Abstract
We herein report two cases of advanced stage rapidly progressive diabetic nephropathy that were effectively treated with combination therapy including renin–angiotensin–aldosterone system (RAS) blocker [angiotensin II receptor blocker (ARB)], glucagon-like peptide-1 (GLP-1) receptor agonist and sodium glucose transporter-2 (SGLT-2) inhibitor. A 30-year-old woman with advanced stage diabetic nephropathy [estimated glomerular filtration rate (eGFR): 20.7 mL/min/1.73 m2; proteinuria: 13.2 g/gCr], showing a rapidly progressive pattern (annual eGFR change: − 60.0 mL/min/1.73 m2/year), had improvement in proteinuria (5.9 g/gCr) and eGFR change (+ 4.3 mL/min/1.73 m2 over 15 weeks) after administration of ARB (irbesartan 25 mg/day), GLP-1 receptor agonist (liraglutide 0.3 mg/day) and SGLT-2 inhibitor (canagliflozin 50 mg/day). A 59-year-old man with advanced stage diabetic nephropathy (eGFR: 32.4 mL/min/1.73 m2; proteinuria: 8.90 g/gCr), showing a rapidly progressive pattern (annual eGFR change: − 21.2 mL/min/1.73 m2/year), had an improvement in proteinuria (0.02 g/gCr) and annual eGFR change (+ 0.1 mL/min/1.73 m2/year) after combination therapy with ARB (olmesartan 40 mg/day), GLP-1 receptor agonist (liraglutide 0.9 mg/day) and SGLT-2 inhibitor (tofogliflozin 10 mg/day). These results suggest that this triple combination therapy has renoprotective effects on advanced stage rapidly progressive diabetic nephropathy.
Keywords: Rapidly progressive diabetic nephropathy, Angiotensin II receptor blocker, Glucagon-like peptide-1 receptor agonist, Sodium glucose transporter-2 inhibitor
Introduction
Several populations of diabetic nephropathy have been reported to show rapid progression, which is defined as a decline rate of the estimate glomerular filtration rate (eGFR) of > 5 mL/min/1.73 m2/year, resulting in a progression to the advanced stage of disease over a short period [1]. The mechanisms of this rapid decline are still under investigation.
Although several classes of drugs such as renin–angiotensin–aldosterone system (RAS) blocker [including angiotensin II receptor blocker (ARB)], glucagon-like peptide-1 (GLP-1) receptor agonist and sodium glucose transporter-2 (SGLT-2) inhibitor have been reported to show renoprotective effects on diabetic nephropathy by monotherapy [2–4] or combination therapy [5, 6], the effects of these drugs on advanced stage rapidly progressive diabetic nephropathy have not been confirmed.
We herein report two cases of advanced stage rapidly progressive diabetic nephropathy that were effectively treated with combination therapy including ARB, GLP-1 receptor agonist and SGLT-2 inhibitor.
