Abstract
We are all too familiar with the events that follow a bee sting—heat, redness, swelling, and pain. These are Celsus' four cardinal signs of inflammation that are driven by very well‐defined signals and hormones. In fact, targeting the factors that drive this onset phase is the basis upon which most current anti‐inflammatory therapies were developed. We are also very well aware that within a few hours, these cardinal signs normally disappear. In other words, inflammation resolves. When it does not, inflammation persists, resulting in damaging chronic conditions. While inflammatory onset is actively driven, so also is its resolution—years of research have identified novel internal counter‐regulatory signals that work together to switch off inflammation. Among these signals, lipids are potent signalling molecules that regulate an array of immune responses including vascular hyper reactivity and pain, as well as leukocyte trafficking and clearance, so‐called resolution. Here, we collate bioactive lipid research to date and summarize the major pathways involved in their biosynthesis and their role in inflammation, as well as resolution.
Linked Articles
This article is part of a themed section on Eicosanoids 35 years from the 1982 Nobel: where are we now? To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.8/issuetoc
Abbreviations
- 15‐epi‐LXs
15 epimeric‐LX
- AA
arachidonic acid
- COX
cyclooxygenase
- CYP450
cytochrome P450
- DHA
docosahexaenoic acid
- DHETs
dihydroxy‐eicosatrienoic acids
- EPA
eicosapentaenoic acid
- LXs
lipoxins
- MaR
maresins
- NSAIDs
nonsteroidal anti‐inflammatory drugs
- PD1
protectin D1
- PDs
protectins
- RvD1
resolvin D1
- Rvs
resolvins
- sEH
soluble epoxide hydrolase
1. INFLAMMATION AND ITS RESOLUTION
Inflammation is a protective response against infection and/or injury. However, when it becomes dysregulated as a consequence of genetic abnormalities, the ageing process or environmental factors, our immune system has the capacity to cause extensive damage. Arthritis, asthma, chronic obstructive pulmonary disease, Alzheimer's disease, atherosclerosis, and even cancer, while aetiologically disparate, are diseases unified by a dysregulated immune component. The current strategy of treating such diseases is based, largely, upon inhibiting the factors that drive acute inflammation such as nonsteroidal anti‐inflammatory drugs (NSAIDs—such as naproxen or diclofenac), steroids (prednisone), and “biological” drugs such as infliximab (anti‐TNF) and anakinra (anti‐IL‐1). Although these medicines ameliorate disease symptoms, they do not bring about a “cure” and are ineffective in a significant subset of patients. Furthermore, side effects can hamper endogenous homeostatic systems, predisposing to infection. Thus, there is a need to develop more efficient and effective therapeutic agents, with one approach being to harness the body's own healing process for therapeutic gain.
Consequently, attention has turned to the other end of the inflammatory spectrum, resolution, in order to understand the endogenous processes that switch off inflammation. Our objective has been to identify novel internal counter‐regulatory systems that terminate inflammation in order to provide new targets that can be harnessed pharmacologically to push ongoing inflammation down a pro‐resolution pathway. As a result, resolution is now been studied in great detail with clear evidence suggesting that resolution is an active process with quantifiable indices and specific requirements. Along these lines, lipid mediators have emerged as internal regulatory signals that activate many aspects of the inflammation and resolution cascade, including terminating leukocyte trafficking into tissue once the inflammatory signal has been removed, scavenging pro‐inflammatory signals as well as clearing dead cells from the resolves site. Hence, in this review, the role of lipids in the resolution cascade will be discussed.
2. CYCLOOXYGENASE AND PROSTANOIDS
The enzyme cyclooxygenase (COX) converts arachidonic acid (AA) to form PGG2 (Pagels et al., 1983) with the peroxidase element of the enzyme further reducing PGG2 to PGH2 (Hamberg & Samuelsson, 1973), which serves as a precursor for all major prostanoid mediators. There are two principal isoforms involved in the conversion of AA to prostanoids, namely, COX‐1 and COX‐2. Unlike COX‐1, which is constitutively expressed in most cells and tissues and is broadly involved in house‐keeping functions, COX‐2 is induced in response to inflammatory stimuli (Dubois et al., 1998) being expressed at sites of infection and injury with the exception of parts of the brain and kidney (Harris et al., 1994). Formation of prostanoids from PGH2 occurs through the actions of downstream synthases that are expressed in a tissue and cell type‐selective fashion including PGD synthase (Shimizu, Yamamoto, & Hayaishi, 1982) PGE synthase 1, 2, and 3 (Tanaka, Ward, & Smith, 1987), PGF synthase (Hayashi, Fujii, Watanabe, Urade, & Hayaishi, 1989), prostacyclin synthase, and thromboxane A synthase (Ullrich & Haurand, 1983), which form PGD2 , PGE2, PGF2α, PGI2 (also known as prostacyclin), and TXA2 respectively. The differential expression of these downstream enzymes within cells determines the profile and levels of prostanoid production generated under resting and inflammatory conditions.
Presently, there are nine known prostanoid receptors in mice and man. These include the PGD receptors, DP1 and DP2; the PGE2 receptors, EP1, EP2, EP3, and EP4; the PGF receptor, FP; the PGI receptor, IP; and the TXA receptor, TP. In addition, there are splice variants of the EP3, FP, and TP receptors differentiated only in their C‐terminal tails. All of these receptors belong to the GPCR superfamily of proteins, with the exception of DP2 (also known as CRTH2), which is a member of the chemokine receptor family (Hirai et al., 2001). The IP, DP1, EP2, and EP4 receptors signal through Gs resulting in an increased intracellular cAMP, whereas the EP3 receptor couples to Gi to reduce cAMP, while EP1, FP, and TP receptors signal through Gq to induce calcium mobilization.
The more common prostanoids, PGE2 and PGI2, both enhance vasodilation (Kaley, Hintze, Panzenbeck, & Messina, 1985), oedema formation, and vascular permeability, particularly in the presence of histamine, bradykinin, and 5‐HT (Hata & Breyer, 2004). Mice that are genetically depleted for their respective receptors (IP, EP2, and EP3) show reduced pleural exudation following treatment with inflammogens including carrageenan and zymosan (Yuhki et al., 2004).
Robust evidence from EP‐deficient mice has shown that the febrile response to PGE2 arises from the actions of PGE2 on its EP3 receptor, which is present on sensory neurons in the periphery and brain (Dantzer, Konsman, Bluthe, & Kelley, 2000). Equally, PGE2 is a potent pyretic agent known with elevated concentrations found in cerebrospinal fluid taken from patients with bacterial or viral infections (Saxena, Beg, Singhal, & Ahmad, 1979). While none of the prostanoids cause pain directly, PGI2 and PGE2 reduce the threshold of nociceptor sensory neurons to stimulation when bound to IP, EP1, EP3, and EP4 receptors respectively (Ahmadi, Lippross, Neuhuber, & Zeilhofer, 2002).
Prostanoids also play an important role in protecting against oxidative injury in cardiac tissue and in maintaining cardiovascular (CV) homeostasis. Indeed, their protective effect has been demonstrated in clinical studies, which found an increased risk of myocardial infarction (MI), stroke, systemic and pulmonary hypertension, thrombosis, and sudden cardiac death following the use of COX‐2‐specific inhibitors (Garcia Rodriguez, Tacconelli, & Patrignani, 2008). Furthermore, deleting specific prostanoid synthases and receptors results in an augmentation of ischaemia–reperfusion injury (Xiao et al., 2001) as well as contributing to the decline in cardiac function following MI. CV health is regulated by vasodilatory PGI2 and pro‐thrombotic TXA2 (Bunting, Moncada, & Vane, 1983), where PGI2 counterbalance the actions of TXA2 (Grosser, Fries, & FitzGerald, 2006). Indeed, endothelial PGI2 along with NO prevents TXA2‐induced platelet aggregation and thrombosis. TXA2 is derived from platelet COX‐1 causing platelet aggregation and vascular smooth muscle contraction (Ellis et al., 1976). Clinical CV diseases including unstable angina, MI, and stroke can arise from overproduction of TXA4. Importantly, the cardio‐protective properties of aspirin can be attributed to the covalent inhibition of COX‐1 (Rocca et al., 2002).
As well as being pro‐inflammatory, many prostanoids up‐regulate intracellular cAMP triggering immuno‐suppressive effects. For example, PGE2 and PGI2 reduce the ability of inflammatory leukocytes to phagocytose and kill microorganisms (Aronoff, Canetti, & Peters‐Golden, 2004), as well as inhibiting the production of downstream pro‐inflammatory mediators (Aronoff et al., 2007) while, in contrast, triggering the synthesis of IL‐10 and IL‐6 (Harizi, Juzan, Pitard, Moreau, & Gualde, 2002). Indeed, in a number of conditions associated with increased susceptibility to infection, including cancer (Starczewski, Voigtmann, Peskar, & Peskar, 1984), ageing (Hayek et al., 1997), and cystic fibrosis (Medjane, Raymond, Wu, & Touqui, 2005), overexpression of PGE2 has been reported. Interestingly, during the very early phase of acute inflammation, PGE2 indirectly exerts pro‐resolution effects by switching on the transcription of enzymes necessary for the generation of lipoxins (LXs; Levy, Clish, Schmidt, Gronert, & Serhan, 2001), resolvins (Rvs), and protectins (PDs; Hong, Gronert, Devchand, Moussignac, & Serhan, 2003). These represent other classes of lipids mediators with pro‐resolution properties.
While PGD2 can elevate cAMP via its DP1 receptors, PGD2 may also act independently of its DP1 and DP2 receptors when it non‐enzymically dehydrates into PGs of the J2 series, such as PGJ2, Δ12,14‐PGJ2, and 15‐deoxy‐Δ12,14‐PGJ2 [15d‐PGJ2]; Clark et al., 2000). These cyclopentenone PGs form covalent attachments with reactive sulphydryl groups on intracellular regulatory proteins, which enables modulation of their function. For instance, 15d‐PGJ2 upon ligation to the nuclear receptor PPAR‐γ (Khan, 1995) decreases pro‐inflammatory cytokine release and modifies gene expression (Jiang, Ting, & Seed, 1998) as well as directly inhibiting the actions of IκB kinase, which is responsible for the activation of NF‐κB (Cernuda‐Morollon, Pineda‐Molina, Canada, & Perez‐Sala, 2001). 15d‐PGJ2, identified in rodent peritonitis resolution exudates (Rajakariar et al., 2007), independently of PPAR‐γ, can preferentially inhibit monocyte, rather than neutrophil, trafficking through differential regulation of cell‐adhesion molecule and chemokine expression (Gilroy et al., 2003); regulate macrophage activation and pro‐inflammatory gene expression (Lawrence, 2002); and induce leukocyte apoptosis through a caspase‐dependent mechanism (Bishop‐Bailey & Hla, 1999). Moreover, PGD2‐derived compounds function as endogenous braking signals for lymphocytes to stimulate resolution (Trivedi et al., 2006). Table 1 summarises the prostanoids, their biological actions and concentrations at sites of inflammation.
Table 1.
