Abstract
Few hydrogen adsorbents balance high usable volumetric and gravimetric capacities. Although metal-organic frameworks (MOFs) have recently demonstrated progress in closing this gap, the large number of MOFs has hindered the identification of optimal materials. Here, a systematic assessment of published databases of real and hypothetical MOFs is presented. Nearly 500,000 compounds were screened computationally, and the most promising were assessed experimentally. Three MOFs with capacities surpassing that of IRMOF-20, the record-holder for balanced hydrogen capacity, are demonstrated: SNU-70, UMCM-9, and PCN-610/NU-100. Analysis of trends reveals the existence of a volumetric ceiling at ∼40 g H2 L−1. Surpassing this ceiling is proposed as a new capacity target for hydrogen adsorbents. Counter to earlier studies of total hydrogen uptake in MOFs, usable capacities in the highest-capacity materials are negatively correlated with density and volumetric surface area. Instead, capacity is maximized by increasing gravimetric surface area and porosity. This suggests that property/performance trends for total capacities may not translate to usable capacities.
Considering the large number of existing synthesised and hypothesised metal-organic frameworks, determining which materials perform best for given applications remains a challenge. Here, the authors screen the usable hydrogen uptake capacities of nearly 500,000 MOFs, and find that three frameworks outperform the current record-holder.
Introduction
Hydrogen is a promising vehicular fuel due to its high specific energy, renewability, and its ability to be produced and oxidized without CO2 emissions1–3. However, due to the low volumetric density of H2 gas, efficient and cost-effective storage of hydrogen remains a challenge1–3. To overcome this challenge, storage in solid adsorbents has received significant attention as an alternative to compression in high-pressure tanks1–4. Adsorbents have the potential to match or surpass the capacities typical of physical storage systems, while doing so at lower pressures and with the potential to reduce cost1.
Metal-organic frameworks (MOFs) are perhaps the most intensively-researched hydrogen adsorbents1,5–17. Microporous crystalline MOFs are formed by the self-assembly of inorganic metal clusters and organic linkers18. The many possible variations of these building blocks allow MOFs to exhibit a wide range of properties, some of which (e.g., surface area) are unmatched by other materials5,19–23. Although this design flexibility allows for the tuning of MOF properties, it also complicates the identification of optimal compositions, because the parameter space that must be searched is very large1.
Computational methods24,25 have been of great value in accelerating this search7,11,16,17,26–36. In the case of hydrogen storage, high-throughput calculations have assisted in the identification of MOFs with the potential to achieve high capacities. These techniques also allow for the identification of property-performance trends, resulting in design guidelines37–39. Table 1 summarizes recent high-throughput studies of hydrogen storage in MOFs. These studies have examined real MOFs (i.e., based on crystal structures of synthesized compounds), and larger collections of hypothetical compounds, which are generated computationally6,17,33–35. For example, a recent study by the present authors identified MOFs that simultaneously exhibit high volumetric and gravimetric hydrogen densities from a database of 5309 real compounds1. Promising MOFs were identified using Grand Canonical Monte Carlo (GCMC) calculations employing the pseudo-Feynman–Hibbs interatomic potential1,40–42. Consistent with the computational predictions, IRMOF-20 was demonstrated experimentally to exhibit an uncommon combination of high usable volumetric (UV) and gravimetric capacities1. Importantly, the measured capacities exceeded those of the benchmark compound MOF-540,43, the previous record-holder for combined volumetric/gravimetric performance1.
Table 1.
Summary of recent high-throughput calculations of hydrogen storage in MOFs
| Database | Number of MOFs screened | H2 storage condition | Literature |
|---|---|---|---|
| CoRE+UM (real) | 5,309 | Usable: (77 K, 100 bar) → (77 K, 5 bar) | Ahmed et al. (2017)1,8,24 |
| Usable: (77 & 298 K, 100 bar) → (77 & 298 K, 1 bar) | Thornton et al. (2017)11,24 | ||
| Northwestern (hypothetical) | 137,953 | Usable: (77 & 298 K, 100 bar) → (77 & 298 K, 1 bar) | Thornton et al. (2017)11,32 |
| Usable: (77 K, 100 bar) → (77 K, 2 bar) | Bobbitt et al. (2016)16,32 | ||
| Total: 1, 50, and 100 atm at 77 K | Gomez et al. (2014)67,32 | ||
| ToBaCCo (hypothetical) | 13, 512 | Total: 100 bar at 130, 200, and 243 K | Colón et al. (2017)6,17 |
| Usable: (77 K, 100 bar) → (77 K, 5 bar) | Gómez-Gualdrón et al. (2016)6,17 | ||
| Mg-MOFs (hypothetical) | 18,383 | Usable: (243 K, 100 bar) → (243 K, 2 bar) | Colón et al. (2014)55 |
| Total: 243 K & 100 bar | |||
| UM (real) | ~4,000 | Total: 77 K & 35 bar | Goldsmith et al. (2013)8 |
The demonstration of IRMOF-20 raises the possibility that other high-capacity MOFs may exist. Indeed, many other databases of MOFs exist beyond those examined in ref. 1. Nevertheless, to our knowledge a systematic assessment of hydrogen capacities across all published databases of real and hypothetical MOFs has not been reported. The present study expands upon prior work by casting a significantly wider net: by assembling a database of databases nearly 500,000 real and hypothetical MOFs (Table 2) have been examined computationally. The most promising materials identified computationally were subsequently synthesized and characterized experimentally. Importantly, three MOFs with usable capacities surpassing that of IRMOF-20 have been demonstrated: SNU-70, UMCM-9, and PCN-610/NU-100. These materials establish a new high-water mark for usable hydrogen capacities in MOFs under physisorptive, pressure-swing (PS) conditions. Analysis of trends across the database reveals the existence of a volumetric ceiling at ~40 g-H2 L−1. This ceiling highlights the need to develop new adsorbents that are specifically constructed to exhibit larger volumetric capacities. Counter to earlier studies of total hydrogen uptake in MOFs8, usable capacities in the highest performing materials identified here were found to be negatively correlated with density and volumetric surface area (VSA). Instead, usable capacities are maximized by increasing gravimetric surface area (GSA) and porosity. These observations suggest that property-performance trends identified for total capacities may not apply to usable capacities.
Results
Computational screening
Fig. 1a shows the calculated UV capacities of 43,777 MOFs examined with GCMC as a function of their usable gravimetric (UG) capacities. These MOFs were down-selected using an initial screen based on crystallographic properties and the Chahine rule8 (see Methods) from a database of 493,458 MOFs described in Table 2 and in Supplementary Tables 1–10. Capacities were evaluated assuming a pressure swing between 100 bar and 5 bar at 77 K. These capacities are compared to that of IRMOF-20, which was identified in prior work as having the best combination of gravimetric & volumetric capacities under these pressure-temperature conditions1, and to MOF-5, a benchmark MOF adopted by the Hydrogen Storage Engineering Center of Excellence (HSECoE)3,43. Irrespective of the database used, Fig. 1a shows that volumetric capacities increase monotonically with increasing gravimetric capacity up to ~10 wt.%, at which point volumetric performance plateaus below ~40 g-H2 L−1. A similar trend was reported in our earlier study, which examined a smaller database containing 5309 real MOFs1. Considering first the performance of the real MOFs8,24,25, only 102 compounds are predicted to exhibit usable capacities greater than that of IRMOF-20 on both a volumetric and gravimetric basis. Supplementary Table 3 lists the 50 highest-capacity MOFs ranked according to volumetric H2 density. Out of the identified compounds, ECOLEP (Cambridge Structural Database (CSD) refcode) exhibits the best UV performance, as well as an appealing UG capacity: 39 g-H2 L−1 & 8.2 wt.%. On the other hand, the highest UG capacity (irrespective of UV) is predicted for MOF-399 (CSD refcode BAZGAM) : 34.3 g-H2 L−1 & 19.3 wt. %.
Fig. 1.
