Abstract
Background
The prognostic significance of paced QRS complex morphology on surface ECG remains unclear. This study aimed to assess long-term outcomes associated with variations in the paced QRS complex.
Methods
Adult patients who underwent dual-chamber pacemaker implantation with 20% or more ventricular pacing and a 12-lead ECG showing a paced complex were included. The paced QRS was analyzed in leads I and aVL. Long-term clinical and echocardiographic outcomes were compared at 5 years.
Results
The study included 844 patients (43.1% female; age 75.0 ± 12.1). Patients with a longer paced QRS (pQRS) duration in lead I had a lower rate of atrial fibrillation (HR 0.80; p = 0.03) and higher rate of systolic dysfunction (HR 1.17; p < 0.001). Total pacing complex (TPC) duration was linked to higher rates of ICD implantation (HR 1.18; p = 0.04) and systolic dysfunction (HR 1.22, p < 0.001). Longer paced intrinsicoid deflection (pID) was associated with less atrial fibrillation (HR 0.75; p = 0.01), more systolic dysfunction (HR 1.17; p < 0.001), ICD implantation (HR 1.23; p = 0.04), and CRT upgrade (HR 1.23; p = 0.03). Exceeding thresholds for TPC, pQRS, and pID of 170, 146, and 112 ms in lead I, respectively, was associated with a substantial increase in systolic dysfunction over 5 years (p < 0.001).
Conclusions
Longer durations of all tested parameters in lead I were associated with increased rates of left ventricular systolic dysfunction. ICD implantation and CRT upgrade were also linked to increased TPC and pID durations. Paradoxically, patients with longer pID and pQRS had less incident atrial fibrillation.
1. Introduction
The surface 12-lead electrocardiogram (ECG) is a widely available, non-invasive, and inexpensive tool; yet the prognostic significance of paced QRS complex morphology is poorly understood in patients with a substantial burden of right ventricular pacing.
Apical RV pacing at a pacing burden as low as 20% has been associated with deleterious long-term clinical outcomes including pacemaker-induced cardiomyopathy [1,2]. The underlying mechanism is thought to be non-physiological electrical activation and resultant inter- and intra-ventricular dyssynchrony [3]. RV pacing sites other than the apex may be more physiological and – based on small studies – may improve clinical outcomes [[4], [5], [6]].
Inadvertent placement of an RV lead in a position other than the intended target is not uncommon [[7], [8], [9]]. The pattern of RV electrical activation on ECG has been correlated with RV lead position [4,[9], [10], [11], [12]] and studies evaluating the accuracy of surface ECG parameters in lead localization have yielded inconsistent results [8,9,11]. In addition, paced QRS (pQRS) duration – especially if greater than 180 ms – has been shown to correlate with reduced left ventricular ejection fraction (<55%) as well as greater end-diastolic and end-systolic diameters by contrast ventriculography performed concurrently with the ECG [13]. In this study, longer pQRS durations resulted in an increase in specificity but a decrease in sensitivity for systolic dysfunction.
The ECG also has shown potential for providing prognostic information in patients with RV pacing. Several studies have shown that long pQRS duration is linked to the future development of left ventricular (LV) systolic dysfunction and clinical heart failure in patients who have no evidence of these complications at baseline [[14], [15], [16], [17], [18], [19]]. Other ECG parameters which assess the myocardial activation sequence with pacing have not been widely studied. One ECG parameter of potential importance is the paced intrinsicoid deflection (pID) – sometimes termed the R wave peak time. This measure reflects the duration of electrical activation in the area of the heart corresponding to the selected surface ECG lead [20]. With regards to pacing, activation is typically most delayed in the basolateral LV wall during RV apical stimulation [21]. Therefore, a longer pID in the corresponding high lateral ECG leads (I and aVL) could be anticipated to correlate with a more dyssynchronous, non-physiological activation state. Similarly, the total pacing complex (TPC) duration (from pacing spike to end of S wave) has not yet been evaluated. This measure should represent the longest time taken for the pacing stimulus to completely activate the transmural myocardium – including local delay after the pacing stimulus not associated with significant voltage on the surface ECG. The TPC may offer additional value over pQRS duration alone.
Reduced mortality but increased atrial fibrillation has been associated with septal pacing when compared to apical and non-septal non-apical (NSNA) pacing [5]. We therefore sought to determine whether the ECG provides mechanistic insight into these clinical sequelae related to the varying degrees of electrical activation of the ventricles. We postulated that septal pacing may substantially improve LV activation time which, in turn, could abbreviate atrial emptying before valve closure. This could lead to higher left atrial pressures, atrial stretch, and – ultimately – atrial fibrillation. We therefore hypothesized that more rapid left ventricular activation as represented on the surface ECG by shorter pQRS, TPC, and pID would result in increased incident atrial fibrillation.
2. Methods
2.1. Participant selection
All patients over the age of 18 who underwent dual-chamber pacemaker implantation at the Mayo Clinic, Rochester, Minnesota from 2004 to 2014 were evaluated for inclusion in the study. In order to reduce heterogeneity, single-chamber pacemakers, biventricular cardiac resynchronization (CRT), and implantable-cardioverter defibrillators (ICD) at initial implant were excluded. In an attempt to omit those with a negligible or unknown pacing burden, patients were also excluded if routine 3 month device interrogation data were unavailable or showed less than 20% pacing burden. In addition, obtaining post-procedural posteroanterior and lateral chest radiographs is routine in our practice and patients were excluded if these images were unavailable or inappropriate for lead position determination. Finally, patients were required to have an ECG showing at least one paced QRS complex in either lead I or aVL within one year of implantation. The study was approved by the Mayo Clinic Institutional Review Board (ID-13-008960).
