Abstract
Inflammatory diseases are a major socio‐economic burden, with the incidence of such conditions on the rise, especially in western societies. For decades, the primary treatment paradigm for many of these conditions was to develop drugs that inhibit or antagonize the production and biological actions of molecules that were thought to be the culprits in propagating disease; these include cytokines and eicosanoids. This approach is effective in controlling disease propagation; however, long‐term exposure to these anti‐inflammatories is also associated with many side effects, some of which are severe, including immune‐suppression. The discovery that termination of self‐limited acute inflammation is an active process orchestrated by endogenous mediators, including the essential fatty acid‐derived resolvins, protectins and maresins, has provided novel opportunities for the design of therapeutics that control inflammation with a lower burden of side effects. This is because at variance to anti‐inflammatories, pro‐resolving mediators do not completely inhibit inflammatory responses; instead, these mediators reprogramme the immune response to accelerate the termination of inflammation, facilitating the regain of function. The scope of this review is to highlight the biological actions of these autacoids and their potential utility as lead compounds in developing resolution pharmacology‐based therapeutics.
Linked Articles
This article is part of a themed section on Eicosanoids 35 years from the 1982 Nobel: where are we now? To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.8/issuetoc
Abbreviations
- 13‐HDPA
13‐(R)‐hydroxy‐7Z,10Z,13R,14E,16Z,19Z‐docosapentaenoic acid
- 13‐HpDPA
13‐(R)‐hydroxyperoxy‐7Z,10Z,13R,14E,16Z,19Z‐docosapentaenoic acid
- 13S, 14S‐epoxy‐MaR
13S,14S‐epoxy‐4Z,7Z,9E,11E,16Z,19Z‐docosahexaenoic acid
- 17R‐RvD3
4S,11R,17R‐trihydroxy‐5Z,7E,9E,13Z,15E,19Z‐docosahexaenoic acid
- ALX
lipoxin A4 receptor
- BLT1 receptor
LTB4 receptor
- DHA
docosahexaenoic acid
- DSS
dextran sodium sulphate
- EPA
eicosapentaenoic acid
- LOX
lipoxygenase
- LXA4
5S,6R,15S‐trihydroxy‐7E,9E,11Z,13E‐eicosatetraenoic acid
- MaR
maresin
- MaR1
7R,14S‐dihydroxy‐4Z,8E,10E,12Z,16Z,19Z‐docosahexaenoic acid
- MaR1n‐3 DPA
7R,14Sdihydroxy‐8E,10E,12Z,16Z,19Z‐docosapentaenoic acid
- MaR2
13R,14S‐dihydroxy‐4Z,7Z,9,11,16Z,19Z‐docosahexaenoic acid
- MaRn‐3 DPA
n‐3docosapentaenoic acid‐derived maresins
- MCTR
maresin conjugates in tissue regeneration
- MCTR1
13R‐glutathionyl,14S‐hydroxy‐4Z,7Z,9E,11E,13R,14S,16Z,19Z‐docosahexaenoic acid
- MCTR2
13R‐cysteinylglycinyl,14S‐hydroxy‐4Z,7Z,9E,11E,13R,14S,16Z,19Z‐docosahexaenoic acid
- MCTR3
13R‐cysteinyl,14S‐hydroxy‐4Z,7Z,9E,11E,13R,14S,16Z,19Z‐docosahexaenoic acid
- miRNA
microRNA
- n‐3 DPA
n‐3 docosapentaenoic acid
- NALP3
NACHT, LRR and PYD domains‐containing protein 3
- NSAIDs
non‐steroidal anti‐inflammatory drugs
- PCTR
protectin conjugates in tissue regeneration
- PCTR1
16R‐glutathionyl,17S‐hydroxy‐4Z,7Z,10Z,12E,14E,19Z‐docosahexaenoic acid
- PD
protectin
- PD1
10R,17S‐dihydroxy‐4Z,7Z,11E,13E,15Z,19Z‐docosahexaenoic acid
- PD1n‐3 DPA
10R,17S‐dihydroxy‐7Z,11E,13E,15Z,19Z‐docosapentaenoic acid
- PDn‐3 DPA
n‐3 docosapentaenoic acid‐derived protectin
- RCTR
resolvin conjugates in tissue regeneration
- Rv
resolvin
- RvD
docosahexaenoic acid‐derived resolvins
- RvD1
7S,8R,17S‐trihydroxy‐4Z,9E,11E,13Z,15E,19Z‐docosahexaenoic acid
- RvD2
7S,16R,17S‐trihydroxy‐4Z,8E,10Z,12E,14E,19Z‐docosahexaenoic acid
- RvD3
4S,11R,17S‐trihydroxy‐5Z,7E,9E,13Z,15E,19Z‐docosahexaenoic acid
- RvD4
4S,5R,17S‐trihydroxy‐6E,8E,10Z,13Z,15E,19Z‐docosahexaenoic acid
- RvD5
7S,17S‐dihydroxy‐4Z,8E,10Z,13Z,15E,19Z‐docosahexaenoic acid
- RvDn‐3 DPA
n‐3 docosapentaenoic acid‐derived resolvins
- RvE
eicosapentaenoic acid derived resolvins
- RvE1
5S,12R,18R‐trihydroxy‐6Z,8E,10E,14Z,16E‐eicosapentaenoic acid
- RvE2
5S,18R‐trihydroxy‐6E,8Z,11Z,14Z,16E‐eicosapentaenoic acid
- RvE3
17,18‐dihydroxy‐5Z,8Z,11Z,13E,15E‐eicosapentaenoic acid
- RvT
thirteen series resolvins
- SPM
specialized pro‐resolvin mediators
- TRPV1
transient receptor potential cation channel subfamily V member 1
During evolution, multicellular organisms arose and cells within an organism became specialized to perform specific tasks leading to the formation of organs and tissues. These specialized organs required protection from invading microbes as well as mechanisms to repair and regenerate damaged tissues and organs, thus paving the way to the evolution of the immune system (Malagoli, 2016). In higher organisms, the immune system is divided into two arms, the innate arm provides the front line defence system and the adaptive arm provides protection from insults and/or pathogens to which the body was previously exposed. In some instances, immune responses may become dysregulated leading to disease. It is now appreciated that a significant portion of diseases that afflict western societies are associated with unbridled inflammation leading to damage of vital organs and tissues resulting in malaise and ultimately death (Kumar et al., 2014; Majno, 1991).
Classically, the inflammatory response has been divided into two phases, the initiation and resolution (or termination) phases (Kumar et al., 2014). Many studies conducted primarily in the last century focused on determining the mechanisms and molecules produced during the initiation phase, demonstrating that this was a tightly orchestrated response with the production of several classes of molecules that are involved in the recruitment of different leukocyte subsets and include the cytokines, chemokines and the classic eicosanoids (Kumar et al., 2014). Interested readers on the biology of these mediators are directed to articles in this special edition as well as to Samuelsson (2012) and Dinarello and Joosten (2016). The identification of these molecules also paved the way to the development of innovative therapeutics for the treatment of many inflammatory diseases that revolutionized medicine and medical practice. The drugs were designed to inhibit or antagonize the biological actions and the production of local mediators that were identified to be important in disease onset and progression. Longitudinal studies on the use of many of these therapeutics demonstrate that while these molecules are effective at inhibiting inflammation, they also come with many side effects. For example, non‐steroidal anti‐inflammatory drugs (NSAIDs) lead to an increased incidence of gastrointestinal bleeds (Goldstein and Cryer, 2015), and anti‐TNF increase the incidence of infections (Minozzi et al., 2016). Thus, these observations underscore an urgent need for the development of alternative approaches, especially in the treatment of chronic diseases, which would not interfere with the immune system and would carry a lower burden of side effects.
For many years, termination of inflammation was thought to occur via the simple dilution of inflammatory signals from the site of injury or infection, leading to the egress of white blood cells and re‐establishment of tissue function (Robbins and Cotran, 1979). Studies focusing on mechanisms that control the termination of inflammation demonstrated that arachidonic acid is not only a substrate in the biosynthesis of inflammation‐initiating molecules, such as PGs and leukotrienes, but is converted to protective and anti‐inflammatory molecules that include the lipoxins (Serhan et al., 1984; Levy et al., 2001) and the cyclopentenone PGs (Gilroy et al., 1999). Identification of these molecules indicated that resolution of inflammation is an active process coordinated by autacoids produced at the site that control immune cell responses. Introducing quantitation indices to mark resolution permitted the delineation of the in vivo biological actions of pro‐resolving mediators, including the lipoxins, resolvins and protectins, during acute inflammation (Bannenberg et al., 2005; Schwab et al., 2007). Detailed studies investigating mechanisms controlling the termination of inflammation also uncovered a link between the inflammation‐initiating eicosanoids PGE2 and PGD2 and lipoxin biosynthesis. These PGs are important in up‐regulating the expression of 15‐lipoxygenase type 1 (15‐LOX‐1), the initiating enzyme in the lipoxin biosynthetic pathway (Levy et al., 2001). Of note, inhibition of PG biosynthesis using NSAIDs is linked to a reduction in the biosynthesis of specialized pro‐resolving mediators and a delay in the termination of inflammation in experimental systems (Fukunaga et al., 2005). Thus, these studies demonstrated that the acute inflammatory response is a coordinated process with the initiation and termination phases being intricately linked.