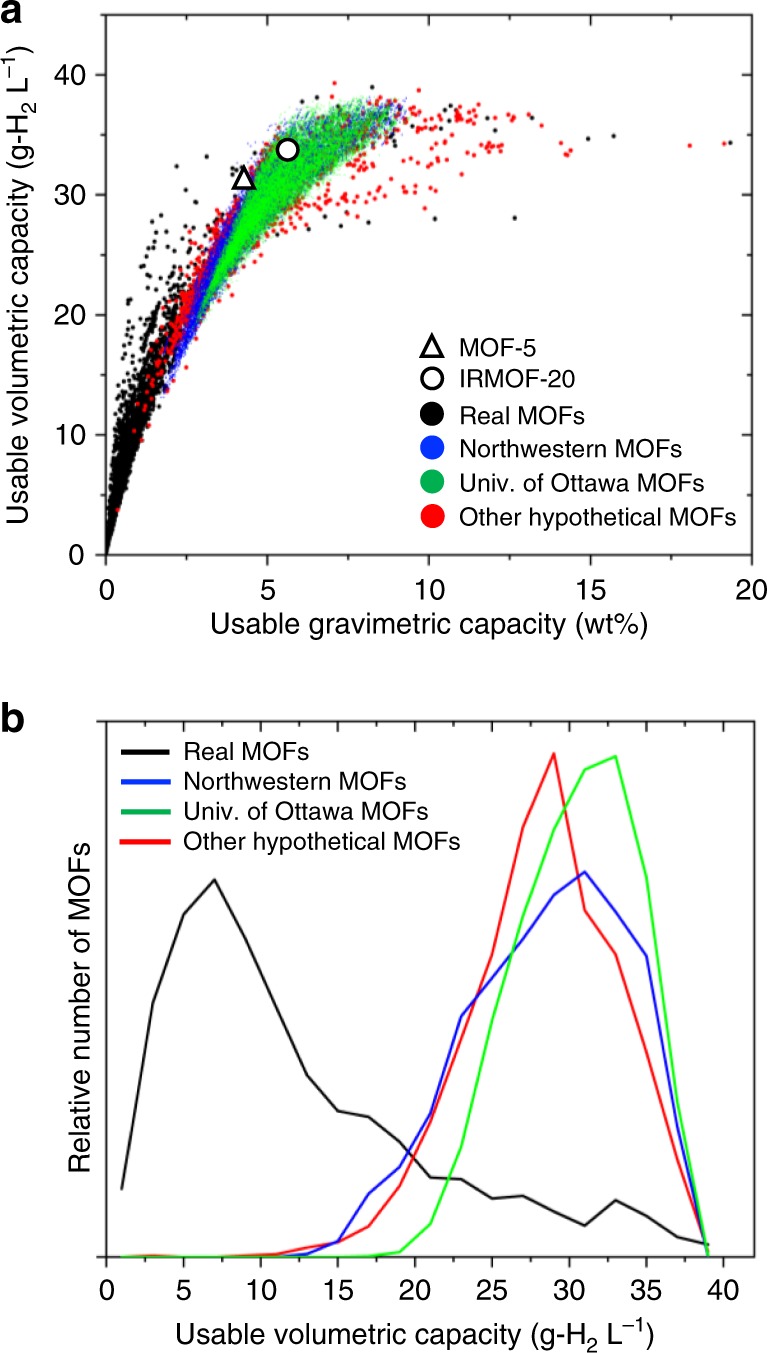
High-throughput screening of MOFs. a Calculated usable hydrogen capacities of 43,777 MOFs compared to the measured capacity of IRMOF-20 (5.7 wt.%, 33.4 g-H2 L−1) and MOF-5 (4.5 wt.% and 31.1 g-H2 L−1). b Relative number of MOFs as a function of usable volumetric capacity and their originating database
Regarding the relative performance of the different databases, the capacities of the real MOFs mostly fall within a lower capacity region between 0–5 wt. % and 0–25 g-H2 L−1. This behavior is again consistent with our prior study1. In contrast, compounds drawn from the hypothetical MOF databases can exhibit much higher capacities. For example, the Univ. of Ottawa database contains the largest number of MOFs that exceed IRMOF-20 in terms of UV and UG capacities; 3581 MOFs surpass this threshold. Similarly, 2154 and 222 MOFs from the Northwestern and remaining (combined) hypothetical MOF databases, respectively, show promise for achieving capacities in excess of IRMOF-2032,44. Supplementary Tables 4–10 list the 20 highest capacity MOFs from each database in Table 2 that exceed the volumetric capacity of IRMOF-20. The synthesizability of these hypothetical MOFs remains unclear.
Table 2.
Details of the MOF database
| Database | No. of MOFs | No. with zero surface area | Capacity exceeds MOF-5 | Exceeds IRMOF-20 |
|---|---|---|---|---|
| Real MOFs:8,24,25 UM+CoRE+CSD | 15,235 | 2950 | 405 | 102 |
| Mail-order35 | 112 | 4 | 30 | 19 |
| In silico deliverable34 | 2816 | 154 | 27 | 6 |
| In silico surface56 | 8, 885 | 283 | 236 | 77 |
| MOF-74 analogs58 | 61 | 0 | 0 | 0 |
| ToBaCCo17 | 13,512 | 214 | 135 | 72 |
| Zr-MOFs27 | 204 | 0 | 126 | 35 |
| Northwestern32 | 137,000 | 30,160 | 4397 | 2154 |
| Univ. of Ottawa44 | 315,615 | 32,993 | 7612 | 3581 |
| In-house | 18 | 0 | 18 | 13 |
| Total | 493,458 | 66,758 | 12,986 | 6059 |
Details of the MOF database, including the number of MOFs in a given database, the number with negligible internal surface area, and the number of compounds identified by GCMC that exceed the usable, pressure-swing capacities of MOF-5 and IRMOF-20. Additional details can be found in Methods Section, Supplementary Methods, Supplementary Figure 1 and Supplementary Tables 1-10
Figure 1b shows the relative number of MOFs having a given UV capacity as a function of the MOF database in which they are found. For the real MOFs the probability distribution is asymmetric and peaked at low capacities: the number of MOFs increases rapidly with UV up to a maximum around 7 g-H2 L−1, but then exhibits a long tail extending out to nearly 40 g-H2 L−1. In contrast, the distributions for the hypothetical MOF databases show opposite behavior: these distributions are skewed to higher UV, with maxima between 28 to 32 g-H2 L−1. As expected from Fig. 1a, the distributions in Fig. 1b all approach zero for UV capacities approaching 40 g-H2 L−1. It is unclear if this volumetric ceiling represents an intrinsic limitation of MOFs, or simply reflects the design decisions made in the assembly of the MOFs present in these databases. A maximum materials-level UV capacity of 40 g-H2 L−1 could in principle surpass the DOE 2020 system-level target of 30 g-H2 L−1, assuming modest volumetric penalties associated with the system. Nevertheless, a MOF below this ceiling could achieve neither the 2025 (40 g-H2 L−1) nor the Ultimate targets (50 g-H2 L−1)4,45. Thus it is suggested that MOF designs that specifically target higher UV be pursued.
MOF Synthesis
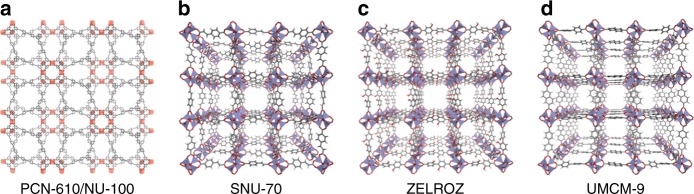
Several of the highest-capacity MOFs predicted by GCMC calculations were targeted for synthesis. These MOFs were selected based on their perceived stability and synthetic accessibility, in addition to their potential to exhibit high capacities exceeding that of IRMOF-20 and MOF-5. These considerations initially resulted in the selection of PCN-610/NU-100 (refcodes HABQUY/GAGZEV), SNU-70/ MOF-5_cooh_2_567_1_basic_opt, and the MOF with CSD refcode ZELROZ. (Although ECOLEP was also predicted to have the highest UV capacity overall, it could not be synthesized in a phase-pure form.) Fig. 2 illustrates the structures of these MOFs. PCN-610/NU-100 is a well-known MOF which was identified from the database of real MOFs8,24,25. To our knowledge its usable capacity for the pressure range 5–100 bar has not been reported. MOF-5_cooh_2_567_1_basic_opt is a hypothetical MOF identified from the mail-order MOF database35. Analysis of its structure revealed that is an ordered variant of the known MOF, SNU-70 (GEBPEK)46. ZELROZ contains 2,2′-dihydroxy-[1,1′-biphenyl]-4,4′-dicarboxylate linkers and is isoreticular with MOF-5 and IRMOF-2047. Although it has a higher projected usable capacity than IRMOF-20, it could not be realized in a fully activated form; only ~70% of the calculated GSA was achieved.
Fig. 2.