Biological actions of lipid mediators and their relative concentrations at sites of inflammation
| Lipid | Biological actions | Concentrations at sites of inflammation (Motwani et al., 2018, Clin Pharm Ther; JCI Insight.) | |||||
|---|---|---|---|---|---|---|---|
| Inhibits granulocyte trafficking | Cytokine scavenging | Dampens pro‐inflammatory signalling | Apoptosis | Efferocytosis | M1‐M2 conversion | ||
| Lipoxins A4 and B4 | ✓ | ✓ | _ | _ | ✓ | ✓ | 3.5–10.0 pg·ml−1 (Human E. coli‐driven blister fluid) |
| Resolvin E1 | ✓ | _ | ✓ | _ | ✓ | ✓ | 2.0 pg·ml−1 (Human E. coli‐driven blister fluid) |
| Resolvin D1 | ✓ | _ | ✓ | _ | ✓ | ✓ | 0.5–3.5 pg·ml−1 (Human E. coli‐driven blister fluid) |
| Resolvin D2 | ✓ | _ | _ | _ | ✓ | _ | 10–15.0 pg·ml−1 (Human E. coli‐driven blister fluid) |
| Maresins | ✓ | _ | _ | _ | ✓ | _ | ~3.5 pg·ml−1 (Human E. coli‐driven blister fluid) |
| Protectin D1 | ✓ | _ | _ | ✓ | _ | _ | ~1.0 pg·ml−1 (Human E. coli‐driven blister fluid) |
| PGD2 | ✓ | _ | _ | _ | _ | _ | ~35.0 pg·ml−1 (Human E. coli‐driven blister fluid) |
| 15‐Deoxy‐Δ12–14‐PGJ2 | ✓ | _ | ✓ | _ | _ | _ | 1,500 pg·ml−1 (mouse peritonitis;Rajakariar et al., 2007, PNAS) |
| PGE2 | Along with PGI2 (~5,000 pg·ml−1 in E. coli‐driven blister fluid), PGE2 enhances vasodilation, oedema formation, and vascular permeability. PGE2 is also a potent pyrogen and dampens innate immune‐mediated responses to bacteria. PGE2 also suppresses tumour immunity and has contrasting roles in the adaptive immune response dampening T cell proliferation in some instances and driving TH‐17 responses in others. | ~700 pg·ml−1 (Human E. coli‐driven blister fluid) | |||||
| PGF2α | PGF2α has potent smooth muscle stimulator, vaso‐ and broncho‐constrictor actions, and is also involved in acute and chronic inflammatory diseases and sub‐chronic inflammation in various cardiovascular dysfunctions. | 75–10,00 pg·ml−1 (Human E. coli‐driven blister fluid) | |||||
| TxA2 | TxA2 is a potent vasoconstrictor and modulates T cell proliferative responses and the spread of bacterial infections | 50–350 pg·ml−1 (Human E. coli‐driven blister fluid) | |||||
Note. E. coli: Escherichia coli.
3. PROSTANOIDS AND POST‐RESOLUTION BIOLOGY
Recently, we demonstrated that classical resolution may not be the end of the local immune response to infection or injury, but rather that a third phase subsequent to these exists: post‐resolution (Motwani et al., 2017). Traditionally, resolution processes were deemed successful if acute inflammation, as described by leukocyte clearance and cytokine catabolism, was terminated. However, they may have a hitherto unappreciated role in controlling adaptive immune responses and maintaining tolerance. Specifically, we found that murine innate immune‐mediated responses to low‐dose yeast cell wall extract (zymosan, administered i.p.) or bacteria (Streptococcus pneumoniae ovalbumin‐labelled, i.p.) were resolved. Interestingly, these low‐dose stimuli elicited a previously overlooked second wave of leukocyte influx into tissues that persisted for weeks. These cells comprised three separate populations of Ly6chi monocyte‐derived macrophages including CD11B+/CD49d+/CD115+/MHC‐II+ myeloid‐derived suppressor cells, F4‐80lo/MHC‐II+/CD11c+ dendritic cells, and F4‐80int/CD11Bhi/CD11c− macrophages. In addition, tissue‐resident (embryonic‐derived) macrophages, which disappear during the acute inflammatory response, re‐appear. These diverse populations of macrophages were observed alongside lymph node expansion and increased numbers of peripheral blood and tissue memory T and B lymphocytes. Polymorphonuclear (PMNs) were not present during this phase. One of the key events in this process is the sustained synthesis of PGE2, which is derived from macrophage COX‐1/mPGES and that is triggered by IFN‐γ. It transpires that this post‐resolution phase of prostanoid biosynthesis creates a window of susceptibility to repeat infections on the one hand, while also controlling local adaptive immune processes on the other (Newson et al., 2017). The nature of these prostanoid/adaptive immune interactions is being investigated.
4. CYTOCHROME P450
Cytochrome P450s (CYP450s) are a family of membrane‐bound, haem‐containing enzymes found in the liver, kidneys, brain, heart, CV system, and lung and are best characterized for the catalysis of NADPH‐dependent oxidation of drugs, chemicals, carcinogens, and hormones (Nelson et al., 1996). The CYP450 family contains 57 genes in humans, and although approximately one quarter of these have been shown capable of metabolizing polyunsaturated fatty acids (PUFAs), the CYP2J2 and CYP2C family members (CYP2C8, 2C9) are thought to be the major enzymes responsible for lipid mediator production (Bishop‐Bailey, Thomson, Askari, Faulkner, & Wheeler‐Jones, 2014). In addition to metabolizing AA (Figure 1), CYP450s also readily metabolize the related ω6 PUFA linoleic acid (Figure 2) and ω3 PUFAs (see below also) docosahexaenoic acid (DHA, Figure 3) and eicosapentaenoic acid (EPA; Figure 4) into a series of related biologically active mediators (Smilowitz et al., 2013). CYP450 are capable or metabolizing PUFA substrates by epoxygenase, lipoxygenase (LOX) and ω‐hydroxylase type of activities (Zeldin, 2001). The epoxygenase activity inserts a single molecular oxygen in one of the double bonds of each PUFA, for example, for AA to form one of four regioisomers of epoxyeicosatrienoic acid (5,6‐, 8,9‐, 11,12‐, or 14,15‐EET; the numbers indicating the double bond in AA subject to epoxygenation; Zeldin, 2001). Each EET can be formed as either an R/S or S/R stereoisomer, with ratios of production depending on the generating CYP450. Stereoisomers of EETs may have different biological activities, but little research exists to understand the extent of these differences. CYP450s can also have LOX activity producing mid‐chain (12[R]‐), and ω‐hydroxylase activity producing terminal (19[S > R]‐, and 20‐) hydroxyeicosatetraenoic acids (HETEs; Roman, 2002). Once formed, epoxygenase products in particular are quickly metabolized by epoxide hydrolases (EH) or reincorporated into membranes (Zeldin, 2001). Soluble EH (sEH) and microsomal EH (encoded by the ephx2 and ephx1, respectively) combine to metabolize virtually all epoxygenase products in vivo (Edin et al., 2018). For example, EETs get converted to dihydroxy‐eicosatrienoic acids. Importantly, a number of sEH inhibitors have been developed that inhibit the breakdown of epoxygenase products to potentiate their signalling (Hwang, Wecksler, Wagner, & Hammock, 2013).
Figure 1.
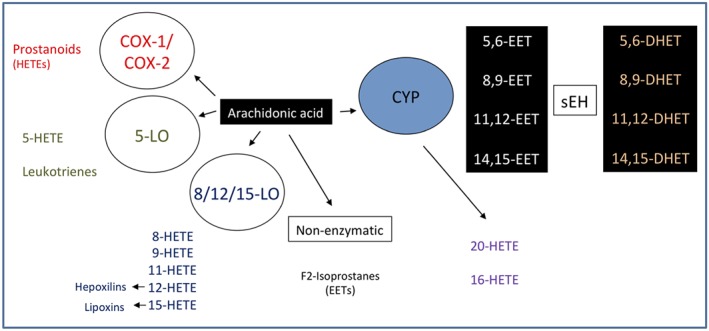
Cytochrome p450 metabolism of arachidonic acid to epoxyeicosatrienoic acids (EETs) and their subsequent conversion by soluble epoxide hydrolase to dihydroxy‐eicosatrienoic acids
Figure 2.
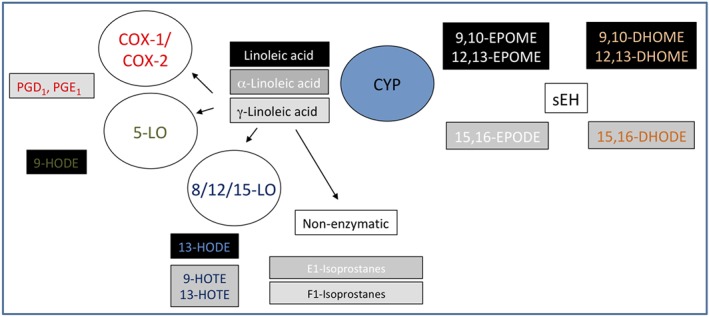
Cytochrome p450 metabolism of linoleic acid to epoxy‐octadecenoic acids (EPOMEs) and dihydroxy‐octadecenoic acids (DHOMEs)
Figure 3.
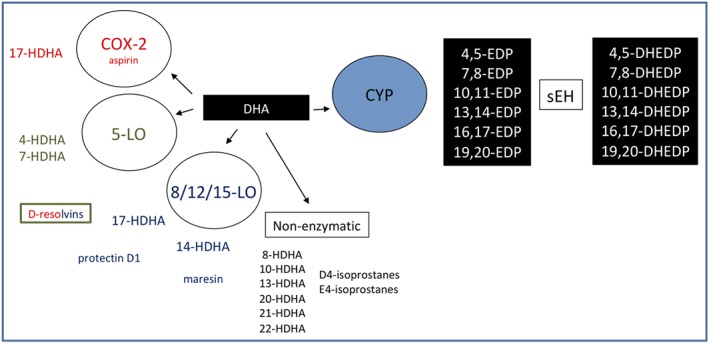
Cytochrome p450 metabolism of docosahexaenoic acid (DHA) to epoxide docosapentaenoic acids
Figure 4.
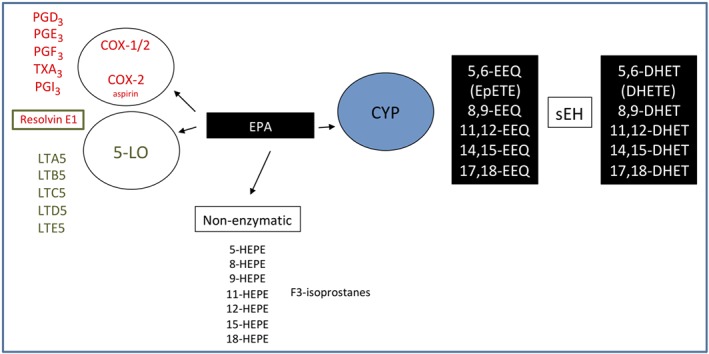
Cytochrome p450 metabolism of docosahexaenoic acid to epoxyeicosatrienoic acids
AA and related PUFA are metabolized by CYP epoxygenase and epoxide hydolases in the vascular endothelium (Roman, 2002; Zhang et al., 2001) and vascular smooth muscle. In vascular smooth muscle, AA is also catalysed by CYP hydroxylases to 20‐HETE (Wang et al., 1999). Indeed, CYP4F3A in myeloid tissue catalyses the ω‐hydroxylation of LTB4 to 20‐hydroxy‐LTB4, an inactivation process that is critical for the regulation of the inflammatory response (Johnson, Edson, Totah, & Rettie, 2015). However, it is unknown whether CYP4F3 is the source of 20‐HETE produced by PMNs (Bednar et al., 2000). These metabolites play a large and complex role in maintaining cardiac, renal, and pulmonary homeostasis by regulating vascular tone and reactivity, ion transport, and renal and pulmonary functions as well as growth responses (Fleming, 2007). Moreover, they have been shown to exert striking anti‐inflammatory actions (Inceoglu et al., 2008), see below.
5. CYP450 AND INFLAMMATION
EETs catalysed by CYPs 2C8, 2C9, and 2J2 inhibit the activation of the transcription factor NF‐κB via the IκB kinase (Node et al., 1999). Consequently, EETs may therefore have the propensity to down‐regulate various cytokine‐induced, pro‐inflammatory, signalling pathways downstream of NF‐κB activation. This may explain how EETs prevent the adhesion of PMNs to the vascular wall by suppressing the expression of cell adhesion molecules, including intracellular adhesion molecule‐1 (ICAM‐1), vascular cell adhesion molecule‐1 (VCAM‐1) and E‐selectin on the surface of endothelial cells in response to cytokines (TNF‐α and IL‐1α), and LPS (Fleming, 2007; Figure 5). We recently reported that epoxygenases are anti‐inflammatory in human primary monocytes and macrophages (Bystrom et al., 2011), regulate M1 and M2 phenotype (Bystrom et al., 2011), and promote bacterial and lipid phagocytosis (Bystrom et al., 2013). In a mouse model of inflammatory resolution, we took this further using a CYP450 epoxygenase inhibitor SKF525A and sEH knockout mice (Gilroy et al., 2016). We reported how CYP450 epoxygenase‐derived mediators play a crucial role in controlling the infiltration of monocytes into sites of inflammation and are essential for the pro‐resolution phenotype of cells of the monocyte lineage (Gilroy et al., 2016) driving macrophage efferocytosis. Additionally, it was recently reported that EETs display analgesic bioactions during experimental inflammatory pain (Inceoglu et al., 2008). In general, CYP450‐derived epoxygenase products are anti‐atherosclerotic, vasodilatory, and anti‐inflammatory (Chaudhary et al., 2013), with the notable exception of linoleic acid‐derived/EH product DiHOMEs. DiHOMEs have recently been shown to mediate thermal hyperalgesia (Zimmer et al., 2018) and, at high levels, are toxic to PMNs and were originally termed “leukotoxins” (Moghaddam et al., 1997).
