Abstract
Rhizopus spp are the most common etiological agents of mucormycosis, causing over 90% mortality in disseminated infection. Key to pathogenesis is the ability of fungal spores to swell, germinate, and penetrate surrounding tissues. Antibiotic treatment in at-risk patients increases the probability of the patient developing mucormycosis, suggesting that bacteria have the potential to control the growth of the fungus. However, research into polymicrobial relationships involving Rhizopus spp has not been extensively explored. Here we show that co-culturing Rhizopus microsporus and Pseudomonas aeruginosa results in the inhibition of spore germination. This inhibition was mediated via the secretion of bacterial siderophores, which induced iron stress on the fungus. Addition of P. aeruginosa siderophores to R. microsporus spores in the zebrafish larval model of infection resulted in inhibition of fungal germination and reduced host mortality. Therefore, during infection antibacterial treatment may relieve bacterial imposed nutrient restriction resulting in secondary fungal infections.
Introduction
Mucormycosis is a life threatening, disfiguring infection caused by ubiquitous environmental fungi belonging to the order Mucorales, with Rhizopus spp. accounting for approximately 70% of infections1,2. In healthy individuals, innate immune cells are capable of controlling spore germination, thus preventing infection3. However, patients with uncontrolled diabetes, cancer, neutropenia, burn/traumatic wounds, post-transplantation and those undergoing corticosteroid therapy or renal dialysis are prone to mucormycosis4,5. Mucorales are inherently resistant to antifungals, requiring surgical debridement of infected tissue followed by an aggressive antifungal regime. As a result, mucormycosis is associated with high mortality rates (up to 96% in disseminated infections), and significant morbidity2.
Mucorales spores enter the body through inhalation or open wounds6. As a result mucormycosis is commonly associated with pulmonary, rhinocerebral, or cutaneous infections7. Germination is key to the pathogenesis of Mucorales, leading to tissue penetration, endothelial angioinvasion, and vessel thrombosis, ultimately resulting in debilitating necrosis4. Traumatic and burn wound infections, including military-associated blast wounds, are known predisposing conditions for mucormycosis in the immunocompetent2, with over 70% of these infections being polymicrobial in nature8,9. Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli are the most commonly co-isolated bacterial species from chronic wounds10,11, and are therefore likely to interact and compete with Mucorales spores. In addition, the emergence of mucormycosis has been associated with broad-spectrum antimicrobial treatment12–14, suggesting that the surrounding microbiome plays a role in controlling fungal growth.
Here we show that P. aeruginosa inhibits the germination, and therefore virulence, of Rhizopus microsporus, a common cause of mucormycosis. This inhibition was mainly caused by bacterial secretion of iron-chelating molecules, which sequester iron from the fungus. Considering the prevalence of opportunistic bacteria and Mucorales in traumatic wounds, antibacterial treatment may reduce the presence of nutrient-restricting molecules like bacterial secreted siderophores in the wound environment rendering the environment more permissive to fungal germination, although we acknowledge that other factors including the immune status of the host also play critical roles in controlling fungal infection.
Results
Pseudomonas aeruginosa inhibits the germination of Rhizopus microsporus
Key to the pathogenesis of mucormycosis is the ability of spores to germinate and penetrate surrounding tissues4. To identify whether bacteria can influence fungal germination, R. microsporus spores were co-cultured with Pseudomonas aeruginosa, Burkholderia cenocepacia, Staphylococcus aureus, and Escherichia coli. Co-culture of R. microsporus with P. aeruginosa at multiplicities of infection (MOI) of 1:50 and 1:100 resulted in 56.8% (+/−8.269, p = 0.0023) and 92% (+/−2.784, p < 0.001) inhibition of fungal germination, respectively (Fig. 1A,B). Conversely, co-culturing R. microsporus spores with S. aureus, E. coli, and B. cenocepacia did not affect fungal growth at any of the MOIs tested (Fig. 1A). Taken together, the results obtained from live co-cultures between R. microsporus and P. aeruginosa indicate that these two microbes undergo a competitive relationship resulting in reduced fungal germination.
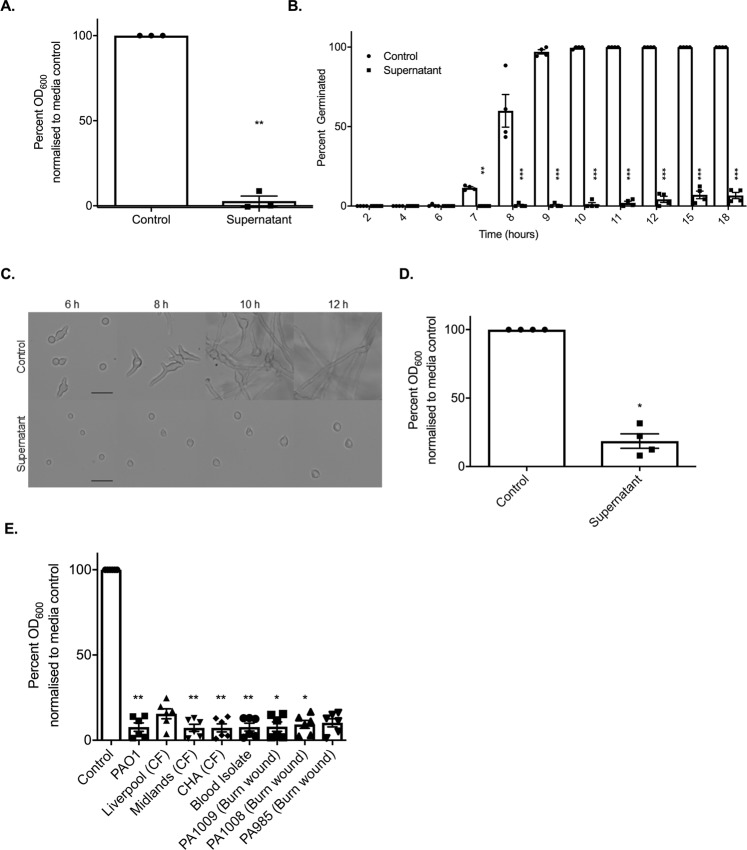
Figure 1.
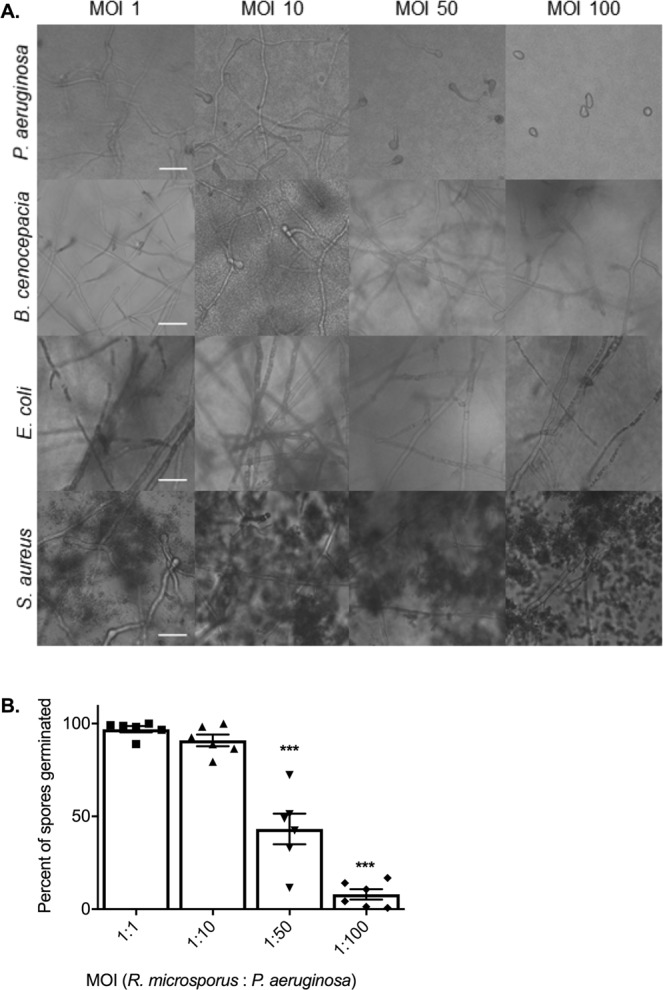
Pseudomonas aeruginosa strongly inhibits the germination of Rhizopus microsporus. R. microsporus spores were incubated with live P. aeruginosa, B. cenocepacia, S. aureus, and E. coli at increasing multiplicities of infection (MOI) for 24 h (A) Representative images after 24 h exposure (37 °C, static). Scale bars depict 50 µm. (B) Per cent of spores germinated after 24 h exposure to P. aeruginosa. One-way ANOVA performed on arcsine transformed data (n = 6). ***p < 0.001. Error bars depict SEM.