Case report
Case 1
A 30-year-old woman was diagnosed with diabetes mellitus by a medical checkup when she was 15 years old. No treatment was conducted according to her choice. Fifteen years later, she was admitted to our hospital because of a vitreous hemorrhage. At that time, her diabetes mellitus control was not good with HbA1c level of 12.1%. Her diabetes mellitus was classified as type 2 because of negative anti-glutamic acid decarboxylase (GAD) antibody and preserved fasting serum C-peptide level of 5.94 ng/mL. She had no family history of diabetes mellitus, so the possible diagnosis of maturity-onset diabetes of the young was excluded. On physical examination, bilateral proliferative diabetic retinopathy with vitreous hemorrhage and loss of Achilles tendon reflex were detected. Additionally, her renal function was impaired with eGFR of 42.3 mL/min/1.73 m2 and proteinuria of 8.1 g/day. Thus, insulin therapy was started to control her diabetes mellitus. Furthermore, amlodipine was initiated because of increase in blood pressure. However, her renal function continued to worsen. Six months later, she was referred to our department for treatment of renal impairment (eGFR: 20.7 mL/min/1.73 m2) with nephrotic range proteinuria (13.2 g/day) (Fig. 1). The decline rate of her renal function before consulting our department was high (annual eGFR change: − 60.0 mL/min/1.73 m2) (Fig. 1). No other pathogenic condition except for diabetic nephropathy explaining her renal disease was found (Table 1). In addition, she had not been taking any medicines including Chinese herbal medicine, supplement, and analgesics that could induce acute kidney injury. On the basis of her clinical course, she was diagnosed with a case of advanced stage rapidly progressive diabetic nephropathy. To attenuate her rapidly declining renal function, ARB (irbesartan 25 mg/day) and SGLT-2 inhibitor (canagliflozin 50 mg/day) were administrated (Fig. 1). Two weeks later, proteinuria was decreased; however, eGFR continued to decrease at the same high speed (Fig. 1). Therefore, GLP-1 receptor agonist (liraglutide 0.3 mg/day) was administrated for further renoprotection (Fig. 1). Thirteen weeks later, her renal function was markedly improved (eGFR change: + 4.3 mL/min/1.73 m2 over 15 weeks) (Fig. 1).
Fig. 1.
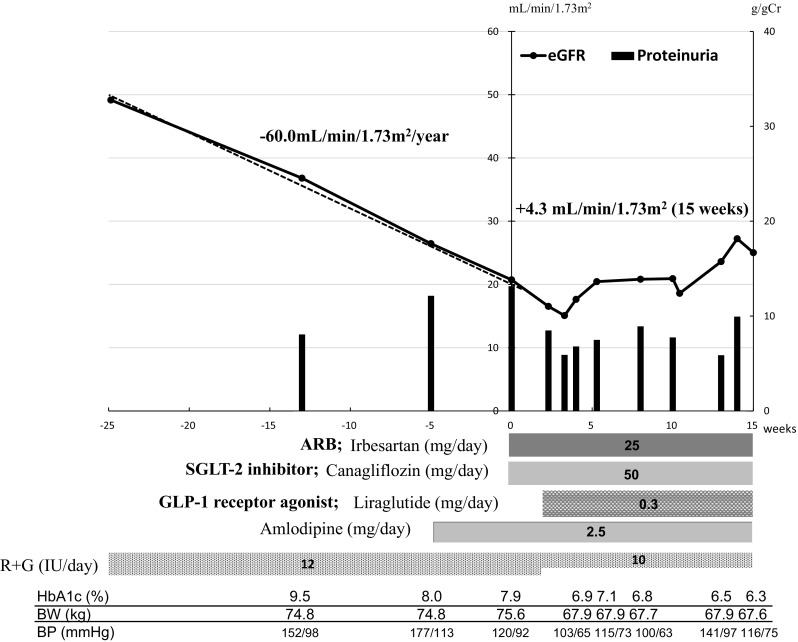
Clinical course of case 1. The x-axis shows the number of months from referral. The y-axis shows the number of eGFR and proteinuria. eGFR estimated glomerular filtration rate, R insulin aspart, G glargine. The annual change in eGFR was determined by linear regression analysis
Table 1.
