Abstract
Ethical restrictions are limitations of in vivo inhalation studies, on humans and animal models. Thus, in vitro or ex vivo anatomical models offer an interesting alternative if limitations are clearly identified and if extrapolation to human is made with caution. This work aimed to develop an ex vivo infant-like respiratory model of bronchopulmonary dysplasia easy to use, reliable and relevant compared to in vivo infant data. This model is composed of a 3D-printed head connected to a sealed enclosure containing a leporine thorax. Physiological data and pleural-mimicking depressions were measured for chosen respiratory rates. Homogeneity of ventilation was assessed by 81mkrypton scintigraphies. Regional radioaerosol deposition was quantified with 99mtechnetium-diethylene triamine pentaacetic acid after jet nebulization. Tidal volumes values are ranged from 33.16 ± 7.37 to 37.44 ± 7.43 mL and compliance values from 1.78 ± 0.65 to 1.85 ± 0.99 mL/cmH2O. Ventilation scintigraphies showed a homogenous ventilation with asymmetric repartition: 56.94% ± 9.4% in right lung and 42.83% ± 9.36 in left lung. Regional aerosol deposition in lungs exerted 2.60% ± 2.24% of initial load of radioactivity. To conclude the anatomical model satisfactorily mimic a 3-months old BPD-suffering bronchopulmonary dysplasia and can be an interesting tool for aerosol regional deposition studies.
Introduction
Bronchopulmonary dysplasia (BPD) is one of the most common pathology of pre-term newborns1–4. This pathology is characterized by the need of supplemental oxygenation or ventilation for at least 28 days of age. Severity is assessed with the remaining need of oxygen at respectively 36 and 56 weeks post-menstrual age (PMA) for infants born before or after 32 weeks of gestation. Physiopathologically, BPD represents the interruption of lung development before the saccular stage, starting at 32 weeks PMA, and corresponds to the formation and architectural development of alveoli5. Risk factors to develop BPD are numerous: internal factors (prematurity, gender, genetics, in utero tobacco exposure), iatrogenic factors (hyperoxia related to mechanical ventilation or/and oxygen supplementation, blood transfusion) or external factors (antenatal or/and postnatal infection)3,6–8.
Treatments of BPD include prophylactic and symptomatic approaches such as aerosol delivery of surfactant, ante- and/or postnatal administration of corticosteroids and ventilation strategies6,9–11. Despite existing therapeutic options, there are still long-term outcomes of BPD with high rates of cognitive or behavioral impairments and reduced lung function12, even impacts on motor skills, cognitive and language developments6,13. Administration of corticosteroids, by systemic or inhaled administration route, remain one of the main strategy to limit outcomes of BPD and to wean infants from ventilation14. Moreover, current inhaled treatments were tested against systemic corticoids. No statistical differences could be found between the two administration routes10. Inhaled corticotherapy could be use as a prophylactic treatment on very low birth weight preterms but failed to show significant efficacy15,16 and as a symptomatic treatment17. However, these approaches are known to impair neurologic development of newborn18 and infants and to increase relative risk associated to death for extremely preterm newborns19. This could be due to a non-optimized nebulization technology, leading to an uncontrolled or uneven deposition of drugs in the lungs. Hence, there is an unmet medical need in the cartography and quantification of pulmonary deposition of aerosolized drugs. All things considered, considering the well known side effects of systemic administration, local delivery of drugs to the lungs are expected to be an interesting route of administration as an alternative to systemic therapies in the management of BPD.
The aerosol deposition cartography depend on airborne particles properties (e.g. aerodynamic size, hydroscopic properties, electrical surface charge, etc.) and also on anatomic and physiologic (e.g. diameter of airways, breathing frequency, tidal volume, etc.)20,21. Nonetheless, as radiolabeled aerosols are the gold standard to assess regional aerosol deposition of inhaled particles within lungs22 in vivo human studies are still scarce due to ethical restrictions. Therefore, as an alternative, in vivo deposition studies using rodent models are frequent23,24. For example, in vivo studies on rats are common and different models were developed especially for BPD studies25. Other in vivo animal models exist, such as baboon26, macaque27, piglet28 or rabbit29, and could be used as infant-like model for aerosol regional deposition studies. However, using animal models is expensive and time-consuming (i.e. very high cost involved in breeding and housing, length of protocols for animal experiments). Also, there are remaining discrepancies to mimic infants/newborns respiratory tracts, such as breathing frequencies or anatomy of lungs. Finally, in vivo human studies of regional deposition assessed by radiolabeled aerosols are focused mainly on adults, thus infant/newborn features are addressed by mathematical modeling30. Deposition models for inhaled particles are based on extrapolation of data from young adults to avoid bias of growth and aging31. These mathematical models predictions trends to overestimate tracheobronchial deposition an to underestimate pulmonary one for children compared to adult32. Hence, a greater pulmonary dose could be administered to infants due to architecture of airways, lung surface area or breathing pattern33. This lack of data concerning inhalation studies focused on infants requires relevant and reliable respiratory models to perform inhalation studies.
