Abstract
The epsilon wave of the electrocardiogram (ECG) together with fragmented QRS (fQRS), the terminal conduction delay, incomplete right bundle branch block (IRBBB) and complete/advanced RBBB (CRBBB) of peripheral origin are part of a spectrum of ventricular depolarization abnormalities of arrhythmogenic cardiomyopathy(AC). Although the epsilon wave is considered a major diagnostic criterion for AC since 2010 (AC Task Force Criteria), its diagnostic value is limited because it is a sign of the later stage of the disease. It would be more appropriate to say that the epsilon wave is a “hallmark” of AC, but is of low diagnostic sensitivity. Although the epsilon wave has high specificity for AC, it can be present in other pathological conditions. In this update we will cover the nomenclature, association with disease states and electrocardiographic aspects of the epsilon wave.
Keywords: Epsilon wave, Ventricular post-excitation wave, Fontaine wave
1. Introduction
1.1. Epsilon wave definition
The epsilon (ε) wave can be defined as an electric signal of depolarization observed between the end of the QRS complex and the beginning of the T wave. The ε wave is found in the right precordial leads, where the QRS complex is broader than the in the left precordial leads (difference ≥25 ms) in arrhythmogenic cardiomyopathy (AC). In patients with AC, who have left ventricular (LV) involvement, the ε wave can be registered in the left and/or inferior leads. The ε wave represents delayed potentials resulting from slow intraventricular conduction due to islands of surviving myocardium interspersed with fatty and fibrous tissue.
This ventricular post-excitation wave consists of a slurring at the end of the QRS complex or an independent potential/s after the return to the isoelectric line. The depolarization abnormality is hardly detectable by the standard 12-lead ECG (S-12-ECG) [1].
Because the ε wave is of low amplitude, it may be affected by ECG filter settings. At the recommended 150-Hz cutoff frequency the ε wave is best detected in the right precordial leads. Currently ECG guidelines recommend a cutoff of 150 Hz for adolescents and adults and 250 Hz for children [2]. The ECG acquisition is often accompanied by high-frequency electromyographic noise. The noise is difficult to filter due to considerable overlapping of its frequency spectrum with the frequency spectrum of the ECG. In clinical practice a 40-Hz cutoff frequency may be used to reduce muscle noise and improve the appearance of the tracing. This approach results in the loss of important information, which was demonstrated in a case report, where ε waves were masked by excessive low-pass filtering in a patient with AC [3] (Fig. 1). Therefore, it is possible that the prevalence of the ε wave in AC patients may be underestimated.
Fig. 1.
ε wave only observed with 150 Hz filter. Low-pass filter cutoff frequency influences the detection of the ε wave in AC: at the recommended 150 Hz cutoff frequency, the ε wave is detected in leads V1-V3. At a 100 Hz cutoff frequency, the ε wave is attenuated in V1-V2 and absent in V3. At 40 Hz, the ε wave disappears from leads V1-V3 (modified from Ref. [3]).
AC can no longer be regarded as an isolated disease of the right ventricle (RV). T-wave inversion in the lateral leads and premature ventricular complexes (PVCs) of LV origin help to identify LV involvement. Timing of the ε wave corresponded to activation of the sub-tricuspid region in patients, who underwent endocardial and epicardial electro-anatomical activation mapping in sinus rhythm [4]. It can also be an aid to the diagnosis of patients with AC, who have other signs or symptoms suggesting the disease, including episodes of myocarditis.
Other terminologies for the epsilon are: ε potentials [5], ventricular post-excitation waves, post-excitation (ε) waves [6], “double potential” [7] or with the merited eponymous Fontaine wave [8].
1.2. Historical aspects
The first recordings of the ε wave were reported by Fontaine et al. in the 1970's [9], and four years later Fontaine called it an ε wave. The reason that led Dr. Fontaine to choose ε is not clear. It could be that its shape reminded him of the Greek letter epsilon (ε) as suggested by Surawicz and Knilans in their classical book on electrocardiography [10]. If so, it should be stated that the ε-like wave is in a horizontal position. Dr. Fontaine considered the letter sequence D, E, G and H in the Greek alphabet. If the additional wave located at the beginning of QRS complex in ventricular pre-excitation is called a delta (δ) wave, the following additional wave in the Greek enumeration could be named according to the alphabetical sequence as an ε wave. Faced with this uncertainty, we decided to ask the author/creator of this nomenclature, Dr. Fontaine, who replied: “The naming of the ECG waves and the reason of their choice is a long story. Dr. Willis Hurst [8] in Circulation published a summary of the naming of the Epsilon waves some years ago. I contributed to do this paper as indicated by Dr. Hurst."
