Introduction
Adenine phosphoribosyltransferase (APRT) deficiency is an autosomal recessive condition that can cause symptoms ranging from recurrent urolithiasis to crystalline nephropathy and may cause irreversible renal damage. The deficiency of the APRT enzyme leads to accumulation of adenine, which is converted by xanthine oxidoreductase to highly insoluble 2,8-dihydroxyadenine (DHA). The DHA is precipitated in urine and may be deposited in the renal tubules and interstitium, leading to wide array of clinical symptoms. The disease is underdiagnosed, and many patients have irreversible kidney damage by the time it is diagnosed.1
Recurrent/multiple kidney stone disease or nephrocalcinosis, particularly in pre-pubertal children, should alert the physician to the possibility of a monogenic underlying disorder, such as APRT deficiency. The lack of recognition and knowledge of monogenic causes of kidney stone disease/crystal nephropathy and chronic kidney disease (CKD), has frequently resulted in an unacceptable delay in diagnosis and treatment, sometimes with serious consequences . Recent reports point toward the risk of renal allograft loss in patients with undiagnosed APRT deficiency. A high index of suspicion coupled with an early diagnosis may reduce or even prevent the serious long-term complications of these "monogenic stone/crystal nephropathy diseases.” We describe here a child with a novel mutation of the aprt gene at the homozygous state (c.526_530del) with a follow-up at 36 months.
Case Presentation
A 10-year-old boy presented to the pediatric emergency with acute retention of urine. The patient had complained of abdomen pain on and off for the past 6 months. There was no history of fever, dysuria, gross hematuria, foul smell in urine, or poor stream of urine. His vitals were as follows: heart rate 90/min, respiratory rate 20/min, blood pressure 102/64 mm Hg. The weight and height of the patient were between −1 SD and −2 SD for age. There were no signs of rickets. The patient was admitted at 5 years of age in another hospital with similar complaints. He did not require catheterization then and had spontaneously passed 4 to 6 stones that were red in color but stone analysis was not done. There was history of second-degree consanguinity among parents. There was no history of renal stone and CKD in the family. Urine routine examination showed full field of red blood cells, 10 to 12 pus cells, no albumin. Serum creatinine was 70.74 μmol/l. The child was found to have bilateral renal calculi. The investigative profile of the patient is described in Table 1.
Table 1.
Investigative profile of the patient
| Investigation | Report |
|---|---|
| Blood urea nitrogen (mmol/l) | 7.14 |
| Serum creatinine (μmol/l) | 70.74 |
| Serum calcium (mmol/l) | 2.2 |
| Serum phosphate (mmol/l) | 1.45 |
| Alkaline phosphatase (IU/l) | 201 |
| Serum uric acid (mmol/l) | 0.21 |
| Ultrasonogram abdomen | Normal-sized left kidney with multiple calculi largest 3.5 cm and hydroureteronephrosis; small-sized right kidney with 13-m calculus at lower pole. |
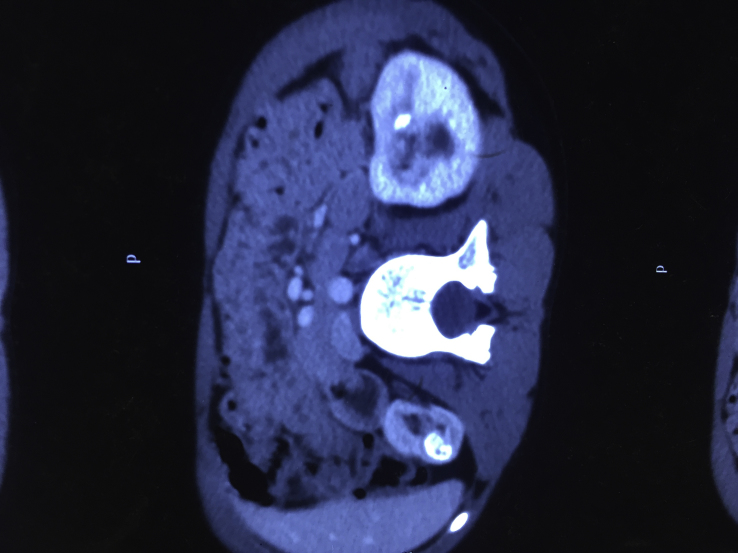
| Noncontrast computed tomogram | Small right kidney with multiple calculi at upper, mid, and lower pole calyx. Large staghorn calculus in left renal pelvis with multiple calculi at upper, mid, and lower pole calyces along with hydroureteronephrosis. |
| 99m Tc-diethylene-triamine-pentaacetate | Differential function of right kidney: 23%; left kidney: 77%; global glomerular filtration rate: 71.6 ml/min. |
| Nephrostogram | Grade 3 hydronephrosis in left kidney with fullness of pelvis with narrowing of caliber of left upper ureter and passage of contrast in distal ureter suggestive of partial stricture in left upper ureter. |