2.2. Data retrieval
Various sources were utilized for obtaining outcomes data depending on the variable. Pacing percentage was obtained from pacemaker interrogation clinical notes at the three month follow-up. ECG tracings and chest radiographs were obtained from the electronic medical record. Our institution has a continuously maintained echocardiography database from which left ventricular ejection fraction (LVEF) and valvular dysfunction data were pulled. Similarly, patients requiring upgrade to an ICD or CRT were found using an institutional device database. Diagnosis of atrial fibrillation was based on International Classification of Diseases billing codes recorded in the electronic medical record. The National Death Index database was queried to verify mortality data.
2.3. Lead position determination
Radiographs were analyzed individually by independent investigators for RV lead tip location using an algorithm based on a grid overlying the heart as described in prior studies from our group [5]. Lead position was categorized into three groups: apical, septal (including RV outflow tract), and NSNA.
2.4. Surface electrocardiogram analysis
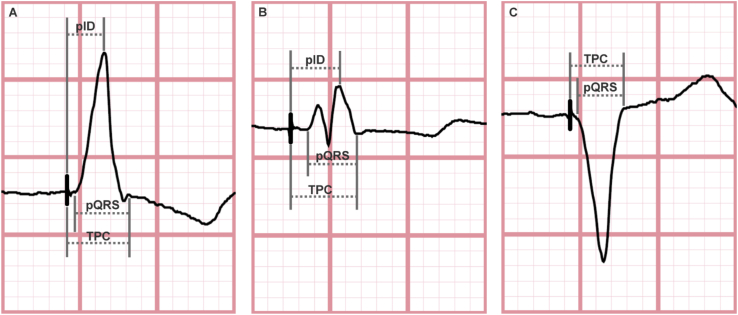
Standard 12-lead ECG's at a paper speed of 25 mm/s and a scale of 10 mm/mV were individually examined by study investigators for parameters representing the pattern of myocardial activation following a pacing spike. The high lateral leads (I and aVL) were chosen based on the hypothesis that these leads best parallel the vector of depolarization from the paced RV to the left ventricle. Width in milliseconds (ms) was recorded for each of the following: paced QRS (pQRS), total pacing complex (TPC), and paced intrinsicoid deflection (pID). These were measured manually according to predetermined and standardized criteria (Fig. 1) by four blinded investigators with each measurement subsequently revalidated by a different investigator. In leads with a predominant R wave, the pID was measured from the pacing spike to the peak of the R wave. In leads with a second dominant positive deflection (RSR′ pattern), the initial R wave was ignored and duration to the R′ wave peak was measured for pID. If no positive deflection was seen in the QRS complex following a pacing spike this was recorded as an absence of pID in the lead of interest.
Fig. 1.
Surface electrocardiograms from three individual patients demonstrating standardized method for measuring parameters of interest. A. Paced QRS (pQRS), total pacing complex (TPC), and paced intrinsicoid deflection (pID) were measured in milliseconds. Width of pQRS was measured from the start of Q or initial R wave to end of S or terminal R wave. TPC was measured from the pacing spike to the end of the S or terminal R wave. pID was measured from the pacing spike to the peak of the R wave. B. If a rSR′ complex was present, the measurement was taken from the peak of the R′ wave – regardless of R wave amplitude. C. If no positive QRS deflection was seen following a pacing spike, pID was noted to be absent in the lead of interest.
2.5. Echocardiogram data
Echocardiogram parameters were recorded in the institutional database from studies performed for diagnostic purposes unrelated to this study. All outcomes relied on interpretation by independent Cardiologists in the echocardiography practice and images were not available for review by the study investigators. In order to minimize inter-observer variability and subtle findings of uncertain clinical significance, only tricuspid and mitral regurgitation of at least moderate severity were reported. Similarly, LVEF of less than or equal to 40% was used as the definition for at least moderate left ventricular systolic dysfunction – a threshold that has been utilized previously in studies and guidelines [22,23].
2.6. Analysis
Outcomes and lead position were compared based on ECG parameters that were predefined prior to analysis. In addition, separate analyses were completed to control for lead tip position as a confounder comparing apical and septal as well as apical and non-septal non-apical lead position. Continuous variables were reported as means and two-sample t-tests were used to compare between groups. Categorical variables were compared using the Chi-square test for independence. The Kaplan-Meier method was used for follow-up events and curves were compared using log-rank tests. The association of each ECG parameter with each endpoint was assessed using Cox proportional hazards models. All outcomes were reported in association with a greater than or equal to 10 ms change in all continuous variable ECG parameters. Best cutoff ECG durations for significance were determined using a changepoint method [24].
3. Results
Over the study period, 844 patients (43.1% female; age 75.0 ± 12.1) were followed for 4.4 ± 2.7 years after undergoing dual-chamber pacemaker implantation. The most common lead tip position as determined by chest radiograph was the RV apex – occurring in 626 (74.2%) patients. Of the remaining, 51 (6.0%) had a septal position (including the RV outflow tract) and 167 (19.8%) had a NSNA position (including the RV free wall and tricuspid valve region).
