Abstract
Measurement of the soluble fms-like tyrosine kinase 1 (sFlt-1)/placental growth factor (PlGF) ratio may be clinically useful to discriminate systemic lupus erythematosus (SLE) from preeclampsia. Here, we present a pregnant woman with new-onset SLE with hypertension, with the measurement of the sFlt-1/PlGF ratio during pregnancy. A 31-year-old Japanese nulliparous woman, who had been diagnosed with idiopathic thrombocytopenic purpura at 10 years, had a systolic blood pressure of 120 mmHg and was negative for proteinuria at 12+1 weeks. Since her blood pressure increased to 159/86 mmHg with 3+ proteinuria at 25+4 weeks, preeclampsia was suspected. Deterioration of the kidney function (creatinine: 0.58 mg/dL at 24+6 weeks to 0.83 mg/dL at 33+6 weeks) necessitated cesarean section at 33+6 weeks. After delivery, she still showed increased creatinine and proteinuria. Therefore, she was transferred to a nephrology specialist in a tertiary center and was finally diagnosed with SLE with lupus nephritis class IV-G(A) (diffuse lupus nephritis). The serum levels of sFlt-1 and the sFlt-1/PlGF ratio, which are usually elevated in preeclampsia, were within normal reference ranges at 27+6, 28+1, and 28+6 weeks of gestation, although the serum levels of PlGF were slightly lower than the normal reference range. In conclusion, measurement of the sFlt-1/PlGF ratio may be clinically useful to discriminate lupus nephritis from preeclampsia.
Keywords: Lupus nephritis, Placental growth factor, Preeclampsia, Pregnancy, Soluble fms-like tyrosine kinase 1, Systemic lupus erythematosus
Introduction
Preeclampsia develops in 2–3% of pregnant women in Japan [1]. Some pregnant women with new-onset thrombotic thrombocytopenic purpura (TTP), hemolytic uremic syndrome (HUS), or acute exacerbation of systemic lupus erythematosus (SLE) show clinical symptoms similar to those of preeclampsia [2, 3]. Since SLE affects multiple organ systems, women with SLE sometimes show both hypertension and proteinuria. Thus, discriminating pregnant women with new-onset SLE with both hypertension and proteinuria from those with preeclampsia is sometimes difficult.
SLE usually occurs in females of reproductive age [2, 4], and thus, it sometimes occurs around or during pregnancy. It is important for obstetricians to accurately diagnose women with new-onset proteinuria during the second half of the pregnancy period with or without hypertension, because the management of new-onset SLE during pregnancy should be different from that for women with preeclampsia.
Soluble fms-like tyrosine kinase 1 (sFlt-1)/placental growth factor (PlGF) ratio has already been an established biomarker of preeclampsia [5]. Therefore, one possible way to discriminate the two conditions may be to measure the levels of sFlt-1/PlGF ratio, because women with preeclampsia often show a very high sFlt-1/PlGF ratio compared with those with chronic kidney disease [6, 7]. Several studies measured circulating levels of sFlt-1 and PlGF, whereby physicians can discriminate preeclampsia from SLE [8–10]. Here, we show a pregnant woman with new-onset lupus nephritis with hypertension during pregnancy, in whom measurement of the sFlt-1/PlGF ratio might have been clinically useful to discriminate lupus nephritis from preeclampsia.
Case report
A 31-year-old Japanese nulliparous woman, who had been diagnosed with idiopathic thrombocytopenic purpura (ITP) at 10 years, was referred from a primary clinic to a regional perinatal center at 12+1 weeks. Her systolic blood pressure (SBP) was 120 mmHg and proteinuria was negative. At 22+0 weeks, she had blood pressure (BP) of 125/76 mmHg with 1+ proteinuria on a dipstick test (Figs. 1, 2). At 24+0 weeks, her BP was slightly increased to 136/83 mmHg with 2 + proteinuria. She was admitted to this hospital at 25+4 weeks. Since her BP was 159/86 mmHg with 3+ proteinuria, she was suspected of having preeclampsia. α-methyldopa at 250 mg/day was started. Since mild hypertension (149/89 mmHg) with 3+ proteinuria (urinary protein-to-creatinine ratio: 0.755 g/g Cr) persisted, she was transferred to our hospital, a tertiary perinatal center, at 26+6 weeks. Because she showed severe hypertension (166/95 mmHg), α-methyldopa was increased to 750 mg/day. However, it was subsequently discontinued, because her BP was normalized at 29 weeks. Interestingly, proteinuria did not resolve (0.6–1.2 g/day) regardless of normalization of the BP. We decided to back-transfer her at 30+2 weeks. At 33+2 weeks, hypertension (154/102 mmHg) recurred, and she was admitted to the initial hospital. α-methyldopa was re-started. Because of a gradual drop in the platelet counts (from 10.5 × 104/µL at 24+6 weeks to 7.6 × 104/µL at 33+2 weeks) and deterioration of the kidney function (creatinine: 0.58 mg/dL at 24+6 weeks to 0.83 mg/dL at 33+6 weeks), emergency cesarean section was performed at 33+6 weeks (Figs. 1, 2). A female infant weighing 2,032 g was delivered.