Laboratory results at the time of referral to our department
| Examination | Case 1 | Case 2 | Reference range |
|---|---|---|---|
| Blood test | |||
| White blood cells (/µL) | 11,530 | 8,820 | 3900–9800 |
| Neutrophil (%) | 78.2 | 60.7 | 40–74 |
| Lymphocytes (%) | 15.4 | 25.9 | 19–48 |
| Monocyte (%) | 3.1 | 5.7 | 3.4–9.0 |
| Eosinophil (%) | 2.0 | 5.0 | 0–7 |
| Basophil (%) | 0.4 | 0.7 | 0–2 |
| Red blood cells (/µL) | 410 × 104 | 361 × 104 | 427–570 × 104 |
| Hemoglobin (g/dL) | 12.5 | 11.0 | 12.0–17.6 |
| Hematocrit (%) | 38.3 | 33.7 | 39.8–51.8 |
| Mean corpuscular volume | 93.4 | 93.7 | 83–101 |
| Platelets (× 104/µL) | 49.6 | 28.5 | 13.0–36.9 |
| Total protein (g/dL) | 6.1 | 5.0 | 6.4–8.2 |
| Albumin (g/dL) | 2.1 | 2.4 | 3.9–5.1 |
| Total bilirubin (mg/dL) | 0.13 | 0.44 | 0.2–1.0 |
| Aspartate aminotransferase (mU/mL) | 13 | 17 | 11–30 |
| Alanine aminotransferase (mU/mL) | 19 | 9 | 4–30 |
| Lactate dehydrogenase (mU/mL) | 166 | 237 | 110–220 |
| Total cholesterol (mg/dL) | 239 | 155 | 142–248 |
| LDL-cholesterol (mg/dL) | 132 | 88 | < 140 |
| HDL-cholesterol (mg/dL) | 39 | 29 | 48–103 |
| Triglyceride (mg/dL) | 199 | 217 | 30–117 |
| Sodium (mEq/L) | 135 | 142 | 138–145 |
| Potassium (mEq/L) | 5.8 | 4.4 | 3.6–4.8 |
| Chloride (mEq/L) | 110 | 111 | 100–110 |
| Calcium (mg/dL) | 8.7 | 8.3 | 8.6–10.1 |
| Phosphate (mg/dL) | 6.2 | 4.0 | 2.7–4.6 |
| Blood urea nitrogen (mg/dL) | 85 | 19 | 8–20 |
| Creatinine (mg/dL) | 2.95 | 1.77 | 0.65–1.07 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 20.7 | 32.4 | |
| C-reactive protein (mg/dL) | 0.47 | 0.87 | < 0.20 |
| Blood glucose (mg/dL) | 198 | 140 | 70–100 |
| HbA1c (%) | 7.9 | 6.4 | 4.6–6.2 |
| IgG (mg/dL) | 1329 | 917 | 870–1700 |
| IgA (mg/dL) | 401 | 163 | 110–410 |
| IgM (mg/dL) | 148 | 27 | 33–190 |
| C3 (mg/dL) | 122 | 93 | 65–135 |
| C4 (mg/dL) | 40 | 28 | 13–35 |
| CH50 (U/mL) | 59.2 | 36.0 | 30.0–45.0 |
| Antinuclear antibody | ≤ 40 | ≤ 40 | ≤ 40 |
| PR3-ANCA (IU/mL) | < 10 | < 1.0 | < 1.0 |
| MPO-ANCA (IU/mL) | < 10 | < 1.0 | < 1.0 |
| Antiglomerular basement membrane antibody (IU/mL) | < 2.0 | < 2.0 | < 3.5 |
| Urine test | |||
| pH | 5.5 | 6.0 | 5.0–7.5 |
| Specific gravity | 1.011 | 1.008 | 1.005–1.025 |
| Protein | 3 + | 3 + | − |
| Glucose | 2 + | − | − |
| Red blood cell (/HPF) | 1–4 | 1–4 | 0–4 |
| White blood cell (/HPF) | 5–9 | 1–4 | 0–4 |
| BJP | + | + | − |
| M protein | − | − | − |
| Proteinuria (g/gCr) | 13.21 | 8.89 | < 0.15 |
ANCA anti-neutrophil cytoplasmic antibody, BJP Bence Jones protein, HbA1c hemoglobin A1c, IgA immunoglobulin A, IgG immunoglobulin G, IgM immunoglobulin M, MPO myeloperoxidase, PR-3 proteinase-3
Case 2