Figure 5.
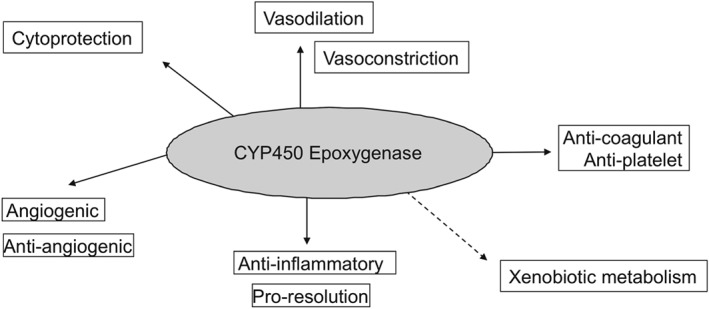
Biological properties of cytochrome p450 metabolites
The use of sEH inhibitors and sEH knockout mice has been invaluable to understanding the in vivo roles or epoxygenase products. Inhibiting sEH has revealed protective roles for epoxygenase products in injury‐induced vascular neointima formation (Revermann et al., 2010), atherosclerosis and aneurysm formation (Zhang et al., 2009), and inflammatory cell recruitment (Gilroy et al., 2016). sEH inhibition or overexpression of producing enzymes such as CYP2J2 are also protective in various acute inflammatory lung injury models (Revermann et al., 2009). EETs released from platelets exert anti‐thrombotic properties by inhibiting platelet aggregation induced by AA and vascular injury (Briggs, Xiao, Parkin, Shen, & Goldman, 2000). EETs can also increase the expression of tissue plasminogen activator in a cAMP‐dependent mechanism, thus suggesting potentially important roles in controlling the fibrinolytic balance at sites of inflammation (Node et al., 2001).
The identification of epoxygenase‐product receptors has almost exclusively focused on AA‐derived EETs and HETEs, with very little research so far on other n3 and n6‐PUFA products. Putative receptor targets include the TRP channels, the PPARs, and GRP40 (Bishop‐Bailey et al., 2014). EETs can directly activate PPAR‐γ in endothelial cells (Liu et al., 2005) and PPAR‐α in monocytes with EET‐mediated anti‐inflammatory effects blocked by PPAR‐γ (Liu et al., 2005) or PPAR‐α antagonists respectively. PPAR activation does not however account for all the anti‐inflammatory effects of EETs. It has been suggested that the anti‐inflammatory properties of EETs occurred through its ligation to a cell surface receptor. It was reported that EETs bind with high affinity to an “EET‐receptor” on the surface of a monocytic cell line, belonging to a specific class of GPCRs (Behm, Ogbonna, Wu, Burns‐Kurtis, & Douglas, 2009). GRP40 can be activated by 14,15‐EET (Ma et al., 2015). However, it must be noted that GRP40 activation only occurred above 10 μM (Ma et al., 2015), whereas most biological effects occur in the nM range. These receptors are not present in all cells, and their known actions do not always correlate with the vascular and anti‐inflammatory activities of epoxygenase products. The identity of this receptor and its role, if any, in initiating the immuno‐modulatory actions of EETs is yet to be determined. By contrast, intracellular signalling pathways are more established. Depending on the model system used, epoxygenases or its products can reduce cellular activation by inhibiting NFκB, inhibiting ERK activation, elevate cAMP, and/or induce cellular hyperpolarization (Thomson, Askari, & Bishop‐Bailey, 2012). Recently, it has also been proposed that inhibiting inflammatory endoplasmic reticulum stress may be critical for the beneficial effects of epoxygenase products, particularly in neuropathic pain.
As stated above, metabolites of the CYP hydroxylases also possess anti‐inflammatory properties. For instance, 16‐HETE can block the adhesion of leukocytes to the microvascular endothelium (Bednar et al., 2000) while also suppressing the synthesis of LTs as well as inhibiting rises in cerebrospinal fluid pressure, which represents index of tissue damage and swelling, in thrombo‐embolic model of stroke in rabbits (Bednar et al., 2000). Moreover, PMN‐derived 20‐HETE and 16‐HETE also counteract TX‐induced platelet aggregation (Hill, Fitzpatrick, & Murphy, 1992). Therefore, it can be surmised that not only do metabolites of CYPs maintain CV and renal systems but also regulate other diverse signalling pathways pertinent to fibrinolysis, platelet aggregation, inflammation, and cellular injury.
6. LEUKOTRIENE AND LXs—BIOSYNTHESIS
LOX enzymes include 5‐, 12‐, or 15‐LOX and are expressed in leukocytes, platelets, and endothelial cells respectively. 5‐LOX, for instance, metabolizes AA to the slow‐reacting substances of anaphylaxis (LTC4, LTD4, and LTE4: potent mediators of the allergic response; Lewis et al., 1980) as well as LTB4, a powerful PMN and eosinophil chemoattractant (Borgeat & Samuelsson, 1979).
To date, four subtypes of LT receptors have been described, namely, the BLT1 and BLT2 and two for the cysteinyl leukotrienes CysLT1 and CysLT2. Once bound, LTs signal via a G‐protein in the cytoplasm to increase intracellular calcium and block formation of cAMP, which then modulates diverse cellular activities ranging from motility to transcriptional activation. While CysLT1 receptors mediate mucus secretion, oedema accumulation, and broncho‐constriction in airways (Lynch et al., 1999), the Cys‐LT2 receptors drive inflammatory responses, tissue fibrosis in the lung, and vascular permeability (Beller et al., 2004). Not surprisingly, CysLT1 receptors are overexpressed in patients with chronic rhinosinusitis or asthma who have aspirin sensitivity (Sousa, Parikh, Scadding, Corrigan, & Lee, 2002). By comparison, the BLT1 receptor is a high‐affinity receptor for LTB4 and mediates its chemo‐attractant and pro‐inflammatory properties (Tager & Luster, 2003). Although BLT2 receptors act in a similar fashion to BLT1 receptors, LTB4 affinity towards BLT1 receptors is much higher.
In contrast, LXs are a series of trihydroxytetraene‐containing bioactive eicosanoids that were first isolated from human leukocytes in the mid‐1980s (Serhan, Hamberg, & Samuelsson, 1984). However, in contrast to LTs, which are manufactured by intracellular biosynthesis, LXs are generated through cell–cell interactions by a process known as transcellular biosynthesis. In different human cell types, during the first biosynthetic step of LX biosynthesis, LOX inserts molecular oxygen into AA. This can be achieved by two major routes—the first pathway occurs in eosinophils, monocytes, or epithelial cells and involves the oxygenation of AA at C‐15 by 15‐LOX yielding 15S‐HPETE. Secreted 15S‐HPETE is then taken up by monocytes or PMNs and converted to 5,6‐epoxytetraene by 5‐LOX, which is then hydrolysed within these cells by either LXA4 or LXB4 hydrolase to LXA4 or LXB4. Activation of this pathway concomitantly reduces LT synthesis, which requires 5‐LOX to convert AA into LTA4 (Claria & Serhan, 1995). The second major route of LX biosynthesis occurs in an LTA4‐dependent manner and involves platelet–leukocyte interactions. 5‐LOX within leukocytes converts AA into LTA4, which when secreted is taken up by platelets adhering on the surface of the leukocyte and is subsequently transformed to LXA4 and LXB4. This occurs via the LX synthase activity of human 12‐LOX (Romano & Serhan, 1992). A third pathway of LX generation was discovered following aspirin ingestion, which irreversibly acetylates COX‐2 in endothelial cells and other activated cell types; this is a property specific to aspirin and not shared with other NSAIDs. Consequently, instead of COX‐2 converting AA into PGG2, aspirin acetylation reprograms the enzyme resulting in the transformation of AA into 15R‐HETE (C‐15 alcohol carried in the R‐configuration). This is then metabolized in a transcellular manner by adherent leukocyte, vascular endothelial, or epithelial 5‐LOX to form 15 epimeric‐LX (15‐epi‐LX) or aspirin‐trigged LXs that carry their C‐15 alcohol in the R configuration rather than 15S native LX. Although initially thought to be only aspirin triggered, a pathway of endogenous 15‐epi‐LX generation has recently been described, where neuronal sphingosine kinase 1 mediates this COX‐2 acetylation (Lee et al., 2018). Aspirin‐triggered LXs share many of the immune regulatory characteristics of native LXs.
7. LXs—RECEPTORS AND BIO‐ACTIONS
The biological actions of LXA4 and 15‐epi‐LXs are mediated through ALX receptors, which are specific GPCRs, isolated and cloned in mouse, human, and rat tissues (Chiang, Takano, Arita, Watanabe, & Serhan, 2003). The ALX receptor is also known as the FPR2 receptor. Human ALX was identified and cloned in various leukocyte populations including T cells (Ariel, Chiang, Arita, Petasis, & Serhan, 2003), monocytes (Maddox et al., 1997), and tissue‐resident macrophages, synovial fibroblasts (Sodin‐Semrl, Taddeo, Tseng, Varga, & Fiore, 2000), and intestinal epithelial cells (Gronert, Gewirtz, Madara, & Serhan, 1998). LXA4 and 15‐epi‐LX A4 (but not LXB4, LTB4, LTD4, or PGE2) show high affinity towards ALX receptors (K d = 1.7 nM). ALX receptors also have the ability to interact with other small peptides/proteins such as Ac2‐26 and glucocorticoid‐derived annexin‐1, which carry out similar anti‐inflammatory effects as the LXs and 15‐epi‐LXs. Studies in transgenic mice over‐expressing human ALX receptors showed that the protective and immune modulatory effects of LXs and 15‐epi‐LXs were ligand‐ and receptor‐dependent (Devchand et al., 2003). In a peritonitis model of zymosan‐induced acute inflammation, infiltration of neutrophils was substantially diminished in ALX transgenic mice compared to their wild‐type equivalents (Devchand et al., 2003) with the site of LX action identified as the leukocyte/endothelial interface mediated by the generation of the anti‐adhesive actions of NO (Paul‐Clark, Van Cao, Moradi‐Bidhendi, Cooper, & Gilroy, 2004).
15‐Epi‐LX analogues also regulate an ALX‐dependent p38/MAPK cascade, known to promote chemotaxis by inhibiting leukocyte‐specific AP‐1 phosphorylation and activation (Ohira et al., 2004). In addition to ALX receptors, LXs also function as partial agonists to a subclass of rhodopsin receptors (Cys‐LT1) more commonly activated by LTs, mediating bioactions in several tissues and cell types other than leukocytes (Badr, DeBoer, Schwartzberg, & Serhan, 1989). At nanomolar concentrations, LXA4 competes for binding with LTD4 on mesangial cells (Badr et al., 1989) and HUVECs (Fiore, Romano, Reardon, & Serhan, 1993) as well as opposing the pro‐inflammatory effects of LTD4. There is also evidence that another intracellular receptor, the Ah receptor, mediates the biological actions of LXs; Ah receptor is a ligand‐activated transcription factor that can trigger such anti‐inflammatory events as the expression of suppressor of cytokine signalling 2 (Aliberti, Serhan, & Sher, 2002).