Fig. 1.
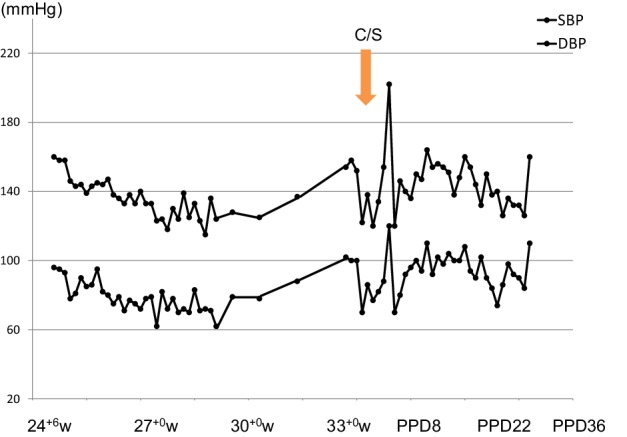
Blood pressure changes during pregnancy and the puerperal period. C/S cesarean section, SBP systolic blood pressure, DBP diastolic blood pressure, w weeks of gestation, PPD postpartum days
Fig. 2.
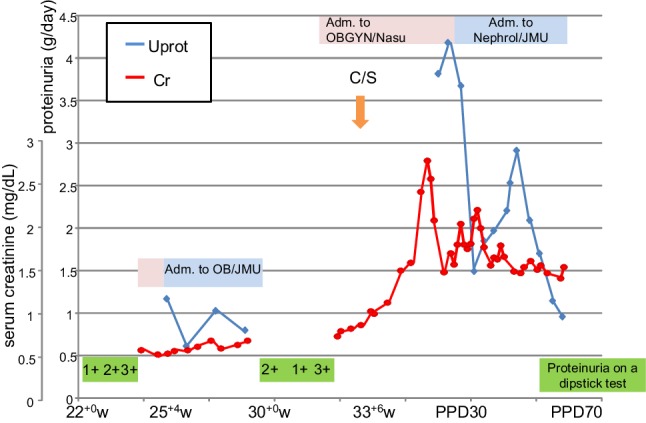
Changes of urine proteinuria and serum levels of creatinine during pregnancy and the puerperal period. C/S cesarean section, Uprot 24-h urine protein test (g/day), Cr serum level of creatinine, Adm. admission, OB/JMU Department of Obstetrics in Jichi Medical University Hospital, OBGYN/Nasu RCH Department of Obstetrics and Gynecology in Nasu Red Cross Hospital, Nephrol/JMU Division of Nephrology in Jichi Medical University Hospital, w weeks of gestation, PPD postpartum days
Proteinuria continued during the puerperal period, and her creatinine level increased to 1.13 mg/dL with granular and red blood cell casts on postpartum day 11; then, she was referred to Division of Nephrology on this hospital. Hemolytic anemia (Hb of 6.0 g/dL) with a haptoglobin level of < 10 mg/dL appeared; then, 4 units of red blood cells were transfused. Since the creatinine level was increased to 2.77 mg/dL on postpartum day 23, she was referred to Urology. She was diagnosed with disturbance of urination; a urinary catheter was inserted and distigmine was prescribed. The catheter was removed on postpartum day 28, because the creatinine level decreased to 1.48 mg/dL. However, proteinuria was continued (4.18 g/day on postpartum day 28). Although proteinuria > 3.5 g/day continued at least > 3 days, serum levels of albumin were almost > 3.0 g/dL; thus, we excluded the possibility of nephrotic syndrome. In addition, she showed low complement and was positive for anti-nuclear antibody (ANA) (x 160). Since the nephrologist suspected atypical hemolytic uremic syndrome (aHUS), SLE, or membranoproliferative glomerulonephritis (MPGN), she was transferred to the Division of Nephrology in our hospital, a tertiary center (Jichi Medical University Hospital) on postpartum day 29.