P. aeruginosa inhibits spore germination through secreted factors
Microbes are able to communicate through the secretion of secondary metabolites, quorum sensing molecules, and metabolic by-products15–20. Therefore, to deduce whether the observed inhibition of R. microsporus germination was a result of direct cell-cell interactions or mediated through secreted products, R. microsporus spores were incubated in P. aeruginosa spent culture supernatants. Incubation of R. microsporus spores with 50% P. aeruginosa supernatant resulted in 94.4% (+/−0.01769, p = 0.0022) inhibition of fungal growth (Fig. 2A), confirming that the inhibitory molecule(s) are secreted by P. aeruginosa. Time-lapse microscopy confirmed that the presence of the supernatant resulted in a significant reduction in spore germination (Fig. 2B,C, Videos 1 and 2), with only 6.7% (+/−3.8, p < 0.0001) of spores germinating after 18 h. However, the inhibition of germination did not affect spore swelling (Videos 1 and 2). To deduce whether P. aeruginosa supernatants are able to inhibit fungal growth after the initiation of germination, spores were pre-germinated, and then subsequently incubated with 50% supernatant. Fungal growth was significantly reduced (by 81.4% +/− 5.252, p = 0.0286) in the presence of the supernatant compared to the media control (Fig. 2D). Therefore, P. aeruginosa supernatants are able to inhibit both germination and growth of R. microsporus.
Figure 2.
P. aeruginosa inhibits spore germination through secreted factors. R. microsporus spores were exposed to 50% P. aeruginosa PAO1 supernatant for 24 h. (A) Fungal growth was measured through absorbance (OD600) and normalised to media control (n = 3). To determine the point of inhibition, spore germination was observed via live-cell imaging and (B) the per cent of spores germinated over time was quantified (n = 4, Two-way ANOVA performed on arcsine transformed data). (C) Representative images of spores germinating over time were collected. Scale bar = 50 µm. (D) To determine whether the supernatant also inhibits the continuation of growth after germination is initiated, spores were incubated in SAB for 4–5 h until germlings emerged, and subsequently exposed to 50% PAO1 supernatant for 18 h (n = 4, Mann-Whitney U test). (E) To test whether this is a lab strain-specific phenomenon, R. microsporus spores were exposed to supernatants from P. aeruginosa clinical isolates for 24 h (n = 6). Fungal growth was determined through absorbance (OD600). All data was analysed by a Kruskal-Wallis test with Dunn’s multiple comparisons test unless indicated otherwise. *p < 0.05, **p < 0.01, ***p < 0.001. Error bars depict SEM.
To determine whether the inhibitory molecule(s) is produced by other P. aeruginosa strains, we tested the ability of supernatants from a series of P. aeruginosa isolates to inhibit spore germination. R. microsporus germination was inhibited in the presence of supernatants from all P. aeruginosa clinical isolates (Fig. 2E), suggesting that the production of this inhibitory molecule is a general trait of P. aeruginosa and is not limited to laboratory-evolved strains.
As fungal germination is dependent on environmental pH and nutrient availability21,22, we assessed whether the addition of the supernatant was inhibiting germination through modulation of these parameters. Addition of the bacterial supernatant to SAB broth resulted in mild alkalisation of the media (pH 7.33 vs. 6.45). However, adjusting the pH of the control media to pH 7.33, to mimic the conditions in media containing the P. aeruginosa supernatant, did not affect R. microsporus germination rates (Supplementary Fig. S1). To elucidate the role of macronutrient restriction, SAB broth was diluted with 50% phosphate buffered solution (PBS) to mimic the nutrient limitation imposed by the addition of 50% supernatant. However, the spores were still able to germinate under these conditions (Supplementary Fig. S1). Therefore, P. aeruginosa secretes a molecule(s) that is able to inhibit R. microsporus germination independent of pH and macronutrient limitation.
Inhibition of R. microsporus germination is not mediated by quorum sensing molecules or pyocyanin
Bacteria secrete a diverse range of proteins and secondary metabolites to aid in host colonisation and inter-species competition. To determine whether the secreted factor responsible for inhibiting spore germination is proteinaceous, P. aeruginosa supernatants were boiled or treated with Proteinase K to degrade any secreted proteins. Supernatants that were boiled or treated with Proteinase K inhibited R. microsporus growth (97.62%, +/−1.558, p = 0.0355 and 99.03%, +/−1.634, p = 0.0140, respectively) (Supplementary Fig. S2A), suggesting that a secreted, heat-stable molecule mediates the observed inhibition of R. microsporus germination.
P. aeruginosa secretes several heat-stable cell density-dependent signalling molecules into the environment to regulate virulence by sensing population density and inducing the expression or inhibition of population-dependent mechanisms23. These quorum sensing molecules (QSMs) are well known to regulate intra- and inter-species interactions including inhibiting the morphological switch of Candida albicans24–27. Therefore, we tested the ability of the major P. aeruginosa QSMs to inhibit R. microsporus germination. Exposure of R. microsporus spores to N-butanoyl-l-homoserine lactone (C4 HSL), N-hexanoyl-DL-homoserine lactone (C6 HSL), and N-octanoyl-L-homoserine lactone (C8 HSL), did not affect fungal growth (Supplementary Fig. S2B–D). At high concentrations (200 μM) N-(3-oxododecanoyl)-L-homoserine lactone (C12 HSL) resulted in 42.1% (+/−0.1518, p = 0.1331) reduction in fungal growth (Supplementary Fig. S2E). Therefore, secreted QSMs appear to not be the major regulators of R. microsporus growth.
Pyocyanin is a heat stable, secreted blue-pigmented toxin, which is known to increase the virulence of P. aeruginosa by depressing the host immune responses through induction of neutrophil apoptosis28,29. Pyocyanin also inhibits the growth and morphogenesis of C. albicans and Aspergillus fumigatus30. Therefore, we determined whether the presence of pyocyanin in the supernatant was inhibiting the germination of R. microsporus. Addition of purified pyocyanin resulted in 31.4% (+/−0.1434, p > 0.9999) inhibition of R. microsporus growth at concentrations above 100 μM (Supplementary Fig. S2F). To deduce whether these pyocyanin concentrations were physiologically relevant the concentration of pyocyanin in the P. aeruginosa supernatants was quantified through absorbance measurement (690 nm) and compared to a standard curve of pre-defined pyocyanin concentrations. Growth of P. aeruginosa in LB media at 200 rpm, 37 °C did not result in the secretion of detectable levels of pyocyanin (not shown). Therefore, the inhibition of R. microsporus growth in the P. aeruginosa supernatant was not due to pyocyanin.
To determine whether the secreted factor is a lipophilic molecule, chloroform extractions were performed. The organic phase of the supernatant did not significantly inhibit spore germination (18% inhibition, +/−3.058, p = 0.0926), while the aqueous phase maintained its inhibitory action (95.1% inhibition, +/−0.9559, p = 0.0079, Supplementary Fig. S2G). Therefore, a secreted, heat-stable, water-soluble molecule(s) inhibits the growth of R. microsporus.
P. aeruginosa inhibits R. microsporus germination via iron sequestration
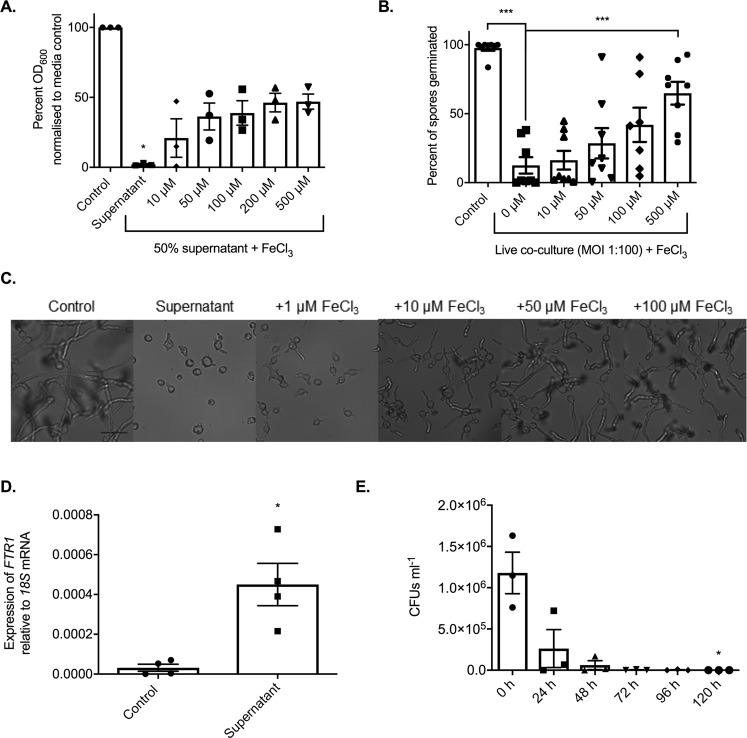
Research has established the importance of metal micronutrients for microbial growth and pathogenicity, and the ability of host metal sequestering proteins to inhibit both fungal and bacterial growth through nutritional immunity31–33. Iron, zinc, copper, and manganese are considered the most important trace metals for the growth of fungi and the availability of iron is key to the pathogenesis of Mucorales34,35. Therefore, we investigated whether the supernatants were imposing micronutrient restriction on R. microsporus. Supplementing the supernatants with iron was able to partially restore fungal growth, resulting in 46.2% R. microsporus growth at concentrations above 200 µM (+/−6.660, Fig. 3A,C) and an insignificant difference as compared to the control (p > 0.9999). However, supplementation with zinc, copper or manganese did not rescue R. microsporus growth (Supplementary Fig. S3). Therefore, the majority of growth inhibition appears to result from the P. aeruginosa supernatants specifically sequestering iron from the environment. However, we acknowledge that other unidentified factors may play a role as supplementation with iron did not completely restore fungal growth. To confirm that the inhibition of spore germination observed in the co-cultures was also attributed to iron restriction, co-cultures of R. microsporus and P. aeruginosa were spiked with iron. Germination and therefore growth of R. microsporus in the co-culture was recovered at concentrations above 100 µM (41.9% +/− 12.46, p = 0.1540) to levels comparable to those observed for iron spiked supernatants (Fig. 3B), confirming that in both scenarios the major contributing factor, resulting in the inhibition of fungal growth, appears to be the sequestration of iron by P. aeruginosa.