Left pyelolithotomy with end trans-anastomotic nephrostomy with bilateral DJ stent insertion was done. Later stents were removed sequentially. The stone removed during surgery was analyzed by Fourier Transform Infrared Spectroscopy and revealed the composition of 100% 2,8-Dihydroxyadenine (Figure 1, Figure 2). APRT activity in red blood cells was undetectable, and the genetic study revealed a homozygous mutation in exon 5 of aprt gene:c.526–530del. This deletion of 5 base pairs creates a frame shift from the codon Leu 76 leading to a STOP codon: p.Leu176Alafs*3. The patient was advised with high fluid intake and oral allopurinol at a dosage of 10 mg/kg per day and left percutaneous nephrolithotomy was done. The child has been asymptomatic for the past 3 years. No crystals were detected on urine examination and he had an estimated glomerular filtration rate of 86 ml/min per 1.73 m2. Repeat ultrasound showed right kidney size of 7.7 × 2.5 cm, with multiple small calculi at lower pole calyx with mild splitting of pelvicalyceal system and left kidney of size 9.0 × 4.7 cm with mild splitting of pelvicalyceal system.
Figure 1.
Red-colored stone, measuring 8 × 10 mm, with a soft friable surface and irregular surface.
Figure 2.
Noncontrast computed tomogram showing renal stones.
Discussion
APRT deficiency is a rare but treatable disorder of crystalline nephropathy, with a wide clinical spectrum. Our case illustrates the recurrent formation of multiple kidney stones in a patient with APRT deficiency and identification of a novel mutation that abolishes the APRT activity. The addition of novel mutation to the biogenomic database of APRT deficiency helps the scientists in assessing the prevalence of APRT deficiency.
APRT deficiency is an underrecognized cause of kidney stones and crystalline nephropathy, which can progress to end-stage renal disease in a significant proportion of untreated individuals.2 Recurrent kidney stones and/or nephrocalcinosis in children should alert the physician to the possibility of an inborn error of purine metabolism as the underlying cause. This is the third pediatric case of APRT deficiency from India. Krishnappa et al.3 described a 2-year-old girl born out of a consanguineous marriage who was diagnosed to have APRT deficiency. The girl presented with gross hematuria and flank pain and 1 episode of febrile urinary tract infection. The diagnosis was established by stone analysis, which showed 100% 2,8-DHA and the presence of 2,8-DHA crystals with a typical maltese cross pattern in urine sediments. The genetic analysis revealed novel mutation in exon 1 of the aprt gene with “c.2T>C” variant.
In another case report, a 3-year-old girl who presented with an acute history of urinary retention and renal failure was diagnosed with APRT deficiency.4 The diagnosis was established by performing stone analysis using Fourier Transform Infrared Spectroscopy, demonstrating 100% 2,8-DHA; however, mutational analysis was not done. All 3 patients, including ours, responded to allopurinol, with improvement in renal function tests but with persistence of small stones in the kidney. The genetic analysis in our case revealed homozygous mutation in exon 5 of the aprt gene: c.526–530del, which has not been described earlier.
A number of cases of children with APRT deficiency have been described in the literature with the age at initial presentation as early as 12 months.5 Study of 21 pediatric patients of APRT deficiency revealed that the median age for diagnosis was 3 years and the clinical symptoms were present in 28% of the patients during infancy. The initial presentation in most children was passage of stones in urine, with the diagnosis established within 3 months of first passage of stones in 75% of the cases.2
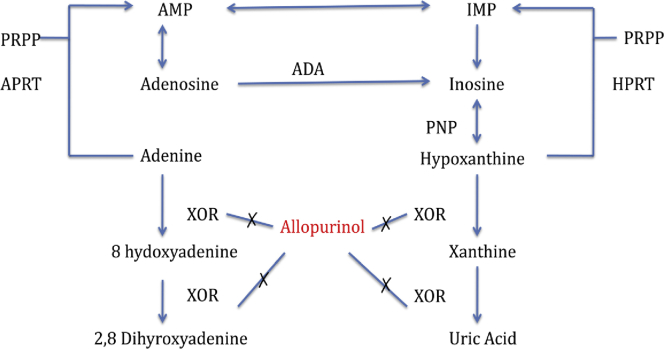
In APRT deficiency, the salvage of the adenine cannot be performed, and it is oxidized to another derivative 2,8-DHA by xanthine oxidoreductase via the 8-hydroxy-intermediate (Figure 3). As 2,8-DHA is extremely insoluble in urine, it is precipitated in urine, forming crystals, which aggregate into stones. The stones could be excreted in urine or may cause obstruction, whereas the crystals may precipitate in either renal parenchyma, interstitium, or tubules causing nephropathy, termed crystalline nephropathy.1
Figure 3.