Thickness of bladder wall and right hydronephrosis without either urinary stone or urinary obstruction was detected on postpartum day 31, and urinary culture was negative finding, suggesting any inflammation in bladder and urinary tracts; then, a double J ureteral stent was inserted in the right ureter. A nephrologist suspected lupus cystitis. She was finally diagnosed with SLE on postpartum day 34, because 4 SLE diagnostic criteria were met: (1) renal disorder (+): increased protein and red blood cell casts; (2) hematologic disorder (+): hemolytic anemia and thrombocytopenia; (3) immunologic disorder (+): increased anti-ds-DNA antibody of 199.3 IU/mL; and (4) ANA (+) [11, 12]. The level of serum C3, C4, and CH50 was 31 mg/dL, 2 mg/dL, and 18.0 U/mL, respectively; hypocomplementemia was one of the well-known feature of SLE. We surveyed the possibility of microangiopathy. We finally excluded the possibility of TTP and HUS. Although she showed the triad of aHUS, renal failure, microangiopathic hemolytic anemia, and thrombocytopenia, we finally concluded that her findings of aHUS might be emerged due to SLE. Anti-phospholipid antibody was negative. Plasma exchange was performed because of hemolytic anemia and thrombocytopenia. Methylprednisolone at 500 mg/day was administered intravenously for 3 days, followed by prednisolone at 60 mg/day, tacrolimus at 2 mg/day, and mizoribine at 200 mg every other day. Since thrombocytopenia was ameliorated, renal biopsy was performed on postpartum day 52.
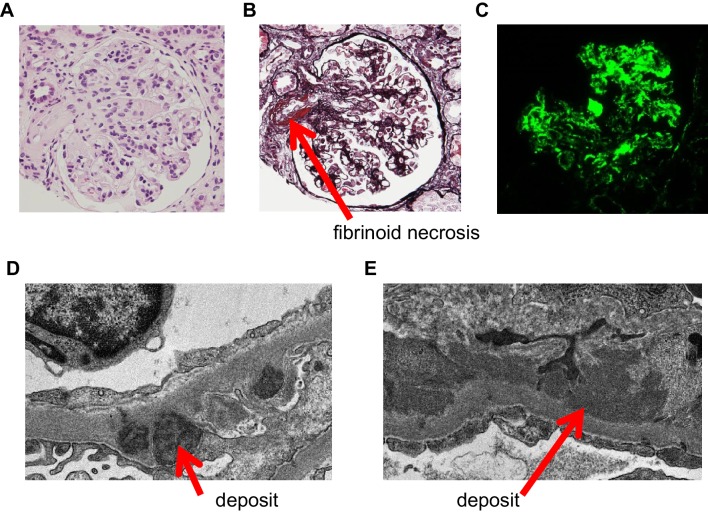
Histological examination confirmed the diagnosis of lupus nephritis class IV-G(A) (diffuse lupus nephritis). The glomeruli showed diffuse and global mesangial hypercellularity and endocapillary proliferation (Fig. 3a). Glomerular sclerosis and crescent formation were absent. Fibrinoid necrosis was observed in several arterioles using PAM stain (Fig. 3b). On immunofluorescence staining, C1q was coarsely deposited in mesangial cells and peripheral glomerular capillaries (Fig. 3c). Although C3, IgG, and IgA were positive in mesangial cells and peripheral glomerular capillaries, their staining intensity was weaker than that of C1q (data not shown). Electron-microscopic examination revealed dense deposits in basement membranes (Fig. 3d, e). The histological and electron-microscopic findings did not show microvascular lesions.
Fig. 3.