These initial observations also raised the question whether novel molecules are produced during acute inflammation to promote its termination and activate reparative and regenerative responses thereby paving the way to the re‐establishment of tissue and organ function. Using a systems approach, we uncovered three novel mediator superfamilies that actively reprogramme the host immune response to halt inflammation and re‐establish organ function (Serhan et al., 2000; 2002; 2009; Dalli et al., 2013; 2014; 2015a). Given their potent biological actions, these mediators were termed as specialized pro‐resolving mediators (SPM). This superfamily is composed of mediators that are produced via the stereoselective conversion of essential fatty acids and include the docosahexaenoic acid derived resolvins (RvD), protectins and maresins and the eicosapentaenoic acid derived resolvins (RvE) (Serhan et al., 2017). The production of these mediators is regulated in a time, organ and stimulus‐dependent manner (see Dalli, 2017), and their relative concentrations to classic eicosanoids are also dependent on these factors. For example, in cerebrospinal fluids from patients with multiple sclerosis PGE2 and RvD1 concentrations were present at similar concentrations (~1 pg·mL−1) (Pruss et al., 2013). In experimental models of eye infections, LXA4 concentrations were between 2‐ and 10‐fold higher than those of PGE2. The production of eicosanoids and SPM is also regulated in a sex‐dependent manner where LXA4 concentrations in females were elevated when compared to males during experimental eye infections (Livne‐Bar et al., 2017), whereas RvD and RvE are elevated during experimental inflammation in humans (Rathod et al., 2017). The scope of the present review is to discuss the evidence underpinning the protective actions of SPM and how insights into their biological actions may provide leads on the utility of harnessing SPM as templates for the development of resolution pharmacology‐based therapeutics that would carry a lower burden of side effects.
The identification and structure elucidation of novel immunoresolvents
The EPA bioactive metabolome
In order to establish whether novel molecules are produced during the resolution phase that actively promote the termination of inflammation, it is essential to employ a systems approach. For this purpose, we developed metrics to measure the kinetics of cellular trafficking as well as tissue regeneration responses (Bannenberg et al., 2005; Schwab et al., 2007). Using this approach, we found that during acute, self‐limited inflammation, eicosapentaenoic acid (EPA) was converted in inflammatory exudates to novel mediators that potently and stereospecifically promoted the termination of inflammation and were thus coined as E‐series resolvins (RvE) (Serhan et al., 2000).
Investigations into the mechanisms activated by these molecules demonstrated that these mediators directly counter‐regulate the production of pro‐inflammatory mediators, including TNF‐α and inflammatory eicosanoids. In addition, the biological actions of RvE1 at controlling leukocyte responses were found to be more potent than those of aspirin and dexamethasone. RvE1 was also found to carry potent antinociceptive actions reducing inflammatory pain by regulating both central and peripheral responses with activities of 10 ng per mouse when administered intrathecally and 285–570 pmol when administered peripherally in vivo. These actions were also displayed in vitro at concentrations as low as 3 nM (Xu et al., 2010; Jo et al., 2016; Fonseca et al., 2017). Of note, the antinociceptive properties of RvE1 are more potent than those exerted by the COX‐2 inhibitor NS398 and morphine (Xu et al., 2010).
In studies assessing the role of omega‐3 supplementation in patients with arthritis, Barden and colleagues (2016) found an association between the synovial fluid concentrations of RvE2 and joint pain, where higher RvE2 concentrations were associated with lower pain scores in these patients. RvE2 is also antidepressant; i.c.v. infusions (10 ng per mouse) reduced LPS‐induced depression in mice via the activation of mTORC1 signalling in the medial prefrontal cortex and hippocampal dentate gyrus (Deyama et al., 2018). Isobe and colleagues also found that the RvE1 and RvE2 precursor 18‐hydroxy‐eicosapentaenoic acid is converted by eosinophils to a novel bioactive mediator denoted as RvE3. This mediator, at 10–100 ng per mouse, displays potent anti‐inflammatory properties characteristic of the SPM family, including the ability to regulate neutrophil recruitment (Isobe et al., 2012; Isobe et al., 2013).
The DHA bioactive metabolome – protectins
EPA is not the only omega‐3 essential fatty acid that is converted during acute self‐limited inflammation to novel bioactive mediators. Docosahexaneoic acid is also a substrate for the formation of two SPM families produced via a key 17‐hydroperoxy‐docosahexaenoic acid intermediate and coined as D‐series resolvins and protectins (Serhan et al., 2017). In peripheral tissues, these mediators are produced by leukocytes and their biosynthesis is temporally regulated (Dalli et al., 2013a; Winkler et al., 2016). In neural tissues, the protectin biosynthetic pathway regulates both stromal and immune cell responses. In retinal pigmented cells, protectin D1 (PD1; 50 nM; referred to as NPD1 in neuronal systems) potently counteracts H2O2/TNF‐α oxidative‐stress‐triggered DNA damage. PD1 also up‐regulates the expression of several anti‐apoptotic proteins including Bcl‐2 and Bcl‐xL and decreases the expression of the pro‐apoptotic factors Bax and Bad as well as the executioner caspase, caspase 3 (Mukherjee et al., 2004). In cytokine‐stressed human neural cells, PD1 formation was associated with an attenuation of amyloid‐β secretion (Lukiw et al., 2005). This SPM was also reduced in the hippocampal cornu ammonis region 1 from patients with Alzheimer's disease but not in the thalamus or occipital lobes from the same brains. Furthermore, the expression of key enzymes in the biosynthesis of PD1, cytosolic PLA2 and 15‐LOX, was altered in the hippocampus of patients with Alzheimer's disease (Lukiw et al., 2005). NPD1 at 300 ng per eye also reduces the severity and incidence of stromal keratitis and corneal neovascularization following herpes simplex virus infections (Rajasagi et al., 2013).
Recent studies have implicated a subset of eosinophils in the biosynthesis of PD1 during the course of self‐limited inflammation. Eosinophil recruitment to inflamed loci during the resolution phase of acute inflammation correlates with an increase in PD1 production (~100 pg per exudate; Yamada et al., 2011). Depletion of eosinophils results in a delay in resolution responses, including an impairment of lymphatic drainage with a reduction in the appearance of phagocytes carrying engulfed zymosan in the draining lymph node and sustained numbers of polymorphonuclear leukocytes in inflamed tissues. The resolution deficit caused by eosinophil depletion was rescued by eosinophil restoration or the administration of PD1, 5 μg per mouse (Yamada et al., 2011). Of note, PD1 production is reduced from 2.23 ± 1.55 ng·mL−1 in healthy volunteers to trace concentrations in exhaled breath from asthmatics (Levy et al., 2007). This reduction in PD1 biosynthesis is also observed in isolated eosinophils from patients with severe asthma, suggesting a role for defective production of this SPM in disease onset and/or propagation (Miyata et al., 2013).
The DHA bioactive metabolome – resolvins
The D‐series resolvins are now thought to regulate host immune responses in a number of disease settings. In Pseudomonas aeruginosa‐mediated lung infection RvD1, at 100 ng per mouse, significantly reduces P. aeruginosa titres, leukocyte infiltration and lung tissue damage (Codagnone et al., 2018). In murine lung macrophages sorted during P. aeruginosa chronic infection, RvD1 regulates the expression of Toll‐like receptors (TLRs), downstream genes, as well as microRNA (miR)‐21 and 155, resulting in a reduction in inflammatory signalling. In vitro, RvD1 up‐regulates P. aeruginosa phagocytosis by both neutrophils and macrophages (Codagnone et al., 2018).
RvD2 was recently identified in skeletal muscle biopsies from humans with peripheral artery disease at concentrations of ~150 pg·g−1 of tissue. When this mediator was administered to mice (100 ng per mouse) during ischaemia/reperfusion, it enhanced perfusion recovery and promoted arteriogenesis (Zhang et al., 2016). In contrast to other strategies used for revascularization that exacerbate inflammation, RvD2 does not enhance vascular permeability, it reduces neutrophil accumulation in the damaged tissues and plasma inflammatory cytokine levels, including TNF‐α and granulocyte macrophage colony‐stimulating factor. RvD2 also increases myocyte regeneration, enhances endothelial cell migration in a Rac‐dependent manner and restores defective revascularization in diabetic mice (Zhang et al., 2016). In alipoliprotein E (ApoE) deficient mice, RvD2 concentrations also correlated with the signs of plaque stability, and an injection of RvD2 (100 ng per mouse) to ApoE−/− mice prevented atheroprogression, reduced macrophage recruitment, increased fibrous cap thickness and smooth muscle cell numbers (Viola et al., 2016).