LXs are anti‐inflammatory at nanomolar concentrations controlling both granulocyte and myeloid cell entry into sites of inflammation. Indeed, the ability of LXs to diminish neutrophil trafficking was corroborated when an analogue of 15‐epi‐LX was intravenously administered to BLT1 receptor knockout mice, that have dramatically elevated neutrophils in the lungs after high limb ischaemia–reperfusion (Chiang et al., 1999). Furthermore, research in our laboratory has found, in humans, that 15‐epi‐LXs regulate PMN influx in forearm blisters, accounting for low‐dose aspirin's anti‐inflammatory properties (Morris et al., 2009). Our additional work on resolving inflammation has revealed that humans fall into two categories, those who resolved their acute inflammatory responses in an immediate manner and those that show a more delayed or prolonged healing process, with the severity and duration controlled by expression of endogenous epi‐LXs or ALX receptors (Morris et al., 2010). Paradoxically, while they inhibit neutrophil and eosinophil transmigration (Maddox et al., 1998), LXs promote monocyte infiltration into sites of inflammation, which when differentiated into macrophages bring about some of the key aspects of resolution and wound healing (Maddox & Serhan, 1996) without inducing neutrophil degranulation or release of other ROS (Jozsef, Zouki, Petasis, Serhan, & Filep, 2002).
Once at the site of inflammation and resolution, monocyte‐derived macrophages are stimulated by LXs to ingest and clear apoptotic neutrophils (Godson et al., 2000), which may be facilitated by changes in the actin cytoskeleton (Maderna et al., 2002). Moreover, LXs increase levels of the anti‐inflammatory cytokine TGF‐β1, which, in turn, dampens a range of pro‐inflammatory pathways (Bannenberg et al., 2005). LXs are also anti‐fibrotic, thereby improving tissue remodelling by reducing the proliferation of fibroblasts and mesanglial cells induced by a numbers of factors, including connective‐tissue growth factor, platelet‐derived growth factor, TNF‐α, LTD4, and TGF‐β (Leonard et al., 2002). 15‐Epi‐LXs exert the same biological effects as endogenously produced LXs, but with additional properties including causing increased vasorelaxation (Serhan, 1994) and endothelial cell production of anti‐inflammatory NO synthesis (Paul‐Clark et al., 2004). In addition, 15‐epi‐LX A4 inhibits TNF‐α‐induced IL‐1β in periodontitis in vivo (Hachicha, Pouliot, Petasis, & Serhan, 1999), down‐regulates suppressor of cytokine signalling 2 signalling (Machado et al., 2006), and dampens TNF‐α‐induced IL‐8 biosynthesis (Gronert et al., 1998). As expected, LXs and 15‐epi‐LXs exert beneficial effects in a range of experimental models of inflammation and human diseases including cystic fibrosis (Karp, Flick, Yang, Uddin, & Petasis, 2005), glomerulonephritis (Munger et al., 1999), periodontitis (Pouliot, Clish, Petasis, Van Dyke, & Serhan, 2000), ischaemia–reperfusion injury (Chiang et al., 1999), various cutaneous inflammation models (Schottelius et al., 2002), pleuritis (Paul‐Clark et al., 2004), asthma (Levy et al., 2005), wound healing processes in the eye (Gronert et al., 2005), colitis, inflammation‐induced hyperalgesia in rats, and microbial infection in mice (Aliberti, Hieny, Reis e Sousa, Serhan, & Sher, 2002). See Table 1 for specialized pro‐resolving lipid mediators, their biological actions and concentrations at sites of inflammation.
8. SPECIALIZED PRO‐RESOLVING LIPID MEDIATORS—BIOSYNTHESIS
Omega‐3 polyunsaturated fatty acids, including EPA and DHA, are known not only to maintain organ function and health but also to reduce the severity of inflammatory reactions and incidences of infection (Arita et al., 2005). Although also now known to be metabolized by COX, LOX, and CYP450 pathways into distinct lipid mediators, a novel series of ω‐3 PUFA products were identified in the resolving exudate of a mouse dorsal air pouch or peritonitis model using lipidomic and bio‐informatic analysis (Lu, Hong, Tjonahen, & Serhan, 2005). These endogenous mediators are called Rvs, PDs, and maresins (MaR).
EPA or DHA generate the Rvs and are categorized as members of the E‐series (from EPA) or D‐series (from DHA). Both series of Rvs were initially isolated from murine dorsal air pouches treated with EPA or DHA as well as aspirin. Transcellular formation of E‐series Rvs occurs with the conversion of EPA to 18R‐hydroxyeicosapentanoic acid by COX‐2 expressed within endothelial cells treated with aspirin. Similar to 15R‐HETE in 15‐epi‐LX formation, 18R‐hydroxyeicosapentanoic acid is released from endothelial cells to neighbouring leukocytes for its conversion by 5‐LOX to either RvE1 or RvE2, via a 5 (Ariel et al., 2006) epoxide‐containing intermediate (Arita, Clish, & Serhan, 2005). This interaction is blocked by selective COX‐2 inhibition but not by indomethacin or paracetamol (Serhan et al., 2000). Although this transcellular route was proposed as the synthetic pathway for Rvs, intracellular production of Rvs and MaR have been observed in macrophages without the need for transcellular interactions. RvE1 is spontaneously produced in healthy subjects with levels increasing after treatment with either aspirin or EPA (Arita et al., 2005). D‐series Rvs, aspirin‐triggered resolvin D1 (RvD1; AT‐RvD1), and RvD1, are synthesized via a pathway involving sequential oxygenations, initiated by 15‐LOX or aspirin‐acetylated COX‐2 in the microvasculature , respectively, followed by 5‐LOX in human neutrophils with an epoxide containing intermediate. For AT‐RvD1s, DHA is initially converted to epimeric 17R‐hydroxydocosahexaenoic acid. In the absence of aspirin, however, DHA is enzymically converted to 17S‐hydroxydocosahexaenoic acid (Hong et al., 2003). Interestingly, generation of E‐series Rvs can also be mediated by microbial and mammalian CYP450 enzymes, which convert EPA into 18‐HEPE. 18‐HEPE can then be transformed by human neutrophils into either RvE1 or RvE2 (Serhan et al., 2000). Hence, it is possible that microbes at sites of infection may contribute to the production of Rvs in a similar pathway.
DHA is also a precursor for the generation of PDs being enzymically converted by 15‐LOX to a 17S‐hydroperoxide‐containing intermediate. This intermediate is then converted by leukocytes into a 16(17)‐epoxide that is subsequently converted in these cells to a 10,17‐dihydroxy‐containing compound (Hong et al., 2003). PDs are distinguished by the presence of a conjugated triene double bond and by their potent bioactivity. One specific DHA‐derived lipid mediator, 10,17S‐docosatriene, was termed protectin D1 (PD1), which when generated in neural tissue is called neuroprotectin D1. Moreover, PD1 exhibits tissue‐specific bioactivity as, in humans, this lipid is synthesized by peripheral blood mononuclear cells and Th2 CD4+ T‐cells, while, in mice, it has been isolated from exudates and brain cells, human microglial cells (Serhan et al., 2002), and in peripheral blood (Hong et al., 2003).
9. SPECIALIZED PRO‐RESOLVING LIPID MEDIATORS IN INFLAMMATION AND RESOLUTION
One of the broader immunomodulatory properties of RvE1 is its ability to inhibit the accumulation of neutrophils and dendritic cells at sites of inflammation. This occurs by blocking trans‐endothelial migration of these cells across the microvascular endothelium as well as enhancing their clearance from inflammatory sites (Arita, Bianchini, et al., 2005). Other actions of RvE1 includes inhibition of ROS production from neutrophils in response to bacterial peptide, fMLP and TNFα (Gronert et al., 2004); inhibition of LTB4‐BLT1 receptor signalling via NF‐κB and hence the biosynthesis of pro‐inflammatory chemokine and cytokines (Arita et al., 2007); enhancement of macrophage efferocytosis of apoptotic bodies (Schwab, Chiang, Arita, & Serhan, 2007); and up‐regulation of the chemokine receptor CCR5 on late apoptotic neutrophils (Ariel et al., 2006), which, in turn, blocks chemokine signalling. RvE1 also regulates leukocyte pro‐inflammatory cell surface markers including L‐selectin, while selectively disrupting TX‐mediated platelet aggregation (Dona et al., 2008), adding further insight into its anti‐inflammatory/pro‐resolution properties. In disease states, RvE1 suppresses Porphyromonas gingivalis‐induced oral inflammation and bone loss during periodontitis (Hasturk et al., 2006), is protective in trinitrobenzene‐sulphonic acid‐induced colitis in rodents (Arita, Yoshida, et al., 2005), and mediates re‐epithelisation of mouse cornea after thermal‐injury (Gronert et al., 2005). Taken together, RvE1 triggers various aspects of the pro‐resolution cascade ranging from the timely inhibition of granulocyte accumulation at sites of inflammation to the efferocytosis or clearance of inflammatory debris (see Serhan, 2008).
RvE1 binds to ChemR23 ( now Chemerin1 receptors, with high affinity (K d = 48.3 nm) resulting in the down‐regulation of NF‐κB activity and consequently the synthesis of pro‐inflammatory cytokines such as TNF‐α as well as modulating pathways involved in MAPK signalling (Arita, Bianchini, et al., 2005). Although it has been found in the kidney, gastrointestinal system, brain, and CV tissue and cells of the myeloid lineage, the levels of ChemR23 receptor expression are highly variable. For example, these receptors are significantly increased on human monocytes but comparatively less so on neutrophils in response to anti‐inflammatory mediators such as TGF‐β. As with ALX receptors, ChemR23 is also a receptor for peptide ligands including chemerin, which also exerts anti‐inflammatory actions (Cash et al., 2008). RvE1 also interacts with the LTB4 receptor, BLT1, and is a partial antagonist preventing neutrophil activation (Arita et al., 2007). Therefore, RvE1 couples to two distinct receptors to suppress pro‐inflammatory mechanisms while enhancing pro‐resolution pathways.
While structurally distinct from RvE1, RvE2 is a second member of the EPA‐derived family of E‐series Rvs. In PMNs from human, it is generated at higher concentrations than RvE1, but is equipotent when given intravenously and additive when administered alongside RvE1 (Tjonahen et al., 2006). RvE2 also suppresses PMN migration into the peritoneum after zymosan (Tjonahen et al., 2006). It is still unclear what receptor RvE2 couples to, as it mediates resolution by activating Chemerin1 receptors and antagonizing the LTB4 receptor BLT1.
There are four members of the D‐series Rvs, namely, RvD1, RvD2, RvD3, and RvD4 (Hong et al., 2003). As with RvE1, RvD1/D2 exerts both anti‐inflammatory and pro‐resolution properties by blocking neutrophil infiltration, while also enhancing macrophage efferocytosis of apoptotic bodies (Krishnamoorthy et al., 2010). The latter occurs via the binding of RvD1 to either ALX receptors or the orphan GPR32, which are present on the surface of monocytes and PMNs, the expression of which is up‐regulated by inflammatory stimuli including granulocyte‐macrophage‐colony‐stimulating factor and zymosan (Krishnamoorthy et al., 2010). Importantly, in a model of cecal ligation and puncture RvD2, whose receptor is GPR18 (Chiang, Dalli, Colas, & Serhan, 2015), markedly reduced PMN accumulation, bacteria numbers and proinflammatory cytokines leading to increased animal survival.
As already mentioned, in addition to D‐series Rvs, DHA also acts as a precursor for the synthesis of PDs. PD1, for instance, is synthesized in the human brain and microglial (Serhan et al., 2002) and peripheral blood mononuclear cells (Hong et al., 2003). As with Rvs, PD1 may also inhibit PMN migration as well as toll‐like receptor‐mediated activation (Duffield et al., 2006) while suppressing Th2 inflammatory cytokines and pro‐inflammatory lipid mediator synthesis (Levy et al., 2007). PD1 also blocks T‐cell migration in vivo and promotes T‐cell apoptosis (Ariel et al., 2005). PD1 is protective in experimental models of oxidative stress (Mukherjee et al., 2007), ischaemic stroke (Marcheselli et al., 2003), ischaemia–reperfusion renal injury (Duffield et al., 2006), asthma (Levy et al., 2007), and Alzheimer's (Lukiw et al., 2005). Indeed, peripheral blood mononuclear cells from Alzheimer's patients given a DHA‐rich dietary supplement show dampened biosynthesis of IL‐1β, IL‐6, and granulocyte‐colony‐stimulating factor (Vedin et al., 2008). As with RvE2, a receptor is yet to be identified. However, it is possible that it couples to a different receptor from that for RvE1, as its anti‐inflammatory effects are additive with those of RvE1 in vivo.