Representative findings of Hematoxylin–Eosin (HE) stain (a), periodic acid–methenamine–silver stain (PAM) stain (b), immunofluorescence (c), and transmission electron microscopy (d, e). a, b Glomeruli showed diffuse and global mesangial hypercellularity and endocapillary proliferation. Glomerular sclerosis and crescent formation were absent. Fibrinoid necrosis (red arrow) was observed in several arterioles. c On immunofluorescence staining, C1q was coarsely deposited in mesangial cells and peripheral glomerular capillaries. Although C3, IgG, and IgA were positive in mesangial cells and peripheral glomerular capillaries, their staining intensity was weaker than that of C1q (data not shown). d, e On electron microscopy, dense deposits (red arrows) were observed in basement membranes, the spaces under the epithelial (d) and endothelial cells (e)
Hypertension was controlled by the administration of enalapril. Because the patient showed diffuse and global hyaline thrombi in glomeruli, dipyridamole was prescribed. She was discharged from our hospital on postpartum day 67, with prescribed drugs of prednisolone at 35 mg/day, tacrolimus at 3 mg/day, mizoribine at 200 mg per every other day, nifedipine at 80 mg/day, enalapril at 2.5 mg/day, and dipyridamole at 50 mg/day.
We had started a study to evaluate the impact of the serial measurements of the sFlt-1/PlGF ratio after the onset of either hypertension and/or proteinuria on the development of a more severe form of PE (I-12-78) [13, 14]. Written informed consent was obtained from the patient. We performed blood sampling at 27+6, 28+1, and 28+6 weeks of gestation. After the puerperal diagnosis of SLE, the serum levels of sFlt-1 and PlGF were measured using Elecsys sFlt-1 and Elecsys PlGF (Roche Diagnostics, Penzberg, Germany). The serum levels of sFlt-1 and sFlt-1/PlGF ratio at 27+6, 28+1, and 28+6 weeks of gestation were within normal reference ranges, although the serum levels of PlGF were slightly lower than the normal reference range (sFlt-1 [pg/mL]: 1,311, 1,311, and 1,208, respectively; PlGF [pg/mL]: 237, 245, and 219, respectively; and sFlt-1/PlGF ratio: 5.5, 5.3, and 5.5, respectively) (Fig. 4A, B, C) [13]. These indicate that measurement of the sFlt-1/PlGF ratio in women with new-onset SLE during pregnancy may be clinically useful to rule out preeclampsia from new-onset lupus nephritis during pregnancy. We did not measure serum levels of soluble endoglin (sEng).
Fig. 4.
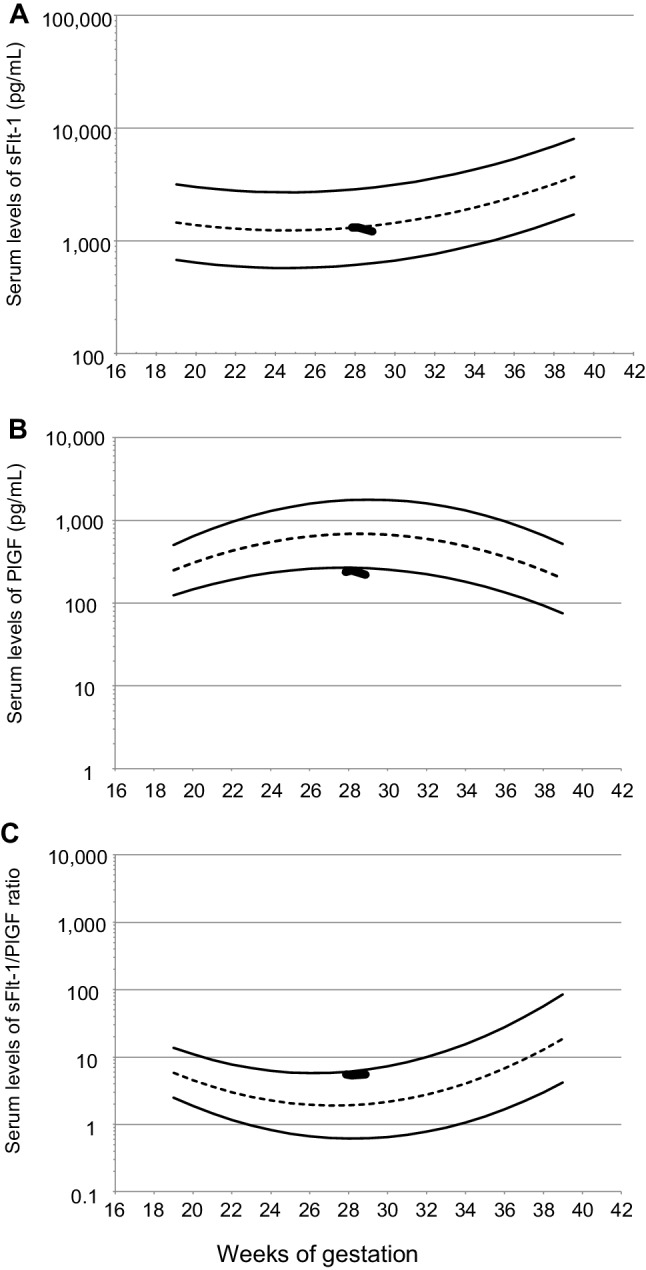
Serum levels of sFlt-1, PlGF, and the sFlt-1/PlGF ratio in a woman with the initial onset of lupus nephritis in pregnancy. Gestational-age-specific reference ranges of serum levels of sFlt-1, PlGF, and the sFlt-1/PlGF ratio during 19–38 weeks of gestation are shown using non-dashed curves representing the 5th and 95th percentiles of the reference ranges, and the short dashed curves represent the mean of the reference ranges. Thick jagged lines represent the values at 27+6, 28+1, and 28+6 weeks of gestation. sFlt-1 soluble fms-like tyrosine kinase 1, PlGF placental growth factor