Additionally, RvD3 displays potent tissue protective actions. RvD3 was identified in uninjured lungs, suggesting that it plays a functional role in regulating tissue resolution tone (Colby et al., 2016). The concentrations of this mediator are rapidly up‐regulated following hydrochloric acid‐initiated injury from ~22 to ~75 pg per lung. Administration of the metabolically stable endogenous isomer of RvD3, 17R‐RvD3 (10 ng per mouse), affords significant tissue protection during hydrochloric acid‐initiated injury, reducing alveolar wall thickening, lung oedema and leukocyte infiltration (Colby et al., 2016). This mediator also increases lung epithelial cell proliferation, bronchoalveolar lavage fluid levels and keratinocyte growth factor promoting cutaneous re‐epithelialization (Colby et al., 2016).
In murine tissues infected with Staphylococcus aureus, recent studies found that RvD4 persists late into the resolution phase at concentrations of 0.75 pg per exudate (Winkler et al., 2016). Administration of this mediator (200 ng per mouse) during S. aureus infection reduces neutrophilic infiltration, increases bacterial clearance and restores resolution responses. RvD4 also enhanced the uptake of apoptotic neutrophils by human dermal fibroblasts at concentrations as low as 0.1 nM (Winkler et al., 2016). RvD5 displays potent anti‐bacterial actions, up‐regulating neutrophil and macrophage phagocytosis of bacteria in a dose‐ and receptor‐dependent manner (Chiang et al., 2012). Of note, RvD biosynthesis in tissues is regulated in both a stimulus‐ and tissue‐dependent manner, thereby demonstrating that these mediators exert distinct biological actions during self‐limited inflammation (Figure 1) (Chiang et al., 2012; Dalli et al., 2013a; Winkler et al., 2016).
Figure 1.
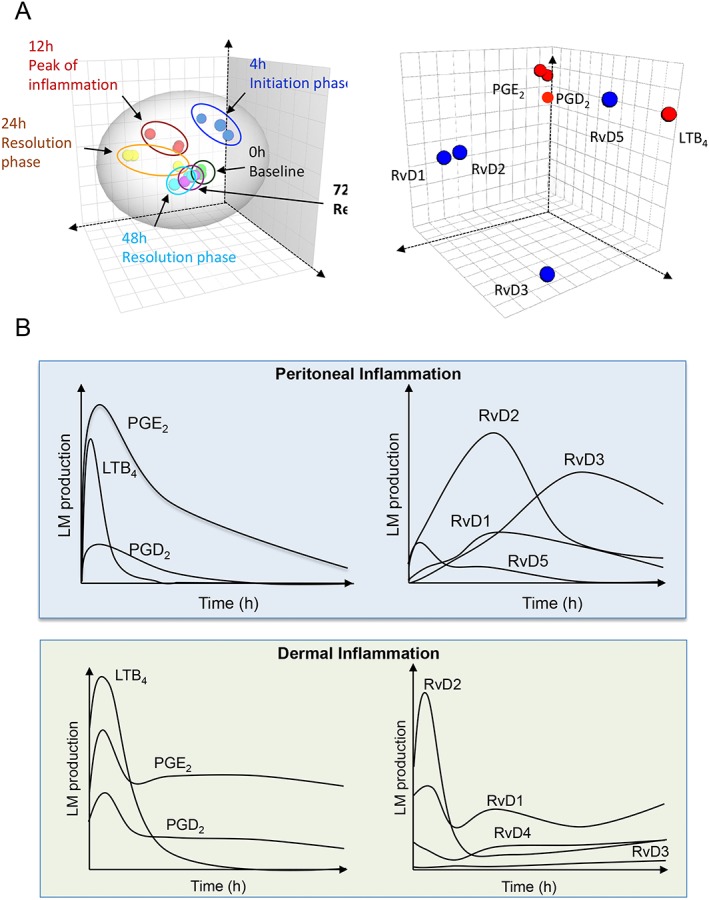
Temporal, tissue and stimulus specific SPM regulation during sterile and infectious inflammation. (A) Mice were challenged with 1 μg of zymosan; inflammatory exudates were collected at the indicated intervals and lipid mediators quantified as in Dalli et al. (2013a). Results were analysed using multivariate analysis. (A, left panel) Depicts the temporal shift in lipid mediator clusters during the course of initiation and resolution. (A, right panel) Denotes the contribution of each mediator to the temporal clustering. (B, top panel) Mice were challenged and lipid mediators were identified and quantified. Panels are illustrations of results presented in Dalli et al. (2013a). (B; bottom panel) Mice were challenged with Staphylococcus aureus and lipid mediators identified and quantified. Panels are illustrations of results presented in Winkler et al. (2016).
The DHA bioactive metabolome – maresins
Macrophages are central players in regulating the host immune response. We recently found that these cells also produce a novel family of mediators termed maresins (macrophage mediators in resolving inflammation) (Serhan et al., 2009). The biosynthesis of this family of mediators is initiated by lipoxygenation and subsequent epoxidation of DHA to yield 13S,14S‐epoxy‐MaR (13S,14S‐eMaR). This product regulates the production of the leukocyte chemoattractant LTB4 via the direct inactivation of the LTA4 hydrolase (Dalli et al., 2013b). 13S,14S‐eMaR (10 nM) also increases the expression of markers associated with an alternatively activated phenotype in human macrophages, including CD163 and CD206, and down‐regulating the expression of markers linked with a classically activated phenotype including CD54 (Dalli et al., 2013b). In addition to directly regulating aspects of the immune response, 13S,14S‐eMaR is also converted to MaR1 and MaR2 via enzyme‐mediated hydrolysis (Serhan et al., 2009; Dalli et al., 2013b; Deng et al., 2014).
MaR1, the first member of this family identified, is antinociceptive regulating the activation of TRPV1 by capsaicin at concentrations as low as 0.5 ng·mL−1. Administration of MaR1 at 10 ng per mouse also inhibits chemotherapy‐elicited neuropathic pain (Serhan et al., 2012). MaR1 exerts protective actions on hepatocytes, reducing lipotoxicity‐induced apoptosis by activating the unfolded protein response pro‐survival mechanisms and limiting the up‐regulation of pro‐apoptotic pathways (Rius et al., 2017). This macrophage‐derived mediator regulates the expression of miRNA targeting both protein folding and apoptosis and enhances the phagocytic capacity of Kupffer cells (Rius et al., 2017).
Recent studies also demonstrate that MaR1 is produced by circulating neutrophil–platelet aggregates. During these heterotypic cell aggregates, DHA is converted to 13S,14S‐eMaR by the platelet 12‐LOX. This intermediate is then donated to neutrophils that, via enzyme‐mediated hydrolysis, produce MaR1 (Abdulnour et al., 2014). The production of MaR1 by these heterotypic cell aggregates in the vasculature is a protective mechanism engaged to limit tissue damage following acid‐induced lung injury and results in a reduction of activated neutrophil recruitment into the damaged tissues (Abdulnour et al., 2014). At the site of infectious inflammation, MaR1 is subjected to further metabolism by exudate leukocytes. Macrophages convert this mediator to 14‐oxo‐MaR1, which displays blunted biological actions in comparison to the parent mediator. Neutrophils convert MaR1 to 22‐OH‐MaR1 that retains the potent biological actions of the parent SPM, including its ability to regulate the activation of the LTB4 receptor (BLT1 receptor) by its cognate ligand (Colas et al., 2016). In the CNS, MaR1 also displays reparative actions, where MaR1 (1 μg per mouse, i.v.) administration to animals subjected to spinal cord injury improved locomotor recovery and reduced secondary injury progression (Francos‐Quijorna et al., 2017).
The DHA bioactive metabolome – sulfido conjugated SPM
In recent studies, we found that 13S,14S‐eMaR is also an intermediate in the biosynthesis of a novel family of peptide‐lipid conjugated molecules termed maresin conjugates in tissue regeneration (MCTR) (Dalli et al., 2014). This family of mediators is evolutionary conserved being identified in planaria, mice and humans. Furthermore, in each of these species, MCTR displays potent tissue protective and tissue regenerative actions. During murine infections, MCTRs are produced late within the resolution phase, coinciding with the turning on of reparative and regenerative responses (Dalli et al., 2014). Administration of MCTRs (100 nM) to planaria following surgical injury leads to the up‐regulation of genes involved in tissue regeneration. In mice following ischaemia–reperfusion mediated injury, MCTR1 and MCTR2 (50 ng per mouse each) up‐regulate the expression of proteins involved in lung repair and regeneration, including Ki67 and R‐spondin 3 (Dalli et al., 2014). Identification of the MCTRs also paved the way to the identification of two novel peptide lipid conjugated mediator families termed as protectin conjugates in tissue regeneration (PCTR) and resolvin conjugates in tissue regeneration (RCTR) (Dalli et al., 2015b). These mediators display tissue regenerative and leukocyte directed actions controlling a host's response to the Gram‐negative bacterium Escherichia coli (Dalli et al., 2015b). PCTR1 production in the murine peritoneum is under neural control (Dalli et al., 2017). This mediator is central in maintaining tissue resolution tone via regulating tissue resident macrophage phenotype and function. Disruption of the vagus nerve or inhibiting 15‐LOX, the initiating enzyme in the PCTR biosynthetic pathway, perturbs PCTR production leading to impaired host responses to E. coli infections, including delayed resolution of self‐limited inflammation and a reduction in the ability of recruited leukocytes to phagocytose and kill bacteria (Dalli et al., 2017).
