthalmol Soc U K 1986;105 ( Pt 2):162–170. [PubMed] [Google Scholar]
- 13.Laatikainen L [Prostaglandins in opthalmology]. Duodecim 1974;90:1199–1201. [PubMed] [Google Scholar]
- 14.Liclican EL, Gronert K. Molecular circuits of resolution in the eye. ScientificWorldJournal 2010;10:1029–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mihail S [Leukotrienes and the eye]. Rev Chir Oncol Radiol O R L Oftalmol Stomatol Ser Oftalmol 1989;33:15–16. [PubMed] [Google Scholar]
- 16.Naveh-Floman N, Moisseiev J. Prostanoids and thromboxane A2 involvement in diabetic retinopathy. Metab Pediatr Syst Ophthalmol 1982;6:321–325. [PubMed] [Google Scholar]
- 17.Nelson EL. Prostaglandins and inflammation in the eye. Mod Probl Ophthalmol 1976;16:125–130. [PubMed] [Google Scholar]
- 18.Rosenberg ES, Asbell PA. Essential fatty acids in the treatment of dry eye. Ocul Surf 2010;8:18–28. [DOI] [PubMed] [Google Scholar]
- 19.Waitzman MB, Colley AM. Prostaglandins in ocular pathophysiology with special reference to diabetic retinopathy and glaucoma. Metab Pediatr Syst Ophthalmol 1983;7:7–23. [PubMed] [Google Scholar]
- 20.Whitelocke RA, Eakins KE, Bennett A. Acute anterior uveitis and prostaglandins. Proc R Soc Med 1973;66:429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodward DF, Regan JW, Lake S, et al. The molecular biology and ocular distribution of prostanoid receptors. Surv Ophthalmol 1997;41 Suppl 2:S15–21. [DOI] [PubMed] [Google Scholar]
- 22.Shim J, Park C, Lee HS, et al. Change in prostaglandin expression levels and synthesizing activities in dry eye disease. Ophthalmology 2012;119:2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masoudi S, Zhao Z, Stapleton F, et al. Contact Lens-Induced Discomfort and Inflammatory Mediator Changes in Tears. Eye Contact Lens 2017;43:40–45. [DOI] [PubMed] [Google Scholar]
- 24.Glasson M, Stapleton F, Willcox M. Lipid, lipase and lipocalin differences between tolerant and intolerant contact lens wearers. Curr Eye Res 2002;25:227–235. [DOI] [PubMed] [Google Scholar]
- 25.Basu S F2-isoprostanes in human health and diseases: from molecular mechanisms to clinical implications. Antioxid Redox Signal 2008;10:1405–1434. [DOI] [PubMed] [Google Scholar]
- 26.Tsukamoto H, Hishinuma T, Mikkaichi T, et al. Simultaneous quantification of prostaglandins, isoprostane and thromboxane in cell-cultured medium using gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2002;774:205–214. [DOI] [PubMed] [Google Scholar]
- 27.Waddington E, Sienuarine K, Puddey I, et al. Identification and quantitation of unique fatty acid oxidation products in human atherosclerotic plaque using high-performance liquid chromatography. Anal Biochem 2001;292:234–244. [DOI] [PubMed] [Google Scholar]
- 28.Yue H, Strauss KI, Borenstein MR, et al. Determination of bioactive eicosanoids in brain tissue by a sensitive reversed-phase liquid chromatographic method with fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci 2004;803:267–277. [DOI] [PubMed] [Google Scholar]
- 29.Honda H, Shibusawa Y, Taniguchi J, et al. Rapid and simple determination of epoxyeicosatrienoic acids in rabbit renal artery by reversed-phase HPLC with fluorescence detection. Vascul Pharmacol 2005;42:163–169. [DOI] [PubMed] [Google Scholar]
- 30.Stenson WF. Measurement of prostaglandins and other eicosanoids. Curr Protoc Immunol 2001;Chapter 7:Unit 7 33. [DOI] [PubMed] [Google Scholar]
- 31.Kortz L, Dorow J, Ceglarek U. Liquid chromatography-tandem mass spectrometry for the analysis of eicosanoids and related lipids in human biological matrices: a review. J Chromatogr B Analyt Technol Biomed Life Sci 2014;964:1–11. [DOI] [PubMed] [Google Scholar]