Crystal structures of MOFs whose hydrogen uptake was assessed experimentally following their identification by computational screening. a PCN-610/NU-100, b SNU-70, c ZELROZ, and d UMCM-9 (C: gray, H: white, O: red, Cu: orange and Zn: blue)
Further, the calculations also identified non-interpenetrated IRMOF-10 as having higher usable capacities than IRMOF-20 (Supplementary Table 1). This MOF is an unfunctionalized variant of ZELROZ, and also contains the 1,1′-biphenyl-4,4′-dicarboxylate linker48. Unfortunately, the structure of IRMOF-10 is missing from the CSD, likely due to the presence of disorder. In addition, IRMOF-10 has never been obtained with high surface area, likely due to interpenetration or pore collapse during activation. As an alternative, a mixed linker-based approach was explored as a means to realize non-interpenetrated MOFs from 1,1′-biphenyl-4,4′-dicarboxylate linkers49. UMCM-9, containing 1,1′-biphenyl-4,4′-dicarboxylate and 2,6-naphthalenedicarboxylate linkers (Fig. 2), is known to have a non-interpenetrated framework with high experimental GSA. Its structure is, however, highly disordered and therefore is not present in the CSD. An ordered structure of the MOF was constructed for GCMC calculations, and favorable capacity predictions from these calculations prompted experimental investigation of its hydrogen storage capacity. The present calculations on UMCM-9 employed a structure developed from powder diffraction data49.
Table 3 summarizes the measured and calculated crystallographic properties of these MOFs. (N2 isotherms used in Brunauer-Emmett-Teller surface area measurements are shown in Supplementary Figures 2–4.) Correlations between these crystallographic properties and usable capacities are discussed below.
Table 3.
Measured and calculated crystallographic properties of high-capacity MOFs examined in this study
| MOF | Gravimetric surface area (m2 g−1) Expt./Calc. | Volumetric surface area (m2 cm−3) Calc. | Pore volume (cm3 g−1) Calc. | Void fraction Calc. |
|---|---|---|---|---|
| MOF-5 | 3512/3563 | 2172 | 1.36 | 0.81 |
| IRMOF-20 | 4073/4127 | 2000 | 1.65 | 0.84 |
| SNU-70 | 4944/4756 | 1905 | 2.14 | 0.86 |
| UMCM-9 | 5039/4847 | 1805 | 2.31 | 0.86 |
| PCN-610/NU-100 | 6050/5777 | 1603 | 3.17 | 0.88 |
Hydrogen capacity—pressure swing
Figure 3 shows the measured H2 adsorption isotherms of PCN-610/NU-100, SNU-70, and UMCM-9 at 77 K. Isotherms for IRMOF-20 and MOF-5 are also shown for comparison1. A comparison between the measured and calculated isotherms are shown in Supplementary Figures 5–7. In general, good agreement is achieved between the measurements and calculations.
Fig. 3.
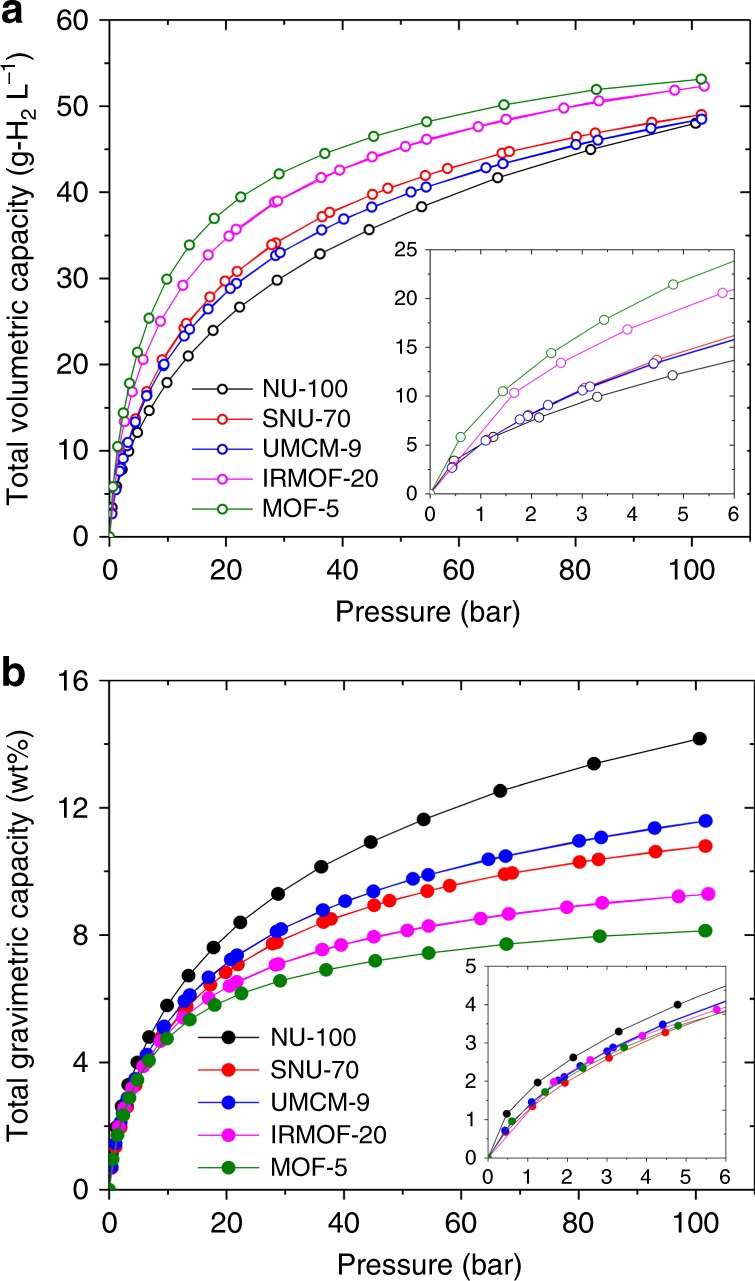
H2 adsorption isotherms. Measured total (a) volumetric and (b) gravimetric H2 adsorption isotherms of NU-100, SNU-70, and UMCM-9 at 77 K. For comparison, isotherms (ref. 1) for the two benchmark MOFs, MOF-5 and IRMOF-20, are also shown. Inset plots illustrate capacities at low pressure
Regarding the total volumetric (TV) capacities of these MOFs, Fig. 3a shows that MOF-5 and IRMOF-20 are, respectively, the two highest capacity MOFs for all pressures greater than ~1 bar. SNU-70, UMCM-9, and PCN-610/NU-100 all exhibit lower total capacities. Nevertheless, these latter three MOFs are superior on a usable basis, as shown in Fig. 4a. UV capacities range from 34.1 to 35.5 g-H2 L−1, which exceed those of MOF-5 and IRMOF-20 (31.1 and 33.4 g-H2 L−1, respectively). To our knowledge these usable capacities are the highest demonstrated for an adsorbent under these conditions. The superior usable performance of these MOFs can be understood by recalling that usable capacity is defined as the difference in total uptake at 100 bar (filled tank condition) and at 5 bar (empty tank). A significantly lower total uptake at 5 bar (compared to MOF-5 & IRMOF-20), combined with a modest difference in uptake at 100 bar, results in a larger usable capacity.
Fig. 4.