Figure 3.
P. aeruginosa inhibits R. microsporus germination via iron sequestration. R. microsporus spores were exposed to (A) 50% P. aeruginosa supernatant and spiked with increasing concentrations of iron chloride for 24 h statically at 37 °C. Fungal growth was measured through absorbance (OD600) and normalised to media control (n = 3, Kruskal-Wallis test with Dunn’s multiple comparisons test). (B) This ability to rescue was confirmed in a live co-culture setting, where the addition of exogenous iron increased the per cent of spores germinated after 24 h in a dose-dependent manner (n = 8). As the addition of iron in 50% supernatant increased overall growth, (C) representative images were collected at 9 h to confirm ability to rescue germination. Scale bar depicts 50 µm. (D) Iron starvation of R. microsporus spores after 7 h exposure to P. aeruginosa supernatant was determined through strong upregulation of the high-affinity iron permease FTR1 (n = 4, Mann-Whitney U test). (E) As iron starvation is associated with Mucorales apoptosis, the viability of spores exposed to 100% P. aeruginosa supernatant over time was quantified by counting colony forming units (CFUs) every 24 h for 120 h (n = 3, Kruskal-Wallis test with Dunn’s multiple comparisons test). *p < 0.05, ***p < 0.001.
Iron starvation has previously been shown to up-regulate the high affinity iron permease FTR1 in other Rhizopus species36. Therefore, to confirm that R. microsporus is undergoing iron starvation in the presence of P. aeruginosa supernatants, the expression levels of FTR1 were determined by qRT-PCR. FTR1 was highly upregulated (10-fold increase, p = 0.0286) when exposed to 50% P. aeruginosa supernatant for 7 h, as compared to the control (Fig. 3D). This confirms that P. aeruginosa mediated iron restriction inhibits R. microsporus growth and germination.
Iron starvation has been shown to induce apoptosis in R. oryzae after prolonged starvation36. Therefore, if spores are undergoing iron starvation when exposed to P. aeruginosa supernatant, prolonged exposure should decrease survival. To isolate the effects of the supernatant, we used 100% P. aeruginosa supernatant to monitor spore survival over time. In this condition, the viability of spores was reduced by 82.40% (+/−13.44) after 24 h, and no viable spores were recovered after 120 h (p = 0.0490, Fig. 3E). This indicates that iron is essential for the survival and pathogenicity of R. microsporus.
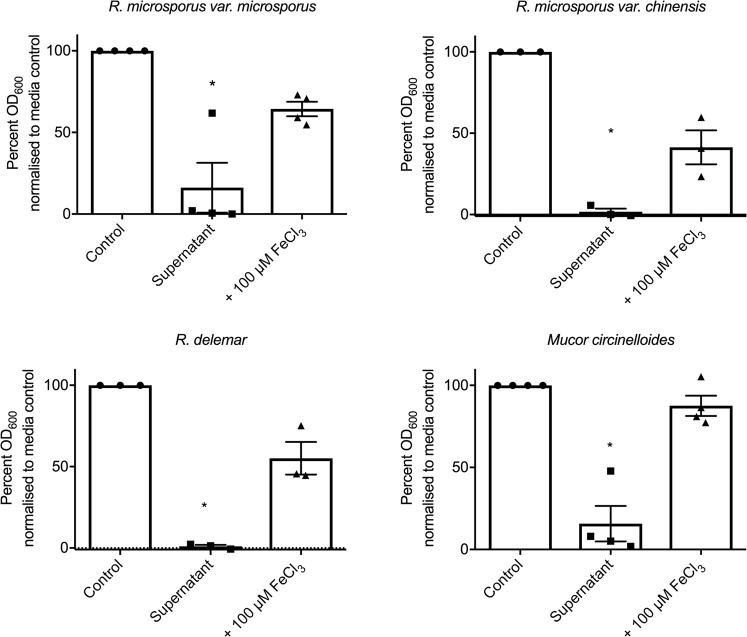
To delineate whether bacteria-associated iron restriction inhibits the growth of Mucorales in general, we tested the ability of P. aeruginosa supernatant to inhibit the growth of R. microsporus var. microsporus, R. microsporus var. chinensis, R. delemar, and Mucor circinelloides. The growth of all isolates was significantly reduced in the presence of P. aeruginosa supernatant [83.85% (+/−15.23), 98.26% (+/−1.977), 99.01% (+/−0.8695), and 87.54% (+/−10.78), respectively], and was significantly rescued by the addition of 100 μM of iron [64.36% (+/−4.450), 41.30% (+/−10.48), 55.14% (+/−10.01), and 87.54% (+/−6.219), respectively]. This confirms that the bacteria-associated inhibition of growth is a general trait of Mucorales and highlights the potential differences between Mucorales strains in response to bacteria (Fig. 4).
Figure 4.
Iron-dependent inhibition of Mucorales by P. aeruginosa is not R. microsporus strain-specific. Most experiments in this study were performed using an R. microsporus clinical isolate. To ensure the inhibitory effect of P. aeruginosa is not limited to this isolate, R. microsporus var. microsporus, R. microsporus var. chinensis, R. delemar, and Mucor circinelloides were exposed to 50% P. aeruginosa supernatant with and without the addition of 100 µM FeCl3. Fungal growth was determined at 24 h by measuring absorbance (OD600) and normalising to control (n = 3). *p < 0.05. All data was analysed by a Kruskal-Wallis test with Dunn’s multiple comparisons test.
Siderophore deficient P. aeruginosa strains lack the ability to suppress germination
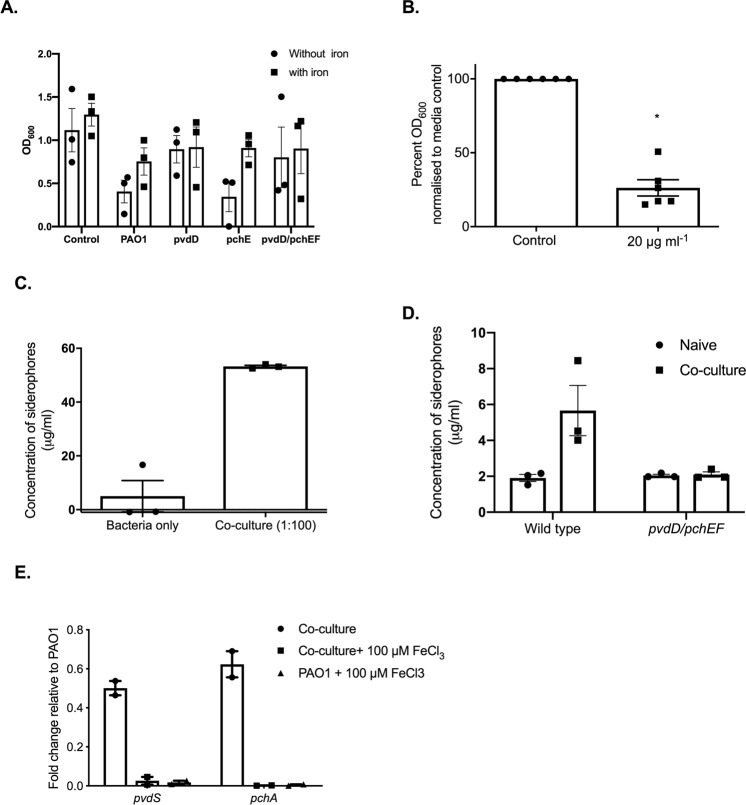
Iron sequestering is mediated via iron binding proteins and molecules known as siderophores. Pyoverdine and pyochelin are the two predominate siderophores produced by P. aeruginosa, with pyoverdine exhibiting the highest affinity for iron37,38. To identify the role of these siderophores in this interaction, we quantified fungal growth in the presence of the supernatants from P. aeruginosa strains deficient in either siderophore alone, or in combination. Growth of R. microsporus was inhibited when incubated with culture supernatants from P. aeruginosa strains defective in pyochelin biosynthesis (∆pchEF), with growth being rescued by exogenous iron (Fig. 5A), suggesting that this siderophore plays a minor role in sequestering iron in these experiments. However, R. microsporus germinated in the presence of bacterial supernatants from P. aeruginosa mutants defective in pyoverdine biosynthesis (∆pvdD) or in pyoverdine and pyochelin biosynthesis (∆pchEF∆pvdD) (Fig. 5A), confirming that, under the tested conditions, P. aeruginosa imposed iron restriction is largely mediated by the secretion of pyoverdine.