A 59-year-old man was admitted to a general hospital because of myocardial infarction. At that time, his HbA1c level was found to be 9.5%. The patient was negative for anti-GAD antibody, and his fasting serum C-peptide level was preserved (6.94 ng/mL). He was also found to have bilateral neuropathy (loss of Achilles tendon reflex), bilateral retinopathy (proliferative diabetic retinopathy) and renal impairment (eGFR: 60.5 mL/min/1.73 m2) with proteinuria (0.99 g/gCr). He was diagnosed with type 2 diabetes mellitus with diabetic neuropathy, retinopathy, and nephropathy. Insulin treatment was started. However, his renal function rapidly decreased year by year (annual eGFR change: − 21.2 mL/min/1.73 m2). Three years later, he was referred to our department for treatment of his renal impairment. At that time, his eGFR was decreased to 32.4 mL/min/1.73 m2. Urinalysis showed nephrotic range proteinuria (8.90 g/gCr). A detailed description of the laboratory data at that time is shown in Table 1. His renal function before consulting our department declined at a high speed (annual eGFR change: − 21.2 mL/min/1.73 m2) (Fig. 2). No other pathogenic condition explaining his renal disease was found (Table 1). Based on his clinical course, he was diagnosed with a case of advanced stage rapidly progressive diabetic nephropathy. To attenuate the rapid decline in his renal function, ARB (olmesartan) was administered at an initial dose of 10 mg/day and was titrated up to 40 mg/day (Fig. 2). Nutrition counseling including salt limitation (3–6 g/day) and protein restriction (0.6–0.8 g/kg/day) was also conducted. After these treatments, his annual eGFR change rate was improved to − 3.9 mL/min/1.73 m2 (Fig. 2). Proteinuria was also decreased to < 0.5 g/gCr (Fig. 2). However, 20 months later, his proteinuria increased to > 5.0 g/gCr (Fig. 2). Therefore, GLP-1 receptor agonist (liraglutide) was administered at an initial dose of 0.3 mg/day and was titrated up to 0.9 mg/day (Fig. 2). After that, his annual eGFR change rate improved to − 1.3 mL/min/1.73 m2 (Fig. 2). Proteinuria also decreased to < 0.2–0.3 g/gCr (Fig. 2). To further improve the rate of decline in renal function, SGLT-2 inhibitor (tofogliflozin) was administered, which improved his annual eGFR change rate to + 0.1 mL/min/1.73 m2 (Fig. 2). Proteinuria also decreased to 0.02 g/gCr (Fig. 2).
Fig. 2.
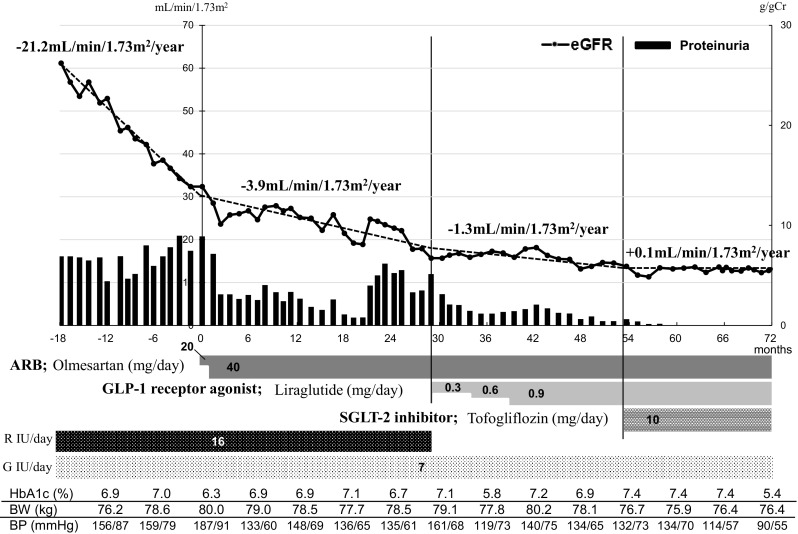
Clinical course of case 2. The x-axis shows the number of weeks from referral. The y-axis shows the number of eGFR and proteinuria. The annual change in eGFR was determined by linear regression analysis. eGFR estimated glomerular filtration rate, R insulin aspart, G glargine
Discussion
We herein described two cases of advanced stage rapidly progressive diabetic nephropathy, which were effectively treated with a combination therapy including RAS blocker (ARB), GLP-1 receptor agonist and SGLT-2 inhibitor. This triple combination therapy appears to be effective against advanced stage rapidly progressive diabetic nephropathy.