Therefore, this study aimed to develop an ex vivo respiratory model of infants, which could be a useful tool for the study of aerosol treatments of BPD. Based on previous work of our laboratory34,35, this chimeric model is composed of a 3D-printed upper airways replica of the Sophia Anatomical Infant Nose-Throat (SAINT) model connected to an ex vivo leporine respiratory thorax placed in a sealed instrumented enclosure specifically designed for that purpose. Leporine lungs are ventilated by applying negative pressure in the enclosure to mimic pleural depression and passive ventilation. Objectives of this work were to validate the likelihood of this innovative ex vivo respiratory model in comparison with infants’ respiratory physiology and existing alternative animal models used in literature. This study is divided into 3 main parts: i) breathing pattern and respiratory parameters, ii) assessment of ventilation and iii) regional aerosol deposition within respiratory tract.
Results and Discussion
Breathing pattern and respiratory parameters
Validation of respiratory parameters was performed using 38 respiratory models with various breathing frequencies in the range 30–40 cycles/min. For each respiratory rate, 6 respiratory parameters were assessed: peak inspiratory flow (PIF), peak expiratory flow (PEF), tidal volume (TV), minute-ventilation (MV), resistances (R) and compliance (C). These parameters were calculated for each respiratory model (n = 38) by collecting data from at least 50 breathing cycles to calculate mean values for each respiratory model. Table 1 and Fig. 1 report mean values and the dispersion of the experimental data collected for each of the 38 respiratory models at various breathing frequencies (30, 35 and 40 cycles/min).
Table 1.
Comparison of respiratory parameters and breathing pattern for each respiratory rate (n = 38; data are presented as mean ± SD [confidence interval 95%]).
| RR | 30 cycles/min | 35 cycles/min | 40 cycles/min |
|---|---|---|---|
| RR (cycles/min) | 29.35 ± 0.83 [29.08; 29.62] | 35.23 ± 1.16 [34.85; 35.61] | 40.31 ± 0.84 [40.03; 40.58] |
| IT (s) | 0.46 ± 0.09 [0.43; 0.49] | 0.46 ± 0.08 [0.43; 0.49] | 0.43 ± 0.07 [0.41; 0.46] |
| ET (s) | 1.58 ± 0.10 [1.54; 1.61] | 1.24 ± 0.09 [1.21; 1.27] | 1.06 ± 0.08 [1.03; 1.08] |
| PIF (mL) | 138.6 ± 21.75 [131.4; 145.7] | 141.2 ± 21.93 [134.0; 148.4] | 134.6 ± 22.23 [127.3; 142.0] |
| PEF (mL) | 31.14 ± 8.13 [28.47; 33.81] | 35.52 ± 11.42 [31.77; 39.28] | 35.59 ± 11.74 [31.73; 39.45] |
| TV (mL) | 36.63 ± 7.31 [34.23; 39.03] | 37.44 ± 7.43 [34.99; 39.88] | 33.16 ± 7.37 [30.74; 35.58] |
| MV (L/min) | 1.07 ± 0.21 [1.01; 1.14] | 1.32 ± 0.25 [1.23; 1.40] | 1.34 ± 0.31 [1.24; 1.44] |
| R (cmH2O/L−1.s−1) | 290.1 ± 92.77 [320.6; 259.6] | 258.0 ± 84.70 [285.8; 230.1] | 253.5 ± 82.50 [280.6; 226.4] |
| C (mL/cmH2O) | 1.85 ± 0.99 [1.52; 2.17] | 1.78 ± 0.65 [1.57; 2.00] | 1.78 ± 0.84 [1.50; 2.06] |
Respiratory rate (RR), inspiratory time (IT), expiratory time (ET), peak inspiratory flow (PIF), peak expiratory flow (PEF), tidal volume (TV), minute ventilation (MV), resistances (R), compliance (C).
Figure 1.
Comparison of respiratory parameters (mean ± SD; n = 38), p < 0.05 is considered as significant. (A) Peak inspiratory flow (PIF). (B) Peak expiratory flow (PEF). (C) Tidal volume (TV). (D) Minute ventilation (MV). (E) Resistances (R). (F) Compliance (C).
Breathing frequency and inspiratory/expiratory time
IT and ET are easily tunable. IT was set at 0.4 s and then ET values vary (set at 1.6 s, 1.3 s and 1.1 s respectively) in order to obtain chosen breathing frequencies (i.e. 30, 35 and 40 cycles/min respectively). This range of respiratory rates (RR) is similar to those found in infants suffering from BPD, 43.7 ± 13.5 cycles/min36. Experimental data collected demonstrated that, as awaited, no significant difference (p = 0.0568) were obtained for the experimental IT when compared to aimed value. RR and ET are close from aimed values meaning that this model is tunable at will for these breathing frequencies.
Peak inspiratory/expiratory flow
PIF values varied between 134.6 ± 22.23 mL and 141.2 ± 21.93 mL, while PEF values spread from 31.14 ± 8.13 mL to 35.59 ± 11.74 mL (Table 1). PIF values are around two times higher than values reported in literature. However, PEF values are almost 2 times lower than in vivo data37. Latzin et al.37 study focused on BPD-suffering infants (113–121 days of age), they found PIF values of 76 ± 16 mL/s and PEF values of 70 ± 17 mL/s. These differences could be explained by the tuning of IT and ET that are slightly different from in vivo pediatric values exerting a longer IT and a shorter ET.