Figure 5.
P. aeruginosa-imposed iron restriction is largely mediated via pyoverdine production. (A) R. microsporus was grown in bacterial supernatants from wild type P. aeruginosa, strains defective in siderophore biosynthesis, or standard LB mixed 25:75 with SAB, with and without iron. R. microsporus spores were exposed to these mixtures for 24 h and fungal growth was determined via absorbance (OD600, n = 3). (B) R. microsporus spores were incubated in SAB with 20 μg ml−1 of exogenous pyoverdine at 37 °C for 24 h. Fungal growth was measured through absorbance (OD600) and normalised to media control (n = 6). (C) P. aeruginosa was exposed to R. microsporus at an MOI of 1:100 (R. microsporus:P. aeruginosa) for 24 h (37 °C). Siderophore production was measured by using the Siderotec Assay (EmerginBio) (n = 3, Mann-Whitney U test). (D) P. aeruginosa strains defective in siderophore biosynthesis were exposed to R. microsporus at an MOI of 1:100 (R. microsporus:P. aeruginosa) for 24 h (37 °C). Siderophore production was measured by using the Siderotec Assay (EmerginBio) (n = 3, Mann-Whitney U test). (E) P. aeruginosa was exposed to R. microsporus at an MOI of 1:100 (R. microsporus:P. aeruginosa) for 7 h, snap frozen and total RNA extracted. The expression levels of PvdS and PchA were quantified by qRT-PCR relative to RpoD and normalised to PAO1 grown in isolation.
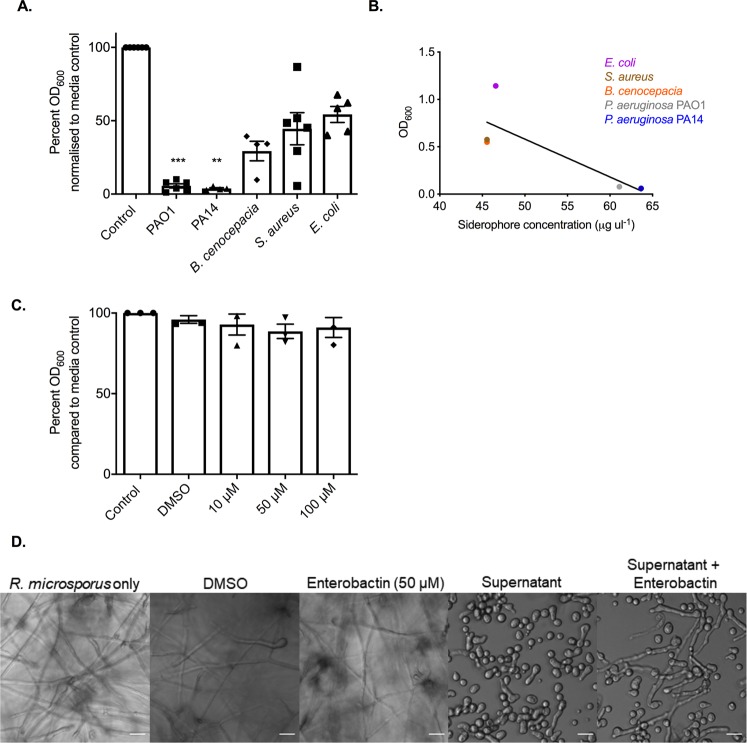
To confirm that pyoverdine alone is sufficient to inhibit R. microsporus growth, spores were exposed to exogenous pyoverdine. P. aeruginosa supernatants contained 58.9 µg ml−1 (+/−1.194) siderophores, making the concentration of siderophores in our assay 29.45 µg ml−1. Therefore, R. microsporus spores were grown in the presence of 20 µg ml−1 purified pyoverdine to resemble siderophore concentrations similar to the culture supernatants. Incubation of fungal spores with pyoverdine significantly reduced fungal growth (73.8%, +/−5.503, p = 0.0022, Fig. 5B). Therefore, pyoverdine alone is sufficient to inhibit R. microsporus growth and germination.
R. microsporus induces iron stress and promotes bacterial siderophore production
C. albicans can decrease P. aeruginosa siderophore production through suppression of the pyoverdine and pyochelin biosynthetic pathways19. To determine whether R. microsporus is also able to interfere with P. aeruginosa siderophore production, the concentration of siderophores after 24 h mono- and co-culture was quantified (Fig. 5C). Surprisingly, the concentration of siderophores in mono-cultures in SAB/LB was lower than in LB (4.99 μg ml−1, +/−5.834, vs 58.9 µg ml−1 (+/−1.194)) suggesting that LB/SAB has a higher iron content, reducing siderophore production. However, in co-cultures, siderophore levels were increased to levels similar to the LB supernatant (53.25 μg ml−1, +/−0.4335 compared to 58.9 µg ml−1 (+/−1.194) indicative of imposed iron stress. This increase in siderophore concentration was not observed in co-cultures containing siderophore deficient P. aeruginosa (Fig. 5D), confirming that the increase in siderophore concentration is likely due to increased bacterial rather than fungal siderophore biosynthesis. In agreement with this, key genes involved in pyoverdine and pyochelin biosynthesis were upregulated during co-culture with R. microsporus. However, this regulation was lost in the presence of exogenous iron (Fig. 5E). These results confirm that during co-culture, the two organisms compete for iron, resulting in the upregulation of bacterial siderophore biosynthesis and P. aeruginosa outcompeting R. microsporus for iron and therefore growth.
The concentration of siderophores produced by bacteria correlates with inhibition of R. microsporus growth
While live E. coli, B. cenocepacia, and S. aureus did not inhibit the growth of R. microsporus, these bacteria all produce siderophores39–41. To determine the ability of secreted factors to inhibit growth, R. microsporus spores were exposed to sterile supernatants from E. coli, B. cenocepacia and S. aureus to determine their ability to inhibit R. microsporus growth as compared to P. aeruginosa PAO1 and PA14. Consistent with the co-culture experiments, P. aeruginosa was the only supernatant able to significantly inhibit growth (Fig. 6A). We further investigated whether this lack of inhibition was associated with insufficient production of iron binding molecules by measuring the amount of siderophores produced after 24 h growth in LB. There was a negative correlation between fungal growth and siderophore production across different bacterial species (p = 0.0029, Fig. 6B), suggesting that siderophore mediated iron restriction may be a common mechanism of bacteria to compete with fungi. However, the presence of supernatants from E. coli had no effect on fungal growth. This was surprising, as E. coli produces enterobactin, a siderophore with a high affinity (1052 M) for iron42. In agreement with this data, exogenous enterobactin did not inhibit R. microsporus growth (91%, +/−6.170, p = 0.936, Fig. 6C), suggesting that R. microsporus may utilise enterobactin as a xenosiderophore. Therefore, the addition of enterobactin in the presence of P. aeruginosa may provide an advantage to R. microsporus. To explore this possibility, we added exogenous enterobactin to R. microsporus-P. aeruginosa co-cultures. However, the presence of the enterobactin appeared to enhance the growth of the P. aeruginosa, presumably because P. aeruginosa can also utilise enterobactin as a xenosiderophore. Therefore, instead we added enterobactin to sterile bacterial supernatants. R. microsporus displayed increased germination in P. aeruginosa supernatants supplemented with enterobactin, although fungal growth was not fully restored (Fig. 6D), presumably due to pyoverdine and pyochelin binding the majority of the free iron. Therefore, taken together this data indicates that R. microsporus can use enterobactin as a xenosiderophore.