Dr. Hurst wrote: “Fontaine discovered and named the ε waves. He chose the ε because it follows delta (δ) in the Greek alphabet and is the mathematical symbol for smallness.” The term "ε" was nice, because it occurs in the Greek alphabet after δ; thus, this letter represents the pre-excitation and ε the post-excitation phenomenon. In addition, ε is also used in mathematics to express a very small phenomenon. Late potentials (LPs) located on the free wall of the RV of patients with AC could be recorded on the body surface by signal-averaged ECG (SAECG) and in some circumstances by increasing the magnification of the standard ECG recording.
1.3. Prevalence of the ε wave
The ECG criteria mentioned in the 2010 AC Task Force Criteria (TFC), namely a low amplitude signal occurring after the QRS complex and before the onset of the T wave in the right precordial leads V1-V3 is only present in a minority of patients with AC. The ε wave as defined by these criteria is observed at advanced AC disease stages [11] and represent late delayed activation of the epicardial right ventricular outflow tract (RVOT) in the peritricuspid region [12].
QRS prolongation >110 ms and an ε wave are strongly indicative of an intraventricular conduction delay [13]. The ε wave is considered an ECG major criterion for the diagnosis of AC with high specificity [14]. Unfortunately, it is an insensitive sign [15]. This cardiomyopathy was reported as the second most common cause of unexpected sudden cardiac death (SCD) among young competitive athletes (aged 12–35 years old) after hypertrophic obstructive cardiomyopathy [16]. In Europe mainly in the Veneto area in Italy and the Greek island Naxos (recessive form) where the entity is endemic, it may even be the most common cause of death in young athletes.
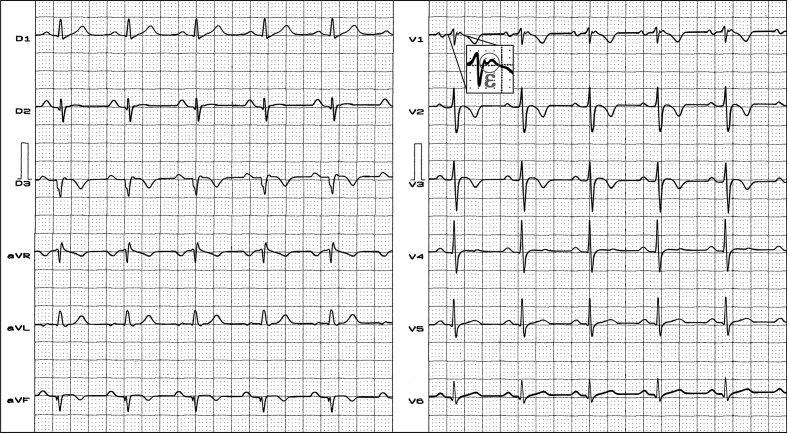
Because the ε wave may be difficult to detect with the S-12-ECG, modified methods for better detection were introduced. The Fontaine bipolar precordial lead ECG (F-ECG) placement is a modified ECG recording, where the left arm electrode is placed on the xiphoid process, the right arm electrode on the manubrium, and the left leg electrode in the location of V4 [17]. The lead placement for the additional right-sided leads (R-ECG) and the F-ECG is demonstrated in Fig. 2. In S-12-ECG, R-ECG and F-ECG, the detection rate of the ε wave is ≈ 15–30%, ≈37%, and ≈57% respectively. The combination of S-ECG, F-ECG, R-ECG and 16-lead high-definition ECG (HD-ECG) in AC [18] was more sensitive than S-12-ECG to record depolarization abnormalities. High anterior lead positioning may increase the detection rate of the ε wave in patients with AC [19]. Additionally, SAECG may help to disclose fragmented low amplitude LPs after the end of the QRS complex [20]. In comparison to S-12-ECG, HD-ECG detects abnormalities at higher rates in carriers of AC (57% vs. 86%). HD-ECG is more sensitive as a screening test than S-12-ECG to detect patients with AC [21]. HD-ECG could even be considered a standard test for the diagnostic evaluation in patients suspected of AC [22].