3.1. Baseline characteristics
Baseline characteristics of each group are shown in Table 1 with comparison based on lead position. Septal lead position had a higher prevalence of tricuspid regurgitation at baseline. There were no other significant differences between groups in age, gender, baseline atrial fibrillation, LVEF, or valvular regurgitation. At 3 months mean ventricular pacing percentages were also not significantly different between apical and septal groups (90.7% vs. 88.1%, respectively, p = 0.45) or apical and NSNA groups (90.7% vs. 85.9%, respectively, p = 0.09).
Table 1.
Baseline clinical and echocardiographic characteristics.
| Variable | Apical (n = 626) | Septal (n = 51) | p value | Apical (n = 626) | Non-septal non-apical (n = 167) | p value |
|---|---|---|---|---|---|---|
| Age, mean | 75.4 ± 14.7 | 74.1 ± 11.6 | 0.96 | 75.4 ± 14.7 | 74.2 ± 13.0 | 0.49 |
| Male gender, n | 344 (55.0%) | 33 (64.7%) | 0.18 | 344 (55.0%) | 104 (61.7%) | 0.12 |
| Atrial fibrillation, n | 350 (55.9%) | 29 (56.9%) | 0.90 | 350 (55.9%) | 91 (54.5%) | 0.74 |
| LVEF (%), mean | 57.9 ± 11.3 | 57.6 ± 10.3 | 0.67 | 57.9 ± 11.3 | 58.9 ± 9.8 | 0.74 |
| MR, n | 35 (9.1%) | 5 (16.1%) | 0.20 | 35 (9.1%) | 5 (4.7%) | 0.14 |
| TR, n | 39 (10.1%) | 8 (25.8%) | 0.01 | 39 (10.1%) | 10 (9.3%) | 0.83 |
| Echocardiogram Unavailable | 240 (38.3%) | 20 (39.2%) | 0.90 | 240 (38.3%) | 60 (35.9%) | 0.57 |
Bold highlights statistical significance with p<0.05
All percentages are of the participants with available data for each variable.
LVEF = left ventricular ejection fraction; MR = moderate or greater mitral regurgitation; TR = moderate or greater tricuspid regurgitation.
3.2. Lead position electrocardiographic characteristics
The surface ECG findings were analyzed based on lead position and results are outlined in Table 2. As compared to the apical group, septal lead position was associated with a shorter TPC in leads I and aVL. In aVL but not lead I, shorter pQRS and absence of pID were associated also with septal rather than apical lead position. There were no significant differences in ECG characteristics between the apical and NSNA groups.
Table 2.
Surface electrocardiographic characteristics by lead position.
| Variable | Apical (n = 626) | Septal (n = 51) | p value | Apical (n = 626) | Non-septal non-apical (n = 167) | p value |
|---|---|---|---|---|---|---|
| Lead I | ||||||
| TPC (ms) | 164.0 ± 26.8 | 153.7 ± 31.6 | 0.01 | 164.0 ± 26.8 | 162.5 ± 26.2 | 0.77 |
| pQRS (ms) | 144.8 ± 30.3 | 141.5 ± 32.2 | 0.50 | 144.8 ± 30.3 | 164.1 ± 25.4 | 0.55 |
| pID (ms) | 110.2 ± 24.1 | 111.1 ± 28.1 | 0.73 | 110.2 ± 24.1 | 107.9 ± 25.2 | 0.16 |
| Absent pID, n |
20 (3.2%) |
3 (5.9%) |
0.31 |
20 (3.2%) |
2 (1.2%) |
0.16 |
| Lead aVL | ||||||
| TPC (ms) | 166.3 ± 27.3 | 153.0 ± 32.3 | 0.005 | 166.3 ± 27.3 | 164.1 ± 25.4 | 0.55 |
| pQRS (ms) | 151.4 ± 28.1 | 139.8 ± 36.6 | 0.03 | 151.4 ± 28.1 | 151.6 ± 25.9 | 0.95 |
| pID (ms) | 105.7 ± 22.6 | 108.1 ± 27.1 | 0.11 | 105.7 ± 22.6 | 103.9 ± 22.7 | 0.25 |
| Absent pID, n | 2 (0.3%) | 10 (19.6%) | < 0.001 | 2 (0.3%) | 3 (1.8%) | 0.06 |
Bold highlights statistical significance with p<0.05
pID = paced intrinsicoid deflection; pQRS = paced QRS; TPC = total pacing complex.
3.3. Long-term clinical and echocardiographic outcomes
Hazard ratios for outcomes associated with ECG parameters are summarized in Table 3. In lead aVL but not lead I, pQRS duration was associated with an increased risk of ICD implantation (HR 1.18, p = 0.04). Otherwise, lead I parameters showed either similar or stronger associations with all long-term outcomes of interest.
Table 3.