- 32.Unterwurzacher I, Koal T, Bonn GK, et al. Rapid sample preparation and simultaneous quantitation of prostaglandins and lipoxygenase derived fatty acid metabolites by liquid chromatography-mass spectrometry from small sample volumes. Clin Chem Lab Med 2008;46:1589–1597. [DOI] [PubMed] [Google Scholar]
- 33.Xu YJ, Ho WE, Xu F, et al. Exploratory investigation reveals parallel alteration of plasma fatty acids and eicosanoids in coronary artery disease patients. Prostaglandins Other Lipid Mediat 2013;106:29–36. [DOI] [PubMed] [Google Scholar]
- 34.Maddipati KR, Zhou SL. Stability and analysis of eicosanoids and docosanoids in tissue culture media. Prostaglandins Other Lipid Mediat 2011;94:59–72. [DOI] [PubMed] [Google Scholar]
- 35.Massey KA, Nicolaou A. Lipidomics of oxidized polyunsaturated fatty acids. Free radical biology & medicine 2013;59:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strassburg K, Huijbrechts AM, Kortekaas KA, et al. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis: application in cardiac surgery. Anal Bioanal Chem 2012;404:1413–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neale JR, Dean BJ. Liquid chromatography-tandem mass spectrometric quantification of the dehydration product of tetranor PGE-M, the major urinary metabolite of prostaglandin E(2) in human urine. J Chromatogr B Analyt Technol Biomed Life Sci 2008;871:72–77. [DOI] [PubMed] [Google Scholar]
- 38.Deems R, Buczynski MW, Bowers-Gentry R, et al. Detection and quantitation of eicosanoids via high performance liquid chromatography-electrospray ionization-mass spectrometry. Methods Enzymol 2007;432:59–82. [DOI] [PubMed] [Google Scholar]
- 39.Montuschi P, Martello S, Felli M, et al. Ion trap liquid chromatography/tandem mass spectrometry analysis of leukotriene B4 in exhaled breath condensate. Rapid Commun Mass Spectrom 2004;18:2723–2729. [DOI] [PubMed] [Google Scholar]
- 40.Dahl SR, Kleiveland CR, Kassem M, et al. Determination of thromboxanes, leukotrienes and lipoxins using high-temperature capillary liquid chromatography-tandem mass spectrometry and on-line sample preparation. J Chromatogr A 2009;1216:4648–4654. [DOI] [PubMed] [Google Scholar]
- 41.Kortz L, Dorow J, Becker S, et al. Fast liquid chromatography-quadrupole linear ion trap-mass spectrometry analysis of polyunsaturated fatty acids and eicosanoids in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2013;927:209–213. [DOI] [PubMed] [Google Scholar]
- 42.Ferreiro-Vera C, Priego-Capote F, Luque de Castro MD. Integrated identification/confirmatory and targeted analysis of epoxyeicosatrienosic acids in human serum by LC-TOF MS and automated on-line SPE-LC-QqQ MS/MS. Talanta 2013;106:440–447. [DOI] [PubMed] [Google Scholar]
- 43.Masoodi M, Eiden M, Koulman A, et al. Comprehensive lipidomics analysis of bioactive lipids in complex regulatory networks. Anal Chem 2010;82:8176–8185. [DOI] [PubMed] [Google Scholar]
- 44.Masoudi S, Zhao Z, Stapleton F, et al. Contact Lens-Induced Discomfort and Inflammatory Mediator Changes in Tears. Eye Contact Lens 2016. [DOI] [PubMed] [Google Scholar]
- 45.Walter SD, Gronert K, McClellan AL, et al. omega-3 Tear Film Lipids Correlate With Clinical Measures of Dry Eye. Invest Ophthalmol Vis Sci 2016;57:2472–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.English JT, Norris PC, Hodges RR, et al. Identification and Profiling of Specialized Pro-Resolving Mediators in Human Tears by Lipid Mediator Metabolomics. Prostaglandins Leukot Essent Fatty Acids 2017;117:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Grauw JC, van de Lest CH, van Weeren PR. A targeted lipidomics approach to the study of eicosanoid release in synovial joints. Arthritis Res Ther 