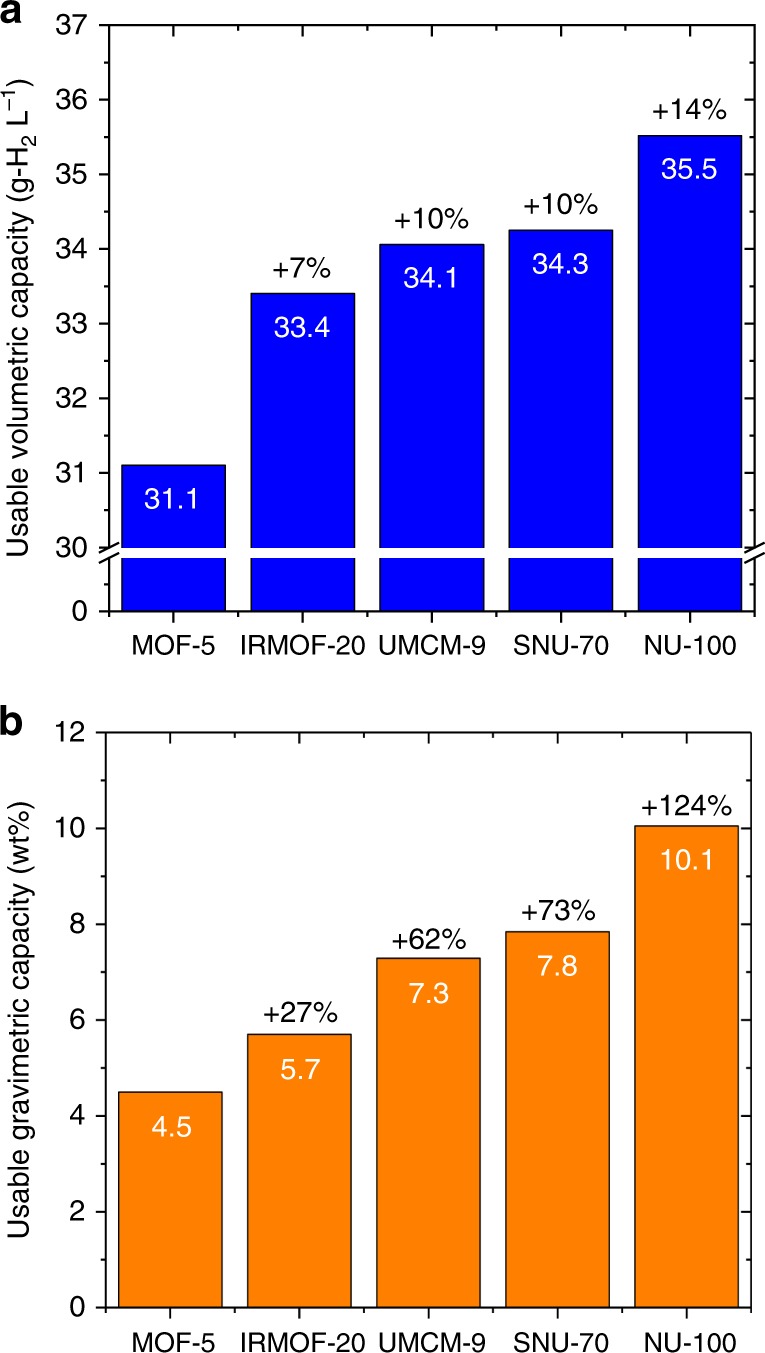
Measured usable H2 storage capacities of MOFs. a Volumetric basis and b gravimetric basis. Capacities are reported for an isothermal pressure swing at 77 K between 5 and 100 bar. Data for MOF-5 and IRMOF-20 are taken from ref. 1. Percentages listed at the top of each bar correspond to improvements over MOF-5
Regarding total gravimetric (TG) capacities, Fig. 3b shows that the trio of MOFs examined here outperform the benchmark MOF-5 and IRMOF-20 compounds for pressures exceeding 10 bar. Below this pressure (Fig. 3b inset) all five MOFs behave similarly, while at high pressures PCN-610/NU-100 is clearly superior. The similar low-pressure total capacities imply that that usable capacities will track with the capacities observed at high pressure. Fig. 4b confirms that this is the case: usable gravimetric capacities range from 7.3 (SNU-70) to 10.1 (PCN-610/NU-100), surpassing those of MOF-5 (4.5 wt.%) and IRMOF-20 (5.7 wt.%).
These data show that PCN-610/NU-100 has the highest usable PS capacity overall, on both a gravimetric and volumetric basis. We previously reported that MOFs with high TG capacities typically exhibit TV capacities that are unexceptional1. Consistent with that trend, Fig. 3a shows that the TG capacity of PCN-610/NU-100 at 100 bar is the largest of the five MOFs examined, yet its TV capacity is the lowest. Nevertheless, on a usable basis PCN-610/NU-100 emerges as a promising MOF due to its low uptake at 5 bar. Thus, we conclude that total capacities can be a poor indicator of useable capacity under PS conditions.
How do these materials-level capacities compare to the DOE system-level targets? Due of penalties associated with the mass and volume of the storage system, to meet these targets a storage material will need to exceed the desired system capacity considerably. The usable gravimetric capacity of PCN-610/NU-100 exceeds the 2020 target4,45 (4.5 wt.%) by 124% and exceeds the Ultimate target4,45(6.5 wt.%) by 55%. These values provide hope that a PCN-610/NU-100-based storage system could meet the targets, even when accounting for the mass of the system. In contrast, meeting the volumetric targets will be more challenging. PCN-610/NU-100 exceeds the DOE’s 2020 system-level target4,45 by only 18%, and falls 29% below the Ultimate targets4,45. Factoring in the volume of components other than the storage medium, we conclude that a PCN-610/NU-100-based system is either unlikely (in the case of the 2020 target4,45) or unable (Ultimate target4,45) to satisfy the DOE goals. This shortcoming points to the importance of emphasizing volumetric hydrogen density in the design of new storage materials50.
Correlations and trends
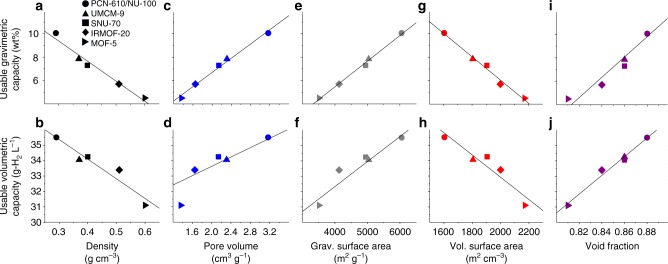
Do the properties of the MOFs characterized here correlate with their capacities? Fig. 5 plots UV and UG capacities as a function of 5 crystallographic features: density (D), pore volume (PV), GSA & VSA, and void fraction (VF). Roughly linear relationships are observed in all cases. Moreover, the same MOFs that bound the capacity range examined in Fig. 4, MOF-5 and PCN-610/NU-100, also bound the range of each crystallographic property. Usable capacities are positively correlated with PV (Fig. 5c, d), GSA (Fig. 5e, f), and VF (5i–j). In contrast, capacities are inversely related to single crystal density (Fig. 5a, b) and volumetric surface area (Fig. 5g, h). Thus, under these conditions, high usable capacities are achieved by maximizing PV, GSA, and VF, while simultaneously minimizing density and volumetric surface area.
Fig. 5.
Relationship between five crystallographic properties and the usable capacities of the highest-capacity MOFs examined in the present study. Capacities are evaluated assuming an isothermal pressure swing between 5 and 100 bar at 77 K. (a, c, e, g, i) usable gravimetric capacities, (b, d, f, h, j) usable volumetric capacities
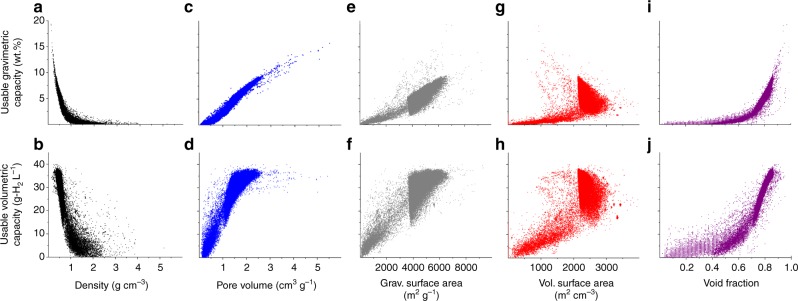
Figure 6 examines these capacity-property trends more broadly across the set of ~43,000 MOFs examined with GCMC calculations. Turning first to the properties which were revealed in Fig. 5 to correlate positively with capacity (PV, GSA, and VF), Fig. 6 demonstrates that these same trends generally hold across the entire MOF dataset: maximizing PV, GSA, and VF maximizes UV and UG capacities. In contrast, Fig. 6 reveals that the inverse correlations that hold for D and VSA for the highest-capacity MOFs (Fig. 5) do not apply generally. For example, Fig. 5a, b suggests that capacity can be maximized by minimizing D. While this is indeed true for the narrow range of D examined in Fig. 5a, b (0.3 to 0.6 g cm−3), Fig. 6a, b shows that capacity decreases for densities outside of this range. Thus, a “sweet-spot” exists for D near 0.6 g cm−3. Similar behavior is observed for VSA: comparing Fig. 5g, h with Fig. 6g, h shows that VSA and usable capacity are in general related in a non-linear fashion. The linear relation observed in Fig. 5g, h is unique to the high-capacity MOFs examined here, and applies across a subset of VSA range from 1600 to 2200 m2 cm−3.
Fig. 6.
Usable capacities of 43,777 MOFs as a function of five crystallographic properties, assuming pressure-swing operation between 100 and 5 bar at 77 K. (a, c, e, g, i) gravimetric capacities, (b, d, f, h, j) volumetric capacities
Hydrogen capacity—temperature & pressure swing
Our analysis has thus far considered PS operation of the storage system. An important advantage of this scheme is its simplicity. Nevertheless, higher capacities can be achieved by adopting a more complex operating scheme. For example, the HSECoE has proposed an alternative operating scenario involving a combined temperature and pressure swing (TPS) from Tmin = 77 K, Pmax = 100 bar (filled) to Tmax = 160 K, Pmin = 5 bar (empty)3,43,51,52. By heating during desorption, the TPS approach increases capacity by minimizing the amount of H2 retained in the MOF in the low-pressure empty state4.