Tidal volume and respiratory minute-ventilation
TV values fluctuated from 33.16 ± 7.37 mL to 37.44 ± 7.43 mL and MV values ranged from 1.07 ± 0.21 L/min to 1.34 ± 0.31 L/min (Table 1). Results found in different papers showed that TV of infants suffering from BPD are often around 30mL37,38. Besides, in another study, they detected lower TV, around 15 mL, but this could be due to body weight of infants, that are lower than those of other studies36. Consequently, MV values obtained with the chimeric model are also in good accordance with data reported in the literature. Hence, the developed model showed TV and MV values very similar to in vivo data.
Resistances and compliance
Lastly, R values are comprised between 253.5 ± 82.50cmH2O/L−1.s−1 and 290.1 ± 92.77cmH2O/ L−1.s−1, while C values covered the range from 1.78 ± 0.65 to 1.85 ± 0.99 mL/cmH2O (Table 1). R values are lower than 383 ± 36 cmH2O/ L−1.s−1 found in the study of Fok et al.39. C values are relatively lower in literature. C values are relatively lower in literature. Indeed, Greenspan et al.40 found C values around 1.51 ± 0.69 mL/cmH2O and Pfenninger et al.41 values were around 1.04 ± 0.42 mL/cmH2O. All things considered, R and C values seem to be in the same order of magnitude of in vivo pediatric data39–44. The chimeric model suffers from few limitations that could impact these results of compliance and resistance such as the lack of lung surfactant and the absence of blood perfusion. Indeed, surfactant is responsible for approximately 50% of compliance of the system44. Perfusion of lungs is also an important parameter related to resistances.
To sum up, all the 6 parameters assessed are found to be very similar to in vivo data reported in literature from infant or animal models frequently used as surrogates. However, due to the narrow dispersion of experimental data collected, significant differences can be observed for some of these 6 parameters measured when the breathing frequency varies (see Fig. 1). All things considered, the respiratory rate of 35 cycles/min appears to be the best compromise. To conclude, the ex vivo pediatric model developed in this study satisfactorily mimic breathing pattern and respiratory parameters of a BPD-suffering infant of 3 to 4 Kg of body weight.
Assessment of ventilation by 81mkrypton (81mKr) scintigraphy
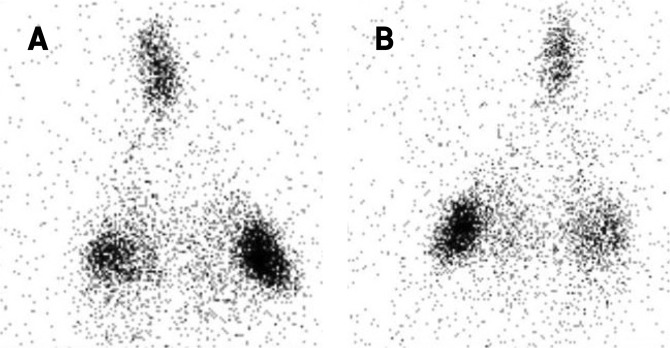
Scintigraphic measurements of ventilation using 81mKr were made on 13 thoraxes. For these nuclear medicine experiments, the RR was set at 35 cycles/min as it appeared to be the best compromise. According to 81mKr half-life, repartition of this gas within lungs is considered to be representative of regional ventilation42. The repartition of total count between right and left lung was studied and compared with pediatric in vivo studies43,45,46 (Fig. 2). Experiments showed an asymmetry in left/right regional ventilation with 56.94% ± 9.4% [51.23%; 62.65%] in right lung and 42.83% ± 9.36 [37.17%; 48.49%] with a significant difference between lungs (p = 0.019). Literature lack of quantitative data because 81mKr ventilation scintigraphies are coupled with 99mTc-albumin scintigraphy to assess ventilation/perfusion mismatch. Hence, comparison with available in vivo data was only qualitative. Ventilation repartition was similar to the one noticed in mild BPD46 with a higher proportion of signal in the right lung.
Figure 2.
81mKrypton (81mKr) scintigraphic images of lungs. (A) anterior view. (B) posterior view.
Regional aerosol deposition
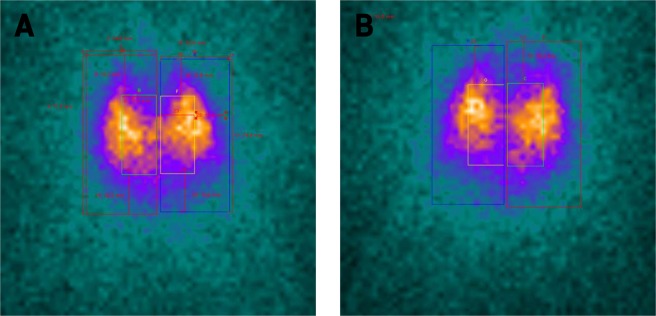
The regional aerosol deposition within the respiratory tract was assessed with 99mtechnetium complexed with diethylene triamine pentaacetic acid (99mTc-DTPA) radioaerosol. The aerosol regional deposition was recorded by planar scintigraphy using gamma-camera imaging (n = 6, using 6 different respiratory models) and 3D scintigraphic images using SPECT-CT (n = 1). Three-dimensional images showed a repartition of nebulized dose in accordance with 2D results (Figs 3 and 4). Deposited fractions along nebulization system and within respiratory tracts were quantified and results are shown in Table 2. The different fractions are expressed as proportions of the initial activity in the nebulizer. Literature of such deposition studies in vivo is very scarce, even more considering pediatric in vivo studies. Most of studies did not use the same nebulizers as ours (Philips Sidestream®), which make results obtained in these studies difficult to compare to our own. Moreover, most of studies focused on intubated infants or surrogate animal models that represents an important bias. Table 3 reports data available in literature. Only studies using a jet nebulizer were selected to allow relevant comparison with these data produced using the chimeric model. Results demonstrated that lung deposited fraction in the ex vivo chimeric model is in good accordance with in vivo data for 3-months old infants. Therefore, the developed model appears to be an interesting tool to successfully assess the regional deposition of aerosol for pediatric model of BPD-suffering infants.