Fig. 2.
Clinical diagnosis: cardiac sarcoidosis. ECG diagnosis: frontal plane QRS axis −60°, negative T wave from V1 to V3, ε wave in V1.
1.4. Ventricular arrhythmias
Three-dimensional electroanatomical endocardial and epicardial mapping studies for ablation of ventricular tachycardia (VT) in AC showed that the ε wave indicates a delayed depolarization of myocardial tissue, which in AC corresponds to the substrate of fibro-fatty infiltration within the RV. The ε wave is a marker of poor prognosis in AC [23]. VT or ventricular fibrillation (VF) may be easily triggered with electrophysiologic testing. Marstrand et al. studied 42 patients admitted with VT or VF, who had undergone both SAECG recording and cardiovascular magnetic resonance imaging (CMRI). Clinical data and CMRI findings were compared in patients with and without LPs. The majority, 26 (62%) patients, were survivors of sudden cardiac death and the remaining 16 (38%) were admitted with VT. After complete diagnostic evaluation, the most common diagnoses in the cohort were idiopathic VT/VF (60%) or cardiomyopathies (26%). There were no significant differences in RV size relative to body surface area (102 ml/m2 vs 92 ml/m2), RV ejection fraction (55% vs 58%), or positive late gadolinium enhancement (29% vs 24%). LPs were present in 69% when using the revised TFC criteria.
The ε wave is a major diagnostic criterion for AC, but remains non-quantifiable, and therefore may leave room for substantial subjective interpretation [11,24]. The presence of ε waves on the S-12-ECG reflects significant RV outflow tract involvement, which is associated with episodes of sustained VT but not with sudden cardiac death. It is possible that lead location of the ε wave may have prognostic significance. Lead aVR ε wave may be a marker of poor prognosis [23,25]. It should be pointed out that the ε wave is only one ECG manifestation of AC [26].
1.5. Possible causes of ε waves in the ECG
1.5.1. Possible physiological ε waves
In a study with elite endurance athletes (190 senior and 157 junior athletes), an ε wave was found in 3/190 senior athletes (1.57%) and in 1/189 individuals from a sedentary control group 31–40 years of age [27]. CMRI showed AC findings in one of the senior athletes.
1.5.2. Pathological ε waves in patients other than in AC
-
a.
Coronary artery disease: the ε wave has been observed in one case of acute inferior myocardial infarction (MI) associated with RV myocardial infarction [28].
-
b.
Uhl's anomaly or “parchment heart”: is an unusual myocardial abnormality first described by Henry Uhl in 1952 [29]. It is characterized by partial or complete absence of the RV myocardium, with severe RV systolic and diastolic impairment. Patients with Uhl's anomaly, who survive to adulthood, may develop right-sided heart failure or arrhythmias [30]. The ECG shows tall and wide P waves, right axis deviation, frequent RBBB, prominent ε waves in all QRS complexes, and signs of severe dilatation of the RV and right atrium.
-
c.
After repair of Fallot's tetralogy [31]: a case report described a patient with tetralogy of Fallot, who showed all features of the familial form of RV, including an ε wave. The patient had heart transplantation because of numerous episodes of ventricular tachycardia, and chronic heart failure, and he had a right ventricular outflow patch aneurysm.
-
d.
Infiltrative diseases: cardiac sarcoidosis may cause the pathological substrate required for production of ε waves [32]. Therefore, differentiating AC from cardiac sarcoidosis is of clinical importance [33]. Fig. 2 shows a single-lead ε wave in a patient with cardiac sarcoidosis.
-
e.
Sickle cell anemia [8]: JW Hurst briefly mentions a probably unpublished observation of epsilon waves in a patient with sickle cell disease with RV hypertrophy due to pulmonary arterial hypertension.
-
f.