Discussion
To the best of our knowledge, we, for the first time, presented a pregnant woman with new-onset lupus nephritis during pregnancy, in whom the measurement of the sFlt-1/PlGF ratio might have been clinically useful to discriminate lupus nephritis from preeclampsia. Although the sFlt-1/PlGF ratio in women with late-onset PE is often within normal reference ranges, the sFlt-1/PlGF ratio in women with early onset PE is very high in almost all cases [15, 16]. Therefore, measurement of the sFlt-1/PlGF ratio at < 34 weeks of gestation may be clinically useful to rule out preeclampsia from not only new-onset lupus nephritis but also other forms of glomerulonephritis with hypertension.
Are there any clinical characteristics of new-onset SLE during pregnancy that can help discriminate new-onset SLE from preeclampsia? The occurrence of new-onset SLE tends to concentrate in the first and second trimesters [3]. The frequencies of the following clinical characteristics were significantly higher, respectively, in new-onset SLE patients than in those with preeclampsia: fever (27.1 vs. 4.2%), malar lesion (35.4 vs. 0%), anemia (77.1 vs. 50.0%), leucopenia (39.6 vs. 0%), thrombocytopenia (39.6 vs. 4.2%), active urinary sediment (39.4 vs. 0%), hypocomplementemia (72.9 vs. 0%), ANA positivity (97.9 vs. 0%), and anti-dsDNA positivity (83.3 vs. 0%) [3]. On the contrary, the frequencies of hypertension and hyperuricemia were significantly lower in new-onset SLE patients than in those with preeclampsia (12.5 vs. 100%, 16.7 vs. 50%, respectively) [3]. If we had examined urinary sediment, the level of complement, ANA, or dsDNA antibody during pregnancy while suspecting new-onset SLE, we could have diagnosed her with SLE during the current pregnancy period. However, we did not suspect new-onset SLE during pregnancy, mainly due to the lack of fever and malar lesions, in addition due to our presumptive diagnosis of preeclampsia.
Measurement of the sFlt-1/PlGF ratio may be useful to discriminate gestational proteinuria without hypertension from new-onset glomerulonephritis only showing proteinuria in pregnancy. In our previous case report, we described a parous woman who developed severe proteinuria (5.8 g/day at 32 weeks of gestation), although she did not show hypertension [17]. Because the sFlt-1 and sEng levels were very high, we decided not to perform a kidney biopsy, and not to prescribe a steroid-based agent [17]. After the cesarean section at 33 weeks, proteinuria decreased to 0.36 g/day in postpartum week 12, and finally disappeared in postpartum week 26, indicating that our presumptive diagnosis of gestational proteinuria in pregnancy was correct [17]. The circulating level of sFlt-1 is sometimes increased in women with gestational proteinuria, similar to those with preeclampsia [18].