Hazard ratios for surface ECG characteristics in relation to clinical and echocardiographic outcomes.
| Characteristic | Mortality | Atrial Fibrillation | ICD Implant | CRT Upgrade | LVEF <40% |
MR | TR |
|---|---|---|---|---|---|---|---|
| Lead I | |||||||
| TPCa | 1.01 (0.97–1.05) | 0.86 (0.69–1.06) | 1.18* (1.01–1.38) | 1.15 (0.99–1.33) | 1.22*** (1.14–1.30) | 1.02 (0.95–1.09) | 0.98 (0.93–1.03) |
| pQRSa | 1.03 (0.99–1.07) | 0.80* (0.65–0.97) | 1.09 (0.94–1.25) | 1.08 (0.95–1.23) | 1.17*** (1.10–1.24) | 1.02 (0.96–1.08) | 1.00 (0.95–1.05) |
| pIDa | 0.98 (0.93–1.02) | 0.75* (0.59–0.94) | 1.23* (1.01–1.49) | 1.23* (1.02–1.47) | 1.17*** (1.08–1.27) | 1.02 (0.95–1.11) | 0.97 (0.91–1.03) |
| Absent pID |
0.67 (0.33–1.35) |
b |
1.30 (0.17–9.69) |
1.07 (0.14–7.86) |
1.26 (0.51–3.07) |
0.73 (0.23–2.31) |
1.61 (0.85–3.06) |
| Lead aVL | |||||||
| TPCa | 1.02 (0.98–1.06) | 0.90 (0.72–1.13) | 1.15 (0.99–1.32) | 1.12 (0.98–1.29) | 1.18*** (1.11–1.25) | 1.03 (0.96–1.10) | 0.97 (0.92–1.03) |
| pQRSa | 1.01 (0.97–1.05) | 0.84 (0.68–1.03) | 1.18* (1.01–1.39) | 1.01 (1.00–1.03) | 1.20*** (1.11–1.28) | 1.01 (0.95–1.08) | 0.95 (0.91–1.00) |
| pIDa | 0.96 (0.91–1.01) | 0.81 (0.62–1.06) | 1.23** (1.06–1.41) | 1.20* (1.04–1.38) | 1.16*** (1.08–1.25) | 1.02 (0.94–1.11) | 0.97 (0.91–1.04) |
| Absent pID | 0.65 (0.21–2.03) | b | b | b | b | 1.09 (0.27–4.41) | 1.76 (0.72–4.28) |
Bold highlights statistical significance with p<0.05
*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Values displayed as hazard ratio (95% confidence interval) for development of incident events.
CRT = cardiac resynchronization therapy; ICD = implantable cardioverter-defibrillation; LVEF = left ventricular ejection fraction; MR = moderate or greater mitral regurgitation; pID = paced intrinsicoid deflection; pQRS = paced QRS; TPC = total pacing complex; TR = moderate or greater tricuspid regurgitation.
Outcomes reported in association with a ≥10 ms change in all continuous variable ECG parameters.
Too few events to draw conclusion.
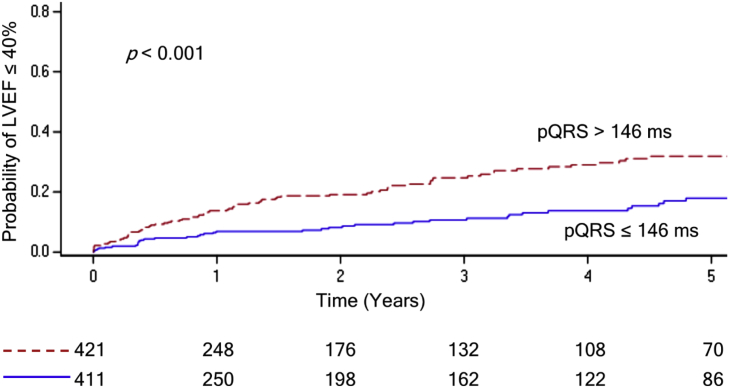
Paced QRS duration in lead I was associated with more frequent incident LVEF ≤40% (HR 1.17, p < 0.001). A cutoff pQRS of greater than 146 ms was found to best predict development of LVEF ≤40% (Fig. 2).
Fig. 2.
Kaplan-Meier estimate of incident left ventricular ejection fraction (LVEF) on echocardiogram over 5 years comparing paced QRS (pQRS) duration cutoff of 146 ms (ms) as a categorical variable.
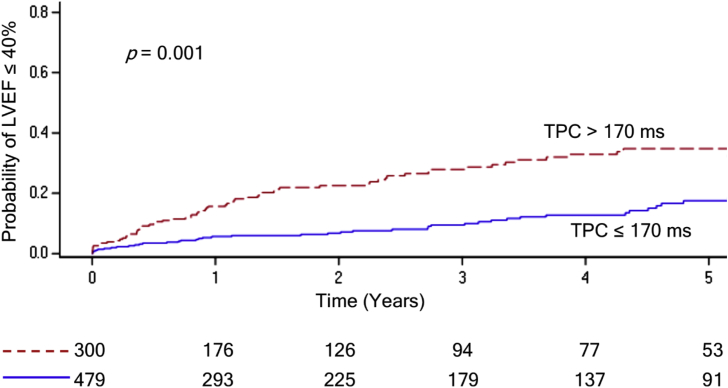
Longer TPC duration in lead I was associated with higher rate of ICD implantation (HR 1.18, p = 0.04) and development of LVEF ≤ 40% (HR 1.22, p < 0.001). A TPC duration cutoff of greater than 170 ms was associated with increased risk of LVEF decline (Fig. 3).
Fig. 3.
Kaplan-Meier estimate of incident left ventricular ejection fraction (LVEF) on echocardiogram over 5 years comparing total pacing complex (TPC) duration cutoff of 170 ms (ms) as a categorical variable.