Figure 7 compares the measured usable TPS capacities of the present MOFs with the two highest-capacity MOFs identified in a recent study by García-Holley et al.53, NU-1103 and NU-125, and with the benchmark compounds MOF-5 and IRMOF-201. Regarding volumetric capacities, Fig. 7a reveals that MOF-5 remains the top MOF (as previously noted)1, followed closely by IRMOF-20. The three MOFs identified in the present study as having high-capacities under PS conditions surpass the TPS capacity of NU-1103, but fall slightly below that of NU-12553. Assuming PS operation, the UV capacity of NU-125 is 24 g-H2 L−1, which is ~30% less than that of SNU-70 and UMCM-9, and ~32% less than that of PCN-610/NU-100. Additionally, the three MOFs synthesized here exhibit TPS volumetric capacities that surpass that of MFU-4l (47 g-H2 L−1), the top performing compound recently identified via machine learning screening of 50,000 MOFs54.
Fig. 7.
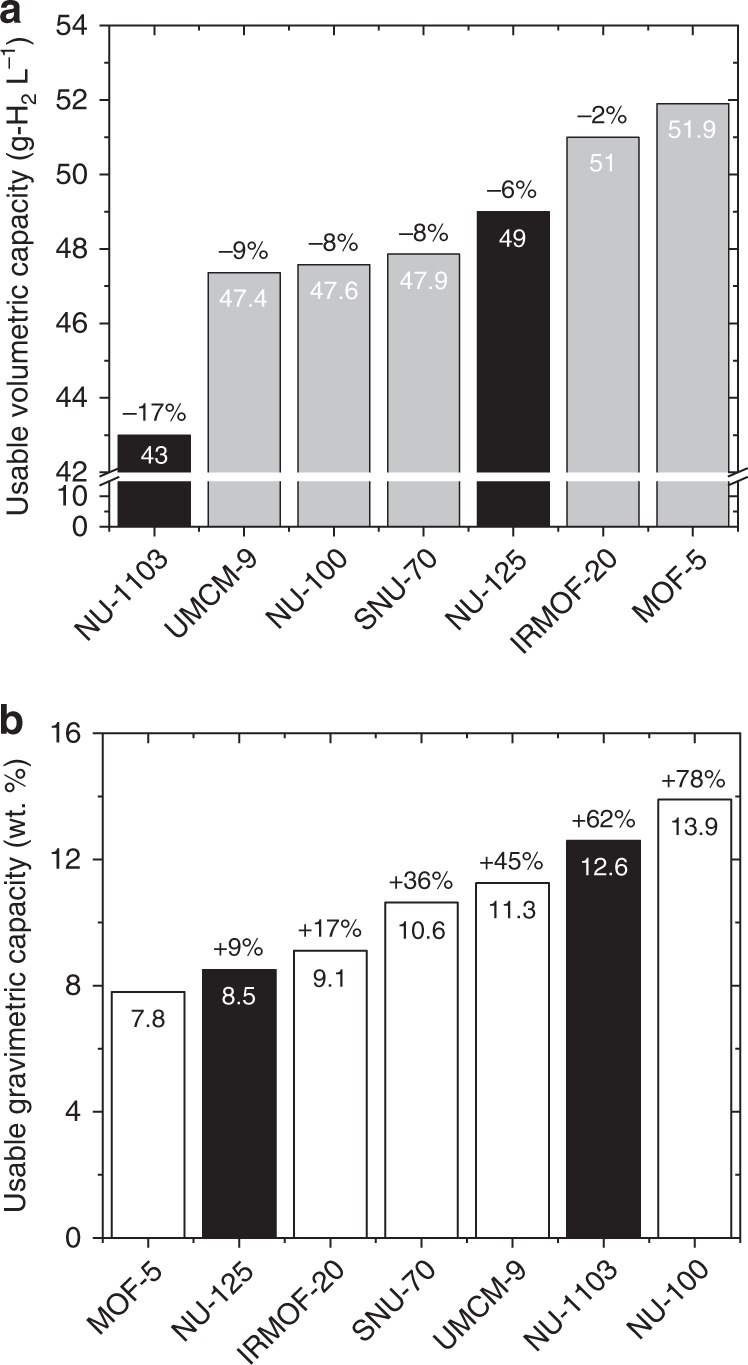
Comparison of measured usable H2 storage capacities of MOFs assuming temperature + pressure-swing operation between 100 bar-77 K and 5 bar-160 K. a Volumetric capacity, and b gravimetric capacity. Grey bars depict the performance of compounds reported in the present study or in the authors’ earlier report1. Black bars depict the performance of two high-capacity MOFs reported in ref. 53. Percentages on top of each bar depict performance relative to MOF-5
Regarding gravimetric TPS capacities, Fig. 7b shows that PCN-610/NU-100 is the top-performer, with a capacity of 13.9 wt.%. This exceptional gravimetric capacity contrasts with its volumetric performance, Fig. 7a, which is comparable to that of UMCM-9 and SNU-70. An even larger tradeoff in gravimetric/volumetric performance is evident for MOF-5, whose gravimetric capacity is the smallest of the 7 MOFs considered here.
More generally, Fig. 7 reveals that no single MOF studied here excels both gravimetrically and volumetrically under TPS operation. This situation differs from PS operation, where Fig. 4 shows that PCN-610/NU-100 is unambiguously the highest-capacity MOF. An ideal MOF would balance gravimetric and volumetric performance. In this regard, IRMOF-20 and SNU-70 both exhibit an appealing combination of volumetric and gravimetric TPS capacities. Furthermore, SNU-70, like UMCM-9, is derived from a commercially-available linker and may, therefore, provide cost advantages relative to MOFs requiring multistep synthesis of linkers.
In summary, a systematic assessment of hydrogen storage capacities in MOFs drawn from several published MOF databases has been presented. The goal was to identify MOFs that exhibit a balance of high volumetric and gravimetric hydrogen capacities under usable, physisorptive operating conditions. In total, nearly 500,000 compounds were screened computationally, and the most promising materials identified were synthesized and assessed experimentally. Three MOFs with usable, PS capacities surpassing that of IRMOF-20, the record-holder for balanced hydrogen capacity, were demonstrated: SNU-70 (identified from a hypothetical, ordered variant), UMCM-9, and PCN-610/NU-100. A similar analysis of the capacities of these MOFs under temperature+PS conditions also revealed them to be high-capacity materials.
Analysis of trends across the database points toward the existence of a volumetric ceiling at a capacity of ~40 g-H2 L−1. Surpassing this ceiling under usable, PS conditions is proposed as a new capacity target for hydrogen adsorbents. Counter to earlier studies of total hydrogen uptake in MOFs, usable capacities in the highest-capacity materials are negatively correlated with density and volumetric surface area. Instead, capacity is maximized by increasing GSA and porosity. These observations suggest that property-performance trends identified for total capacities may not translate to usable capacities.
Methods
Computational methods
A database of 493,458 MOF crystal structures, summarized in Table 2, was compiled by combining 11 published databases8,17,24,25,27,34,35,44,55–57. This meta-database consists of both real and hypothetical MOFs: 15,235 real MOFs were included from the UM8, CoRE24, and CSD25 databases; 478,205 hypothetical MOFs were aggregated from the Northwestern32, University of Ottawa44, mail-order35, in silico deliverable34, in silico surface56, MOF-74 analogs58, ToBaCCo17, and Zr-MOFs27 databases. Eighteen additional MOFs were included based on “in-house” designs. These latter compounds include hypothetical functionalized MOFs, as well as modeled crystal structures of real MOFs (such as UMCM-9) whose structures exhibit extensive disorder and are absent from the CSD. Additional details regarding the in-house database is available in Supplementary Table 1.
Crystallographic properties of all MOFs—single crystal density, gravimetric and volumetric surface areas, PV, VF, largest pore diameter, and pore limiting diameter—were calculated using the Zeo++ code (Supplementary Method 1.2)59.