Figure 3.
Diethylene triamine pentaacetic acid (99mTc-DTPA) planar scintigraphic images of the ex vivo pediatric model developed in this study. (A) anterior view. (B) posterior view.
Figure 4.
Diethylene triamine pentaacetic acid (99mTc-DTPA) images after three dimension reconstruction of the ex vivo model developed in this study. (A) tomography images. (B) fusion of tomography and scintigraphic images. 1: transversal view. 2: coronal view. 3: sagittal view.
Table 2.
Deposited fractions along nebulization system as a proportion of initial activity in the nebulizer (mean ± SD, n = 6).
| Nebulized activity | Interface | Replica | Expiratory filter | Lungs | Pump filter |
|---|---|---|---|---|---|
| 50.33 ± 6.75% | 7.82% ± 6.91% | 15.34% ± 10.20% | 23.57% ± 13.87% | 2.60% ± 2.24% | 1.00% ± 1.38% |
Interface: naso-buccal mask. Replica: 3D-printed infant nasal replica with connecting tube. Expiratory filter: collection of exhaled aerosols. Pump filter: security filter to avoid contamination of the depression generator.
Table 3.
Comparison of published studies using jet nebulizers in pediatric modelss. NA: not available.
| Study | Model | Age | Weight | Nebulizer | Lung deposited fraction |
|---|---|---|---|---|---|
| Montigaud et al. | Ex vivo chimeric model | Around 3 months | Between 3–4Kg | Philips Sidestream | 2.60% ± 2.24% |
| Watterberg et al.51 | Intubated infants/toddlers | 9–36 months | NA | Travenol #2C7161 | ≤1% |
| Fok et al.52 | Intubated infants | Around 2.5 months | Around 1.7Kg | MedicAid Sidestream | 0.4 to 2.62% |
| Non-intubated infants | Around 3 months | Around 2.4Kg | 0.87 to 3.43% | ||
| Fok et al.53 | Ventilated rabbits | NA | Around 3Kg | Hudson RCI Flo Thru® | 3.34% |
| Dubus et al.27,28 | Ventilated macaques | 44 months | 2.5 to 2.8Kg | MistyNeb | 0.4 to 1.4% |
| Ventilated piglets | 2 days | 1.7Kg ± 0.3Kg | 0.50 to 7.70% | ||
| Cameron et al.54 | Ventilated rabbits | NA | 1.5Kg | Mallinckrodt Ultravent® | 2.8% |
However, this model raises few limitations, such as the supine position during nebulization process that decreases penetration of airborne particles and obviously the lack a physiopathological features of the disease. Thus, regional aerosol deposition could be slightly modified.
Conclusion
BPD induced mortality, as one of the most common diseases of preterm infants, decreased since neonatal care unit became more and more qualified. However, new techniques induced a modification of the physiopathology and morbidity of this disease partly due to side effects of mechanical ventilation and oxygen supplementation. In response, some drugs are used to wean prematurely born infants from ventilation or oxygen supplementation but they have adverse effects. Inhaled therapies could be an interesting route of administration for such treatments, aiming the specifically defective organ. However, uneven and/or uncontrolled deposition of drugs along respiratory tract is one the remaining challenge to improve inhaled therapies.
In this study, an ex vivo respiratory model of BPD was developed to assess aerosol regional deposition. This chimeric model consists in a 3D-printed infant upper airways replica connected to a leporine thorax placed in a sealed enclosure producing pleural depression. This method mimic passive ventilation by inducing negative pressure within the enclosure. Breathing pattern and parameters are easily and reproducibly controllable. Three RR were tested (i.e. 30, 35 and 40 cycles/min) that led to experimentally measure 6 parameters. Results obtained clearly demonstrated that the breathing pattern obtained for the chimeric model was very similar compared to in vivo data of 3-months old BPD-suffering infants available in the literature. Then, using 81mKr planar scintigraphies, homogeneity of ventilation of the developed model was proved to be comparable to ventilation behavior observed in infants. Lastly, a regional aerosol deposition with 99mTc-DTPA planar and 3D SPECT/CT experiments was performed. The results obtained were analogous to those found for in vivo studies from the literature. However, the developed model suffers from limitations, among others, the supine position and the lack of lung surfactant and blood perfusion. To counterbalance these limitations, this ex vivo chimeric model presents many advantages. It perfectly fits the 3R guidelines (Refine, Reduce and Replace) as a good and relevant surrogate model to animal experiments. Secondly, this model is relatively costs saving. Indeed, it does not need any animal husbandry (nor animals). Lastly, this model is easy to use and could be tuned as wanted. Hence, this study demonstrated that this new ex vivo model could reliably simulate BPD with less ethical restrictions and allowing an alternative to animal experiments.