The n‐3 docosapentaenoic acid bioactive metabolome
We recently found that n‐3 docosapentaenoic acid (DPA) was not simply a precursor in the biosynthesis of DHA from EPA. This essential fatty acid is converted by leukocytes to several mediator families that are congeners to those produced when DHA is the substrate (Figure 2) (Dalli et al., 2013). In murine plasma and inflammatory exudates following ischaemia–reperfusion injury, we found that n‐3 DPA is converted to the D‐series resolvins, RvD1n‐3 DPA, RvD2n‐3 DPA and RvD5n‐3 DPA as well as PD1n‐3 DPA with concentrations at the site of inflammation for these SPM ranging between 20 and 100 pg per exudate (Dalli et al., 2013). These new mediators each display potent leukocyte‐directed properties, reducing neutrophil recruitment during acute peritonitis and leukocyte‐mediated lung damage during ischaemia–reperfusion (Dalli et al., 2013; Aursnes et al., 2014). Human leukocytes, in addition to producing RvDn‐3 DPA and PDn‐3 DPA, also convert n‐3 DPA to MaRn‐3 DPA, which displays leukocyte and endothelial directed actions reducing the expression of CD54 in TNF‐α activated endothelial cells (Dalli et al., 2013; Tungen et al., 2014). The biosynthesis of RvD5n‐3 DPA and PD1n‐3 DPA is disrupted in colonic biopsies from patients with inflammatory bowel disease as well as in mice given dextran sodium sulphate (DSS) (Gobbetti et al., 2017). Administration of either of these mediators provided significant protection against DSS‐initiated colon inflammation. Furthermore, increasing the endogenous production of these mediators in mice during DSS‐initiated colitis also led to a significant reduction in tissue damage (Gobbetti et al., 2017). In this context, recent studies in healthy volunteers found that supplementation with n‐3 DPA increased plasma RvD5n‐3 DPA concentrations (Markworth et al., 2016). Using a lipid mediator profiling approach, we found that in humans systemic concentrations of RvDn‐3 DPA diurnally regulated and their concentrations correlate with markers of both platelet and leukocyte activation including CD11b and CD62P (Colas et al., 2018).
Figure 2.
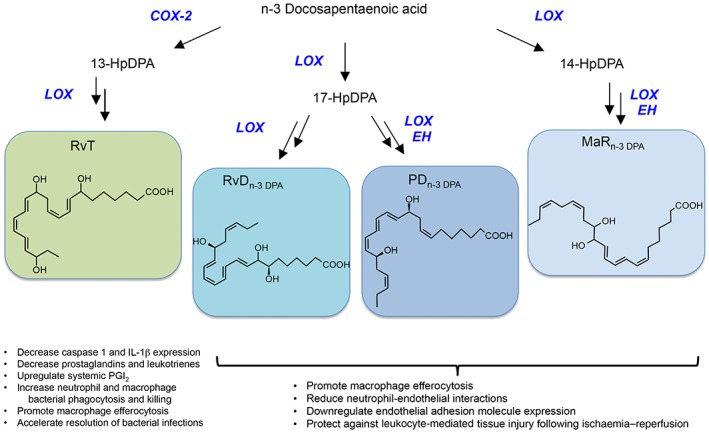
Novel immunoresolvents biosynthesized from n‐3 docosapentaenoic. In the vasculature, n‐3 docosapentaenoic acid is the substrate for conversion, by endothelial COX‐2, to 13‐HDPA that is then donated to neutrophils and converted to RvT that display potent protective actions in infectious inflammation (Dalli et al., 2015a). The n‐3 docosapentaenoic is also converted by leukocytes to 17‐HpDHA that is a precursor to RvDn‐3 DPA and PDn‐3 DPA. Conversion of n‐3 DPA via 14‐lipoxygenation yields 14‐HpDPA that is converted to MaRn‐3 DPA. Each of these mediator families displays potent leukocyte‐directed and host‐protective actions (Dalli et al., 2013; Gobbetti et al., 2017).
In the vasculature during ongoing inflammation, n‐3 DPA is also converted to the 13‐series resolvins (RvT) (Dalli et al., 2015a). The biosynthesis of these mediators is initiated by endothelial COX‐2 that converts the essential fatty acid to 13‐(R)‐hydroxyperoxy‐7Z,10Z,13R,14E,16Z,19Z‐docosapentaenoic acid (13‐HpDPA). This or its reduced form, namely, 13‐HDPA, are then donated to neutrophils for transcellular biosynthesis to produce RvT (Dalli et al., 2015a; Primdahl et al., 2016). These mediators are produced during the early stages of self‐limited bacterial infections with plasma concentrations reaching concentrations of ~30–50 pg·mL−1. These mediators regulate the ability of leukocytes to uptake and kill bacteria. RvT also down‐regulate the activation of the NACHT, LRR and PYD domain‐containing protein 3 (NALP3) inflammasome in murine and human macrophages, reducing the expression of caspase 1 and the production of IL‐1β as well as the release of LDH, a hallmark of pyroptosis (Dalli et al., 2015a). In addition, RvTs are also important in mediating the biological actions of atorvastatin and pravastatin (Dalli et al., 2015a; Walker et al., 2017). Administration of these statins regulates COX‐2 activity by up‐regulating the NOS‐mediated nitrosylation of the enzyme, increasing its catalytic turnover and 13‐HDPA formation (Dalli et al., 2015a). Inhibition of either NOS or COX‐2 using specific pharmacological inhibitors led to a reversal of the protective actions of atorvastatin and pravastatin in both infectious inflammation and rheumatoid arthritis (Dalli et al., 2015a; Walker et al., 2017).
How do the SPM exert their biological actions?
Agonists of GPCRs
The biological actions of SPM are stereoselective and are mediated via the activation of cognate receptors and signalling pathways. The first of these receptors to be identified was the LXA4 receptor, which up until then was thought to be a low affinity receptor for endogenous formylated peptides (Fiore et al., 1994). The LXA4 receptor (ALX) is part of the GPCR family (Figure 3). Its activation by cognate endogenous ligands, including LXA4 and the pro‐resolving protein annexin A1, displays a characteristic bell shape, where at either end of the dose range, the ability of the ligand to activate the receptor is significantly reduced (Hayhoe et al., 2006; Rovira et al., 2010). Of note, ALX receptor activation by its pro‐resolving ligands leads to distinct downstream signalling pathways that are cell specific. For example, LXA4 increases intracellular calcium levels in conjunctival goblet cells via the ALX receptor (Hodges et al., 2016); whereas activation of this receptor by LXA4 does not elicit a calcium response in human neutrophils (Fiore and Serhan, 1995). Studies conducted by Cooray and colleagues demonstrate that the ALX receptor on cell membranes is expressed as a homo‐ or heterodimer with the formyl peptide receptor FPR1 or FPR3 (the two other family members) and operates in a ligand‐biased fashion. Furthermore, signalling pathways activated by these different dimerization states are distinct (Cooray et al., 2013). This process of dimerization is at least in part influenced by the ligands themselves whereby annexin A1 promotes homodimerization and the activation of p38/MAPK‐activated protein kinase/heat shock protein 27 signalling and IL‐10 up‐regulation (Cooray et al., 2013). On the other hand, the pro‐inflammatory acute phase protein serum amyloid protein A did not lead to receptor homodimerization. These findings shed light on how one receptor can mediate the biological actions of functionally distinct molecules (Cooray et al., 2013). In addition to mediating the biological actions of LXA4 and its aspirin triggered epimer (Ortiz‐Munoz et al., 2014; Wang et al., 2014; Romano et al., 2015), the ALX receptor also mediates the actions of the D‐series resolvins RvD1, RvD3 and their aspiring trigger epimers (Krishnamoorthy et al., 2012; Arnardottir et al., 2016; de Oliveira et al., 2017; Mottola et al., 2017; Serhan et al., 2017).
Figure 3.