In adenine phosphoribosyltransferase (APRT) deficiency, the adenine cannot be converted to adenosine. The alternative pathway converts the adenine to 2,8-dihydroxyadenine (2,8-DHA), which is highly insoluble in urine and precipitates in urine causing urolithiasis and in tubules causing DHA nephropathy. Allopurinol inhibits the xanthine oxidoreductase (XOR), thus preventing the conversion to 2,8 DHA. ADA, adenosine deaminase; AMP, adenine monophosphate; HPRT, hypoxaanthinephophoribosyltransfrase; IMP, inosinemonophosphate; PNP, purine nucleoside phosphorylase; PRPP, 5-phosphoribosyl-1-pyrophosphte.
Because of the highly insoluble nature of the DHA crystals, they precipitate in renal tubules and interstitium causing impaired renal function. This crystalline nephropathy has been termed “DHA nephropathy.” Acute kidney injury may be present, especially in conditions of dehydration, which causes oliguria, urinary super saturation with DHA, and massive precipitation of crystals. In some patients, bilateral stones may be present, which can cause obstruction and acute kidney injury.2 However, the more common presentation of DHA nephropathy is progressive decline of renal function over years. DHA nephropathy usually occurs in patients who are not diagnosed timely or where appropriate treatment is not initiated, but may occur in patients who have experienced only a few episodes. In a recent observational study of patients enrolled in the APRT Deficiency Registry of Rare Kidney Stone Consortium,6 it was found that in 20 patients of APRT deficiency with CKD stage 3 to 5, 45% of patients did not have any history of kidney stones.
Clinical recognition of the APRT deficiency is challenging because of the rarity of the disease and a variable course. Frequent lack of awareness and the delay in diagnosis, as seen in our case, leads to CKD and its complications in children with APRT deficiency. Either stone analysis or examination of crystals in urine for the demonstration of DHA is useful for the diagnosis of APRT deficiency. DHA stones are radiolucent and they need to be differentiated from uric acid stones. Presence of calcium may cause some of the DHA stones to be radiopaque. Biochemical stone analysis fails to differentiate uric acid stones from DHA stones, making it an unreliable tool for diagnosis of APRT deficiency. The stone analysis is done by using a stereomicroscope for morphological examination and infrared spectroscopy.7 If the stone is not available for analysis, detection of 2,8-DHA crystals in urine can point to the diagnosis. Crystals are most likely to be detected on the first voided morning urine samples, as they are the most concentrated. The crystals are round and reddish brown on regular light microscopy, and polarized light microscopy reveals the characteristic maltese cross pattern. Crystal identification is an inexpensive, noninvasive, and very useful tool for the identification of DHA crystals. As identification of urinary DHA crystals is subject to error, it is generally considered suggestive of the disorder, not confirmatory. Quantification can be done by counting the number of crystals per unit volume, which is high in untreated patients. A newer technique of identifying DHA crystals using high performance liquid chromatography–linked assay and liquid chromatography-electrospray assay tandem mass spectrometry can be used, but none of these have been established for use in clinical practice. Recently an ultra-performance liquid chromatography-tandem mass spectrometry has been developed for absolute quantification of urinary DHA, using isotopically labeled DHA as an internal standard.8 The gold standard technique for diagnosing APRT deficiency is measuring APRT enzyme activity, and detection of bi-allelic pathogenic mutations can also confirm the diagnosis.
Renal biopsy is not required for the diagnosis if 2,8-DHA crystals are identified in the urine. However, in cases with unexplained renal failure, it often leads to the diagnosis of DHA nephropathy. Renal biopsy shows tubular damage and precipitation of crystals in tubular lumen and renal interstitium. The best method is to characterize crystals in renal biopsy using polarizing microscopy and Fourier transform infrared microscopy.
Molecular genetic testing for detection of mutations in APRT gene by sequencing of polymerase chain reaction–amplified DNA is used for the confirmation of diagnosis. As it is an autosomal recessive condition, the siblings of the proband have a 25% chance of being affected, hence they should be investigated, whether they are symptomatic or not. The siblings should be screened for crystalluria, and APRT assays should be done, if possible. Urine microscopy should be performed in all the at-risk relatives, but it should not be used alone as a screening tool. In individuals with biallelic mutations, assessment of renal functions and imaging studies are warranted.