MaR1 and MaR2 are produced in tissues by macrophages via the actions of 12‐LOX, through a 13,14‐epoxide intermediate (Serhan et al., 2009). MaR1 can also be generated at sites of vascular inflammation during human platelet–neutrophil interactions via platelet 12‐LOX conversion of DHA to 13S,14S‐epoxy‐MaR, followed by neutrophil conversion to MaR1 (Abdulnour et al., 2014). The receptors for MaR have yet to be identified. Although MaR have only been recently discovered, it has been reported similar to Rvs and PD1, MaR1 blocks the infiltration of PMNs while stimulating macrophage phagocytosis of apoptotic PMNs or zymosan.
10. SUMMARY
Inflammation is a good thing; it kills bacteria and helps to heal wounds while imparting long‐term memory against inciting antigens. Lipids play a key role in these events and come in many forms, including those that drive the cardinal signs of inflammation and those that help to restrain it and bring the response to a timely end. In fact, studying lipids and their inhibitors, NSAIDs, has given us a great deal of insight into homeostasis, immune responses to infection/injury and the wound healing process. Indeed, inflammatory onset has been a historical point of interest for the development of anti‐inflammatory drug therapies. Research on the other end of the inflammatory spectrum, resolution, has provided the opportunity to harness endogenous mediators and their receptors to help drive ongoing inflammation down a pro‐resolution pathway. Moreover, this is achievable without compromising host defence. Such complex manipulation of the immune system provides new opportunities to develop further pro‐resolution therapies based upon what we have learned from studying lipids in this setting.
10.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos et al., 2017; Alexander, Cidlowski et al., 2017; Alexander, Striessnig et al., 2017).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Gilroy DW, Bishop‐Bailey D. Lipid mediators in immune regulation and resolution. Br J Pharmacol. 2019;176:1009–1023. 10.1111/bph.14587
REFERENCES
- Abdulnour, R. E. , Dalli, J. , Colby, J. K. , Krishnamoorthy, N. , Timmons, J. Y. , Tan, S. H. , … Levy, B. D. (2014). Maresin 1 biosynthesis during platelet‐neutrophil interactions is organ‐protective. Proceedings of the National Academy of Sciences of the United States of America, 111, 16526–16531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi, S. , Lippross, S. , Neuhuber, W. L. , & Zeilhofer, H. U. (2002). PGE(2) selectively blocks inhibitory glycinergic neurotransmission onto rat superficial dorsal horn neurons. Nature Neuroscience, 5, 34–40. 10.1038/nn778 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. British Journal of Pharmacology, 174, S208–S224. 10.1111/bph.13880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Striessnig, J. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. British Journal of Pharmacology, 174, S160–S194. 10.1111/bph.13884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliberti, J. , Hieny, S. , Reis e Sousa, C. , Serhan, C. N. , & Sher, A. (2002). Lipoxin‐mediated inhibition of IL‐12 production by DCs: A mechanism for regulation of microbial immunity. Nature Immunology, 3, 76–82. 10.1038/ni745 [DOI] [PubMed] [Google Scholar]
- Aliberti, J. , Serhan, C. , & Sher, A. (2002). Parasite‐induced lipoxin A4 is an endogenous regulator of IL‐12 production and immunopathology in Toxoplasma gondii infection. The Journal of Experimental Medicine, 196, 1253–1262. 10.1084/jem.20021183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel, A. , Chiang, N. , Arita, M. , Petasis, N. A. , & Serhan, C. N. (2003). Aspirin‐triggered lipoxin A4 and B4 analogs block extracellular signal‐regulated kinase‐dependent TNF‐alpha secretion from human T cells. Journal of Immunology, 170, 6266–6272. [DOI] [PubMed] [Google Scholar]
- Ariel, A. , Fredman, G. , Sun, Y. P. , Kantarci, A. , Van Dyke, T. E. , Luster, A. D. , & Serhan, C. N. (2006). Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nature Immunology, 7, 1209–1216. 10.1038/ni1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel, A. , Li, P. L. , Wang, W. , Tang, W. X. , Fredman, G. , Hong, S. , … Serhan, C. N. (2005). The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. The Journal of Biological Chemistry, 280, 43079–43086. [DOI] [PubMed] [Google Scholar]
- Arita, M. , Bianchini, F. , Aliberti, J. , Sher, A. , Chiang, N. , Hong, S. , … Serhan, C. N. (2005). Stereochemical assignment, antiinflammatory properties, and receptor for the omega‐3 lipid mediator resolvin E1. The Journal of Experimental Medicine, 201, 713–722. 10.1084/jem.20042031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita, M. , Clish, C. B. , & Serhan, C. N. (2005). The contributions of aspirin and microbial oxygenase to the biosynthesis of anti‐inflammatory resolvins: Novel oxygenase products from omega‐3 polyunsaturated fatty acids. Biochemical and Biophysical Research Communications, 338, 149–157. 10.1016/j.bbrc.2005.07.181 [DOI] [PubMed] [Google Scholar]
- Arita, M. , Ohira, T. , Sun, Y. P. , Elangovan, S. , Chiang, N. , & Serhan, C. N. (2007). Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. Journal of Immunology, 178, 3912–3917. 10.4049/jimmunol.178.6.3912 [DOI] [PubMed] [Google Scholar]
- Arita, M. , Yoshida, M. , Hong, S. , Tjonahen, E. , Glickman, J. N. , Petasis, N. A. , … Serhan, C. N. (2005). Resolvin E1, an endogenous lipid mediator derived from omega‐3 eicosapentaenoic acid, protects against 2,4,6‐trinitrobenzene sulfonic acid‐induced colitis. Proceedings of the National Academy of Sciences of the United States of America, 102, 7671–7676. 10.1073/pnas.0409271102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff, D. M. , Canetti, C. , & Peters‐Golden, M. (2004). Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E‐prostanoid 2 receptor‐mediated increase in intracellular cyclic AMP. Journal of Immunology, 173, 559–565. 10.4049/jimmunol.173.1.559 [DOI] [PubMed] [Google Scholar]
- Aronoff, D. M. , Peres, C. M. , Serezani, C. H. , Ballinger, M. N. , Carstens, J. K. , Coleman, N. , … Peters‐Golden, M. (2007). Synthetic prostacyclin analogs differentially regulate macrophage function via distinct analog‐receptor binding specificities. Journal of Immunology, 178, 1628–1634. 10.4049/jimmunol.178.3.1628 [DOI] [PubMed] [Google Scholar]
- Badr, K. F. , DeBoer, D. K. , Schwartzberg, M. , & Serhan, C. N. (1989). Lipoxin A4 antagonizes cellular and in vivo actions of leukotriene D4 in rat glomerular mesangial cells: Evidence for competition at a common receptor. Proceedings of the National Academy of Sciences of the United States of America, 86, 3438–3442. 10.1073/pnas.86.9.3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannenberg, G. L. , Chiang, N. , Ariel, A. , Arita, M. , Tjonahen, E. , Gotlinger, K. H. , … Serhan, C. N. (2005). Molecular circuits of resolution: Formation and actions of resolvins and protectins. Journal of Immunology, 174, 4345–4355. 10.4049/jimmunol.174.7.4345 [DOI] [PubMed] [Google Scholar]
- Bednar, M. M. , Gross, C. E. , Balazy, M. K. , Belosludtsev, Y. , Colella, D. T. , Falck, J. R. , & Balazy, M. (2000). 16(R)‐hydroxy‐5,8,11,14‐eicosatetraenoic acid, a new arachidonate metabolite in human polymorphonuclear leukocytes. Biochemical Pharmacology, 60, 447–455. [DOI] [PubMed] [Google Scholar]
- Behm, D. J. , Ogbonna, A. , Wu, C. , Burns‐Kurtis, C. L. , & Douglas, S. A. (2009). Epoxyeicosatrienoic acids function as selective, endogenous antagonists of native thromboxane receptors: Identification of a novel mechanism of vasodilation. The Journal of Pharmacology and Experimental Therapeutics, 328, 231–239. [DOI] [PubMed] [Google Scholar]
- Beller, T. C. , Friend, D. S. , Maekawa, A. , Lam, B. K. , Austen, K. F. , & Kanaoka, Y. (2004). Cysteinyl leukotriene 1 receptor controls the severity of chronic pulmonary inflammation and fibrosis. Proceedings of the National Academy of Sciences of the United States of America, 101, 3047–3052. 10.1073/pnas.0400235101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop‐Bailey, D. , & Hla, T. (1999). Endothelial cell apoptosis induced by the peroxisome proliferator‐activated receptor (PPAR) ligand 15‐deoxy‐Delta12, 14‐prostaglandin J2. The Journal of Biological Chemistry, 274, 17042–17048. 10.1074/jbc.274.24.17042 [DOI] [PubMed] [Google Scholar]
- Bishop‐Bailey, D. , Thomson, S. , Askari, A. , Faulkner, A. , & Wheeler‐Jones, C. (2014). Lipid‐metabolizing CYPs in the regulation and dysregulation of metabolism. Annual Review of Nutrition, 34, 261–279. 10.1146/annurev-nutr-071813-105747 [DOI] [PubMed] [Google Scholar]
- Borgeat, P. , & Samuelsson, B. (1979). Arachidonic acid metabolism in polymorphonuclear leukocytes: Effects of ionophore A23187. Proceedings of the National Academy of Sciences of the United States of America, 76, 2148–2152. 10.1073/pnas.76.5.2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs, W. H. , Xiao, H. , Parkin, K. L. , Shen, C. , & Goldman, I. L. (2000). Differential inhibition of human platelet aggregation by selected Allium thiosulfinates. Journal of Agricultural and Food Chemistry, 48, 5731–5735. 10.1021/jf0004412 [DOI] [PubMed] [Google Scholar]
- Bunting, S. , Moncada, S. , & Vane, J. R. (1983). The prostacyclin–thromboxane A2 balance: Pathophysiological and therapeutic implications. British Medical Bulletin, 39, 271–276. [DOI] [PubMed] [Google Scholar]
- Bystrom, J. , Thomson, S. J. , Johansson, J. , Edin, M. L. , Zeldin, D. C. , Gilroy, D. W. , … Bishop‐Bailey, D. (2013). Inducible CYP2J2 and its product 11,12‐EET promotes bacterial phagocytosis: A role for CYP2J2 deficiency in the pathogenesis of Crohn's disease? PLoS One, 8, e75107 10.1371/journal.pone.0075107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrom, J. , Wray, J. A. , Sugden, M. C. , Holness, M. J. , Swales, K. E. , Warner, T. D. , … Bishop‐Bailey, D. (2011). Endogenous epoxygenases are modulators of monocyte/macrophage activity. PLoS One, 6, e26591 10.1371/journal.pone.0026591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash, J. L. , Hart, R. , Russ, A. , Dixon, J. P. , Colledge, W. H. , Doran, J. , … Greaves, D. R. (2008). Synthetic chemerin‐derived peptides suppress inflammation through ChemR23. The Journal of Experimental Medicine, 205, 767–775. 10.1084/jem.20071601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernuda‐Morollon, E. , Pineda‐Molina, E. , Canada, F. J. , & Perez‐Sala, D. (2001). 15‐Deoxy‐delta 12,14‐prostaglandin J2 inhibition of NF‐kappaB‐DNA binding through covalent modification of the p50 subunit. The Journal of Biological Chemistry, 276, 35530–35536. 10.1074/jbc.M104518200 [DOI] [PubMed] [Google Scholar]