Figure 6.
R. microsporus can utilise enterobactin as a xenosiderophore. (A) R. microsporus spores were exposed to 50% supernatants harvested from P. aeruginosa PAO1, P. aeruginosa PA14, B. cenocepacia, S. aureus, and E. coli for 24 h. Fungal growth was determined by absorbance (OD600) and normalised to control. (n = 6). (B) Concentration of siderophores produced by bacteria was determined by using the Siderotec Assay (EmerginBio). Correlation between total siderophore production and fungal growth was determined by performing a linear regression with Pearson correlation (n = 3). (C) R. microsporus spores were exposed to varying concentrations of purified enterobactin for 24 h at 37 °C (n = 3). Fungal growth was determined by absorbance (OD600) and normalised to control. (D) Enterobactin was added to PAO1 sterile supernatants diluted 50% with SAB to a final concentration of 50 μM and incubated at 37 °C for 24 h. Scale bar represents 20 μm. All data was analysed by a Kruskal-Wallis test with Dunn’s multiple comparisons test unless indicated otherwise. *p < 0.05, **p < 0.01, ***p < 0.001.
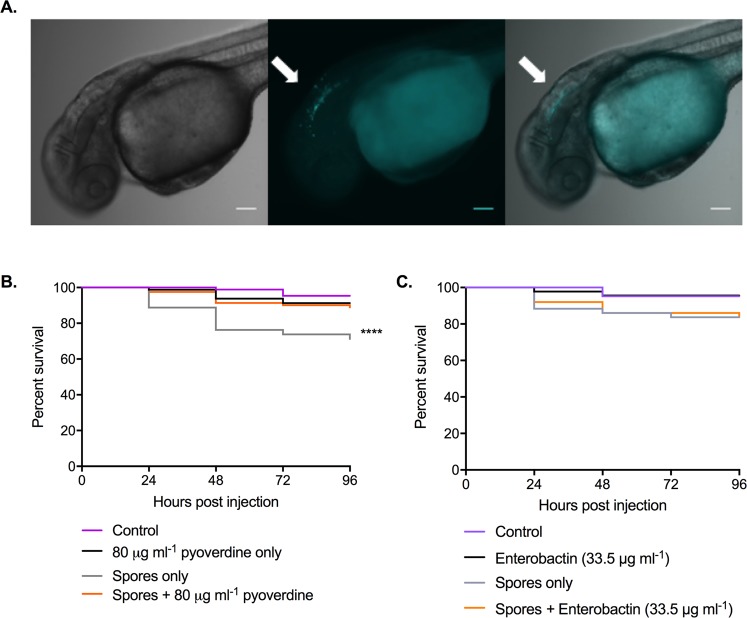
The bacterial siderophore, pyoverdine, reduces the virulence of R. microspores
To determine whether the effects of the bacterial siderophores have a role in controlling fungal infection in the host, we utilised the zebrafish larval model (Fig. 7A). Co-injection of R. microsporus with pyoverdine (80 µg ml−1) resulted in a mild but significant increase in fish survival when compared to spores alone (Fig. 7B) with 89% (+/−6.377) of fish surviving across a 96-h time course. As a control to rule out any impact of the siderophore on innate immune cell function, fish were also infected with R. microsporus in the presence of enterobactin, which should induce iron restriction on the host, but not on the pathogen due to its ability to use enterobactin as a xenosiderophore. Unlike pyoverdine, the addition of enterobactin did not increase the survival of the larvae compared to larvae infected with R. microsporus alone (Fig. 7C). Together these data confirm that the presence of pyoverdine is sufficient to reduce host damage caused by R. microsporus infection.
Figure 7.
The bacterial siderophore, pyoverdine, reduces R. microsporus virulence in a zebrafish model of infection. To determine the impact of pyoverdine on fungal virulence within a host, zebrafish larvae were injected in the hindbrain with 50 spores +/−80 μg ml−1 pyoverdine. (A) Representative images of zebrafish larvae at 0 hpi. White arrows indicate R. microsporus spores (Calcofluor White stain, cyan pseudo-coloured) located within hindbrain compartment. Scale bars depict 100 μm. (B) Survival of larvae was quantified over time. Shown are data pooled from four separate experiments with a total of 87, 80, 80, and 81 fish for control, pyoverdine only, spores only, and spores + pyoverdine, respectively. Data analysed with Mantel-Cox log-rank test. (C) Survival of larvae was quantified over time. Data shown is pooled from two independent experiments with a total of 42, 44, 46, 43, and 50 fish for control, enterobactin only, spores only, and spores + enterobactin, respectively. Data analysed with Mantel-Cox log-rank test.
Discussion
Mucormycosis is a lethal infection with high mortality rates and lack of treatment options due to intrinsic antifungal resistance43. Our current understanding of the pathogenesis is incomplete, especially when compared to other opportunistic fungal pathogens such as C. albicans and A. fumigatus. Because of this, it is important to understand the pressures Mucorales encounter within the human body. This not only includes pressures from the host, but also from the microbiota. Here we identify that P. aeruginosa is able to inhibit the germination of R. microsporus through the secretion of siderophores.
Pseudomonas species interact with and control the growth of a variety of fungal species including important plant and animal pathogens26,44,45. These interactions have been linked to a variety of contact dependent46 and bacterial secreted factors26,44,45. The most characterised secreted molecules known to affect fungal growth and morphology are the phenazines47,48 and the homoserine lactones26,44. For example, in C. albicans low levels of phenazines inhibit filamentation and biofilm formation, and are fungicidal at high concentrations49. Furthermore, the quorum sensing molecule, 3-oxo-C12-homoserine lactone (C12 HSL) induces apoptosis in C. albicans26 and A. fumigatus44. Despite this, these molecules had negligible impact on the growth of R. microsporus, confirming that other bacterial secreted factors control the growth of R. microsporus. However, high concentrations of C12 HSL (200 µM) had a marginal effect on R. microsporus growth, indicating that intra-species QS may play a role in polymicrobial biofilms.
Instead we identified that this antagonistic relationship between P. aeruginosa and R. microsporus to be the result of competition for iron. Iron acquisition is key to Mucorales pathogenesis50. For example, medical conditions (i.e. diabetic ketoacidosis) that result in increased serum levels of iron predispose individuals to mucormycosis51, whereas iron chelation therapy, or reduction in fungal iron acquisition mechanisms reduce mortality in murine models of mucormycosis50,52. P. aeruginosa secretes several iron binding molecules, with pyoverdine being the major siderophore with a high affinity for iron. In agreement with this, we found that exogenous pyoverdine, at concentrations equal to those secreted by P. aeruginosa in our culture conditions, was sufficient to inhibit the growth of R. microsporus to levels comparable to the bacterial supernatant. In addition, deletion of key enzymes in the biosynthesis pathways of the major P. aeruginosa siderophores was sufficient to reduce the effect of the bacterial supernatant, confirming a role for these siderophores in controlling fungal growth.
The presence of fungi has been shown to modulate the expression of siderophore biosynthetic genes in P. aeruginosa. For instance, C. albicans was described as down-regulating the production of pyoverdine and pyochelin through secreted proteins19. Conversely, this study has found the production of pyoverdine to be increased in response to P. aeruginosa co-cultured with R. microsporus. This is clinically important, as pyoverdine production is directly linked to virulence of P. aeruginosa and is shown to modulate the production of other toxins53,54.
The addition of exogenous iron or the inhibition of bacterial siderophore production only resulted in the restoration of approximately 50% fungal growth compared to media only controls, suggesting that other factors also contribute to this inhibition. However, in Rhizopus oryzae, iron starvation induces apoptosis36, suggesting that spore viability may also be affected. In agreement with this, growth of R. microsporus in 100% P. aeruginosa supernatant decreased spore viability. As such, it is possible that reduced viability may account for the inability to completely rescue fungal growth. Interestingly though, exogenous iron was able to fully restore the growth of Mucor circinelloides, suggesting that M. circinelloides is less susceptible to apoptosis induced by iron starvation. Differences in the ability of iron to rescue growth between Mucorales strains also suggests the presence of other potential interactions beyond iron starvation.