The mainstay of treatment is medical treatment with allopurinol. It blocks the xanthine oxidoreductase enzyme and hence prevents the formation of 2,8-DHA. In patients with established DHA nephropathy, treatment with therapeutic doses of allopurinol can preserve or even improve kidney function in some cases.1 The recovery of the renal function depends on the amount of interstitial damage and tubular injury at presentation before starting the treatment. Allopurinol should be administered even to the patients who are asymptomatic, as they are at risk for DHA nephropathy. The sufficient dose of allopurinol to halt the progression of the disease has not been established. In a recent clinical trial, it was revealed that even patients treated with 400 mg of allopurinol had a quantifiable amount of DHA in their urine.9 Patients with this disorder are successfully treated with up to 600 mg of allopurinol per day. Further testing is needed to establish an adequate dosage of allopurinol required for patients with APRT deficiency. Allopurinol should be started with low doses, as higher doses may increase the risk for allopurinol hypersensitivity syndrome. Dosing adaptation is required in patients with impaired renal function tests. Treatment is generally well tolerated and lifelong. The side effects include itching, hair loss, and ocular symptoms. In patients who are intolerant to allopurinol, febuxostat may be used as an alternative. For therapeutic monitoring, assessment of DHA crystalluria is used. The absence of DHA crystals is considered indicative of adequate treatment. Recurrence occurs very rarely in patients who receive adequate treatment. Poor compliance with the medical treatment and inadequate dosing are the major reasons for recurrence in most of the cases and should be checked.
Conclusion
APRT is a rare, but easily treatable disorder. The lack of recognition and knowledge often lead to a missed or late diagnosis of the disease. Early diagnosis and treatment initiation can reduce the new stone formation and the rate of CKD progression or may even improve the kidney function in patients with APRT deficiency, hence the necessity for a timely diagnosis. Some learning points are listed in Table 2.
Table 2.
Teaching points in the case
|
|
|
|
|
Disclosure
All the authors declared no competing interests.
References
- 1.Bollée G., Dollinger C., Boutaud L. Phenotype and genotype characterization of adenine phosphoribosyltransferase deficiency. J Am Soc Nephrol. 2010;21:679–688. doi: 10.1681/ASN.2009080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harambat J., Bollée G., Daudon M. Adenine phosphoribosyltransferase deficiency in children. Pediatr Nephrol. 2012;27:571–579. doi: 10.1007/s00467-011-2037-0. [DOI] [PubMed] [Google Scholar]
- 3.Krishnappa P., Krishnamoorthy V., Gowda K.K. Dihydroxyadenine stone with adénine phosphoribosyltransferase deficiency: a case report. Indian J Urol. 2017;33:246–248. doi: 10.4103/iju.IJU_419_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sreejith P., Narasimnah K.L., Sakhuja V. 2,8 Dihydroxyadenine urolithiasis: a case report and review of literature. Indian J Nephrology. 2009;19:34–36. doi: 10.4103/0971-4065.50680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakanishi S., Saito R., Mizuno K. A case of bilateral renal calculi in a 1 yr old female with adenine phosphoribosyl transférase partial deficiency. Hinyokika Kiyo. 2011;52:551–554. [PubMed] [Google Scholar]
- 6.Runolfsdottir H.L., Palsson R., Agustsdottir I.M. Kidney disease in adenine phosphoribosyltransferase deficiency. Am J Kidney Dis. 2016;67:431–438. doi: 10.1053/j.ajkd.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daudon M., Bader C.A., Jungers P. Urinary calculi: review of classification methods and correlations with etiology. Scanning Microsc. 1993;7:1081–1104. discussion 1104–1106. [PubMed] [Google Scholar]
- 8.Thorsteinsdottir M., Thorssteinsdottir U.A., Eiriksson F.F. Quantitative UPLC–MS/MS assay of urinary 2,8-dihydroxyadenine for diagnosis and management of adenine phosphoribosyltransferase deficiency. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1036–1037:170–177. doi: 10.1016/j.jchromb.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edvardsson V.O., Runolfsdottir H.L., Thorsteinsdottir U.A. Comparison of the effect of allopurinol and febuxostat on urinary 2,8 dihydroxyadenine excretion in patients with APRT deficiency: a clinical trial. Eur J Intern Med. 2018;48:75–79. doi: 10.1016/j.ejim.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]