- Chaudhary, K. R. , Zordoky, B. N. , Edin, M. L. , Alsaleh, N. , El‐Kadi, A. O. , Zeldin, D. C. , & Seubert, J. M. (2013). Differential effects of soluble epoxide hydrolase inhibition and CYP2J2 overexpression on postischemic cardiac function in aged mice. Prostaglandins Other Lipid Mediat, 104‐105, 8–17. 10.1016/j.prostaglandins.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, N. , Dalli, J. , Colas, R. A. , & Serhan, C. N. (2015). Identification of resolvin D2 receptor mediating resolution of infections and organ protection. The Journal of Experimental Medicine, 212, 1203–1217. 10.1084/jem.20150225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, N. , Gronert, K. , Clish, C. B. , O'Brien, J. A. , Freeman, M. W. , & Serhan, C. N. (1999). Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin‐triggered lipoxins in reperfusion. The Journal of Clinical Investigation, 104, 309–316. 10.1172/JCI7016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, N. , Takano, T. , Arita, M. , Watanabe, S. , & Serhan, C. N. (2003). A novel rat lipoxin A4 receptor that is conserved in structure and function. British Journal of Pharmacology, 139, 89–98. 10.1038/sj.bjp.0705220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claria, J. , & Serhan, C. N. (1995). Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell‐leukocyte interactions. Proceedings of the National Academy of Sciences of the United States of America, 92, 9475–9479. 10.1073/pnas.92.21.9475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, R. B. , Bishop‐Bailey, D. , Estrada‐Hernandez, T. , Hla, T. , Puddington, L. , & Padula, S. J. (2000). The nuclear receptor PPAR gamma and immunoregulation: PPAR gamma mediates inhibition of helper T cell responses. Journal of Immunology, 164, 1364–1371. 10.4049/jimmunol.164.3.1364 [DOI] [PubMed] [Google Scholar]
- Dantzer, R. , Konsman, J. P. , Bluthe, R. M. , & Kelley, K. W. (2000). Neural and humoral pathways of communication from the immune system to the brain: Parallel or convergent? Autonomic Neuroscience, 85, 60–65. 10.1016/S1566-0702(00)00220-4 [DOI] [PubMed] [Google Scholar]
- Devchand, P. R. , Arita, M. , Hong, S. , Bannenberg, G. , Moussignac, R. L. , Gronert, K. , & Serhan, C. N. (2003). Human ALX receptor regulates neutrophil recruitment in transgenic mice: Roles in inflammation and host defense. The FASEB Journal, 17, 652–659. 10.1096/fj.02-0770com [DOI] [PubMed] [Google Scholar]
- Dona, M. , Fredman, G. , Schwab, J. M. , Chiang, N. , Arita, M. , Goodarzi, A. , … Serhan, C. N. (2008). Resolvin E1, an EPA‐derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood, 112, 848–855. 10.1182/blood-2007-11-122598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois, R. N. , Abramson, S. B. , Crofford, L. , Gupta, R. A. , Simon, L. S. , Van De Putte, L. B. , & Lipsky, P. E. (1998). Cyclooxygenase in biology and disease. The FASEB Journal, 12, 1063–1073. 10.1096/fasebj.12.12.1063 [DOI] [PubMed] [Google Scholar]
- Duffield, J. S. , Hong, S. , Vaidya, V. S. , Lu, Y. , Fredman, G. , Serhan, C. N. , & Bonventre, J. V. (2006). Resolvin D series and protectin D1 mitigate acute kidney injury. Journal of Immunology, 177, 5902–5911. [DOI] [PubMed] [Google Scholar]
- Edin, M. L. , Hamedani, B. G. , Gruzdev, A. , Graves, J. P. , Lih, F. B. , Arbes, S. J. 3rd , … Zeldin, D. C. (2018). Epoxide hydrolase 1 (EPHX1) hydrolyzes epoxyeicosanoids and impairs cardiac recovery after ischemia. The Journal of Biological Chemistry, 293, 3281–3292. 10.1074/jbc.RA117.000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, E. F. , Oelz, O. , Roberts, L. J. 2nd , Payne, N. A. , Sweetman, B. J. , Nies, A. S. , & Oates, J. A. (1976). Coronary arterial smooth muscle contraction by a substance released from platelets: Evidence that it is thromboxane A2. Science, 193, 1135–1137. 10.1126/science.959827 [DOI] [PubMed] [Google Scholar]
- Fiore, S. , Romano, M. , Reardon, E. M. , & Serhan, C. N. (1993). Induction of functional lipoxin A4 receptors in HL‐60 cells. Blood, 81, 3395–3403. [PubMed] [Google Scholar]
- Fleming, I. (2007). DiscrEET regulators of homeostasis: Epoxyeicosatrienoic acids, cytochrome P450 epoxygenases and vascular inflammation. Trends in Pharmacological Sciences, 28, 448–452. 10.1016/j.tips.2007.08.002 [DOI] [PubMed] [Google Scholar]
- Garcia Rodriguez, L. A. , Tacconelli, S. , & Patrignani, P. (2008). Role of dose potency in the prediction of risk of myocardial infarction associated with nonsteroidal anti‐inflammatory drugs in the general population. Journal of the American College of Cardiology, 52, 1628–1636. 10.1016/j.jacc.2008.08.041 [DOI] [PubMed] [Google Scholar]
- Gilroy, D. W. , Colville‐Nash, P. R. , McMaster, S. , Sawatzky, D. A. , Willoughby, D. A. , & Lawrence, T. (2003). Inducible cyclooxygenase‐derived 15‐deoxy (delta)12‐14PGJ2 brings about acute inflammatory resolution in rat pleurisy by inducing neutrophil and macrophage apoptosis. The FASEB Journal, 17, 2269–2271. 10.1096/fj.02-1162fje [DOI] [PubMed] [Google Scholar]
- Gilroy, D. W. , Edin, M. L. , De Maeyer, R. P. , Bystrom, J. , Newson, J. , Lih, F. B. , … Bishop‐Bailey, D. (2016). CYP450‐derived oxylipins mediate inflammatory resolution. Proceedings of the National Academy of Sciences of the United States of America, 113, E3240–E3249. 10.1073/pnas.1521453113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson, C. , Mitchell, S. , Harvey, K. , Petasis, N. A. , Hogg, N. , & Brady, H. R. (2000). Cutting edge: Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte‐derived macrophages. Journal of Immunology, 164, 1663–1667. [DOI] [PubMed] [Google Scholar]
- Gronert, K. , Gewirtz, A. , Madara, J. L. , & Serhan, C. N. (1998). Identification of a human enterocyte lipoxin A4 receptor that is regulated by interleukin (IL)‐13 and interferon gamma and inhibits tumor necrosis factor alpha‐induced IL‐8 release. The Journal of Experimental Medicine, 187, 1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronert, K. , Kantarci, A. , Levy, B. D. , Clish, C. B. , Odparlik, S. , Hasturk, H. , … Serhan, C. N. (2004). A molecular defect in intracellular lipid signaling in human neutrophils in localized aggressive periodontal tissue damage. Journal of Immunology, 172, 1856–1861. 10.4049/jimmunol.172.3.1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronert, K. , Maheshwari, N. , Khan, N. , Hassan, I. R. , Dunn, M. , & Laniado Schwartzman, M. (2005). A role for the mouse 12/15‐lipoxygenase pathway in promoting epithelial wound healing and host defense. The Journal of Biological Chemistry, 280, 15267–15278. [DOI] [PubMed] [Google Scholar]
- Grosser, T. , Fries, S. , & FitzGerald, G. A. (2006). Biological basis for the cardiovascular consequences of COX‐2 inhibition: Therapeutic challenges and opportunities. The Journal of Clinical Investigation, 116, 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachicha, M. , Pouliot, M. , Petasis, N. A. , & Serhan, C. N. (1999). Lipoxin (LX)A4 and aspirin‐triggered 15‐epi‐LXA4 inhibit tumor necrosis factor 1alpha‐initiated neutrophil responses and trafficking: Regulators of a cytokine‐chemokine axis. The Journal of Experimental Medicine, 189, 1923–1930. 10.1084/jem.189.12.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg, M. , & Samuelsson, B. (1973). Detection and isolation of an endoperoxide intermediate in prostaglandin biosynthesis. Proceedings of the National Academy of Sciences of the United States of America, 70, 899–903. 10.1073/pnas.70.3.899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harizi, H. , Juzan, M. , Pitard, V. , Moreau, J. F. , & Gualde, N. (2002). Cyclooxygenase‐2‐issued prostaglandin e(2) enhances the production of endogenous IL‐10, which down‐regulates dendritic cell functions. Journal of Immunology, 168, 2255–2263. 10.4049/jimmunol.168.5.2255 [DOI] [PubMed] [Google Scholar]
- Harris, R. C. , McKanna, J. A. , Akai, Y. , Jacobson, H. R. , Dubois, R. N. , & Breyer, M. D. (1994). Cyclooxygenase‐2 is associated with the macula densa of rat kidney and increases with salt restriction. The Journal of Clinical Investigation, 94, 2504–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasturk, H. , Kantarci, A. , Ohira, T. , Arita, M. , Ebrahimi, N. , Chiang, N. , … van Dyke, T. E. (2006). RvE1 protects from local inflammation and osteoclast‐mediated bone destruction in periodontitis. The FASEB Journal, 20, 401–403. 10.1096/fj.05-4724fje [DOI] [PubMed] [Google Scholar]
- Hata, A. N. , & Breyer, R. M. (2004). Pharmacology and signaling of prostaglandin receptors: Multiple roles in inflammation and immune modulation. Pharmacology & Therapeutics, 103, 147–166. 10.1016/j.pharmthera.2004.06.003 [DOI] [PubMed] [Google Scholar]
- Hayashi, H. , Fujii, Y. , Watanabe, K. , Urade, Y. , & Hayaishi, O. (1989). Enzymatic conversion of prostaglandin H2 to prostaglandin F2 alpha by aldehyde reductase from human liver: Comparison to the prostaglandin F synthetase from bovine lung. The Journal of Biological Chemistry, 264, 1036–1040. [PubMed] [Google Scholar]
- Hayek, M. G. , Mura, C. , Wu, D. , Beharka, A. A. , Han, S. N. , Paulson, K. E. , … Meydani, S. N. (1997). Enhanced expression of inducible cyclooxygenase with age in murine macrophages. Journal of Immunology, 159, 2445–2451. [PubMed] [Google Scholar]
- Hill, E. , Fitzpatrick, F. , & Murphy, R. C. (1992). Biological activity and metabolism of 20‐hydroxyeicosatetraenoic acid in the human platelet. British Journal of Pharmacology, 106, 267–274. 10.1111/j.1476-5381.1992.tb14327.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai, H. , Tanaka, K. , Yoshie, O. , Ogawa, K. , Kenmotsu, K. , Takamori, Y. , … Nagata, K. (2001). Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven‐transmembrane receptor CRTH2. The Journal of Experimental Medicine, 193, 255–261. 10.1084/jem.193.2.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, S. , Gronert, K. , Devchand, P. R. , Moussignac, R. L. , & Serhan, C. N. (2003). Novel docosatrienes and 17S‐resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti‐inflammation. The Journal of Biological Chemistry, 278, 14677–14687. 10.1074/jbc.M300218200 [DOI] [PubMed] [Google Scholar]
- Hwang, S. H. , Wecksler, A. T. , Wagner, K. , & Hammock, B. D. (2013). Rationally designed multitarget agents against inflammation and pain. Current Medicinal Chemistry, 20, 1783–1799. 10.2174/0929867311320130013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu, B. , Jinks, S. L. , Ulu, A. , Hegedus, C. M. , Georgi, K. , Schmelzer, K. R. , … Hammock, B. D. (2008). Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proceedings of the National Academy of Sciences of the United States of America, 105, 18901–18906. 10.1073/pnas.0809765105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, C. , Ting, A. T. , & Seed, B. (1998). PPAR‐gamma agonists inhibit production of monocyte inflammatory cytokines. Nature, 391, 82–86. 10.1038/34184 [DOI] [PubMed] [Google Scholar]