Brugada syndrome (BrS): it is believed that BrS and AC are different clinical entities with respect to the clinical presentation and the genetic predisposition. The coexistence of these two relatively rare clinical entities has been reported [34]. There may be cases where the differential diagnosis is not clear [35]. ε waves appear to be rare in BrS, and were found in 2 of 47 patients by Letsas et al. [36], and in 1 of a total of 12 unrelated index BrS cases included in the study by Yu et al. [37].
1.6. New names for the identification of depolarization abnormalities?
Initially, depolarization abnormalities occurring at the beginning, inside or at the end of the QRS complex were classified as ε waves [26]. These changes could also be defined as QRS fragmentation. Later on, it was considered preferable to use the term ε wave only for LPs occurring after the QRS for clinical use. Li et al. proposed to use the terms Presilon, Topsilon and Postsilon for depolarization abnormalities occurring at the beginning, in the top of and at the end of the QRS complex, respectively [26] (Fig. 3).
Fig. 3.
The figure shows the three possibilities of fragmented QRS in AC: at the beginning (presilon), in the middle (topsilon) and at the end (postsilon) of the QRS complex, and when the ε wave is located after the J-point and the beginning of the ST-segment. Although the ε wave is a depolarization abnormality (late potential), it is recorded at the beginning of repolarization.
Note: Our concern with regard to the ε wave is how to differentiate this phenomenon from QRS fragmentation. It is clear if there is a space between the end of the QRS complex and the ε waves especially in V1-V3. However, how can one differentiate an ε wave from fractionated potentials within the QRS? New studies are necessary to clarify this problem.
1.7. Triggers for the appearance of ε waves
-
a)
During exercise stress test in asymptomatic gene carriers: in asymptomatic AC gene carriers, ε waves were found to develop more frequently (4/28 = 14%) during exercise stress tests compared with healthy controls (0/30) [38]. Adler et al. showed to that ε waves may be uncovered during exercise in asymptomatic patients carrying mutations in the PKP2 gene. This finding suggests that the exercise stress test may increase the sensitivity for the diagnosis of AC and that exercise-induced ε waves may be found in various genetic subtypes of this disease [39].
-
b)
Ajmaline-induced ε wave: Therase et al. demonstrated that ajmaline challenge has a very high sensitivity to exclude the diagnosis of BrS. Conversely, a negative flecainide test does not eliminate the possibility of BrS inheritance and risk of sudden cardiac death. This may suggest systematic use of ajmaline drug challenge [40]. The elimination of the ECG type 1 Brugada ECG pattern with radiofrequency catheter ablation focused on the area of the free wall of the RV outflow tract epicardium supports the depolarization theory. Catheter ablation offers an alternative therapy for patients with BrS, especially when implantable cardioverter defibrillator shocks are encountered as a consequence of electrical storm. After this procedure, surviving cells surrounded by fibrosis were found to be responsible for late dromotropic disturbance (after the J point) and reentry in inhomogeneous scars detected by the intracardiac electrogram (IEGM) as delayed or isolated conduction, LPs or diastolic IEGMs indicative of ε waves [41]. The ajmaline these might unmask not only the type 1 Brugada ECG pattern, but also concealed slow late conduction features in risk stratification of BrS patients [35].
-
c)
Ablation-induced ε wave [41]: in a young man with AC, VT ablation was performed because of repeated shocks from an implantable cardioverter-defibrillator. Post-ablation new ε waves were evident in the right precordial leads, and they remained static at 12-month follow-up.
2. Highlights
Intrinsic features: ε waves consist of a slurring at the end of the QRS complex or an independent potential after the return to the isoelectric line [1].
Location in the ECG: after the QRS and before the T wave.
Lead locations: observed in right precordial leads. However, ε waves could be found in the leads of the frontal plane, especially in the inferior leads, when the LV is involved.
Frequency in AC: approximately 15–30% of cases in 12-lead S-12-ECG. This percentage increases with the use of modified ECG recordings, such as R-ECG, F-ECG, and HR-ECG (Fig. 4).
Fig. 4.
Leads I, II and III with S-ECG and F-ECG. Typical example of AC with LV involvement. Note the ε wave (arrows) observed only with F-ECG in the left (I) and inferior leads (II, III).