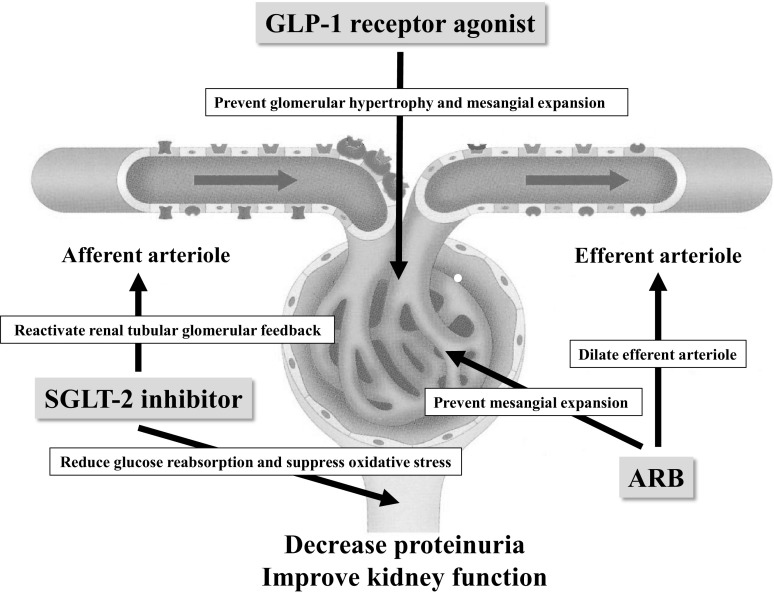
We diagnosed these two cases with advanced stage rapidly progressive diabetic nephropathy based on their renal function, clinical course and eGFR decline rate [1]. The mechanisms and treatment of advanced stage rapidly progressive diabetic nephropathy have not been established, yet. RAS blockers, GLP-1 receptor agonists and SGLT-2 inhibitors have been reported to have certain renoprotective effects on diabetic nephropathy [2, 3, 7]. Recently, the positive effects of combination therapies with RAS blockers and GLP-1 receptor agonists or SGLT-2 inhibitors have been reported for diabetic nephropathy [5, 6], as they have different renoprotective mechanisms (Fig. 3) [8–12]. SGLT-2 inhibitors have been suggested to have renoprotective effects in patients with advanced stage diabetic nephropathy [13]. GLP-1 receptor agonists have been shown to be superior in terms of renoprotection compared with other classes of glucose-lowering agents in diabetes mellitus with renal impairment [14].
Fig. 3.
Suggested mechanisms of the nephroprotective effects of the triple combination therapy with ARB, GLP-1 receptor agonist and SGLT-2 inhibitor. ARB angiotensin II receptor blocker, GLP-1 glucagon-like peptide-1, SGLT-2 sodium glucose transporter-2
In this case report, a full combination therapy including RAS blocker (ARB), GLP-1 receptor agonist and SGLT-2 inhibitor showed significant renoprotection effects in two cases of rapidly progressive advanced stage diabetic nephropathy. In both cases, we started with SGLT-2 inhibitor at a lower dose because of the concern that concomitant use of ARB and SGLT-2 inhibitor may increase the risk for acute kidney injury [15]. We did not observe any adverse events including hyperkalemia, dehydration and urinary tract infection in both cases. These results suggest that the combination therapy of RAS blocker (ARB), GLP-1 receptor agonist and SGLT-2 inhibitor has strong renoprotective effects on advanced stage rapidly progressive diabetic nephropathy. However, the effects of this triple therapy on advanced stage rapidly progressive diabetic nephropathy should also be investigated by clinical studies.