- Johnson, A. L. , Edson, K. Z. , Totah, R. A. , & Rettie, A. E. (2015). Cytochrome P450 omega‐hydroxylases in inflammation and cancer. Advances in Pharmacology, 74, 223–262. 10.1016/bs.apha.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozsef, L. , Zouki, C. , Petasis, N. A. , Serhan, C. N. , & Filep, J. G. (2002). Lipoxin A4 and aspirin‐triggered 15‐epi‐lipoxin A4 inhibit peroxynitrite formation, NF‐kappa B and AP‐1 activation, and IL‐8 gene expression in human leukocytes. Proceedings of the National Academy of Sciences of the United States of America, 99, 13266–13271. 10.1073/pnas.202296999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaley, G. , Hintze, T. H. , Panzenbeck, M. , & Messina, E. J. (1985). Role of prostaglandins in microcirculatory function. Advances in Prostaglandin, Thromboxane, and Leukotriene Research, 13, 27–35. [PubMed] [Google Scholar]
- Karp, C. L. , Flick, L. M. , Yang, R. , Uddin, J. , & Petasis, N. A. (2005). Cystic fibrosis and lipoxins. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 73, 263–270. 10.1016/j.plefa.2005.05.015 [DOI] [PubMed] [Google Scholar]
- Khan, M. M. (1995). Regulation of IL‐4 and IL‐5 secretion by histamine and PGE2. Advances in Experimental Medicine and Biology, 383, 35–42. 10.1007/978-1-4615-1891-4_5 [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy, S. , Recchiuti, A. , Chiang, N. , Yacoubian, S. , Lee, C. H. , Yang, R. , … Serhan, C. N. (2010). Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proceedings of the National Academy of Sciences of the United States of America, 107, 1660–1665. 10.1073/pnas.0907342107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, T. (2002). Modulation of inflammation in vivo through induction of the heat shock response, effects on NF‐kappaB activation. Inflammation Research, 51, 108–109. 10.1007/BF02684012 [DOI] [PubMed] [Google Scholar]
- Lee, J. Y. , Han, S. H. , Park, M. H. , Baek, B. , Song, I. S. , Choi, M. K. , … Jin, H. K. (2018). Neuronal SphK1 acetylates COX2 and contributes to pathogenesis in a model of Alzheimer's disease. Nature Communications, 9, 1479 10.1038/s41467-018-03674-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard, M. O. , Hannan, K. , Burne, M. J. , Lappin, D. W. , Doran, P. , Coleman, P. , … Brady, H. R. (2002). 15‐Epi‐16‐(para‐fluorophenoxy)‐lipoxin A(4)‐methyl ester, a synthetic analogue of 15‐epi‐lipoxin A(4), is protective in experimental ischemic acute renal failure. J Am Soc Nephrol, 13, 1657–1662. 10.1097/01.ASN.0000015795.74094.91 [DOI] [PubMed] [Google Scholar]
- Levy, B. D. , Bonnans, C. , Silverman, E. S. , Palmer, L. J. , Marigowda, G. , & Israel, E. (2005). Diminished lipoxin biosynthesis in severe asthma. American Journal of Respiratory and Critical Care Medicine, 172, 824–830. 10.1164/rccm.200410-1413OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, B. D. , Clish, C. B. , Schmidt, B. , Gronert, K. , & Serhan, C. N. (2001). Lipid mediator class switching during acute inflammation: Signals in resolution. Nature Immunology, 2, 612–619. 10.1038/89759 [DOI] [PubMed] [Google Scholar]
- Levy, B. D. , Kohli, P. , Gotlinger, K. , Haworth, O. , Hong, S. , Kazani, S. , … Serhan, C. N. (2007). Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. Journal of Immunology, 178, 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, R. A. , Austen, K. F. , Drazen, J. M. , Clark, D. A. , Marfat, A. , & Corey, E. J. (1980). Slow reacting substances of anaphylaxis: Identification of leukotrienes C‐1 and D from human and rat sources. Proceedings of the National Academy of Sciences of the United States of America, 77, 3710–3714. 10.1073/pnas.77.6.3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Zhang, Y. , Schmelzer, K. , Lee, T. S. , Fang, X. , Zhu, Y. , … Shyy, J. Y. J. (2005). The antiinflammatory effect of laminar flow: The role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proceedings of the National Academy of Sciences of the United States of America, 102, 16747–16752. 10.1073/pnas.0508081102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y. , Hong, S. , Tjonahen, E. , & Serhan, C. N. (2005). Mediator‐lipidomics: Databases and search algorithms for PUFA‐derived mediators. Journal of Lipid Research, 46, 790–802. 10.1194/jlr.D400020-JLR200 [DOI] [PubMed] [Google Scholar]
- Lukiw, W. J. , Cui, J. G. , Marcheselli, V. L. , Bodker, M. , Botkjaer, A. , Gotlinger, K. , … Bazan, N. G. (2005). A role for docosahexaenoic acid‐derived neuroprotectin D1 in neural cell survival and Alzheimer disease. The Journal of Clinical Investigation, 115, 2774–2783. 10.1172/JCI25420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, K. R. , O'Neill, G. P. , Liu, Q. , Im, D. S. , Sawyer, N. , Metters, K. M. , … Evans, J. F. (1999). Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature, 399, 789–793. 10.1038/21658 [DOI] [PubMed] [Google Scholar]
- Ma, S. K. , Wang, Y. , Chen, J. , Zhang, M. Z. , Harris, R. C. , & Chen, J. K. (2015). Overexpression of G‐protein‐coupled receptor 40 enhances the mitogenic response to epoxyeicosatrienoic acids. PLoS One, 10, e0113130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado, F. S. , Johndrow, J. E. , Esper, L. , Dias, A. , Bafica, A. , Serhan, C. N. , & Aliberti, J. (2006). Anti‐inflammatory actions of lipoxin A(4) and aspirin‐triggered lipoxin are SOCS‐2 dependent. Nature Medicine, 12, 330–334. 10.1038/nm1355 [DOI] [PubMed] [Google Scholar]
- Maddox, J. F. , Colgan, S. P. , Clish, C. B. , Petasis, N. A. , Fokin, V. V. , & Serhan, C. N. (1998). Lipoxin B4 regulates human monocyte/neutrophil adherence and motility: Design of stable lipoxin B4 analogs with increased biologic activity. The FASEB Journal, 12, 487–494. 10.1096/fasebj.12.6.487 [DOI] [PubMed] [Google Scholar]
- Maddox, J. F. , Hachicha, M. , Takano, T. , Petasis, N. A. , Fokin, V. V. , & Serhan, C. N. (1997). Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP‐1 cells via a G‐protein‐linked lipoxin A4 receptor. The Journal of Biological Chemistry, 272, 6972–6978. 10.1074/jbc.272.11.6972 [DOI] [PubMed] [Google Scholar]
- Maddox, J. F. , & Serhan, C. N. (1996). Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: Selective inactivation by dehydrogenation and reduction. The Journal of Experimental Medicine, 183, 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maderna, P. , Cottell, D. C. , Berlasconi, G. , Petasis, N. A. , Brady, H. R. , & Godson, C. (2002). Lipoxins induce actin reorganization in monocytes and macrophages but not in neutrophils: Differential involvement of rho GTPases. The American Journal of Pathology, 160, 2275–2283. 10.1016/S0002-9440(10)61175-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheselli, V. L. , Hong, S. , Lukiw, W. J. , Tian, X. H. , Gronert, K. , Musto, A. , … Bazan, N. G. (2003). Novel docosanoids inhibit brain ischemia‐reperfusion‐mediated leukocyte infiltration and pro‐inflammatory gene expression. The Journal of Biological Chemistry, 278, 43807–43817. 10.1074/jbc.M305841200 [DOI] [PubMed] [Google Scholar]
- Medjane, S. , Raymond, B. , Wu, Y. , & Touqui, L. (2005). Impact of CFTR DeltaF508 mutation on prostaglandin E2 production and type IIA phospholipase A2 expression by pulmonary epithelial cells. American Journal of Physiology. Lung Cellular and Molecular Physiology, 289, L816–L824. 10.1152/ajplung.00466.2004 [DOI] [PubMed] [Google Scholar]
- Moghaddam, M. F. , Grant, D. F. , Cheek, J. M. , Greene, J. F. , Williamson, K. C. , & Hammock, B. D. (1997). Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nature Medicine, 3, 562–566. 10.1038/nm0597-562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, T. , Stables, M. , Colville‐Nash, P. , Newson, J. , Bellingan, G. , de Souza, P. M. , & Gilroy, D. W. (2010). Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proceedings of the National Academy of Sciences of the United States of America, 107, 8842–8847. 10.1073/pnas.1000373107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, T. , Stables, M. , Hobbs, A. , de Souza, P. , Colville‐Nash, P. , Warner, T. , … Gilroy, D. W. (2009). Effects of low‐dose aspirin on acute inflammatory responses in humans. Journal of Immunology, 183, 2089–2096. [DOI] [PubMed] [Google Scholar]
- Motwani, M. P. , Colas, R. A. , George, M. J. , Flint, J. D. , Dalli, J. , Richard‐Loendt, A. , … Gilroy, D. W. (2018). Pro‐resolving mediators promote resolution in a human skin model of UV‐killed Escherichia coli‐driven acute inflammation. JCI Insight, 3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motwani, M. P. , Newson, J. , Kwong, S. , Richard‐Loendt, A. , Colas, R. , Dalli, J. , & Gilroy, D. W. (2017). Prolonged immune alteration following resolution of acute inflammation in humans. PLoS One, 12, e0186964 10.1371/journal.pone.0186964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee, P. K. , Marcheselli, V. L. , Barreiro, S. , Hu, J. , Bok, D. , & Bazan, N. G. (2007). Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proceedings of the National Academy of Sciences of the United States of America, 104, 13152–13157. 10.1073/pnas.0705949104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger, K. A. , Montero, A. , Fukunaga, M. , Uda, S. , Yura, T. , Imai, E. , … Badr, K. F. (1999). Transfection of rat kidney with human 15‐lipoxygenase suppresses inflammation and preserves function in experimental glomerulonephritis. Proceedings of the National Academy of Sciences of the United States of America, 96, 13375–13380. 10.1073/pnas.96.23.13375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, D. R. , Koymans, L. , Kamataki, T. , Stegeman, J. J. , Feyereisen, R. , Waxman, D. J. , … Nebert, D. W. (1996). P450 superfamily: Update on new sequences, gene mapping. Accession Numbers and Nomenclature. Pharmacogenetics, 6, 1–42. [DOI] [PubMed] [Google Scholar]
- Newson, J. , Motwani, M. P. , Kendall, A. C. , Nicolaou, A. , Muccioli, G. G. , Alhouayek, M. , … Gilroy, D. W. (2017). Inflammatory resolution triggers a prolonged phase of immune suppression through COX‐1/mPGES‐1‐derived prostaglandin E2. Cell Reports, 20, 3162–3175. 10.1016/j.celrep.2017.08.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Node, K. , Huo, Y. , Ruan, X. , Yang, B. , Spiecker, M. , Ley, K. , … Liao, J. K. (1999). Anti‐inflammatory properties of cytochrome P450 epoxygenase‐derived eicosanoids. Science, 285, 1276–1279. 10.1126/science.285.5431.1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Node, K. , Ruan, X. L. , Dai, J. , Yang, S. X. , Graham, L. , Zeldin, D. C. , & Liao, J. K. (2001). Activation of Galpha s mediates induction of tissue‐type plasminogen activator gene transcription by epoxyeicosatrienoic acids. The Journal of Biological Chemistry, 276, 15983–15989. [DOI] [PubMed] [Google Scholar]
- Ohira, T. , Bannenberg, G. , Arita, M. , Takahashi, M. , Ge, Q. , Van Dyke, T. E. , … Badwey, J. A. (2004). A stable aspirin‐triggered lipoxin A4 analog blocks phosphorylation of leukocyte‐specific protein 1 in human neutrophils. Journal of Immunology, 173, 2091–2098. 10.4049/jimmunol.173.3.2091 [DOI] [PubMed] [Google Scholar]