Measurements of sFlt-1, PlGF, and the sFlt-1/PlGF ratio may be useful to diagnose superimposed preeclampsia on chronic glomerulonephritis. Circulating sFlt-1 and PlGF levels were measured in 5, 5, and 5 women with superimposed preeclampsia on chronic glomerulonephritis, those with severe proteinuria without hypertension, and those with normal clinical course, respectively; the sFlt-1 levels in women with superimposed preeclampsia on chronic glomerulonephritis were significantly higher than in those with severe proteinuria, or those with a normal clinical course; in addition, the circulating PlGF levels in women with superimposed preeclampsia on chronic glomerulonephritis were significantly lower than in those with severe proteinuria, or those with a normal clinical course [7].
Measurement of sFlt-1, PlGF, sFlt-1/PlGF ratio, and sEng may be clinically useful to predict and diagnose preeclampsia in pregnant women with anti-phospholipid syndrome (APS) and/or SLE. These biomarkers were measured prospectively at 4-week intervals in 17 women with primary APS (PAPS), 18 women with secondary APS (SAPS), and 23 women with SLE; women who developed preeclampsia showed significantly higher levels of sFlt-1, sFlt-1/PlGF ratio, and sEng, and lower levels of PlGF, than women who did not; in addition, these changes occurred at 12 weeks for sFlt-1, PlGF and sEng [8]. However, it is not determined whether these measurements could be useful to discriminate new onset of SLE showing both hypertension and proteinuria during pregnancy from superimposed preeclampsia.
In conclusion, measurement of the sFlt-1/PlGF ratio may be clinically useful to discriminate new-onset lupus nephritis in pregnancy from preeclampsia. However, it was not elucidated whether measurement of the sFlt-1/PlGF ratio at the appearance of either proteinuria with or without hypertension could generally be used to discriminate the occurrence of preeclampsia from new-onset glomerulonephritis in pregnancy. Reports on the sFlt-1/PlGF ratio measured at the appearance of proteinuria with or without hypertension in pregnant women with a final diagnosis of chronic nephritis should be accumulated, which will help answer this important clinical question.
Acknowledgements
We thank Roche Diagnostics K. K. for the offer of reagents (sFlt-1 and PlGF), and the measurements of sFlt-1 and PlGF conducted free of charge under collaborative research with Roche Diagnostics K. K. (I-12-78.) Toshimitsu Ohtani, Kanako Nakamura, Chihiro Kamosawa, Keizou Yoshida, and Hideki Yano of Nasu Red Cross Hospital contributed to the detection and diagnosis of SLE.
Funding
None.
Conflict of interest
Measurements of serum levels of sFlt-1, PlGF, and the sFlt-1/PlGF ratio in this case were performed in collaborative research with Roche Diagnostics K. K. (I-12-78.)
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee at which the studies were conducted (IRB approval number: I-12-78) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. We also obtained written informed consent for this case report from the patient.
Informed consent
We also obtained written informed consent for this case report from the patient.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shiozaki A, Matsuda Y, Satoh S, Saito S. Comparison of risk factors for gestational hypertension and preeclampsia in Japanese singleton pregnancies. J Obstet Gynaecol Res. 2013;39:492–499. doi: 10.1111/j.1447-0756.2012.01990.x. [DOI] [PubMed] [Google Scholar]
- 2.Sibai BM. Imitators of severe pre-eclampsia. Semin Perinatol. 2009;33:196–205. doi: 10.1053/j.semperi.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Zhao C, Zhao J, Huang Y, Wang Z, Wang H, Zhang H, Xu H, Yang N. New-onset systemic lupus erythematosus during pregnancy. Clin Rheumatol. 2013;32:815–822. doi: 10.1007/s10067-013-2180-z. [DOI] [PubMed] [Google Scholar]
- 4.Cortés-Hernández J, Ordi-Ros J, Paredes F, Casellas M, Castillo F, Vilardell-Tarres M. Clinical predictors of fetal and maternal outcome in systemic lupus erythematosus: a prospective study of 103 pregnancies. Rheumatology. 2002;41:643–650. doi: 10.1093/rheumatology/41.6.643. [DOI] [PubMed] [Google Scholar]