Materials and Methods
Materials
Upper airways and larynx are composed of a stereolithographied infant nasal replica obtained by reconstruction from CT scan images of a SAINT model47 and by three-dimensional printing technology. It integrated laryngeal structures to mimic vocal folds resistances. This 3D-printed replica is well adapted and was previously used for nasal and pulmonary deposition studies26,47–49. Rabbit thoraxes are obtained from leporine slaughterhouses (Rouget Volailles, La Talaudière France), satisfying French sanitary controls, and were used within 24 hours or frozen at −20 °C depending on availability. Visual control of wound was performed and assessment of recruitment of each lung was carried out. Thoraxes were placed in a sealed enclosure and ventilated using a depression generator SuperDimension® (Covidien, Dusseldorf, Germany) mimicking pleural depression similarly to in vivo passive ventilation. A picture of the setup is available online as Supplementary Figs S1 and S2.
Forty leporine thoraxes were used to analyze respiratory parameters. Twelves of them (30%) were used within 48 h, others were frozen and thawed at least 12 hours before experiments due to supplier unavailability. Among 43 thoraxes, 5 (5%) were excluded due to major lung injuries that impaired ventilation. Thoraxes were not used for more than 8 hours. A video of ventilating model is available as Supplementary Video SV1. Before slaughter, rabbits were around 3Kg of bodyweight.
The 3R guidelines (Refine, Reduce and Replace) are 50 years old framework focused on ethical considerations of animal use in research. It defines ways to reduce animal suffering and increase reliability of produced data. Moreover, it recommends using substitutive methods to animal experiments. As our ex vivo model uses wastes of food industry instead of live animals, it fits in these guidelines and represents a good alternative to animal experiments.
Passive ventilation assessment
Instrumentation of the enclosure was realized with a Biopac® system (Biopac, Goleta, USA) composed of a pneumotachograph (TSD 117) and a differential pressure transducer (TSD160D), which are connected to amplifier (DA100C) plugged on unit acquisition (M160). This system allowed a real-time follow-up of depression in the enclosure and airflow at the replica. AcqKnowledge® 5.0 software was used to determine respiratory parameters for each cycle: peak inspiratory flow (PIF), peak expiratory flow (PEF), tidal volume (TV), minute-ventilation (MV), respiratory rate (RR), inspiratory time (IT), expiratory time (ET), total time (TT), resistances (R) and compliance (C).
Data were recorded for 3 minutes with fixed IT of 0.4 s and tuned ET to reach 30, 35 and 40 respiratory rates (1.6 s, 1.3 s and 1.1 s respectively). Average, standard deviation and 95% confidence interval were calculated for at least 50 cycles to obtain a representative values for each thorax. Breathing patterns and parameters were chosen to represent infants with BPD at rest.
Ventilation and regional aerosol deposition
Regional ventilation was assessed using 81mkrypton (81mKr) scintigraphy50 while regional aerosol deposition was observed with 99mtechnetium complexed with diethylene triamine pentaacetic acid (99mTc-DTPA) scintigraphy. Aerosol was generated from a 100MBq in 3 mL solution of 99mTc-DTPA using a Sidestream® jet nebulizer (Philips Healthcare, Suresnes, France) connected to the infant nasal replica with a naso-buccal mask (Ambu®, Bordeaux, France).
For ventilation scintigraphies, an acquisition was made for each RR with a naso-buccal mask. Two different regions of interest (ROI) were identified on ventilation scintigraphies to define left and right lung. Additional details about the method are provided in the online data supplement. Scintigraphic measurements of ventilation using 81mKr were made on 14 thoraxes but 1 (7.1%) had to be censored due to obstruction of a primary bronchi leading to biased repartition of the gaz.
For aerosol regional deposition study, 6 thoraxes were used for 2D scintigraphies. These planar scintigraphic images (matrix 256*256) were recorded with a variable angle dual detector Single Photon Emission Computed TomographY/Computed Tomography(SPECT/ CT, SYMBIA T2; Siemens, Knoxville, TN) equipped with a low-energy, high-resolution collimator (FWHM 8.3 mm at 10 cm); tested weekly for uniformity (UFOV 533 mm × 387 mm, CFOV 400 mm × 290 mm). Before conducting the inhalation experiments, the initial radioactive doses filled in the nebulizer were quantified (scintigraphic images, 60-sec anterior/posterior, were acquired corresponding to the full and empty syringe). Once the inhalation experiments were performed, 180-sec anterior/posterior images of the experimental setup were acquired for each element: empty nebulizer, expiratory filter, infant nasal replica and lungs. An interest ROI was delimited on the images with a correction of the background radiation using the mean of three external ROIs. Results were expressed in terms of the activity loaded into the nebulizer.