Value of criterion: the ε wave is considered as a major criterion for diagnosis by the 2010 TFC for AC diagnosis. However, the ε wave is a late manifestation of the disease and it is unlikely to contribute significantly to the diagnosis, because at the time when ε waves becomes apparent on the S-ECG, other AC manifestations are evident and are sufficient for establishing an AC diagnosis regardless of the ε wave [11].
Pathognomonic character: despite the characteristics of AC, ε waves are not pathognomonic, since they have been described in other physiological and pathological circumstances.
Meaning: late posterior potentials that occur in the RV free wall in patients with AC. Inversion of the T wave in leads V1-V3 and/or ε wave is found in 70% of patients with AC. Epicardial electrophysiological studies in dysplastic areas reveal that LPs occurring at the end of the QRS complex, at the J point, or at the onset of the ST segment, are explained by fibro-fatty substitution of myocardial tissue.
3. Conclusion
The ε wave belongs to the depolarization abnormality spectrum characteristic of AC. ε waves have high specificity but are not pathognomonic. The sensitivity of ε waves is low with S-12-ECG alone. If ε waves are suspected from the S-12-ECG, it is recommended to use R-ECG, F-ECG, 16-lead HD-ECG, as well as HR-ECG.
The ε wave is considered a major sign of AC according to the 2010 TFC. However, it is relatively rare and present in advanced cases. It is indicative of a worse prognosis. Since it is present in advanced disease of AC, the ε wave may contribute little to the diagnosis.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Fontaine G.H., Duthoit G., Li G., Andreoletti L., Gandjbakhch E., Frank R. Epsilon wave on an electronic loop in a case of arrhythmogenic right ventricular dysplasia with myocarditis: an updated definition of the Epsilon wave. Europace. 2017;19:1084–1090. doi: 10.1093/europace/euw320. [DOI] [PubMed] [Google Scholar]
- 2.Kligfield P., Gettes L.S., Bailey J.J. Recommendations for the standardization and interpretation of the electrocardiogram: part I: the electrocardiogram and its technology a scientific statement from the American heart association electrocardiography and arrhythmias committee, council on clinical cardiology; the American college of cardiology foundation; and the heart rhythm society endorsed by the international society for computerized electrocardiology. J Am Coll Cardiol. 2007;49:1109–1127. doi: 10.1016/j.jacc.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Niebla J., Baranchuk A., Bayes de Luna A. Epsilon wave in the 12-lead electrocardiogram: is its frequency underestimated? Rev Esp Cardiol (Engl Ed). 2016;69:438. doi: 10.1016/j.rec.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Tanawuttiwat T., Te Riele A.S., Philips B. Electroanatomic correlates of depolarization abnormalities in arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Cardiovasc Electrophysiol. 2016;27:443–452. doi: 10.1111/jce.12925. [DOI] [PubMed] [Google Scholar]
- 5.Peters S., Trummel M., Koehler B., Westermann K.U. The value of different electrocardiographic depolarization criteria in the diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Electrocardiol. 2007;40:34–37. doi: 10.1016/j.jelectrocard.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Okano Y. [Electrocardiographic findings in arrhythmogenic right ventricular dysplasia (ARVD) evaluated by body surface mapping] Nihon Rinsho. 1995;53:230–238. [PubMed] [Google Scholar]
- 7.Fontaine G., Guiraudon G., Frank R. Surgical management of ventricular tachycardia not related to myocardial ischemia. In: Josephson M.E., Wellens H.J.J., editors. Tachycardias: mechanism, diagnosis, treatment. Lea & Febiger; Philadelphia, PA: 1984. pp. 451–473. [Google Scholar]
- 8.Hurst J.W. Naming of the waves in the ECG, with a brief account of their genesis. Circulation. 1998;98:1937–1942. doi: 10.1161/01.cir.98.18.1937. [DOI] [PubMed] [Google Scholar]