Higher rates of ICD implantation (HR 1.23, p = 0.04), CRT upgrade (HR 1.23, p = 0.03), and LVEF ≤ 40% (1.17, p < 0.001) occurred in patients with increased pID in lead I. A cutoff of greater than 112 ms was found to increase risk of LVEF ≤40% (Fig. 4).
Fig. 4.
Kaplan-Meier estimate of incident left ventricular ejection fraction (LVEF) on echocardiogram over 5 years comparing paced intrinsicoid deflection (pID) duration cutoff of 112 ms (ms) as a categorical variable.
Interestingly, longer pQRS (HR 0.80, p = 0.03) and pID (HR 0.75, p = 0.01) appeared to be protective for incident atrial fibrillation diagnosis. Too few patients had an absent pID to draw conclusions regarding many of the outcomes studied. No ECG parameters were associated with development of tricuspid or mitral regurgitation. Similarly, no ECG parameters predicted mortality, however, pQRS duration did demonstrate a statistically non-significant trend towards increased mortality (HR 1.03, 95% CI 0.99–1.07, p = 0.13).
4. Discussion
Several associations between paced surface ECG parameters and long-term clinical and echocardiographic outcomes were identified in this study revealing important prognostic implications in patients with at least a moderate burden of right ventricular pacing – adding value to this widely available, non-invasive, and inexpensive tool. Prolonged pID duration in high lateral leads was associated with deterioration of left ventricular function (increased rates of ICD implantation, CRT upgrade, and left ventricular ejection fraction decline) over time but was paradoxically linked to a lower rate of incident atrial fibrillation. Exceeding thresholds for TPC, pQRS, and pID of 170, 146, and 112 ms in lead I, respectively, were associated with a substantial increase in rate of developing moderate to severe LV systolic dysfunction (LVEF ≤40%) over 5 years.
Several studies have demonstrated higher rates of incident left ventricular systolic dysfunction in RV paced patients with a prolonged pQRS – an association which was corroborated by the findings of this study. Likely due to differences in study design and definitions of systolic dysfunction, threshold pQRS durations for significance have varied widely from 130 to 180 ms [[15], [16], [17], [18], [19]]. The largest of these studies, a prospective trial of 194 patients with RV apical pacing, found that a pQRS duration of ≥165 ms had a sensitivity of 79% for development of pacemaker-induced cardiomyopathy over 5 years [19]. All of these studies either failed to disclose how pQRS was measured or utilized the broadest pQRS in all 12 leads. This limits use of this parameter in real-world settings given the time constraints of clinical practice. In addition, the pQRS complex ignores local myocardial activation of a voltage too low to be detected by surface electrodes – as would expected in the presence of a scarred and diseased ventricle. The TPC assumes that myocardial activation starts immediately following the pacing spike and aims to capture additional delay that may not be obvious on surface ECG.
Utilizing pID in addition to pQRS and TPC in a single lead, specifically lead I, may offer the ability to obtain additional prognostic information efficiently from the surface ECG. A positive deflection is recorded as an activation wave front approaches a unipolar lead followed by a sudden change in polarity when the wave front reaches the point nearest that same lead. As such, intrinsicoid deflection aims to measure the time of electrical activation to the myocardium beneath the surface ECG lead of interest. Intrinsicoid deflection has been used to help distinguish conduction abnormalities [25,26] and, more recently, was shown to predict response to CRT in advanced heart failure [26]. In high lateral ECG leads, longer values of intrinsicoid deflection have been suggested to reflect delayed LV activation and may be less influenced by an underlying cardiomyopathic process than the QRS duration [26]. The measure has not been previously studied in paced ECG tracings but it can be inferred that pID in leads I and aVL represents the duration from initiation of pacing to activation of the lateral LV free wall – thereby, defining the ventricular activation sequence and, ultimately, interventricular synchrony with pacing.
Longer durations of pQRS and pID (measured in lead I) were found to be associated with a reduced incidence of atrial fibrillation. Increased atrial fibrillation as a result of excessive RV pacing is well documented [27,28] and is postulated to develop through the downstream effects of pacing dyssynchrony leading to systolic and diastolic left ventricular dysfunction and resultant increases in atrial pressures and dilatation [28,29]. Shorter pID and pQRS durations, however, more closely resemble a native QRS complex without pacing or conduction disease and more physiological left ventricular activation could be expected as a result. Furthermore, this study found an increase in LV dysfunction with longer, not shorter, durations of pID, pQRS, and TPC. Therefore, an increase risk of atrial fibrillation cannot be explained by the left ventricular dysfunction mechanism alone.
Septal pacing was associated with shorter pQRS duration in lead aVL. Unlike the narrow QRS produced with normal conduction or synchronized biventricular pacing, the mechanism of a narrow pQRS in these patients may be a reflection of earlier LV activation due to RV pacing nearer to the base of the heart rather than the apex. Early LV activation can curtail atrial emptying and – through inadequate ventricular diastolic filling – may potentially result in increased atrial pressures, atrial dilatation, and atrial fibrillation. Truncation of ventricular diastolic filling is a well-recognized complication with short atrioventricular (AV) delays nominally programmed to improve pacing percentages [29,30], yet further echocardiographic and clinical data will be needed to fully evaluate this putative mechanism with non-apical RV pacing. With the increasing focus on more physiological pacing techniques, including His-bundle and conduction system pacing, it will be important to consider atrial fibrillation as a possible long-term consequence of alternate pacing sites. Individually adjusting AV conduction delays may be an important component in optimizing LV diastolic filling in these patients.