- Pagels, W. R. , Sachs, R. J. , Marnett, L. J. , Dewitt, D. L. , Day, J. S. , & Smith, W. L. (1983). Immunochemical evidence for the involvement of prostaglandin H synthase in hydroperoxide‐dependent oxidations by ram seminal vesicle microsomes. The Journal of Biological Chemistry, 258, 6517–6523. [PubMed] [Google Scholar]
- Paul‐Clark, M. J. , Van Cao, T. , Moradi‐Bidhendi, N. , Cooper, D. , & Gilroy, D. W. (2004). 15‐Epi‐lipoxin A4‐mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. The Journal of Experimental Medicine, 200, 69–78. 10.1084/jem.20040566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot, M. , Clish, C. B. , Petasis, N. A. , Van Dyke, T. E. , & Serhan, C. N. (2000). Lipoxin A(4) analogues inhibit leukocyte recruitment to Porphyromonas gingivalis: A role for cyclooxygenase‐2 and lipoxins in periodontal disease. Biochemistry, 39, 4761–4768. [DOI] [PubMed] [Google Scholar]
- Rajakariar, R. , Hilliard, M. , Lawrence, T. , Trivedi, S. , Colville‐Nash, P. , Bellingan, G. , … Gilroy, D. W. (2007). Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15‐deoxyDelta12 14 PGJ2. Proceedings of the National Academy of Sciences of the United States of America, 104, 20979–20984. 10.1073/pnas.0707394104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revermann, M. , Barbosa‐Sicard, E. , Dony, E. , Schermuly, R. T. , Morisseau, C. , Geisslinger, G. , … Brandes, R. P. (2009). Inhibition of the soluble epoxide hydrolase attenuates monocrotaline‐induced pulmonary hypertension in rats. Journal of Hypertension, 27, 322–331. 10.1097/HJH.0b013e32831aedfa [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revermann, M. , Schloss, M. , Barbosa‐Sicard, E. , Mieth, A. , Liebner, S. , Morisseau, C. , … Brandes, R. P. (2010). Soluble epoxide hydrolase deficiency attenuates neointima formation in the femoral cuff model of hyperlipidemic mice. Arteriosclerosis, Thrombosis, and Vascular Biology, 30, 909–914. 10.1161/ATVBAHA.110.204099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca, B. , Secchiero, P. , Ciabattoni, G. , Ranelletti, F. O. , Catani, L. , Guidotti, L. , … Patrono, C. (2002). Cyclooxygenase‐2 expression is induced during human megakaryopoiesis and characterizes newly formed platelets. Proceedings of the National Academy of Sciences of the United States of America, 99, 7634–7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman, R. J. (2002). P‐450 metabolites of arachidonic acid in the control of cardiovascular function. Physiological Reviews, 82, 131–185. [DOI] [PubMed] [Google Scholar]
- Romano, M. , & Serhan, C. N. (1992). Lipoxin generation by permeabilized human platelets. Biochemistry, 31, 8269–8277. 10.1021/bi00150a021 [DOI] [PubMed] [Google Scholar]
- Saxena, P. N. , Beg, M. M. , Singhal, K. C. , & Ahmad, M. (1979). Prostaglandin‐like activity in the cerebrospinal fluid of febrile patients. The Indian Journal of Medical Research, 70, 495–498. [PubMed] [Google Scholar]
- Schottelius, A. J. , Giesen, C. , Asadullah, K. , Fierro, I. M. , Colgan, S. P. , Bauman, J. , … Parkinson, J. F. (2002). An aspirin‐triggered lipoxin A4 stable analog displays a unique topical anti‐inflammatory profile. Journal of Immunology, 169, 7063–7070. 10.4049/jimmunol.169.12.7063 [DOI] [PubMed] [Google Scholar]
- Schwab, J. M. , Chiang, N. , Arita, M. , & Serhan, C. N. (2007). Resolvin E1 and protectin D1 activate inflammation‐resolution programmes. Nature, 447, 869–874. 10.1038/nature05877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan, C. N. (1994). Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochimica et Biophysica Acta, 1212, 1–25. 10.1016/0005-2760(94)90185-6 [DOI] [PubMed] [Google Scholar]
- Serhan, C. N. (2008). Systems approach with inflammatory exudates uncovers novel anti‐inflammatory and pro‐resolving mediators. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 79, 157–163. 10.1016/j.plefa.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan, C. N. , Clish, C. B. , Brannon, J. , Colgan, S. P. , Chiang, N. , & Gronert, K. (2000). Novel functional sets of lipid‐derived mediators with antiinflammatory actions generated from omega‐3 fatty acids via cyclooxygenase 2‐nonsteroidal antiinflammatory drugs and transcellular processing. The Journal of Experimental Medicine, 192, 1197–1204. 10.1084/jem.192.8.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan, C. N. , Hamberg, M. , & Samuelsson, B. (1984). Trihydroxytetraenes: A novel series of compounds formed from arachidonic acid in human leukocytes. Biochemical and Biophysical Research Communications, 118, 943–949. 10.1016/0006-291X(84)91486-4 [DOI] [PubMed] [Google Scholar]
- Serhan, C. N. , Hong, S. , Gronert, K. , Colgan, S. P. , Devchand, P. R. , Mirick, G. , & Moussignac, R. L. (2002). Resolvins: A family of bioactive products of omega‐3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. The Journal of Experimental Medicine, 196, 1025–1037. 10.1084/jem.20020760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan, C. N. , Yang, R. , Martinod, K. , Kasuga, K. , Pillai, P. S. , Porter, T. F. , … Spite, M. (2009). Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. The Journal of Experimental Medicine, 206, 15–23. 10.1084/jem.20081880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, T. , Yamamoto, S. , & Hayaishi, O. (1982). Purification of PGH‐PGD isomerase from rat brain. Methods in Enzymology, 86, 73–77. 10.1016/0076-6879(82)86171-5 [DOI] [PubMed] [Google Scholar]
- Smilowitz, J. T. , Zivkovic, A. M. , Wan, Y. J. , Watkins, S. M. , Nording, M. L. , Hammock, B. D. , & German, J. B. (2013). Nutritional lipidomics: Molecular metabolism, analytics, and diagnostics. Molecular Nutrition & Food Research, 57, 1319–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodin‐Semrl, S. , Taddeo, B. , Tseng, D. , Varga, J. , & Fiore, S. (2000). Lipoxin A4 inhibits IL‐1 beta‐induced IL‐6, IL‐8, and matrix metalloproteinase‐3 production in human synovial fibroblasts and enhances synthesis of tissue inhibitors of metalloproteinases. Journal of Immunology, 164, 2660–2666. 10.4049/jimmunol.164.5.2660 [DOI] [PubMed] [Google Scholar]
- Sousa, A. R. , Parikh, A. , Scadding, G. , Corrigan, C. J. , & Lee, T. H. (2002). Leukotriene‐receptor expression on nasal mucosal inflammatory cells in aspirin‐sensitive rhinosinusitis. The New England Journal of Medicine, 347, 1493–1499. 10.1056/NEJMoa013508 [DOI] [PubMed] [Google Scholar]
- Spite, M. , Norling, L. V. , Summers, L. , Yang, R. , Cooper, D. , Petasis, N. A. , … Serhan, C. N. (2009). Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature, 461, 1287–1291. 10.1038/nature08541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starczewski, M. , Voigtmann, R. , Peskar, B. A. , & Peskar, B. M. (1984). Plasma levels of 15‐keto‐13,14‐dihydro‐prostaglandin E2 in patients with bronchogenic carcinoma. Prostaglandins, Leukotrienes, and Medicine, 13, 249–258. 10.1016/0262-1746(84)90037-4 [DOI] [PubMed] [Google Scholar]
- Tager, A. M. , & Luster, A. D. (2003). BLT1 and BLT2: The leukotriene B(4) receptors. Prostaglandins, Leukotrienes, and Essential Fatty Acids, 69, 123–134. 10.1016/S0952-3278(03)00073-5 [DOI] [PubMed] [Google Scholar]
- Tanaka, Y. , Ward, S. L. , & Smith, W. L. (1987). Immunochemical and kinetic evidence for two different prostaglandin H‐prostaglandin E isomerases in sheep vesicular gland microsomes. The Journal of Biological Chemistry, 262, 1374–1381. [PubMed] [Google Scholar]
- Thomson, S. J. , Askari, A. , & Bishop‐Bailey, D. (2012). Anti‐inflammatory effects of epoxyeicosatrienoic acids. Int J Vasc Med, 2012, 605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjonahen, E. , Oh, S. F. , Siegelman, J. , Elangovan, S. , Percarpio, K. B. , Hong, S. , … Serhan, C. N. (2006). Resolvin E2: Identification and anti‐inflammatory actions: Pivotal role of human 5‐lipoxygenase in resolvin E series biosynthesis. Chemistry & Biology, 13, 1193–1202. [DOI] [PubMed] [Google Scholar]
- Trivedi, S. G. , Newson, J. , Rajakariar, R. , Jacques, T. S. , Hannon, R. , Kanaoka, Y. , … Gilroy, D. W. (2006). Essential role for hematopoietic prostaglandin D2 synthase in the control of delayed type hypersensitivity. Proceedings of the National Academy of Sciences of the United States of America, 103, 5179–5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich, V. , & Haurand, M. (1983). Thromboxane synthase as a cytochrome P450 enzyme. Advances in Prostaglandin, Thromboxane, and Leukotriene Research, 11, 105–110. [PubMed] [Google Scholar]
- Vedin, I. , Cederholm, T. , Freund Levi, Y. , Basun, H. , Garlind, A. , Faxen Irving, G. , … Palmblad, J. (2008). Effects of docosahexaenoic acid‐rich n‐3 fatty acid supplementation on cytokine release from blood mononuclear leukocytes: The OmegAD study. The American Journal of Clinical Nutrition, 87, 1616–1622. 10.1093/ajcn/87.6.1616 [DOI] [PubMed] [Google Scholar]
- Wang, M. H. , Guan, H. , Nguyen, X. , Zand, B. A. , Nasjletti, A. , & Laniado‐Schwartzman, M. (1999). Contribution of cytochrome P‐450 4A1 and 4A2 to vascular 20‐hydroxyeicosatetraenoic acid synthesis in rat kidneys. The American Journal of Physiology, 276, F246–F253. [DOI] [PubMed] [Google Scholar]
- Xiao, C. Y. , Hara, A. , Yuhki, K. , Fujino, T. , Ma, H. , Okada, Y. , … Ushikubi, F. (2001). Roles of prostaglandin I(2) and thromboxane A(2) in cardiac ischemia‐reperfusion injury: A study using mice lacking their respective receptors. Circulation, 104, 2210–2215. 10.1161/hc4301.098058 [DOI] [PubMed] [Google Scholar]
- Yuhki, K. , Ueno, A. , Naraba, H. , Kojima, F. , Ushikubi, F. , Narumiya, S. , & Oh‐ishi, S. (2004). Prostaglandin receptors EP2, EP3, and IP mediate exudate formation in carrageenin‐induced mouse pleurisy. The Journal of Pharmacology and Experimental Therapeutics, 311, 1218–1224. 10.1124/jpet.104.071548 [DOI] [PubMed] [Google Scholar]
- Zeldin, D. C. (2001). Epoxygenase pathways of arachidonic acid metabolism. The Journal of Biological Chemistry, 276, 36059–36062. 10.1074/jbc.R100030200 [DOI] [PubMed] [Google Scholar]
- Zhang, L. N. , Vincelette, J. , Cheng, Y. , Mehra, U. , Chen, D. , Anandan, S. K. , … Wang, Y. X. (2009). Inhibition of soluble epoxide hydrolase attenuated atherosclerosis, abdominal aortic aneurysm formation, and dyslipidemia. Arteriosclerosis, Thrombosis, and Vascular Biology, 29, 1265–1270. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Oltman, C. L. , Lu, T. , Lee, H. C. , Dellsperger, K. C. , & VanRollins, M. (2001). EET homologs potently dilate coronary microvessels and activate BK (Ca) channels. American Journal of Physiology. Heart and Circulatory Physiology, 280, H2430–H2440. 10.1152/ajpheart.2001.280.6.H2430 [DOI] [PubMed] [Google Scholar]
- Zimmer, B. , Angioni, C. , Osthues, T. , Toewe, A. , Thomas, D. , Pierre, S. C. , … Sisignano, M. (2018). The oxidized linoleic acid metabolite 12,13‐DiHOME mediates thermal hyperalgesia during inflammatory pain. Biochimica et Biophysica Acta, 1863, 669–678. 10.1016/j.bbalip.2018.03.012 [DOI] [PubMed] [Google Scholar]