After acquiring 2D images with the same gamma camera (SYMBIA T2; Siemens, Knoxville, TN), SPECT and CT acquisitions were performed immediately for attenuation correction and anatomical mapping. A 3D SPECT acquisition of the lungs was performed with 64 (2 × 32) projection images, each 30 s. Finally, a CT was performed with the following parameters: 130 kV, 90mAs, 1.25 mm slice thickness, 0.9 mm increment, 1.6 mm pitch, and rotation time of 1.5 s. A multimodality computer platform (Symbia net; Siemens) was used for image reviews and manipulations. Both the transmission and emission scans were reconstructed using 3D OSEM by default (8 subsets, 5 iterations), with pre-reconstruction smoothing using a 3D Butterworth filter (cutoff: 0.45 cycles/cm; order 5), a 128 × 128 image matrix, a 1.23 zoom, and a pixel size of 3.9 mm. SPECT images were reconstructed using scatter correction (scatter energy window) and CT attenuation correction. CT and SPECT images were matched and fused into trans-axial images. The tracheobronchial area included the trachea and the left and right main bronchi. There, 1 thorax was used to collect data.
Statistical analyses
Results are expressed as mean ± standard deviation (95% confidence interval). For respiratory parameters, Gaussian distribution was assessed with a Shapiro-Wilk normality test. If Gaussian distribution was validated, a repeated-measure one-way ANOVA with Tukey’s multiple comparison post hoc test was used. If distribution was not Gaussian, Friedmann’s test with Dunn’s multiple comparison post hoc test was performed. For ventilation studies, a paired t-test was used to compare left and right lung fraction of total counted radioactivity. All tests were two-sided and p < 0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism® 7 (GraphPad Software, La Jolla, CA, USA).
Supplementary information
Author Contributions
Y.M., S.P.R., N.P. and J.P. designed experiments. Y.M., S.P.R., L.L., M.S., C.G., A.C., N.P. and J.P. conducted the experiments. All authors analyzed the data. Y.M. wrote the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-42103-2.
References
- 1.Kair LR, Leonard DT, Anderson JM, Med Bronchopulmonary Dysplasia. Pediatr. Rev. 2012;33:255–264. doi: 10.1542/pir.33-6-255. [DOI] [PubMed] [Google Scholar]
- 2.Jensen EA, Schmidt B. Epidemiology of bronchopulmonary dysplasia. Birt. Defects Res. A. Clin. Mol. Teratol. 2014;100:145–157. doi: 10.1002/bdra.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalikkot Thekkeveedu R, Guaman MC, Shivanna B. Bronchopulmonary dysplasia: A review of pathogenesis and pathophysiology. Respir. Med. 2017;132:170–177. doi: 10.1016/j.rmed.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bronchopulmonary dysplasia: Pathophysiology and potential anti-inflammatory therapies. Paediatr. Respir. Rev. 10.1016/j.prrv.2018.07.007 (2018). [DOI] [PubMed]
- 5.Hadchouel A, Franco-Montoya M-L, Delacourt C. Altered lung development in bronchopulmonary dysplasia. Birt. Defects Res. A. Clin. Mol. Teratol. 2014;100:158–167. doi: 10.1002/bdra.23237. [DOI] [PubMed] [Google Scholar]
- 6.Ali Z, Schmidt P, Dodd J, Jeppesen DL. Bronchopulmonary dysplasia: a review. Arch. Gynecol. Obstet. 2013;288:325–333. doi: 10.1007/s00404-013-2753-8. [DOI] [PubMed] [Google Scholar]
- 7.Keszler M, Sant’Anna G. Mechanical Ventilation and Bronchopulmonary Dysplasia. Clin. Perinatol. 2015;42:781–796. doi: 10.1016/j.clp.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Simpson SJ, Hall GL, Wilson AC. Lung function following very preterm birth in the era of ‘new’ bronchopulmonary dysplasia. Respirology. 2015;20:535–540. doi: 10.1111/resp.12503. [DOI] [PubMed] [Google Scholar]
- 9.Amin RS, Rutter MJ. Airway Disease and Management in Bronchopulmonary Dysplasia. Clin. Perinatol. 2015;42:857–870. doi: 10.1016/j.clp.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Cole CH. Postnatal glucocorticoid therapy for prevention of bronchopulmonary dysplasia: routes of administration compared. Semin. Neonatol. 2001;6:343–350. doi: 10.1053/siny.2001.0069. [DOI] [PubMed] [Google Scholar]
- 11.Darlow BA, Morley CJ. Oxygen Saturation Targeting and Bronchopulmonary Dysplasia. Clin. Perinatol. 2015;42:807–823. doi: 10.1016/j.clp.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 12.McEvoy CT, Aschner JL. The Natural History of Bronchopulmonary Dysplasia: The Case for Primary Prevention. Clin. Perinatol. 2015;42:911–931. doi: 10.1016/j.clp.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhandari A, McGrath-Morrow S. Long-term pulmonary outcomes of patients with bronchopulmonary dysplasia. Semin. Perinatol. 2013;37:132–137. doi: 10.1053/j.semperi.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Delara, M. et al. Efficacy and safety of pulmonary application of corticosteroids in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch. Dis. Child. - Fetal Neonatal Ed. fetalneonatal-2017-314046, 10.1136/archdischild-2017-314046 (2018). [DOI] [PubMed]
- 15.Onland, W., Offringa, M. & Kaam, A. van. Late (≥ 7 days) inhalation corticosteroids to reduce bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 10.1002/14651858.CD002311.pub4 (2017).