Although lead aVL appeared to more accurately predict lead position, lead I parameters offered more prognostic associations. This may be a factor of the vector of lead I more closely paralleling the direction of activation from RV to LV during univentricular pacing. The pattern of RV electrical activation on ECG has been studied as a tool for confirming RV lead position but results have been inconsistent. The largest prospective evaluation of criteria to differentiate RV lead position (SPICE ECG sub-study) proposed a step-wise algorithm including pQRS positivity in lead V6, pQRS positivity in inferior leads, and a paced QR pattern in aVL to favor mid-septal over apical lead position [9]. In 227 patients, this algorithm showed a high sensitivity (87%) and specificity (90%) for determining septal lead positioning with echocardiographic confirmation.
Supporting the results of this study, SPICE ECG and several smaller investigations consistently demonstrated that shorter pQRS duration correlated with septal and RV outflow tract pacing [[9], [10], [11], [12],18]. A shorter TPC and the absence of pID in lead aVL were both stronger predictors of septal pacing location in this study than pQRS which has been evaluated by other trials. Incorporating these variables may offer increased accuracy in determining lead position in future algorithms.
5. Limitations
The retrospective study was performed at a single tertiary referral center and some clinical events may have been managed locally – resulting in some missed outcomes. Device interrogation data was not available to the investigators. Mortality data, however, was obtained from national sources and not limited by patient follow-up.
ECG measurements were made utilizing two leads (I and aVL) in an attempt to create a manual measure that can be efficiently translated to clinical practice based on the premise that these high-lateral leads would reflect activation and synchrony from the right ventricular pacing impulse to the left ventricle – a hypothesis that was supported by the outcomes. Other leads may have added value but were not evaluated due to the large sample size and resource constraints. Manual measurements are also vulnerable to inter- and intra-observer variability [31]. An attempt was made to reduce variability by using predefined measurement criteria and having a second investigator revalidate all measurements. All studies that analyze the surface ECG during pacing are also limited by the inability to completely exclude fusion between intrinsic atrioventricular conduction in a proportion of tracings as opposed to pure ventricular capture.
Variability in cardiac rotation can impact accurate lead position by chest radiographs in a subset of patients with nonstandard or distorted anatomy. In the absence of cardiac CT or MRI for all patients, this approach (previously published by our group) [5] is reproducible, unbiased, and blinded from the location intended by the implanting operator.
The numbers of patients in septal and NSNA groups were substantially lower than the apical lead position group which limits the power of observations – this likely reflects a real world distribution of these pacing sites over the last decade. In addition, pacing percentage was documented at 3 months and could potentially have changed during subsequent follow-up. This element was minimized by excluding patients with low pacing burden resulting in a high (over 90%) baseline pacing percentage.
Atrial fibrillation (defined by clinical diagnoses) does not account for subclinical episodes. Unfortunately, device recorded episodes were not readily available for the purposes of this study. This limitation applied to all groups and would not be expected to impact between group comparisons.
6. Conclusions
Surface ECG parameters offer important prognostic information in patients with at least moderate right ventricular pacing burden. Longer durations of all tested parameters in lead I were associated with increased rates of left ventricular systolic dysfunction. Paced intrinsicoid deflection duration, an understudied parameter, also appears to be a marker associated with higher rates of CRT upgrade and ICD implantation. The parameters are easily identified on the surface ECG in a single lead, and whether using these measures at the time of standard pacemaker implant aids the operator in the finer selection of pacing site remains to be prospectively evaluated. Less atrial fibrillation associated with longer pID and pQRS is intriguing and suggests the importance of optimized AV conduction timing on diastolic filling.
Disclosures
None.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Kiehl E.L., Makki T., Kumar R., Gumber D., Kwon D.H., Rickard J.W., Kanj M., Wazni O.M., Saliba W.I., Varma N., Wilkoff B.L., Cantillon D.J. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm. 2016;13:2272–2278. doi: 10.1016/j.hrthm.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 2.Healey J.S., Toff W.D., Lamas G.A., Andersen H.R., Thorpe K.E., Ellenbogen K.A., Lee K.L., Skene A.M., Schron E.B., Skehan J.D., Goldman L., Roberts R.S., Camm A.J., Yusuf S., Connolly S.J. Cardiovascular outcomes with atrial-based pacing compared with ventricular pacing: meta-analysis of randomized trials, using individual patient data. Circulation. 2006;114:11–17. doi: 10.1161/CIRCULATIONAHA.105.610303. [DOI] [PubMed] [Google Scholar]