As an initial screen, TG and TV H2 capacities were estimated at 77 K and 35 bar using the semi-empirical Chahine rule8. MOFs that surpassed the predicted capacity of MOF-5 under these conditions (TG > 8.4 wt.% and TV > 54.4 g-H2 L−1)8, amounting to 43,777 compounds, underwent further evaluation using GCMC calculations (see Supplementary Methods 1.3 & 1.4 for details), as described in an earlier study60. Usable capacities were calculated by GCMC assuming an isothermal PS between 5 and 100 bar at 77 K. MOFs predicted to have usable capacities exceeding MOF-5 (4.5 wt.% and 31.1 g-H2 L−1) and IRMOF-20 (5.7 wt.% and 33.4 g-H2 L−1) were identified and assessed for possible experimental characterization. Finally, the capacities for a subset of MOFs were predicted using GCMC under temperature+PS conditions between 77 K/100 bar and 160 K/5 bar. Additional details regarding these calculations can be found in Supplementary Mothods 1.3 & 1.4.
Experimental methods
Promising MOFs identified by computation were synthesized and evaluated with respect to their hydrogen capacities. These included: SNU-70 (CSD refcode GEBPEK), PCN-610/NU-100 (CSD refcodes: HABQUY/GAGZEV), and UMCM-9 (Crystallographic Information File available in the Supplementary Information)46,49,61,62. With the exception of the PCN-610/NU-100 linker, all the metal salts and organic linkers were obtained from commercial sources. The linker for PCN-610/NU-100 was synthesized following the reaction scheme shown in Supplementary Figure 861,62. Notably, this synthetic scheme involves only three steps (Supplementary Figures 8–11 show ligand characterization via NMR spectroscopy) leading to much higher yield of the final linker as compared to both reported procedures61,62. SNU-70 and PCN-610/NU-100 were activated by flowing supercritical (SC) CO263. UMCM-9 was activated by successive exchanges of the guest solvent in the MOF pores with DMF, DCM, and n-hexane, and subsequently applying vacuum64.
UMCM-9 was synthesized following the literature procedure49. In five 60 mL glass jars with teflon-lined lids were added naphthalene-2,6-dicarboxylic acid (H2NDC, 0.0285 mg, 0.131 mmol), 1,1′-biphenyl-4,4′-dicarboxylic acid (H2BPDC, 0.0354 mg, 0.146 mmol), 6.7 mL of DEF, and 13.3 mL of N-methylpyrrolidone, and the solids were dissolved in the solvent mixtures by sonication. Subsequently, Zn(NO3)2·6H2O (0.235 g, 0.790 mmol) was added to the solution and the mixture was sonicated until transparent solutions were obtained. The reaction mixtures were heated to 85 °C for four days. Cubic crystals of UMCM-9 were formed at the inner surface of the vials along with minor amount of flocculent precipitate. After cooling to room temperature the mother liquor was decanted, the precipitate was removed by multiple DMF washes, and the crystals were collected together in a different vial. The MOF crystals were immersed in DMF for three days (washed several times with fresh DMF), then in dichloromethane for 18 h (washed with DCM, 20 mL × 8), and finally, in dry n-hexane for 12 h (washed with dry n-hexane 20 mL × 4). Subsequently, the solvent was decanted, the vial was placed in a vacuum chamber, and exposed to vacuum very slowly at room temperature. Finally, the material was activated under high vacuum (below 10−4 torr) for 26 h to yield clear pale yellow crystals (average yield 0.0523 g, 38%, based on H2NDC).
PCN-610/NU-100 was synthesized following the literature procedure61. 1,3,5-Tris[(1,3-carboxylic acid-5-(4-(ethynyl)phenyl)) ethynyl]benzene (LH6) (0.300 g, 0.32 mmol) and Cu(NO3)2·2.5H2O (0.600 g, 2.579 mmol) were dissolved in 36 mL DMF in a glass vial. Subsequently, 0.2 mL HBF4 was added to the solution, and the color of the solution turned teal. The solution was divided into thirty 4 mL vials (1.2 mL solution in each vial), and the vials were heated to 75 °C for 20 h. Teal colored octahedral crystals were formed at the bottom of the vial, which were collected together in a 60 mL jar, immersed in DMF for 1 day, and the supernatant liquid was replaced with fresh DMF (20 mL × 4) in this time. Subsequently, the MOF was immersed in ethanol for another day, and the liquid was replaced with fresh ethanol four times (20 mL × 4). The compound was then activated by flowing liquid CO2 at 2 mL min−1 flow rate for 1 h at room temperature, subsequently by supercritical CO2 at 2 mL min−1 flow rate for 2 h at 55 °C, and finally by supercritical CO2 at 1 mL min−1 flow rate for 6 h at 55 °C to result a purple solid (0.123 g, 34.4 % based on LH6).
SNU-70 was synthesized following the reported procedure with slight modification46. (E)-4-(2-Carboxyvinyl)benzoic acid (0.075 g, 0.390 mmol) and Zn(NO3)2·6H2O (0.150 g, 0.504 mmol) were dissolved in 25 mL DEF in a 60 mL glass jars with a teflon-lined lid. Six such reaction mixtures were heated to 105 °C for 12.5 h. At the end of this period, the glass jars were removed from the oven, and allowed to cool down to room temperature. Colorless cubic crystals (along with some fluffy precipitate) were formed at the bottom and the wall of the jars. The fluffy precipitate was removed from the MOF crystals by multiple wash with DMF. The remaining crystals were then collected together in a 60 mL glass vial, soaked in DMF and kept emerged for 2 d. The supernatant liquid was replaced with fresh DMF six times (20 mL each) in this time. The material was activated by SC CO2 flow by the same procedure as NU-100 (0.567 g, 51%).
MOFs were characterized by powder X-ray diffraction (PXRD) and surface areas were calculated from measured N2 isotherms following the recommendations by Rouquerol and co-workers65. Supplementary Figures 2–4 show the N2 isotherms used in Brunauer-Emmett-Teller (BET) surface area measurements. Comparisons of measured and simulated powder X-ray diffraction patterns (Supplementary Figures 12–14) confirm the crystallinity and phase purity of all three compounds.
Hydrogen adsorption and desorption measurements were performed using a manometric Sievert’s-type instrument (HPVA-200, Micromeritics Instrument Corporation)66. Additional details on MOF synthesis, activation, and characterization are provided in Supplementary Methods 4 & 5.
Supplementary information
Acknowledgements
A.A. acknowledges Profs. Randall Snurr and Tom Woo for providing access to their MOF databases. A.A. also acknowledges Dr. Maciej Haranczyk for use of the Zeo++ code and the mail-order MOF database. Financial support for this study was provided by the US Department of Energy, Office of Energy Efficiency and Renewable Energy, Grant no. DE-EE0007046. Computing resources were provided by NSF grant MRI-1531752: Acquisition of Conflux, A Novel Platform for Data-Driven Computational Physics (Tech. Monitor: Ed Walker). The model structure for UMCM-9 was constructed by Dr. Kyoungmoo Koh.
Author contributions
A.A. conducted the computational components of the project. S.S., A.G.W.-F. and A.J.M. conducted MOF synthesis and characterization experiments. J.P. and M.V. performed H2 uptake measurements. All authors contributed to the drafting of the manuscript. D.J.S. conceived the project idea.
Data availability
All calculated/measured crystallographic properties, adsorption isotherms, and characterization data sets generated and/or analyzed during the current study are available at the HyMARC Data Hub (https://datahub.hymarc.org) or from the corresponding author upon reasonable request. A portion of the MOF crystal structures used in generating the calculated data sets are accessible from the Cambridge Structural Database (CSD). The remaining crystal structures are either publicly available as Supplementary Information from published papers, or can be requested from the authors of the respective databases presented in Table 2.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Alauddin Ahmed and Saona Seth.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-09365-w.