In conclusion, we presented two cases of advanced stage rapidly progressive diabetic nephropathy that were successfully treated with a combination therapy of RAS blocker (ARB), GLP-1 receptor agonist and SGLT-2 inhibitor. Thus, this triple therapy may be effective for renoprotection against advance stage rapidly progressive diabetic nephropathy.
Conflict of interest
The authors have declared that no conflict of interest exists.
Human and animal rights statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from the patients in these case reports.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) Evaluation and Management of Chronic Kidney Disease Work Group Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/kisup.2012.73. [DOI] [Google Scholar]
- 2.Palmer SC, Mavridis D, Navarese E, Craig JC, Tonelli M, Salanti G, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385(9982):2047–2056. doi: 10.1016/S0140-6736(14)62459-4. [DOI] [PubMed] [Google Scholar]
- 3.Tuttle KR, Lakshmanan MC, Rayner B, Busch RS, Zimmermann AG, Woodward DB, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605–617. doi: 10.1016/S2213-8587(18)30104-9. [DOI] [PubMed] [Google Scholar]
- 4.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 5.Imamura S, Hirai K, Hirai A. The glucagon-like peptide-1 receptor agonist, liraglutide, attenuates the progression of overt diabetic nephropathy in type 2 diabetic patients. Tohoku J Exp Med. 2013;231(1):57–61. doi: 10.1620/tjem.231.57. [DOI] [PubMed] [Google Scholar]
- 6.Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6(9):691–704. doi: 10.1016/S2213-8587(18)30141-4. [DOI] [PubMed] [Google Scholar]
- 7.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 8.Zatz R, Dunn BR, Meyer TW, Anderson S, Rennke HG, Brenner BM. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Investig. 1986;77(6):1925–1930. doi: 10.1172/JCI112521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hostetter TH, Rosenberg ME, Ibrahim HN, Juknevicius I. Aldosterone in renal disease. Curr Opin Nephrol Hypertens. 2001;10(1):105–110. doi: 10.1097/00041552-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Kodera R, Shikata K, Kataoka HU, Takatsuka T, Miyamoto S, Sasaki M, et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia. 2011;54(4):965–978. doi: 10.1007/s00125-010-2028-x. [DOI] [PubMed] [Google Scholar]
- 11.Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129(5):587–597. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 12.Ishibashi Y, Matsui T, Yamagishi S, Tofogliflozin A highly selective inhibitor of SGLT2 blocks proinflammatory and proapoptotic effects of glucose overload on proximal tubular cells partly by suppressing oxidative stress generation. Horm Metab Res. 2016;48(3):191–195. doi: 10.1055/s-0035-1555791. [DOI] [PubMed] [Google Scholar]
- 13.Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2(5):369–384. doi: 10.1016/S2213-8587(13)70208-0. [DOI] [PubMed] [Google Scholar]
- 14.Boye KS, Botros FT, Haupt A, Woodward B, Lage MJ. Glucagon-like peptide-1 receptor agonist use and renal impairment: a retrospective analysis of an electronic health records database in the US population. Diabetes Ther. 2018;9(2):637–650. doi: 10.1007/s13300-018-0377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn K, Ejaz AA, Kanbay M, Lanaspa MA, Johnson RJ. Acute kidney injury from SGLT2 inhibitors: potential mechanisms. Nat Rev Nephrol. 2016;12(12):711–712. doi: 10.1038/nrneph.2016.159. [DOI] [PubMed] [Google Scholar]