- 3.Ebrille E., DeSimone C.V., Vaidya V.R., Chahal A.A., Nkomo V.T., Asirvatham S.J. Ventricular pacing - electromechanical consequences and valvular function. Indian Pacing Electrophysiol J. 2016;16:19–30. doi: 10.1016/j.ipej.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hillock R.J., Stevenson I.H., Mond H.G. The right ventricular outflow tract: a comparative study of septal, anterior wall, and free wall pacing. Pacing Clin Electrophysiol: PACE (Pacing Clin Electrophysiol) 2007;30:942–947. doi: 10.1111/j.1540-8159.2007.00790.x. [DOI] [PubMed] [Google Scholar]
- 5.Witt C.M., Lenz C.J., Shih H.H., Ebrille E., Rosenbaum A.N., van Zyl M., Aung H., Manocha K.K., Deshmukh A.J., Hodge D.O., Mulpuru S.K., Cha Y.M., Espinosa R.E., Asirvatham S.J., McLeod C.J. Right ventricular pacemaker lead position is associated with differences in long-term outcomes and complications. J Cardiovasc Electrophysiol. 2017;28:924–930. doi: 10.1111/jce.13256. [DOI] [PubMed] [Google Scholar]
- 6.Zou C., Song J., Li H., Huang X., Liu Y., Zhao C., Shi X., Yang X. Right ventricular outflow tract septal pacing is superior to right ventricular apical pacing. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaye G.C., Linker N.J., Marwick T.H., Pollock L., Graham L., Pouliot E., Poloniecki J., Gammage M., Protect-Pace trial i Effect of right ventricular pacing lead site on left ventricular function in patients with high-grade atrioventricular block: results of the Protect-Pace study. Eur Heart J. 2015;36:856–862. doi: 10.1093/eurheartj/ehu304. [DOI] [PubMed] [Google Scholar]
- 8.Sharma G., Salahuddin S., Sanders P., Gupta H., Gulati G., Jagia P., Bahl V.K. Inadequacy of fluoroscopy and electrocardiogram in predicting septal position in RVOT pacing - validation with cardiac computed tomography. Indian Heart J. 2016;68:174–180. doi: 10.1016/j.ihj.2015.10.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrikopoulos G., Tzeis S., Asbach S., Semmler V., Lennerz C., Solzbach U., Grebmer C., Kloppe A., Klein N., Pastromas S., Biermann J., Kolb C., Investigators S.S. A stepwise electrocardiographic algorithm for differentiation of mid-septal vs. apical right ventricular lead positioning: the SPICE ECG substudy. Europace : European pacing, arrhythmias, and cardiac electrophysiology. J Work Groups Cardiac Pacing Arrhythm Cardiac Cell Electrophysiol Eur Soc Cardiol. 2015;17:915–920. doi: 10.1093/europace/euu344. [DOI] [PubMed] [Google Scholar]
- 10.Balt J.C., van Hemel N.M., Wellens H.J., de Voogt W.G. Radiological and electrocardiographic characterization of right ventricular outflow tract pacing. Europace : european pacing, arrhythmias, and cardiac electrophysiology. J Work Groups Cardiac Pacing Arrhythm Cardiac Cell Electrophysiol Eur Soc Cardiol. 2010;12:1739–1744. doi: 10.1093/europace/euq341. [DOI] [PubMed] [Google Scholar]
- 11.Burri H., Park C.I., Zimmermann M., Gentil-Baron P., Stettler C., Sunthorn H., Domenichini G., Shah D. Utility of the surface electrocardiogram for confirming right ventricular septal pacing: validation using electroanatomical mapping. Europace : European pacing, arrhythmias, and cardiac electrophysiology. J Work Groups Cardiac Pacing Arrhythm Cardiac Cell Electrophysiol Eur Soc Cardiol. 2011;13:82–86. doi: 10.1093/europace/euq332. [DOI] [PubMed] [Google Scholar]
- 12.McGavigan A.D., Roberts-Thomson K.C., Hillock R.J., Stevenson I.H., Mond H.G. Right ventricular outflow tract pacing: radiographic and electrocardiographic correlates of lead position. Pacing Clin Electrophysiol: PACE (Pacing Clin Electrophysiol) 2006;29:1063–1068. doi: 10.1111/j.1540-8159.2006.00499.x. [DOI] [PubMed] [Google Scholar]
- 13.Recke S., Göhl K., Gansser R., von der Emde J. Assessment of left heart failure from the pacemaker QRS duration. Eur J Cardiac Pacing Electrophysiol. 1991;1:16–20. [Google Scholar]
- 14.Pan W., Su Y., Gong X., Sun A., Shu X., Ge J. Value of the paced QRS duration. J Card Fail. 2009;15:347–352. doi: 10.1016/j.cardfail.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Khurshid S., Liang J.J., Owens A., Lin D., Schaller R., Epstein A.E., Marchlinski F.E., Frankel D.S. Longer paced QRS duration is associated with increased prevalence of right ventricular pacing-induced cardiomyopathy. J Cardiovasc Electrophysiol. 2016;27:1174–1179. doi: 10.1111/jce.13045. [DOI] [PubMed] [Google Scholar]
- 16.Sharma G., Shetkar S.S., Patel C.D., Singh H., Naik N., Roy A., Juneja R., Sanders P. Paced QRS duration predicts left ventricular function in patients with permanent pacemakers - one-year follow-up study using equilibrium radionuclide angiography (ERNA) Indian Pacing Electrophysiol J. 2015;15:90–95. doi: 10.1016/j.ipej.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shukla H.H., Hellkamp A.S., James E.A., Flaker G.C., Lee K.L., Sweeney M.O., Lamas G.A., Mode Selection Trial I. Heart failure hospitalization is more common in pacemaker patients with sinus node dysfunction and a prolonged paced QRS duration. Heart Rhythm. 2005;2:245–251. doi: 10.1016/j.hrthm.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Chan N.Y., Yuen H.C., Choy C.C., Mok N.S., Tsui P.T., Lau C.L., Lo Y.K., Chu P.S., Chow H.F. Left ventricular volumes and systolic function after long-term right ventricular pacing may be predicted by paced QRS duration, but not pacing site. Heart Lung Circ. 2014;23:43–48. doi: 10.1016/j.hlc.2013.04.122. [DOI] [PubMed] [Google Scholar]