- 9.Fontaine G., Frank R., Bonnet M., Cabrol C., Guiraudon G. [Experimental and clinical study of Wolff-Parkinson-White and myocardial ischemia syndromes by cartography of epicardial ventricular depolarization] Coeur Med Interne. 1973;12:105–113. [PubMed] [Google Scholar]
- 10.Surawicz B., Knilans T.K. fifth ed. WB Saunders Company; USA: 2001. Chou's Electrocardiography in clinical practice. Adult and pediatric. [Google Scholar]
- 11.Platonov P.G., Calkins H., Hauer R.N. High interobserver variability in the assessment of epsilon waves: implications for diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2016;13:208–216. doi: 10.1016/j.hrthm.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 12.Fontaine G., Guiraudon G., Frank R. Stimulation studies and epicardial mapping in ventricular tachycardia: study of mechanisms and selection for surgery. In: Kulbertus H., editor. Re-entrant arrhythmias : mechanisms and treatment. University Park Press; Baltimore, USA: 1977. pp. 334–350. [Google Scholar]
- 13.Fontaine G. Arrhythmogenic right ventricular dysplasia. Curr Opin Cardiol. 1995;10:16–20. doi: 10.1097/00001573-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Marcus F.I., McKenna W.J., Sherrill D. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nunes de Alencar Neto J., Baranchuk A., Bayes-Genis A., Bayes de Luna A. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: an electrocardiogram-based review. Europace. 2018;20:f3–f12. doi: 10.1093/europace/eux202. [DOI] [PubMed] [Google Scholar]
- 16.Maron B.J., Maron B.A. Revisiting athlete's heart versus pathologic hypertrophy: ARVC and the right ventricle. JACC Cardiovasc Imag. 2017;10:394–397. doi: 10.1016/j.jcmg.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottschalk B., Gysel M., Barbosa-Barros R. The use of fontaine leads in the diagnosis of arrhythmogenic right ventricular dysplasia. Ann Noninvasive Electrocardiol. 2014;19:279–284. doi: 10.1111/anec.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li G.L., Saguner A.M., Akdis D., Fontaine G.H. Value of a novel 16-lead High-Definition ECG machine to detect conduction abnormalities in structural heart disease. Pacing Clin Electrophysiol. 2018;41:643–655. doi: 10.1111/pace.13338. [DOI] [PubMed] [Google Scholar]
- 19.Wu S., Wang P., Hou Y., Yang P., Xiao Y., Zhan X. Epsilon wave in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Pacing Clin Electrophysiol. 2009;32:59–63. doi: 10.1111/j.1540-8159.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 20.Turrini P., Corrado D., Basso C., Nava A., Bauce B., Thiene G. Dispersion of ventricular depolarization-repolarization: a noninvasive marker for risk stratification in arrhythmogenic right ventricular cardiomyopathy. Circulation. 2001;103:3075–3080. doi: 10.1161/01.cir.103.25.3075. [DOI] [PubMed] [Google Scholar]
- 21.Sekiguchi K., Miya Y., Kaneko Y. Evaluation of signal-averaged electrocardiography for clinical diagnosis in arrhythmogenic right ventricular dysplasia. Jpn Heart J. 2001;42:287–294. doi: 10.1536/jhj.42.287. [DOI] [PubMed] [Google Scholar]
- 22.Nasir K., Rutberg J., Tandri H., Berger R., Tomaselli G., Calkins H. Utility of SAECG in arrhythmogenic right ventricle dysplasia. Ann Noninvasive Electrocardiol. 2003;8:112–120. doi: 10.1046/j.1542-474X.2003.08204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallo C., Blandino A., Giustetto C. Arrhythmogenic right ventricular cardiomyopathy: ECG progression over time and correlation with long-term follow-up. J Cardiovasc Med (Hagerstown). 2016;17:418–424. doi: 10.2459/JCM.0000000000000354. [DOI] [PubMed] [Google Scholar]