R. microsporus can utilise some bacterial siderophores as sources of iron within the host, such as deferoxamine (a siderophore produced by some actinomycetes) to promote its growth and virulence55. However, unlike deferoxamine, and potentially enterobactin, R. microsporus cannot scavenge iron from pyoverdine, which suggests that molecules with similar structure may have the potential to be used to control mucormycosis. While utilising pyoverdine itself would be problematic due to its ability to enhance P. aeruginosa virulence54, this siderophore could provide a starting point for the development of novel iron chelators. Given that pyoverdine has also been shown to limit the growth of other invasive fungi, such as A. fumigatus18,56, molecules based on pyoverdine may have wide implications for the treatment of a range of invasive fungal diseases. This is further enforced by the fact that the presence of pyoverdine in our zebrafish larval model of infection was able to reduce mortality. Similar effects have also be observed in mouse models of infection where deferasirox protects against mycormycosis52. Therefore, iron chelation therapy could be an important preventative treatment for mucormycosis. However, it should be noted that iron is not only important for microbial growth, but also plays essential roles in immunity57. Consequently, iron chelation therapy may have unexpected effects on host immunity. For example, in C. elegans, pyoverdine has been shown to induce mitochondrial damage trigging autophagy and an altered host immune response58. Therefore, it is important to understand the consequences these iron scavenging molecules have on the host before such therapies are applied.
Taken together, our results agree with the current understanding of Mucorales pathogenesis where iron availability is considered essential for pathogenesis. However, here we present this in a different scenario where iron availability is controlled by surrounding bacteria. Given that a high percentage of invasive mucormycosis results from burn and blast wound infections, where iron availability will be high due to tissue damage, we propose that opportunistic bacteria like P. aeruginosa will sequester iron away from the fungus restricting fungal growth. In agreement with this, burn wound exudate enhances P. aeruginosa siderophore production59 resulting in high concentrations of pyoverdine in the wound. However, antibiotic treatment would reduce this competition for iron, and promote fungal germination. This, coupled with natural immunosuppression following trauma could lead to aggressive secondary mucormycosis60. Therefore, patients that have potentially been exposed to fungal spores (i.e. soldiers with blast wounds where significant environmental contamination of the wound has occurred) should be closely monitored for secondary fungal infections. The discovery of suitable iron chelators that do not promote bacterial virulence would be advantageous in this setting to help prevent fungal infection.
Methods
Ethics
Zebrafish care and experiments were performed under Home Office project license P51AB7F76 and personal license I5B923969 in accordance with the Animal Scientific Procedures Act 1986.
Strains and culture conditions
All media and chemicals were purchased from Sigma-Aldrich unless stated otherwise. For details of fungal and bacterial strains used, please see (Table 1). R. microsporus was routinely sub-cultured and maintained on Sabouraud 4% dextrose agar (SAB, Merck Millipore, Germany) and incubated for 10–14 days before use (25 °C). Bacteria were maintained on Lysogeny broth (LB) with 2% agar.
Table 1.
Strains used in this study.
| Strain | Characteristics | Source |
|---|---|---|
| Rhizopus microsporus 12.6652333 | Clinical isolate | Queen Elizabeth Hospital Birmingham |
| R. microsporus var. microsporus CBS 699.68 | Clinical isolate | Westerdijk Fungal Biodiversity Institute |
| R. microsporus var. chinensis CBS 631.82 | Clinical isolate | Westerdijk Fungal Biodiversity Institute |
| R. delemar RA 99–880 | Clinical isolate | Fungal Genetics Stock Centre |
| Mucor circinelloides NRRL3631 | Clinical isolate | ARS Culture Collection (NRRL) |
| Burkholderia cenocepecia K56–2 | Clinical isolate from cystic fibrosis | Amy Dumigan, Queen’s University Belfast |
| Staphylococcus aureus MRSA | Wild type | Anne-Marie Krachler, University of Texas |
| Escherichia coli MG1655 | Wild type | Anne-Marie Krachler, University of Texas |
| Pseudomonas aeruginosa PAO1 ATCC15692 | Wild type | ATCC |
| P. aeruginosa PAO1 | Wild type | 62, 63 |
| P. aeruginosa ΔpchEF | PAO1, deleted pyochelin | 63 |
| P. aeruginosa ΔpvdD | PAO1, deleted pyoverdine | 63 |
| P. aeruginosa ΔpchEFΔpvdD | PAO1, deleted pyochelin and pyoverdine | 63 |
Live co-cultures
LB broth was inoculated with P. aeruginosa, B. cenocepecia, E. coli, or S. aureus and incubated for 24 h (37 °C, 200 rpm). Bacteria were washed three times with phosphate buffered solution (PBS). R. microsporus sporangiospores were harvested through flooding with PBS, washed once, and counted via haemocytometer. Spores (1 × 104 spores/ml) were added to 50% SAB, 50% LB in a 96-well plate. Bacteria were added to each well at a multiplicity of infection (MOI) ratio of 1:1, 1:10, 1:50, and 1:100 and incubated for 24 h (static, 37 °C). Wells were imaged using an inverted Zeiss AxioObserver microscope (20x magnification) and the number of germinated spores per field of view quantified. Germination was defined as the point in which the germ tube reached the same size as the spore diameter.
Spore germination when exposed to bacterial supernatants
Bacterial cultures were prepared as previously detailed and grown to at least stationary phase (OD600 > 3.0). Cultures were centrifuged (3220 × g, 10 min) and the resulting supernatant filter sterilised. Sterile supernatants were stored at −80 °C until required. Spores (1 × 104/ml) were added to a 96-well plate containing either 50% SAB and 50% LB broth, or 50% SAB and 50% supernatant. Fungal growth was determined by endpoint analysis using OD600 as a quantifier of growth (FLUOstar Omega plate reader).
To investigate the role of iron restriction, ferric chloride (100 mM) was diluted to 1, 10, 50, 100, 200, and 500 µM in supernatants. The iron was allowed to associate with any chelating molecules for 15 min before the addition of an equal volume of SAB. Wells containing the SAB/supernatant mixture without iron were included as controls.
Live cell imaging
Live-cell imaging was performed for 12–18 h at 37 °C with humidity using a Zeiss AxioObserver microscope (20x magnification). Images were taken every 10 min to create a time-lapse movie, and the percentage of germinated spores in each field of view was determined.
Exposure of pre-germinated spores to P. aeruginosa supernatant
Spores were harvested and added to 500 µl of SAB broth at a concentration of 1 × 106 spores/ml in triplicate in a 24 well plate. Spores were incubated statically for 4–5 h at 37 °C until germlings emerged and then either 500 µl of P. aeruginosa supernatant or 500 µl LB was added. The plate was incubated for 18 h at 37 °C, and the endpoint absorbance (OD600) of each well measured.
Viability of spores exposed to P. aeruginosa supernatant
R. microsporus spores (1 × 106 spores/ml) were exposed to 100% P. aeruginosa supernatant for 96 h (statically, 37 °C). Every 24 h, 100 spores were plated on SAB agar and incubated at 25 °C for 24 h. Following incubation, the number of viable spores were counted and compared to 0 h control plates.
Pyocyanin secretion
P. aeruginosa supernatants were prepared as described previously. Absorbance (690 nm) was measured using a FLUOstar Omega plate reader and compared to a pyocyanin standard curve.
RNA extraction of fungi
R. microsporus spores (2.5 × 106 spores/ml) were exposed to SAB/LB (50:50) (media only control) or 50% PAO1 supernatant. Flasks were incubated statically at 37 °C for 7 h. Spores were centrifuged (1,811 × g, 3 min), snap frozen in liquid nitrogen, and stored at −80 °C. When ready to extract RNA, 1 ml of TRIzol (Invitrogen) was added to each sample and thawed on ice. These samples were homogenised as before. Chloroform (200 µl) was added to each sample, vortexed thoroughly, and centrifuged at 4 °C, 9,400 × g for 15 min. The aqueous layer was collected, and an equal volume of 100% ethanol was added. 700 µl of this was transferred to RNeasy columns, and the RNeasy Mini Plus Kit (Qiagen) protocol followed according to manufacturer guidelines. The RNA concentration and quality were measured using a spectrophotometer.
RNA extraction of bacteria
P. aeruginosa (1 × 108 CFUs/ml) were exposed to 50:50 SAB/LB (+/−100 μM FeCl3) or R. microsporus spores (1 × 106 spores/ml, +/−100 μM FeCl3). Flasks were incubated at 37 °C and 50 rpm for 7 h. Cultures were centrifuged (6,000 × g for 3 min), snap frozen in liquid nitrogen, and stored at −80 °C. The RNeasy Mini Plus Kit (Qiagen) protocol for purification of total RNA from bacteria was followed according to manufacturer guidelines. The RNA concentration and quality were measured using a spectrophotometer.
Quantitative Reverse Transcriptase PCR
qRT-PCR was performed using an iTaq Universal SYBR Green One-Step Kit (Bio Rad) using 50 ng RNA with a total reaction volume of 20 μl. Protocol was followed according to manufacturer’s recommendations. FTR1 was amplified using the forward primer (5′-GTGGTGTCTCCTTGGGTGTT-3′) and reverse primer (5′-CCACCACGGTAGATGAGGA-3′). This was normalised to 18 s rRNA using the forward primer (5′-GGCGACGGTCCACTCGATTT-3′) and reverse primer (5′-TCACTACCTCCCCGTGTCGG-3′).