- 5.Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, Olovsson M, Brennecke SP, Stepan H, Allegranza D, Dilba P, Schoedl M, Hund M, Verlohren S. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:13–22. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- 6.Rolfo A, Attini R, Nuzzo AM, Piazzese A, Parisi S, Ferraresi M, Todros T, Piccoli GB. Chronic kidney disease may be differentially diagnosed from preeclampsia by serum biomarkers. Kidney Int. 2013;83:177–181. doi: 10.1038/ki.2012.348. [DOI] [PubMed] [Google Scholar]
- 7.Masuyama H, Suwaki N, Nakatsukasa H, Masumoto A, Tateishi Y, Hiramatrsu Y. Circulating angiogenic factors in preeclampsia, gestational proteinuria, and preeclampsia superimposed on chronic glomerulonephritis. Am J Obstet Gynecol. 2006;194:551–556. doi: 10.1016/j.ajog.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Mayer-Pickel K, Stern C, Eberhard K, Lang U, Obermayer-Pietsch B, Cervar-Zivkovic M. Angiogenic factors in pregnancies of women with antiphospholipid syndrome and systemic lupus erythematosus. J Reprod Immunol. 2018;127:19–23. doi: 10.1016/j.jri.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Leaños-Miranda A, Campos-Galicia I, Berumen-Lechuga MG, Molina-Pérez CJ, García-Paleta Y, Isordia-Salas I, Ramírez-Valenzuela KL. Circulating angiogenic factors and the risk of preeclampsia in systemic lupus erythematosus pregnancies. J Rheumatol. 2015;42:1141–1149. doi: 10.3899/jrheum.141571. [DOI] [PubMed] [Google Scholar]
- 10.Qazi U, Lam C, Karumanchi SA, Petri M. Soluble Fms-like tyrosine kinase associated with preeclampsia in pregnancy in systemic lupus erythematosus. J Rheumatol. 2008;35:631–634. [PubMed] [Google Scholar]
- 11.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 12.Hartman EAR, van Royen-Kerkhof A, Jacobs JWG, Welsing PMJ, Fritsch-Stork RDE. Performance of the 2012 Systemic Lupus International Collaborating Clinics classification criteria versus the 1997 American College of Rheumatology classification criteria in adult and juvenile systemic lupus erythematosus. A systematic review and meta-analysis. Autoimmun Rev. 2018;17:316–322. doi: 10.1016/j.autrev.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki H, Hirashima C, Nagayama S, Takahashi K, Yamamoto T, Matsubara S, Ohkuchi A. Increased serum levels of sFlt-1/PlGF ratio in preeclamptic women with onset at < 32 weeks compared with ≥ 32 weeks. Pregnancy Hypertens. 2018;12:96–103. doi: 10.1016/j.preghy.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki H, Nagayama S, Hirashima C, Takahashi K, Takahashi H, Ogoyama M, Nagayama M, Shirasuna K, Matsubara S, Ohkuchi A. Markedly higher sFlt-1/PlGF ratio in a woman with acute fatty liver of pregnancy compared with HELLP syndrome. J Obstet Gynaecol Res. 2018 doi: 10.1111/jog.13786. [DOI] [PubMed] [Google Scholar]
- 15.Ohkuchi A, Hirashima C, Matsubara S, Suzuki H, Takahashi K, Arai F, Watanabe T, Kario K, Suzuki M. Alterations in placental growth factor levels before and after the onset of preeclampsia are more pronounced in women with early onset severe preeclampsia. Hypertens Res. 2007;30:151–159. doi: 10.1291/hypres.30.151. [DOI] [PubMed] [Google Scholar]
- 16.Ohkuchi A, Hirashima C, Suzuki H, Takahashi K, Yoshida M, Matsubara S, Suzuki M. Evaluation of a new and automated electrochemiluminescence immunoassay for plasma sFlt-1 and PlGF levels in women with preeclampsia. Hypertens Res. 2010;33:422–427. doi: 10.1038/hr.2010.15. [DOI] [PubMed] [Google Scholar]
- 17.Ohmaru T, Ohkuchi A, Muto S, Hirashima C, Matsubara S, Suzuki M. Increased antiangiogenetic factors in severe proteinuria without hypertension in pregnancy: is kidney biopsy necessary? CEN Case Rep. 2014;3:86–89. doi: 10.1007/s13730-013-0092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohkuchi A, Hirashima C, Matsubara S, Suzuki H, Takahashi K, Usui R, Suzuki M. Serum sFlt1:PlGF ratio, PlGF, and sEng levels in gestational proteinuria. Hypertens Pregnancy. 2009;28:95–108. doi: 10.1080/10641950802419895. [DOI] [PubMed] [Google Scholar]