References
- 1.Ahmed A, et al. Balancing gravimetric and volumetric hydrogen density in MOFs. Energy Environ. Sci. 2017;10:2459–2471. doi: 10.1039/C7EE02477K. [DOI] [Google Scholar]
- 2.Yang J, Sudik A, Wolverton C, Siegel DJ. High capacity hydrogen storage materials: attributes for automotive applications and techniques for materials discovery. Chem. Soc. Rev. 2010;39:656–675. doi: 10.1039/B802882F. [DOI] [PubMed] [Google Scholar]
- 3.Siegel, D. J. & Hardy, B. Engineering an Adsorbent-Based Hydrogen Storage System: What Have We Learned?https://energy.gov/sites/prod/files/2015/02/f19/fcto_h2_storage_summit_siegel.pdf (United States Department of Energy, USA, 2014)
- 4.Allendorf MD, et al. An assessment of strategies for the development of solid-state adsorbents for vehicular hydrogen storage. Energy Environ. 2018;11:2784–2812. doi: 10.1039/C8EE01085D. [DOI] [Google Scholar]
- 5.Wong-Foy AG, Matzger AJ, Yaghi OM. Exceptional H2 saturation uptake in microporous metal-organic frameworks. J. Am. Chem. Soc. 2006;128:3494–3495. doi: 10.1021/ja058213h. [DOI] [PubMed] [Google Scholar]
- 6.Gómez-Gualdrón DA, et al. Evaluating topologically diverse metal–organic frameworks for cryo-adsorbed hydrogen storage. Energy Environ. Sci. 2016;9:3279–3289. doi: 10.1039/C6EE02104B. [DOI] [Google Scholar]
- 7.Witman M, et al. Rational design of a low-cost, high-performance metal-organic framework for hydrogen storage and carbon capture. J. Phys. Chem. C. 2017;121:1171–1181. doi: 10.1021/acs.jpcc.6b10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldsmith J, Wong-Foy AG, Cafarella MJ, Siegel DJ. Theoretical limits of hydrogen storage in metal–organic frameworks: opportunities and trade-offs. Chem. Mater. 2013;25:3373–3382. doi: 10.1021/cm401978e. [DOI] [Google Scholar]
- 9.Rowsell JLC, Yaghi OM. Strategies for hydrogen storage in metal-organic frameworks. Angew. Chemie Int. Ed. 2005;44:4670–4679. doi: 10.1002/anie.200462786. [DOI] [PubMed] [Google Scholar]
- 10.Kaye SS, Dailly A, Yaghi OM, Long JR. Impact of preparation and handling on the hydrogen storage properties of Zn4O(1,4-Benzenedicarboxylate)3 (MOF-5) J. Am. Chem. Soc. 2007;129:14176–14177. doi: 10.1021/ja076877g. [DOI] [PubMed] [Google Scholar]
- 11.Thornton AW, et al. Materials genome in action: identifying the performance limits of physical hydrogen storage. Chem. Mater. 2017;29:2844–2854. doi: 10.1021/acs.chemmater.6b04933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broom DP, et al. Outlook and challenges for hydrogen storage in nanoporous materials. Appl. Phys. A. 2016;122:151. doi: 10.1007/s00339-016-9651-4. [DOI] [Google Scholar]
- 13.Gomez-Gualdron DA, et al. Understanding volumetric and gravimetric hydrogen adsorption trade-off in metal−organic frameworks. ACS Appl. Mater. Interfaces. 2017;9:33419–33428. doi: 10.1021/acsami.7b01190. [DOI] [PubMed] [Google Scholar]
- 14.Collins DJ, Zhou HC. Hydrogen storage in metal–organic frameworks. J. Mater. Chem. 2007;17:3154–3160. doi: 10.1039/b702858j. [DOI] [Google Scholar]
- 15.Rosi NL, et al. Hydrogen storage in microporous metal-organic frameworks. Science. 2003;300:1127–1129. doi: 10.1126/science.1083440. [DOI] [PubMed] [Google Scholar]
- 16.Bobbitt NS, Chen J, Snurr RQ. High-throughput screening of metal–organic frameworks for hydrogen storage at cryogenic temperature. J. Phys. Chem. C. 2016;120:27328–27341. doi: 10.1021/acs.jpcc.6b08729. [DOI] [Google Scholar]
- 17.Colón YJ, Gómez-Gualdrón DA, Snurr RQ. Topologically guided, automated construction of metal–organic frameworks and their evaluation for energy-related applications. Cryst. Growth Des. 2017;17:5801–5810. doi: 10.1021/acs.cgd.7b00848. [DOI] [Google Scholar]
- 18.Batten SR, et al. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013) Pure Appl. Chem. 2013;85:1715–1724. doi: 10.1351/PAC-REC-12-11-20. [DOI] [Google Scholar]
- 19.Howarth AJ, et al. Best practices for the synthesis, activation, and characterization of metal−organic frameworks. Chem. Mater. 2017;29:26–39. doi: 10.1021/acs.chemmater.6b02626. [DOI] [Google Scholar]
- 20.Kim HK, et al. A chemical route to activation of open metal sites in the copper-based metal–organic framework materials HKUST-1 and Cu-MOF-2. J. Am. Chem. Soc. 2015;137:10009–10015. doi: 10.1021/jacs.5b06637. [DOI] [PubMed] [Google Scholar]
- 21.Rowsell JLC, Yaghi OM. Effects of functionalization, catenation, and variation of the metal oxide and organic linking units on the low-pressure hydrogen adsorption properties of metal−organic frameworks. J. Am. Chem. Soc. 2006;128:1304. doi: 10.1021/ja056639q. [DOI] [PubMed] [Google Scholar]
- 22.Yuan S, et al. Construction of hierarchically porous metal–organic frameworks through linker labilization. Nat. Commun. 2017;8:15356. doi: 10.1038/ncomms15356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu W, et al. Tuning the structure and function of metal–organic frameworks via linker design. Chem. Soc. Rev. 2014;43:5561–5593. doi: 10.1039/C4CS00003J. [DOI] [PubMed] [Google Scholar]
- 24.Chung YG, et al. Computation-ready, experimental metal−organic frameworks: a tool to enable high-throughput screening of nanoporous crystals. Chem. Mater. 2014;26:6185–6192. doi: 10.1021/cm502594j. [DOI] [Google Scholar]
- 25.Moghadam PZ, et al. Development of a cambridge structural database subset: a collection of metal–organic frameworks for past, present, and future. Chem. Mater. 2017;29:2618–2625. doi: 10.1021/acs.chemmater.7b00441. [DOI] [Google Scholar]
- 26.Chung YG, et al. In silico discovery of metal-organic frameworks for precombustion CO2 capture using a genetic algorithm. Sci. Adv. 2016;2:e1600909. doi: 10.1126/sciadv.1600909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez-Gualdron DA, et al. Computational design of metal–organic frameworks based on stable zirconium building units for storage and delivery of methane. Chem. Mater. 2014;26:5632–5639. doi: 10.1021/cm502304e. [DOI] [Google Scholar]
- 28.Getman RB, Bae YS, Wilmer CE, Snurr RQ. Review and analysis of molecular simulations of methane, hydrogen, and acetylene storage in metal–organic frameworks. Chem. Rev. 2012;112:703–723. doi: 10.1021/cr200217c. [DOI] [PubMed] [Google Scholar]
- 29.Huong TTT, Thanh PN, Huynh NTX, Son DN. Metal–organic frameworks: state-of-the-art material for gas capture and storage. VNU J. Sci. Math. Phys. 2016;32:67–85. [Google Scholar]
- 30.Müller K, Felderhoff F. Special issue: application of hydrogen storage materials, carriers, and processes. Energy Technol. 2018;6:443–444. doi: 10.1002/ente.201800106. [DOI] [Google Scholar]
- 31.Groom CR, Bruno IJ, Lightfoot MP, Ward SC. The cambridge structural. Database Acta Cryst. 2016;72:171–179. doi: 10.1107/S2052520616003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilmer CE, et al. Large-scale screening of hypothetical metal–organic frameworks. Nat. Chem. 2011;4:83–89. doi: 10.1038/nchem.1192. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez M, Boyd PG, Daff TD, Aghaji MZ, Woo TK. Rapid and accurate machine learning recognition of high performing metal organic frameworks for CO2 capture. J. Phys. Chem. Lett. 2014;5:3056–3060. doi: 10.1021/jz501331m. [DOI] [PubMed] [Google Scholar]
- 34.Bao Y, et al. In silico discovery of high deliverable capacity metal–organic frameworks. J. Phys. Chem. C. 2015;119:186–195. doi: 10.1021/jp5123486. [DOI] [Google Scholar]
- 35.Martin RL, Lin LC, Jariwala K, Smit B, Haranczyk M. Mail-order metal–organic frameworks (MOFs): Designing isoreticular MOF-5 analogues comprising commercially available organic molecules. J. Phys. Chem. C. 2013;117:12159–12167. doi: 10.1021/jp401920y. [DOI] [Google Scholar]
- 36.Martin RL, Simon CM, Smit B, Haranczyk M. In silico design of porous polymer networks: high-throughput screening for methane storage materials. J. Am. Chem. Soc. 2014;136:5006–5022. doi: 10.1021/ja4123939. [DOI] [PubMed] [Google Scholar]
- 37.Slater A. G., Cooper A. I. Function-led design of new porous materials. Science. 2015;348(6238):aaa8075–aaa8075. doi: 10.1126/science.aaa8075. [DOI] [PubMed] [Google Scholar]
- 38.Smit B. Screening materials relevant for energy technologies. Chim. Int. J. Chem. 2015;69:248–252. doi: 10.2533/chimia.2015.248. [DOI] [PubMed] [Google Scholar]
- 39.Simon CM, et al. The Materials genome in action: identifying the performance limits for methane storage. Energy Environ. Sci. 2015;8:1190–1199. doi: 10.1039/C4EE03515A. [DOI] [Google Scholar]
- 40.Anton, D.L., & Motyka, T.. Hydrogen Storage Engineering Center of Excellence. (United States Department of Energy, USA, 2015).