PvdS was amplified using the forward primer (5′-ACCGTACGATCCTGGTGAAG-3′) and reverse primer (5′- TGAACGACGAAGTGATCTGC-3′). PchA was amplified using the forward primer (5′- CTGCCTGTACTGGGAACAGC-3′) and reverse primer (5′-GCAGAGCAATTGCCAGTTTT-3′). These were normalised to rpoD using the forward primer (5′-GGGCGAAGAAGGAAATGGTC-3′) and the reverse primer (5′-CAGGTGGCGTAGGTGGAGAA-3′).
Quantification of overall siderophore production
Siderophore concentrations in bacterial supernatants were quantified by using the SideroTec Assay Kit (Emergen Bio) according to the manufacturer recommendations.
Zebrafish infections
Adult wild type (AB) Danio rerio zebrafish were maintained at the University of Birmingham Aquatic Facility in recirculating tanks with 14 h light/10 h dark cycles at 28 °C. Adult zebrafish naturally spawned overnight in groups of 11 fish (six female, five males). Embryos were transferred to E3 medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, pH 7) with 0.3 µg ml−1 methylene blue and 0.003% 1-phenyl-2-thiourea (PTU) for the first 24 hours post fertilisation (hpf) and maintained at 32 °C.
Hindbrain injections were performed as previously described61. Sample sizes were calculated via power analysis using an alpha value of 0.05, power of 80%, mean effect size of 4.2%, and standard deviation of 8%, based on preliminary data and standards accepted by the zebrafish infection community. At 24 hpf larvae were manually dechorionated and anaesthetised (160 µg ml−1 Ethyl 3-aminobenzoate methanesulfonate salt [Tricaine]). R. microsporus spores were suspended in either polyvinylpyrrolidone (PVP, 10% in PBS + 0.05% phenol red), PVP + 80 µg ml−1 pyoverdine, dimethyl sulfoxide (DMSO, solvent for enterobactin) or 33.5 μg ml−1 enterobactin at a concentration of 5 × 106 spores/ml. Suspended spores (2 nl) were injected into the hindbrain via the otic vesicle to achieve a dose of 50 spores/larva. Control larvae were injected with either PVP only or PVP + 80 µg ml−1 pyoverdine. Any fish that did not survive the injection process were removed. Survival was recorded every 24 h until larvae were sacrificed at 5 dpf (96 hours post infection) through 10x overdose of Tricaine. For the pyoverdine experiment, data were pooled from four separate experiments with a total of 87, 80, 80, and 81 fish for control, pyoverdine only, spores only, and spores + pyoverdine, respectively. For the enterobactin experiment, data were pooled from two separate experiments with a total of 42, 44, 46, 43, and 50 fish for control, DMSO, enterobactin only, spores only, and spores + enterobactin, respectively.
Statistical analysis
Each experiment was performed with at least two technical and two biological replicates. Microsoft Excel 2016 and GraphPad Prism 6 were used to record and analyse data. Statistical tests used are indicated in figure legends. All analysis was performed on non-normalised raw data or arcsine transformed data where appropriate. A p-value of p < 0.05 was considered to indicate statistical significance. Statistical significance is indicated by *p < 0.05, **p < 0.01, and ***p < 0.001.
Supplementary information
Acknowledgements
We would like to acknowledge Steve Diggle, Anne-Marie Krachler, Amy Dumigan, and Mark Webber for the generous gifts of bacterial strains; Francisco Fernandez-Trillo, and Oliver Creese for assistance with the organic extractions; Kevin Waldron and Daniel Stones for valuable consultation regarding experimental design with metals; Fabien Cottier for critical input while preparing the manuscript; Elizabeth Ballou for help with power calculations for animal studies; and the Host and Pathogen Interaction laboratory at the University of Birmingham for helpful discussion and valuable support. C.K is funded by the Darwin Trust of Edinburgh. R.A.H, C.C and S.S are funded by the Medical Research Council (MR/L00903X/1).
Author Contributions
C.K., C.C. and S.S. acquired and analysed the data. C.K. and R.H. wrote the manuscript. R.H. and K.V. conceptualised and designed the study.
Data Availability
The datasets generated during this study are available from the corresponding author upon reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kerstin Voelz, Email: K.Voelz@bham.ac.uk.
Rebecca A. Hall, Email: r.a.hall@bham.ac.uk
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-42175-0.
References
- 1.Alvarez E, et al. Spectrum of zygomycete species identified in clinically significant specimens in the United States. J. Clin. Microbiol. 2009;47:1650–1656. doi: 10.1128/JCM.00036-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roden MM, et al. Epidemiology and outcome of zygomycosis: A Review of 929 reported cases. Clin. Infect. Dis. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 3.Waldorf AR, Levitz SM, Diamond RD. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J. Infect. Dis. 1984;150:752–760. doi: 10.1093/infdis/150.5.752. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin. Infect. Dis. 2012;54:1–7. doi: 10.1093/cid/cir865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: Pathophysiology, presentation, and management. Clinical Microbiology Reviews. 2005;18:556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rammaert, B. et al. Healthcare-associated mucormycosis. Clin. Infect. Dis. 54 (2012). [DOI] [PubMed]
- 7.Torres-Narbona M, et al. Impact of zygomycosis on microbiology workload: A survey study in Spain. J. Clin. Microbiol. 2007;45:2051–2053. doi: 10.1128/JCM.02473-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warkentien TE, et al. Impact of Mucorales and other invasive molds on clinical outcomes of polymicrobial traumatic wound infections. J. Clin. Microbiol. 2015;53:2262–2270. doi: 10.1128/JCM.00835-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akers, K. S. et al. Biofilms and persistent wound infections in United States military trauma patients: A case-control analysis. BMC Infect. Dis. 14 (2014). [DOI] [PMC free article] [PubMed]
- 10.Gjødsbøl, K. et al. Multiple bacterial species reside in chronic wounds: A longitudinal study. Int. Wound J. 3 (2006). [DOI] [PMC free article] [PubMed]
- 11.Kalan L, et al. Redefining the chronic-wound microbiome: Fungal communities are prevalent, dynamic, and associated with delayed healing. MBio. 2016;7:1–12. doi: 10.3391/mbi.2016.7.1.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Struck MF, Gille J. Fungal infections in burns: A comprehensive review. Ann. Burns Fire Disasters. 2013;26:147–153. [PMC free article] [PubMed] [Google Scholar]
- 13.Baker R. Mucormycosis; a new disease? J Am Med Assoc. 1957;163:805–808. doi: 10.1001/jama.1957.02970450007003. [DOI] [PubMed] [Google Scholar]
- 14.Nash G, et al. Fungal burn wound infection. J. Am. Med. Assoc. 1971;215:1664–6. doi: 10.1001/jama.1971.03180230072017. [DOI] [PubMed] [Google Scholar]
- 15.Jabra-Rizk MA, Meiller TF, James CE, Shirtliff ME. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob Agents Chemother. 2006;50:1463–9. doi: 10.1128/AAC.50.4.1463-1469.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peleg AY, et al. Prokaryote-eukaryote interactions identified by using Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2008;105:14585–14590. doi: 10.1073/pnas.0805048105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boon C, et al. A novel DSF-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME J. 2008;2:27–36. doi: 10.1038/ismej.2007.76. [DOI] [PubMed] [Google Scholar]
- 18.Penner JC, et al. Pf4 bacteriophage produced by Pseudomonas aeruginosa inhibits Aspergillus fumigatus metabolism via iron sequestration. Microbiol. (United Kingdom) 2016;162:1583–1594. doi: 10.1099/mic.0.000344. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Medina, E. et al. Candida albicans inhibits Pseudomonas aeruginosa virulence through suppression of pyochelin and pyoverdine biosynthesis. PLoS Pathog. 11 (2015). [DOI] [PMC free article] [PubMed]
- 20.Hogan DA. Pseudomonas-Candida Interactions: An ecological role for virulence factors. Science (80-.). 2002;296:2229–2232. doi: 10.1126/science.1070784. [DOI] [PubMed] [Google Scholar]
- 21.Buffo J, Herman MA, Soll DR. A characterization of pH-regulated dimorphism in Candida albicans. Mycopathologia. 1984;85:21–30. doi: 10.1007/BF00436698. [DOI] [PubMed] [Google Scholar]
- 22.Singh P, Paul S, Shivaprakash MR, Chakrabarti A, Ghosh AK. Stress response in medically important Mucorales. Mycoses. 2016;59:628–635. doi: 10.1111/myc.12512. [DOI] [PubMed] [Google Scholar]