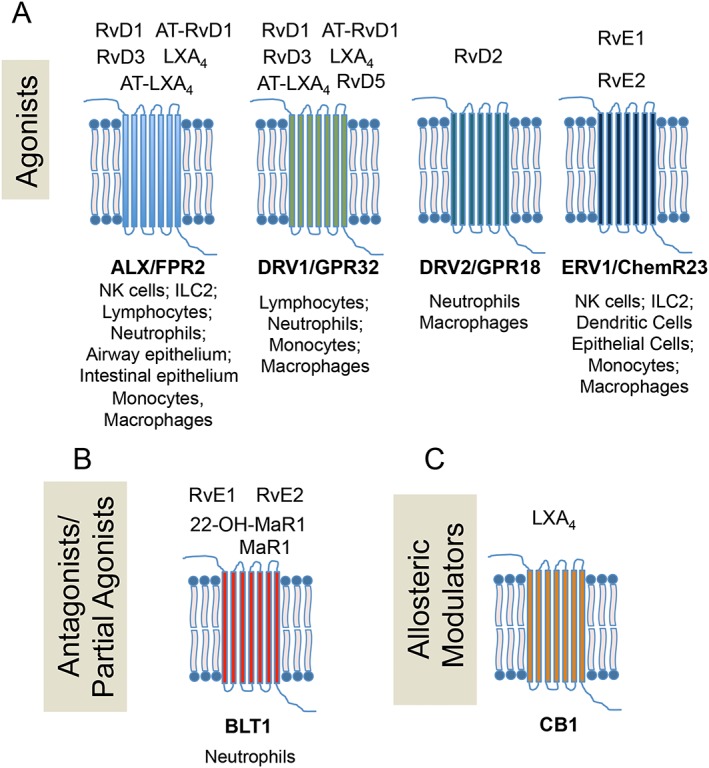
Distinct interactions between SPM and GPCRs in controlling host responses. (A) Depicts the receptors activated by select pro‐resolving mediators and the target cells in which these receptors mediate the actions of their cognate SPM. (B,C) Depicts the receptors at which each SPM acts as a (B) antagonists/partial agonist or (C) allosteric modulator.
Activation of the orphan receptor GPR32 by RvD1 leads to the regulation of a number of miRNA involved in the orchestration of acute inflammation, including miR‐146b, miR‐208a and miR‐219 (Krishnamoorthy et al., 2012; Serhan et al., 2017). This receptor also mediates the biological actions of RvD5 in the context of bacterial infections, whereby its activation by RvD5 leads to enhanced bacterial phagocytosis in human macrophages and a down‐regulation of several pro‐inflammatory genes, including NF‐κB and TNF‐α (Chiang et al., 2012). In lung cancer cells, GPR32 also mediates the biological actions of RvD1 in preventing TGFβ1‐induced epithelial‐mesenchymal‐transition (Lee et al., 2013). Additionally, this receptor is involved in regulating macrophage phenotype and function, whereby it mediates RvD1‐initiated regulation of pro‐inflammatory cytokines IL‐1β and IL‐8. GPR32 is also involved in mediating the inhibitory actions of RvD1 on macrophage chemotaxis towards chemerin, fMLF and monocyte chemoattractant protein‐1 (also known as CCL2) (Schmid et al., 2016).
The biological actions of the E‐series resolvins, RvE1 and RvE2, are mediated by chemerin receptor 2 /resolvin E1 (chemerin1) receptor (ERV1; Arita et al., 2005; Oh et al., 2012). RvE1 signalling via the chemerin1 receptor (ERV1) leads to the activation of PI3K and ERK resulting in the phosphorylation of both Akt and the ribosomal protein S6 (Arita et al., 2005). The expression of this receptor in human diabetic patients is up‐regulated. Of note, despite increased expression of the RvE receptor in diabetic neutrophils, the biological actions of RvE1 in these patients are blunted suggesting that impaired/defective signalling of this receptor in diabetes may be a component in the ethiopathogenis of the disease (Freire et al., 2017). The expression of chemerin1 receptors (ERV1) in human monocyte‐derived macrophages was recently shown to be regulated by LPS and IFN‐γ, where incubation of monocyte‐derived macrophages with these inflammatory signals activates the promoter of these receptors (Herova et al., 2015).
GPR18 (resolvin D2 receptor; DRV2) is a receptor for RvD2. The binding of the agonist to its receptor leads to an increase in intracellular cAMP as well as CREB, ERK1/2 and STAT3 phosphorylation. Loss of this receptor in transgenic animals is associated with delayed resolution response to both sterile and infectious inflammatory insults (Chiang et al., 2015; Zhang et al., 2016) as well as increased mortality during polymicrobial sepsis (Chiang et al., 2017). Silencing of GPR18 (DRV2) on human macrophages also leads to a loss in their ability to clear bacteria and apoptotic cells, two key resolution responses, thereby underscoring the role of this RvD2‐GPR18 axis in restabilising tissue function following an inflammatory insult (Chiang et al., 2015; 2017).
SPM as allosteric modulators and antagonists
In addition to activating cognate receptors, SPM also regulate the activity of receptors for other endogenous mediators. LXA4 was recently found to be an endogenous allosteric modulator of the cannabinoid CB1 receptor (Pamplona et al., 2012). This SPM enhanced the affinity of anandamide at the CB1 receptor potentiating its biological actions without competing for the orthosteric binding site of the CB1 receptor and altering endocannabinoid metabolism (Figure 3). In addition, LXA4 displayed protective actions against β‐amyloid (1–40)‐induced spatial memory impairment, actions that were CB1 receptor‐dependent (Pamplona et al., 2012).
Some SPM also act as antagonists to receptors of pro‐inflammatory eicosanoids. RvE1, MaR1 and its further metabolite 22‐OH‐MaR1 are all competitive antagonists of BLT1 receptors (Figure 3) inhibiting the signalling and biological actions of the potent leukocyte chemoattractant LTB4 (Arita et al., 2005; El Kebir et al., 2012; Oh et al., 2012; Colas et al., 2016). In human neutrophils, BLT1 antagonism enhances neutrophil apoptosis (El Kebir et al., 2012). Of note, during self‐limiting infectious inflammation, MaR1 concentrations reach a maximum early on in the course of the inflammatory process, concomitant with a rise in LTB4 (Colas et al., 2016). This suggests that, MaR1 production may play an important role in antagonizing the actions of LTB4, thereby limiting neutrophil recruitment. These results also indicate that disruptions in the biosynthesis of this mediator may lead to delayed resolution of responses. Together, these findings highlight the utility of SPM as templates for the development of novel receptor modulators.
Harnessing SPM biology towards resolution pharmacology
SPM display potent biological actions without the side effects displayed by traditional anti‐inflammatories. The reason for this lack of unwanted side effects may be attributable to the mechanisms activated by SPM, which are distinct from those regulated by anti‐inflammatories. Indeed, SPM do not completely abolish the production and actions of molecules and cells considered to be inflammatory. Instead, these mediators reprogramme both the immune response and stromal cells limiting the production of inflammatory molecules and up‐regulate the expression of protective mediators (Mukherjee et al., 2004; Pamplona et al., 2012; Lee et al., 2013; Miyata et al., 2013; Dalli et al., 2014; Herova et al., 2015; Colby et al., 2016; Codagnone et al., 2018; de Oliveira et al., 2017). This reprogramming of the immune system is also linked to the apparent lack of immunosuppressive action. Thus, prompted by these observations, several studies have explored the potential of harnessing these actions to control excessive inflammation characteristic of many diseases that afflict modern societies (Takano et al., 1997; Leonard et al., 2002; Mitchell et al., 2002; Guilford et al., 2004).
From these efforts, several generations of analogues and mimetics are now developed (see Table 1) with the aim of obtaining molecules with enhanced pharmacokinetics and pharmacodynamics that are also amenable to scaled‐up synthesis (Takano et al., 1997; Leonard et al., 2002; Mitchell et al., 2002; Guilford et al., 2004). The first of these developed was the lipoxin analogues that retain the biological actions of their parent molecules but with enhanced stability. One of these analogues, a benzo‐LXA4, displays potent host protective actions that include the ability to regulate neutrophil responses and promote tissue repair and regeneration in periodontal disease (Van Dyke et al., 2015). These observations have now been extended to humans where the protective actions of this analogue are being investigated in the context of human periodontal disease (ClinicalTrials.gov Identifier: NCT02342691). A later generation analogue of RvE1 displays potent protective actions in dry eye syndrome in humans. In a phase 2 trial, this RvE1 analogue significantly reduced disease incidence, controlling dry eye‐related inflammation, findings that are under further investigation in a phase 3 trial (Wire, 2009.).
Table 1.