- 16.Shah SS, Ohlsson A, Halliday HL, Shah VS. Inhaled versus systemic corticosteroids for preventing bronchopulmonary dysplasia in ventilated very low birth weight preterm neonates. Cochrane Database Syst. Rev. 2017 doi: 10.1002/14651858.CD002058.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah SS, Ohlsson A, Halliday HL, Shah VS. Inhaled versus systemic corticosteroids for the treatment of bronchopulmonary dysplasia in ventilated very low birth weight preterm infants. Cochrane Database Syst. Rev. 2017 doi: 10.1002/14651858.CD002057.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onland W, Jaegere APD, Offringa M, Kaam Avan. Systemic corticosteroid regimens for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst. Rev. 2017 doi: 10.1002/14651858.CD010941.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassler D, et al. Long-Term Effects of Inhaled Budesonide for Bronchopulmonary Dysplasia. N. Engl. J. Med. 2018 doi: 10.1056/NEJMoa1708831. [DOI] [PubMed] [Google Scholar]
- 20.Schüepp KG, et al. In Vitro Determination of the Optimal Particle Size for Nebulized Aerosol Delivery to Infants. J. Aerosol Med. 2005;18:225–235. doi: 10.1089/jam.2005.18.225. [DOI] [PubMed] [Google Scholar]
- 21.Geiser M, Kreyling WG. Deposition and biokinetics of inhaled nanoparticles. Part. Fibre Toxicol. 2010;7:2. doi: 10.1186/1743-8977-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baskin MI. Regional Deposition of Aerosolized Pentamidine: Effects of Body Position and Breathing Pattern. Ann. Intern. Med. 1990;113:677. doi: 10.7326/0003-4819-113-9-677. [DOI] [PubMed] [Google Scholar]
- 23.Oosthuizen MA, Oberholzer HM, Scriba MR, van der Spuy WJ, Pretorius E. Evaluation of the morphological changes in the lungs of BALB/c mice after inhalation of spherical and rod-shaped titanium nanoparticles. Micron. 2012;43:863–869. doi: 10.1016/j.micron.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 24.McKinney W, et al. Pulmonary and cardiovascular responses of rats to inhalation of a commercial antimicrobial spray containing titanium dioxide nanoparticles. Inhal. Toxicol. 2012;24:447–457. doi: 10.3109/08958378.2012.685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Reilly M, Thébaud B. Animal models of bronchopulmonary dysplasia. The term rat models. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2014;307:L948–L958. doi: 10.1152/ajplung.00160.2014. [DOI] [PubMed] [Google Scholar]
- 26.Albuquerque-Silva I, et al. Particle Deposition in a Child Respiratory Tract Model: In Vivo Regional Deposition of Fine and Ultrafine Aerosols in Baboons. PLOS ONE. 2014;9:e95456. doi: 10.1371/journal.pone.0095456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubus JC, et al. Aerosol Deposition in Neonatal Ventilation. Pediatr. Res. 2005;58:10–14. doi: 10.1203/01.PDR.0000156244.84422.55. [DOI] [PubMed] [Google Scholar]
- 28.Dubus J-C, et al. Lung Deposition of HFA Beclomethasone Dipropionate in an Animal Model of Bronchopulmonary Dysplasia. Pediatr. Res. 2007;61:21–25. doi: 10.1203/01.pdr.0000250055.26148.42. [DOI] [PubMed] [Google Scholar]
- 29.Dubus J-C, Rhem R, Monkman S, Dolovich M. Delivery of Salbutamol Pressurized Metered-Dose Inhaler Administered Via Small-Volume Spacer Devices in Intubated, Spontaneously Breathing Rabbits. Pediatr. Res. 2001;50:384–389. doi: 10.1203/00006450-200109000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Köbrich R, Rudolf G, Stahlhofen W. A Mathematical Model of Mass Deposition in Man. Ann. Occup. Hyg. 1994;38:15–23. [Google Scholar]
- 31.Phalen RF, Oldham MJ. Methods for modeling particle deposition as a function of age. Respir. Physiol. 2001;128:119–130. doi: 10.1016/S0034-5687(01)00270-5. [DOI] [PubMed] [Google Scholar]
- 32.Phalen RF, Oldham MJ, Beaucage CB, Crocker TT, Mortensen J. Postnatal enlargement of human tracheobronchial airways and implications for particle deposition. Anat. Rec. 1985;212:368–380. doi: 10.1002/ar.1092120408. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann W. Mathematical model for the postnatal growth of the human lung. Respir. Physiol. 1982;49:115–129. doi: 10.1016/0034-5687(82)90106-2. [DOI] [PubMed] [Google Scholar]
- 34.Perinel, S. et al. Development of an ex vivo human-porcine respiratory model for preclinical studies. Sci. Rep. 7 (2017). [DOI] [PMC free article] [PubMed]
- 35.Perinel, S. et al. Micron-sized and submicron-sized aerosol deposition in a new ex vivo preclinical model. Respir. Res. 17 (2016). [DOI] [PMC free article] [PubMed]