- 23.Waters CM, Bassler BL. QUORUM SENSING: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 24.Enjalbert B, Whiteway M. Release from quorum-sensing molecules triggers hyphal formation during Candida albicans resumption of growth. Eukaryot. Cell. 2005;4:1203–1210. doi: 10.1128/EC.4.7.1203-1210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies DG. The Involvement of cell-to-cell Signals in the development of a bacterial biofilm. Science (80-.). 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 26.Hogan DA, Vik Å, Kolter R. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol. Microbiol. 2004;54:1212–1223. doi: 10.1111/j.1365-2958.2004.04349.x. [DOI] [PubMed] [Google Scholar]
- 27.Cruz MR, Graham CE, Gagliano BC, Lorenz MC, Garsin DA. Enterococcus faecalis inhibits hyphal morphogenesis and virulence of Candida albicans. Infect. Immun. 2013;81:189–200. doi: 10.1128/IAI.00914-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen L, et al. Pyocyanin production by Pseudomonas aeruginosa induces neutrophil apoptosis and impairs neutrophil-mediated host defenses in vivo. J. Immunol. 2005;174:3643–3649. doi: 10.4049/jimmunol.174.6.3643. [DOI] [PubMed] [Google Scholar]
- 29.Prince LR, et al. Subversion of a lysosomal pathway regulating neutrophil apoptosis by a major bacterial toxin, pyocyanin. J Immunol Ref. J. Immunol. Osaka Univ. Libr. 2013;180:3502–3511. doi: 10.4049/jimmunol.180.5.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau GW, Hassett DJ, Ran H, Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends in Molecular Medicine. 2004;10:599–606. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg ED. Nutritional immunity: Host’s attempt to withhold iron from microbial invaders. JAMA J. Am. Med. Assoc. 1975;231:39–41. doi: 10.1001/jama.1975.03240130021018. [DOI] [PubMed] [Google Scholar]
- 32.Corbin BD, et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science (80). 2008;319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 33.Foster J. The heavy metal nutrition of fungi. Bot Rev. 1939;5:207–239. doi: 10.1007/BF02878490. [DOI] [Google Scholar]
- 34.Ballou ER, Wilson D. The roles of zinc and copper sensing in fungal pathogenesis. Curr. Opin. Microbiol. 2016;32:128–134. doi: 10.1016/j.mib.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibrahim AS, Spellberg B, Edwards J. Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr. Opin. Infect. Dis. 2008;21:620–625. doi: 10.1097/QCO.0b013e3283165fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shirazi F, Kontoyiannis DP, Ibrahim AS. Iron starvation induces apoptosis in Rhizopus oryzae in vitro. Virulence. 2015;6:121–126. doi: 10.1080/21505594.2015.1009732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braud A, Hannauer M, Mislin GLA, Schalk IJ. The Pseudomonas aeruginosa pyochelin-iron uptake pathway and its metal specificity. J. Bacteriol. 2009;191:3517–3525. doi: 10.1128/JB.00010-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braud A, Hoegy F, Jezequel K, Lebeau T, Schalk IJ. New insights into the metal specificity of the Pseudomonas aeruginosa pyoverdine-iron uptake pathway. Environ. Microbiol. 2009;11:1079–1091. doi: 10.1111/j.1462-2920.2008.01838.x. [DOI] [PubMed] [Google Scholar]
- 39.Courcol RJ, Lambert Pa, Fournier P, Martin GR, Brown MR. Effects of iron depletion and sub-inhibitory concentrations of antibodies on siderophore production by Staphylococcus aureus. J. Antimicrob. Chemother. 1991;28:663–668. doi: 10.1093/jac/28.5.663. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien IG, Cox GB, Gibson F. Biologically active compounds containing 2,3-dihydroxybenzoic acid and serine formed by Escherichia coli. Biochim. Biophys. Acta. 1970;201:453–60. doi: 10.1016/0304-4165(70)90165-0. [DOI] [PubMed] [Google Scholar]
- 41.Darling P, Chan M, Cox AD, Sokol PA. Siderophore production by cystic fibrosis isolates of Burkholderia cepacia. Infect. Immun. 1998;66:874–877. doi: 10.1128/iai.66.2.874-877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carrano CJ, Raymond KN. Kinetics and mechanism of iron removal from transferrin by enterobactin and synthetic tricatechols. J. Am. Chem. Soc. 1979;101:5401–5404. doi: 10.1021/ja00512a047. [DOI] [Google Scholar]
- 43.Sun QN, Fothergill AW, McCarthy DI, Rinaldi MG, Graybill JR. In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes. Antimicrob. Agents Chemother. 2002;46:1581–1582. doi: 10.1128/AAC.46.5.1581-1582.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mowat E, et al. Pseudomonas aeruginosa and their small diffusible extracellular molecules inhibit Aspergillus fumigatus biofilm formation. FEMS Microbiology Letters. 2010;313:96–102. doi: 10.1111/j.1574-6968.2010.02130.x. [DOI] [PubMed] [Google Scholar]
- 45.Wallace RL, Hirkala DL, Nelson LM. Efficacy of Pseudomonas fluorescens for control of Mucor rot of apple during commercial storage and potential modes of action. Can J Microbiol. 2018;e-First ar:1–12. doi: 10.1139/cjm-2017-0776. [DOI] [PubMed] [Google Scholar]
- 46.Hogan D. a & Kolter, R. Pseudomonas-Candida interactions: an ecological role for virulence factors. Science. 2002;296:2229–32. doi: 10.1126/science.1070784. [DOI] [PubMed] [Google Scholar]
- 47.Morales, D. K. et al. Control of Candida albicans metabolism and biofilm formation by Pseudomonas aeruginosa phenazines. MBio4 (2013). [DOI] [PMC free article] [PubMed]
- 48.Briard, B. et al. Pseudomonas aeruginosa manipulates redox and iron homeostasis of its microbiota partner Aspergillus fumigatus via phenazines. Sci. Rep. 5 (2015). [DOI] [PMC free article] [PubMed]
- 49.Gibson J, Sood A, Hogan DA. Pseudomonas aeruginosa-Candida albicans interactions: Localization and fungal toxicity of a phenazine derivative. Appl. Environ. Microbiol. 2009;75:504–513. doi: 10.1128/AEM.01037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ibrahim AS, et al. The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis. Mol. Microbiol. 2010;77:587–604. doi: 10.1111/j.1365-2958.2010.07234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Artis WM, Fountain JA, Delcher HK, Jones HE. A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: Transferrin and iron availability. Diabetes. 1982;31:1109–1114. doi: 10.2337/diacare.31.12.1109. [DOI] [PubMed] [Google Scholar]
- 52.Ibrahim AS, et al. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J. Clin. Invest. 2007;117:2649–2657. doi: 10.1172/JCI32338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyer JM, Neely A, Stintzi A, Georges C, Holder IA. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect. Immun. 1996;64:518–523. doi: 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamont IL, Beare PA, Ochsner U, Vasil AI, Vasil ML. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2002;99:7072–7. doi: 10.1073/pnas.092016999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boelaert JR, et al. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection: In vitro and in vivo animal studies. J. Clin. Invest. 1993;91:1979–1986. doi: 10.1172/JCI116419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sass, G. et al. Studies of Pseudomonas aeruginosa mutants indicate pyoverdine as the central factor in inhibition of Aspergillus fumigatus biofilm. J. Bacteriol. 200 (2018). [DOI] [PMC free article] [PubMed]
- 57.Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nature Rev. Microbiol. 2012;10:525–537. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang, D., Kirienko, D. R., Webster, P., Fisher, A. L. & Kirienko, N. V. Pyoverdine, a siderophore from Pseudomonas aeruginosa, translocates into C. elegans, removes iron, and activates a distinct host response. Virulence, 10.1080/21505594.2018.1449508 (2018). [DOI] [PMC free article] [PubMed]
- 59.Gonzalez MR, et al. Effect of human burn wound exudate on Pseudomonas aeruginosa virulence. mSphere. 2016;1:1–14. doi: 10.1128/mSphere.00111-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Miyazaki M. Immunosuppression following surgical and traumatic injury. Surgery Today. 2010;40:793–808. doi: 10.1007/s00595-010-4323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voelz K, Gratacap RL, Wheeler RT. A zebrafish larval model reveals early tissue-specific innate immune responses to Mucor circinelloides. Dis. Model. Mech. 2015;8:1375–88. doi: 10.1242/dmm.019992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holloway BW. Genetic recombination in Pseudomonas aeruginosa. J Gen Microbiol. 1955;13:572–581. doi: 10.1099/00221287-13-3-572. [DOI] [PubMed] [Google Scholar]
- 63.Ghysels B, et al. FpvB, an alternative type I ferripyoverdine receptor of Pseudomonas aeruginosa. Microbiology. 2004;150:1671–1680. doi: 10.1099/mic.0.27035-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during this study are available from the corresponding author upon reasonable request.