Biological actions of SPM analogues
| Analogue | Biological action | Dose | Reference |
|---|---|---|---|
| Lipoxin analogues | |||
| 15(R/S)‐methyl‐LXA4 |
Inhibits vascular permeability change and PMN infiltration Stimulates phagocytosis of apoptotic PMN by macrophages in vivo |
3–130 nM 2.5–10 μg·kg−1 |
(Takano et al., 1998) (Mitchell et al., 2002) |
| 16‐phenoxy‐LXA4 | Inhibits neutrophil infiltration in response to LTB4 | 240 nM | (Takano et al., 1997) |
| 15‐epi,16‐phenoxy‐LXA4 | Inhibits neutrophil infiltration in response to LTB4 | 240 nM | (Takano et al., 1997) |
| 16‐parafluoro‐phenoxy‐LXA4 | Inhibits PMN infiltration | 26 nM | (Takano et al., 1998) |
| 5(S)‐methyl‐LXB4 | Inhibits PMN infiltration | 26 nM | (Takano et al., 1998) |
| 15‐epi, 16‐para‐fluorophenoxy‐LXA4‐methyl ester |
Tissue protection and reduced neutrophil infiltration during kidney ischaemia–reperfusion. Reduces tissue IL‐1β, IL‐6, and GRO1 mRNA levels Prevents airway hyper‐responsiveness to methacholine Reduces eosinophils and lymphocytes infiltration Protects against vascular injury Reduces skin inflammation Inhibits human neutrophil chemotaxis Reduces colon inflammation Inhibits VEGF‐induced EC migration and proliferation |
15 μg per mouse 10 μg per mouse 100–1000 μg·cm−2 0.1 nM–1 μM 10 μg·mL−1 (p.o) 1–100 nM |
(Leonard et al., 2002) (Levy et al., 2002) (Schottelius et al., 2002) (Gewirtz et al., 2002) (Cezar‐de‐Mello et al., 2008) |
| o‐[9,12]‐benzo‐ω6‐epi‐lipoxin A4 |
Reducec leukocyte infiltration into the tempo mandibular joint following CFA administration Reduces neutrophil recruitment in response to zymosan Promotes regeneration of hard and soft tissues irreversibly lost to periodontitis in the Hanford miniature pig |
10 ng per mouse 100 ng per mouse 1 μg per site |
(Norling et al., 2011) (Van Dyke et al., 2015) |
| D‐series resolvin analogues | |||
| 7R/S methyl RvD1 methyl ester |
Reduces DC expression of MHC II, CD40 and IL‐12 following LPS stimulation Reduces allosensitization during corneal transplant and enhances graft survival and angiogenesis |
100 μg per mouse | (Hua et al., 2014) |
| Benzo‐diacetylenic‐17R‐RvD1‐methyl ester |
Shortens the resolution interval, Ri, during E. coli peritonitis Reduces ischaemia‐reperfusion‐initiated second organ injury |
100 ng per mouse 1 μg per mouse |
(Orr et al., 2015) |
| E‐series resolvin analogues | |||
| RX‐10045 |
Substrate/inhibitor for efflux transporters multidrug resistance‐associated protein, breast cancer‐resistant protein and organic cation transporter‐1 Reduces corneal opacity after haze‐generating after opacity‐generating high correction photorefractive keratectomy Reduction from baseline in controlled adverse environment‐induced staining of the central cornea Improvement in human dry eye disease symptoms, including dryness, stinging, burning, grittiness and ocular discomfort |
50–300 μM 0.01% solution 0.05–0.1% nanomicellar solution |
(Cholkar et al., 2015) (Torricelli et al., 2014) (Wire, 2009) |
|
α‐cyclopropane resolvin E2 |
Reduces the number of exudate leukocytes in response to Propionibacterium acnes infection | 300 fg–3 ng per mouse | (Fukuda et al., 2016) |
|
β‐cyclopropane resolvin E2 |
Reduces the number of exudate leukocytes in response to P. acnes infection | 300 fg–3 ng per mouse | (Fukuda et al., 2016) |
Additionally, recent studies have demonstrated the protective actions of analogues of RvD1 (Orr et al., 2015) and RvE2 (Fukuda et al., 2016). The RvD1 analogues retain the ability to activate GPR32 (DRV1) as well as the tissue protective actions of the parent SPM (Orr et al., 2015). Enhanced biological actions are also displayed the cyclopropane congeners of RvE2 (Fukuda et al., 2016), thereby supporting a role for these analogues as novel therapeutics.
Conclusion
The appreciation that resolution of inflammation is an active process and SPM are central in controlling both cellular trafficking and responses has opened up new and exciting horizons for the development of new therapeutics. Given that resolution pharmacology‐based medicines will harness endogenous reparative responses, it is anticipated that they will be burdened with lower side effects since they will not interfere with natural host immunity, potentially also increasing patient compliance. Furthermore, these findings shed new light on the roles of omega‐3 essential fatty acids in the control of acute inflammation. The protective actions observed in many clinical studies using these fatty acids are not simply due to the inhibition of inflammatory eicosanoid formation by competing for the biosynthetic enzymes. Indeed, these substrates are precursors to structurally unique molecules, the SPM, that carry potent tissue protective actions. Thus, these mediators may represent novel markers to determine the pharmacodynamics and pharmacokinetics of omega‐3 supplements and their ability to influence the host response in both healthy people as well as patients with inflammatory diseases. While the evidence in humans for the utility of this approach is still being investigated, results from animal and initial human experiments are encouraging (Dalli et al., 2013; Arnardottir et al., 2016; Barden et al., 2016; Markworth et al., 2016; Gobbetti et al., 2017). Therefore, resolution‐based therapeutics provide an exciting new paradigm for personalized medicine where supplementation can be utilized to maintain/boost endogenous SPM levels to preserve tissue resolution tone and health. Whereas in disease settings, SPM‐based drugs may be useful in regulating host responses to both local and systemic inflammation. In this context, nanomedicines enriched in SPM or their analogues/mimetics (Norling et al., 2011) may provide a tractable system for targeted tissue delivery that can control both inflammation and promote tissue repair and regeneration (Van Dyke et al., 2015). Hence, resolution pharmacology could provide the basis for reprograming host immunity in order to expedite microbial clearance, limit collateral tissue damage and stimulate tissue regeneration.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018) and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c,d).
Conflict of interest
The authors declare no financial conflicts of interest.
Acknowledgements
Studies reviewed here from the authors' laboratories were supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (grant number 107613/Z/15/Z) and the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant number 677542) to J.D. Studies reviewed from the CN Serhan laboratories were supported by National Institutes of Health, USA (grant numbers R01GM38765 and P01GM095467).
Dalli, J. , and Serhan, C. N. (2019) Identification and structure elucidation of the pro‐resolving mediators provides novel leads for resolution pharmacology. British Journal of Pharmacology, 176: 1024–1037. 10.1111/bph.14336.
References
- Abdulnour RE, Dalli J, Colby JK, Krishnamoorthy N, Timmons JY, Tan SH et al (2014). Maresin 1 biosynthesis during platelet‐neutrophil interactions is organ‐protective. Proc Natl Acad Sci U S A 111: 16526–16531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. Br J Pharmacol 174: S225–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017d). The Concise Guide to PHARMACOLOGY 2017/18: Overview. Br J Pharmacol 174: S1–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S et al (2005). Stereochemical assignment, antiinflammatory properties, and receptor for the omega‐3 lipid mediator resolvin E1. J Exp Med 201: 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnardottir HH, Dalli J, Norling LV, Colas RA, Perretti M, Serhan CN (2016). Resolvin D3 is dysregulated in arthritis and reduces arthritic inflammation. J Immunol 197: 2362–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aursnes M, Tungen JE, Vik A, Colas R, Cheng CY, Dalli J et al (2014). Total synthesis of the lipid mediator PD1n‐3 DPA: configurational assignments and anti‐inflammatory and pro‐resolving actions. J Nat Prod 77: 910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH et al (2005). Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol 174: 4345–4355. [DOI] [PubMed] [Google Scholar]
- Barden AE, Moghaddami M, Mas E, Phillips M, Cleland LG, Mori TA (2016). Specialised pro‐resolving mediators of inflammation in inflammatory arthritis. Prostaglandins Leukot Essent Fatty Acids 107: 24–29. [DOI] [PubMed] [Google Scholar]
- Cezar‐de‐Mello PF, Vieira AM, Nascimento‐Silva V, Villela CG, Barja‐Fidalgo C, Fierro IM (2008). ATL‐1, an analogue of aspirin‐triggered lipoxin A4, is a potent inhibitor of several steps in angiogenesis induced by vascular endothelial growth factor. Br J Pharmacol 153: 956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Dalli J, Colas RA, Serhan CN (2015). Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med 212: 1203–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, de la Rosa X, Libreros S, Serhan CN (2017). Novel resolvin D2 receptor axis in infectious inflammation. J Immunol 198: 842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA et al (2012). Infection regulates pro‐resolving mediators that lower antibiotic requirements. Nature 484: 524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholkar K, Trinh HM, Vadlapudi AD, Wang Z, Pal D, Mitra AK (2015). Interaction studies of resolvin E1 analog (RX‐10045) with efflux transporters. J Ocul Pharmacol Ther 31: 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codagnone M, Cianci E, Lamolinara A, Mari VC, Nespoli A, Isopi E et al (2018). Resolvin D1 enhances the resolution of lung inflammation caused by long‐term Pseudomonas aeruginosa infection. Mucosal Immunol 11: 35–49. [DOI] [PubMed] [Google Scholar]