2011;13:R123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 49.Prasain JK, Arabshahi A, Taub PR, et al. Simultaneous quantification of F2-isoprostanes and prostaglandins in human urine by liquid chromatography tandem-mass spectrometry. Journal of chromatography B, Analytical technologies in the biomedical and life sciences 2013;0:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore C, Li D, Liu A, et al. Improved Sensitivity for the Selective Quantitation of LTB4 in Human Plasma and Sputum via UPLC-MS/MS., AAPS Annual Meeting 2014. San Diego, CA; 2014. [Google Scholar]
- 51.Benito MJ, González-García MJ, Tesón M, et al. Intra- and inter-day variation of cytokines and chemokines in tears of healthy subjects. Experimental Eye Research 2014;120:43–49. [DOI] [PubMed] [Google Scholar]
- 52.Rohit A, Stapleton F, Brown SH, et al. Comparison of tear lipid profile among basal, reflex, and flush tear samples. Optom Vis Sci 2014;91:1391–1395. [DOI] [PubMed] [Google Scholar]
- 53.Puppolo M, Varma D, Jansen SA. A review of analytical methods for eicosanoids in brain tissue. J Chromatogr B Analyt Technol Biomed Life Sci 2014;964:50–64. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki N Mass spectrometry-based quantitative analysis and biomarker discovery. Yakugaku Zasshi 2011;131:1305–1309. [DOI] [PubMed] [Google Scholar]
- 55.Sharma M, Hucke B, Lianos EA. Separation of Hydroxyeicosatetraenoic Acids and Leukotrienes Originating from Kidney Tissue by C-18 Reverse-Phase High-Pressure Liquid Chromatography. In: Lianos EA (ed), Eicosanoid Protocols; Totowa, NJ: Humana Press; 1999:45–54. [DOI] [PubMed] [Google Scholar]
- 56.Nourooz-Zadeh J, Pereira P. F2 Isoprostanes, Potential Specific Markers of Oxidative Damage in Human Retina. Ophthalmic Research 2000;32:133–137. [DOI] [PubMed] [Google Scholar]
- 57.Koliakos GG, Konstas AGP, Schlötzer-Schrehardt U, et al. 8-Isoprostaglandin F<sub>2a</sub> and ascorbic acid concentration in the aqueous humour of patients with exfoliation syndrome. British Journal of Ophthalmology 2003;87:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nichols KK, Ham BM, Nichols JJ, et al. Identification of Fatty Acids and Fatty Acid Amides in Human Meibomian Gland Secretions. Investigative Ophthalmology & Visual Science 2007;48:34–39. [DOI] [PubMed] [Google Scholar]
- 59.Chen J, Green-Church KB, Nichols KK. Shotgun Lipidomic Analysis of Human Meibomian Gland Secretions with Electrospray Ionization Tandem Mass Spectrometry. Invest Ophthalmol Vis Sci 2010;51:6220–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J, Green-Church KB, Nichols KK. Shotgun lipidomic analysis of human meibomian gland secretions with electrospray ionization tandem mass spectrometry. Invest Ophthalmol Vis Sci 2010;51:6220–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Butovich IA, Wojtowicz JC, Molai M. Human tear film and meibum. Very long chain wax esters and (O-acyl)-omega-hydroxy fatty acids of meibum. J Lipid Res 2009;50:2471–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schuett BS, Millar TJ. An investigation of the likely role of (O-acyl) omega-hydroxy fatty acids in meibomian lipid films using (O-oleyl) omega-hydroxy palmitic acid as a model. Exp Eye Res 2013;115:57–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mass spectra for tears extracted using the Folch method and analyzed using (time-of-flight) – mass spectrometry (TOF-MS). A. Extracted Ion Chromatogram (XIC) for all lipid species. B. Spectrum for cholesteryl esters observed during MS/MSall in positive-ion mode. C. Spectrum for wax esters observed during MS/MSall in positive-ion mode. D. Spectrum for phospholipids observed during MS/MSall in positive-ion mode.