- 24.Saguner A.M., Ganahl S., Baldinger S.H. Usefulness of electrocardiographic parameters for risk prediction in arrhythmogenic right ventricular dysplasia. Am J Cardiol. 2014;113:1728–1734. doi: 10.1016/j.amjcard.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 25.Pei H., Yu Q., Su X. New features of electrocardiogram in a case report of arrhythmogenic right ventricular cardiomyopathy: a care-compliant article. Medicine (Baltim) 2016;95:e3442. doi: 10.1097/MD.0000000000003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G.L., Saguner A.M., Fontaine G.H., Frank R. Epsilon waves: milestones in the discovery and progress. Ann Noninvasive Electrocardiol. 2018;23(6) doi: 10.1111/anec.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macarie C., Stoian I., Dermengiu D. The electrocardiographic abnormalities in highly trained athletes compared to the genetic study related to causes of unexpected sudden cardiac death. J Med Life. 2009;2:361–372. [PMC free article] [PubMed] [Google Scholar]
- 28.Zorio E., Arnau M.A., Rueda J. The presence of epsilon waves in a patient with acute right ventricular infarction. Pacing Clin Electrophysiol. 2005;28:245–247. doi: 10.1111/j.1540-8159.2005.40021.x. [DOI] [PubMed] [Google Scholar]
- 29.Uhl H.S. A previously undescribed congenital malformation of the heart: almost total absence of the myocardium of the right ventricle. Bull Johns Hopkins Hosp. 1952;91:197–209. [PubMed] [Google Scholar]
- 30.Cooper J.M., Gerasimon G.G., Deyell M.W. Uhl's anomaly: cardiac features and ICD implantation. JACC Clin Electrophysiol. 2016;2:393–394. doi: 10.1016/j.jacep.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 31.George B.A., Ko J.M., Lensing F.D., Kuiper J.J., Roberts W.C. Repaired" tetralogy of fallot mimicking arrhythmogenic right ventricular cardiomyopathy (another phenocopy) Am J Cardiol. 2011;108:326–329. doi: 10.1016/j.amjcard.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 32.Santucci P.A., Morton J.B., Picken M.M., Wilber D.J. Electroanatomic mapping of the right ventricle in a patient with a giant epsilon wave, ventricular tachycardia, and cardiac sarcoidosis. J Cardiovasc Electrophysiol. 2004;15:1091–1094. doi: 10.1046/j.1540-8167.2004.03708.x. [DOI] [PubMed] [Google Scholar]
- 33.Khaji A., Zhang L., Kowey P., Martinez-Lage M., Kocovic D. Mega-epsilon waves on 12-lead ECG--just another case of arrhythmogenic right ventricular dysplasia/cardiomyopathy? J Electrocardiol. 2013;46:524–527. doi: 10.1016/j.jelectrocard.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Hoogendijk M.G. Diagnostic dilemmas: overlapping features of brugada syndrome and arrhythmogenic right ventricular cardiomyopathy. Front Physiol. 2012;3:144. doi: 10.3389/fphys.2012.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozeke O., Aras D., Cay S., Ozcan F., Acar B., Topaloglu S. Ajmaline-induced Epsilon wave: its role is not only for diagnosis but also for risk stratification. Int J Cardiol. 2018;264:99. doi: 10.1016/j.ijcard.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 36.Letsas K.P., Efremidis M., Weber R. Epsilon-like waves and ventricular conduction abnormalities in subjects with type 1 ECG pattern of Brugada syndrome. Heart Rhythm. 2011;8:874–878. doi: 10.1016/j.hrthm.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 37.Yu J., Hu J., Dai X. SCN5A mutation in Chinese patients with arrhythmogenic right ventricular dysplasia. Herz. 2014;39:271–275. doi: 10.1007/s00059-013-3998-5. [DOI] [PubMed] [Google Scholar]
- 38.Perrin M.J., Angaran P., Laksman Z. Exercise testing in asymptomatic gene carriers exposes a latent electrical substrate of arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2013;62:1772–1779. doi: 10.1016/j.jacc.2013.04.084. [DOI] [PubMed] [Google Scholar]
- 39.Adler A., Perrin M.J., Spears D., Gollob M.H. Epsilon wave uncovered by exercise test in a patient with desmoplakin-positive arrhythmogenic right ventricular cardiomyopathy. Can J Cardiol. 2015;31:819 e1–2. doi: 10.1016/j.cjca.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 40.Therasse D., Sacher F., Babuty D. Value of the sodium-channel blocker challenge in Brugada syndrome. Int J Cardiol. 2017;245:178–180. doi: 10.1016/j.ijcard.2017.05.099. [DOI] [PubMed] [Google Scholar]
- 41.Caldwell J., Redfearn D., Chiale P.A., Baranchuk A. Ablation-induced epsilon wave. Heart Rhythm. 2013;10:1737–1738. doi: 10.1016/j.hrthm.2012.08.038. [DOI] [PubMed] [Google Scholar]