- Colas RA, Dalli J, Chiang N, Vlasakov I, Sanger JM, Riley IR et al (2016). Identification and actions of the maresin 1 metabolome in infectious inflammation. J Immunol 197: 4444–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas RA, Souza PR, Walker ME, Burton M, Marques RM, Zasłona Z Curtis AM et al (2018). Impaired production and diurnal regulation of vascular RvDn‐3 DPA increase systemic inflammation and cardiovascular disease. Circ Res 122: 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby JK, Abdulnour RE, Sham HP, Dalli J, Colas RA, Winkler JW et al (2016). Resolvin D3 and aspirin‐triggered resolvin D3 are protective for injured epithelia. Am J Pathol 186: 1801–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooray SN, Gobbetti T, Montero‐Melendez T, McArthur S, Thompson D, Clark AJ et al (2013). Ligand‐specific conformational change of the G‐protein‐coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci U S A 110: 18232–18237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J (2017). Does promoting resolution instead of inhibiting inflammation represent the new paradigm in treating infections? Mol Aspects Med 58: 12–20. [DOI] [PubMed] [Google Scholar]
- Dalli J, Chiang N, Serhan CN (2014). Identification of 14‐series sulfido‐conjugated mediators that promote resolution of infection and organ protection. Proc Natl Acad Sci U S A 111: E4753–E4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Chiang N, Serhan CN (2015a). Elucidation of novel 13‐series resolvins that increase with atorvastatin and clear infections. Nat Med 21: 1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Colas RA, Arnardottir H, Serhan CN (2017). Vagal regulation of group 3 innate lymphoid cells and the immunoresolvent PCTR1 controls infection resolution. Immunity 46: 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Colas RA, Serhan CN (2013). Novel n‐3 immunoresolvents: structures and actions. Sci Rep 3: 1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Ramon S, Norris PC, Colas RA, Serhan CN (2015b). Novel proresolving and tissue‐regenerative resolvin and protectin sulfido‐conjugated pathways. FASEB J 29: 2120–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, Chiang N et al (2013a). Resolvin D3 and aspirin‐triggered resolvin D3 are potent immunoresolvents. Chem Biol 20: 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Zhu M, Vlasenko NA, Deng B, Haeggstrom JZ, Petasis NA et al (2013b). The novel 13S,14S‐epoxy‐maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J 27: 2573–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira JR, da Silva PR, Rogerio AP (2017). AT‐RvD1 modulates the activation of bronchial epithelial cells induced by lipopolysaccharide and Dermatophagoides pteronyssinus. Eur J Pharmacol 805: 46–50. [DOI] [PubMed] [Google Scholar]
- Deng B, Wang CW, Arnardottir HH, Li Y, Cheng CY, Dalli J et al (2014). Maresin biosynthesis and identification of maresin 2, a new anti‐inflammatory and pro‐resolving mediator from human macrophages. PLoS One 9: e102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyama S, Shimoda K, Suzuki H, Ishikawa Y, Ishimura K, Fukuda H et al (2018). Resolvin E1/E2 ameliorate lipopolysaccharide‐induced depression‐like behaviors via ChemR23. Psychopharmacology (Berl) 235: 329–336. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Joosten LA (2016). Inflammation in rheumatology in 2015: new tools to tackle inflammatory arthritis. Nat Rev Rheumatol 12: 78–80. [DOI] [PubMed] [Google Scholar]
- El Kebir D, Gjorstrup P, Filep JG (2012). Resolvin E1 promotes phagocytosis‐induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc Natl Acad Sci U S A 109: 14983–14988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore S, Maddox JF, Perez HD, Serhan CN (1994). Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med 180: 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore S, Serhan CN (1995). Lipoxin A4 receptor activation is distinct from that of the formyl peptide receptor in myeloid cells: inhibition of CD11/18 expression by lipoxin A4‐lipoxin A4 receptor interaction. Biochemistry 34: 16678–16686. [DOI] [PubMed] [Google Scholar]
- Fonseca FC, Orlando RM, Turchetti‐Maia RM, de Francischi JN (2017). Comparative effects of the omega3 polyunsaturated fatty acid derivatives resolvins E1 and D1 and protectin DX in models of inflammation and pain. J Inflamm Res 10: 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francos‐Quijorna I, Santos‐Nogueira E, Gronert K, Sullivan AB, Kopp MA, Brommer B et al (2017). Maresin 1 promotes inflammatory resolution, neuroprotection, and functional neurological recovery after spinal cord injury. J Neurosci 37: 11731–11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire MO, Dalli J, Serhan CN, Van Dyke TE (2017). Neutrophil resolvin E1 receptor expression and function in type 2 diabetes. J Immunol 198: 718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H, Muromoto R, Takakura Y, Ishimura K, Kanada R, Fushihara D et al (2016). Design and synthesis of cyclopropane congeners of resolvin E2, an endogenous proresolving lipid mediator, as its stable equivalents. Org Lett 18: 6224–6227. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD (2005). Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J Immunol 174: 5033–5039. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, Collier‐Hyams LS, Young AN, Kucharzik T, Guilford WJ, Parkinson JF et al (2002). Lipoxin a4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate‐induced colitis. J Immunol 168: 5260–5267. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Colville‐Nash PR, Willis D, Chivers J, Paul‐Clark MJ, Willoughby DA (1999). Inducible cyclooxygenase may have anti‐inflammatory properties. Nat Med 5: 698–701. [DOI] [PubMed] [Google Scholar]
- Gobbetti T, Dalli J, Colas RA, Federici Canova D, Aursnes M, Bonnet D et al (2017). Protectin D1n‐3 DPA and resolvin D5n‐3 DPA are effectors of intestinal protection. Proc Natl Acad Sci U S A 114: 3963–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Cryer B (2015). Gastrointestinal injury associated with NSAID use: a case study and review of risk factors and preventative strategies. Drug Healthc Patient Saf 7: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford WJ, Bauman JG, Skuballa W, Bauer S, Wei GP, Davey D et al (2004). Novel 3‐oxa lipoxin A4 analogues with enhanced chemical and metabolic stability have anti‐inflammatory activity in vivo. J Med Chem 47: 2157–2165. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayhoe RP, Kamal AM, Solito E, Flower RJ, Cooper D, Perretti M (2006). Annexin 1 and its bioactive peptide inhibit neutrophil‐endothelium interactions under flow: indication of distinct receptor involvement. Blood 107: 2123–2130. [DOI] [PubMed] [Google Scholar]
- Herova M, Schmid M, Gemperle C, Hersberger M (2015). ChemR23, the receptor for chemerin and resolvin E1, is expressed and functional on M1 but not on M2 macrophages. J Immunol 194: 2330–2337. [DOI] [PubMed] [Google Scholar]
- Hodges RR, Li D, Shatos MA, Serhan CN, Dartt DA (2016). Lipoxin A4 counter‐regulates histamine‐stimulated glycoconjugate secretion in conjunctival goblet cells. Sci Rep 6: 36124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Jin Y, Chen Y, Inomata T, Lee H, Chauhan SK et al (2014). The resolvin D1 analogue controls maturation of dendritic cells and suppresses alloimmunity in corneal transplantation. Invest Ophthalmol Vis Sci 55: 5944–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe Y, Arita M, Iwamoto R, Urabe D, Todoroki H, Masuda K et al (2013). Stereochemical assignment and anti‐inflammatory properties of the omega‐3 lipid mediator resolvin E3. J Biochem 153: 355–360. [DOI] [PubMed] [Google Scholar]
- Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H et al (2012). Identification and structure determination of novel anti‐inflammatory mediator resolvin E3, 17,18‐dihydroxyeicosapentaenoic acid. J Biol Chem 287: 10525–10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YY, Lee JY, Park CK (2016). Resolvin E1 inhibits substance P‐induced potentiation of TRPV1 in primary sensory neurons. Mediators Inflamm 2016: 5259321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN (2012). Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol 180: 2018–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Abbas AK, Aster JC (2014). Robbins and Cotran Pathologic Basis of Disease, Ninth edn., Elsevier: Philadelphia USA. [Google Scholar]
- Lee HJ, Park MK, Lee EJ, Lee CH (2013). Resolvin D1 inhibits TGF‐beta1‐induced epithelial mesenchymal transition of A549 lung cancer cells via lipoxin A4 receptor/formyl peptide receptor 2 and GPR32. Int J Biochem Cell Biol 45: 2801–2807. [DOI] [PubMed] [Google Scholar]
- Leonard MO, Hannan K, Burne MJ, Lappin DW, Doran P, Coleman P et al (2002). 15‐Epi‐16‐(para‐fluorophenoxy)‐lipoxin A(4)‐methyl ester, a synthetic analogue of 15‐epi‐lipoxin A(4), is protective in experimental ischemic acute renal failure. J Am Soc Nephrol 13: 1657–1662. [DOI] [PubMed] [Google Scholar]
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN (2001). Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol 2: 612–619. [DOI] [PubMed] [Google Scholar]
- Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA et al (2002). Multi‐pronged inhibition of airway hyper‐responsiveness and inflammation by lipoxin A(4). Nat Med 8: 1018–1023. [DOI] [PubMed] [Google Scholar]
- Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S et al (2007). Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol 178: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livne‐Bar I, Wei J, Liu HH, Alqawlaq S, Won GJ, Tuccitto A et al (2017). Astrocyte‐derived lipoxins A4 and B4 promote neuroprotection from acute and chronic injury. J Clin Invest 127: 4403–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K et al (2005). A role for docosahexaenoic acid‐derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest 115: 2774–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G (1991). The ancient riddle of sigma eta psi iota sigma (sepsis). J Infect Dis 163: 937–945. [DOI] [PubMed] [Google Scholar]