- 36.Patzak A, Foitzik B, Mrowka R, Schmalisch G. Time of measurement influences the variability of tidal breathing parameters in healthy and sick infants. Respir. Physiol. 2001;128:187–194. doi: 10.1016/S0034-5687(01)00277-8. [DOI] [PubMed] [Google Scholar]
- 37.Latzin, P. et al. Lung Volume, Breathing Pattern and Ventilation Inhomogeneity in Preterm and Term Infants. PLoS ONE4 (2009). [DOI] [PMC free article] [PubMed]
- 38.Olden, C., Symes, E. & Seddon, P. Measuring tidal breathing parameters using a volumetric vest in neonates with and without lung disease. Pediatr. Pulmonol. 45, 1070–1075 [DOI] [PubMed]
- 39.Fok TF, et al. Randomised crossover trial of salbutamol aerosol delivered by metered dose inhaler, jet nebuliser, and ultrasonic nebuliser in chronic lung disease. Arch. Dis. Child. - Fetal Neonatal Ed. 1998;79:F100–F104. doi: 10.1136/fn.79.2.F100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenspan JS, Wolfson MR, Locke RG, Allen JL, Shaffer TH. Increased Respiratory Drive and Limited Adaptation to Loaded Breathing in Bronchopulmonary Dysplasia. Pediatr. Res. 1992;32:356–359. doi: 10.1203/00006450-199209000-00022. [DOI] [PubMed] [Google Scholar]
- 41.Pfenninger J, Aebi C. Respiratory response to salbutamol (albuterol) in ventilator-dependent infants with chronic lung disease: pressurized aerosol delivery versus intravenous injection. Intensive Care Med. 1993;19:251–255. doi: 10.1007/BF01690544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.West, J. B. & West, J. B. Pulmonary pathophysiology: the essentials. (Wolters Kluwer/Lippincott Williams & Wilkins Health, 2012).
- 43.Bajc M, et al. EANM guidelines for ventilation/perfusion scintigraphy. Eur. J. Nucl. Med. Mol. Imaging. 2009;36:1356–1370. doi: 10.1007/s00259-009-1170-5. [DOI] [PubMed] [Google Scholar]
- 44.Li DK, et al. Krypton-81m: A Better Radiopharmaceutical for Assessment of Regional Lung Function in Children. Radiology. 1979;130:741–747. doi: 10.1148/130.3.741. [DOI] [PubMed] [Google Scholar]
- 45.Björkman KC, et al. Postoperative regional distribution of pulmonary ventilation and perfusion in infants with congenital diaphragmatic hernia. J. Pediatr. Surg. 2011;46:2047–2053. doi: 10.1016/j.jpedsurg.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 46.Kjellberg M, Björkman K, Rohdin M, Sanchez-Crespo A, Jonsson B. Bronchopulmonary dysplasia: Clinical grading in relation to ventilation/perfusion mismatch measured by single photon emission computed tomography. Pediatr. Pulmonol. 2013;48:1206–1213. doi: 10.1002/ppul.22751. [DOI] [PubMed] [Google Scholar]
- 47.Janssens HM, et al. The Sophia Anatomical Infant Nose-Throat (Saint) Model: A Valuable Tool to Study Aerosol Deposition in Infants. J. Aerosol Med. 2001;14:433–441. doi: 10.1089/08942680152744640. [DOI] [PubMed] [Google Scholar]
- 48.Laube BL, et al. Deposition of Albuterol Aerosol Generated by Pneumatic Nebulizer in the Sophia Anatomical Infant Nose-Throat (SAINT) Model. Pharm. Res. 2010;27:1722–1729. doi: 10.1007/s11095-010-0171-1. [DOI] [PubMed] [Google Scholar]
- 49.Réminiac F, et al. Nasal high flow nebulization in infants and toddlers: An in vitro and in vivo scintigraphic study. Pediatr. Pulmonol. 2017;52:337–344. doi: 10.1002/ppul.23509. [DOI] [PubMed] [Google Scholar]
- 50.Fazio F, Jones T. Assessment of regional ventilation by continuous inhalation of radioactive krypton-81m. Br. Med. J. 1975;3:673–676. doi: 10.1136/bmj.3.5985.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watterberg KL, Clark AR, Kelly HW, Murphy S. Delivery of aerosolized medication to intubated babies. Pediatr. Pulmonol. 1991;10:136–141. doi: 10.1002/ppul.1950100217. [DOI] [PubMed] [Google Scholar]
- 52.Fok TF, et al. Efficiency of aerosol medication delivery from a metered dose inhaler versus jet nebulizer in infants with bronchopulmonary dysplasia. Pediatr. Pulmonol. 1996;21:301–309. doi: 10.1002/(SICI)1099-0496(199605)21:5<301::AID-PPUL5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 53.Fok T-F, et al. Pulmonary Deposition of Salbutamol Aerosol Delivered by Metered Dose Inhaler, Jet Nebulizer, and Ultrasonic Nebulizer in Mechanically Ventilated Rabbits. Pediatr. Res. 1997;42:721. doi: 10.1203/00006450-199711000-00027. [DOI] [PubMed] [Google Scholar]
- 54.Cameron D, Arnot R, Clay M, Silverman M. Aerosol delivery in neonatal ventilator circuits: A rabbit lung model. Pediatr. Pulmonol. 1991;10:208–213. doi: 10.1002/ppul.1950100314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.