- 41.Fischer M, Hoffmann F, Fröba M. Preferred hydrogen adsorption sites in various MOFs—a comparative computational study. Chemphyschem. 2009;10:2647–2657. doi: 10.1002/cphc.200900459. [DOI] [PubMed] [Google Scholar]
- 42.Feynman RP, Hibbs AR. Quantum Mechanics and Path Integrals. New York: McGraw-Hill; 1965. [Google Scholar]
- 43.Veenstra, M., et al. Ford/BASF-SE/UM Activities in Support of the Hydrogen Storage Engineering Center of Excellence. https://www.hydrogen.energy.gov/pdfs/review14/st010_veenstra_2014_o.pdf (United States Department of Energy, Hydrogen and Fuel Cells Program 2014 Annual Merit Review Proceedings: Project ST010, USA, 2014).
- 44.Aghaji MZ, Fernandez M, Boyd PG, Daff TD, Woo TK. Quantitative structure-property relationship models for recognizing metal organic frameworks (MOFs) with high CO2 working capacity and CO2/CH4 selectivity for methane purification. Eur. J. Inorg. Chem. 2016;2016:4505–4511. doi: 10.1002/ejic.201600365. [DOI] [Google Scholar]
- 45.United States Driving Research and Innovation for Vehicle efficiency and Energy sustainability. Target Explanation Document: Onboard Hydrogen Storage for Light-Duty Fuel Cell Vehicles.https://www.energy.gov/sites/prod/files/2017/05/f34/fcto_targets_onboard_hydro_storage_explanation.pdf (U.S. Drive, USA, 2017).
- 46.Prasad TK, Suh MP. Control of interpenetration and gas-sorption properties of metal-organic frameworks by a simple change in ligand design. Chemistry. 2012;18:8673–8680. doi: 10.1002/chem.201200456. [DOI] [PubMed] [Google Scholar]
- 47.Rankine D, Avellaneda A, Hill MR, Doonan CJ, Sumby CJ. Control of framework interpenetration for in situ modified hydroxyl functionalised IRMOFs. Chem. Commun. 2012;48:10328–10330. doi: 10.1039/c2cc35565e. [DOI] [PubMed] [Google Scholar]
- 48.Eddaoudi M, et al. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science. 2002;295:469–472. doi: 10.1126/science.1067208. [DOI] [PubMed] [Google Scholar]
- 49.Koh K, Van Oosterhout JD, Roy S, Wong-Foy AG, Matzger AJ. Exceptional surface area from coordination copolymers derived from two linear linkers of differing lengths. Chem. Sci. 2012;3:2429. doi: 10.1039/c2sc20407j. [DOI] [Google Scholar]
- 50.Mason JA, Veenstra M, Long JR. Evaluating metal–organic frameworks for natural gas storage. Chem. Sci. 2014;5:32–51. doi: 10.1039/C3SC52633J. [DOI] [Google Scholar]
- 51.Kapelewski MT, et al. Record high hydrogen storage capacity in the metal–organic framework Ni 2 (m -Dobdc) at near-ambient temperatures. Chem. Mater. 2018;30:8179–8189. doi: 10.1021/acs.chemmater.8b03276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson G, Schweitzer B, Anderson R, Gómez-Gualdrón DA. Attainable volumetric targets for adsorption-based hydrogen storage in porous crystals: molecular simulation and machine learning. J. Phys. Chem. C. 2019;123:120–130. doi: 10.1021/acs.jpcc.8b09420. [DOI] [Google Scholar]
- 53.García-Holley P, et al. Benchmark study of hydrogen storage in metal–organic frameworks under temperature and pressure swing conditions. ACS Energy Lett. 2018;3:748–754. doi: 10.1021/acsenergylett.8b00154. [DOI] [Google Scholar]
- 54.Bucior BJ, et al. Energy-based descriptors to rapidly predict hydrogen storage in metal-organic frameworks. Mol. Syst. Des. Eng. 2019;4:162–174. doi: 10.1039/C8ME00050F. [DOI] [Google Scholar]
- 55.Colón YJ, Fairen-Jimenez D, Wilmer CE, Snurr RQ. High-throughput screening of porous crystalline materials for hydrogen storage capacity near room temperature. J. Phys. Chem. C. 2014;118:5383–5389. doi: 10.1021/jp4122326. [DOI] [Google Scholar]
- 56.Bao Y, Martin RL, Haranczyk M, Deem MW. In silico prediction of MOFs with high deliverable capacity or internal surface area. Phys. Chem. Chem. Phys. 2015;17:11962–11973. doi: 10.1039/C5CP00002E. [DOI] [PubMed] [Google Scholar]
- 57.Gómez-Gualdrón DA, Wilmer CE, Farha OK, Hupp JT, Snurr RQ. Exploring the limits of methane storage and delivery in nanoporous materials. J. Phys. Chem. C. 2014;118:6941–6951. doi: 10.1021/jp502359q. [DOI] [Google Scholar]
- 58.Hyeon S, Kim YC, Kim J. Computational prediction of high methane storage capacity in V-MOF-74. Phys. Chem. Chem. Phys. 2013;19:21132–21139. doi: 10.1039/C7CP03605A. [DOI] [PubMed] [Google Scholar]
- 59.Willems TF, Rycroft CH, Kazi M, Meza JC, Haranczyk M. AlgorithMs And Tools For High-throughput Geometry-based Analysis Of Crystalline Porous Materials. Microporous Mesoporous Mater. 2012;149:134–141. doi: 10.1016/j.micromeso.2011.08.020. [DOI] [Google Scholar]
- 60.Dubbeldam D, Calero S, Ellis DE, Snurr RQ. RASPA: Molecular Simulation Software For Adsorption And Diffusion In Flexible Nanoporous. Mater. Mol. Simul. 2016;42:81–101. doi: 10.1080/08927022.2015.1010082. [DOI] [Google Scholar]
- 61.Farha OK, et al. De novo synthesis of a metal–organic framework material featuring ultrahigh surface area and gas storage capacities. Nat. Chem. 2010;2:944–948. doi: 10.1038/nchem.834. [DOI] [PubMed] [Google Scholar]
- 62.Yuan D, Zhao D, Sun D, Zhou HC. An isoreticular series of metal-organic frameworks with dendritic hexacarboxylate ligands and exceptionally high gas-uptake capacity. Angew. Chem. Int. Ed. 2010;49:5357–5361. doi: 10.1002/anie.201001009. [DOI] [PubMed] [Google Scholar]
- 63.Mondloch JE, Karagiaridi O, Farha OK, Hupp JT. Activation of metal–organic framework materials. CrystEngComm. 2013;15:9258–9264. doi: 10.1039/c3ce41232f. [DOI] [Google Scholar]
- 64.Ma J, Kalenak AP, Wong-Foy AG, Matzger AJ. Rapid guest exchange and ultra-low surface tension solvents optimize metal-organic framework activation. Angew. Chemie Int. Ed. 2017;56:14618–14621. doi: 10.1002/anie.201709187. [DOI] [PubMed] [Google Scholar]
- 65.Thommes M, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report) Pure Appl. Chem. 2015;87:1051–1069. doi: 10.1515/pac-2014-1117. [DOI] [Google Scholar]
- 66.Parilla PA, Gross K, Hurst K, Gennett T. Recommended volumetric capacity definitions and protocols for accurate, standardized and unambiguous metrics for hydrogen storage materials. Appl. Phys. A. 2016;122:201. doi: 10.1007/s00339-016-9654-1. [DOI] [Google Scholar]
- 67.Fischer RA, Schwedler I. Terminologie von metall-organischen gerüstverbindungen und koordinationspolymeren (IUPAC-Empfehlungen 2013) Angew. Chem. 2014;126:7209–7214. doi: 10.1002/ange.201400619. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All calculated/measured crystallographic properties, adsorption isotherms, and characterization data sets generated and/or analyzed during the current study are available at the HyMARC Data Hub (https://datahub.hymarc.org) or from the corresponding author upon reasonable request. A portion of the MOF crystal structures used in generating the calculated data sets are accessible from the Cambridge Structural Database (CSD). The remaining crystal structures are either publicly available as Supplementary Information from published papers, or can be requested from the authors of the respective databases presented in Table 2.