- Malagoli D (2016). The Evolution of the Immune System: Conservation and Diversification, Elsevier: London, UK. [Google Scholar]
- Markworth JF, Kaur G, Miller EG, Larsen AE, Sinclair AJ, Maddipati KR et al (2016). Divergent shifts in lipid mediator profile following supplementation with n‐3 docosapentaenoic acid and eicosapentaenoic acid. FASEB J 30: 3714–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minozzi S, Bonovas S, Lytras T, Pecoraro V, Gonzalez‐Lorenzo M, Bastiampillai AJ et al (2016). Risk of infections using anti‐TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta‐analysis. Expert Opin Drug Saf 15: 11–34. [DOI] [PubMed] [Google Scholar]
- Mitchell S, Thomas G, Harvey K, Cottell D, Reville K, Berlasconi G et al (2002). Lipoxins, aspirin‐triggered epi‐lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol 13: 2497–2507. [DOI] [PubMed] [Google Scholar]
- Miyata J, Fukunaga K, Iwamoto R, Isobe Y, Niimi K, Takamiya R et al (2013). Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. J Allergy Clin Immunol 131: 353–360 e351–e352. [DOI] [PubMed] [Google Scholar]
- Mottola G, Chatterjee A, Wu B, Chen M, Conte MS (2017). Aspirin‐triggered resolvin D1 attenuates PDGF‐induced vascular smooth muscle cell migration via the cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) pathway. PLoS One 12: e0174936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG (2004). Neuroprotectin D1: a docosahexaenoic acid‐derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A 101: 8491–8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN (2011). Cutting edge: humanized nano‐proresolving medicines mimic inflammation‐resolution and enhance wound healing. J Immunol 186: 5543–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, Serhan CN (2012). Resolvin E2 formation and impact in inflammation resolution. J Immunol 188: 4527–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SK, Colas RA, Dalli J, Chiang N, Serhan CN (2015). Proresolving actions of a new resolvin D1 analog mimetic qualifies as an immunoresolvent. Am J Physiol Lung Cell Mol Physiol 308: L904–L911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz‐Munoz G, Mallavia B, Bins A, Headley M, Krummel MF, Looney MR (2014). Aspirin‐triggered 15‐epi‐lipoxin A4 regulates neutrophil‐platelet aggregation and attenuates acute lung injury in mice. Blood 124: 2625–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona FA, Ferreira J, Menezes de Lima O Jr, Duarte FS, Bento AF, Forner S et al (2012). Anti‐inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc Natl Acad Sci U S A 109: 21134–21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primdahl KG, Aursnes M, Walker ME, Colas RA, Serhan CN, Dalli J et al (2016). Synthesis of 13(R)‐Hydroxy‐7Z,10Z,13R,14E,16Z,19Z docosapentaenoic acid (13R‐HDPA) and its biosynthetic conversion to the 13‐series resolvins. J Nat Prod 79: 2693–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss H, Rosche B, Sullivan AB, Brommer B, Wengert O, Gronert K et al (2013). Proresolution lipid mediators in multiple sclerosis – differential, disease severity‐dependent synthesis – a clinical pilot trial. PLoS One 8: e55859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasagi NK, Reddy PB, Mulik S, Gjorstrup P, Rouse BT (2013). Neuroprotectin D1 reduces the severity of herpes simplex virus‐induced corneal immunopathology. Invest Ophthalmol Vis Sci 54: 6269–6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathod KS, Kapil V, Velmurugan S, Khambata RS, Siddique U, Khan S et al (2017). Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. J Clin Invest 127: 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rius B, Duran‐Guell M, Flores‐Costa R, Lopez‐Vicario C, Lopategi A, Alcaraz‐Quiles J et al (2017). The specialized proresolving lipid mediator maresin 1 protects hepatocytes from lipotoxic and hypoxia‐induced endoplasmic reticulum stress. FASEB J 31: 5384–5398. [DOI] [PubMed] [Google Scholar]
- Robbins SL, Cotran RS (1979). Pathologic Basis of Disease, 2nd edn. W. B. Saunders Co.: Philadelphia. [Google Scholar]
- Romano M, Cianci E, Simiele F, Recchiuti A (2015). Lipoxins and aspirin‐triggered lipoxins in resolution of inflammation. Eur J Pharmacol 760: 49–63. [DOI] [PubMed] [Google Scholar]
- Rovira X, Pin JP, Giraldo J (2010). The asymmetric/symmetric activation of GPCR dimers as a possible mechanistic rationale for multiple signalling pathways. Trends Pharmacol Sci 31: 15–21. [DOI] [PubMed] [Google Scholar]
- Samuelsson B (2012). Role of basic science in the development of new medicines: examples from the eicosanoid field. J Biol Chem 287: 10070–10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Gemperle C, Rimann N, Hersberger M (2016). Resolvin D1 polarizes primary human macrophages toward a proresolution phenotype through GPR32. J Immunol 196: 3429–3437. [DOI] [PubMed] [Google Scholar]
- Schottelius AJ, Giesen C, Asadullah K, Fierro IM, Colgan SP, Bauman J et al (2002). An aspirin‐triggered lipoxin A4 stable analog displays a unique topical anti‐inflammatory profile. J Immunol 169: 7063–7070. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN (2007). Resolvin E1 and protectin D1 activate inflammation‐resolution programmes. Nature 447: 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Dalli J (2017). New pro‐resolving n‐3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol Aspects Med . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K (2000). Novel functional sets of lipid‐derived mediators with antiinflammatory actions generated from omega‐3 fatty acids via cyclooxygenase 2‐nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med 192: 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ et al (2012). Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J 26: 1755–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hamberg M, Samuelsson B (1984). Trihydroxytetraenes: a novel series of compounds formed from arachidonic acid in human leukocytes. Biochem Biophys Res Commun 118: 943–949. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G et al (2002). Resolvins: a family of bioactive products of omega‐3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 196: 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF et al (2009). Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med 206: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Clish CB, Gronert K, Petasis N, Serhan CN (1998). Neutrophil‐mediated changes in vascular permeability are inhibited by topical application of aspirin‐triggered 15‐epi‐lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest 101: 819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN (1997). Aspirin‐triggered 15‐epi‐lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti‐inflammatory receptors. J Exp Med 185: 1693–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AA, Santhanam A, Agrawal V, Wilson SE (2014). Resolvin E1 analog RX‐10045 0.1% reduces corneal stromal haze in rabbits when applied topically after PRK. Mol Vis 20: 1710–1716. [PMC free article] [PubMed] [Google Scholar]
- Tungen JE, Aursnes M, Dalli J, Arnardottir H, Serhan CN, Hansen TV (2014). Total synthesis of the anti‐inflammatory and pro‐resolving lipid mediator MaR1n‐3 DPA utilizing an sp(3) ‐sp(3) Negishi cross‐coupling reaction. Chemistry 20: 14575–14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke TE, Hasturk H, Kantarci A, Freire MO, Nguyen D, Dalli J et al (2015). Proresolving nanomedicines activate bone regeneration in periodontitis. J Dent Res 94: 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola JR, Lemnitzer P, Jansen Y, Csaba G, Winter C, Neideck C et al (2016). Resolving lipid mediators maresin 1 and resolvin D2 prevent atheroprogression in mice. Circ Res 119: 1030–1038. [DOI] [PubMed] [Google Scholar]
- Walker ME, Souza PR, Colas RA, Dalli J (2017). 13‐Series resolvins mediate the leukocyte‐platelet actions of atorvastatin and pravastatin in inflammatory arthritis. FASEB J 31: 3636–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZF, Li Q, Liu SB, Mi WL, Hu S, Zhao J et al (2014). Aspirin‐triggered Lipoxin A4 attenuates mechanical allodynia in association with inhibiting spinal JAK2/STAT3 signaling in neuropathic pain in rats. Neuroscience 273: 65–78. [DOI] [PubMed] [Google Scholar]
- Winkler JW, Orr SK, Dalli J, Cheng CY, Sanger JM, Chiang N et al (2016). Resolvin D4 stereoassignment and its novel actions in host protection and bacterial clearance. Sci Rep 6: 18972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wire B (2009). Resolvyx announces positive data from Phase 2 clinical trial of the reesolvin RX‐10045 in patients with dry eye syndrome. Available at: http://www. businesswire.com/news/home/20090824005320/en/Resolvyx‐AnnouncesPositive‐Data‐Phase‐20090824005322‐Clinical.
- Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R et al (2010). Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med 16: 592–597 591p following 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Tani Y, Nakanishi H, Taguchi R, Arita M, Arai H (2011). Eosinophils promote resolution of acute peritonitis by producing proresolving mediators in mice. FASEB J 25: 561–568. [DOI] [PubMed] [Google Scholar]
- Zhang MJ, Sansbury BE, Hellmann J, Baker JF, Guo L, Parmer CM et al (2016). Resolvin D2 enhances postischemic revascularization while resolving inflammation. Circulation 134: 666–680. [DOI] [PMC free article] [PubMed] [Google Scholar]