- 19.Chen S., Yin Y., Lan X., Liu Z., Ling Z., Su L., Kiuchi M.G., Li X., Zhong B., Krucoff M.W., group PR-HFsi Paced QRS duration as a predictor for clinical heart failure events during right ventricular apical pacing in patients with idiopathic complete atrioventricular block: results from an observational cohort study (PREDICT-HF) Eur J Heart Fail. 2013;15:352–359. doi: 10.1093/eurjhf/hfs199. [DOI] [PubMed] [Google Scholar]
- 20.Dower G.E. In defence of the intrinsic deflection. Br Heart J. 1962;24:55–60. doi: 10.1136/hrt.24.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghani A., Delnoy P.P., Ottervanger J.P., Ramdat Misier A.R., Smit J.J., Elvan A. Assessment of left ventricular dyssynchrony in pacing-induced left bundle branch block compared with intrinsic left bundle branch block. Europace : European pacing, arrhythmias, and cardiac electrophysiology. J Work Groups Cardiac Pacing Arrhythm Cardiac Cell Electrophysiol Eur Soc Cardiol. 2011;13:1504–1507. doi: 10.1093/europace/eur117. [DOI] [PubMed] [Google Scholar]
- 22.Ebert M., Jander N., Minners J., Blum T., Doering M., Bollmann A., Hindricks G., Arentz T., Kalusche D., Richter S. Long-term impact of right ventricular pacing on left ventricular systolic function in pacemaker recipients with preserved ejection fraction: results from a large single-center registry. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheitlin M.D., Armstrong W.F., Aurigemma G.P., Beller G.A., Bierman F.Z., Davis J.L., Douglas P.S., Faxon D.P., Gillam L.D., Kimball T.R., Kussmaul W.G., Pearlman A.S., Philbrick J.T., Rakowski H., Thys D.M., Antman E.M., Smith S.C., Jr., Alpert J.S., Gregoratos G., Anderson J.L., Hiratzka L.F., Hunt S.A., Fuster V., Jacobs A.K., Gibbons R.J., Russell R.O., American College of C., American Heart A., American Society of E. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American college of cardiology/American heart association task force on practice guidelines (ACC/AHA/ASE committee to update the 1997 guidelines for the clinical application of echocardiography) Circulation. 2003;108:1146–1162. doi: 10.1161/01.CIR.0000073597.57414.A9. [DOI] [PubMed] [Google Scholar]
- 24.Contal C., O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999;30:253–270. [Google Scholar]
- 25.Medrano G.A., Brenes C., De Micheli A., Sodi-Pallares D. Clinical electrocardiographic and vectorcardiographic diagnosis of the left anterior subdivision block isolated or associated with RBBB. Am Heart J. 1972;83:447–458. doi: 10.1016/0002-8703(72)90034-8. [DOI] [PubMed] [Google Scholar]
- 26.Munoz F.D., Powell B.D., Cha Y.M., Wiste H.J., Redfield M.M., Friedman P.A., Asirvatham S.J. Delayed intrinsicoid deflection onset in surface ECG lateral leads predicts left ventricular reverse remodeling after cardiac resynchronization therapy. Heart Rhythm. 2013;10:979–987. doi: 10.1016/j.hrthm.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 27.Connolly S.J., Kerr C.R., Gent M., Roberts R.S., Yusuf S., Gillis A.M., Sami M.H., Talajic M., Tang A.S., Klein G.J., Lau C., Newman D.M. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physiologic Pacing Investigators. N Engl J Med. 2000;342:1385–1391. doi: 10.1056/NEJM200005113421902. [DOI] [PubMed] [Google Scholar]
- 28.Kristensen L., Nielsen J.C., Mortensen P.T., Pedersen O.L., Pedersen A.K., Andersen H.R. Incidence of atrial fibrillation and thromboembolism in a randomised trial of atrial versus dual chamber pacing in 177 patients with sick sinus syndrome. Heart. 2004;90:661–666. doi: 10.1136/hrt.2003.016063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen J.C., Kristensen L., Andersen H.R., Mortensen P.T., Pedersen O.L., Pedersen A.K. A randomized comparison of atrial and dual-chamber pacing in 177 consecutive patients with sick sinus syndrome: echocardiographic and clinical outcome. J Am Coll Cardiol. 2003;42:614–623. doi: 10.1016/s0735-1097(03)00757-5. [DOI] [PubMed] [Google Scholar]
- 30.Jones R.C., Svinarich T., Rubin A., Levin V., Phang R., Murillo J., Sambelashvili A. Optimal atrioventricular delay in CRT patients can be approximated using surface electrocardiography and device electrograms. J Cardiovasc Electrophysiol. 2010;21:1226–1232. doi: 10.1111/j.1540-8167.2010.01807.x. [DOI] [PubMed] [Google Scholar]
- 31.Tomlinson D.R., Bashir Y., Betts T.R., Rajappan K. Accuracy of manual QRS duration assessment: its importance in patient selection for cardiac resynchronization and implantable cardioverter defibrillator therapy. Europace : european pacing, arrhythmias, and cardiac electrophysiology. J Work Groups Cardiac Pacing Arrhythm Cardiac Cell Electrophysiol Eur Soc Cardiol. 2009;11:638–642. doi: 10.1093/europace/eup001. [DOI] [PubMed] [Google Scholar]